Abstract
Although cerebellar alterations have been consistently noted in the addiction literature, the pathophysiology of this link remains unclear. The cerebellum is commonly classified as a motor structure, but human functional neuroimaging along with clinical observations in cerebellar stroke patients and anatomical tract tracing in non-human primates suggest its involvement in cognitive and affective processing. A comprehensive literature search on the role of the cerebellum in addiction was performed. This review article (1) considers the potential role of the cerebellum in addiction, (2) summarizes the cerebellar structural alterations linked to addiction, (3) presents the functional neuroimaging evidence linking the cerebellum with addiction, and (4) proposes a model for addiction that underscores the role of the cerebellum. The data implicate the cerebellum as an intermediary between motor and reward, motivation and cognitive control systems, as all are relevant etiologic factors in addiction. Furthermore, consideration of these findings could contribute to deeper and more sophisticated insights into normal reward and motivational function. The goal of this review is to spread awareness of cerebellar involvement in addictive processes, and to suggest a preliminary model for its potential role.
Keywords: PET, MRI, craving, opioids, cocaine, marijuana, alcohol
1. Introduction
Over the past two decades, a convergence of clinical (Manto, 2012; Phillips et al., 1987) and basic research investigations of addictive substances (Kühn et al., 2012; Miquel et al., 2009; Strick et al., 2009; Sullivan et al., 2000; Volkow et al., 1997) have implicated the cerebellum in addiction. Though cerebellar findings are frequent in such studies, they are often marginalized, as the structure is not typically thought of as having a key role in addiction. In more extreme cases, the cerebellum is not even captured in some neuroimaging studies, eliminating it entirely from scientific consideration. With the availability of freely available cerebellar spatial registration software (Diedrichsen et al., 2011; Diedrichsen et al., 2010), improved acquisition parameters designed to capture the whole brain (Uğurbil et al., 2013), and cerebellar neuroimaging atlases (Diedrichsen et al., 2009; Diedrichsen, 2006), few barriers remain to consider significant imaging effects often found in this structure. This paper will review the structural and functional neuroimaging literature that implicates the cerebellum in addiction, and will discuss these findings in the context of contemporary theories of affective and cognitive cerebellar function. Although the cerebellum has received little attention in the context of addiction, this review turns the focus towards the cerebellum to highlight its potential involvement. A model for addiction will be presented, that features the cerebellum as a key modulator of behavior.
The role the cerebellum may play in addiction may be inferred from its involvement in non-motor function, which has been extensively reviewed (Ramnani, 2012; Schmahmann, 2004; Stoodley, 2012; Strick et al., 2009). In brief, the cerebellum receives extensive afferent input from the pontine nuclei, which receive input from prefrontal and association cortices in the cerebrum. Lesions to the posterior cerebellar hemispheres can lead to cerebellar cognitive affective syndrome, which features higher-level deficits beyond motor planning and execution. Resting state neuroimaging has shown that the cerebellum contains a variety of functional networks associated with many parts of the neocortex, beyond motor areas. fMRI studies have shown that the cerebellum also activates with a variety of functions, even those unassociated with a motor component. Cerebellar mechanisms of addictive drug action have been previously reviewed in the context of molecular and cellular investigations (Miquel et al., 2009). Collectively, the evidence suggests that the cerebellum may be intertwined with brain processes related to addiction, such as reward, motivational drive, saliency, inhibitory control, and insight. As summarized here, morphological and functional studies on human subjects with compulsive drug abuse indicate that the cerebellum is prominently involved in addiction.
2. Gross cerebellar anatomy
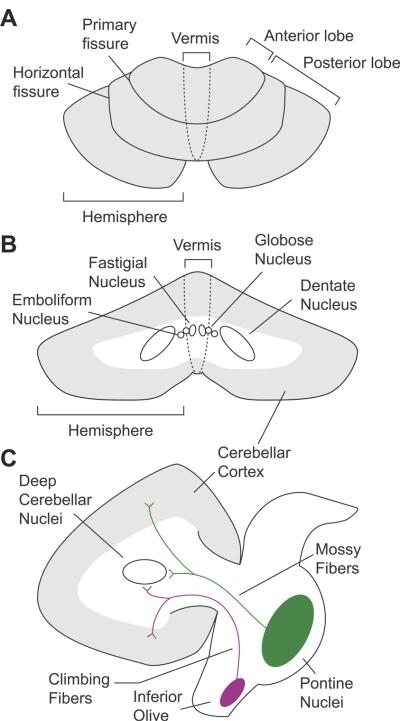
In the human, the cerebellum consists of two hemispheres connected by a midline structure, the vermis (Figure 1). These hemispheres are divided into lobules, with the primary fissure defining anterior and posterior lobes. The lobule immediately anterior to the horizontal fissure is identified as crus I, with crus II on the opposite side of the fissure. Like the cerebral cortex, the cerebellum has an outer cortical layer made up of gray matter, and with an inner layer of white matter scaffolding. The white matter tracts appear like stalks of broccoli, with the highly convoluted cortical gray matter extruding as if they were leaves, leading to the term cerebellar folia. The cerebellar peduncles form a thick trunk of white matter that connects the cerebellum to the brainstem. The cerebellar cortex has a highly conserved lattice structure consisting of three layers (Molecular, Purkinje cell, and Granular layers) with different cytoarchitectural features. Purkinje cells are inhibitory neurons that project to the deep cerebellar neurons, and regulate their efferent signals output to the brainstem and cerebral cortex. Currently, standard neuroimaging methods are able to differentiate gray and white matter macrostructure, and can define the paths of white matter tracts.
Figure 1.
Cerebellar anatomy. A) Top-down axial view. B) Coronal view of the deep cerebellar nuclei. C) Sagittal view highlighting the major afferent pathways into the cerebellum. Mossy fibers project to granule cells in the cerebellar cortex, and send collaterals to the deep cerebellar nuclei. Climbing fibers extend to Purkinje cells, and also have collateral projections to deep cerebellar nuclei. The axons of the deep cerebellar nuclei form the primary output channels away from the cerebellum, and to the brainstem and cerebral cortex.
The cerebellum receives input through two major brainstem afferent relays, the inferior olive and the pontine nuclei, through the climbing fibers and mossy fiber pathways respectively (Figure 1). The climbing fiber pathway enters the cerebellum through the inferior cerebellar peduncle, and the mossy fiber pathway enters through the middle cerebellar peduncle. Both pathways convey projections from the cerebral cortex to the cerebellum, with the pontine nuclei receiving a larger proportion of input from non-motor cortical regions (please see (Schmahmann, 1996) for detailed review), but also showing reciprocal connections with the basal ganglia (Bostan & Strick, 2010).
3. Cerebellar mechanisms related to addiction
Cerebellar function has traditionally been ascribed to motor coordination, including postural control, eye movements, motor learning, and time keeping associated with motor tasks. In this capacity, the cerebellum receives sensory inputs from the spinal cord and integrates them with cortical inputs to execute complex motor tasks (Courchesne & Allen, 1997; Doyon et al., 2003; Ito, 2006; Ivry, 1997). Dysmetria, a symptom of cerebellar dysfunction, reflects an inability to execute intended motor tasks due to a lack of sensory-motor coordination. The cerebellum's role in motor learning, as studied in the context of classical conditioning, has also been extended to include reinforcement learning and the establishment of motivational behavior (Bauer et al., 2011; Swain et al., 2011; Thoma et al., 2008).
The cerebellum has been hypothesized to have a single generalizable function (D'Angelo & Casali, 2012; Ito, 2006; Schmahmann, 2004), given its strikingly uniform arrangement of neurons. This singular function has been theorized to be modulation, which would extend to different domains based on the variety of functional inputs the cerebellum receives. This theory suggests that the cerebellum could optimize performance by modulating behavior according to context, acting as an oscillation dampener. For example, the cerebellum may modulate emotional processes by integrating positive and negative affective inputs in the same way that it modulates fine motor control by integrating sensory inputs. Different parts of the cerebellum receive inputs from different parts of the brain, leading to a functional topography within the cerebellum that may reflect compartmentalization of modulatory processes (Stoodley & Schmahmann, 2009). For example, depending on the location, cerebellar lesions can result not only in movement disorders such as ataxia and dysmetria, but also disrupted affect and cognitive ability (Schmahmann & Sherman, 1998; Schmahmann, 1991; Schmahmann, 2004). Similarly, cerebellar abnormalities are observed in patients with obsessive-compulsive disorder and attention deficit hyperactivity disorder (Seidman et al., 2011; Zarei et al., 2011). Given their relationship with cerebellar abnormalities, comorbidities between these disorders and substances of abuse may produce interactions that manifest in the cerebellum.
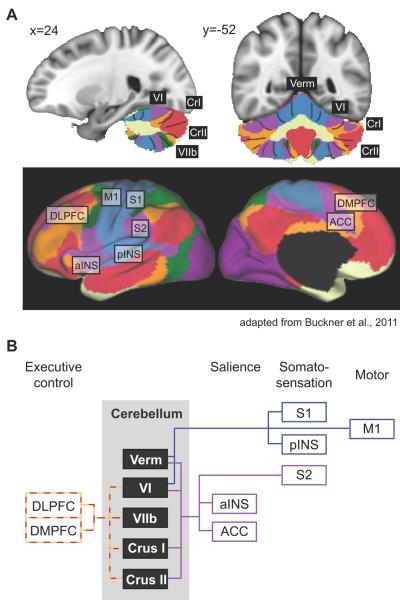
The cerebellar areas Crus I, Lobule VI, and VIIb are associated with functional resting state networks (RSNs) that are related to executive and associative processing in the cerebral cortex (Buckner et al., 2011). RSNs represent coherent signal fluctuations that are measured across different networks at rest that imply functional relationships between brain regions (Figure 2). In a dataset of 1000 healthy subjects, Buckner and colleagues identified functional connectivity between the cerebellum and 7 major RSNs in the cerebrum. Crus I, Lobule VI, and VIIb were all correlated with brain regions related to cognitive control, such as the dorsolateral prefrontal cortex and the dorsomedial prefrontal cortex. Functional correlations between these areas in the cerebellum and the prefrontal cortex have also been observed in other functional connectivity studies using fewer subjects (Habas et al., 2009; Krienen & Buckner, 2009; O'Reilly et al., 2010; Sang et al., 2012), which complements anatomical tracer studies in non-human primates that show reciprocal connections between these areas (Kelly & Strick, 2003; Middleton & Strick, 1994, 2001). Furthermore, both Crus I and Lobule VI were correlated with an RSN that included the anterior insula and anterior cingulate cortex, structures related to stimulus salience (Habas et al., 2009; Mouraux et al., 2011) and, in the case of the insula, interoception (Craig, 2009). The cerebellum may play an influential modulatory role in reward/saliency, as it also shares reciprocal connections with dopaminergic systems in the basal ganglia (Bostan & Strick, 2010). The functional correlations between these cerebellar areas with cerebral structures related to executive control, drug craving, response selection, and salience (Goldstein & Volkow, 2002, 2011; Habas et al., 2009; Volkow et al., 2010) further highlight the cerebellum's potential role in addiction.
Figure 2. Cerebellar functional connectivity to cerebral resting state networks.
A) Colors in the cerebellum correspond to areas functionally correlated to cerebral resting state networks. Coordinates correspond to MNI atlas space. Figure adapted from Buckner et al., 2011. B) Schematic of cerebral connectivity in cerebellar areas with gray matter deficits in addiction, and fMRI activation in response to acute drug exposure and drug craving. Colored lines correspond to the colored resting state networks in panel A. Each cerebral area is classified based on its contribution to different functional processes (i.e. cognitive control, salience, etc.). ACC, anterior cingulate cortex; aINS, anterior insula; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; M1, primary motor cortex; pINS, posterior insula; S1, primary somatosensory cortex; S2, secondary somatosensory cortex.
Addiction includes a breakdown in motivational homeostasis, a disruption in reward and aversive processing and their modulation (Koob, 1996). Recent functional neuroimaging findings indicate that the cerebellum may modulate motivational processing (Moulton et al., 2011). In healthy volunteers, aversive heat and unpleasant pictures activated overlapping areas in the cerebellum, specifically in Crus I, Lobule VI, and VIIb. This functional overlap between different unpleasant sensory modalities suggests that the cerebellum contains specific regions in encoding generalized aversive process. In this case, cerebellar activation of these areas was interpreted as reflecting aversion rather than stimulus salience, as a control analysis of pleasant pictures did not replicate activation observed with unpleasant pictures. Not only were these areas commonly activated by aversive stimuli, but their activity coincided with decreased activity in brain areas related to emotional and motivational processing, including the hypothalamus, the subgenual anterior cingulate cortex, and the parahippocampal gyrus. This response pattern suggests that the cerebellum could modulate the flow of aversive information and motivational processing (Figure 3), complementing clinical observations of disrupted affective and cognitive processing in stroke patients (Schmahmann & Sherman, 1998; Schmahmann, 1991; Schmahmann, 2004).
Figure 3. Model of cerebellar modulation of circuitry related to aversion and addiction.
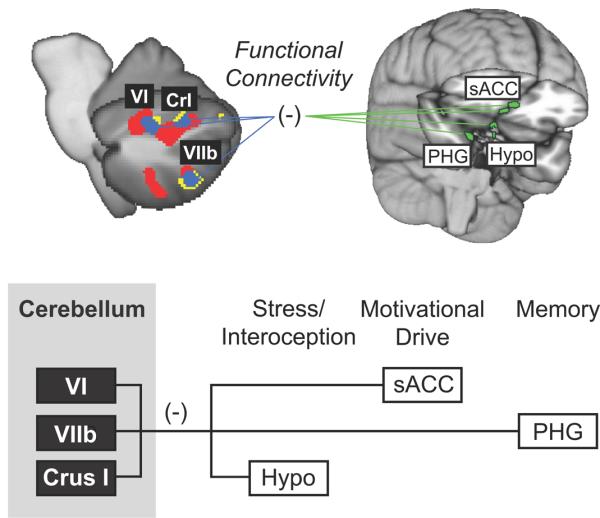
Aversive stimuli in the form of pain (red) and unpleasant pictures (yellow) produce overlapping fMRI activation (blue) in posterior cerebellar hemispheres Lobule VI, VIIb, and Crus I. Activation in these overlapping cerebellar areas is inversely related to activation in limbic areas in the brain, including the hypothalamus (Hypo), parahippocampal gyrus (PHG), and subgenual anterior cingulate cortex (sACC). These limbic structures have been associated with networks for salience, memory, and motivational drive. Figure adapted from Moulton et al., 2011.
Interestingly, prescription opioid-dependent patients have decreased functional connectivity at rest between reward structures and the cerebellum, including Crus I (Upadhyay et al., 2010). In opioid dependent patients, cerebellar functional connectivity with the nucleus accumbens and the amygdala decreased relative to age-gender matched controls. This change in functional correlation suggests that these reward regions are decoupled from cerebellar activation, indicating that opioid dependent patients have disrupted communication between these regions.
4. Addiction and neuroimaging measures of cerebellar structure
The primary neuroimaging techniques to assess changes in cerebellar structure in vivo in humans are magnetic resonance imaging (MRI) based methods, such as volumetric MRI, and diffusion tensor imaging (DTI). Volumetric MRI is a morphometric approach that uses 3D T1-weighted images to measure changes in the volume of specific anatomical structures, either in terms of total volume, grey matter, or white matter. One type of method to analyze volumetric MRI is voxel-based morphometry (VBM), a method that compares grey or white matter volume between brain states (e.g. addicted vs. healthy) based on MRI signal intensity in a voxel-by-voxel manner. Another volumetric method measures cortical thickness, a gray matter measure of the distance between the pial surface and the underlying white matter, calculated using computationally rendered 3D brain surfaces based on T1-weighted images. Though a refined measure, cortical thickness has not been practically applied to the cerebellum, primarily due to the lack of adequate spatial resolution to capture the exquisite complexity of cerebellar folia. In addition to measures of brain volume, MRI can be used to measure the axonal organization of white matter tracts with DTI. DTI acquisitions measure the directional diffusion of water, which is highly directional in proximity to white matter tracts. The hydrophobic phospholipid bilayer of the myelin sheath restricts the flow of water perpendicular to axonal processes, which results in anisotropic diffusion. DTI can be used to calculate measures of diffusion, such as fractional anisotropy (FA), which reflects white matter integrity or structure.
Volumetric MRI studies have documented that users of addictive substances tend to have smaller cerebellums (Table 1). Previous studies that have used whole-brain neuroimaging to identify addiction-related circuits have, with few exceptions, downplayed findings in the cerebellum in favor of better-understood motivational pathways. In the context of structural changes, significant decreases in gray matter in the cerebellum have been detected with long-term exposure to cocaine (Barrós-Loscertales et al., 2011; Sim et al., 2007), heroin (Lin et al., 2012; Walhovd et al., 2007), nicotine (Brody et al., 2004; Gallinat et al., 2006; Kühn et al., 2012), and alcohol (Chanraud et al., 2007; Chanraud et al., 2010; Mechtcheriakov et al., 2007; Shear et al., 1996; Sullivan et al., 2000; Sullivan et al., 2010). These gray matter decreases occur in the cerebellar cortices, but have not been linked to specific cerebellar cell groups given the spatial resolution limitations of MRI. A few studies have proposed that these cerebellar deficits could relate to motor deficits (Sullivan et al., 2000; Sullivan et al., 2010), executive function deficits (Chanraud et al., 2007; Lin et al., 2012; Sim et al., 2007; Sullivan et al., 2000), or dysfunctional processing of drug-related cues (Kühn et al., 2012). The structural differences in the posterior cerebellum are reoccurring across volumetric MRI studies, suggesting that (1) the cerebellum either reduces in volume as a consequence of chronic drug use, or (2) small cerebellums are a risk factor in the development drug addictions.
Table 1.
Cerebellar structure and chronic drug exposure.
| Paper | Method | Subjects | Structural difference | CB Localization |
|---|---|---|---|---|
| Cocaine | ||||
| Sim et al., 2007 | 1.5T MRI | 40 (13F) dependent vs. 41 (15F) healthy | Lower GM | R CrII: 12,−85,−30 (Tal) |
| Lower GM | L CrI: −16,−85,−30 (Tal) | |||
| Lower WM | R CrII: 25,−67,−41 (Tal) | |||
| Barros-Loscertales et al., 2011 | 1.5T MRI | 20M dependent vs. 16M healthy | Lower GM w/ usage | R VI: 32,−38,−33 (MNI) |
| Heroin | ||||
| Walhovd et al., 2007 | 1.5T MRI | 14 (7F) prenatally exposed vs 14 (5F) healthy | Lower GM | CB Cortex ROI |
| Lin et al., 2012 | 3T MRI | 27 (1F) methadone patients vs. 23 (11F) healthy | Lower GM | L CrI: −17,−78,−27 (MNI) |
| Bora et al., 2012 | 3T MRI | 24 (13F) dependent vs. 29 (15F) healthy | Lower WM | R VIIIa: 25,−54,−47 (MNI) |
| Lower WM | L Sup CB Ped: −5,−34,−28 (MNI)a | |||
| Lower WM | L Mid CB Ped: −12,−22,−25 (MNI)a | |||
| Nicotine | ||||
| Brody et al., 2004 | 1.5T MRI | 19 (8F) dependent vs. 17 (7F) healthy | Lower GM | R VI: 16,−56,−26 (MNI) |
| Gallinat et al., 2006 | 3T MRI | 22 (10F) dependent vs. 23 (11F) healthy | Lower GM | R VI: 14,−59,−20 (Tal) |
| L VI: −33,−37,−32 (Tal) | ||||
| Kuhn et al., 2012 | 3T MRI | 33 (20F) dependent vs. 22 (11F) healthy | Lower GM | L CrI: −32,−71,−32 (MNI) |
| Alcohol | ||||
| Gallucci et al., 1989 | 0.5T MRI | 35 (?F) dependent vs. 35 (?F) healthy | Lower WM | CB ROI |
| Shear et al., 1996 | 1.5T MRI | 33M non-amnesic dependent vs. 20M healthy | Lower ROI volume | CB Hemispheres ROI |
| Lower ROI volume | CB Vermis ROI | |||
| Sullivan et al., 2000 | 1.5T MRI | 25M dependent vs. 61M healthy | Lower GM/WM | Vermis I–V ROI |
| Lower GM/WM | Vermis VI–VII ROI | |||
| Lower GM | CB Hemispheres ROI | |||
| De Bellis et al., 2005 | 1.5T MRI | 14 (6F) dependent vs. 28 (12F) healthy | Lower WM (M only) | CB ROI |
| Chanraud et al., 2007 | 1.5T MRI | 31M dependent vs. 28M healthy | Lower GM | L CrII: −48,−60,−47 (MNI) |
| Lower WM | L VI: −32,−47,−37 (MNI) | |||
| Lower WM | R VI: 33,−47,−38 (MNI) | |||
| Lower WM | L I–IV: −15,−29,−18 (MNI) | |||
| Mechtcheriakov et al., 2007 | 1.5T MRI | 22 (8F) dependent vs. 22 (8F) healthy | Lower GM | R VIIb: 40,−47,−51 (MNI) |
| Lower GM | L VIIb: −42,−48,−51 (MNI) | |||
| Lower WM | 57,−7,34 (MNI)* | |||
| Lower WM | −57,−11,31 (MNI)* | |||
| Chanraud et al., 2010 | 3T MRI | 17 (?F) dependent vs. 31 (?F) healthy | Lower ROI volume | Vermis I–V ROI |
| Sullivan et al., 2010 | 1.5T MRI | 95 (31F) dependent vs. 105 (55F) healthy | Lower ROI volume | Vermis, anterior ROI |
| Lower ROI volume | Vermis, posterior ROI | |||
| Lower ROI volume | Vermis, inferior ROI | |||
| Marijuana | ||||
| Block et al., 2000a | 1.5T MRI | 18 (9F) users vs. 13 (7F) healthy | None | N/A |
| Matochik et al., 2005 | 1.5T MRI | 11M users vs. 8M healthy | None | N/A |
| Tzilos et al., 2005 | 1.5T MRI | 22 (6F) users vs. 26 (7F) healthy | None | N/A |
| Medina et al., 2010 | 1.5T MRI | 16 (4F) users vs. 16 (6F) healthy | Greater ROI volume | Vermis VIII-–X ROI |
| Solowij et al., 2011 | 3T MRI | 15M users vs. 16M healthy | Lower WM | CB ROI |
| Cousijn et al., 2012 | 3T MRI | 33 (12F) users vs. 42 (16F) healthy | Greater GM | L I–IV: −8,−43,−20 (MNI) |
Coordinate localization was determined using the SUIT probabilistic atlas of the human cerebellum (Diedrichsen et al., 2009). For this purpose, Talaraich coordinates were converted to MNI space using the Brett transformation, though the corresponding MNI coordinates are not shown.
CB, cerebellum; CrI, Crus I; CrII, Crus II; GM, Gray Matter; L, Left; Mid CB Ped, middle cerebellar peduncle; ROI, region of interest; MNI, Montreal Neurological Institute space; R, Right; Sup CB Ped, superior cerebellar peduncle; Tal, Talaraich space; WM, White Matter
Reported coordinates outside of cerebellum.
Identified as listed in source article.
4.1 Cocaine exposure
Cocaine is a crystalline tropane alkaloid derived from the coca plant, and acts as a serotonin-norepinephrine-dopamine reuptake inhibitor. Both serotonin receptors and norepinephrine receptors are expressed in the cerebellum. A VBM study on cocaine use and brain structure (Sim et al., 2007) suggests that drug use impacts specific regions within the cerebellum. This study compared gray and white matter in 40 cocaine abusers and 41 matched healthy controls, and found that abusers had significantly reduced gray matter in Lobule VI, Crus I, and Crus II. The finding of reduced gray matter in Lobule VI in cocaine abusers was also corroborated recently in another VBM study (Barrós-Loscertales et al., 2011). Lobule VI, Crus I, and Crus II are located in the posterior cerebellar hemispheres, which have been related to modulation of affect and cognition in clinical studies on cerebellar stroke (Schmahmann & Sherman, 1998).
4.2 Opioid exposure
Opioids such as heroin act on opioid receptors in the brain, of which mu- and kappa-varieties are present in the human cerebellum (Schadrack et al., 1999). Regarding the impact of opiates on structure, the volume of cerebellar cortex is significantly reduced in children who were exposed to heroin in utero (Walhovd et al., 2007). In this study, gray matter differences were evaluated in 14 exposed vs. 14 control children using a region of interest analysis. When accounting for age, gestational age, and gender, cerebellar white matter and cerebellar cortex showed a significant loss in gray matter, along with the accumbens, putamen, pallidum, amygdala, and cerebral cortex overall. Though these structural changes were reported as the main findings, the only significant effect was in the cerebellar cortex when intracranial volume was included as a factor. Considering that intracranial volume is highly variable in children of different ages, this serves to highlight the vulnerability of the cerebellum to heroin exposure during prenatal development.
A recent DTI study in ex-heroin users reported a reduction in mean FA in the cerebellum (Bora et al., 2012). Using DTI, 30 ex-heroin users on either methadone or buprenorphine maintenance medication were compared with 29 age-gender matched healthy controls. Bora et al. found lower FA, degraded white matter integrity, in the superior and middle cerebellar peduncles. These cerebellar peduncles serve as major pathways for afferent and efferent neural information flow between the cerebellum and the midbrain, including the pons. The implication that these white matter tracts are degraded with a history of heroin abuse suggests that addiction is related to a disruption in cerebellar processing and connectivity with other brain regions.
4.3 Nicotine exposure
Nicotine is a parasympathomimetic alkaloid, which acts as a nicotinic acetylcholine receptor agonist. In the cerebellum, these receptors control the release of the neurotransmitters glutamate, gamma-aminobutryic acid (GABA), and norepinephrine. VBM studies in cigarette smokers vs. healthy controls have demonstrated reduced gray matter in the posterior cerebellar hemispheres, specifically in Lobule VI (Brody et al., 2004; Gallinat et al., 2006) and Crus I (Kühn et al., 2012). Gray matter volume in Crus I was also found to be correlated negatively to the magnitude of nicotine dependence (Kühn et al., 2012). The effect of nicotine on cerebellar structure has recently been reviewed in detail (Gallinat et al., 2006).
4.4 Alcohol exposure
Ethanol binds to the GABA-A receptor to increase the inhibitory effects of the neurotransmitter GABA, and GABA-A receptors are expressed in the cerebellum. Cerebellar structural deficits have been frequently characterized clinically in alcoholism, with structural MRI studies (Chanraud et al., 2007, 2010; De Bellis et al., 2005; Gallucci et al., 1989; Mechtcheriakov et al., 2007; Shear et al., 1996; Sullivan et al., 2000; Sullivan et al., 2010) and computed tomography studies in living patients (Haubek & Lee, 1979; Hillbom et al., 1986; Ramos et al., 1987) complementing findings from post-mortem studies (Phillips et al., 1987; Yokota et al., 2006). In these studies, chronic alcoholism is linked to gray and white matter deficits in the anterior superior cerebellar vermis, though deficits in the inferior vermis and the cerebellar hemispheres have also been reported. A VBM study found that patients with severe alcohol dependence showed significant gray matter deficits in Crus II, along with white matter deficits in Lobule VI (Chanraud et al., 2007). These deficits were correlated with the neuropsychological measures of degraded executive function. Note that the deficits observed with chronic alcoholism in Crus II and Lobule VI, located in the posterior cerebellar hemispheres, have also been observed with cocaine and heroin use, as noted above. As a caveat, these structural changes may also be associated with vitamin B deficiency and other aspects of poor nutritional status (Martin et al., 2006).
4.5 Marijuana exposure
The cerebellum contains the cannabinoid receptors CB1 and CB2, inhibitory G-protein coupled receptors that bind to cannabinoids, the psychoactive chemical in marijuana. However, a consistent pattern of cerebellar changes with marijuana usage has not been apparent across structural studies (Block et al., 2000; Cousijn et al., 2012; Matochik et al., 2005; Medina, Nagel, & Tapert, 2010; Solowij et al., 2011; Tzilos et al., 2005; Wilson et al., 2000), nor across resting state metabolism studies using PET (Block et al., 2000; Sneider et al., 2008). When cerebellar structural changes are detected, they appear as increases in the anterior cerebellum (Cousijn et al., 2012) and the inferior posterior vermis (Medina et al., 2010), decreases in white-matter volume (Solowij et al., 2011). Some evidence suggests that the posterior cerebellum has lower resting cerebral blood flow in marijuana users after monitored abstinence vs. controls (Block et al., 2000).
4.6 Premorbid Cerebellar Volume and Addiction
Recent structural evidence indicates that abnormal cerebellar volume may reflect genetic risk to addiction. Fifty siblings of stimulant-dependent individuals compared with fifty unrelated healthy controls had more cerebellar gray matter (Ersche et al., 2012). These siblings were not users of addictive substances, yet they showed significant differences in the posterior cerebellar hemispheres compared to healthy volunteers. This suggests that the cerebellar abnormalities may identify individuals genetically at risk to develop dependence. However, stimulant-dependent individuals themselves showed no significant differences with controls in the cerebellum. In either case, the data from this study indicate that cerebellar structure may influence the potential to develop addiction. This has also been suggested with alcoholism (Benegal et al., 2007; Hill et al., 2011; Tessner & Hill, 2010), where individuals at high risk for developing alcohol dependence have altered cerebellar gray matter volume relative to controls. Cerebellar indications are also observed in neurological assessments of patients with substance dependence to cocaine and heroin as well (Manto, 2012).
5. Addiction and neuroimaging measures of cerebellar function
The neuroimaging techniques frequently used to measure functional activation are positron emission tomography (PET) and functional MRI (fMRI). PET relies on radiolabelled tracers to localize measures of brain activity. Examples of such tracers include radiolabelled water to measure regional cerebral blood flow (rCBF), and fluorodeoxyglucose (FDG) to measure glucose metabolism. Instead of radioactive tracers, fMRI uses the magnetic properties of oxygenated as compared to deoxygenated hemoglobin to track neural activity. This indirect measure is also referred to as the blood oxygen level dependent (BOLD) signal. Both of these techniques have measured physiological responses in the cerebellum in response to drugs of abuse or drug cues.
5.1 Acute drug exposure
While cerebellar structural deficits could be argued to be non-specific, as certain commonly used drugs (e.g. alcohol, nicotine) share a similar pattern of deficits, the cerebellum's rapid functional response to the exposure of a wide variety of drugs suggest specific acute effects. Functional imaging findings indicate that the cerebellum is activated acutely by drugs of abuse (Table 2), including cocaine (Risinger et al., 2005), methylphenidate (Volkow et al., 1997, 1999; Volkow et al., 2003; Volkow et al., 2006), marijuana (Mathew et al., 1998; Mathew et al., 2002; Volkow et al., 1991, 1996), and nicotine (Domino et al., 2000; Zubieta et al., 2005). Few studies comment on cerebellar involvement, though a role in conditioned responses has been suggested (Bonson et al., 2002; Schneider et al., 2001; Volkow et al., 2003; Volkow et al., 2006; Wang et al., 1999). These studies suggested that cerebellar responses were related to drug conditioning, based on the finding that cocaine abusers, and not non-drug abusing subjects, showed a cerebellar response to a drug expectation condition. However, the functional implication of conditioned responses in the cerebellum remains unclear.
Table 2.
Cerebellar functional responses to acute drug exposure.
| Paper | Method | Subjects | Drug delivery | CB Localization |
|---|---|---|---|---|
| Cocaine | ||||
| Risinger et al., 2005 | 1.5T MRI | 8M dependent | IV (6x, 20mg/70kg) | L CrI: −45,−57,−23 (Tal) |
| R I–IV: 12,−38,−14 (Tal) | ||||
| Methylphenidate | ||||
| Volkow et al., 1997 | FDG PET | 15M healthy | IV (0.5+0.25mg/kg) | CB ROI |
| Volkow et al., 1999 | FDG PET | 20M cocaine dependent | IV (0.5+0.25mg/kg) | CB ROI |
| Volkow et al., 2003 | FDG PET | 25 (4F) cocaine dependent | IV (0.5mg/kg) | R VIIIa: 17,−63,−33 (Tal) |
| L VIIIa: −15,−63,−33 (Tal) | ||||
| Vermis VIIIb: 3,−63,−29 (Tal) | ||||
| Vermis VIIIa: −7,−63,−29 (Tal) | ||||
| Volkow et al., 2006 | FDG PET | 15M healthy; not expecting MP | IV (0.5mg/kg) | Vermis VIIIb: −4,−64,−34 (Tal) |
| R VIIb: 40,−54,−38 (Tal) | ||||
| 15M healthy; expecting MP | IV (0.5mg/kg) | LIX: −2,−54,−36 (Tal) | ||
| R CrI: 36,−62,−30 (Tal) | ||||
| Volkow et al., 1991 | FDG PET | 8M users | IV (2mg) | Left CB ROI |
| Right CB ROI | ||||
| Delta-9-tetrahrdocannabinol | ||||
| Volkow et al., 1996 | FDG PET | 8M dependent | IV (2mg) | CB ROI |
| 8M healthy | CB ROI | |||
| Mathew et al., 1998 | FDG PET | 46 (24F) dependent | IV (0.25mg/min) | Left CB ROI |
| Right CB ROI | ||||
| Mathew et al., 2002 | FDG PET | 47 (21F) dependent | IV (0.25mg/min) | Left CB ROI |
| Right CB ROI | ||||
| Nicotine | ||||
| Domino et al., 2000 | rCBF PET | 18 (9F) users | Nasal spray | R VIIb: 28,−72,−40 (Tal) |
| Zubieta et al., 2005 | rCBF PET | 19 (11F) users | Cigarette | L CrI: −38,−68,−28 (Tal) |
| R CrII: 24,−84,−28 (Tal) |
Coordinate localization was determined using the SUIT probabilistic atlas of the human cerebellum (Diedrichsen et al., 2009). For this purpose, Talaraich coordinates were converted to MNI space using the Brett transformation, though the corresponding MNI coordinates are not shown.
CB, cerebellum; CrI, Crus I; CrII, Crus II; FDG, fluorodeoxyglucose; GM, Gray Matter; L, Left; MNI, Montreal Neurological Institute space; MP, methylphenidate; PET, positron emission tomography; R, Right; rCBF, regional cerebral blood flow; ROI, region of interest; Tal, Talaraich space; Verm. Lob., Vermal Lobule; WM, White Matter
5.1.1 Methylphenidate (cocaine analog)
The cerebellum was brought front and center into the addiction literature by its conspicuous response to methylphenidate (Volkow et al., 1997, 1999, 2003, 2006), a psychostimulant similar to cocaine in that it inhibits the reuptake of dopamine and norepinephrine. Intravenous cocaine itself has also been reported to activate the cerebellum (Risinger et al., 2005). In a series of PET studies using FDG as a tracer, the largest effects of intravenous infusion of methylphenidate occur in and throughout the entire cerebellum. These cerebellar responses occur regardless if the drug is administered in plain view or with a hidden infusion, suggesting that the response is driven pharmacologically rather than by expectation. The cerebellum is mostly devoid of dopamine transporters, and is not considered as part of the mesocorticolimbic reward circuitry. Based on this, the authors suggest that methylphenidate's cerebellar effects are either a downstream effect of activation of the striatum, or are due to blockade of cerebellar norepinephrine transporters. The robust responses to methylphenidate and cocaine suggest that the cerebellum is acutely affected by these drugs, and could play a central role in the mechanisms underlying addiction.
Methamphetamine, a pharmacologically-related psychostimulant, has yet to be related to cerebellar effects in humans, but has been shown to enhance tyrosine hydroxylase expression in the cerebellar cortex of mice (Ferrucci et al., 2006), and chronic treatment significantly depresses the discharge rate of cerebellar Purkinje neurons in rats (Sorensen et al., 1982).
5.1.2 Marijuana
Infusion of delta-9-tetrahydrocannabinol (THC), the primary psychoactive constituent of cannabis, has repeatedly been reported in PET studies to produce increases in cerebellar activity in healthy subjects (Volkow et al., 1991, 1996) and marijuana users (Mathew et al., 1998; Mathew et al., 2002; Volkow et al., 1996). This is consistent with animal and human studies that demonstrate that cannabinoid (CB1) receptors are localized to the molecular layer of the cerebellum (Herkenham et al., 1990), and are found on inhibitory interneurons that modulate Purkinje cells (Ashton et al., 2004). As measured with FDG, increases in cerebellar metabolism were significantly correlated with subjective reports of THC intoxication and blood plasma THC concentration (Volkow et al., 1991, 1996), and not motor incoordination.
Perhaps incidentally, a few case reports indicate that acute marijuana use can result in cerebellar infarctions in teenage males (Geller et al., 2004). The incidence of stroke in this age group is very low, about 6 per 100,000 per year. At one hospital, three cases over the course of five years were documented of cerebellar stroke associated with marijuana use. Interestingly, the focal acute hemorrhages were localized to the posterior cerebellar hemispheres.
5.1.3 Nicotine
More precise localization of cerebellar responses was observed with nicotine than with cocaine, methylphenidate, and marijuana (Domino et al., 2000; Zubieta et al., 2005). A PET study contrasted rCBF in smokers after their first cigarette of the day following 12 hours of overnight abstinence vs. baseline (Zubieta 2005). In this contrast, the posterior cerebellum showed elevated rCBF, localized to Lobule VI, Crus I, and Crus II. These functional activations complement the spatial localization of the gray matter deficits reported with cocaine and alcohol abuse, as well as the acute cerebellar infarctions observed with marijuana use. Co-localization of acute functional activation and gray matter deficits suggest that repetitive activation of these regions by these agents may result in neuronal excitotoxicity. This possibility has not yet been evaluated.
5.2 Drug craving
A cerebellar role in drug craving has been demonstrated with cues for heroin (Lou et al., 2012; Sell et al., 2000), cocaine (Anderson et al., 2006; Bonson et al., 2002; Grant et al., 1996; Kilts et al., 2001; Risinger et al., 2005; Wang et al., 1999), and alcohol (Schneider et al., 2001)(Table 3). On the few occasions when spatial coordinates are reported for cerebellar responses to craving, the activations are reported in the posterior cerebellum, similar to acute drug use. Cerebellar activity has been correlated with self-reports of “feeling tense” and “withdrawal symptoms” during cue evoked craving in heroin users (Sell et al., 2000). Again, limited interpretations for cerebellar involvement are suggested, with the predominant explanations touching on conditioned responses (Bonson et al., 2002; Schneider et al., 2001; Volkow et al., 2003; Volkow et al., 2006; Wang et al., 1999) and selective attention (Anderson et al., 2006).
Table 3.
Cerebellar functional responses to drug craving.
| Paper | Method | Subjects | Cue | CB Localization |
|---|---|---|---|---|
| Heroin | ||||
| Sell et al., 2000 | rCBF PET | 10M dependent: detoxing | Video of use | Left hemisphere ROI |
| Right hemisphere ROI | ||||
| Lou et al., 2012 | 1.5T fMRI | 19M dependent: abstaining | Images of use | L CrI: −33,−72,−24 (Tal) |
| R CrI: 42,−63,−21 (Tal) | ||||
| Cocaine | ||||
| Grant et al., 1996 | FDG PET | 13 (1F) dependent | Multimedia | CB ROI |
| Wang et al., 1999 | 1.5T fMRI | 13 (5F) dependent | Drug interview | CB ROI |
| Kilts et al., 2001 | rCBF PET | 8M dependent | Audio script | CB ROI |
| Bonson et al., 2002 | FDG PET | 10 (4F) dependent | Multimedia | R Dentate: 24,−50,−42 (MNI)a |
| Risinger et al., 2005 | 1.5T fMRI | 8M dependent | Button press | R Dentate: 18,−47,−25 (Tal)a |
| L V: −18,−46,−13 (Tal) | ||||
| Anderson et al., 2006 | 1.5T fMRI | 10 (4F) dependent | Audio/Video of use | Vermis II–III ROI |
| Vermis.VIII–IX ROI | ||||
| Alcohol | ||||
| Schneider et al., 2001 | 1.5T fMRI | 10M dependent: abstaining | Odor | L CrI: −28,−64,−24 (Tal) |
| L CrII: −12,−80,−20 (Tal) |
Coordinate localization was determined using the SUIT probabilistic atlas of the human cerebellum (Diedrichsen et al., 2009). For this purpose, Talaraich coordinates were converted to MNI space using the Brett transformation, though the corresponding MNI coordinates are not shown.
CB, cerebellum; CrI, Crus I; CrII, Crus II; FDG, fluorodeoxyglucose; GM, Gray Matter; L, Left; MNI, Montreal Neurological Institute space; PET, positron emission tomography; R, Right; rCBF, regional cerebral blood flow; ROI, region of interest; Tal, Talaraich space; WM, White Matter
Identified using the MRI Atlas of the Cerebellum (Schmahmann et al., 2000).
Craving is particularly acute in heroin users, and evidence suggests that cerebellum has a role in opioid craving. A PET study in 10 male active heroin users measured rCBF responses to drug related and neutral video cues presented in separate scans (Sell et al., 2000). After each scan, subjective ratings were collected on a scale from 1–10 for a variety of sensations, including withdrawal symptoms, and feeling tense. Across both drug and neutral video cues, cerebellar blood flow was correlated with self-reports of severity of withdrawal symptoms and feeling tense. These findings suggest that cerebellar activation may reflect aversive sensations that are not specific to drug craving per se. A recent fMRI study compared responses to drug image cues in short-term vs. long-term abstinence in 19 male heroin users (Lou et al., 2012). Greater cerebellar activation was found to heroin vs. neutral cues with short-term abstinence, yet cerebellar deactivation was observed with long-term abstinence. The authors suggested that this meant that long-term abstinence decreased the salience of conditioned cues, with the cerebellar activation presumably reflecting stimulus salience. Though neither study explicitly related cerebellar responses to craving, they did relate them to drug-related cues.
An fMRI study found posterior cerebellar BOLD responses in abstinent alcoholics while presented with ethanol odors to induce craving (Schneider 2001). An rCBF PET study that induced craving for cocaine also found a response in the left posterior cerebellar hemisphere (Kilts 2001). Aside from the posterior hemispheres, the cerebellar vermis has also been found to respond to cues related to cocaine (Anderson 2006), methylphenidate (Volkow 2003), and alcohol (Schneider 2001) in cocaine dependent subjects and alcoholics.
Converging evidence from structural and functional neuroimaging studies across a variety of drug conditions indicates that the cerebellar posterior hemispheres are significantly different in addicted subjects vs. healthy controls. Yet to be determined is whether these differences in the cerebellum promote the development addiction or are simply a reflection of the pharmacological effects of drug use. The wide variety of cerebellar findings presented thus far suggest that this structure should receive greater consideration in future addiction studies.
6. A model for cerebellar systems in addiction
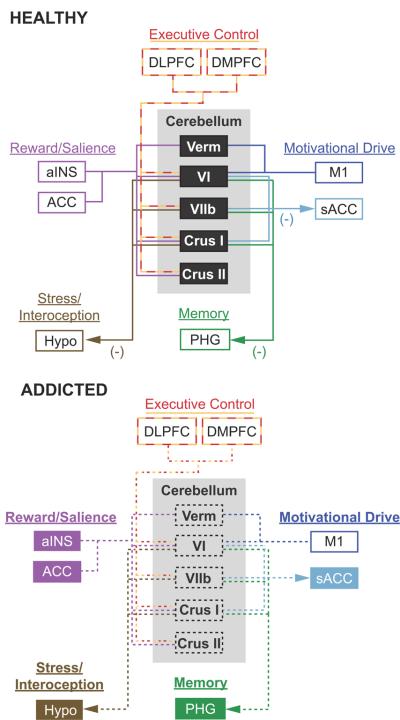
Given the supporting evidence, the cerebellum's role as a modulator can be readily adapted to existing models of addiction. In the context of neuroimaging, a major conceptual model for addiction is the i-RISA (Impaired Response Inhibition and Salience Attribution) model (Goldstein & Volkow, 2002, 2011), also described as a four-circuit model (Volkow et al., 2003; Volkow et al., 2010). Briefly, the model consists of four inter-connected circuits relating to memory, reward/saliency, executive control, and motivation/drive. The response to a reward is mediated by the interactions of these four components.
In the addicted brain, the homeostatic balance is drastically altered, such that the components for memory, reward/saliency, and motivation/drive are amplified, and cognitive control is diminished. Different brain regions have been assigned to each circuit, such as the dorsolateral prefrontal cortex for control and the nucleus accumbens for reward. With the cerebellum's postulated function as a multimodal modulator of cognition, affect, and aversion, the cerebellum is optimally configured to regulate brain processes directly involved in addiction (Figure 4). This cerebellar model for addiction proposes that the cerebellum plays an influential role in maintaining the homeostatic balance of the four circuits. In the addicted brain, structural gray matter deficits in Lobule VI, VIIb, Crus I, Crus II, and the vermis implicate changes in the cerebellum's ability to interact and communicate with brain regions related to the four circuits.
Figure 4. A cerebellar model for addiction.
The top panel highlights the cerebellum's role as a modulator of affective and cognitive function in the healthy brain. This schematic highlights the four major brain networks proposed to be affected by addiction (adapted from Volkow et al., 2010). The components within each network are color-coded to match the cerebral resting state networks in Figure 2 (adapted from Buckner et al., 2011). Arrows show the proposed direction of cerebellar down-modulation of specific brain regions within each circuit (Moulton et al., 2011). The bottom panel proposes how cerebellar dysfunction may impact the addicted brain. The black dashed boxes highlight structural degradation of specifically localized cerebellar structures with addiction. The dashed lines show disrupted functional connectivity and cerebellar modulation of specific brain regions related to executive control, reward/salience, motivational drive, and memory. Filled colored boxes and bold type represent brain circuits released from cerebellar modulation, leading to an uninhibited and sensitized neurological state. Note that all cerebellar outputs project through the deep cerebellar nuclei (refer to text for details). ACC, anterior cingulate cortex; aINS, anterior insula; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; Hypo, hypothalamus; M1, primary motor cortex; PHG, parahippocampal gyrus; sACC, subgenual anterior cingulate cortex; Verm, vermis; VI, cerebellar hemispheric lobule VI; VIIb, cerebellar hemispheric lobule VIIb.
These brain regions receive inputs from the deep cerebellar nuclei, the main output channels of the cerebellum. From the cerebellar cortex, the dentate and fastigial nuclei receive inputs from the lateral hemisphere and vermis, respectively. Within the dentate nuclei, separate cerebellar output channels have been identified that project to areas related to cognitive (BA 9 and 46 in the dorsolateral prefrontal cortices), skeletomotor (motor and premotor cortices), and oculomotor function (frontal eye fields) (Middleton & Strick, 2000; Strick et al., 2009). The fastigial nuclei have an output channel that relates to limbic processing, with projections to the hippocampus, amygdala, and the cingulate cortex (Strick et al., 2009). However, not all of the output channels through the deep cerebellar nuclei have been mapped.
In addiction, decreased inhibition of brain regions such as the hypothalamus, subgenual anterior cingulate, and parahippocampal gyrus would result in a net excitatory effect in brain circuits related to reward/salience, motivational drive, and memory, as well as stress and interoception. Likewise, the reciprocal pathways between brain circuits related to executive control would be disrupted, interfering with the ability of the prefrontal cortex to inhibit unwanted drug seeking behavior. Addiction has conventionally been assumed to impair the cerebellum, but the notion that the cerebellum may serve a more active role has not been thoroughly examined. This model is meant to inspire hypotheses to test the assertion that the cerebellum may or may not have a generative role in addictive processes.
To our knowledge, no prospective lesion or developmental studies in humans have been published to address the potential causal role of the cerebellum in addiction. However, animal models for motivational behavior suggest that lesions to the dentate deep cerebellar nuclei impair the development of reinforcement learning (Bauer et al., 2011; Thoma et al., 2008). In the human studies presented here, the posterior hemispheres tend to be affected, suggesting that abnormalities in this region are a vulnerability factor for addiction. However, given that only a handful of studies exist for each substance, we were unable to perform a quantitative neuroimaging meta-analysis to investigate whether cause and effect varies across substances.
7. Conclusions
The cerebellum is potentially a key modulator that impacts and is impacted by addiction. Specific structures in the posterior cerebellar hemispheres, such as hemispheric lobule VI and Crus I, are particularly salient as contributing factors. Despite its relatively low profile in the literature, evidence of the cerebellum's involvement in addiction continues to accumulate. While neuroimaging has revealed the cerebellum to be unexpectedly related to addiction, further investigations are needed to directly assess its physiological relevance.
The coalescence of preclinical, neuroimaging, and clinical data suggests that cerebellar alterations both predispose and may play a key role in the course of addictive disorder. The existing models of addiction do not take into account the potential involvement of the cerebellum, yet it may be involved in the opponent vs. proponent effects of addictive drugs, along with incentive sensitization, and aberrant learning mechanisms (Berridge & Robinson, 2003; Hyman et al., 2006).
If confirmed in clinical studies, our insights could have important implications for prevention and treatment of substance use disorders and behavioral addictions, such as pathological gambling and internet addiction. Specifically, if cerebellar vulnerability factors for addiction could be defined, they might be used to target susceptible individuals for early interventions. On the other hand, abnormalities arising in the context of ongoing addictive disorder may be used for diagnostic purposes and/or for a choice of a proper therapeutic agent in conjunction with monitoring of therapeutic response. The proposed model may also provide important leads for recognition and treatment of reward deficiency syndrome typical of patients with other disorders where cerebellar abnormalities were also noted, including depression, schizophrenia, and post-traumatic stress disorder.
Acknowledgements
This work was supported by the National Institutes of Health (National Institute on Drug Abuse Grant K01DA024289 to E.A.M; National Institute of Neurological Disorders and Stroke Grant K24NS064050 to D.B.; National Institute on Drug Abuse Grants R01DA023579 and R21DA034954 to R.Z.G.).
Footnotes
Conflict of Interest Statement: The authors claim no conflicts of interest.
Author contribution EM and DB conceptualized the review. EM drafted the manuscript. DB, RZ, IE, and LB provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Anderson CM, Maas LC, Frederick B. deB, Bendor JT, Spencer TJ, Livni E, Lukas ES, Fischman AJ, Madras BK, Renshaw PF, Kaufman MJ. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2006;31(6):1318–1326. doi: 10.1038/sj.npp.1300937. [DOI] [PubMed] [Google Scholar]
- Ashton JC, Appleton I, Darlington CL, Smith PF. Immunohistochemical localization of cannabinoid CB1 receptor in inhibitory interneurons in the cerebellum. Cerebellum. 2004;3(4):222–226. doi: 10.1080/14734220410019011. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, Parcet MA, Avila C. Reduced striatal volume in cocaine-dependent patients. NeuroImage. 2011;56(3):1021–1026. doi: 10.1016/j.neuroimage.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Kerr AL, Swain RA. Cerebellar dentate nuclei lesions reduce motivation in appetitive operant conditioning and open field exploration. Neurobiology of learning and memory. 2011;95(2):166–175. doi: 10.1016/j.nlm.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction biology. 2007;12(1):122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends in neurosciences. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Block RI, O'Leary DS, Ehrhardt JC, Augustinack JC, Ghoneim MM, Arndt S, Hall JA. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11(3):491–496. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- Block RI, O'Leary DS, Hichwa RD, Augustinack JC, Ponto LL, Ghoneim MM, Arndt S, Ehrhardt JC, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Cerebellar hypoactivity in frequent marijuana users. Neuroreport. 2000;11(4):749–753. doi: 10.1097/00001756-200003200-00019. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26(3):376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bora E, Yücel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L, Pell G, Lubman DI. White matter microstructure in opiate addiction. Addiction biology. 2012;17(1):141–148. doi: 10.1111/j.1369-1600.2010.00266.x. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychology review. 2010;20(3):261–270. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Batzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biological psychiatry. 2004;55(1):77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin H-J, Reynaud M, Martinot J-L. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32(2):429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel A-L, Rohlfing T, Pfefferbaum A, Sullivan EV. Dual tasking and working memory in alcoholism: relation to frontocerebellar circuitry. Neuropsychopharmacology. 2010;35(9):1868–1878. doi: 10.1038/npp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Allen G. Prediction and preparation, fundamental functions of the cerebellum. Learning & memory. 1997;4(1):1–35. doi: 10.1101/lm.4.1.1. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. NeuroImage. 2012;59(4):3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature reviews neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- D'Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Frontiers in neural circuits. 2012;6:116. doi: 10.3389/fncir.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism, clinical and experimental research. 2005;29(9):1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Maderwald S, Küper M, Thürling M, Rabe K, Gizewski ER, Ladd ME, Timmann D. Imaging the deep cerebellar nuclei: A probabilistic atlas and normalization procedure. Neuroimage. 2011;54(3): 1786–1794. doi: 10.1016/j.neuroimage.2010.10.035. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage. 2006;33(1):127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- Diedrichsen Jörn, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46(1):39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Verstynen T, Schlerf J, Wiestler T. Advances in functional imaging of the human cerebellum. Current opinion in neurology. 2010;23(4):382–387. doi: 10.1097/WCO.0b013e32833be837. [DOI] [PubMed] [Google Scholar]
- Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, Koeppe RA, Zubieta JK. Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse. 2000;38(3):313–321. doi: 10.1002/1098-2396(20001201)38:3<313::AID-SYN10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the corticostriatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41(3):252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335(6068):601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Ferrucci M, Busceti CL, Falleni A, Giorgi FS, Ruggieri S, Fornai F. Effects of methamphetamine on the cerebellar cortex: a preliminary study. Annals of the New York Academy of Sciences. 2006;1074:149–153. doi: 10.1196/annals.1369.014. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M. Smoking and structural brain deficits: a volumetric MR investigation. The European journal of neuroscience. 2006;24(6):1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Gallucci M, Amicarelli I, Rossi A, Stratta P, Masciocchi C, Zobel BB, Casacchia M, Passariello R. MR imaging of white matter lesions in uncomplicated chronic alcoholism. Journal of computer assisted tomography. 1989;13(3):395–398. doi: 10.1097/00004728-198905000-00004. [DOI] [PubMed] [Google Scholar]
- Geller T, Loftis L, Brink DS. Cerebellar infarction in adolescent males associated with acute marijuana use. Pediatrics. 2004;113(4):e365–370. doi: 10.1542/peds.113.4.e365. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. The American journal of psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews neuroscience. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. PNAS. 1996;93(21):12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. Journal of neuroscience. 2009;29(26):8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubek A, Lee K. Computed tomography in alcoholic cerebellar atrophy. Neuroradiology. 1979;18(2):77–79. doi: 10.1007/BF00344826. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. PNAS. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, Tessner K, Holmes B, McDermott M, Zezza N, Stiffler S. Cerebellum volume in high-risk offspring from multiplex alcohol dependence families: association with allelic variation in GABRA2 and BDNF. Psychiatry research. 2011;194(3):304–313. doi: 10.1016/j.pscychresns.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillbom M, Muuronen A, Holm L, Hindmarsh T. The clinical versus radiological diagnosis of alcoholic cerebellar degeneration. Journal of the neurological sciences. 1986;73(1):45–53. doi: 10.1016/0022-510x(86)90062-6. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual review of neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Progress in neurobiology. 2006;78(3–5):272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Ivry R. Cerebellar timing systems. International review of neurobiology. 1997;41:555–573. [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. Journal of neuroscience. 2003;23(23):8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Archives of general psychiatry. 2001;58(4):334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kohlmeyer K, Stober B, Jennen C. Computed tomography in chronic alcoholism. Acta radiologica. Supplementum. 1986;369:393–395. [PubMed] [Google Scholar]
- Koob GF. Drug addiction: the yin and yang of hedonic homeostasis. Neuron. 1996;16(5):893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cerebral cortex. 2009;19(10):2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Romanowski A, Schilling C, Mobascher A, Warbrick T, Winterer G, Gallinat J. Brain grey matter deficits in smokers: focus on the cerebellum. Brain structure & function. 2012;217(2):517–522. doi: 10.1007/s00429-011-0346-5. [DOI] [PubMed] [Google Scholar]
- Lin W-C, Chou K-H, Chen H-L, Huang C-C, Lu C-H, Li S-H, Wang Y-L, Cheng Y-F, Lin C-C, Chen C-C. Structural deficits in the emotion circuit and cerebellum are associated with depression, anxiety and cognitive dysfunction in methadone maintenance patients: a voxel-based morphometric study. Psychiatry research. 2012;201(2):89–97. doi: 10.1016/j.pscychresns.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Lou M, Wang E, Shen Y, Wang J. Cue-elicited craving in heroin addicts at different abstinent time: an fMRI pilot study. Substance use & misuse. 2012;47(6):631–639. doi: 10.3109/10826084.2011.646381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manto M. Toxic agents causing cerebellar ataxias. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 103. 2012. pp. 201–213. [DOI] [PubMed] [Google Scholar]
- Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol research & health. 2003;27(2):134–142. [PMC free article] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Turkington TG, Coleman RE. Cerebellar activity and disturbed time sense after THC. Brain research. 1998;797(2):183–189. doi: 10.1016/s0006-8993(98)00375-8. [DOI] [PubMed] [Google Scholar]
- Mathew Roy J, Wilson WH, Turkington TG, Hawk TC, Coleman RE, DeGrado TR, Provenzale J. Time course of tetrahydrocannabinol-induced changes in regional cerebral blood flow measured with positron emission tomography. Psychiatry research. 2002;116(3):173–185. doi: 10.1016/s0925-4927(02)00069-0. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet J-L, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug and alcohol dependence. 2005;77(1):23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Mechtcheriakov S, Brenneis C, Egger K, Koppelstaetter F, Schocke M, Marksteiner J. A widespread distinct pattern of cerebral atrophy in patients with alcohol addiction revealed by voxel-based morphometry. Journal of neurology, neurosurgery, and psychiatry. 2007;78(6):610–614. doi: 10.1136/jnnp.2006.095869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry research. 2010;182(2):152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266(5184):458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain research. Brain research reviews. 2000;31(2–3):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. Journal of neuroscience. 2001;21(2):700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel M, Toledo R, García LI, Coria-Avila GA, Manzo J. Why should we keep the cerebellum in mind when thinking about addiction? Current drug abuse reviews. 2009;2(1):26–40. doi: 10.2174/1874473710902010026. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. Journal of neuroscience. 2011;31(10):3795–3804. doi: 10.1523/JNEUROSCI.6709-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix.”. NeuroImage. 2011;54(3):2237–2249. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ. Susceptibility of the cerebellum to thiamine deficiency. Cerebellum. 2006;5(1):55–63. doi: 10.1080/14734220600551707. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cerebral cortex. 2010;20(4):953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SC, Harper CG, Kril J. A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain. 1987;110(Pt 2):301–314. doi: 10.1093/brain/110.2.301. [DOI] [PubMed] [Google Scholar]
- Ramnani N. Frontal lobe and posterior parietal contributions to the corticocerebellar system. Cerebellum. 2012;11(2):366–383. doi: 10.1007/s12311-011-0272-3. [DOI] [PubMed] [Google Scholar]
- Ramos A, Quintana F, Díez C, Leno C, Berciano J. CT findings in spinocerebellar degeneration. AJNR. 1987;8(4):635–640. [PMC free article] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage. 2005;26(4):1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Sang L, Qin W, Liu Y, Han W, Zhang Y, Jiang T, Yu C. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. NeuroImage. 2012;61(4):1213–1225. doi: 10.1016/j.neuroimage.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Schadrack J, Willoch F, Platzer S, Bartenstein P, Mahal B, Dworzak D, Wester HJ, Zieglgansberger W, Tölle TR. Opioid receptors in the human cerebellum: evidence from [11C]diprenorphine PET, mRNA expression and autoradiography. Neuroreport. 1999;10(3):619–624. doi: 10.1097/00001756-199902250-00032. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Archives of neurology. 1991;48(11):1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Human brain mapping. 1996;4(3):174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain: a journal of neurology. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schmahmann Jeremy D. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. Journal of neuropsychiatry and clinical neurosciences. 2004;16(3):367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. The American journal of psychiatry. 2001;158(7):1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Liang L, Valera EM, Monuteaux MC, Brown A, Kaiser J, Spencer T, Faraone SV, Makris N. Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biological psychiatry. 2011;69(9):857–866. doi: 10.1016/j.biopsych.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, Frackowiak RSJ, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug and Alcohol Dependence. 2000;60(2):207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Shear PK, Sullivan EV, Lane B, Pfefferbaum A. Mammillary body and cerebellar shrinkage in chronic alcoholics with and without amnesia. Alcoholism, clinical and experimental research. 1996;20(8):1489–1495. doi: 10.1111/j.1530-0277.1996.tb01153.x. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32(10):2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Sneider JT, Pope HG, Jr, Silveri MM, Simpson NS, Gruber SA, Yurgelun-Todd DA. Differences in regional blood volume during a 28-day period of abstinence in chronic cannabis smokers. European neuropsychopharmacology. 2008;18(8):612–619. doi: 10.1016/j.euroneuro.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Yücel M, Respondek C, Whittle S, Lindsay E, Pantelis C, Lubman DI. Cerebellar white-matter changes in cannabis users with and without schizophrenia. Psychological medicine. 2011;41(11):2349–2359. doi: 10.1017/S003329171100050X. [DOI] [PubMed] [Google Scholar]
- Sorensen SM, Johnson SW, Freedman R. Persistent effects of amphetamine on cerebellar Purkinje neurons following chronic administration. Brain research. 1982;247(2):365–371. doi: 10.1016/0006-8993(82)91262-8. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11(2):352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annual review of neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, Pfefferbaum A. Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Archives of general psychiatry. 2000;57(9):894–902. doi: 10.1001/archpsyc.57.9.894. [DOI] [PubMed] [Google Scholar]
- Sullivan Edith V, Rohlfing T, Pfefferbaum A. Pontocerebellar volume deficits and ataxia in alcoholic men and women: no evidence for “telescoping.”. Psychopharmacology. 2010;208(2):279–290. doi: 10.1007/s00213-009-1729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain RA, Kerr AL, Thompson RF. The cerebellum: a neural system for the study of reinforcement learning. Frontiers in behavioral neuroscience. 2011;5:8. doi: 10.3389/fnbeh.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessner KD, Hill SY. Neural circuitry associated with risk for alcohol use disorders. Neuropsychology review. 2010;20(1):1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma P, Bellebaum C, Koch B, Schwarz M, Daum I. The cerebellum is involved in reward-based reversal learning. Cerebellum. 2008;7(3):433–443. doi: 10.1007/s12311-008-0046-8. [DOI] [PubMed] [Google Scholar]
- Tzilos GK, Cintron CB, Wood JBR, Simpson NS, Young AD, Pope HG, Jr, Yurgelun-Todd DA. Lack of hippocampal volume change in long-term heavy cannabis users. The American journal on addictions. 2005;14(1):64–72. doi: 10.1080/10550490590899862. [DOI] [PubMed] [Google Scholar]
- Uğurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, Lenglet C, Wu X, Schmitter S, Van de Moortele PF, Strup J, Sapiro G, Martino FD, Wang D, Harel N, Garwood M, CHen L, Feinberge DA, Smith SM, Miller KL, Sotiropoulos SN, Jbabh S, Andersson JLR, Behrens TEJ, Glasser MF, Van Essen DC, Yacoud E. Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project. NeuroImage. 2013;80(15):80–104. doi: 10.1016/j.neuroimage.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133(t 7):2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Ivanovic M, Hollister L. Cerebellar metabolic activation by delta-9-tetrahydro-cannabinol in human brain: a study with positron emission tomography and 18F-2-fluoro-2-deoxyglucose. Psychiatry research. 1991;40(1):69–78. doi: 10.1016/0925-4927(91)90030-t. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry research. 1996;67(1):29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding YS, Pappas N. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. The American journal of psychiatry. 1999;156(1):19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Angrist B, Hitzemann R, Lieberman J, Pappas N. Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. The American journal of psychiatry. 1997;154(1):50–55. doi: 10.1176/ajp.154.1.50. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G-J. The addicted human brain: insights from imaging studies. Journal of clinical investigation. 2003;111(10):1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. BioEssays. 2010;32(9):748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Ma Y, Fowler JS, Wong C, Jayne M, Telang F, Swanson JM. Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. NeuroImage. 2006;32(4):1782–1792. doi: 10.1016/j.neuroimage.2006.04.192. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Ma Y, Fowler JS, Zhu W, Maynard L, Telang F, Vaska P, DIng YS, Wong C, Swanson JM. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. Journal of neuroscience. 2003;23(36):11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Moe V, Slinning K, Due-Tønnessen P, Bjørnerud A, Dale AM, van der Kouwe A, Quinn BT, Kosofsky B, Greve D, Fischl B. Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. NeuroImage. 2007;36(4):1331–1344. doi: 10.1016/j.neuroimage.2007.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life sciences. 1999;64(9):775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. Journal of addictive diseases. 2000;19(1):1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- Yokota O, Tsuchiya K, Terada S, Oshima K, Ishizu H, Matsushita M, Kuroda S, Akiyama H. Frequency and clinicopathological characteristics of alcoholic cerebellar degeneration in Japan: a cross-sectional study of 1,509 postmortems. Acta neuropathologica. 2006;112(1):43–51. doi: 10.1007/s00401-006-0059-7. [DOI] [PubMed] [Google Scholar]
- Zarei M, Mataix-Cols D, Heyman I, Hough M, Doherty J, Burge L, Winmill L, Nijhawan S, Matthews P, James A. Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biological psychiatry. 2011;70(11):1083–1090. doi: 10.1016/j.biopsych.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Zubieta J-K, Heitzeg MM, Xu Y, Koeppe RA, Ni L, Guthrie S, Domino EF. Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. The American journal of psychiatry. 2005;162(3):567–577. doi: 10.1176/appi.ajp.162.3.567. [DOI] [PubMed] [Google Scholar]