Abstract
3,4-(±)-Methylenedioxymethamphetamine (MDMA) is a ring-substituted amphetamine derivative with potent psychostimulant properties. The neuropharmacological effects of MDMA are biphasic in nature, initially causing synaptic monoamine release, primarily of serotonin (5-HT), inducing thermogenesis and hyperactivity (5-HT syndrome). The long-term effects of MDMA manifest as a prolonged depletion in 5-HT, and structural damage to 5-HT nerve terminals. MDMA toxicity is in part mediated by an ability to inhibit the presynaptic 5-HT reuptake transporter (SERT). Using a SERT-knockout (SERT-KO) rat model, we determined the impact of SERT deficiency on thermoregulation, locomotor activity, and neurotoxicity in SERT-KO or Wistar-based wild-type (WT) rats exposed to MDMA. WT and SERT-KO animals exhibited the highest thermogenic responses to MDMA (four times 10 mg/kg, sc at 12 h intervals) during the diurnal (first and third) doses according to peak body temperature and area under the curve (∑°C × h) analysis. Although no differences in peak body temperature were observed between MDMA-treated WT and SERT-KO animals, ∑°C × h following the first MDMA dose was reduced in SERT-KO rats. Exposure to a single dose of MDMA stimulated horizontal velocity in both WT and SERT-KO rats, however, this effect was delayed and attenuated in the KO animals. Finally, SERT-KO rats were insensitive to MDMA-induced long-term (7 days) depletions in 5-HT and its metabolite, 5-hydroxyindole acetic acid, in both cortex and striatum. In conclusion, SERT deficiency modulated MDMA-mediated thermogenesis, hyperactivity and neurotoxicity in KO rats. The data confirm that the SERT is essential for the manifestation of the acute and long-term toxicities of MDMA.
Keywords: MDMA, SERT, thermoregulation, locomotor activity, neurotoxicity
The serotonergic system participates in a variety of behaviors, including mood, appetite, sleep, cognition, and in regulating the gastrointestinal and endocrine systems (Schloss and Williams, 1998). Disturbed serotonin (5-HT) homeostasis has been implicated in many disorders, mostly of a psychiatric nature, such as schizophrenia, eating disorders, obsessive compulsive disorders, and drug addiction (Murphy et al., 2004). 5-HT homeostasis is primarily regulated by the action of the SERT. This reuptake transporter belongs to the neurotransmitter transporter family that shares a 12 transmembrane domain structure. The extended N- and C-termini, localized in the cytoplasm, display putative phosphorylation sites that regulate transport activity. A long extracellular loop, containing N-glycosylation sites, is important for transport assembly (Blakely et al., 1994). The SERT exchanges one internal K+ ion for a molecule of 5-HT and NaCl, mediating the reuptake of 5-HT from the synaptic space following its release during neurotransmission (Rudnick and Clark, 1993). Similar to other biogenic amine transporters, the SERT is the site of action for many antidepressant compounds, such as, fluoxetine (Prozac) (Schloss and Williams, 1998), in addition to psychostimulant drugs including 3,4-(±)-methylenedioxymethamphetamine (MDMA) (Rothman and Baumann, 2003).
The SERT has long been implicated in the mechanism of action of a number of 5-HT depleting agents, including p-chloroamphetamine, fenfluramine, 3,4-methylenedioxyamphetamine (MDA), and MDMA. These compounds share the ability to elicit 5-HT release (Berger et al., 1992; Crespi et al., 1997), with subsequent sustained depletions in 5-HT and 5-hydroxyindole acetic acid (5-HIAA) (Clineschmidt et al., 1976; Ricaurte et al., 1985). SERT inhibitors prevent amphetamine-stimulated synaptosomal 5-HT release (Berger et al., 1992). Coadministration of MDMA with benzylpiperazine derivatives, which are weak SERT inhibitors, attenuates 5-HT deficiency in vivo (Hashimoto et al., 1992), suggesting that MDMA interacts with the SERT at the 5-HT transport site. Pretreatment of rats with fluoxetine, a potent SERT blocker, 2 and 4 days prior to MDMA administration completely protected against 5-HT depletion and reductions in 5-HT uptake sites (Sanchez et al., 2001). The acute psychological effects of MDMA in humans were also reduced by citalopram, another 5-HT reuptake inhibitor (Liechti et al., 2000). Collectively, the studies suggest the participation of the SERT in the acute and long-term effects of MDMA on the serotonergic neurotransmitter system. Furthermore, it is postulated that the SERT serves as a carrier for the entry of MDMA, and other amphetamines, into serotonergic neurons, and mediates the rapid efflux of 5-HT into the synapse (Fuller, 1980). Morphological damage to serotonergic nerve terminals in various regions of the central nervous system can also be a consequence of exposure to these amphetamines O'Hearn et al., 1988(Callahan et al., 2001).
To determine the effects of SERT function in the acute and long-term toxicities of MDMA, we used a SERT knockout (SERT-KO, SERT−/−) rat model previously generated by N-ethyl-N nitrosurea (ENU)-driven targeted selected mutagenesis (Smits et al., 2006). Biochemical analysis revealed that mutant SERT mRNA is targeted for nonsense-mediated decay, and [3H] citalopram binding to brain slices is absent in SERT-KO rats, suggesting that SERT expression and function is completely abolished. SERT-KO animals exhibit a reduction in the maximal rate of 5-HT uptake into hippocampal synaptosomes, a ninefold increase in basal extracellular 5-HT levels, and decreases in tissue 5-HT content and depolarization-induced 5-HT efflux. In contrast, no changes are observed in 5-HT synthesis and degradation or major adaptations in other monoamine neurotransmitter systems (Homberg et al., 2007).
The restriction of neurobiological changes in SERT-KO rats to the serotonergic system provides a unique research tool to examine the effect of constitutive SERT dysfunction in MDMA toxicity, furthering our understanding of this protein in the mechanism of action of MDMA. Acute monoamine release and subsequent activation of postsynatic monoamine receptors following MDMA treatment is accompanied by significant increases in body temperature (Shioda et al., 2008) and hyperactivity (Ball et al., 2003; Ball and Rebec, 2005) in rats. Evidence suggests that body temperature elevation in response to MDMA requires the concomitant activation of sympathetic nervous system pathways and the hypothalamic-pituitary-adrenal axis, reflective of a thermogenic response (Mills et al., 2004). In the present study, we compared the effects of MDMA on body temperature and locomotor activity in WT and SERT-KO animals. MDMA neurotoxicity in SERT-deficient rats was determined by measuring indoleamine (5-HT and 5-HIAA) concentrations in serotonergic-rich brain regions, 7 days after MDMA treatment, since neurochemical changes appear as early as 1 week after MDMA exposure (Schmidt, 1987).
MATERIALS AND METHODS
Subjects
Male Wistar wild-type (SERT+/+) rats were obtained from Harlan Laboratories (Indianapolis, IN) and SERT knockout (SERT−/−) Wistar rats (both 150–200 g) were purchased from GENOWAY S.A. (France). Male rats were exclusively used in this study, based on previous biochemical and functional characterization of the genetic SERT-KO male rat model (Homberg et al., 2007). Moreover, sex differences in response to MDMA toxicity have been well-described (Walker et al., 2007; Wallinga et al., 2011). Animals were housed in groups of three per cage and maintained on a 12 h light/dark cycle. Food and water were provided ad libitum. Animals were given a week to acclimate prior to initiating experiments. Procedures were carried out in accordance with the University of Arizona Institutional Animal Care and Use Committee.
Dose regimen
(±)-MDMA-HCl was obtained from the National Institute on Drug Abuse (NIDA) (Bethesda, MD) and Sigma-Aldrich (St Louis, MO). Wistar WT and SERT-KO rats received four doses of either MDMA (10 mg/kg, sc) or 0.9% saline 12 h apart. The number of animals used for each treatment group were as follows: WT saline (n = 6–10), WT MDMA (n = 6–10), SERT-KO saline (n = 4–7) and SERT-MDMA (n = 6–8). Principles of interspecies scaling predict a dose of 1.28 mg/kg (96 mg/75 kg) in humans to be equivalent to a neurotoxic dose of MDMA of 20 mg/kg in rats (Ricaurte et al., 2000), which is twice the amount used for this study; however, a single dose of 10 mg/kg is sufficient to induce MDMA neurotoxicity (Schmidt, 1987). Recreational doses of MDMA have been found to range from 75 to 125 mg of (±)-MDMA for a single dose, which are found within the neurotoxic threshold dictated by interspecies scaling (Ricaurte et al., 2000). Multiple neurotoxic doses of MDMA were used to simulate party raves and similar weekend-long gatherings, where users ingest multiple drug doses.
Body temperature
Mini subcue dataloggers from SubCue (Calgary, Canada) were implanted into the peritoneal cavity of rats. Animals were anesthetized with isoflurane (5% in air) and kept under isoflurane (2% in air) for the duration of the surgery. A small incision (1.5 cm) was made along the top of the right leg, where the subcue datalogger was inserted. Rats were given a 3–5-day recovery period. Body temperature was monitored for 1 h prior to any treatment to establish a baseline, and 48 h posttreatment in intervals of 3 min. Ambient temperature was kept at ∼22°C prior and during the entire treatment course.
Locomotor activity
Animals were placed inside open-field activity cages (Coulbourn Instruments) consisting of a clear Plexiglas square box (3.25 in. × 10 in. × 7 in.) crisscrossed by three photobeam sensor rings. Rats were kept in these cages for 30 min prior to any data recording to allow them to acclimate to the new environment. Computer software supplied by the manufacturer recorded locomotor activity for ∼3 h in 15 min bins, which included a 45 min baseline period and a 2 h posttreatment period. Both horizontal and vertical activities were recorded after the first dose of MDMA. Horizontal activity was measured as floor plane movement velocity (centimeter per minute) and vertical activity is reported as the number of interruptions of rearing photobeams (entries).
Neurotransmitter isolation
To access long-term MDMA neurotoxicity in SERT-deficient rats, indoleamine concentrations were measured in serotonergic-rich brain regions, 7 days after MDMA treatment. Neurochemical changes appear as early as 1 week after MDMA exposure (Schmidt, 1987). However, these adverse changes can persist for months, and even up to 1 year posttreatment 5-HT content remains 40–50% reduced in rat cortex (De Souza et al., 1990). Moreover, disruption of tissue indoleamine levels serve as indirect markers of MDMA serotonergic neurodegeneration, paralleling histological abnormalities (Fischer et al., 1995) and functional deficits (Marston et al., 1999). Thus, animals were euthanized via CO2 asphyxiation followed by decapitation, 1 week after the final dose of MDMA. Brains were excised and microdissected, collecting the frontal cortex and striatum for neurotransmitter concentration analysis. Tissue was weighed and suspended in 10 volumes of ice-cold 0.1M perchloric acid (134μM EDTA and 263μM octane-sulfonic acid sodium salt). The tissue was then sonicated for 15 s followed by centrifugation at 16,000 × g (4°C) for 20 min. Supernatant was filtered at 0.45 μm and used for monoamine detection via high performance liquid chromatography coupled to a coulometric electrode array system (HPLC-CEAS).
HPLC-CEAS
Neurotransmitter content was assayed using a Shimadzu 10ADvp system equipped with an ESA C-18, 3 μm, 4.6 × 80 mm column (Dionex, MA) coupled to an ESA Model 5600A CoulArray system (Dionex). Mobile phase consisted of 35mM citric acid, 54mM sodium acetate, 324μM octane-sulfonic acid sodium salt, 171μM EDTA, 3% (vol/vol) methanol, 3% (vol/vol) acetonitrile, pH 4.0. Flow rate was 0.8 ml/min. Potentials were set at +50, +150, +300, and +450 mV. Tissue samples (50 μl per injection) were analyzed, and peak areas were compared with a standard curve of dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), 5-HT, and 5-HIAA to achieve quantitation.
Statistics
Results are presented as absolute values and expressed as the mean ± SE (n = 4–10). Effects of MDMA on core body temperature were analyzed by summarizing individual temperature responses after each MDMA dose as peak body temperature and area under the curve (∑°C × h) in order to avoid discrepancies in the timing response of individual animals. ∑°C × h following MDMA-related temperature spikes was estimated using GraphPad Prism 5. Effects of MDMA on peak temperature and ∑°C × h after each MDMA dose were analyzed by one-way ANOVA and subsequent “protected” Fisher-Hayter pairwise comparisons. To ensure the nominal 5% significance level, we applied a Bonferroni p-value correction to the tests associated with overall model significance for each dose period (i.e., significance level for individual tests was 0.05/4 = 0.0125 for each dose period). Once this standard was met, we proceeded hierarchically toward more specific comparisons. Effects of MDMA on locomotor activity were examined by two-way ANOVA (treatment group × time interval) with time as a repeated measure, followed by Bonferroni's post hoc multiple comparisons. Total horizontal velocity, total vertical activity, and neurotransmitter concentrations were analyzed by one-way ANOVA followed by Fisher-Hayter post hoc tests. All analyzes were performed using Stata 11.0 and GraphPad Prism 5 softwares.
RESULTS
SERT Deficiency Attenuates ∑°C × h but not Peak Body Temperature in SERT-KO Rats after Multiple MDMA Dosing
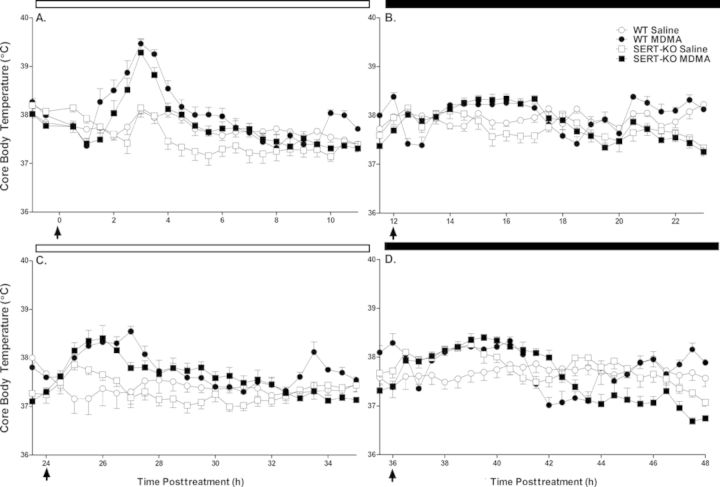
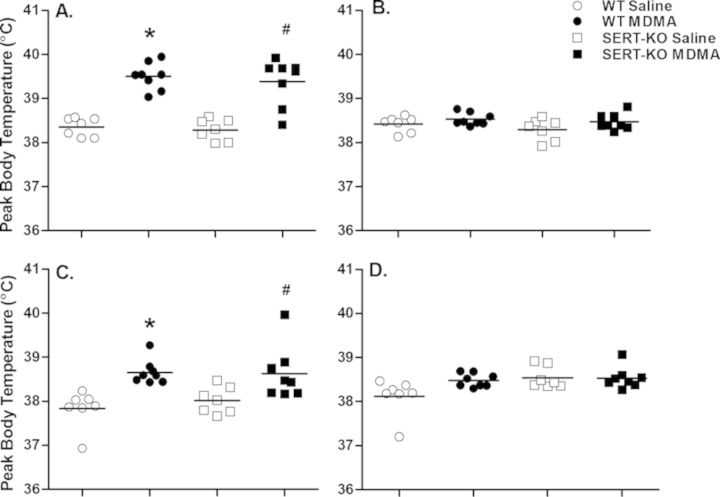
Saline control animals showed normal fluctuations in body temperature over the 48 h course of treatment, in synchrony with the rodent circadian cycle. During the dark phase, core body temperature slightly rises as a consequence of the increase in physiological and behavioral activity of these nocturnal animals, whilst modestly decreasing during the light, and less active phase. MDMA (10 mg/kg, sc) induced an acute increase in core body temperature in both WT and SERT-KO rats upon exposure to multiple doses (Fig. 1). MDMA-mediated thermogenesis was most prominently observed after the first and third MDMA doses, reaching peak body temperature 2–3 h following drug administration. One-way ANOVA of peak body temperature calculated after each MDMA dose revealed significant differences between treatment groups after the first and third diurnal MDMA doses [dose 1: F(3, 27) = 27.3, p < 0.0001; dose 3: F(3, 27) = 7.3, p < 0.001] (Fig. 2). No significant differences in peak body temperature between the saline and MDMA groups were observed for either the WT or SERT-KO animals after the doses administered during the dark phase of the rodent life cycle [dose 2: F(3, 27) = 2.2, p = 0.12; dose 4: F(3, 27) = 2.9, p = 0.057]. Fisher-Hayter post hoc tests revealed that peak body temperatures following the first (39.5°C ± 0.1°C, p < 0.05) and third (38.7°C ± 0.1°C, p < 0.05) MDMA injections were significantly higher in MDMA-treated WT animals compared with their saline counterparts (38.3°C ± 0.07°C, 37.9°C ± 0.1ºC, respectively). Similarly, SERT-KO MDMA rats reached higher peak body temperatures after the first (39.4°C ± 0.2ºC, p < 0.05) and third (38.6°C ± 0.2ºC, p < 0.05) MDMA injections in comparison to SERT-KO saline controls (38.3°C ± 0.09°C, 38.0°C ± 0.1ºC, respectively). Post hoc tests revealed no significant differences in peak body temperature between the WT MDMA and the SERT-KO MDMA groups. Finally, peak body temperature was not significantly different between WT and SERT-KO control rats at any of the dose intervals.
FIG. 1.
MDMA-induced thermogenesis occurs in both WT and SERT-KO rats after repeated drug administration. Animals received four consecutive doses of MDMA (10 mg/kg, sc) at 12 h intervals. Core body temperature was recorded using subcue dataloggers implanted into the peritoneal cavity of experimental animals as previously described in the Materials and Methods section. Data points display the recorded body temperatures averaged over 30 min intervals for the first (A), second (B), third (C), and fourth (D) MDMA doses. Black arrows indicate the times of drug administration, zero denoting the start of the MDMA treatment course. White and black bars at the top of the graphs indicate the duration of the light and dark phases of the 12 h light/dark rodent cycle. Results are expressed as mean ± SE (n = 7–8).
FIG. 2.
MDMA increases peak body temperature in Wistar rats regardless of genotype. Data points show individual peak body temperatures and horizontal lines represent the mean peak body temperature (n = 7–8) for the different treatment groups following the first (A), second (B), third (C), and fourth (D) sc MDMA doses. Values are significantly different from WT saline at *p < 0.05, and from SERT-KO saline at #p < 0.05.
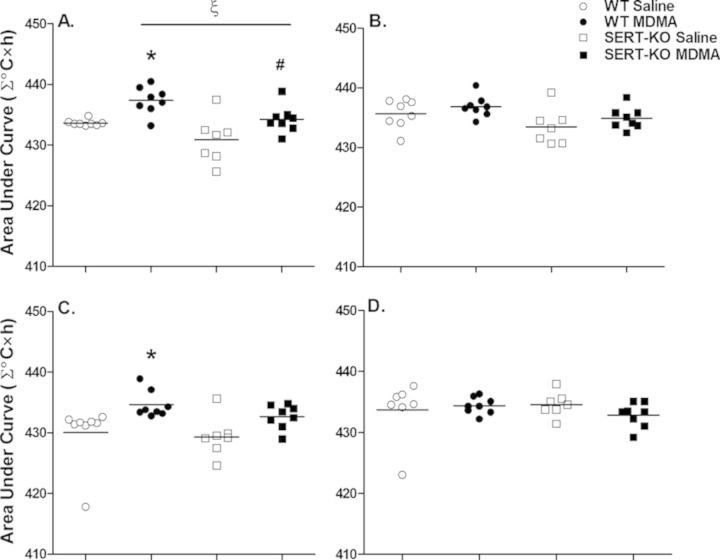
Area under the curve (∑°C × h) over a 12 h treatment period was estimated following each MDMA dose for further analysis of the MDMA-induced thermogenic effect (Fig. 3). One-way ANOVA showed significant overall differences in ∑°C × h between the treatment groups after the first and third MDMA doses [dose 1: F(3, 27) = 8.9, p = 0.0003; dose 3: F(3, 27) = 4.1, p < 0.01]. Nocturnal MDMA doses showed no significant differences in ∑°C × h among the treatment groups [dose 2: F(3, 27) = 2.9, p = 0.056; dose 4: F(3, 26) = 0.56, p = 0.65]. Post-hoc analysis revealed that WT MDMA rats exhibited higher ∑°C × h after the first (437.4 ± 0.8 ∑°C × h, p < 0.05) and third (434.6 ± 0.8 ∑°C × h, p < 0.05) MDMA-mediated temperature elevations than their saline controls (433.7 ± 0.2, 430.0 ± 1.8 ∑°C × h, respectively). SERT-KO animals exhibited significantly higher ∑°C × h following the first (434.3 ± 0.8 ∑°C × h, p < 0.05) MDMA temperature boost compared with their saline counterparts (430.9 ± 1.5 ∑°C × h). However, when compared with MDMA-treated WT rats, ∑°C × h at this MDMA dose was significantly lower in the SERT-KO rats (p < 0.05). No significant differences in ∑°C × h were detected between WT and SERT-KO saline animals throughout the 48 h treatment course. In summary, both WT and SERT-KO rats displayed a similar thermoregulatory response to multiple MDMA dosing in relation to peak body temperature, showing a greater MDMA-related effect after the two diurnal doses. MDMA-treated WT rats had similar increases in ∑°C × h after these MDMA doses; however, ∑°C × h in SERT-KO MDMA animals was overall attenuated.
FIG. 3.
SERT-KO rats display attenuated ∑°C × h after multiple MDMA doses. Data shows the average (n = 7–8) ∑°C × h over the 12 h treatment period following the first (A), second (B), third (C), and fourth (D) MDMA-mediated body temperature spikes. Values are significantly different from WT saline values at *p < 0.05, from SERT-KO saline values at #p < 0.05, and from WT MDMA values at ξp < 0.05.
SERT Deficiency Ameliorates MDMA-Induced Hyperactivity in SERT-KO Rats
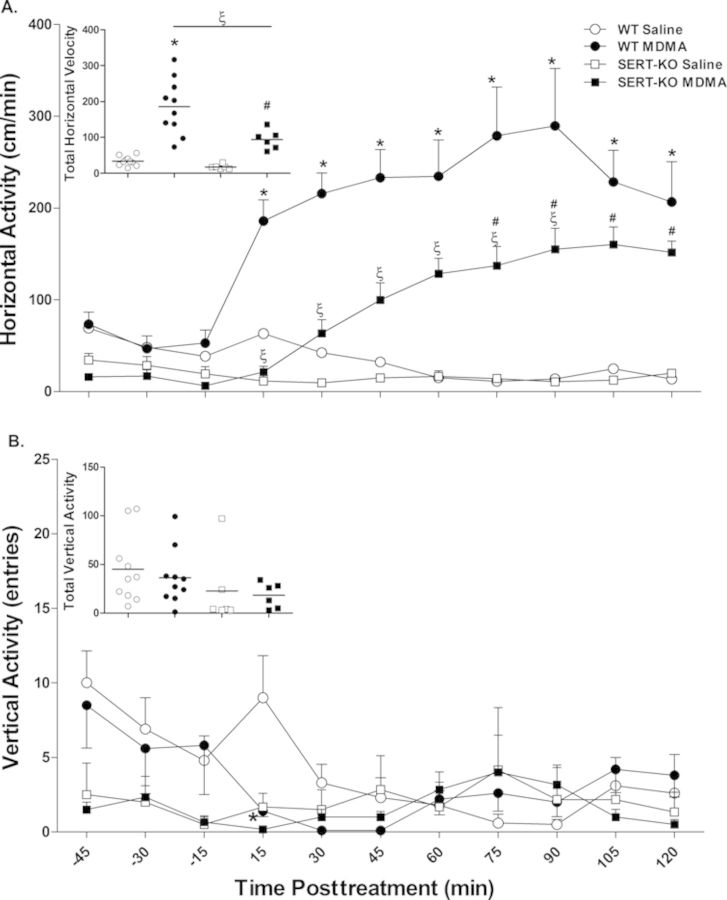
MDMA-mediated hyperactivity was assessed using activity chambers that recorded horizontal and vertical locomotor activities after the first MDMA dose (Fig. 4). Horizontal locomotor activity was sharply stimulated in WT Wistar rats within 15 min of MDMA (10 mg.kg, sc) administration, reaching maximal horizontal velocity (289 ± 63 cm/min) 90 min posttreatment and gradually declining thereafter (Fig. 4A). SERT-KO animals also displayed an increase in horizontal activity upon MDMA dosing; however, this effect was delayed by 60 min. Horizontal velocity increased more gradually in MDMA-treated SERT-KO animals compared with WT counterparts, achieving maximal velocity (160 ± 19 cm/min) 105 min after drug administration. Two-way repeated measures ANOVA of horizontal velocity revealed significant main effects of treatment group [F(3, 280) = 24.7, p < 0.0001], significant main effects of time interval [F(10, 280) = 10.4, p < 0.0001], and a significant treatment group × time interval interaction [F(30, 280) = 8.7, p < 0.0001]. Further Bonferroni's post hoc comparisons confirmed significant increases in horizontal velocity in MDMA-treated WT rats compared with their saline counterparts throughout the posttreatment period (15–120 min, p < 0.001). SERT-KO MDMA rats displayed significant increases in horizontal velocity compared with their saline controls 75–120 min (p < 0.05) after drug administration. However, MDMA-mediated induction in horizontal velocity was significantly lower in SERT-KO rats in contrast to WT counterparts between 15 and 90 min posttreatment (p < 0.05). No significant differences in horizontal velocity were observed between WT and SERT-KO saline rats during the pre- and post- treatment periods. Similarly, one-way ANOVA revealed significant differences in total horizontal velocity averaged over the entire measurement period between the treatment groups [F(3, 28) = 24.4, p < 0.0001]. Fisher-Hayter post hoc comparisons showed MDMA significantly induced total horizontal velocity in both WT (186 ± 24 cm/min, p < 0.05) and SERT-KO (94 ± 11 cm/min, p < 0.05) rats compared with their saline controls (34 ± 4 and 17 ± 3 cm/min, respectively; Fig. 4A insert). However, the effects of MDMA on total horizontal velocity were significantly higher in WT rats in comparison to SERT-KO counterparts (p < 0.05). Overall, the results showed that MDMA-mediated induction in horizontal velocity is delayed and attenuated in SERT-deficient Wistar rats.
FIG. 4.
SERT-KO rats display delayed and attenuated horizontal movement velocity after a single dose of MDMA. Locomotor activity was measured by placing animals into open-field activity chambers. Baseline levels of locomotion were measured for 45 min, followed by a 120 min posttreatment period in which MDMA (10 mg/kg, sc) was administered. Figures show the mean (SE ± 6–10) horizontal (A) and vertical (B) activities at each time interval. Scatter plots display total horizontal velocity and total vertical activity over the entire 165 min treatment course. Values are significantly different from WT saline values at *p < 0.05, from SERT-KO saline values at #p < 0.05, and from WT MDMA values at ξp < 0.05.
Figure 4B illustrates changes in vertical locomotor activity during the pre- and post- treatment periods in WT and SERT-KO male rats. Two-way repeated measures ANOVA of vertical activity revealed significant time interval effects [F(10, 280) = 2.2, p = 0.018] and a treatment group × time interval interaction [F(30, 280) = 1.8, p = 0.0076] but no treatment group effects [F(3, 280) = 1.2, p = 0.32]. Post-hoc analysis showed a significant decrease in vertical activity in MDMA-treated WT rats compared with their saline counterparts 15 min (p < 0.05) after drug administration. No changes in vertical activity were detected in SERT-KO MDMA animals compared with their saline controls or WT counterparts. Vertical activity was not significantly different between WT and SERT-KO saline rats throughout the treatment course. Furthermore, one-way ANOVA analysis revealed no significant differences in total vertical activity among the treatment groups [F(3, 28) = 1.2, p = 0.32] (Fig. 4B insert). In summary, MDMA induced a transient decrease in vertical activity in WT rats and no changes in vertical locomotion in SERT-KO rats.
SERT Deficiency Prevents Indoleamine Depletion with MDMA Treatment in SERT-KO Rats
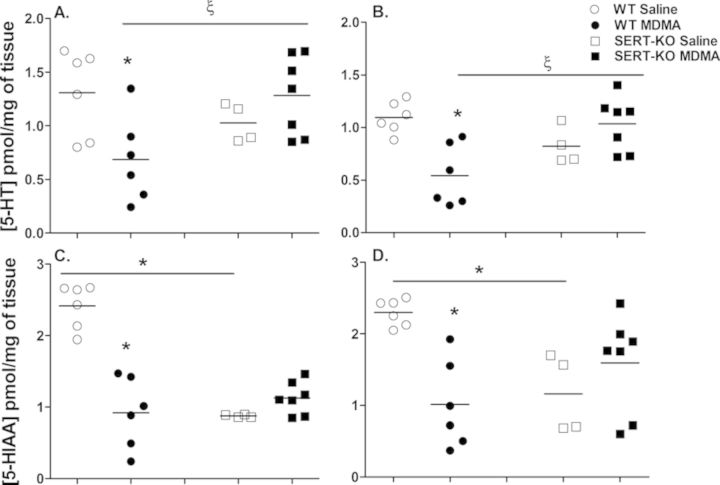
In order to evaluate the effect of SERT protein dysfunction on long-term MDMA neurotoxicity, neurotransmitter concentrations were determined in brain regions heavily innervated by serotonergic nerve terminals, 7 days after the last dose of MDMA. Saline controls showed normal indoleamine concentrations in the cortex (Cx) and striatum (St) of WT [5-HTCx = 1.31 ± 0.16, 5-HTSt = 1.095 ± 0.061, 5-HIAACx = 2.41 ± 0.13, 5-HIAASt = 2.30 ± 0.076 pmol/mg of tissue] and SERT-KO [5-HTCx = 1.03 ± 0.089, 5-HTSt = 0.82 ± 0.088, 5-HIAACx = 0.88 ± 0.0097, 5-HIAASt = 1.16 ± 0.27 pmol/mg of tissue] animals (Fig. 5). One-way ANOVA analysis revealed significant differences in 5-HT and 5-HIAA levels in both brain regions among treatment groups [5-HTCx (F(3, 19) = 3.9, p = 0.025), 5-HTSt (F(3, 19) = 7.1, p = 0.0021), 5-HIAACx (F(3, 19) = 29.0, p < 0.0001), 5-HIAASt (F(3, 19) = 6.4, p = 0.0036)]. SERT deficient rats exhibited lower basal levels of 5-HT (Cx = 21%, St = 25%) and 5-HIAA (Cx = 64%, p < 0.05; St = 49%, p < 0.05; Fisher-Hayter post hoc tests) in these two brain regions in contrast to the WT control group. Reduced tissue levels of 5-HT and 5-HIAA in serotonergic-rich brain regions of SERT-KO rats are consistent with previous reports (Homberg et al., 2007). Fisher-Hayter post hoc tests also confirmed significant decreases in cortical and striatal 5-HT concentrations with MDMA (four times 10 mg/kg, sc) treatment of 48% (p < 0.05) and 50% (p < 0.05), respectively and in cortical and striatal 5-HIAA concentrations of 62% (p < 0.05) and 56% (p < 0.05) in WT rats. In contrast, MDMA-treated SERT-KO rats exhibited higher 5-HT (Cx = 25%, St = 26%) and 5-HIAA (Cx = 29%, St = 37%) concentrations in these brain regions compared with their saline counterparts, however, these changes were not significant by post-hoc analysis. Moreover, tissue indoleamine levels were higher in SERT-KO MDMA rats in both cortex (5-HT = 87%, p < 0.05; 5-HIAA = 23%) and striatum (5-HT = 91%, p < 0.05; 5-HIAA = 58%) in comparison to WT counterparts. Overall, neurotransmitter analyses revealed that SERT-KO animals are protected against MDMA-mediated depletion of indoleamines in serotonergic-rich brain regions.
FIG. 5.
MDMA-mediated 5-HT and 5-HIAA depletion is abolished in SERT-KO rats following multiple drug administrations. 5-HT and 5-HIAA levels were measured from cortex (A and C) and striatum (B and D) 7 days after the last of four MDMA (10 mg/kg, sc) injections by HPLC-CEAS as described in the Materials and Methods section. Results show individual concentrations of these indoleamines in each treatment group (n = 4–7). Values are significantly different from WT saline values at *p < 0.05, and from WT MDMA values at ξp < 0.05.
Long-term neurochemical changes in the dopaminergic system of WT and SERT-KO male rats were determined by measuring striatal DA and DOPAC concentrations 7 days after the last MDMA dose (Fig. 6). MDMA neurotoxicity selectively damages the serotonergic neurotransmitter system in rats (Callahan et al., 2001; Ricaurte et al., 1985), therefore, evaluation of tissue catecholamine levels is used as a control. Saline animals showed baseline levels of DA and DOPAC in the striatum of WT [DA = 55.50 ± 2.91, DOPAC = 15.39 ± 0.59 pmol/mg of tissue] and SERT-KO rats [DA = 61.78 ± 2.92, DOPAC = 17.84 ± 4.49 pmol/mg of tissue]. One-way ANOVA analysis revealed no significant differences in striatal catecholamine content among the different treatment groups [DA: F(3, 19) = 1.6, p = 0.21, DOPAC: F(3, 19) = 1.9, p = 0.17]. In summary, MDMA had no effect on tissue DA and DOPAC concentrations in both WT and SERT-KO rats.
FIG. 6.
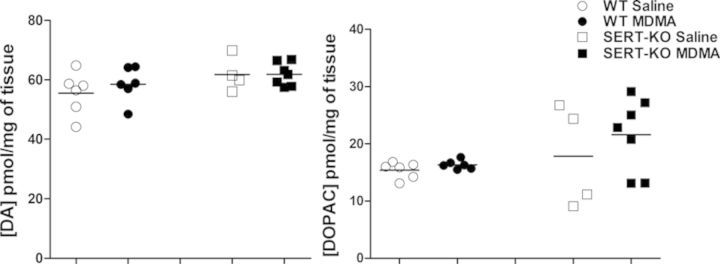
MDMA had no effect on tissue DA and DOPAC levels in WT and SERT-KO rats following multiple drug administrations. Striatal DA and DOPAC concentrations were measured 7 days after the last of four MDMA (10 mg/kg, sc) injections by HPLC-CEAS as described in the Materials and Methods section. Results show individual concentrations of these catecholamines in each treatment group (n = 4–7).
DISCUSSION
The primary objective of this study was to determine the influence of SERT deficiency and of disturbances in 5-HT homeostasis on the physiological, behavioral, and neurochemical effects of MDMA. Our data reveal that SERT gene deletion modulates the acute effects of MDMA including thermoregulation (Figs. 1–3) and hyperactivity (Fig. 4), whilst completely abolishing the long-term neurotoxic effects (Fig. 5).
MDMA causes dose-dependent increases in body temperature that can lead to hyperthermia (Dafters, 1994), and multiple doses of MDMA increased body temperature in both WT and SERT-KO rats; however, this thermogenic response was most pronounced after the first and third MDMA doses, as measured by peak body temperature and ∑°C × h. Differences in the body temperature response after multiple MDMA doses, in both WT and SERT-KO animals, correlated with thermogenic fluctuations of the circadian cycle. In laboratory rats maintained in a 12 h light/dark cycle, circadian changes in body temperature and locomotor activity occur in synchrony, and their acrophase (time period in the cycle at which the peak happens) occurs during nighttime (Benstaali et al., 2001). It is possible that the thermogenic effect of nocturnal MDMA doses was diminished in both WT and SERT-KO rats compared with diurnal MDMA doses due to parallel increases in body temperature related to circadian rhythmicity in these animals.
Overall, SERT-KO animals displayed a different thermoregulatory effect than their WT counterparts in response to repeated MDMA dosing (four times 10 mg/kg, sc). Indeed, similar increases in peak body temperature occurred following the first and third, diurnal MDMA doses 2; however, ∑°C × h was significantly attenuated in MDMA-treated SERT-KO rats 3. The findings suggest that SERT-KO rats are exposed to reduced periods of body temperature elevation following multiple MDMA challenges, which could render them less susceptible to the long-term neurotoxic effects of this drug. Despite a lack of consensus in the literature on the precise role of increased body temperature in MDMA neurotoxicity, some studies report a positive correlation between the magnitude of the thermogenic response and the degree of neurotoxicity (Broening et al., 1995; Malberg and Seiden, 1998). In particular, Malberg and Seiden (1998) demonstrated that low ambient temperatures (20–24°C) attenuate area under the curve (AUC) and prevent 5HT and 5-HIAA depletions, whereas elevated ambient temperatures (28–30°C) potentiate both AUC and the neurotoxic response to MDMA.
SERT-KO rats are insensitive to d-fenfluramine-induced hypothermia (Homberg et al., 2007), a process driven by the interaction of d-fenfluramine with the SERT. Similarly, the thermogenic effects of MDMA are in part mediated by its interaction with the SERT at presynaptic 5-HT terminals, and the subsequent release of 5-HT into the synaptic cleft. Although MDMA is mainly an indirect 5-HT agonist, it also acts on DA and norepinephrine (NE) reuptake transporters, causing DA and NE efflux (Fitzgerald and Reid, 1993). Indeed, pretreatment with D1 receptor antagonists prevents MDMA-mediated hyperthermia in rats, highlighting the importance of DA in MDMA thermogenesis (Shioda et al., 2008). MDMA-evoked 5-HT efflux may indirectly induce DA release via interaction with 5-HT2 receptors, and MDMA-mediated hyperthermia is inhibited with 5-HT2A receptor antagonist pretreatment (Shioda et al., 2008). Thus, it is possible that the attenuation in ∑°C × h in SERT-KO rats following the diurnal MDMA-induced temperature spikes results from a disturbance in 5-HT release in the absence of functional SERT. The similar peak body temperature response in KO and WT animals after the diurnal MDMA doses likely reflects the maintenance of MDMA-mediated release of other monoamines in SERT-KO rats. Consistent with this view, studies in SERT-KO mice revealed minimal to no changes in extracellular 5-HT concentrations in the prefrontal cortex after MDMA treatment, with similar increases in extracellular striatal DA levels ocurring in WT and SERT-KO mice (Trigo et al., 2007). In vivo microdialysis studies measuring extracellular monoamine levels in MDMA-treated SERT-KO rats are necessary to confirm this hypothesis.
MDMA-mediated monoamine release also results in dose-dependent behavioral changes that include enhanced locomotion, and all major features of the 5-HT syndrome. The increased locomotor activity observed with MDMA is likely a consequence of striatal DA release. Studies in freely moving rats have shown that DA neurotransmission and locomotor activity stimulation are blocked by treatment with D2 receptor antagonists prior to MDMA exposure (Ball et al., 2003). The role of 5-HT release in MDMA-induced locomotion is thought to occur via indirect modulation of the striatal DA system. Pharmacological blockade of 5-HT2A receptors decreases MDMA-mediated striatal excitation in parallel with a suppression in locomotor activity (Ball and Rebec, 2005). Our results reveal a significant delay, and attenuation, in MDMA-induced horizontal velocity in SERT-deficient rats compared with WT counterparts (Fig. 4A). We hypothesize that direct MDMA-evoked DA efflux is most likely unaltered in SERT-KO rats, whereas perturbations in carrier-mediated 5-HT release in these animals affects striatal DA neurotransmission, which is manifested as a reduction in MDMA-induced horizontal hyperactivity. In contrast, a single dose of MDMA had no effect on total vertical activity in both WT and SERT-KO male rats compared with saline controls (Fig. 4B), which is consistent with previous findings that demonstrate minimal to no changes in vertical locomotion with MDMA treatment in male rats compared with female counterparts and saline controls (Palenicek et al., 2005; Walker et al., 2007). However, MDMA-treated WT male rats displayed an acute decrease in vertical locomotion, a phenomenon that has been previously reported (Timar et al., 2003). Finally, in agreement with a study analyzing cocaine supersensitivity in SERT-KO rats (Homberg et al., 2008), our results show baseline locomotor activity levels in SERT-KO rats were similar to the levels in WT animals despite their depressive phenotype (Olivier et al., 2008).
SERT deficiency in SERT-KO rats had profound effects on the long-term neurotoxicity of MDMA. MDMA selectively targets the serotonergic system in both humans (Bolla et al., 1998) and rats (Callahan et al., 2001). The biochemical and histological hallmarks of MDMA neurotoxicity manifest as depletions in 5-HT and 5HIAA levels in neocortex, striatum, and hippocampus, and decreases in presynaptic SERT protein (Xie et al., 2006). Immunohistochemical analysis reveal degeneration of serotonergic nerve terminals, showing decreases in terminal density, thickening of preterminal fibers, and fragmentation of 5-HT axons in serotonergic-rich brain regions with no aberrant anatomical changes to cell bodies in the raphe nuclei (O’Hearn et al., 1988).
MDMA-mediated indoleamine depletion was completely abolished in SERT-KO rats in both cortex and striatum (Fig. 5). In fact, 5-HT and 5-HIAA levels were slightly higher in MDMA-treated SERT-KO animals compared with saline controls. The present findings therefore support the critical role for the SERT in the neurotoxic response to MDMA. Although many hypotheses on the molecular mechanism of long-term MDMA neurotoxicity have been put forward, the involvement of the SERT in this process is indisputable. The fact that reductions in presynaptic SERT protein in 5-HT neurons are one of the hallmarks of MDMA neurotoxicity is perhaps most revealing. The selectivity of MDMA toward the serotonergic system can in part be attributed to its higher affinity for the SERT compared with the DA transporter (DAT) or the NE transporter (NET) in rat synaptomes (Rothman et al., 2001). In vitro studies have demonstrated MDMA inhibits the SERT and reverses its function, causing major neurotransmitter efflux via calcium dependent (Crespi et al., 1997) and calcium independent (Johnson et al., 1986) mechanisms, to a greater extent than the DAT. Furthermore, 5-HT uptake inhibitors, such as fluoxetine, can block in vivo MDMA-evoked 5-HT and DA release (Gudelsky and Nash, 1996) and neurotoxicity in rat brain (Sanchez et al., 2001) but DAT uptake inhibitors, such as GRB 12909, fail to prevent MDMA-mediated DA release (Mechan et al., 2002).
Neurotransmitter analyses also confirmed disturbances in 5-HT homeostasis of SERT-KO control rats, with significant reductions in basal levels of 5-HIAA in both cortex and striatum (Fig. 5). No differences were found between basal levels of striatal DA or DOPAC in WT and KO control animals (Fig. 6), suggesting no major adaptations in the dopaminergic system of SERT-KO rats, and most likely in other nonserotonergic neurotransmitter systems, as previously demonstrated (Homberg et al., 2007). Furthermore, MDMA had no effect on tissue catecholamine content in the striatum, confirming that MDMA-induced neurotoxicity was restricted to the serotonergic system in both WT and SERT-deficient rats. Overall, it can be concluded that prevention of long-term MDMA neurotoxicity in SERT-KO rats is primarily due to the absence of functional SERT. A detailed immunohistological analysis of serotonergic neurons is necessary to confirm whether MDMA-mediated neurodegeneration is abolished in SERT-KO animals, as suggested by the neurotransmitter assay findings.
In summary, acute studies revealed prominent changes in the thermoregulatory and hyperlocomotor activity response in SERT-KO rats, presumably due to blockade of MDMA-mediated 5-HT release. Moreover, although SERT modulation and enhanced 5-HT release have been suggested to play a minor if any role in MDMA thermoregulation and increased locomotor activity, our findings clearly demonstrate that the SERT is indeed involved in these acute MDMA responses. Finally, SERT-KO animals exhibited resistance to long-term MDMA neurotoxicity, confirming previous pharmacological studies with selective 5-HT reuptake inhibitors. Disturbances in 5-HT homeostasis due to SERT deficiency can impact the immediate and long-lasting toxicities of MDMA in SERT-KO rats.
FUNDING
National Institute on Drug Abuse (DA023525); Environmental Toxicology of Complex Diseases Training Grant (5T32ES007091 to L.E.L.) from the National Institute of Environmental Health Sciences; Integrative Health Sciences Facility Core at the Southwest Environmental Health Sciences Center (P30 ES006694).
Acknowledgments
We thank the NIDA Drug Supply Program for kindly providing (±)-MDMA. We also thank Dr Gregory O. Dussor from the Department of Medical Pharmacology, College of Pharmacy at the University of Arizona for providing the locomotor activity chambers used in the behavioral studies. Special thanks to Dr Dean Billheimer from the Southwest Environmental Health Sciences Center, College of Pharmacy at the University of Arizona for his help and advice with the biostatistical analysis.
REFERENCES
- Ball K. T., Budreau D., Rebec G. V. Acute effects of 3,4-methylenedioxymethamphetamine on striatal single-unit activity and behavior in freely moving rats: Differential involvement of dopamine D1 and D2 receptors. Brain Res. 2003;994:203–215. doi: 10.1016/j.brainres.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Ball K. T., Rebec G. Role of 5-HT2A and 5-HT2C/B receptors in the acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on striatal single-unit activity and locomotion in freely moving rats. Psychopharmacology. 2005;181:676–687. doi: 10.1007/s00213-005-0038-z. [DOI] [PubMed] [Google Scholar]
- Benstaali C., Mailloux A., Bogdan A., Auzéby A., Touitou Y. Circadian rhythms of body temperature and motor activity in rodents: Their relationships with the light-dark cycle. Life Sci. 2001;68:2645–2656. doi: 10.1016/s0024-3205(01)01081-5. [DOI] [PubMed] [Google Scholar]
- Berger U. V., Gu X. F., Azmitia E. C. The substituted amphetamines 3,4-methylenedioxymethamphetamine, methamphetamine, p-chloroamphetamine and fenfluramine induce 5-hydroxytryptamine release via a common mechanism blocked by fluoxetine and cocaine. Eur. J. Pharmacol. 1992;215:153. doi: 10.1016/0014-2999(92)90023-w. [DOI] [PubMed] [Google Scholar]
- Blakely R. D., De Felice L. J., Hartzell H. C. Molecular physiology of norepinephrine and serotonin transporters. J. Exp. Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- Bolla K. I., McCann U. D., Ricaurte G. A. Memory impairment in abstinent MDMA (“Ecstasy”) users. Neurology. 1998;51:1532–537. doi: 10.1212/wnl.51.6.1532. [DOI] [PubMed] [Google Scholar]
- Broening H. W., Bowyer J. F., Slikker W., Jr Age-dependent sensitivity of the serotonergic neurotoxicant (+/−)-3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J. Pharmacol. Exp. Ther. 1995;275:325–333. [PubMed] [Google Scholar]
- Callahan B. T., Cord B. J., Ricaurte G. A. Long-term impairment of anterograde axonal transport along fiber projections originating in the rostral raphe nuclei after treatment with fenfluramine or methylenedioxymethamphetamine. Synapse. 2001;40:113–121. doi: 10.1002/syn.1032. [DOI] [PubMed] [Google Scholar]
- Clineschmidt B. V., Totaro J. A., McGuffin J. C., Pflueger A. B. Fenfluramine: Long-term reduction in brain serotonin (5-hydroxytryptamine) Eur. J. Pharmacol. 1976;35:211–214. doi: 10.1016/0014-2999(76)90318-6. [DOI] [PubMed] [Google Scholar]
- Crespi D., Mennini T., Gobbi M. Carrier-dependent and Ca2+-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3,4-methylendioxymethamphetamine, p-chloroamphetamine and (+)-fenfluramine. Br. J. Pharmacol. 1997;121:1735–1743. doi: 10.1038/sj.bjp.0701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafters R. I. Effect of ambient temperature on hyperthermia and hyperkinesis induced by 3,4-methylenedioxymethamphetamine (MDMA or “ecstacy”) in rats. Psychopharmacology. 1994;114:505–508. doi: 10.1007/BF02249342. [DOI] [PubMed] [Google Scholar]
- Fischer C., Hatzidimitriou G., Wlos J., Katz J., Ricaurte G. Reorganization of ascending 5-HT axon projections in animals previously exposed to the recreational drug (+/−)3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) J. Neurosci. 1995;15:5476–5485. doi: 10.1523/JNEUROSCI.15-08-05476.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J.L., Reid J. J. Interactions of methylenedioxymethamphetamine with monoamine transmitter release mechanisms in rat brain slices. Naunyn Schmiedebergs Arch. Pharmacol. 1993;347:313–323. doi: 10.1007/BF00167451. [DOI] [PubMed] [Google Scholar]
- Fuller R. W. Mechanism by which uptake inhibitors antagonizep-chloroamphetamine-induced depletion of brain serotonin. Neurochem. Res. 1980;5:241–245. doi: 10.1007/BF00964612. [DOI] [PubMed] [Google Scholar]
- Gudelsky G. A., Nash J. F. Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: Implications for serotonin-dopamine interactions. J. Neurochem. 1996;66:243–249. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Maeda H., Goromaru T. Effects of benzylpiperazine derivatives on the neurotoxicity of 3,4-methylenedioxymethamphetamine in rat brain. Brain Res. 1992;590:341–344. doi: 10.1016/0006-8993(92)91119-y. [DOI] [PubMed] [Google Scholar]
- Homberg J. R., De Boer S. F., Raaso H. S., Olivier J. D., Verheul M., Ronken E., Cools A. R., Ellenbroek B. A., Schoffelmeer A. N., Vanderschuren L. J., et al. Adaptations in pre- and postsynaptic 5-HT1A receptor function and cocaine supersensitivity in serotonin transporter knockout rats. Psychopharmacology (Berl.) 2008;200:367–380. doi: 10.1007/s00213-008-1212-x. [DOI] [PubMed] [Google Scholar]
- Homberg J. R., Olivier J., Smits B. M. G., Mul J. D., Mudde J., Verheul M., Nieuwenhuizen O. F. M., Cools A. R., Ronken E., Cremers T., et al. Characterization of the serotonin transporter knockout rat: A selective change in the functioning of the serotonergic system. Neuroscience. 2007;146:1662–1676. doi: 10.1016/j.neuroscience.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Johnson M. P., Hoffman A. J., Nichols D. E. Effects of enantiomers of MDA, MDMA and related analogues on 3H serotonin and 3H dopamine release from superfused rat brain slices. Eur. J. Pharmacol. 1986;132:269–276. doi: 10.1016/0014-2999(86)90615-1. [DOI] [PubMed] [Google Scholar]
- Liechti M. E., Baumann C., Gamma A., Vollenweider F. X. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology. 2000;22:513–521. doi: 10.1016/S0893-133X(99)00148-7. [DOI] [PubMed] [Google Scholar]
- Malberg J. E., Seiden L. S. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J. Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston H. M., Reid M. E., Lawrence J. A., Olverman H. J., Butcher S. P. Behavioral analysis of the acute and chronic effects of MDMA treatment in the rat. Psychopharmacology. 1999;144:67–76. doi: 10.1007/s002130050978. [DOI] [PubMed] [Google Scholar]
- Mechan A. O., Esteban B., O’Shea E., Elliott J. M., Colado M. I., Green A. R. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) to rats. Br. J. Pharmacol. 2002;135:170–180. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E. M., Rusyniak D. E., Sprague J. E. The role of the sympathetic nervous system and uncoupling proteins in the thermogenesis induced by 3,4-methylenedioxymethamphetamine. J. Mol. Med. (Berl.) 2004;82:787–799. doi: 10.1007/s00109-004-0591-7. [DOI] [PubMed] [Google Scholar]
- Murphy D. L., Lerner A., Rudnick G., Lesch K. P. Serotonin transporter: Gene, genetic disorders, and pharmacogenetics. Mol. Interv. 2004;4:109–123. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- Olivier J. D., Van Der Hart M. G., Van Swelm R. P., Dederen P. J., Homberg J. R., Cremers T., Deen P. M., Cuppen E., Cools A. R., Ellenbroek B. A. A study in male and female 5-HT transporter knockout rats: An animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- O’Hearn E., Battaglia G., De Souza E. B., Kuhar M. J., Molliver M. E. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: Immunocytochemical evidence for neurotoxicity. J. Neurosci. 1988;8:2788–2803. doi: 10.1523/JNEUROSCI.08-08-02788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palenicek T., Votava M., Bubenikova V., Horacek J. Increased sensitivity to the acute effects of MDMA (“ecstasy”) in female rats. Physiol. Behav. 2005;86:546–553. doi: 10.1016/j.physbeh.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Ricaurte G., Bryan G., Strauss L., Seiden L., Schuster C. Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals. Science. 1985;229:986–988. doi: 10.1126/science.4023719. [DOI] [PubMed] [Google Scholar]
- Ricaurte G. A., Yuan J., McCann U. D. (+/−)3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: Studies in animals. Neuropsychobiology. 2000;42:5–10. doi: 10.1159/000026664. [DOI] [PubMed] [Google Scholar]
- Rothman R. B., Baumann M. H. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rothman R. B., Baumann M. H., Dersch C. M., Romero D. V., Rice K. C., Carroll F. I., Partilla J. S. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rudnick G., Clark J. From synapse to vesicle: The reuptake and storage of biogenic amine neurotransmitters. Biochim. Biophys. Acta. 1993;4:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Sanchez V., Camarero J., Esteban B., Peter M. J., Green A. R., Colado M. I. The mechanisms involved in the long-lasting neuroprotective effect of fluoxetine against MDMA (‘ecstasy’)-induced degeneration of 5-HT nerve endings in rat brain. Br. J. Pharmacol. 2001;134:46–57. doi: 10.1038/sj.bjp.0704230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss P., Williams D. C. The serotonin transporter: A primary target for antidepressant drugs. J. Psychopharmacol. 1998;12:115–121. doi: 10.1177/026988119801200201. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J. Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 1987;240:1–7. [PubMed] [Google Scholar]
- Shioda K., Nisijima K., Yoshino T., Kuboshima K., Iwamura T., Yui K., Kato S. Risperidone attenuates and reverses hyperthermia induced by 3,4-methylenedioxymethamphetamine (MDMA) in rats. Neurotoxicology. 2008;29:1030–1036. doi: 10.1016/j.neuro.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Smits B. M., Mudde J. B., van de Belt J., Verheul M., Olivier J., Homberg J., Guryev V., Cools A. R., Ellenbroek B. A., Plasterk R. H., et al. Generation of gene knockouts and mutant models in the laboratory rat by ENU-driven target-selected mutagenesis. Pharmacogenet. Genomics. 2006;16:159–169. doi: 10.1097/01.fpc.0000184960.82903.8f. [DOI] [PubMed] [Google Scholar]
- De Souza E. B., Battaglia G., Insel T. R. Neurotoxic effect of MDMA on brain neurons: Evidence from neurochemical and radioligand binding studies. Ann. N. Y. Acad. Sci. 1990;600:682–698. doi: 10.1111/j.1749-6632.1990.tb16918.x. [DOI] [PubMed] [Google Scholar]
- Timar J., Gyarmati S., Szabo A., Furst S. Behavioural changes in rats treated with a neurotoxic dose regimen of dextrorotatory amphetamine derivatives. Behav. Pharmacol. 2003;14:199–206. doi: 10.1097/00008877-200305000-00003. [DOI] [PubMed] [Google Scholar]
- Trigo J. M., Renoir T., Lanfumey L., Hamon M., Lesch K.-P., Robledo P., Maldonado R. 3,4-Methylenedioxymethamphetamine self-administration is abolished in serotonin transporter knockout mice. Biol. Psychiatry. 2007;62:669–679. doi: 10.1016/j.biopsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Walker Q. D., Williams C., Jotwani R., Waller S., Francis R., Kuhn C. Sex differences in the neurochemical and functional effects of MDMA in Sprague–Dawley rats. Psychopharmacology. 2007;189:435–445. doi: 10.1007/s00213-006-0531-z. [DOI] [PubMed] [Google Scholar]
- Wallinga A. E., Grahlmann C., Granneman R. A., Koolhaas J. M., Buwalda B. Gender differences in hyperthermia and regional 5-HT and 5-HIAA depletion in the brain following MDMA administration in rats. Brain Res. 2011;1398:13–20. doi: 10.1016/j.brainres.2011.04.039. [DOI] [PubMed] [Google Scholar]
- Xie T., Tong L., McLane M. W., Hatzidimitriou G., Yuan J., McCann U., Ricaurte G. Loss of serotonin transporter protein after MDMA and other ring-substituted amphetamines. Neuropsychopharmacology. 2006;31:2639–2651. doi: 10.1038/sj.npp.1301031. [DOI] [PubMed] [Google Scholar]