Abstract
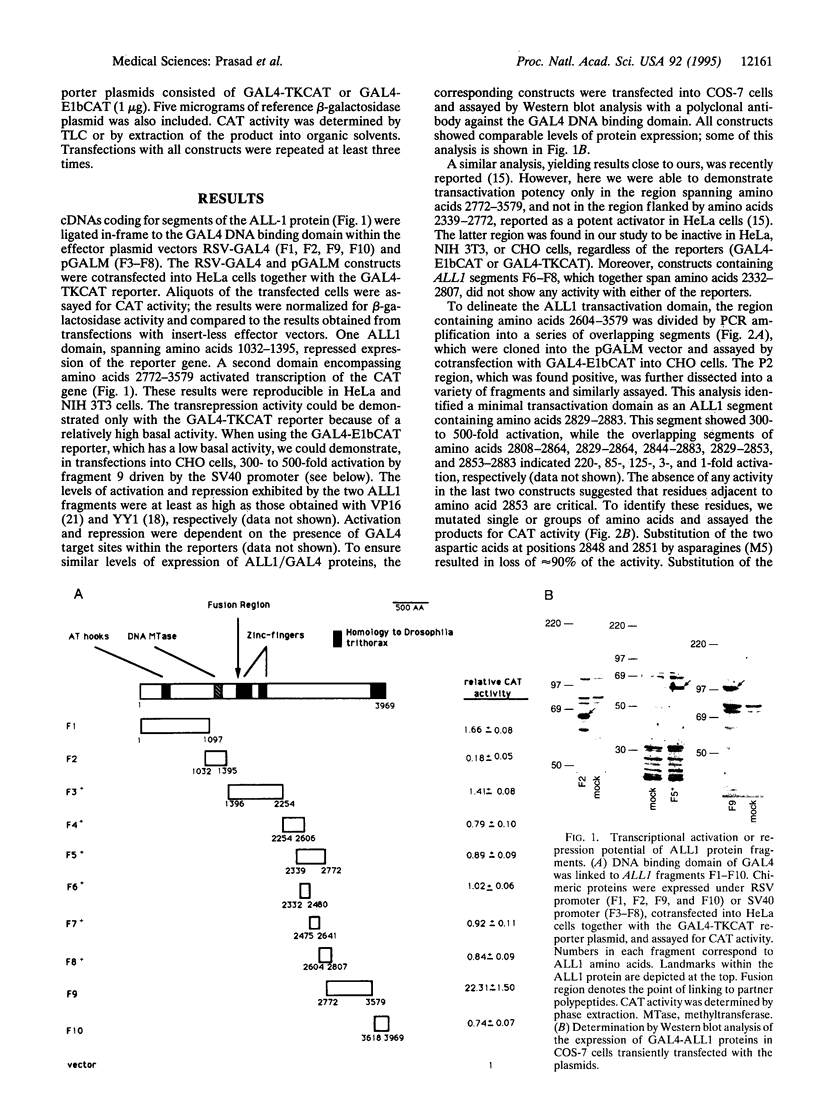
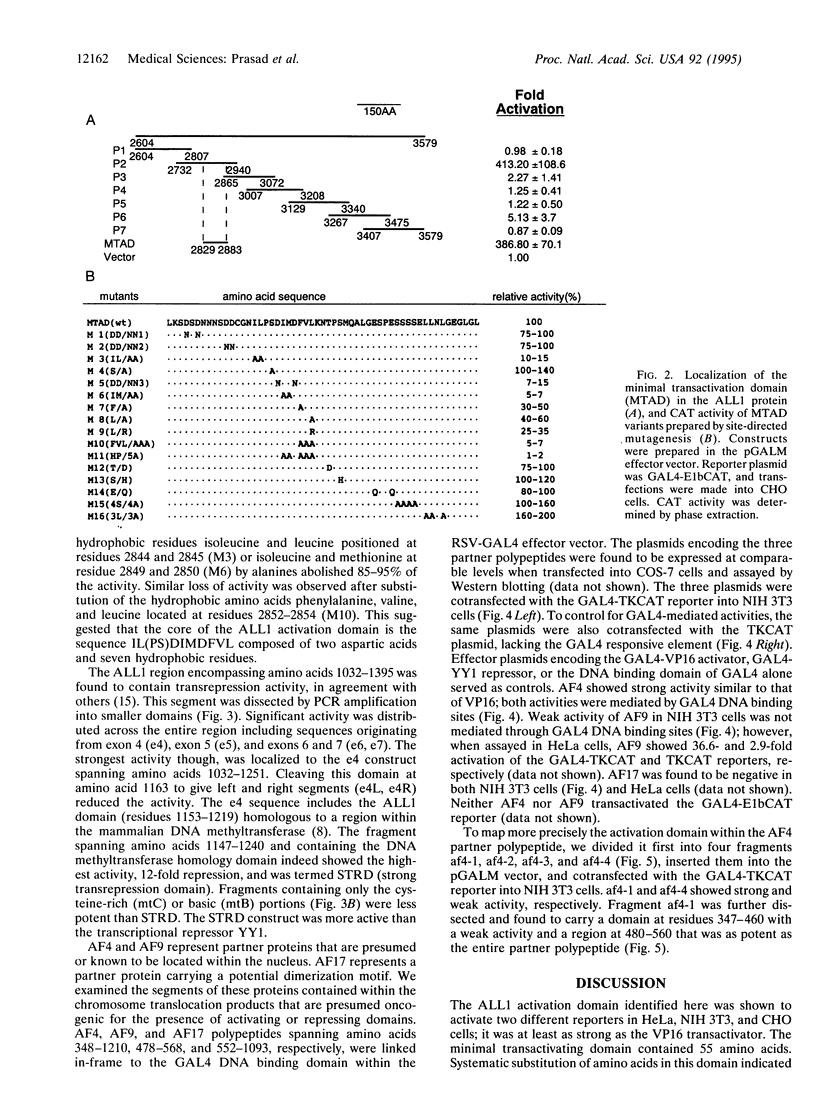
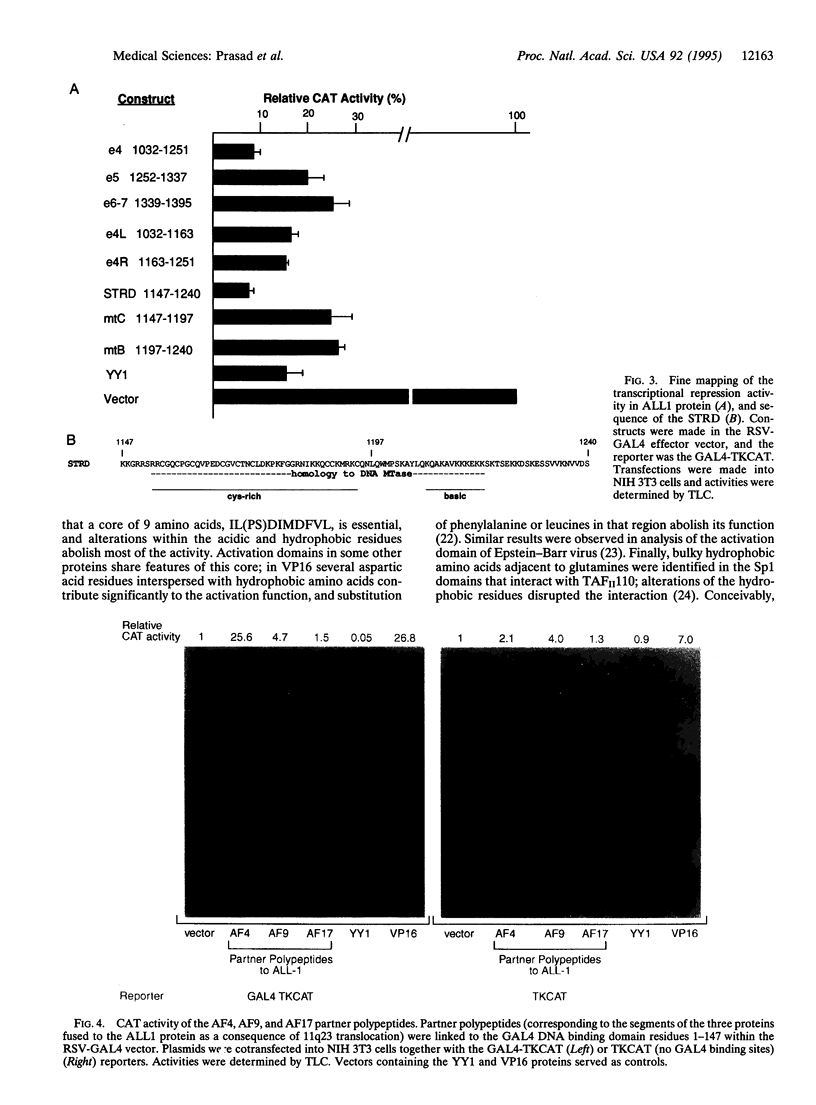
The ALLI gene, located at chromosome band 11q23, is involved in acute leukemia through a series of chromosome translocations and fusion to a variety of genes, most frequently to A4 and AF9. The fused genes encode chimeric proteins proteins. Because the Drosophila homologue of ALL1, trithorax, is a positive regulator of homeotic genes and acts at the level of transcription, it is conceivable that alterations in ALL1 transcriptional activity may underlie its action in malignant transformation. To begin studying this, we examined the All1, AF4, AF9, and AF17 proteins for the presence of potential transcriptional regulatory domains. This was done by fusing regions of the proteins to the yeast GAL4 DNA binding domain and assaying their effect on transcription of a reporter gene. A domain of 55 residues positioned at amino acids 2829-2883 of ALL1 was identified as a very strong activator. Further analysis of this domain by in vitro mutagenesis pointed to a core of hydrophobic and acidic residues as critical for the activity. An ALL1 domain that repressed transcription of the reporter gene coincided with the sequence homologous to a segment of DNA methyltransferase. An AF4 polypeptide containing residues 480-560 showed strong activation potential. The C-terminal segment of AF9 spanning amino acids 478-568 transactivated transcription of the reporter gene in HeLa but not in NIH 3T3 cells. These results suggest that ALL1, AF4, and probably AF9 interact with the transcriptional machinery of the cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronheim A., Shiran R., Rosen A., Walker M. D. The E2A gene product contains two separable and functionally distinct transcription activation domains. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8063–8067. doi: 10.1073/pnas.90.17.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Nowell P. C., Croce C. M. Molecular genetics of 11q23 chromosome translocations. Adv Cancer Res. 1995;66:213–234. doi: 10.1016/s0065-230x(08)60255-9. [DOI] [PubMed] [Google Scholar]
- Chaplin T., Ayton P., Bernard O. A., Saha V., Della Valle V., Hillion J., Gregorini A., Lillington D., Berger R., Young B. D. A novel class of zinc finger/leucine zipper genes identified from the molecular cloning of the t(10;11) translocation in acute leukemia. Blood. 1995 Mar 15;85(6):1435–1441. [PubMed] [Google Scholar]
- Djabali M., Selleri L., Parry P., Bower M., Young B. D., Evans G. A. A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nat Genet. 1992 Oct;2(2):113–118. doi: 10.1038/ng1092-113. [DOI] [PubMed] [Google Scholar]
- Gill G., Pascal E., Tseng Z. H., Tjian R. A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):192–196. doi: 10.1073/pnas.91.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Nakamura T., Alder H., Prasad R., Canaani O., Cimino G., Croce C. M., Canaani E. The t(4;11) chromosome translocation of human acute leukemias fuses the ALL-1 gene, related to Drosophila trithorax, to the AF-4 gene. Cell. 1992 Nov 13;71(4):701–708. doi: 10.1016/0092-8674(92)90603-a. [DOI] [PubMed] [Google Scholar]
- Hardwick J. M., Tse L., Applegren N., Nicholas J., Veliuona M. A. The Epstein-Barr virus R transactivator (Rta) contains a complex, potent activation domain with properties different from those of VP16. J Virol. 1992 Sep;66(9):5500–5508. doi: 10.1128/jvi.66.9.5500-5508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunger S. P., Tkachuk D. C., Amylon M. D., Link M. P., Carroll A. J., Welborn J. L., Willman C. L., Cleary M. L. HRX involvement in de novo and secondary leukemias with diverse chromosome 11q23 abnormalities. Blood. 1993 Jun 15;81(12):3197–3203. [PubMed] [Google Scholar]
- Kato G. J., Barrett J., Villa-Garcia M., Dang C. V. An amino-terminal c-myc domain required for neoplastic transformation activates transcription. Mol Cell Biol. 1990 Nov;10(11):5914–5920. doi: 10.1128/mcb.10.11.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt O., Grunert H. P., Hahn U. A general method for rapid site-directed mutagenesis using the polymerase chain reaction. Gene. 1990 Nov 30;96(1):125–128. doi: 10.1016/0378-1119(90)90351-q. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M. R. Transcription activation by the adenovirus E1a protein. Nature. 1989 Mar 2;338(6210):39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- Mitani K., Kanda Y., Ogawa S., Tanaka T., Inazawa J., Yazaki Y., Hirai H. Cloning of several species of MLL/MEN chimeric cDNAs in myeloid leukemia with t(11;19)(q23;p13.1) translocation. Blood. 1995 Apr 15;85(8):2017–2024. [PubMed] [Google Scholar]
- Parry P., Wei Y., Evans G. Cloning and characterization of the t(X;11) breakpoint from a leukemic cell line identify a new member of the forkhead gene family. Genes Chromosomes Cancer. 1994 Oct;11(2):79–84. doi: 10.1002/gcc.2870110203. [DOI] [PubMed] [Google Scholar]
- Prasad R., Leshkowitz D., Gu Y., Alder H., Nakamura T., Saito H., Huebner K., Berger R., Croce C. M., Canaani E. Leucine-zipper dimerization motif encoded by the AF17 gene fused to ALL-1 (MLL) in acute leukemia. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8107–8111. doi: 10.1073/pnas.91.17.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier J. L., Shen F., Triezenberg S. J. Pattern of aromatic and hydrophobic amino acids critical for one of two subdomains of the VP16 transcriptional activator. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):883–887. doi: 10.1073/pnas.90.3.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz J. E., Morrissey J., Savage P. A., Cleary M. L. ENL, the gene fused with HRX in t(11;19) leukemias, encodes a nuclear protein with transcriptional activation potential in lymphoid and myeloid cells. Blood. 1994 Sep 15;84(6):1747–1752. [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988 Oct 6;335(6190):563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Thirman M. J., Gill H. J., Burnett R. C., Mbangkollo D., McCabe N. R., Kobayashi H., Ziemin-van der Poel S., Kaneko Y., Morgan R., Sandberg A. A. Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations. N Engl J Med. 1993 Sep 23;329(13):909–914. doi: 10.1056/NEJM199309233291302. [DOI] [PubMed] [Google Scholar]
- Thirman M. J., Levitan D. A., Kobayashi H., Simon M. C., Rowley J. D. Cloning of ELL, a gene that fuses to MLL in a t(11;19)(q23;p13.1) in acute myeloid leukemia. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12110–12114. doi: 10.1073/pnas.91.25.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachuk D. C., Kohler S., Cleary M. L. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocations in acute leukemias. Cell. 1992 Nov 13;71(4):691–700. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- Tse W., Zhu W., Chen H. S., Cohen A. A novel gene, AF1q, fused to MLL in t(1;11) (q21;q23), is specifically expressed in leukemic and immature hematopoietic cells. Blood. 1995 Feb 1;85(3):650–656. [PubMed] [Google Scholar]
- Zeleznik-Le N. J., Harden A. M., Rowley J. D. 11q23 translocations split the "AT-hook" cruciform DNA-binding region and the transcriptional repression domain from the activation domain of the mixed-lineage leukemia (MLL) gene. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10610–10614. doi: 10.1073/pnas.91.22.10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemin-van der Poel S., McCabe N. R., Gill H. J., Espinosa R., 3rd, Patel Y., Harden A., Rubinelli P., Smith S. D., LeBeau M. M., Rowley J. D. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10735–10739. doi: 10.1073/pnas.88.23.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]






