Abstract
The use of brominated flame retardants and incineration of bromine-containing materials has lead to an increase in polybrominated dibenzo-p-dioxins and dibenzofurans (PBDD/Fs) in the environment. Measurable amounts of PBDD/Fs have been detected in soil, seafood, and human breast milk and serum. Studies indicate that the relative potencies of some PBDD/Fs based on enzyme induction are equivalent to those of some polychlorinated dibenzo-p-dioxins and dibenzofurans. To assess the humoral immunity relative potencies of PBDD/Fs and compare them to their chlorinated analogs, female B6C3F1/N mice received a single oral exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 2,3,7,8-tetrabromodibenzofuran (TBDF), 2,3,7,8-tetrachlorodibenzofuran (TCDF), 1,2,3,7,8-pentabromodibenzofuran (1PeBDF), 1,2,3,7,8-pentachlorodibenzofuran (1PeCDF), 2,3,4,7,8-pentabromodibenzofuran (4PeBDF), 2,3,4,7,8-pentachlorodibenzofuran (4PeCDF), 2,3-dibromo-7,8-dichlorodibenzo-p-dioxin (DBDCDD), or 2,3,7-tribromodibenzo-p-dioxin (TriBDD). Inhibition of the immunoglobulin M (IgM) antibody forming cell response was measured 4 days following immunization with sheep red blood cells. The data were fit to a Hill model to estimate the ED50 for inhibition. Expression of xenobiotic metabolizing enzyme (XME) and thyroxine transport protein (Ttr) genes in liver was measured by PCR to assess aryl hydrocarbon-mediated responses. TCDD, TBDF, TCDF, 1PeBDF, 4PeBDF, 4PeCDF, and DBDCDD suppressed the IgM antibody response and Ttr gene expression, and upregulated phase I XME genes. 1PeCDF suppressed the IgM antibody response but only upregulated phase I XME genes; TriBDD had no effect on antibody response. The rank order of potency (ED50) for these chemicals was TCDD>TBDF>4PeBDF>TCDF/4PeCDF/1PeBDF>1PeCDF. Whereas TCDD was the most potent compound tested, the brominated analogs were more potent than their chlorinated analogs, suggesting that these compounds should be considered in toxic equivalency factor evaluation and risk assessment.
Keywords: 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin, chlorinated furans, brominated dioxins, brominated furans, relative potency, IgM antibody forming cell, toxic equivalency factor, TEF
Polybrominated dibenzo-p-dioxins and dibenzofurans (PBDD/Fs) are contaminants in brominated flame retardants (BFRs) used in a variety of commercial products, including electronics, appliances, carpet, upholstery, and paint. Environmental contamination by PBDD/Fs, polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs), and mixed bromo-/chloro-congeners can occur through the leaching of the chemical from treated materials, and in airborne dust and fly ash resulting from incineration of BFRs and chemical precursor-containing materials. Human exposure is primarily through food and occupational exposure (soil/dust ingestion, dermal exposure, and inhalation) (Ma et al., 2009; Rose and Fernandes, 2010). Measurable amounts of these compounds have been detected in electronic waste, shop dust, soil, sediment, vegetation, fish, shellfish, meat, and human serum (Birnbaum et al., 2003; Fernandes et al., 2011; Ma et al., 2009) and adipose tissue (Choi et al., 2003; Jogsten et al., 2010).
The World Health Organization (WHO) and the United States Environmental Protection Agency evaluate the potential adverse health effects from exposure to PCDD/Fs using a toxic equivalency factor (TEF) methodology, in which potencies for these dioxin-like congeners are expressed relative to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the most potent of the dioxin-like compounds (TEF = 1.0) (van den Berg et al., 2006). 1,2,3,7,8-pentachlorodibenzofuran (1PeCDF), 2,3,4,7,8-pentachlorodibenzofuran (4PeCDF), and 2,3,7,8-tetrachlorodibenzofuran (TCDF) have been assigned TEF values of 0.03, 0.3, and 0.1, respectively, based largely on in vivo data (van den Berg et al., 2006). The WHO does not currently include the PBDD/Fs in the TEF approach. A panel of experts has recommended including the PBDD/Fs in TEF evaluations, but due to limited mammalian in vivo data, the panel recommended using the interim TEF values of the chlorinated dioxins for their brominated analogues (van den Berg et al., 2013).
While limited in vivo studies have evaluated the relative potency of PBDD/Fs, both in vivo and in vitro studies have demonstrated that PBDD/Fs have aryl hydrocarbon (Ah) agonist properties, induce dioxin-like biological and toxic effects, have a structural relationship to classic chlorinated dioxins, and persist in the environment and bioaccumulate; all of which are criteria for inclusion in the TEF concept (van den Berg et al., 1998, 2006). In vitro evidence from EROD (ethoxy-resorufin-O-deethylase) and CALUX (Chemical Activated LUciferase gene eXpression) cell-based bioassays, which measure Cyp1A1 induction and induction of the firefly luciferase gene coupled to dioxin-responsive elements respectively, suggests that some PBDD/Fs are equally as potent as, and possibly more potent than, the chlorinated analogues (Behnisch et al., 2003; Samara et al., 2009). Mixed bromo/chloro orthologues also appear to be highly active and follow the structure-activity rules of the PCDD/Fs (Behnisch et al., 2001,2003; Samara et al., 2009), whereas some studies indicate that addition of a non-lateral halogen group or deletion of one of the four lateral halogens (positions 2, 3, 7, and 8) results in reduced potency (Mason et al., 1987).
Dioxins, dibenzofurans, and some polyhalogenated aromatic hydrocarbons (PHAHs) are potent immunotoxicants (Davis and Safe, 1988). Inhibition of the antibody response to sheep red blood cells (SRBCs) in mice following an acute single dose of dioxins is a sensitive measure of altered humoral immunity and a consistent indicator of toxicity for PHAHs (Davis and Safe, 1988; Johnson et al., 2000). The T-cell-dependent antibody response requires T cell, B cell, and antigen presenting cells working in concert to produce immunoglobulin M (IgM) in response to an antigen. The antibody forming cell (AFC) assay has been shown to be the most reliable single measure for predicting immunotoxicity (Luster et al., 1992).
The objective of this study was to evaluate the relative potencies of three brominated dibenzofurans and their chlorinated analogs, a mixed halogenated dioxin, and a tribrominated dioxin, in order to assess whether there is a need to generate additional toxicological data on the brominated dioxins and dibenzofurans for use in TEF evaluation. This was accomplished by examining the capacity of each compound to alter humoral immunity in B6C3F1/N mice through measurement of antigen-specific antibody responses. In addition, expression of phase I xenobiotic metabolizing enzyme (XME) genes was measured in liver to confirm activation of the aryl hydrocarbon receptor (AhR). In addition, other XME, immunoresponsive, and thyroid hormone related genes were evaluated in liver to provide further insight into the mechanisms of toxicity and provide additional endpoints to develop relative potencies. The chemicals were selected based on evidence of human exposure. It has been estimated that 2,3,7,8-tetrabromodibenzofuran (TBDF), 1,2,3,7,8-pentabromodibenzofuran (1PeBDF), and 2,3,4,7,8-pentachlorodibenzofuran (4PeBDF) contribute up to 15% of the total dioxin toxicity equivalents (TEQ) in human tissues (Jogsten et al., 2010). 2,3,7-tribromodibenzo-p-dioxin (TriBDD) and 2,3-dibromo-7,8-dichlorodibenzo-p-dioxin (DBDCDD) were detected in fish and shellfish (Fernandes et al., 2009, 2011), and DBDCDD contributes to the dioxin load in contaminated soil following industrial fires (Myers et al., 2012).
MATERIALS AND METHODS
Animals and treatment
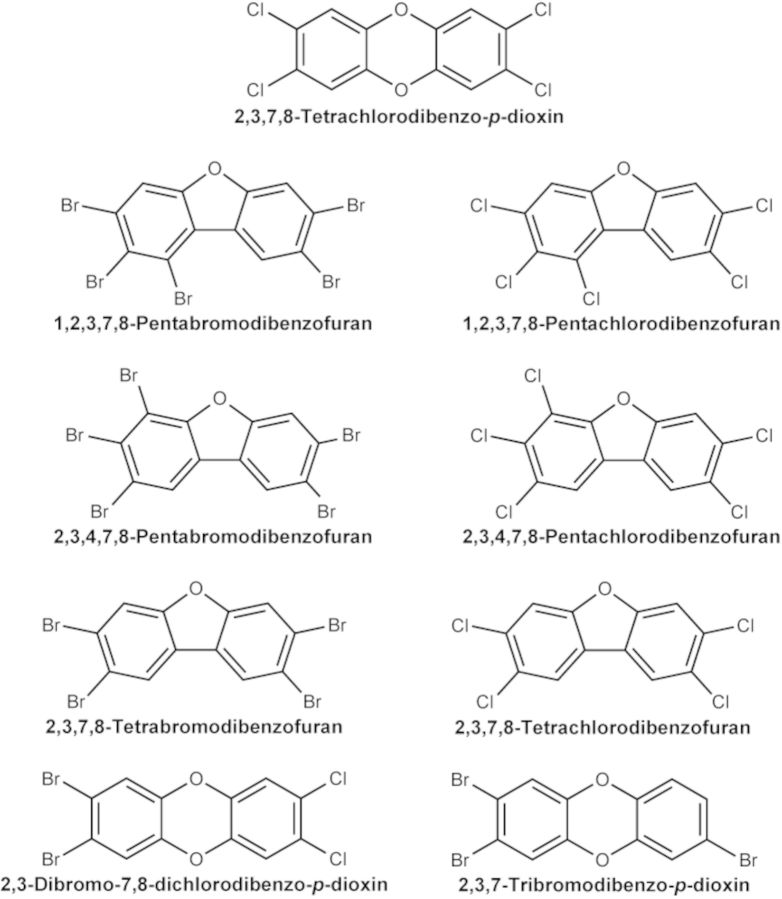
Female pathogen-free B6C3F1/N mice were obtained from Taconic (Rockville, MD) at 4–6 weeks of age and maintained on a 12-h light/dark cycle at 20–22°C in an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility. All experiments were conducted under an approved Virginia Commonwealth University (VCU) Animal Care and Use Committee protocol, and animals were treated humanely and with regard for alleviation of pain and distress. The animals received NTP-2000 diet (Ziegler Brothers, Inc., Gardner, PA) and water ad libitum. At 8–9 weeks of age (18–24 g), the mice were weighed, randomized, identified by tattoo, and placed on treatment (N = 14 per group). All animals received a single dose of one of the test chemicals (Table 1, Fig. 1), or corn oil as the vehicle control, in a volume of 0.1 ml/10g body weight, by oral gavage on day 0. The doses selected for evaluation were based on published toxicity data (Johnson et al., 2000), in vitro Ah receptor activity, projected potency based on mass to chlorinated congener, and published guidelines for a dose response study for determination of an in vivo relative effect potency (Van den Berg et al., 2006). Johnson et al. (2000) reported significant suppression of the AFC response at ≥0.5 μg TCDD/kg, and a similar magnitude of suppression at ≥15.0 μg 4PeCDF/kg in B6C3F1 female mice. All furans were evaluated at the same doses, to enable direct comparison of biologic effects within the group. In vitro evidence (Behnisch et al., 2003) suggests that the Ah receptor activity of DBDCDD is approximately equal to that of TCDD, therefore, DBDCDD was evaluated at the same doses as TCDD. TriBDD was approximately 0.1 times as potent at TCDD in vitro, and was evaluated in this study at doses that were 10 times higher than TCDD.
TABLE 1. Compounds and Dosages.
| Chemical | Doses | TEFa |
|---|---|---|
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) | 0.1, 0.3, 0.5, 1, 3 μg TCDD/kg | 1.0 |
| 2,3,7,8-Tetrabromodibenzofuran (TBDF) | 3, 9, 15, 30, 90 μg TBDF/kg | |
| 2,3,7,8-Tetrachlorodibenzofuran (TCDF) | 3, 9, 15, 30, 90 μg TCDF/kg | 0.1 |
| 1,2,3,7,8-Pentabromodibenzofuran (1PeBDF) | 3, 9, 15, 30, 90 μg 1PeBDF/kg | |
| 1,2,3,7,8-Pentachlorodibenzofuran (1PeCDF) | 3, 9, 15, 30, 90 μg 1PeCDF/kg | 0.03 |
| 2,3,4,7,8-Pentabromodibenzofuran (4PeBDF) | 3, 9, 15, 30, 90 μg 4PeBDF/kg | |
| 2,3,4,7,8-Pentachlorodibenzofuran (4PeCDF) | 3, 9, 15, 30, 90 μg 4PeCDF/kg | 0.3 |
| 2,3-Dibromo-7,8-dichlorodibenzo-p-dioxin (DBDCDD) | 0.1, 0.3, 0.5, 1, 3 μg DBDCDD/kg | |
| 2,3,7-Tribromodibenzo-p-dioxin (TriBDD) | 1, 3, 5, 10, 30 μg TriBDD/kg |
Note. N = 6 female B6C3F1/N mice for gene expression assays, N = 8 for immune assays.
aVan den Berg et al. (2006).
FIG. 1.
Chemical structures for the dioxins and furans evaluated in this study.
All test articles were procured from Cerillient Corporation (Round Rock, TX) at a purity of 99%, as determined by the manufacturer, and used as purchased to prepare the dose formulations (Table 1). The specific dose formulations were prepared through a National Toxicology Program (NTP) analytical chemistry contract at Battelle (Columbus, OH) and shipped to VCU. All test articles were dissolved in n-nonane, the required amounts were aliquoted into glass bottles, the solvent was evaporated under a nitrogen stream to dryness, and the chemical was dissolved through extensive mixing in corn oil. The test articles were assayed in pairs, with matching bromo- and chloro-congeners evaluated simultaneously. There was a single matched vehicle control for each bromo/chloro pair. Based on the dose response curve from the TCDD AFC studies, a group of animals treated with 1 μg/kg TCDD was included in the AFC response assay as a positive control for each pair of test articles. Cyclophosphamide (50 mg/kg) was included in the AFC response assay as a positive control to assess assay quality.
On day 3, six animals per group were euthanized by CO2 asphyxiation, and the liver was excised and weighed. The left and median lobes were flash frozen in liquid nitrogen for genomic analysis. On day 7, eight animals per group were immunized with 7.5×107 SRBCs by intravenous injection. On day 11, those animals were anesthetized (CO2 inhalation) and blood was collected from the retro-orbital sinus for hematological evaluation. The animals were euthanized (CO2 asphyxiation) and blood was collected by cardiac puncture to obtain serum. The liver, spleen, lungs, thymus, and kidneys with adrenal glands were excised and weighed, and the left and median lobes of the liver were flash frozen in liquid nitrogen for potential future evaluation. The spleen was macerated in Earle's Balanced Salt Solution and prepared as described below.
Primary IgM response to SRBCs
The spleen AFC response was enumerated using a modified hemolytic plaque assay (Jerne and Nordin, 1963; White et al., 2010) as previously described. An aliquot of spleen cells (day 11) was mixed with guinea pig complement, SRBCs and warm agar, and incubated in a petri dish under a glass cover slip at 37°C for 3 h. Plaques resulted from complement-mediated antibody lysis of SRBCs produced by antigen-specific plasma cells, and were counted using a Bellco plaque viewer (Bellco Glass Company, Vineland, NJ). The specific activity (AFC/106 spleen cells) and total spleen activity (AFC/spleen) were calculated.
Serum IgM antibody titers to SRBCs were evaluated using an enzyme-linked immunosorbent assay as previously described (Temple et al., 1993). Briefly, serum samples (day 11) were incubated with SRBC membrane antigens (1:100 dilution) adsorbed to microtiter plates, affinity purified horseradish peroxidase-conjugated goat anti-mouse IgM antibody, and the peroxidase substrate (2,2′-azino-bis[3-ethyl-benzthiazoline-6-sulfonic acid]). The assay was read at 405 nm on a Molecular Devices plate reader and the titer determined using SoftMax (v. 2.32, Molecular Devices Corp., Sunnyvale, CA).
Hematology
A panel of hematological parameters was analyzed in blood from the day 11 animals using a Hemavet 1500FS (Drew Scientific, Waterbury, CT). The following hematology parameters were evaluated: erythrocyte number, hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and platelet number. Reticulocytes were evaluated using Retic-COUNT™ stain and FACScan flow cytometer (Becton Dickinson, San Jose, CA), according to the manufacturer's directions. Peripheral blood leukocyte differentials were also determined for the absolute numbers and percentages of lymphocytes, neutrophils, monocytes, basophils, and eosinophils.
Gene expression analysis
Liver samples (60–100 mg) from day 3 mice were pulverized in liquid nitrogen, and total RNA was isolated using the Qiagen RNeasy Midi Kit (Qiagen, Valencia, CA). RNA quantity and A260/280 ratios, a measure of RNA purity, were measured using the Nanodrop-1000 (Thermo Scientific, Wilmington, DE), whereas the RNA Integrity Numbers were measured using the Agilent 2100 Bioanalyzer and the Agilent RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA). Reverse Transcriptase PCR was performed using the RT2 HT First Strand Kit (Qiagen). The cDNA was amplified via quantitative real-time PCR (qRT-PCR) for each gene of interest plus controls using the RT2 SYBR Green ROX qPCR Mastermix (Qiagen), SABiosciences custom arrays (Qiagen), and ViiA 7 RT-PCR System (Applied Biosystems, Foster City, CA). All products were used according to the manufacturer's directions. Each sample was run in duplicate on two different plates. Quantification of mRNA levels for each gene was determined using the threshold cycle values (CT) for the target genes of interest and the CT value for the endogenous reference gene (Hprt). The fold change in a target gene from a treated sample compared with the vehicle control group was determined by the comparative CT method, where fold change = 2−(ΔΔCT). This produced a relative quantitation (RQ) value that was used for statistical analysis. In some cases, fold change values are based on single replicates due to no amplification in one of the replicates.
The custom array consisted of the following genes:
Phase I XMEs: cytochrome P450 (Cyp) enzymes Cyp1a1 and Cyp1a2
Phase II XMEs: uridinediphosphate-glucuronosyltransferases (Ugt) Ugt1a1 and Ugt1a6; sulfotransferases (Sult) Sult1c1 and Sult1e1
Phase III XMEs: ATP-binding cassette (ABC) transporters Abcb1a, Abcb1b, Abcb4, Abcc2, and Abcc3; solute carriers (Slc) Slco1b2, Slc22a7, and Slc16a2
Thyroid Responsive: thyroid transport protein transthyretin (Ttr), deiodinase I (Dio1)
Housekeeping: glyceraldehyde 3-phosphate dehydrogenase (Gapdh), 18S ribosomal RNA (18SrRNA), hypoxanthine-guanine phosphoribosyltransferase (Hprt), beta-actin (Actβ)
Immune: cysteine-rich protein 2 (Crip2); interferon gamma (Ifnγ); interleukins (Il) Il1α, Il1β, Il4, Il6, Il10, and Il12b; nuclear factor kappa B (Nfκb); tumor necrosis factor (Tnfα)
Determination of relative potency
A Hill model (Reeve and Turner, 2013) was fit, using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA), to the full dose response data for changes in IgM response to SRBCs and expression of Cyp1a1 and Cyp1a2 genes to estimate the ED50. Each endpoint was evaluated independently of the other endpoints (e.g., absolute AFC/spleen, Cyp1a1 gene expression, etc.). For each endpoint, the entire dataset (e.g., all chemicals, all doses) was modeled under uniform constraints. These constraints were necessary to prevent mathematical extrapolation of variables beyond the biologically relevant, dynamic range of the data. Relative potency factors (RPFs) were estimated by the ratio of the ED50 estimated from the Hill model relative to the full dose response data for TCDD, i.e., ED50 TCDD/ED50 brominated PBDD/Fs. The equation for the model was: Y = E0 ± [(emax * Xn) / (bn + Xn)], where E0 = basal response, emax = maximum response, X = dose, n = Hill coefficient, and b = ED50. In all cases the Hill coefficient was constrained to n > 0.8 and shared among all chemicals. The E0 was constrained to 100 for the downward sloping (inhibition) IgM responses to SRBCs, and to >1.0 for the upward sloping (induction) Cyp gene expression data. The emax was constrained to <100 and shared among all chemicals for the IgM responses to SRBC and serum titers, to <13,000 and shared among all chemicals for Cyp1a1 gene expression, and to <50 and shared among all chemicals for Cyp1a2 gene expression. The resulting emax values were 90.27 for AFC/106 spleen cells, 91.60 for AFC/spleen, 43.92 for serum titers, 6794 for Cyp1a1 gene expression, and 37.54 for Cyp1a2 gene expression. Note that in the equation described above, for the immunological responses E0 is 100 and the function, [(emax * Xn) / (bn + Xn)], was subtracted from E0. When emax was unconstrained, for some chemicals it was greater than 100. This resulted in a negative value for the IgM response. Because a negative IgM is not biologically plausible, we constrained emax to less than 100. For enzyme induction, the function [(emax * Xn) / (bn + Xn)] was added to E0. When emax was not constrained for enzyme induction, for chemicals with no clear maximal response, the model estimated emax at values far outside of the experimental data. Thus, emax for enzyme induction was constrained to the maximal response for TCDD.
Not all chemicals were evaluated at sufficient exposures to clearly demonstrate a maximum response. In the analysis it was assumed that all chemicals have the same maximum in order to derive relative potencies. The primary assumption of the TEF method is the same as for the Hill parameters, except ED50, and therefore this assumption was used, even in the absence of an apparent observed emax.
Data analysis
All data were evaluated for homogeneity of variances using Bartlett's test. Homogeneous data were evaluated using a parametric analysis of variance (ANOVA) and Dunnett's t-test. Nonhomogeneous data were evaluated using Welch ANOVA, the Wilcoxon Rank Sum Test, and a pairwise Wilcoxin Rank Sum Test. Student's t-test was used to compare the vehicle and positive controls. Data that were different from control at a level of p ≤ 0.05 were considered statistically significant.
RESULTS
General Parameters of Toxicity
There were very few differences observed in body weight between treated and control animals in the 11-day studies, and no differences in the 3-day studies (see Supplementary table 1A and B). Notably, both the final body weight and overall weight gain were higher for mice exposed to 9, 15, and 30 μg TBDF/kg at day 11 compared with control mice. Transient differences were noted at day 7 (data not shown), but not at day 11, for mice exposed to 9 and 30 μg 4PeCDF/kg and 5 μg TriBDD/kg. The relative (i.e., % of body weight) spleen weight was decreased at ≥9 μg TBDF/kg, 90 μg 1PeBDF/kg, and 90 μg 4PeBDF/kg (see Supplementary table 1C and D). The absolute spleen weight was also decreased by 30 μg TBDF/kg and 90 μg 4PeBDF/kg. Absolute and relative liver weights were increased at ≥15 μg/kg in animals exposed to 4PeBDF and 4PeCDF, and in animals exposed to 90 μg TBDF/kg, 3 μg DBDCDD/kg, and 3 μg TCDD/kg. Relative kidney weight was decreased at 30 μg TCDF/kg; however, there were no other changes associated with exposure in thymus, lung, or kidney weight.
TCDD induced an increase in the MCV at ≥1.0 μg TCDD/kg (see Supplementary table 2). Isolated, single-dose increases were noted for MCV (3 μg 4PeBDF/kg; 30 μg 4PeCDF/kg), erythrocyte number (1 μg DBDCDD/kg), hematocrit (1 μg DBDCDD/kg; 0.1 μg TCDD/kg), and platelet number (1.0 μg TCDD/kg). Peripheral blood leukocyte differentials in mice exposed to 3, 9, 15, and 90 μg 4PeCDF/kg exhibited a decrease in the percentage of monocytes (relative to total leukocytes), whereas mice exposed to 4PeBDF exhibited a decrease in the percentage of neutrophils at ≥30 μg/kg (see Supplementary table 3A and B). Altered specific leukocyte populations were also observed for 90 μg 1PeBDF/kg (basophils), 90 μg 1PeCDF/kg (basophils and neutrophils), and 90 μg 4PeBDF/kg (lymphocytes, monocytes). Single lower doses of 4PeCDF, TBDF, and TCDD also shifted the percentages of certain cell types. Because TCDD did not produce consistent dose-dependent changes in any of these responses, relative potencies for the hematology parameters were not derived.
Primary IgM Response to SRBCs
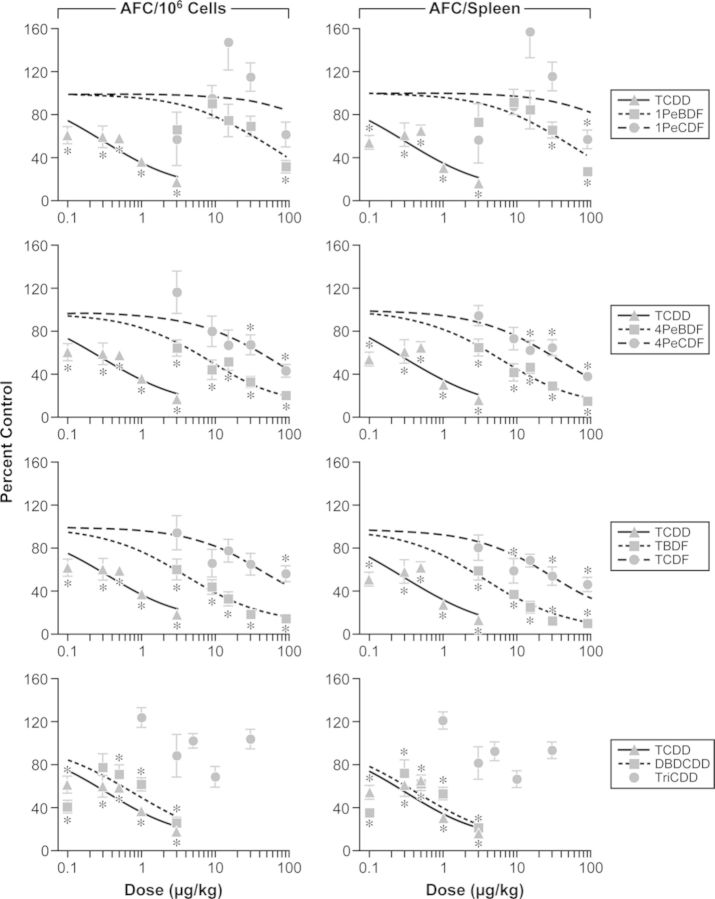
The AFC response was measured 4 days post-immunization to capture the peak IgM response. The serum IgM antibody titers to SRBCs were measured in the same mice for efficient use of animals. The reference compound, TCDD, induced a significant reduction in both specific activity (AFC/106 spleen cells) and total activity (AFC/Spleen) at ≥0.1 μg/kg. Exposure to all of the other compounds tested, with the exception of TriBDD, resulted in a reduction in AFC activity, although to a lesser degree than TCDD (Fig. 2). DBDCDD (3 μg/kg) maximally suppressed the specific and total activity by 72% and 79%, respectively, compared with TCDD, which suppressed the specific and total activity by 82% and 84%, respectively, at the same dose level. 4PeBDF suppressed specific and total AFC activity at ≥3 μg/kg; the chlorinated analog 4PeCDF suppressed specific activity at ≥30 μg/kg and total activity at ≥15 μg/kg. Similarly, TBDF suppressed specific and total activity at ≥3 μg/kg, whereas TCDF suppressed specific activity at 90 μg/kg and total activity at 9, 30, and 90 μg/kg. 1PeBDF suppressed specific activity at 90 μg/kg and total activity at ≥30 μg/kg; the chlorinated analog 1PeCDF suppressed total activity at 90 μg/kg but did not affect specific activity. Only two chemicals, 90 μg 4PeBDF/kg and 0.1 and 0.5–3.0 μg DBDCDD/kg, induced a reduction in spleen cellularity (data not shown). The serum IgM antibody titers to SRBCs mirrored the AFC specific activity response in mice exposed to 1PeBDF, TBDF, and DBDCDD, and was unaffected by treatment with 1PeCDF and TriBDD. The titer was also reduced following exposure to ≥0.5 μg TCDD/kg, ≥9 μg 4PeBDF/kg, 90 μg 4PeCDF/kg, and 30 μg TCDF/kg.
FIG. 2.
Effect of PBDD/Fs and PCDD/Fs on the IgM AFC response to SRBCs 4 days post-immunization. Data points represent percent of control (mean ± SEM, n = 8); lines represent projected best-fit curve from Hill model. TriBDD was not included in the RPF assessment due to the lack of a significant dose-dependent effect. *p ≤ 0.05 versus matched vehicle control.
Expression of Genes Associated with Induction of XME, Thyroid Response, and Immune Responses
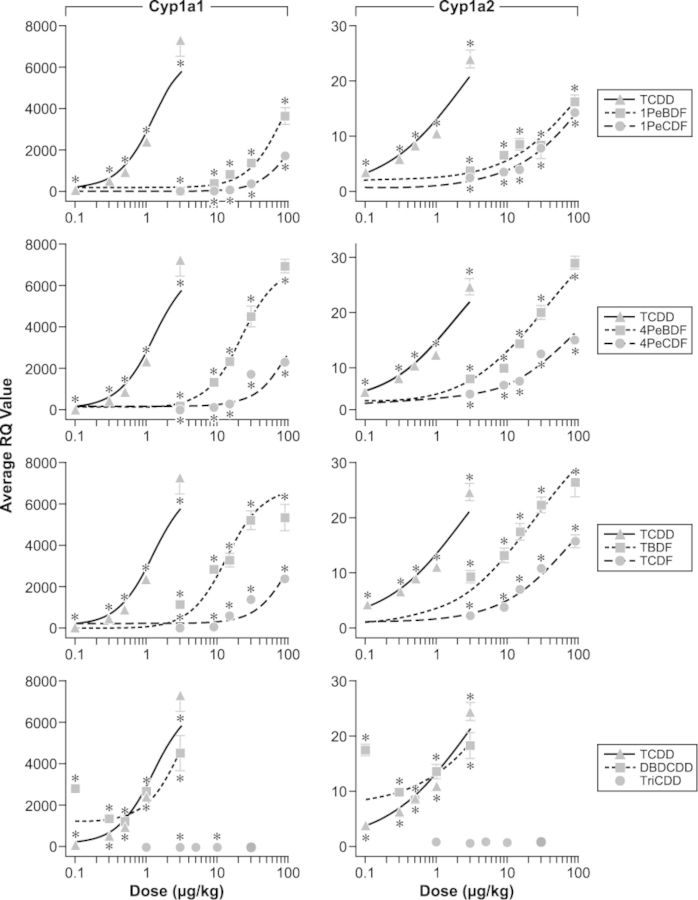
Expression of genes associated with hepatic xenobiotic metabolism pathways was measured in liver 3 days post-exposure to assess potential chemical-induced regulation of enzymes involved with the hydroxylation (phase I), conjugation (phase II), and transport (phase III) of xenobiotics. Statistically significant changes are reported here and in Table 2; full gene expression results (RQ values) are reported in the Supplementary table 4A, B, C, and D. All chemicals tested, except TriBDD, upregulated phase I Cyp1a1 and Cyp1a2 genes in a dose-dependent manner (Fig. 3). TriBDD downregulated these genes at 3 and 10 μg/kg. Although these values were statistically significant, there is negligible dose response for TriBDD, and the magnitude of the change (less than 50%) is inconsistent with the magnitude of the change observed for TCDD (>1700%) and the other test articles (>256%). 3 μg TCDD/kg, ≥15 μg 4PeBDF/kg, ≥30 μg 4PeCDF/kg, and 90 μg TBDF/kg upregulated phase II Ugt1a1, whereas DBDCDD upregulated Ugt1a1 only at 0.1 μg/kg and 3 and 9 μg TCDF/kg downregulated Ugt1a1 (Supplementary table 4A). Similarly, 3 μg TCDD/kg and 90 μg 4PeBDF/kg upregulated Ugt1a6 expression, whereas DBDCDD upregulated Ugt1a6 expression at lower doses (0.1 and 0.5 μg/kg), and 3–15 μg TCDF/kg downregulated Ugt1a6 expression. Exposure to ≥1 μg TCDD/kg, ≥15 μg 1PeBDF/kg, and ≥30 μg TBDF significantly downregulated Sult1e in the liver. None of the chemicals tested altered expression of the Sult1c gene. 1PeCDF and TriBDD had no effect on any of the phase II metabolism genes. The effects on phase III XME gene expression were more varied. TCDD, 4PeBDF, TBDF, TCDF, and DBDCDD all altered one or more genes from the solute carrier family and from the ABC family (Table 2, Supplementary table 4B). 1PeBDF, 1PeCDF, 4PeCDF, and TriBDD had no effect on any of the phase III metabolism genes.
TABLE 2. Summary of the Effect of Brominated and Chlorinated Dioxins and Furans on Gene Expression.
| TCDD | 1PeBDF | 1PeCDF | 4PeBDF | 4PeCDF | TBDF | TCDF | DBDCDD | TriBDD | |
|---|---|---|---|---|---|---|---|---|---|
| Dose range (μg/kg) | |||||||||
| 0.1–3 | 3–90 | 3–90 | 3–90 | 3–90 | 3–90 | 3–90 | 0.1–3 | 1–30 | |
| Gene | |||||||||
| Phase I XMEs | |||||||||
| Cyp1a1 | ↑≥0.1 | ↑≥3 | ↑≥9 | ↑≥3 | ↑≥3 | ↑ ≥3 | ↑≥3 | ↑≥0.1 | ↓3, 10 |
| Cyp1a2 | ↑≥0.1 | ↑≥3 | ↑≥3 | ↑≥3 | ↑≥3 | ↑≥3 | ↑ ≥3 | ↑ ≥0.1 | – |
| Phase II XMEs | |||||||||
| Ugt1a1 | ↑3 | – | – | ↑≥15 | ↑ ≥30 | ↑ 90 | ↓3, 9 | ↑0.1 | – |
| Ugt1a6 | ↑3 | – | – | ↑ 90 | – | – | ↓3 - 15 | ↑0.1, 0.5 | – |
| Sult1c | – | – | – | – | – | – | – | – | – |
| Sult1e | ↓ ≥1 | ↓ ≥15 | – | – | – | ↓≥30 | – | – | – |
| Phase III XMEs | |||||||||
| Abcb1a | – | – | – | – | – | ↓3, 15, 30, 90 | – | – | – |
| Abcb1b | ↓≥1 | – | – | – | – | ↓ ≥3 | ↓≥3 | – | – |
| Abcb4 | – | – | – | – | – | – | ↓9, 15 | ↓3 | – |
| Abcc2 | – | – | – | – | – | ↓≥3 | ↓3, 15, 30, 90 | ↓3 | – |
| Abcc3 | ↑3 | – | – | ↑≥15 | – | – | ↓9, ↑90 | ↑0.1, 3 | – |
| Slco1b | ↓3 | – | – | – | – | ↓ ≥3 | ↓ 90 | ↓3 | – |
| Slc22a7 | ↑3 | – | – | – | – | ↑ 9, 30 | ↑3, 30, 90 | – | – |
| Slc16a2 | ↓3 | – | – | ↓≥30 | – | ↓≥9 | – | ↓3 | – |
| Thyroid Responsive | |||||||||
| Ttr | ↓≥1 | ↓ ≥3 | – | ↓ ≥3 | ↓≥15 | ↓≥9 | ↓≥30 | ↓ 3 | – |
| Dio1 | – | – | – | – | – | ↓9, 15, 90 | ↓ 3, 9, 15, 90 | – | – |
| Housekeeping | |||||||||
| Gapdh | – | – | – | – | – | ↓≥3 | ↓ ≥3 | – | – |
| 18srRNA | – | – | – | – | – | – | – | ↓3 | – |
| ActB | – | ↓90 | – | – | – | ↓ ≥3 | ↓≥3 | ↓3 | – |
| Immune | |||||||||
| Crip2 | ↑3 | – | – | – | – | ↓ ≥9 | ↓≤15 | ↓3 | – |
| Ifnγ | – | – | – | ↓90 | – | ↓≥9 | ↓9, 90 | – | – |
| Il1α | ↑0.1, 0.3, 3 | – | – | – | – | – | – | ↓3 | – |
| Il1β | – | – | – | ↓3, 9, 30, 90 | – | ↓ ≥3 | ↓3, 9, 30, 90 | – | – |
| Il4 | – | – | – | – | – | – | – | ↓0.1, 0.5, 1, 3 | ↓≤3 |
| Il6 | – | – | – | ↓3, 30, 90 | – | ↓15, 90 | ↓≥30 | – | – |
| Il10 | – | – | – | – | – | – | – | – | – |
| Il12β | – | – | – | – | – | – | – | – | – |
| Nfκb | – | ↓15, 90 | – | ↓≥30 | – | ↓≥15 | ↓ 3, 9, 30, 90 | ↓0.3, 3 | ↓5, 30 |
| Tnfα | – | – | – | – | – | ↓ 9, 15 | ↓9, 90 | ↓ ≥1 | ↓ 3 |
Note. This table represents a summary of specific chemical treatments with statistically significant effects. The numbers represent the dosage at which effects were observed. See Supplementary table 4A, B, C, and D for RQ values.
FIG. 3.
Effect of PBDD/Fs and PCDD/Fs on Cyp1a1 and Cyp1a2 gene expression. Data points represent percent of control (mean ± SEM, n = 6); lines represent projected best-fit curve from Hill model. TriBDD was not included in the RPF assessment due to the lack of a significant dose-dependent effect. *p ≤ 0.05 versus matched vehicle control.
The expression of two genes (Ttr and Dio1) involved in transport and modification of thyroid hormones was measured. The expression of Ttr was downregulated by all of the chemicals tested except 1PeCDF and TriBDD; only TBDF and TCDF downregulated Dio1 (Table 2, Supplementary table 4D). A panel of immunoresponsive genes that included Th1 and Th2 cytokines and inflammatory molecules was measured in liver to assess potential immunomodulatory effects in that tissue (Table 2, Supplementary table 4C and D). TCDD upregulated Crip2 and Il1α gene expression. 4PeBDF, TBDF, TCDF, and DBDCDD had the most pronounced effect on immunoregulatory gene expression. 4PeBDF, TBDF, and TCDF downregulated both the Th1 Ifnγ and Th2 Il6 genes, whereas DBDCDD downregulated the Th2 Il4 gene. Each of these chemicals also downregulated one of the Il1 genes and Nfκb. The remaining alterations in gene expression were varied. At high doses, TCDF and DBDCDD downregulated Tnfα, TBDF and DBDCDD downregulated Crip2, and 1PeBDF and TriBDD downregulated Nfκb. At low to mid doses only, TBDF and TriBDD downregulated Tnfα, TCDF downregulated Crip2, and TriBDD downregulated Il4. 1PeCDF and 4PeCDF did not alter any of the immune genes in the panel.
Relative Potency
The IgM AFC data and the Cyp1a1 and Cyp1a2 gene expression data demonstrated a consistent dose-dependent effect by TCDD and other chemicals, excluding TriBDD. A Hill model provided a good fit for the TCDD data and was, therefore, used to evaluate the IgM antibody forming response, and the Cyp1a1 and Cyp1a2 gene expression data, to estimate the ED50 of each chemical for these responses. The chemical doses used in this study were selected based on published reports of the IgM antibody forming response induced by TCDD- and TCDD-like congeners (Johnson et al., 2000), with the goal of obtaining the optimal TCDD-induced dose-dependent effect on AFC formation in the spleen. However, some of the TCDD-induced alterations in phase II or III XME, thyroid related, or immunoresponsive genes were observed only at the high dose, which did not allow for dose-dependent modeling and/or the estimation of relative potency with any accuracy. The rank orders of potency for each assay are shown in Table 3. The ED50 and relative potency for TriBDD could not be calculated because there was no statistically significant trend in the data. TriBDD did not significantly alter antibody forming response or Cyp1a1 and Cyp1a2 gene expression. The relative potency for DBDCDD could not be calculated due to a nonmonotonic dose response for all endpoints, which resulted in poor fits of the Hill model. Treatment with 0.1 μg DBDCDD/kg consistently resulted in unexplained, unexpectedly high effect levels that were similar to 0.3 μg DBDCDD/kg. Such effects were consistent across endpoints, including AFC response, serum titers, and XME induction. It is possible that this effect resulted from an experimental error; additional investigation would be required to determine the veracity of the 0.1 μg DBDCDD/kg data. Therefore, it was decided to make full disclosure of the data, to acknowledge the immunosuppression observed at the remaining doses, and to exclude the DBDCDD data from relative potency calculations.
TABLE 3. Relative Potency Factors (RPFs)a and Rank Order of Potency.
| Most potent |  |
Least potent | |||||
|---|---|---|---|---|---|---|---|
| AFC/106 spleen cells: | |||||||
| Chemical | TCDD | TBDF | 4PeBDF | 1PeBDF | TCDF | 4PeCDF | 1PeCDF |
| ED50 | 0.34 | 3.87 | 8.17 | 42.79 | 55.3 | 59.96 | 696.4 |
| RPF | 1.0 | 0.09 | 0.04 | 0.008 | 0.006 | 0.006 | 0.0005 |
| AFC/Spleen: | |||||||
| Chemical | TCDD | TBDF | 4PeBDF | TCDF | 4PeCDF | 1PeBDF | 1PeCDF |
| ED50 | 0.32 | 3.61 | 6.07 | 34.12 | 35.92 | 45.63 | 573.5 |
| RPF | 1.0 | 0.088 | 0.052 | 0.009 | 0.009 | 0.007 | 0.0006 |
| Serum IgM antibody titer to SRBCs: | |||||||
| Chemical | TCDD | 4PeBDF | TBDF | 4PeCDF | TCDF | 1PeBDF | 1PeCDF |
| ED50 | 1.87 | 16.89 | 19.31 | 126.3 | 180.2 | 181.1 | 529.0 |
| RPF | 1.0 | 0.110 | 0.097 | 0.015 | 0.010 | 0.010 | 0.004 |
| Cyp1a1 expression: | |||||||
| Chemical | TCDD | TBDF | 4PeBDF | 1PeBDF | TCDF | 4PeCDF | 1PeCDF |
| ED50 | 1.22 | 13.39 | 21.84 | 83.74 | 128.6 | 129.2 | 169.1 |
| RPF | 1.0 | 0.091 | 0.056 | 0.015 | 0.010 | 0.009 | 0.007 |
| Cyp1a2 expression: | |||||||
| Chemical | TCDD | TBDF | 4PeBDF | TCDF | 1PeBDF | 4PeCDF | 1PeCDF |
| ED50 | 2.43 | 23.17 | 33.06 | 138.7 | 161.5 | 184.5 | 189.8 |
| RPF | 1.0 | 0.11 | 0.074 | 0.018 | 0.015 | 0.013 | 0.013 |
Note. TriBDD was not included in the RPF assessment due to a lack of a statistically significant effect. DBDCDD was not included in the RPF assessment due to a nonmonotonic dose response.
aRelative potency factors were calculated as the ED50 (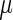 g/kg) value (from the Hill model) for TCDD/ED50 (
g/kg) value (from the Hill model) for TCDD/ED50 (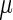 g/kg) value for the test chemical.
g/kg) value for the test chemical.
DISCUSSION
Reports of the presence of brominated dioxins and dibenzofurans in the environment, food, and human tissues are increasing, however, there is limited toxicological data on these compounds. This study was undertaken as part of the National Toxicology Program's TEF studies to investigate relative potencies of brominated dioxins and dibenzofurans as compared with their chlorinated analogs using endpoints whose sensitivity to these halogenated chemicals is well established.
With the exception of TriBDD, the brominated dioxins evaluated in the present study suppressed the humoral immune response as reflected by the AFC response and serum IgM titers. Of the eight test compounds, DBDCDD-induced suppression (four of five doses) of the antibody forming response most closely resembled TCDD-induced suppression. 4PeBDF and TBDF produced dose-dependent decreases that paralleled the TCDD response and that were greater than their chlorinated counterparts, with TBDF inducing the largest differential between the brominated and chlorinated congeners. At the high dose, 1PeBDF also suppressed antibody formation to a greater degree than 1PeCDF. The serum titer responses paralleled the AFC responses, but were lower in magnitude of suppression.
Cyp1a1 and Cyp1a2 enzymes are phase I XMEs and induction of these genes is a sensitive biomarker of AhR activation and dioxin-like effects, and dysregulation of Cyp1a1 and Cyp1a2 gene expression has long been used to evaluate those markers (Bock and Kohle, 2009; Ma, 2001). All compounds tested, except TriBDD, significantly upregulated hepatic Cyp1a1 and Cyp1a2 gene expression in a dose-dependent manner similar to TCDD. Calculation of the ED50 and relative potency for antibody formation and Cyp1a1 and Cyp1a2 data resulted in consistent patterns for AFC activity and gene expression. Of those compounds for which ED50 determinations were possible (see Table 3), the most potent compounds were TCDD, TBDF, and 4PeBDF, whereas 1PeCDF was consistently the least potent compound. The dose response curves for TCDF, 1PeBDF, and 4PeCDF were very similar and their potencies were also similar across endpoints. Although published reports indicate that TriBDD can exert effects in rat liver and hepatoma cells (Mason et al., 1987; Yang et al., 1983), in this study it did not affect antibody formation or Cyp1a1 gene expression.
Martin et al. (2012) proposed that TCDD decreases circulating T4 through increased biliary excretion (Martin et al., 2012). It has been suggested that induction of UGTs, which are AhR regulated genes (Bock and Kohle, 2009), and subsequent glucuronidation of T4 lead to increased excretion of the hormone. However, glucuronidation alone does not account for increased excretion of T4 for all organohalogens, and sulfotransferases and sulfation may also contribute to altered homeostasis. All of the compounds tested, except 1PeCDF and TriBDD, downregulated the gene for T4 serum transport protein (Ttr), which could indicate chemical-induced disruption in thyroxine transport. Phase III ABC transporters and solute carriers may also be involved in the increased elimination of thyroid hormones following dioxin exposure (Martin et al., 2012; Mustacich et al., 2009; Szabo et al., 2009).
Calculation of RPF requires a dose response from the reference and test compounds. Since TCDD induced limited effects on phase II and III XME, thyroid transport, and immune gene expression, relative potencies could not be calculated for those gene sets. At the highest tested dose(s), TCDD, TBDF, 4PeBDF, and 4PeCDF upregulated UGT genes, suggesting possible alterations in glucuronidation; however, induction of UGT activity in mice typically requires doses approximately 10–25 times higher than CYP1A1 induction (Craft et al., 2002). TCDD, 4PeBDF, TBDF, TCDF, and DBDCDD altered at least one of the phase III ABC transporter and solute carrier genes, with the greatest impact on gene expression observed from TBDF and TCDF.
Ligand-activated AhR can bind directly to specific dioxin response elements of target genes, resulting in potential transcriptional regulation. In addition to the XME genes described above, AhR can modulate gene expression of cytokines that anchor a complex network of molecules involved in antibody formation, inflammation, modulation of NFκB protein activity, and intercellular communication (Yoshioka et al., 2011). Although a potent immunotoxicant, TCDD had very limited effect on expression of immune genes in the liver. This was similar to what was observed in thymus in previous studies (Frawley et al., 2011). Vogel et al. (2013) reported that TCDD may regulate cytokine and chemokine gene expression in a tissue-specific manner. Published reports (Li et al., 2010; Yoshioka et al., 2011) have examined molecular targets and alterations in inflammatory responses and other specific endpoints following TCDD exposure, and proposed both direct genomic and non-genomic protein-protein interaction pathways initiated by the AhR as mechanisms for modulation of these endpoints. Although alterations in gene expression are not absolute proof of altered protein expression, it is notable that in this study the immune genes altered by one or more of the test compounds were consistently downregulated. Although cytokine gene expression was changed in the liver as a whole, it is not known which specific cell populations were affected. 4PeBDF, TBDF, and TCDF downregulated both the Th1 Ifnγ and Th2 Il6 genes, whereas DBDCDD downregulated the Th2 Il4 gene. These four chemicals also downregulated one of the Il1 genes and Nfκb. At high doses, TCDF and DBDCDD downregulated Tnfα, and 1PeBDF and TriBDD downregulated Nfκb.
The position of halogens in dioxin and dibenzofurans influences their affinity for the AhR and subsequent biologic activity, with the most active compounds being those that are halogenated only in the four lateral (2, 3, 7, and 8) positions (Martin et al., 2012; Mason et al., 1987; Safe, 1986). Halogenation at non-lateral sites or removal of lateral substituents decrease the potency of the compound (Behnisch et al., 2003; Mason et al., 1987). Increasing chlorine substitution at non-lateral positions decreases coplanarity due to steric crowding, and decreases receptor binding capabilities (Safe, 1986). Consistent with the coplanar theory, four of the five compounds that most profoundly reduced antibody formation (TCDD, DBDCDD, TBDF, 4PeBDF, and TCDF), and the three compounds with the largest induction of the Cyp1a1 and Cyp1a2 genes (TCDD, DBDCDD, and TBDF), are brominated or chlorinated only at the lateral positions. 4PeCDF, 1PeBDF, and 1PeCDF, each of which contains a non-lateral addition, were the least potent compounds. TriBDD, which lacks a halogen at the 8 position, had no impact on antibody formation or XME gene expression. In addition, the tetra-halogenated compounds TBDF, TCDF, and DBDCDD consistently and significantly suppressed expression of phase III XME and immune genes that were, for the most part, unaffected by the penta- and tri-halogenated compounds. 4PeBDF was the most potent of the penta-halogenated compounds tested, more potent than TCDF with regard to the AFC response and Cyp1a1 and Cyp1a2 expression. The effect of 4PeBDF on expression of phase III XME and immune genes was moderate compared with the tetra-halogenated compounds. TCDF is eliminated from the body faster than 4PeCDF or 1PeCDF (Kuroki et al., 1980; Zwiernik et al., 2008), but disposition and elimination of brominated congeners was not evaluated. The relationships between tissue accumulation, elimination, and potency of dioxin-like compounds have been reviewed (van Ede et al., 2013) in multiple species, with the suggestion that pharmacokinetic differences play a role in potency (DeVito and Birnbaum, 1995).
Although the studies presented here compare the relative potencies of brominated dioxins with their chlorinated analogs, there are several uncertainties in the application of this data to human health. First, relative potency values are not TEFs. TEFs are assigned values based on all available data, whereas RPFs are for a single endpoint from a single study. The exposure paradigm employed a single oral treatment in contrast to human exposure, which is most likely more frequent, and through the consumption of animal fat. Studies comparing the relative potencies of dioxins using a single exposure are very sensitive to pharmacokinetic differences between chemicals and may not be equivalent to relative potencies from chronic studies (DeVito and Birnbaum, 1995). Not all the chemicals induced maximal effects on the endpoints evaluated. Thus, based on this data alone, it is not clear if the chemicals are full or partial agonists and their interaction with chlorinated dioxins in mixtures is untested. Presently, exposure to brominated dioxins by the general population is limited compared with their chlorinated analogs. However, should exposure to brominated dioxins continue to increase, the present data would support the development of additional studies on this class of chemicals.
The present study suggests that some brominated dioxins and furans are as potent as, if not more potent than, their chlorinated counterparts. Cyp1a1 and Cyp1a2 gene expression indicated that 1PeBDF, 4PeBDF, TBDF, and DBDCDD are potent activators of the AhR; inhibition of antibody formation demonstrated that these compounds induce toxicological effects consistent with TCDD and other classical dioxin-like compounds, essential WHO criteria for classification as a dioxin (van den Berg et al., 2006). DBDCDD (four of five doses) demonstrated immunosuppressive capability nearly as potent as TCDD. TriBDD did not demonstrate any significant AhR activity. The gene expression patterns of 4PeBDF, TBDF, TCDF, and DBDCDD in the liver suggest immunomodulating capacity. Collectively, these data suggest that specific brominated analogs have potent dioxin-like activities that should be considered in TEF evaluation and risk assessment.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health, National Institute of Environmental Health Sciences (NIEHS) and National Cancer Institute; NIEHS Research and Development Contracts (N01-ES-55538, NO1-ES-00005).
Supplementary Material
Acknowledgments
We thank Deborah Musgrove, Ronnetta Brown, Dr Keith Shockley, and Dr Sue Edelstein for valuable assistance with this project, and Dr Cynthia Rider and Dr Michael Wyde for their thoughtful review of this manuscript. Conflict of interest statement. None declared.
REFERENCES
- Behnisch P. A., Hosoe K., Sakai S. Bioanalytical screening methods for dioxins and dioxin-like compounds—a review of bioassay/biomarker technology. Environ. Int. 2001;27:413–439. doi: 10.1016/s0160-4120(01)00028-9. [DOI] [PubMed] [Google Scholar]
- Behnisch P. A., Hosoe K., Sakai S. Brominated dioxin-like compounds: In vitro assessment in comparison to classical dioxin-like compounds and other polyaromatic compounds. Environ. Int. 2003;29:861–877. doi: 10.1016/s0160-4120(03)00105-3. [DOI] [PubMed] [Google Scholar]
- Van den Berg M., Birnbaum L., Bosveld A. T. C., Brunstrom B., Cook P., Feeley M., Geisy J. P., Hanberg A., Hasegawa R., Kennedy S. W., et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ. Health Perspect. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M., Birnbaum L. S., Denison M., DeVito M., Farland W., Feeley M., Fiedler H., Hakansson H., Hanberg A., Haws L., et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M., Denison M. S., Birnbaum L. S., DeVito M. J., Feidler H., Falandysz J., Rose M., Schrenk D., Safe S., Tohyama C., et al. Polybrominated dibenzo-p-dioxins, dibenzofurans, and biphenyls: Inclusion in the toxicity equivalency factor concept for dioxin-like compounds. Toxicol. Sci. 2013;133:197–208. doi: 10.1093/toxsci/kft070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum L. S., Staskal D. F., Diliberto J. J. Health effects of polybrominated dibenzo-p-dioxins (PBDDs) and dibenzofurans (PBDFs) Environ. Int. 2003;29:855–860. doi: 10.1016/S0160-4120(03)00106-5. [DOI] [PubMed] [Google Scholar]
- Bock K. W., Kohle C. The mammalian aryl hydrocarbon (Ah) receptor: From mediator of dioxin toxicity toward physiological functions in skin and liver. Biol. Chem. 2009;390:1225–1235. doi: 10.1515/BC.2009.138. [DOI] [PubMed] [Google Scholar]
- Choi J. W., Fujimaki T. S., Kitamura K., Hashimoto S., Ito H., Suzuki N., Sakai S., Morita M. Polybrominated dibenzo-p-dioxins, dibenzofurans, and diphenyl ethers in Japanese human adipose tissue. Environ. Sci. Technol. 2003;37:817–821. doi: 10.1021/es0258780. [DOI] [PubMed] [Google Scholar]
- Craft E. S., DeVito M. J., Crofton K. M. Comparative responsiveness of hypothyroxinemia and hepatic enzyme induction in Long-Evans rats versus C57BL/6J mice exposed to TCDD-like and phenobarbital-like polychlorinated biphenyl congeners. Toxicol. Sci. 2002;68:372–380. doi: 10.1093/toxsci/68.2.372. [DOI] [PubMed] [Google Scholar]
- Davis D., Safe S. Immunosuppressive activities of polychlorinated dibenzofuran congeners: Quantitative structure-activity relationships and interactive effects. Toxicol. Appl. Pharmacol. 1988;94:141–149. doi: 10.1016/0041-008x(88)90344-4. [DOI] [PubMed] [Google Scholar]
- DeVito M. J., Birnbaum L. S. The importance of pharmacokinetics in determining the relative potency of 2,3,7,8-tetrachlorodibenzo-p-dioxin and 2,3,7,8-tetrachlorodibenzofuran. Fundam. Appl. Toxicol. 1995;24:145–148. doi: 10.1006/faat.1995.1016. [DOI] [PubMed] [Google Scholar]
- Van Ede K. I., Aylward L. L., Andersson P. L., van den Berg M., van Duursen M. B. Tissue distribution of dioxin-like compounds: Potential impacts on systemic relative potency estimates. Toxicol. Lett. 2013;220:294–302. doi: 10.1016/j.toxlet.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Fernandes A., Mortimer D., Gem M., Dicks P., Smith F., White S., Rose M. Brominated dioxins (PBDD/Fs) and PBDEs in marine shellfish in the UK. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2009;26:918–927. doi: 10.1080/02652030902803026. [DOI] [PubMed] [Google Scholar]
- Fernandes A., Rose M., Mortimer D., Carr M., Panton S., Smith F. Mixed brominated/chlorinated dibenzo-p-dioxins, dibenzofurans and biphenyls: Simultaneous congener-selective determination in food. J. Chromatogr. A. 2011;1218:9279–9287. doi: 10.1016/j.chroma.2011.10.058. [DOI] [PubMed] [Google Scholar]
- Frawley R., White K. Jr., Brown R., Musgrove D., Walker N., Germolec D. Gene expression alterations in immune system pathways in the thymus after exposure to immunosuppressive chemicals. Environ. Health Perspect. 2011;119:371–376. doi: 10.1289/ehp.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963;140(3565) [PubMed] [Google Scholar]
- Jogsten I. E., Hagberg J., Lindstrom G., van Bavel B. Analysis of POPs in human samples reveal a contribution of brominated dioxin of up to 15% of the total dioxin TEQ. Chemosphere. 2010;78:113–120. doi: 10.1016/j.chemosphere.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Johnson C. W., Williams W. C., Copeland C. B., DeVito M. J., Smialowicz R. J. Sensitivity of the SRBC PFC assay versus ELISA for detection of immunosuppression by TCDD and TCDD-like congeners. Toxicology. 2000;156:1–11. doi: 10.1016/s0300-483x(00)00330-9. [DOI] [PubMed] [Google Scholar]
- Kuroki H., Masuda Y., Yoshihara S., Yoshimura H. Accumulation of polychlorinated dibenzofurans in the livers of monkeys and rats. Food Cosmet. Toxicol. 1980;18:387–392. doi: 10.1016/0015-6264(80)90195-9. [DOI] [PubMed] [Google Scholar]
- Li W. L., Vogel C. F. A., Wu D., Matsumura F. Non-genomic action of TCDD to induce inflammatory responses in HepG2 human hepatoma cells and in liver of C57BL6J mice. Biol. Chem. 2010;391:1205–1219. doi: 10.1515/BC.2010.126. [DOI] [PubMed] [Google Scholar]
- Luster M,I., Portier C., Pait D. G., White K. L., Jr, Gennings C., Munson A. E., Rosenthal G. J. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 1992;18:200–210. doi: 10.1016/0272-0590(92)90047-l. [DOI] [PubMed] [Google Scholar]
- Ma Q. Induction of Cyp1a1. The Ahr/DRE paradigm: transcription, receptor regulation, and expanding biological roles. Curr. Drug Metab. 2001;2:149–164. doi: 10.2174/1389200013338603. [DOI] [PubMed] [Google Scholar]
- Ma J., Addink R., Yun S., Cheng J., Wang W, Kannan K. Polybrominated dibenzo-p-dioxins/dibenzofurans and polybrominated diphenyl ethers in soil, vegetation, workshop-floor dust, and electronic shredder residue from an electronic waste recycling facility and in soils from a chemical industrial complex in eastern China. Environ. Sci. Technol. 2009;43:7350–7356. doi: 10.1021/es901713u. [DOI] [PubMed] [Google Scholar]
- Martin L. A., Wilson D. T., Reuhl K. R., Gallo M. A., Klassen C. D. Polychlorinated biphenyl congeners that increase the glucuronidation and biliary excretion of thyroxine are distinct from the congeners that enhance the serum disappearance of thyroxine. Drug Metab. Dispos. 2012;40:588–595. doi: 10.1124/dmd.111.042796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason G., Zacharewski T., Denomme M. A., Safe L, Safe S. Polybrominated dibenzo-p-dioxins and related compounds : quantitative in vivo and in vitro structure-activity relationships. Toxicology. 1987;44:245–255. doi: 10.1016/0300-483x(87)90027-8. [DOI] [PubMed] [Google Scholar]
- Mustacich D. J., Gohil K., Bruno R. S., Yan M., Leonard S. W., Ho E., Cross C. E., Traber M. G. Alpha-tocopherol modulates genes involved in hepatic xenobiotic pathways in mice. J. Nutr. Biochem. 2009;20:469–476. doi: 10.1016/j.jnutbio.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A. L., Mabury S. A., Reiner E. J. Analysis of mixed halogenated dibenzo-p-dioxins and dibenzofurans (PXDD/PXDFs) in soil by gas chromatography tandem mass spectrometry (GC_MS/MS) Chemosphere. 2012;87:1063–1069. doi: 10.1016/j.chemosphere.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Reeve R., Turner J. R. Pharmacodynamic models: Parameterizing the Hill equation, Michaelis-Menten, the logistic curve, and relationships among these models. J. Biopharm. Stat. 2013;23:648–661. doi: 10.1080/10543406.2012.756496. [DOI] [PubMed] [Google Scholar]
- Rose M., Fernandes A. Proceeding of the 5th International Symposium on Brominated Flame Retardants. Kyoto, Japan: 2010. Are BFRs responsible for brominated dioxins and furans (PBDD/Fs) in food? 7–9 April 2010. [Google Scholar]
- Safe S. H. Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Ann. Rev. Pharmacol. Toxicol. 1986;26:371–399. doi: 10.1146/annurev.pa.26.040186.002103. [DOI] [PubMed] [Google Scholar]
- Samara F., Gullett B. K., Harrison R. O., Chu A., Clark G. Determination of relative assay response factors for toxic chlorinated and brominated dioxins/furans using an enzyme immunoassay (EIA) and a chemically-activated luciferase gene expression cell bioassay (CALUX) Environ. Int. 2009;35:588–593. doi: 10.1016/j.envint.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Szabo D. T., Richardson V. M., Ross D. G., Diliberto J., Kodavanti P. R. S, Birnbaum L. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol. Sci. 2009;107:27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple L., Kawabata T. T., Munson A. E., White K. L. Comparison of ELISA and Plaque-forming cell assays for measuring the humoral immune response to SRBC in rats and mice treated with Benzo[a]pyrene or cyclophosphamide. Fundam. Appl. Toxicol. 1993;21:412–419. doi: 10.1006/faat.1993.1116. [DOI] [PubMed] [Google Scholar]
- Vogel C., Wu D., Baeck J., Kado S., Singh T., Yang G., Leung P., Gershwin E., Herrmann-Stemmann T., Matsumura F. Tissue-specific expression of cytokines and chemokines in TCDD-treated B6 and AhR-Repressor transgenic mice. The Toxicologist. 2013;132 [Google Scholar]
- White K. L., Musgrove D. L., Brown R. D. The sheep erythrocyte T-dependent antibody response (TDAR) In: Dietert R. R., editor. Immunotoxicity Testing: Methods and Protocols, Methods in Molecular Biology. Vol. 598. New Jersey, NJ: Humana Press; 2010. pp. 173–184. [DOI] [PubMed] [Google Scholar]
- Yang K. H., Yoo B. S., Choe S. Y. Effects of halogenated dibenzo-p-dioxins on plasma disappearance and biliary excretion of ouabain in rats. Toxicol. Lett. 1983;15:259–264. doi: 10.1016/0378-4274(83)90225-4. [DOI] [PubMed] [Google Scholar]
- Yoshioka W., Peterson R.E., Tohyama C. Molecular targets that link dioxin exposure to toxicity phenotypes. J Steroid Biochem. Mol. Biol. 2011;127:96–101. doi: 10.1016/j.jsbmb.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiernik M. J., Bursian S., Aylward L. L., Kau D. P., Moore J., Rowlands C., Woodburn K., Shotwell M., Khim J. S., Geisy J. P., et al. Toxicokinetics of 2,3,7,8-TCDF and 2,3,4,7,8-PeCDF in mink (Mustela vison) at ecologically relevant exposures. Toxicol. Sci. 2008;105:33–43. doi: 10.1093/toxsci/kfn118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.