To the Editor—Eighteen million US healthcare workers (HCWs) undergo mandatory annual screening for latent tuberculosis [1]. Screening is also performed in tuberculosis contacts, immunocompromised patients, and other individuals employed in the public sector. Many institutions have switched from the tuberculin skin test to the Quantiferon (QFT) assay for latent tuberculosis screening. In recent months, we experienced a sharp increase in false-positive QFT results. We want to ensure that other institutions using QFT testing are aware of the potential problem of false-positive results.
Our institution began using the QFT test in 2008, screening approximately 10 000 HCWs annually. Due to the detection of transient increases in the daily positivity rate, the clinical laboratory implemented a surveillance program in 2010 for tracking daily positivity rates. As we have reported previously [2], in November 2011, the proportion of positive results significantly increased, from a mean baseline of 9% to a mean of 31%. The elevated proportion of positive QFTs returned to baseline after switching to a new lot of tuberculosis antigen tubes. Direct comparison of the tubes with tubes from a different lot identified that a lot-specific problem with the tuberculosis antigen tubes was the root cause of false-positive results. The manufacturer reportedly instituted stricter quality assurance measures prior to distribution of the QFT assays.
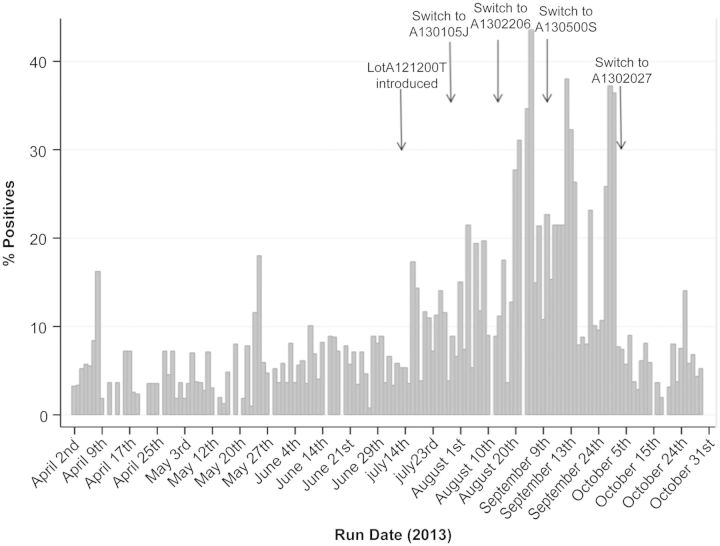
We recently again detected a significant increase in the daily QFT positivity rate following the introduction of lot A121200T to a mean of 16.2% during the period of 17 July to 2 October 2013, compared with a 5.7% baseline rate in January through June 2013 (P < .001, χ2 test). Throughout August and September, multiple different lots were implemented in hopes of correcting the problem. However, the elevated rate persisted until lot A1302027 was put into use on 3 October 2013 and the average daily positivity rate throughout the remainder of October declined to 5.4% (Figure 1). An investigation into the QFT practices at our institution in conjunction with the manufacturer did not identify any preanalytical or analytical errors. Within-subjects comparison of QFT results (n = 19) with tuberculosis antigen tubes of the suspect lot (A130500S) and the new lot (A1302027) showed a significant increase in the proportion of positive results with the suspect lot (57.9% vs 0%; P < .001, McNemar test). The median tuberculosis antigen minus nil values were significantly higher in the suspect lot (0.52 IU/mL) than in the new lot (0 IU/mL; P < .001).
Figure 1.
The Quantiferon (QFT) surveillance graph showing daily positive rates. The bar graph displays the proportion of total QFT tests that were positive on each day the QFT was run in April through October 2013. The first arrow indicates the date a new lot was introduced and an increase in the positive test rate was noted. In August and September, lots A130105J, A1302206, and A130500S were used without a decline in the positivity rate (dates of switch denoted by arrows). The final arrow indicates the date the switch to lot A1302027 was made, resulting in a return to the baseline positivity rate.
The impact on the occupational health program and HCWs was significant. During the episode described, 544 individuals with positive QFT results were asked to return for retesting. The additional cost for the retesting materials and chest radiographs alone was estimated in excess of $30 000 (not accounting for productivity loss and labor). Fortunately, unnecessary treatment of patients with false-positive results was avoided. Through personal communication, we are aware of 7 other healthcare centers in the United States reporting elevated positive QFT rates in July–October 2013.
This most recent episode of false-positive QFTs reemphasizes the need for more rigorous quality assurance by the manufacturer.
Note
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Workplace safety and health topics. 2013. Available at: www.cdc.gov/niosh/topics/healthcare/ . Accessed 1 January 2013.
- 2.Slater M, Parsonnet J, Banaei N. Investigation of false-positive results given by the QuantiFERON-TB Gold In-Tube assay. J Clin Microbiol. 2012;50:3105–7. doi: 10.1128/JCM.00730-12. [DOI] [PMC free article] [PubMed] [Google Scholar]