Abstract
Following the 2009 and 2010 dengue-1 (DENV-1) outbreaks in Key West, FL, we used Florida Aedes aegypti (L.) mosquitoes and DENV-1 isolated from Key West in 2010 to test the hypothesis that if the 2009 and 2010 DENV-1 genome sequences are similar, then vertical transmission of DENV-1 from infected Ae. aegypti female mosquitoes to their eggs could have served as an interepidemic reservoir between outbreaks. We also investigated the ability of Florida Aedes albopictus (Skuse) mosquitoes to vertically transmit DENV-1. In addition, we determined the rates of infection and dissemination of these Florida mosquito species for DENV-1 and the effect of DENV-1 infection on oviposition success and number of mosquito eggs laid by females. Vertical transmission of DENV-1 was documented, with rates of 11.11% (2 out of 18) for Ae. albopictus and 8.33% (3 out of 36) for Ae. aegypti. Approximately 93% (111 out of 119) of Ae. aegypti that fed on DENV-1 in blood became infected, and 80% (89 out of 111) of infections were disseminated. Similarly, 93% of Ae. albopictus became infected (53 out of 57), and 85% (45 out of 53) of infections were disseminated. No significant differences were detected in numbers of eggs laid by either species after imbibing DENV-1 in blood, suggesting little cost of infection on number of eggs laid. Our results demonstrate that Florida Ae. aegypti and Ae. albopictus mosquitoes are competent vectors for DENV-1, whose maintenance between the 2009 and 2010 Key West outbreaks may have been facilitated by vertical transmission.
Keywords: interepidemic dengue transmission, arbovirus, container mosquito, cost of infection, fecundity
Dengue virus (DENV, family Flaviviridae, genus Flavivirus) is responsible for causing dengue fever and dengue hemorrhagic fever (DHF), currently the most important arthropod-borne (arbo) virus-associated diseases affecting humans. More than 40% of the world's population is at risk of acquiring a dengue infection, and the World Health Organization (WHO 2009) estimates that 50–100 million infections occur every year in tropical and subtropical regions. However, the results of a recent study conducted by Bhatt et al. (2013) suggest that the world's dengue burden may be closer to 390 million infections per year. In addition to its public health impact, the economic impact of dengue infections is staggering. For example, Shepard et al. (2011) estimated that the economic burden of dengue illness in the Americas alone from 2000 to 2007 was US$2.1 billion per year on average.
In the >100 tropical countries where DENV is endemic, temporal patterns of horizontal transmission between Aedes aegypti (L.) and Aedes albopictus (Skuse) mosquitoes, the primary DENV vectors, and humans vary (Gubler 2002, Adams and Boots 2010). Horizontal transmission may occur throughout the year, in sporadic outbreaks years apart, or yearly in a cyclical seasonal pattern (Adams and Boots 2010). In endemic areas where horizontal transmission is not apparent throughout the year, the ability of DENV to persist in the environment, even after long periods of few or no documented human cases, is not clearly understood. One mechanism that may allow DENV to remain in an environment during interepidemic periods is vertical transmission, the transfer of virions from an infected female mosquito to her progeny (Adams and Boots 2010). Infected eggs can potentially survive for months, and the female mosquitoes that hatch and develop from them are able to transmit DENV to humans during probing and blood-feeding events (Mourya et al. 2001).
The vertical transmission success of a pathogen such as DENV is a function of the number of infected offspring produced by a female mosquito and can be influenced by mosquito life history traits and mosquito–pathogen interactions (Agnew and Koella 1999). One such life history trait that can impact DENV vertical transmission success is the fecundity of an adult female mosquito, which is strongly influenced by adult size (Christophers 1960, Agnew and Koella 1999). In addition to being influenced by size, mosquito fecundity may also be influenced by interactions with pathogens. Specifically, DENV infection has been shown elsewhere to reduce Ae. aegypti fecundity (Joshi et al. 2002, Maciel–de-Freitas et al. 2011, Sylvestre et al. 2013). However, more studies are needed to better understand how mosquito–DENV interactions may affect mosquito life history traits such as fecundity and DENV vertical transmission success (Maciel–de-Freitas et al. 2011).
While DENV transmission primarily occurs in tropical regions, local transmission between Ae. aegypti and humans was documented within subtropical Key West in Monroe County, FL, in 2009 and 2010 for the first time in over 50 yr, resulting in 27 and 63 human cases in 2009 and 2010, respectively (Centers for Disease Control [CDC] 2010, Graham et al. 2011). In addition to the confirmed cases, a random serosurvey conducted in September 2009 suggested that ≈600– 1,000 Key West residents were infected with DENV during the 2009 outbreak (Radke et al. 2012). Following these outbreaks, we used Florida Ae. aegypti mosquitoes and DENV-1 isolated from Key West in 2010 to test the hypothesis that if 2009 and 2010 DENV-1 genome sequences are similar, then vertical transmission of DENV-1 from infected Ae. aegypti female mosquitoes to their eggs could have served as an inter-epidemic reservoir between outbreaks. We also investigated the ability of Florida Ae. albopictus (Skuse) mosquitoes to vertically transmit DENV-1, because although it is not currently known to be established in Monroe County, FL, this species is an important vector of DENV elsewhere in its range and is common throughout peninsular Florida (Richards et al. 2012). In addition, we determined the rates of infection and dissemination of these Florida mosquito species for DENV-1 and the effect of DENV-1 infection on ovi-position success and number of eggs laid by females.
Materials and Methods
Mosquitoes
Mosquitoes were reared in an insectary maintained at 24 ± 0.5°C and a photoperiod of 14:10 (L:D) h. The Ae. albopictus used were F5 progeny of larvae collected on the campus of the Florida Medical Entomology Laboratory (FMEL) in Vero Beach, FL. The Ae. aegypti used were F2 progeny of larvae collected from Key West, FL. To stimulate hatching, eggs on papers were added to enamel pans (24 by 36 by 5 cm) with 1.0 liter tap water and 0.2 g of 1:1 brewer's yeast and lactalbumin. After hatching, first instar larvae were redistributed to a density of 200 larvae per enamel pan. Supplemental resources (0.2 g of brewer's yeast and lactalbumin) were added to each pan every other day for ≈10 d until all individuals had pupated or died.
Pupae were transferred to water-filled cups and placed by species into 0.3-m3 adult cages. Pupae collected for three consecutive days were placed in one cage, which narrowed the ages of adult mosquitoes in each cage to within a 3-d period. These methods enabled control of adult age to ensure infectious bloodmeals were provided to similarly aged adult females. After emergence, male and female mosquitoes were kept within the cages for approximately 7 d to allow for mating. During this time, the mosquitoes were provided with 20% sucrose solution and water. Approximately 48 h before administering infectious bloodmeals, females were cold-anesthetized (4°C), transferred to 1-liter cylindrical cartons at a density of 100 per cage, and provided with water but not sucrose solution.
Oral Infection of Mosquitoes
Propagation of DENV-1 for bloodmeals followed previously established methods (Alto et al. 2008). Briefly, tissue culture flasks (175 cm2) with confluent monolayers of African green monkey kidney (Vero) cells were inoculated with 250 μl DENV-1 (strain BOL-KW010 originally isolated from a human infected in Key West, FL, in 2010 [Florida Department of Health] and passaged three times in African green monkey kidney [Vero] cells) at a multiplicity of infection (number of viruses to host cells) of 0.0004. After 1 h of incubation at 35°C and 5% CO2 atmosphere, 25 ml media (199 media, 10% fetal bovine serum, 0.2% antimycotic, and 2% penicillin–streptomycin) were added to each flask. After 7 d, media and virus were collected from flasks and combined with bovine blood containing sodium citrate as an anticoagulant (Hemostat, Dixon, CA) in a 1:1 ratio of media + virus:blood. Females that were 8–10 d old were given the opportunity to feed on DENV-1-infected blood using an artificial membrane feeding system (Hemotek, Accrington, United Kingdom) for ≈30 min. Samples of DENV-1-infected blood were taken just before feeding trials, and viral titers were determined later using quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). After the delivery of the infectious bloodmeal, females were cold-anesthetized, and fully engorged mosquitoes were housed together in cages. Females that did not initially feed were given subsequent opportunities to feed on infectious blood daily for the next 3 d.
Maintenance of Adults
During and after feeding on the infectious bloodmeal, females were held within incubators at 30 ± 0.5°C on a daily photoperiod of 14:10 (L:D) h. We deliberately chose to maintain adults during the feeding trials at a higher temperature, which increases rates of DENV-1 dissemination relative to cooler temperatures (Alto and Bettinardi 2013). After blood feeding, females were provided with 20% sucrose and water. Because vertical transmission of DENV in the first gonotrophic cycle is rare (Mourya et al. 2001), females were allowed to lay eggs during their first gonotrophic cycle, but those eggs were not assayed for virus. After the first oviposition, female mosquitoes were provided with a noninfectious bloodmeal to stimulate a second gonotrophic cycle. The noninfectious bloodmeal was given ≈12 d after the infectious bloodmeal, which approximates the length of the extrinsic incubation period for DENV at 30°C (Watts et al. 1987, Chan and Johansson 2012).
After the noninfectious bloodmeal, mosquitoes were cold-anesthetized and separated based on abdominal distension from a bloodmeal. Fully engorged mosquitoes were placed individually in 37-ml plastic tubes (h by d: 8 by 3 cm), which were covered with screen and lined internally with moist germination paper (h by w: 7 by 5 cm) as an oviposition site. A cotton ball soaked with 20% sucrose solution was daily placed on top of the screen of each tube, providing a carbohydrate source for each female. After females had laid eggs during their second gonotrophic cycle, they were individually stored in microcentrifuge tubes at –80°C. All eggs on germination papers were counted and held in an incubator at 85% relative humidity (RH) until the infection status of each adult female was determined.
Determination of Adult Infection Status
The legs of adult females were dissected from bodies, and the legs and body of each female were tested separately for the presence of DENV-1 RNA. The body and legs from each female mosquito were separately homogenized with a TissueLyser (Qiagen, Valencia, CA) in 2-ml flat-bottom vials containing 0.9 ml BA-1diluent and two zinc-plated steel BBs. The homogenized samples were then clarified by centrifugation (3,148 × g for 4 min at 4°C). A 250 μl aliquot was taken from each sample and dispensed into a microcentrifuge tube containing 250 μl of lysis buffer.
Total nucleic acids were extracted from each sample using a MagNA Pure Total Nucleic Acid kit (Roche Diagnostics, Chicago, IL) and a MagNA Pure LC robot (Roche Diagnostics). Following extraction of total nucleic acids, qRT-PCR was conducted on the samples using SuperScript III Platinum one step qRT-PCR (Invitrogen Company, Carlsbad, CA) and fluorogenic probe hydrolysis (TaqMan, Applied Biosystems, Foster City, CA) technology. Quantitative RT-PCR reactions were performed in 96-well reaction plates, with each containing 0.4 μl SuperScript III RT/Platinum Taq mix, 10 μl 2× reaction mix, 1 μl DENV-1-specific forward primer (10 μmol/liter), 1 μl DENV-1-specific reverse primer (10 μmol/liter), 0.4 μl fluorogenic probe (10 μmol/liter), and 2.2 μl H2O, and 5 μl sample. Amplification and quantification of DENV-1 RNA in positive samples were performed by running the 96-well reaction plate in a LC480 thermocycler machine (Roche Diagnostics, Chicago, IL) programmed for the following: 60°C for 30 min, 2 min at 95°C, followed by 40 cycles of PCR (15 s at 95°C and 1 min at 60°C), and 1 s at 45°C (Callahan et al. 2001). A negative control (water) and positive control standard (DENV-1, strain BOL-KW010 RNA, 10–2 dilution) were included on each 96-well reaction plate. The titer of each sample in plaque-forming unit equivalents (PFUeq) per milliliter was calculated by a standard curve method that compares the known titer of the positive standard control with the titer of unknown samples (Alto et al. 2008). DENV-1 RNA detected in the body but not in the legs of a female mosquito represented a nondisseminated infection. DENV-1 RNA detected in both the body and legs of a female was considered a disseminated infection (Turell et al. 1984).
Determination of Progeny Infection Status
Using only egg batches from females with a disseminated virus infection, hatching was stimulated by immersing germination papers with eggs and 0.1 g of brewer's yeast and lactalbumin to individual 0.47-liter water-filled plastic containers. The progeny from each fe male were provided with larval food every other day until they developed to late larval instars, then killed by freezing, pooled, and stored in a microcentrifuge tube at –80°C. The larval pools of progeny were then tested for the presence of DENV-1 RNA using the same methods that were used to test for the presence of DENV-1 RNA in the parental generation.
Statistical Analysis
All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC). We divided females into three categories of infection status (uninfected, nondisseminated infection, or disseminated infection) based on the presence or absence of DENV RNA in body and leg samples. The proportions of Ae. aegypti and Ae. albopictus adult female mosquitoes in each infection category were compared using maximum likelihood categorical analyses of contingency tables (PROC CATMOD, SAS 1999). As data transformations did not satisfy requirements for parametric statistics, the effects of species and infection category on body titer were analyzed using a nonparametric Kruskal–Wallis test. Significant effects were further analyzed by all possible pairwise comparisons of distributions using nonparametric Wilcoxon two-sample tests (α value adjusted for multiple comparisons using the sequential Bonferroni method [Rice 1989]). The proportions of females in each infection category that oviposited were compared using categorical data modeling. In addition, the effects of species and infection stage on number of eggs laid were analyzed using a Kruskal–Wallis test. Spearman nonparametric correlations were used to examine the relationships between fecundity, species, body titer, and infection category.
Results
Viral Infection and Dissemination
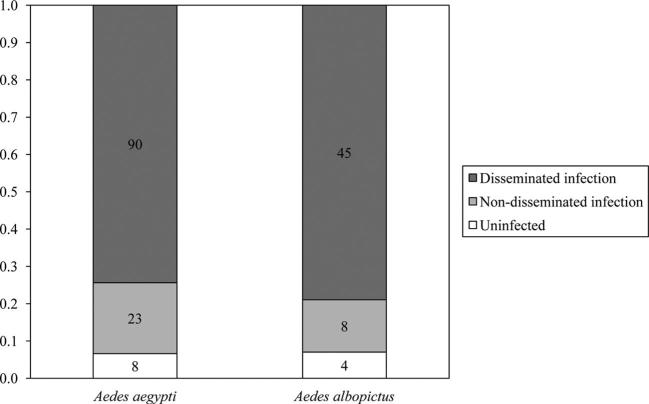
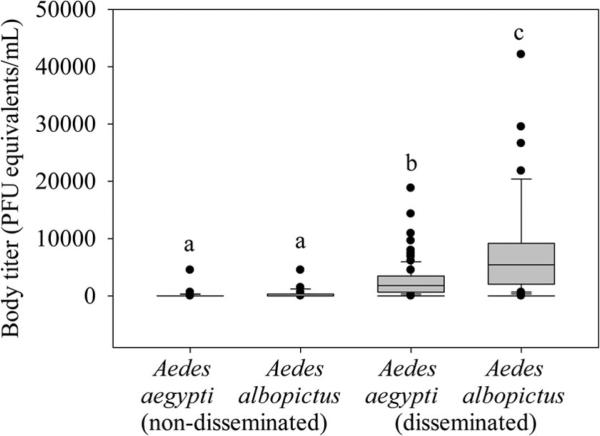
The mean viral titer of the infectious bloodmeals provided to mosquitoes was 6.3 ± 0.2 Log10 PFUeq DENV/ml, which is within the range of viremia levels experienced by infected humans (e.g., Gubler et al. 1981, Vaughn et al. 2000, Stramer et al. 2012). Approximately 93% (111 out of 119) of exposed Ae. aegypti mosquitoes were determined as infected by qRT-PCR, and 80% (89 out of 111) of those infections were disseminated (Table 1). The same percentage (93%) of exposed Ae. albopictus mosquitoes were shown to be infected by qRT-PCR (53 out of 57), and 85% (45 out of 53) of those infections were disseminated (Table 1). The proportions of Ae. aegypti and Ae. albopictus mosquitoes in each infection category (uninfected, nondisseminated infection, and disseminated infection) did not significantly differ (χ2 = 0.66; df = 2; P = 0.72; Fig. 1). However, body viral titer was significantly affected by species and infection category (χ2 = 68.90; df = 3; P < 0.01). The viral titer in the bodies of all mosquitoes with disseminated infections was significantly higher than the viral titer in the bodies of mosquitoes with non-disseminated infections, regardless of species (Fig. 2). The viral titer in the bodies of Ae. albopictus mosquitoes with disseminated infections was significantly higher than that of Ae. aegypti mosquitoes with disseminated infections (Fig. 2).
Table 1.
The mean titers (Log10 PFUeq DENV/ml ± SE) and rates of infection (% with DENV-positive bodies), dissemination (% infected with DENV-positive legs), and vertical transmission (% of females with disseminated infections and DENV-positive progeny) for Ae. aegypti and Ae. albopictus fed DENV-1-infected blood
| Species | No. adults tested | No. body infected (%) | No. leg infected (%) | No. progeny pools tested | No. progeny pools infected (%) | Body titer | Leg titer | Progeny titer |
|---|---|---|---|---|---|---|---|---|
| Ae. aegypti | 119 | 111 (93) | 89 (80) | 36 | 3 (8) | 2.5 ± 0.1 | 1.5 ± 0.1 | 1.8 ± 1.5 |
| Ae. albopictus | 57 | 53 (93) | 45 (85) | 18 | 2 (11) | 3.1 ± 0.2 | 1.8 ± 0.1 | 1.6 ± 0.6 |
Fig. 1.
The proportions of Ae. aegypti and Ae. albopictus mosquitoes in each infection category (uninfected, nondisseminated infection, or disseminated infection), after imbibing DENV-1-infected blood, which did not significantly differ (χ2 = 0.66; df = 2; P = 0.72).
Fig. 2.
Medians with 75 and 25 quartiles (±SE) for body titer of Ae. aegypti and Ae. albopictus mosquitoes with non-disseminated and disseminated infections. Different letters indicate significant differences determined by Wilcoxon two-sample tests with Bonferroni corrections (all P ≤ 0.0003).
Vertical Transmission
DENV-1 vertical transmission by both Aedes species was documented, under laboratory conditions, with rates of 11.11% (2 out of 18) for Ae. albopictus and 8.33% (3 out of 36) for Ae. aegypti (Table 1).
Oviposition
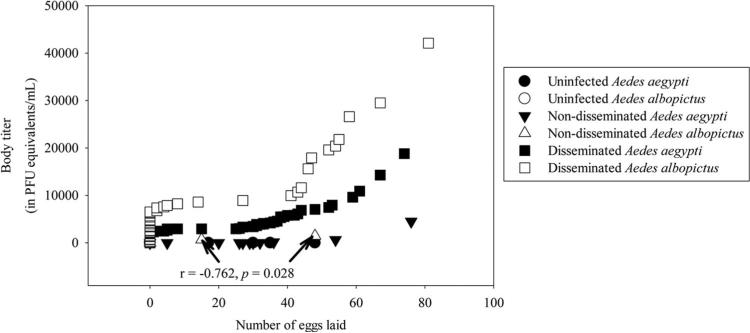
In total, 51 Ae. aegypti and 20 Ae. albopictus mosquitoes laid eggs. Regardless of species, categorical data modeling showed that oviposition was not significantly associated with infection category (uninfected, nondisseminated infection, and disseminated infection). A Kruskal–Wallis test revealed no significant differences in numbers of eggs laid by females in each infection category (χ2 = 3.79; df = 5; P = 0.58). The only significant Spearman nonparametric correlation was a negative relationship between the body titer of Ae. albopictus mosquitoes with nondisseminated infections and number of eggs laid (r = –0.76; P = 0.03) (Fig. 3).
Fig. 3.
Relationship between body titer and number of eggs laid by infected Ae. aegypti and Ae. albopictus mosquitoes with detectable levels of DENV-1 RNA (crossing point ≤35). Spearman nonparametric correlations between fecundity, species, body titer, and infection category were statistically not significant (P ≥ 0.17), except for a negative relationship between the body titer of Ae. albopictus mosquitoes with nondisseminated infections and number of eggs laid (r =–0.76, P = 0.03).
Discussion
The results of this study show that Florida Ae. aegypti and Ae. albopictus mosquitoes are competent vectors of the DENV-1 strain isolated from Key West, FL. Richards et al. (2012) is the only prior study that has attempted to evaluate the vector competence of Florida mosquitoes for DENV-1. The results of the current study, which was executed with larger sample sizes than the prior study, are in agreement with the results of Richards et al. (2012) and therefore affirm conclusions made by the authors about the vector competence of Florida Ae. aegypti and Ae. albopictus mosquitoes for DENV-1. Interestingly, the collective results from our study and Richards et al. (2012) suggest that Florida Ae. albopictus mosquitoes could be as or potentially even more competent than Florida Ae. aegypti mosquitoes, because viral infection rates were similar in both species but dissemination rates were higher in Ae. albopictus. In addition, the body titer of Florida Ae. albopictus mosquitoes with disseminated infections was statistically significantly higher than their Ae. aegypti cohorts in our study. The vector competence of Florida Ae. albopictus may potentially prove important in future DENV outbreaks because of the wide geographic range of Ae. albopictus and the projected altered distribution of DENV because of climate change (Erickson et al. 2012).
In addition to vector competence, the reproductive output of a mosquito population can also impact local arbovirus transmission dynamics by affecting mosquito population size. Therefore, any pathogen-induced changes in the fecundity of an infected mosquito could have an important bearing on transmission dynamics (Hurd et al. 1995). We found that horizontally transmitted DENV-1 infection had no significant effect on the number of eggs laid by Ae. albopictus and Ae. aegypti compared with those that fed on DENV-1-infected blood but did not become infected. These results suggest that while DENV-1-infected female mosquitoes bear the burden of the metabolic demands of developing virions in addition to their own reproductive effort (Hurd et al. 1995), DENV-1 infections may not have a significant negative impact on number of eggs laid and potentially mosquito population size and vectorial capacity. However, the current study did not expose females to bloodmeals lacking DENV-1. Thus, we are unable to determine whether ingestion of DENV-1-infected blood may elicit an immune response that may be metabolically costly and reduce the number of eggs laid. That is, exposure to virus even in the absence of any infection may come at a metabolic cost (Schmid–Hempel 2005). For example, Maciel–de-Freitas et al. (2011) and Sylvestre et al. (2013) reported that dengue-2 virus (DENV-2) exposed Ae. aegypti resulting in infected and uninfected mosquitoes were less fecund than mosquitoes fed blood lacking DENV-2 (controls).
While studies on the impact of DENV infection on mosquito fecundity are limited, multiple studies have shown that vertical transmission of DENV by Ae. aegypti and Ae. albopictus mosquitoes is possible both in nature and experimental studies in the laboratory (Shroyer 1990, Bosio et al. 1992, Joshi et al. 2002, de Castro et al. 2004, Guo et al. 2007, Angel and Joshi 2008, Figueiredo et al. 2010, Le Goff et al. 2011). The average DENV vertical transmission rates recorded in prior experimental studies have been ≈1% for Ae. albopictus mosquitoes and 3% for Ae. aegypti mosquitoes (Adams and Boots 2010). Vertical transmission rates of 11% for Ae. albopictus mosquitoes and 8% for Ae. aegypti mosquitoes in our experiment may, in part, be higher than rates previously documented, because we waited 12 d after providing mosquitoes with a DENV-infected bloodmeal before providing an uninfected bloodmeal to stimulate production of second gonotrophic cycle eggs. Furthermore, our higher rates of DENV vertical transmission may be because of the greater sensitivity of our virus detection method, qRT-PCR, compared with other methods of virus detection. However, the vertical transmission rates documented in our study should be interpreted cautiously because of the small number of mosquitoes that survived through their second gonotrophic cycle and comprised the vertical transmission sample sizes. In addition, laboratory experiments cannot truly duplicate the complex interactions that occur between the environment, mosquitoes, and viruses in nature (Saiyasombat et al. 2011, Richards et al. 2012). Nonetheless, our findings are potentially significant in light of results of sequencing performed by Munoz–Jordan and others (2013), which revealed that the 2009 and 2010 Key West DENV-1 strains are highly genetically similar and suggest that the 2010 cases were a continuation of the 2009 outbreak. Given the findings of Munoz–Jordan et al. (2013), our study shows that vertical transmission of DENV-1 from infected Ae. aegypti female mosquitoes to their eggs may have served as an interepidemic reservoir between outbreaks in successive years in Key West, FL. Furthermore, vertical transmission of DENV-1 from infected Ae. aegypti and Ae. albopictus females to their progeny may potentially serve as interepidemic reservoirs between any future outbreaks within the continental United States.
Acknowledgments
Dengue-1 virus (strain BOL-KW010) was kindly provided by the Florida Department of Health Bureau of Laboratories. We kindly thank three anonymous reviewers, whose comments greatly improved this manuscript. The research was funded by Florida Department of Agricultural and Consumer Services Project No. 00090369 and National Institutes of Health grant R01 (AI)-044793.
References Cited
- Adams B, Boots M. How important is vertical transmission in mosquitoes for the persistence of dengue? Insights from a mathematical model. Epidemics. 2010;2:1–10. doi: 10.1016/j.epidem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Agnew P, Koella JC. Constraints on the reproductive value of vertical transmission for a microsporidian parasite and its female-killing behaviour. J. Anim. Ecol. 1999;68:1010–1019. [Google Scholar]
- Alto B, Bettinardi D. Temperature and dengue virus infection in mosquitoes: independent effects on the immature and adult stages. Am. J. Trop. Med. Hyg. 2013;88:497–505. doi: 10.4269/ajtmh.12-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Lounibos LP, Mores CN, Reiskind MH. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc. R. Soc. B Biol. Sci. 2008;275:463–471. doi: 10.1098/rspb.2007.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel B, Joshi V. Distribution and seasonality of vertically transmitted dengue viruses in Aedes mosquitoes in arid and semi-arid areas of Rajasthan, India. J. Vector Borne Dis. 2008;45:56–59. [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosio C, Thomas R, Grimstad P, Rai K. Variation in the efficiency of vertical transmission of dengue-1 virus by strains of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 1992;29:985–989. doi: 10.1093/jmedent/29.6.985. [DOI] [PubMed] [Google Scholar]
- Callahan J, Wu S, Dion–Schultz AB, Mangold L, Peruski D, Watts K, Porter G, Murphy W, Suharyono C, King, et al. Development and evaluation of serotypeand group-specific fluorogenic reverse transcriptase PCR (TaqMan) assays for dengue virus. J. Clin. Microbiol. 2001;39:4119–4124. doi: 10.1128/JCM.39.11.4119-4124.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (CDC) Centers for Disease Control Locally acquired dengue-Key West, Florida, 2009–2010. Morb. Mortal Wkly. Rep. 2010;59:577–581. [PubMed] [Google Scholar]
- Chan M, Johansson MA. The incubation periods of dengue viruses. PLoS ONE. 2012;7:e50972. doi: 10.1371/journal.pone.0050972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophers SR. Aedes aegypti (L.), the Yellow Fever Mosquito; its life history, bionomics, and structure. University Press; Cambridge, England, United Kingdom: 1960. [Google Scholar]
- de Castro MC, Nogueira RMR, Schatzmayr HG, Miagostovich MP, Lourenco-de-Oliveira R. Dengue virus detection by using reverse transcription-polymerase chain reaction in saliva and progeny of experimentally infected Aedes albopictus from Brazil. Mem. Inst. Oswaldo Cruz. 2004;99:809–814. doi: 10.1590/s0074-02762004000800005. [DOI] [PubMed] [Google Scholar]
- Erickson RA, Hayhoe K, Presley SM, Allen LJS, Long KR, Cox SB. Potential impacts of climate change on the ecology of dengue and its mosquito vector the Asian tiger mosquito (Aedes albopictus). Environ. Res. Lett. 2012;7:034003. [Google Scholar]
- Figueiredo M, Gomes A, Amarilla A, Leandro A, Or-rico A, de Araujo R, Castro J, Durigon E, Aquino V, Figueiredo L. Mosquitoes infected with dengue viruses in Brazil. Virol. J. 2010;7:152. doi: 10.1186/1743-422X-7-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AS, Pruszynski CA, Hribar LJ, DeMay DJ, Tambasco AN, Hartley AE, Fussell EM, Michael SF, Isern S. Mosquito-associated dengue virus, Key West, Florida, USA, 2010. Emerg. Infect. Dis. 2011;17:2074–2075. doi: 10.3201/eid1711.110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- Gubler DJ, Suharyono W, Tan R, Abidin M, Sie A. Viremia in patients with naturally acquired dengue infection. Bull. W.H.O. 1981;59:623–630. [PMC free article] [PubMed] [Google Scholar]
- Guo XX, Zhao TY, Dong YD, Lu BL. Survival and replication of dengue-2 virus in diapausing eggs of Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2007;44:492–497. doi: 10.1603/0022-2585(2007)44[492:sarodv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Hurd H, Hogg JC, Renshaw M. Interactions between blood feeding, fecundity and infection in mosquitoes. Parasitol. Today. 1995;11:411–416. [Google Scholar]
- Joshi V, Mourya DT, Sharma RC. Persistence of dengue-3 virus through transovarial transmission passage in successive generations of Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg. 2002;67:158–161. doi: 10.4269/ajtmh.2002.67.158. [DOI] [PubMed] [Google Scholar]
- Le Goff G, Revollo J, Guerra M, Cruz M, Simon Z, Roca Y, Flores J, Herve J. Natural vertical transmission of dengue viruses by Aedes aegypti in Bolivia. Parasite. 2011;18:277–280. doi: 10.1051/parasite/2011183277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel–de-Freitas R, Koella JC, Lourenco–de-Oliveira R. Lower survival rate, longevity and fecundity of Aedes aegypti (Diptera: Culicidae) females orally challenged with dengue virus serotype 2. Trans. R. Soc. Trop. Med. Hyg. 2011;105:452–458. doi: 10.1016/j.trstmh.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Munoz–Jordan JL, Santiago GA, Margolis H, Stark L. Genetic relatedness of dengue viruses in Key West, Florida, USA, 2009–2010. Emerg. Infect. Dis. 2013;19:652–654. doi: 10.3201/eid1904.121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourya D, Gokhale M, Basu A, Barde P, Sapkal G, Padbidri V, Gore M. Horizontal and vertical transmission of dengue virus type 2 in highly and lowly susceptible strains of Aedes aegypti mosquitoes. Acta Virol. 2001;45:67–71. [PubMed] [Google Scholar]
- Radke EG, Gregory CJ, Kintziger KW, Sauber–Schatz EK, Hunsperger EA, Gallagher GR, Barber JM, Biggerstaff BJ, Stanek DR, Tomashek KM, et al. Dengue outbreak in Key West, Florida, USA, 2009. Emerg. Infect. Dis. 2012;18:135–137. doi: 10.3201/eid1801.110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Richards SL, Anderson SL, Alto BW. Vector competence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) for dengue virus in the Florida Keys. J. Med. Entomol. 2012;49:942–946. doi: 10.1603/me11293. [DOI] [PubMed] [Google Scholar]
- Saiyasombat R, Bolling B, Brault A, Bartholomay L, Blitvich B. Evidence of efficient transovarial transmission of Culex Flavivirus by Culex pipiens (Diptera: Culicidae) J. Med. Entomol. 2011;48:1031–1038. doi: 10.1603/me11043. [DOI] [PubMed] [Google Scholar]
- SAS Institute . 8th ed. SAS Institute; Cary, NC: 1999. The CATMOD procedure user's guide. [Google Scholar]
- Schmid–Hempel P. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 2005;50:529–551. doi: 10.1146/annurev.ento.50.071803.130420. [DOI] [PubMed] [Google Scholar]
- Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. Am. J. Trop. Med. Hyg. 2011;84:200–207. doi: 10.4269/ajtmh.2011.10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroyer D. Vertical maintenance of dengue-1 virus in sequential generations of Aedes albopictus. J. Am. Mosq. Control Assoc. 1990;6:312–314. [PubMed] [Google Scholar]
- Stramer SL, Linnen JM, Carrick JM, Foster GA, Krysztof DE, Zou SM, Dodd RY, Tirado–Marrero LM, Hunsperger E, Santiago GA, et al. Dengue viremia in blood donors identified by RNA and detection of dengue transfusion transmission during the 2007 dengue outbreak in Puerto Rico. Transfusion. 2012;52:1657–1666. doi: 10.1111/j.1537-2995.2012.03566.x. [DOI] [PubMed] [Google Scholar]
- Sylvestre G, Gandini M, Maciel–de-Freitas R. Age-dependent effects of oral infection with dengue virus on Aedes aegypti (Diptera: Culicidae) feeding behavior, survival, oviposition success and fecundity. PLoS ONE. 2013;8:e59933. doi: 10.1371/journal.pone.0059933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turell MJ, Gargan TP, Bailey CL. Replication and dissemination of Rift Valley fever virus in Culex pipiens. Am. J. Trop. Med. Hyg. 1984;33:176–181. doi: 10.4269/ajtmh.1984.33.176. [DOI] [PubMed] [Google Scholar]
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, Suntayakorn S, Endy TP, Raengsakulrach B, Rothman AL, Ennis FA, et al. Dengue viremia titer, antibody response pattern, and virus sero-type correlate with disease severity. J. Infect. Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue-2 virus. Am. J. Trop. Med. Hyg. 1987;36:143–152. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- (WHO) World Health Organization Dengue and Dengue Haemorrhagic Fever. Fact sheet N°117. 2009 ( http://www.who.int/mediacentre/factsheets/fs117/en/)