Abstract
Although language difficulties are common in children born prematurely, robust neuroanatomical correlates of these impairments remain to be established. This study investigated whether the greater prevalence of language problems in preterm (versus term-born) children might reflect injury to major intra- or interhemispheric white matter pathways connecting frontal and temporal language regions. To investigate this, we performed a comprehensive assessment of language and academic abilities in a group of adolescents born prematurely, some of whom had evidence of brain injury at birth (n = 50, mean age: 16 years, mean gestational age: 27 weeks) and compared them to a term-born control group (n = 30). Detailed structural magnetic resonance imaging and diffusion-tractography analyses of intrahemispheric and interhemispheric white matter bundles were performed. Analysis of intrahemispheric pathways included the arcuate fasciculus (dorsal language pathway) and uncinate fasciculus/extreme capsule (ventral language pathway). Analysis of interhemispheric pathways (in particular, connections between the temporal lobes) included the two major commissural bundles: the corpus callosum and anterior commissure. We found language impairment in 38% of adolescents born preterm. Language impairment was not related to abnormalities of the arcuate fasciculus (or its subsegments), but was associated with bilateral volume reductions in the ventral language pathway. However, the most significant volume reduction was detected in the posterior corpus callosum (splenium), which contains interhemispheric connections between the occipital, parietal and temporal lobes. Diffusion tractography showed that of the three groups of interhemispheric fibres within the splenium, only those connecting the temporal lobes were reduced. Crucially, we found that language impairment was only detectable if the anterior commissure (a second temporal lobe commissural pathway) was also small. Regression analyses showed that a combination of anatomical measures of temporal interhemispheric connectivity (through the splenium of the corpus callosum and anterior commissure) explained 57% of the variance in language abilities. This supports recent theories emphasizing the importance of interhemispheric connections for language, particularly in the developing brain.
Keywords: language impairment, preterm, arcuate fasciculus, uncinate fasciculus, corpus callosum
Introduction
Language difficulties are among the most commonly reported cognitive deficits in children born prematurely (Barre et al., 2011). Delays in the development of expressive and receptive language (Stolt et al., 2007; Guarini et al., 2009; Woodward et al., 2009; Foster-Cohen et al., 2010) as well as more persistent language problems have been identified in early childhood (Briscoe et al., 1998; Sansavini et al., 2010), which may impact upon social competence (Redmond and Rice, 1998) and future academic attainments (Johnson et al., 2011). However, it remains unclear to what degree these impairments persist beyond childhood because to date, limited assessments of language skills in adolescence have been reported (Luu et al., 2011; Mullen et al., 2011). Furthermore, although alterations in the functional organization of language networks have been well studied in this population (Gozzo et al., 2009; Ment et al., 2009; Schafer et al., 2009; Myers et al., 2010), few have examined the structural brain correlates of language impairment (Frye et al., 2010; Mullen et al., 2011).
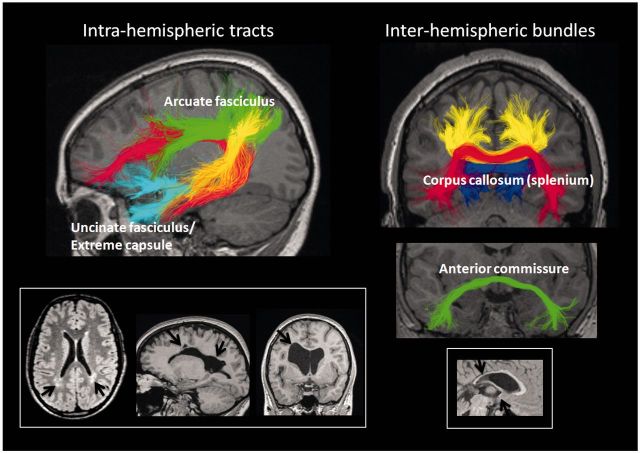
The greater prevalence of language problems in preterm (versus term-born) children might reflect injury to the periventricular white matter or corpus callosum because abnormalities in these areas are among the most commonly reported findings on conventional MRI in preterm individuals (Skranes et al., 2005). The periventricular white matter contains major intrahemispheric connections between frontal and temporal language regions, most notably the arcuate fasciculus. Ischaemic injury or haemorrhagic lesions associated with preterm birth may therefore disrupt the integrity of this important language pathway (see Fig. 1 for example cases), compounded by the fact that injury is frequently bilateral (Volpe, 2009). The corpus callosum is also of interest, as it contains the majority of interhemispheric fibre connections necessary for bi-hemispheric cooperation between language areas in the dominant hemisphere and their non-dominant homologues (Zaidel and Iacoboni, 2003; Friederici and Alter, 2004; Jung-Beeman, 2005). In children born preterm, the corpus callosum has been shown to be smaller than controls at term-age (Thompson et al., 2011), and this finding persists into childhood and adolescence (Nosarti et al., 2004; Caldu et al., 2006). The posterior portion (splenium) of the corpus callosum, which contains the fibres connecting language regions in the temporal lobes (Hofer and Frahm, 2006), is often most severely affected (Nosarti et al., 2004; Caldu et al., 2006; Thompson et al., 2011) (Fig. 1). This finding is of particular relevance given the recent evidence for a critical role of interhemispheric splenial connections in the interaction of syntax and prosody in the adult brain (Friederici et al., 2007; Sammler et al., 2010). Furthermore, it appears that prosodic cues play an important role in early language acquisition (Jusczyk, 1999), though it remains to be determined whether early injury to the splenium interferes with subsequent language development. Indeed, Nosarti et al. (2004) previously reported a correlation between posterior CC size and both verbal fluency and intelligence scores in male preterm participants; however, it is uncertain whether this finding extends to other core language functions or whether it applies to the wider preterm population.
Figure 1.
Language-relevant white matter pathways and regions of vulnerability in the preterm brain. Left: Intrahemispheric language tracts, the uncinate fasciculus/extreme capsule, arcuate fasciculus and its subsegments (red: direct segment; green: anterior segment; yellow: posterior segment). Bottom left: Example cases with periventricular white matter injury in proximity to the arcuate fasciculus (arrows). Right: Interhemispheric bundles traversing the splenium of the corpus callosum (red: connecting the temporal lobes; blue: connecting the occipital lobes; yellow: between parietal lobes) and the anterior commissure (connecting the anterior temporal lobes of both hemispheres). Bottom right: Example case with severe reduction of the posterior corpus callosum, including the splenium (upper arrow), and of the anterior commissure (lower arrow).
A second commissural pathway that might also be involved in interhemispheric communication between language areas is the anterior commissure. This substantial white matter bundle is of potential significance, as its fibres largely connect the anterior and lateral portions of the temporal lobes, and changes in anterior commissure size have been noted in developmental groups with abnormalities in the corpus callosum (Paul, 2011). Nevertheless, this white matter pathway has received very little attention in the preterm literature (Counsell et al., 2008).
Modern diffusion-weighted imaging (DWI) methods allow in vivo visualization and quantification of parameters that are assumed to reflect the microstructural integrity of white matter tracts (Catani and Thiebaut de, 2008; Friederici, 2009; Tournier et al., 2011). DWI-based tractography has shown that the arcuate fasciculus has a number of subcomponents. In addition to a long segment that directly connects Broca’s area with the temporal association cortex, there is an indirect pathway composed of two separate parts: a posterior segment extending from the temporal association cortex to the inferior parietal region and an anterior segment extending between the inferior parietal lobule and Broca’s area (Catani et al., 2005). In addition to this well-known dorsal language pathway, more recent work has identified a separate ventral route connecting the anterior temporal lobes with the inferior frontal gyrus via the uncinate fasciculus/extreme capsule fibre system (Anwander et al., 2007; Saur et al., 2008). The respective functional roles of these pathways are still under debate, but it is suggested that the dorsal path subserves auditory–motor integration, whereas the ventral path is more involved in the semantic and syntactic aspects of language comprehension (Hickok and Poeppel, 2007; Friederici, 2012). A comprehensive examination of intrahemispheric language pathways should therefore consider the possibility that the ventral white matter bundles traversing the extreme capsule might theoretically be able to compensate for periventricular arcuate fasciculus damage.
Finally, whole-brain, voxel-based methods have also been used to identify preterm-birth-associated abnormalities that would not be visible on conventional MRI and have revealed volume reduction in both temporal lobes, most notably in the middle temporal gyrus (Nosarti et al., 2008; Nagy et al., 2009; Soria-Pastor et al., 2009; Zubiaurre-Elorza et al., 2009). The role of these temporal lobe regions in receptive language functions is well established (Price, 2010), and structural changes in these areas have been implicated in children with developmental language disorders (Jancke et al., 2007). However, it is not currently known whether the reported volume reductions are responsible for language dysfunction in the preterm population.
In this study, we carried out a comprehensive assessment of language skills and academic ability in a group of adolescents born very prematurely, some of whom had evidence of brain injury at birth, and compared them with a term-born control group. This allowed us to identify a group of children with suboptimal language performance: the ‘LI’ group. Neuroimaging (including structural MRI and DWI) was used to examine the contribution of white matter pathways to language impairment in this cohort. We examined both intrahemispheric pathways (including the white matter tracts of the dorsal and ventral routes) and interhemispheric pathways (via the corpus callosum and anterior commissure) (summarized in Fig. 1). First, we assessed abnormalities at the whole-brain level using voxel-based methods, followed by a more focused quantification of damage to the intra- and interhemispheric connections. Importantly, we removed global volume effects from these analyses to evaluate language specificity, since general cognitive abilities (as measured by IQ) have been shown to strongly correlate with volume of total brain white matter in this population (Soria-Pastor et al., 2008; Northam et al., 2011). Finally, although changes in functional connectivity of language were not the focus of this study, a simple functional MRI task was used to determine language lateralization in all participants.
Materials and methods
Participants
Fifty adolescents (mean age ± SD, 16 ± 1 years) were recruited from a prospective follow-up study of preterm children (born at <33 weeks gestation) at the Neonatal Intensive Care Unit, University College Hospital, London, UK [see Northam et al. (2011) for further details of the recruitment]. Twenty-eight children had positive cranial ultrasound findings and 22 had normal scans. The mean gestational age of the total group was 27 ± 2 weeks. The mean birth weight was 1081 ± 385 g. A control group of 30 term-born adolescents was recruited from siblings, school friends and by advertisement. These were group-matched for age and sex and maternal education (years of additional schooling beyond the age of 16, representing the end of compulsory education in the UK).
Magnetic resonance imaging acquisition
All participants were scanned with a 1.5-T Avanto Siemens scanner. Conventional T2-weighted images were acquired using an axial multi-slice sequence (repetition time = 4920 ms, echo time = 101 ms, field of view = 220 mm, slice thickness = 4 mm, slices = 25, matrix size = 384 × 384). Three-dimensional data sets were acquired using a T1-weighted 3D-FLASH sequence (repetition time = 11 ms, echo time = 4.94 ms, flip angle = 15°, field of view = 256 mm, matrix size = 256 × 256) and a T2-weighted FLAIR sequence (repetition time = 6000 ms, echo time = 353 ms, flip angle = 150°, field of view = 256 mm, matrix = 256 × 256).
DWI data were acquired in all participants using an eddy-current-nulled twice-refocused EPI sequence with high-angular resolution (b-value = 3000 s/mm2, echo time = 128 ms, 60 diffusion-weighted directions, in-plane resolution 2.1 × 2.1 mm2, 3 mm slice thickness, 37 contiguous axial slices, acquisition time ∼9 min). DWI data sets were preprocessed for tractography using the MRTrix software suite (Tournier et al., 2012), to obtain whole-brain FA, eigen-vector and constrained spherical deconvolution maps. The order of MRI acquisition for all participants was as follows: structural MRI, language functional MRI (see Supplementary material for details) and DWI.
Neuropsychological, behavioural and academic assessment
The interval between MRI scanning and neuropsychological assessment was short; for the majority of participants all tests were completed on the same day of scanning, 10 children returned within 1 month and three within 6 months to complete the assessment. The Wechsler Abbreviated Scale of Intelligence was used to calculate separate verbal and non-verbal/performance scores and a combined full-scale intelligence quotient. The expressive and receptive scales from the Clinical Evaluation of Language Fundamentals (CELF-3UK) (Semel et al., 2000) provided global language measures, assessing a broad range of expressive and receptive skills. The Expressive and Receptive One-Word Picture Vocabulary tests (Brownell, 2000a, b) were administered to provide a measure of one-word vocabulary. The Test for Reception of Grammar (Bishop, 2003) was used to assess each child’s ability to understand grammatical contrasts of increasing difficulty. Literacy skills were measured using the Wechsler Objective Reading Dimensions (Wechsler, 1993), providing a separate score for spelling, reading and comprehension abilities. The Comprehensive Test of Phonological Processing (Wagner et al., 1999) was used to measure phonological awareness (the ability to perceive and manipulate sounds of spoken words) and phonological memory (the ability to code information phonologically for temporary storage in working or short-term memory). The digit span test from the Wechsler Intelligence Scale (Wechsler, 1992) was administered to provide a short-term memory measure.
Additional assessments included the test for Auditory Processing Disorders in Adolescents and Adults (SCAN-A) (Keith, 1994) and standard neurological examination. School performance was evaluated by the number of achieved or predicted passes in final school exams at the age of 16 years (General Certificate of Secondary Education). Behaviour was assessed with the Strengths and Difficulties Questionnaire (Goodman et al., 1998) where a higher score indicates greater emotional and behavioural difficulties. Developmental quotient at 1 year was assessed with the Griffiths Developmental Scale (Griffths, 1954). The same group was also assessed using tests of speech and oromotor praxis (Northam et al., 2012).
Definition of language impairment
Children who scored more than −1.5 z-scores (derived from the mean and SD of the control group) on the CELF-3UK total language score were classified as ‘language impaired’. The CELF-3UK test was chosen as it encompasses a wide range of receptive and productive language skills and is an established clinical tool for the identification and diagnosis of children and adolescents with language skill deficits.
Whole-brain measures
Tissue segmentation and total brain volumes
Volumes of CSF, grey matter and white matter were calculated from 3D-FLASH images, using the Voxel-Based Morphometry toolbox (C. Gaser, http://dbm.neuro.uni-jena.de/vbm/) for Statistical Parametric Mapping 8 (SPM8; Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). In contrast to the default SPM8 algorithm, the final tissue probabilities were estimated without a spatial template of tissue distribution (‘priors’), which allows reliable segmentation of grossly abnormal brains (in particular those with ventricular dilatation). This was achieved by using the unified segmentation procedure implemented in SPM8, modified to include a Hidden Markov Field model as an additional spatial constraint (http://dbm.neuro.uni-jena.de/vbm/markov-random-fields/). For consistency, this method was applied to all preterm and term-born control brains. All tissue segments were visually inspected for accuracy. Areas of high signal on T2-weighted images (presumed to represent gliosis) were automatically excluded from the white matter volume if the signal change was detectable on 3D-FLASH images. For further details, see Northam et al. (2011).
Qualitative neuroradiological review
All scans were evaluated using a standardized protocol by an experienced paediatric neuroradiologist (W.K.C.). This included the assessment of the degree and laterality of periventricular white matter reduction and gliosis, in addition to ratings of the presence and extent of thinning of the corpus callosum. Details of the findings are reported elsewhere (Northam et al., 2011).
Voxel-based morphometry
A group-level comparison was performed after spatially normalizing grey and white matter segments to a study-specific template created using the TMP-O-Matic toolbox (Wilke et al., 2008) and smoothing using an 8 mm full-width at half-maximum Gaussian kernel. Global white or grey matter volume, age and sex were included in the statistical model as covariates of no interest. After the normalization step, four preterm participants were excluded due to residual ventricular dilatation. Group comparisons were performed: first, between all preterm brains versus controls (reported in Supplementary material) for comparison with previous VBM studies (Nosarti et al., 2008; Nagy et al., 2009) and second, between language-impaired and unimpaired preterm brains. The statistical threshold of P < 0.05, family-wise error-corrected for multiple comparisons across the whole brain, was used for regional differences where there was no prior hypothesis. Regions where there was an a priori hypothesis (i.e. in periventricular regions and the corpus callosum) were evaluated at threshold of P < 0.001, uncorrected for multiple comparisons. The minimum cluster size was 50.
Integrity of intrahemispheric language pathways
DWI-tractography of the arcuate fasciculus and its segments
White matter tracts were dissected using probabilistic streamline fibre-tracking based on constrained spherical deconvolution (Tournier et al., 2007). For every tract, streamlines (n = 100 000) were generated from a seed region of interest. Only those streamlines that connected with the second (target) region of interest were retained. The frontal seed region for the arcuate fasciculus was identified lateral to the pyramidal tract according to the method of Catani et al. (2005). The temporal lobe target region was defined by tracing the extent of the temporal lobes on a single coronal slice just posterior to the transverse temporal gyri. The parietal region of interest was determined using the boundaries of the inferior parietal lobule (supramarginal and angular gyri) identified on co-registered eigen-vector maps and T1-weighted images, aided by an anatomical atlas (Oishi et al., 2010). Two-ROI tractography was performed as in Catani et al. (2005, 2007): the direct arcuate fasciculus segment connected the frontal and temporal regions, while the anterior indirect segment connected the frontal and parietal regions. The posterior indirect segment [corresponding to the middle longitudinal fasciculus (Schmahmann et al., 2007)] was created by tracking between the parietal and temporal regions. The resulting tracts were transformed into volumes, where each voxel represents the proportion of streamlines retained in relation to the overall number generated. In other words, the voxel value is the probability that streamlines passing through this voxel will connect both regions of interest. These raw tract volumes were subjected to thresholding (i.e. converted into a binary mask) to reduce the contribution of spurious connections (Johansen-Berg et al., 2007). The probability threshold was set to 0.001 so that each voxel contained at least 100 streamlines. The total apparent volume and mean fractional anisotropy were determined within the binary mask in each hemisphere. Intra-rater reliability for total arcuate fasciculus tract volume was excellent in both hemispheres (left single measures intra-class coefficient = 0.90; right intra-class correlation coefficient = 0.84; in 20 cases).
DWI-tractography of the uncinate fasciculus/extreme capsule fibre system
As the uncinate fasciculus and extreme capsule fibre system have largely overlapping termination fields in anterior temporal and inferior frontal lobes (Schmahmann et al., 2007), and in view of the limited resolution of DWI, we combined them into one uncinate fasciculus/extreme capsule fibre system (Friederici, 2012). The uncinate fasciculus was determined using the recommendations of Catani and Thiebaut de Schotten (2008), where a seed region of interest was placed on four adjacent axial slices in the white matter of anterior temporal lobe. The more dorsal fibres of the extreme capsule fibre system were collected from the white matter of the superior temporal gyrus anterior to Heschl’s gyrus according to method of Frey et al. (2008). The target region of interest for both tracts was the white matter of the extreme capsule, identified on four axial slices (Catani and de Schotten, 2008). The calculation of volume and mean anisotropy was performed as mentioned above.
Integrity of interhemispheric pathways
Corpus callosum measurements
The corpus callosum was measured manually by tracing the total area on the mid-sagittal slice (Giedd et al., 1999) on 3D-FLASH images with MRIcron software (C. Rorden, www.sph.sc.edu/comd/rorden/mricron). Intra-rater reliability was excellent (single measures intra-class correlation coefficient = 0.99; in 21 cases). Area measurements of five subdivisions of the corpus callosum were performed according to the revised anatomical model of Hofer and Frahm (2006). Mean fractional anisotropy was also measured on the mid-saggital slice, avoiding partial volume effects by only including voxels well within the boundary of the corpus callosum.
DWI-tractography of transcallosal fibres
The transcallosal fibres of the occipital, parietal and temporal lobes were identified using a modified procedure proposed by Hofer and Frahm (2006). The seed region of interest for all three groups of fibres was identical and included the posterior half of the corpus callosum, traced in the mid-sagittal plane. Boundaries between the lobes were defined using anatomical landmarks similar to Behrens et al. (2003), aided by an anatomical white matter atlas (Oishi et al., 2010). For each lobe, planar or quasi-planar target regions of interest were placed in each hemisphere. The occipital lobe regions were traced in a coronal plane approximately equidistant between the anterior and posterior lobar boundaries. The parietal lobe regions were traced on consecutive sagittal slices, excluding the primary somatosensory cortices (S1), in order to include only those connections passing through the most posterior corpus callosum subregion (region V), which are the focus of this study. The temporal lobe target regions of interest were determined as above for the arcuate fasciculus. Streamlines connecting both the target regions in each hemisphere, through the seed region, were selected (Johansen-Berg et al., 2007; Westerhausen et al., 2009). The raw tract volumes were thresholded with a P > 0.001.
Measurement of the anterior commissure
The anterior commissure could be identified confidently on T1- and T2-weighted images in all participants, and the cross-sectional area was determined using the method of Patel et al. (2010). Briefly, the maximal extension of the anterior commissure was determined on coronal and axial planes, and this information was used to calculate the mean ellipsoid area. This same procedure was repeated for the whole sample. Measurement intra-rater reliability was excellent (single measures intra-class correlation coefficient = 0.83; in all cases). Tractography of the anterior commissure across the mid-saggital plane (Catani et al., 2002) was not possible in a large number of participants owing to the limited resolution of DWI, and these data will not be reported here.
Functional magnetic resonance imaging to determine language lateralization
Functional MRI was used to investigate hemispheric language lateralization in all preterm and control participants using a covert verb generation task. This is a reliable task used clinically (Baldeweg and Liegeois, 2010) and was used here to assess whether LI individuals differed from their peers in language lateralization in the inferior frontal and temporal regions. See the online Supplementary material for further details on the task and functional MRI results.
Statistical analysis
Neuropsychological performance between the total preterm sample and the control group was first compared using standardized test scores. Then z-scores for the preterm group were calculated from the mean and SD of the control group (Supplementary Table 1). The computed z-scores were then compared between the LI and unimpaired preterm groups using: (i) t-tests; and (ii) analyses of covariance (ANCOVA) with performance IQ as a covariate. The effect size in each test was also computed before and after removing performance IQ effects. To assess the differences in intra- and interhemispheric white matter between preterm groups (with and without language impairment) and controls, ANCOVAs were used (covariates: age, sex and intracranial volumes/total white matter, where appropriate). Tukey’s honestly significant difference test was used for post hoc comparisons. Data that were not normally distributed were converted before analysis using a logarithmic transformation. Principle component analysis was conducted on all neuropsychological measures to reduce the number of variables for correlation analyses (see Supplementary material for further details). Pearson’s correlations were used to demonstrate the relationship between brain measures and neuropsychological components extracted. A logistic regression analysis was performed to determine the significant predictors of the presence of language impairment, and stepwise linear regression analyses were performed to identify predictors of the severity of the language impairment (both before and after removing performance IQ effects). Diagnostic analyses included examination of influential data points, normality of residuals and multi-collinearity. Model selection was performed in a stepwise fashion using the likelihood ratio statistic for logistic regression and using the R2 change statistic for multiple linear regression (Field, 2005).
Ethics
Ethical approval for the study was obtained from Great Ormond Street Hospital for Children/UCL Institute of Child Health Research Ethics Committee, and written informed consent was obtained from all participants or their parents (depending on age at assessment).
Results
Language outcome and school performance
Language outcome
The preterm group showed wide variation in language abilities, with scores ranging from exceptionally low to high-average (Supplementary Table 1). However, at the group level, adolescents born preterm scored significantly lower than controls on measures of expressive and receptive language, spelling and reading. After Bonferroni correction for multiple comparisons, only scores on the expressive and receptive scales of the CELF-3UK remained significantly lower than controls.
Language impairment
Nineteen preterm participants (38%) were classified as LI based on a total CELF-3UK language score falling below the control group mean by 1.5 z-scores or more. Verbal and performance IQ scores were also significantly lower than those of the unimpaired group. However, in 12 of the 19 cases with language impairment, the language score was at least 10 points lower than the non-verbal IQ (with a discrepancy of up to 31 points). Importantly, performance on all language measures remained significantly worse in the LI group (compared with preterm peers) even after adjusting for performance IQ (all P ≤ 0.001, Supplementary Table 2).
Academic ability
After controlling for performance IQ, the LI group scored significantly lower in tests of spelling, reading and comprehension; they also passed fewer school-leavers’ exams. A larger proportion of the LI group also had (i) a history of speech and language problems; and (ii) had been receiving additional help at school (Table 1).
Table 1.
Characteristics and performance of the preterm groups with and without language impairment
| Characteristics and performance | Unimpaired language (n = 31) | Impaired language (n = 19) | Statistical comparison |
|---|---|---|---|
| Total Language Score (CELF-3UK) | 102 (10) | 68 (10) | t(48) = 11.9, P < 0.0001* |
| Verbal IQ | 100 (10) | 77 (9) | t(48) = 8.3, P < 0.0001 |
| Non-verbal IQ | 100 (14) | 81 (13) | t(48) = 4.6, P < 0.0001 |
| Demographics | |||
| Age (years) | 16.2 (1.2) | 16.1 (1.6) | t(48) = 0.3, P = 0.767 |
| Sex ratio (male:female) | 11:20 | 8:11 | χ2(1, n = 50) = 0.2, P = 0.431 |
| Maternal education (years) | 2.5 (3.3) | 2.1 (3.2) | t(48) = 0.4, P = 0.714 |
| Abnormal MRI at follow-up, n (%) | 16 (52) | 17 (89) | χ2(1, n = 50) = 4.5, P = 0.032 |
| Abnormal/suspicious neurological outcome, n (%) | 7 (23) | 10 (53) | χ2 (2, n = 50) = 4.7, P = 0.031 |
| Perinatal and developmental variables | |||
| Cranial ultrasound lesion, n (%) | χ2 (2, n = 50) = 1.7, P = 0.430 | ||
| Minor | 11 (35) | 6 (32) | |
| Major | 5 (16) | 6 (32) | |
| Gestation (weeks) | 28 (2) | 26 (2) | t(48) = 3.0, P = 0.004 |
| Birth weight (g) | 1203 (420) | 883 (210) | t(48) = 3.6, P = 0.001 |
| Development quotient at 1 year | 119 (16) | 110 (12) | t(46) = 2.1, P = 0.041 |
| History of speech and/or language delay, n (%) | 6 (19) | 13 (68) | χ2(1, n = 50) = 6.0, P = 0.014 |
| School performance and behaviour | |||
| Total behavioural score | 7.4 (5.7) | 12.0 (3.6) | t(41) = 2.9, P = 0.007 |
| Additional help at school, n (%) | 2 (7) | 15 (75) | χ2 (1, n = 50) = 28.0, P < 0.0001 |
| School leavers exam passes (grades A–C) | 8 (3) | 2 (2) | t(38) = 6.7, P < 0.0001* |
Table displays mean values and standard deviation in parenthesis unless otherwise stated. Values in bold are significant.
* Significantly different after controlling for non-verbal IQ (P < 0.0001).
Whole-brain comparisons
Brain volumes
The LI group had reduced total brain white matter [F(2,72) = 4.7, P = 0.012] compared with the unimpaired preterm group (LI group = 394 ml, unimpaired = 439 ml, adjusted for intracranial volume) and with the term-born control group (467 ml). There were no differences in adjusted grey matter volumes [F(2,72) = 0.3, P = 0.718].
Radiological assessment
Comparing the two preterm groups, there were no differences in the frequency or severity of abnormalities detected on cranial ultrasound at birth (Table 1), but significantly more cases with LI had an abnormal MRI in adolescence. The difference between the groups was due to an excess of bilateral periventricular white matter reduction [χ2(1,50) = 11.2, P = 0.004] or thinning of the corpus callosum in the LI group [χ2(1,50) = 9.6, P = 0.008].
Voxel-based comparisons
Voxel-based morphometry analysis of the entire preterm group (compared with controls) revealed foci of grey matter and white matter reductions in both temporal lobes (Supplementary material and Supplementary Fig. 1). Direct comparison of the two preterm groups (LI versus non-LI) showed two foci of white matter reduction, but no grey matter differences (Fig. 2). The white matter reductions were in the posterior callosal region and left temporal lobe. These findings were supported by tract-based spatial statistics (TBSS) analysis, showing significant fractional anisotropy reductions in similar regions of the corpus callosum and temporal white matter (Supplementary material and Supplementary Fig. 2).
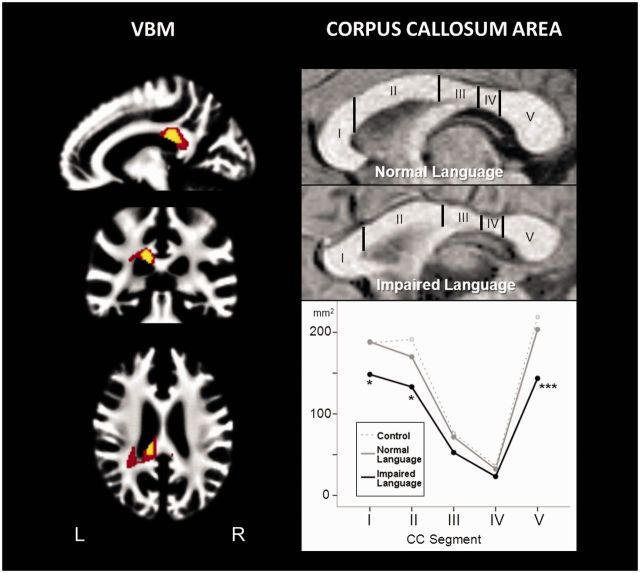
Figure 2.
Corpus callosum reduction in the language-impaired preterm group. Left: Voxel-based morphometry (VBM) analysis identified two focal regions of white matter volume reduction in language-impaired individuals in the region of the posterior corpus callosum (splenium) extending into the left temporal lobe. At lower thresholds, this cluster also extended into the right temporal white matter. Colour coding indicates different statistical thresholds: yellow: P < 0.05, family wise error-corrected for multiple comparisons, red: P < 0.001, uncorrected. Right: Manual measurements of the area of corpus callosum segments [according to Hofer and Frahm (2006)] confirmed pronounced reduction in segment V. Post hoc comparisons between preterm groups with and without language impairment (covariate: global white matter volume) are indicated by **P < 0.0001, *P < 0.05 (all Bonferroni corrected). CC = corpus callosum.
Integrity of intrahemispheric language pathways
Arcuate fasciculus and its segments
Volumes of the direct arcuate fasciculus segments were significantly lower in the LI group in both cerebral hemispheres, compared with term-born controls (Table 2). There were no differences in the indirect segments or the total arcuate fasciculus volume (combining all three segments) between groups. Comparison of LI versus unimpaired preterm groups also showed a significant reduction of the right direct arcuate fasciculus in the LI group, together with a trend for reduction in the left hemisphere. However, this difference disappeared when the contribution of total brain white matter was removed, suggesting that the observed arcuate fasciculus reduction was in proportion to overall white matter loss. Closer, case by case inspection of the relationship between severity of periventricular white matter injury, arcuate fasciculus integrity and language outcome did not reveal a systematic association (Fig. 3). A group comparison of laterality indices for both arcuate fasciculus volume and FA was also not significant, and there were no differences in mean anisotropy across all groups.
Table 2.
Intrahemispheric language tract measurements in both preterm groups and controls
| Tract | Segment | Controls | Preterm |
ANCOVA [1] |
ANCOVA [2] |
||||
|---|---|---|---|---|---|---|---|---|---|
| Unimpaired language | Impaired language | Covariates: age and sex/age, sex and ICV | Post hoc: preterm groups | Covariate: age, sex, total WM | Post hoc: preterm groups | ||||
| Left AF | Direct | Volume (cm3) | 11.8 (2.3) | 11.1(2.6) | 9.6 (2.9)* | F(2,71) = 2.3, P = 0.117 | P = 0.093 | F(2,71) = 0.4, P = 0.694 | P = 0.451 |
| FA | 0.284 (0.02) | 0.295 (0.02) | 0.286 (0.02) | F(2,73) = 1.5, P = 0.237 | P = 0.369 | - | - | ||
| Anterior | Volume (cm3) | 8.2 (2.2) | 9.2 (3.5) | 8.2 (3.1) | F(2,70) = 0.9, P = 0.390 | P = 0.476 | F(2,70) = 0.7, P = 0.490 | P = 0.337 | |
| FA | 0.286 (0.02) | 0.284 (0.02) | 0.282 (0.03) | F(2,71) = 0.3, P = 0.719 | P = 0.718 | - | - | ||
| Posterior | Volume (cm3) | 4.2 (2.8) | 4.7 (3.3) | 4.8 (3.3) | F(2,70) = 0.4, P = 0.656 | P = 0.751 | F(2,70) = 0.3, P = 0.745 | P = 0.787 | |
| FA | 0.342 (0.03) | 0.346 (0.04) | 0.339 (0.03) | F(2,71) = 0.2, P = 0.799 | P = 0.525 | - | - | ||
| Right AF | Direct | Volume (cm3) | 10.89 (2.3) | 10.82 (2.3) | 9.29 (1.9)* | F(2,71) = 2.1, P = 0.131 | P = 0.047 | F(2,70) = 0.9, P = 0.38 | P = 0.191 |
| FA | 0.28 (0.02) | 0.29 (0.02) | 0.28 (0.02) | F(2,70) = 1.2, P = 0.305 | P = 0.438 | - | - | ||
| Anterior | Volume (cm3) | 13.0 (2.2) | 12.5 (2.5) | 12.4 (3.7) | F(2,71) = 0.06, P = 0.945 | P = 0.747 | F(2.70) = 0.3, P = 0.750 | P = 0.492 | |
| FA | 0.279 (0.02) | 0.280 (0.02) | 0.269 (0.02) | F(2,70) = 1.6, P = 0.205 | P = 0.105 | - | - | ||
| Posterior | Volume (cm3) | 3.8 (2.7) | 4.7 (3.2) | 4.9 (2.7) | F(2,71) = 0.5, P = 0.624 | P = 0.902 | F(2,70) = 0.8, P = 0.442 | P = 0.738 | |
| FA | 0.341 (0.04) | 0.342 (0.04) | 0.327 (0.04) | F(2,70) = 1.0, P = 0.360 | P = 0.177 | - | - | ||
| Left UF/ECFS | Volume (cm3) | 5.1 (2.2) | 3.7 (1.8) | 2.5 (1.5)** | F(2,71) = 7.2, P = 0.001 | P = 0.014 | F(2,70) = 4.1, P = 0.020 | P = 0.042 | |
| FA | 0.261 (0.02) | 0.260 (0.02) | 0.260 (0.02) | F(2,70) = 0.005, P = 0.995 | P = 0.992 | - | - | ||
| Right UF/ECFS | Volume (cm3) | 5.7 (1.8) | 4.9 (2.1) | 3.2 (1.7)*** | F(2,71) = 6.5, P = 0.003 | P = 0.003 | F(2,70) = 4.0, P = 0.023 | P = 0.011 | |
| FA | 0.254 (0.02) | 0.253 (0.02) | 0.253 (0.01) | F(2,70) = 0.05, P = 0.947 | P = 0.895 | - | - | ||
Table displays mean unadjusted values and standard deviation in parenthesis. Value in bold are significant.
Different from controls *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, - = not performed.
AF = arcuate fasciculus; FA = fractional anisotropy; ICV = intracranial volume; WM = white matter volume; UF/ECFS = uncinate fasciculus/ extreme capsule fibre system.
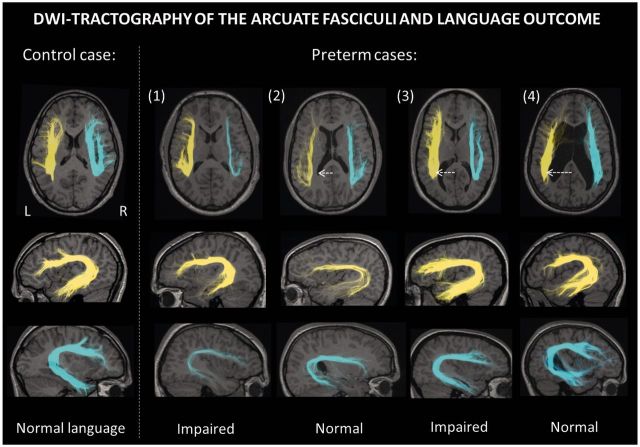
Figure 3.
DWI-tractography of the arcuate fasciculus. Identification of the direct segment in representative cases illustrates the lack of a robust relationship between tract volume and language outcome in the preterm cohort. Arcuate fasciculi in both hemispheres are shown for one control (left side) and four preterm adolescents (Cases 1–4). The projection of the whole tract is shown on representative slices of T1-weighted MRI scans. Preterm cases had varying degrees of ventricular dilatation (white arrows). Language impairment was found in cases with no MRI abnormalities and normal arcuate fasciculus volume in the left hemisphere (Case 1).
Uncinate fasciculus/ECFS
The volumes of the combined uncinate fasciculus/extreme capsule fibre system were reduced bilaterally in the LI group compared with unimpaired preterms (Table 2), even when global white matter reduction was accounted for. There were no anisotropy differences in this pathway between groups.
Integrity of interhemispheric pathways
Corpus callosum
Total corpus callosum area was significantly lower in the LI group compared with both unimpaired preterms and controls, even after removing global white matter effects (Table 3). This difference was driven by a marked reduction in segment five (V) in the posterior (splenial) region, followed by more anterior reductions in segments one and two (I/II), corresponding to the rostrum and genu (Fig. 2). Group differences in anisotropy were also evident, but were entirely in keeping with corpus callosum area reductions.
Table 3.
Corpus callosum and interhemispheric tract measurements in both preterm groups and controls
| Controls | Preterm |
ANCOVA [1] |
ANCOVA [2] |
|||||
|---|---|---|---|---|---|---|---|---|
| Unimpaired language | Impaired language | Covariates: age, sex, ICV/age, sex | Post hoc: preterm groups | Covariates: age, sex, total WM | Post hoc: preterm groups | |||
| Total corpus callosum | Area (mm2) | 709 (104) | 671 (112) | 500 (95)*** | F(2,72) = 20.5, P < 0.0001 | P < 0.0001 | F(2,73) = 10.3, P < 0.0001 | P < 0.0001 |
| FA | 0.53 (0.04) | 0.51 (0.07) | 0.46 (0.08)*** | F(2,74) = 7.3, P = 0.001 | P = 0.006 | - | - | |
| Segment of corpus callosum (area, mm2) | I | 18.7 (3.4) | 18.8 (3.3) | 14.8 (2.5)** | F(2,72) = 9.9, P < 0.0001 | P < 0.0001 | F(2,73) = 6.1, P = 0.004 | P = 0.004 |
| II | 19.1 (2.7) | 17.3 (3.2) | 13.3 (3.1)*** | F(2,72) = 16.3, P < 0.0001 | P < 0.0001 | F(2,73) = 4.7, P = 0.012 | P = 0.004 | |
| III | 7.6 (1.5) | 7.3 (2.0) | 5.3 (1.4)*** | F(2,72) = 8.7, P < 0.0001 | P < 0.0001 | F(2,73) = 3.2, P = 0.045 | P = 0.017 | |
| IV | 3.6 (1.5) | 3.3 (0.8) | 2.3 (0.9)** | F(2,72) = 6.7, P = 0.002 | P = 0.004 | F(2,73) = 2.6, P = 0.085 | P = 0.04 | |
| V | 21.9 (4.2) | 20.7 (4.4) | 14.4 (4.0)*** | F(2,72) = 16.5, P < 0.0001 | P < 0.0001 | F(2,73) = 6.9, P = 0.002 | P < 0.0001 | |
| Occipital transcallosal fibres | Volume (cm3) | 39.5 (7.1) | 37.7 (11.4) | 35.9 (15.9) | F(2,70) = 0.6, P = 0.570 | P = 0.575 | F(2,70) = 0.8, P = 0.446 | P = 0.541 |
| FA | 0.30 (0.03) | 0.30 (0.02) | 0.29 (0.04) | F(2,72) = 0.7, P = 0.509 | P = 0.251 | - | - | |
| Parietal transcallosal fibres | Volume (cm3) | 62.4 (16.5) | 56.6 (24.9) | 53.0 (18.7) | F(2,70) = 1.3, P = 0.271 | P = 0.582 | F(2,70) = 0.6, P = 0.581 | P = 0.413 |
| FA | 0.29 (0.03) | 0.29 (0.03) | 0.28 (0.03) | F(2,72) = 1.2, P = 0.304 | P = 0.18 | - | - | |
| Temporal transcallosal fibres | Volume (cm3) | 12.5 (9.5) | 12.2 (10.5) | 2.3 (3.0)*** | F(2,70) = 8.6, P < 0.0001 | P = 0.001 | F(2,70) = 7.9, P = 0.001 | P = 0.001 |
| FA | 0.37 (0.04) | 0.37 (0.08) | 0.38 (0.11) | F(2,72) = 0.04, P = 0.959 | P = 0.86 | - | - | |
Table displays mean unadjusted values and standard deviation in parenthesis. Value in bold are significant.
Different from controls **P ≤ 0.01, ***P ≤ 0.001, - = not performed.
FA = fractional anisotropy; ICV = intracranial volume; WM = white matter volume.
Posterior CC connectivity
In the LI group, DWI-based tractography showed that of the three groups of interhemispheric fibres within posterior segment V (occipital, parietal, temporal), only those connecting the temporal lobes were reduced in volume (Table 3). Representative example cases are shown in Fig. 4.
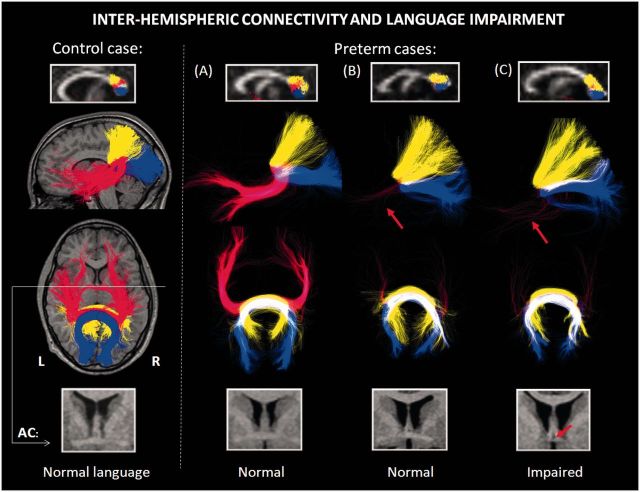
Figure 4.
Interhemispheric connectivity and language impairment. DWI-tractography of the posterior transcallosal fibre connections between the parietal lobes (shown in yellow), occipital lobes (blue) and temporal lobes (red) in one term-born control case (displayed on a T1-weighted MRI scan on left side) and three preterm adolescents (Cases A–C), all males aged 16–18 years. The projections of the whole tracts are shown. The mid-saggital section of the corpus callosum and the distribution of the fibre bundles are displayed on fractional anisotropy maps (top row). The size the anterior commissure (AC) is also shown on T1-weighted MRI (bottom row). Case A: Language outcome was unimpaired (CELF-3UK language score = 101, 11 points higher than performance IQ). Both interhemispheric connections between the temporal lobes were intact. Case B: Despite severely reduced temporal lobe fibres (red arrow), language was not impaired (CELF-3UK language score = 99, 13 points higher than performance IQ). The size of anterior commissure was intact. Note, this patient is also shown in Fig. 2 (Case 2) with bilaterally reduced arcuate fasciculi. Case C: Language was impaired (CELF-3UK language score = 78, 23 points lower than performance IQ). Reduction of both temporal lobe fibres and the anterior commissure is visible, with intact parietal and occipital connections. This case is also shown in Fig. 2 (Case 1), with intact left arcuate fasciculus.
Anterior commissure
Cross-sectional area of the anterior commissure was also reduced in the LI group in excess of global white matter reduction (mean area: 2.5 mm2, SD 1.4 mm2) compared with controls (6.4 mm2, SD 3.2 mm2) and unimpaired preterms (7.7 mm2, SD 3.4 mm2) [F(2,73) = 13.7, P < 0.0001]. Three example preterm cases are shown in Fig. 4.
Predictors of language impairment in the total preterm group
Having identified differences in intra- as well as interhemispheric white matter connectivity between the LI and unimpaired preterm adolescents, we used regression analyses to determine which of those independently predicted (i) the presence/absence of LI defined using a clinical criterion; and (ii) the severity/degree of impairment (CELF-3UK total language score) in the preterm cohort. In each case, correlation coefficients between predictor variables were <0.6, tolerance values exceeded 0.67 and variance inflation factors were <1.5, indicating no multicollinearity (Field, 2005).
Presence of language impairment
Stepwise logistic regression was conducted using area/volume measurements of the following: combined left and right arcuate fasciculi (direct segment), combined left and right uncinate fasciculus/extreme capsule fibre system, anterior commissure, corpus callosum segment V and temporal lobe interhemispheric fibres (Table 4). The final model (Step 2) predicted the presence of LI with a 91% success rate (P < 0.0001, sensitivity 94%, specificity 89%) using two variables: size of the anterior commissure and volume of the temporal lobe interhemispheric fibres.
Table 4.
Logistic regression analysis
| Logistic regression: Presence of language impairment (CELF-3UK score <1.5 z-scores) | |||||
|---|---|---|---|---|---|
| B (SE) | Exp(B) | P | change in −2 × log likelihood | ||
| Step 1 | |||||
| Predictors: | AC | −0.90 (0.28) | 0.41 | 0.001 | 31.6, P < 0.0001 |
| Step 2 (final model) | |||||
| Predictors: | AC | −0.90 (0.32) | 0.41 | 0.005 | 7.4, P = 0.006 |
| TL fibre volume | −0.27 (0.16) | 0.76 | 0.088 | ||
AC = anterior commissure; TL = temporal lobe.
Severity of language impairment
A stepwise linear regression model using the same variables explained 57% of the variance in CELF-3UK scores [R2adjusted = 0.57, F(2,43) = 29.7, P < 0.0001], with the size of the anterior commissure and area of corpus callosum segment V as predictors (Table 5). The analysis was repeated in an attempt to predict the degree of LI in excess of any non-verbal IQ deficit, which was determined by first removing the contribution of performance IQ to CELF-3UK scores in an initial linear regression. The model explained 32% of the variance in the discrepancy, with the anterior commissure as the only predictor [R2adjusted = 0.32, F(1,43) = 21.4, P < 0.0001].
Table 5.
Linear regression analyses
| B (SE) | Beta | P | R2 change | ||
|---|---|---|---|---|---|
| Linear regression: Severity of language impairment (total CELF-3uk score) | |||||
| Step 1 | |||||
| Predictors: | AC | 2.87 (0.47) | 0.68 | <0.0001 | 0.451, P < 0.0001 |
| Step 2 (final model) | |||||
| Predictors: | AC | 1.84 (0.51) | 0.44 | 0.001 | 0.122, P = 0.001 |
| CC segment V | 1.57 (0.44) | 0.43 | 0.001 | ||
| Linear regression: CELF-3UK residuals (after removing PIQ) | |||||
| Step 1 (final model) | |||||
| Predictors: | AC | 1.73 (0.37) | 0.58 | <0.0001 | 0.32, P < 0.0001 |
AC = anterior commissure; CC = corpus callosum; PIQ = performance IQ.
Predictors of language impairment in IQ-matched groups and controls
To provide additional confirmation that the brain correlates of LI identified above are truly specific to language abilities, we conducted analyses in two subgroups of preterm individuals, matched for non-verbal IQ scores.
A LI subgroup (n = 10, non-verbal IQ = 92 ± 7, total language score = 73 ± 7) was compared (on all brain measures) with a preterm group with normal language abilities (n = 15, non-verbal IQ = 90 ± 7, total language score = 97 ± 8). The findings replicated the group comparisons, showing significant differences only for anterior commissure size [F(1,23) = 26.0, P < 0.0001], total corpus callosum area [F(1,23) = 7.6, P = 0.01], uncinate fasciculus/extreme capsule fibre system volume [right hemisphere only: F(1,23) = 10.3, P = 0.003] and temporal lobe interhemispheric fibre volume [F(1,23) = 11.0, P = 0.003].
In term-born controls, correlations were also evident (covaried for sex, age and white matter volume) between the size of the anterior commissure and verbal IQ [r(25) = 0.49, P = 0.009] and the CELF-3UK receptive language score [r(25) = 0.42, P = 0.028]. The volume of temporal lobe interhemispheric fibres also correlated with verbal IQ [r(25) = 0.43, P = 0.026]. Correlations with performance IQ were not significant.
Language domains and their white matter tract correlates
To explore in more detail the respective contributions of interhemispheric and intrahemispheric (dorsal and ventral) fibre systems to LI in this population, we analysed the relationship between the volume of these tracts and a range of more specific language skills. We first performed data reduction using principal component analysis of all language test scores (Supplementary material), which identified three independent components: (i) a ‘complex language’ factor (reflecting a composite of CELF-3UK, receptive grammar and literacy scores, accounting for 47% of variance); (ii) a ‘phonological processing’ factor (reflecting the phonological awareness, phonological memory and SCAN-A scores, 13% of variance); and (iii) a ‘vocabulary’ factor (receptive and expressive vocabulary scores, 9% of variance). We found correlations between the ‘complex language’ factor and the uncinate fasciculus/extreme capsule fibre system bilaterally [left side: r(43) = 0.35, P = 0.025, right side: r(43) = 0.36, P = 0.019] as well as the temporal lobe interhemispheric pathways: anterior commissure [r(43) = 0.46, P = 0.002] and splenial fibre volume [r(43) = 0.36, P = 0.023]. In contrast, the ‘phonological processing’ factor correlated only with the arcuate fasciculus direct segment volumes in the left [r(43) = 0.41, P = 0.007] and right hemispheres [r(43) = 0.34, P = 0.027]. The ‘vocabulary’ factor correlated only with anterior commissure size [r(43) = 0.40, P = 0.009].
Discussion
The key finding of this study is that reduced interhemispheric temporal lobe connectivity strongly predicts LI in adolescents born preterm, accounting for nearly 60% of the variance in language scores. Examination of the integrity of intrahemispheric connectivity showed no compelling evidence that injury to the dorsal language pathway (the arcuate fasciculus and its segments) independently predicts LI. This is an unexpected finding given the well-established role of this white matter tract in language (Catani et al., 2005; Friederici, 2009). In contrast, significant bilateral reductions were found within the ventral pathway (incorporating fibres within the uncinate fasciculus and extreme capsule). Nevertheless, the most robust predictors of LI were interhemispheric (commissural). These consisted of two anatomically separate white matter bundles connecting the left and right temporal lobes: (i) a more rostral pathway, linking the anterior temporal lobes via the anterior commissure; and (ii) a caudal pathway, connecting the posterior temporal region via the splenium of the corpus callosum.
The profile of language impairment
This study used an extensive battery of language assessments in adolescents born preterm to demonstrate persistence of deficits that have previously been reported only in childhood (Wolke and Meyer, 1999; Wolke et al., 2008; Luu et al., 2009; Foster-Cohen et al., 2010). It has also shown that despite receiving additional educational support, these children continue to perform less well in final school exams and literacy. In our study, LI was defined using a clinical tool that assesses complex language skills (including semantics, morphology and syntax, CELF-3UK). However, when examining the profile of impairment across domains (Supplementary Table 1), it is clear that preterm children are impaired on a wider range of tests, including vocabulary, literacy, receptive grammar and phonological processing. Nevertheless, deficits in the CELF-3UK were most apparent, as ∼40% of the preterm sample fell in the impaired range in this test. This finding is in line with a recent meta-analysis suggesting that complex language is most affected in preterm children and that these deficits are likely to increase as children grow up (van Noort-van der Spek et al., 2012).
Interhemispheric connections
The CC/splenium
The corpus callosum is the principal commissural (interhemispheric) white matter bundle connecting the left and right cerebral hemispheres and, although not traditionally associated with language, it has more recently been suggested to play an important role in language comprehension (Friederici and Alter, 2004). This hypothesis was directly supported in our sample by VBM comparison and manual tracing of the cross-sectional area of the corpus callosum showing that LI adolescents have a significantly reduced corpus callosum, which was most pronounced in the posterior (splenial) region.
Correlations between verbal IQ/fluency and the cross-sectional area of the posterior corpus callosum (Nosarti et al., 2004; Narberhaus et al., 2007) have previously been reported in cohorts of adolescent preterms, but similar relationships have also been documented with non-verbal IQ (Peterson et al., 2000; Allin et al., 2007; Narberhaus et al., 2007) and visuo-motor abilities (Rademaker et al., 2004). In agreement with these findings, we found in our cohort that the size of corpus callosum segment V (splenial region) correlated with both verbal IQ and performance IQ. This is perhaps unsurprising given that this region contains substantial transcallosal connections between the occipital, parietal and temporal lobes. In the preterm population, reduced anisotropy and volume of transcallosal fibres has been reported in infancy (Hasegawa et al., 2011; Thompson et al., 2011) but until now this had not been investigated in childhood or adolescence. Using in vivo tractography, we were able to dissect these transcallosal projections into parietal, occipital and temporal components and show that LI is specifically associated with reduction of fibres connecting the temporal lobes. These findings are in line with studies in term-born groups, which have also demonstrated a relationship between diffusion properties of temporal lobe corpus callosum fibres and verbal abilities such as reading and phonological awareness (Ben-Shachar et al., 2007; Dougherty et al., 2007; Carreiras et al., 2009).
The transcallosal (splenial) fibres form dense connections between the superior and middle temporal cortices of both hemispheres (Turken and Dronkers, 2011). Bilateral superior temporal gyrus/superior temporal sulcus regions are involved in the early (phoneme-level) stages of speech comprehension, with the left hemisphere preferentially responding to phonetic features of speech and the right to prosodic and intonational cues (Scott and Wise, 2004). In addition, the middle temporal gyrus appears to be an important cortical ‘hub’ for lexical access (Hickok and Poeppel, 2007; Buckner et al., 2009, Turken and Dronkers, 2011). It is therefore conceivable that reduction in the transcallosal connections between these regions, in addition to the focal grey matter reductions observed (Nosarti et al., 2008; Nagy et al., 2009), may contribute to language deficits in preterm children by disturbing the initial stages of speech perception (degraded and dichotic speech processing) and word-level comprehension.
Furthermore, transcallosal temporal lobe connections appear to play a critical role in the integration of syntax and prosody, whereby right-hemisphere specialization for prosodic cues interacts with left-hemisphere morpho-syntactic processing. This has been shown in event-related potential studies in patients with adult onset corpus callosum lesions (Friederici et al., 2007; Sammler et al., 2010). It would be interesting to determine whether the anterior commissure also mediates such prosody–syntax interaction, in addition to the posterior corpus callosum, in particular in cases of early injury to splenial fibres.
The anterior commissure
In our cohort, LI was found to be present only when both the anterior commissure and the corpus callosum temporal lobe fibre connections were reduced. This situation presumably reflects severe limitation of interhemispheric communication between temporal lobe language areas. The anterior commissure fibres predominantly interconnect the anterior temporal lobes (Demeter et al., 1990; Di Virgilio et al., 1999) in contrast with the splenium of the corpus callosum, which links the more posterior temporal regions. Investigations using diffusion tractography (Catani et al., 2002) have demonstrated that the territory of temporal lobe cortex interlinked by anterior commissure fibres is much more extensive than previously thought, consistent with a major role in interhemispheric communication (Peltier et al., 2011).
Nevertheless, the robust contribution of the anterior commissure to language performance demonstrated in this cohort is somewhat surprising. However, it is important to note that the majority of anterior commissure fibres interconnect the cortical regions of the anterior temporal lobes, and that the importance of both the left and right anterior temporal lobes in semantic aspects of language comprehension is well documented (Patterson et al., 2007), although some differences in their respective roles have been suggested (Jung-Beeman, 2005; Hickok and Poeppel, 2007). Furthermore, there is clinical evidence in support of the functional importance of anterior temporal lobe connectivity, as it has been shown to predict language comprehension ability after stroke in adults (Warren et al., 2009).
The anterior commissure may compensate for reduced callosal connectivity
In patients with callosal agenesis, the cross-sectional area of the anterior commissure is reportedly increased (Herweh et al., 2009), possibly due to greater retention of connections that would otherwise have been lost in a competitive process during callosal development (Bossy, 1970). We found evidence supporting the concept of compensatory anterior commissure increase in our preterm children who did not have language problems. It remains unclear whether this apparent compensation might take place at an early stage (in infancy) or at a later point (e.g. during language acquisition). An alternative explanation for the increase in anterior commissure size, which is likely to be relevant in at least a proportion of preterms, might be the relative preservation of this structure due to its early development in fetal life; this is completed by gestational week 13, well before the vulnerable preterm period (Raybaud, 2010).
Degree of prematurity and risk of language impairment
The mean gestational age of the LI preterms was significantly lower than those without language problems. This raises the possibility that infants born earlier may be more vulnerable to anterior commissure and posterior callosal injury, with subsequent temporal lobe and language abnormalities. In keeping with this suggestion, the size of the posterior corpus callosum and volume of the lateral temporal lobes have previously been show to correlate with degree of prematurity (Ball et al., 2012; Hasegawa et al., 2011; Thompson et al., 2011). In our cohort, there were correlations not only between gestational age and size of the posterior splenial region but also with the anterior commissure.
Selective vulnerability of callosal fibres connecting the temporal lobes might reflect their close proximity to the germinal (subependymal) matrix that lines the walls of the lateral ventricles (Vasung et al., 2011) and is prominent in the roof of the temporal horns. This highly vascular structure is well-known to be susceptible to injury, particularly in the most preterm infants or those of very low birth weight (Perlman and Volpe, 1986).
The anterior commissure may also be affected due to (i) its proximity to the thalamostriate groove, which is the most common site for germinal matrix haemorrhage; and (ii) its position in the anterior wall of the third ventricle, in direct contact with CSF (containing blood and blood breakdown products in the context of intraventricular haemorrhage). We noted that in our cohort, the children with the greatest anterior commissure reduction had a history of germinal matrix haemorrhage and blood within the ventricular system. Post-mortem studies in cases of intraventricular haemorrhage support this contention, showing associated damage to adjacent white and grey matter (Rutherford et al., 2010; Del Bigio, 2011).
Relevance to other developmental language disorders
The corpus callosum abnormalities identified in the LI preterm group show parallels with other developmental language disorders. Although total corpus callosum size is not reduced in such disorders, there is some evidence that the splenium may be smaller (Preis et al., 2000). It has been suggested (Paul, 2011) that this accords with the robust behavioural evidence (dichotic listening) of ‘callosal dysfunction’ in this group (Njiokiktjien et al., 1994; Fabbro et al., 2002). In line with this, we also found auditory speech perception deficits (using the SCAN-A) in our LI cases that were confined to comprehension of dichotically presented single words and sentences (data not shown).
Finally, we observed that the group with LI showed more bilateral activation in both frontal and temporal language regions on functional MRI (Supplementary material and Supplementary Fig. 3). A direct relationship between the degree of LI (CELF-3UK total score) and extent of right-hemisphere (atypical) lateralization was also evident. Myers et al. (2010) also showed that engagement of right-hemisphere language regions was associated with poorer performance on comprehension and vocabulary measures in preterm adolescents. Differences in the functional asymmetry of language are also well-described in developmental language disorders (Whitehouse and Bishop, 2008; de Guibert et al., 2011). One interpretation of our data is that compromised interhemispheric connectivity incurred early in life impedes the development of typical left-hemisphere language specialization, with consequences for the development of normal language skills.
Intrahemispheric pathways: dorsal and ventral language streams
To examine the dorsal language pathway in detail, we used a fine-grained segmentation of the arcuate fasciculus (Catani et al., 2005) and found bilateral reduction in our language-impaired group of the long arcuate fasciculus segment, which directly connects the inferior frontal regions with the temporal lobes. Although on a group-level this was calculated to be in keeping with the total white matter reduction, there was a proportion of children (with brain injury at birth) in whom arcuate fasciculus volumes were reduced by up to 33% (data not shown). This suggests that although injury to this pathway did occur in our sample, it does not robustly predict the presence of LI as defined by our clinical criterion. A possible explanation for the lack of group differences in the indirect segments of the arcuate fasciculus could be their more lateral position within the subcortical white matter (Catani et al., 2005), further from the vulnerable periventricular white matter region.
To examine the ventral language pathway, we considered the fibres passing in the uncinate fasciculus together with those traversing the extreme capsule, and in contrast to the dorsal pathway, there were bilateral volume differences in the uncinate fasciculus/extreme capsule fibre system, which could also be observed on VBM at a lower statistical threshold. Reductions in the anisotropy of the uncinate fasciculus/extreme capsule fibre system have previously been reported using a region of interest method in preterm adolescents and related to vocabulary skills (Constable et al., 2008; Mullen et al., 2011). Although the anterior temporal lobes and their connections to the frontal cortex are not usually regarded as directly vulnerable in association with preterm birth, recent neuroimaging studies have shown volume loss in this region extending into the temporal stem and this correlates with degree of prematurity in both infants (Ball et al., 2012) and adolescents (Constable et al., 2008; Nagy et al., 2009).
More detailed analyses of the white matter correlates of language abilities in our study are consistent with previous findings in preterm adolescents who did not have overt brain injury, which showed associations between arcuate fasciculus integrity and reading/phonological abilities (Frye et al., 2010; Mullen et al., 2011). Such findings broadly support the concept of a functional distinction between the dorsal and ventral language routes, which is seen in functional MRI studies in healthy adults (Saur et al., 2008; Lopez-Barroso et al., 2011), but maybe most discernible after brain injury, for instance as a consequence of preterm birth or temporal lobe surgery (Yogarajah et al., 2010).
More complex language deficits in our cohort correlated with markers of integrity of both the anterior commissure and the uncinate fasciculus/extreme capsule fibre system. The associations with the uncinate fasciculus/extreme capsule fibre system are in line with the proposal that this ventral route may also subserve the integration of semantic and syntactic aspects of language (involving anterior temporal and inferior frontal regions) that are necessary for comprehension of sentential information (Friederici, 2012). However, our study suggests that the anatomical connections between the left and right anterior temporal lobes (via the anterior commissure) may represent an important ‘bottleneck’ and that the strength of these connections may be critical for the development of normal language ability. The anterior commissure (anterior interhemispheric) and UF/extreme capsule fibre system (ventral intrahemispheric) pathways could be jointly vulnerable due to the mutual influences of callosal and cortical development (de Lacoste et al., 1985; Moses et al., 2000) or as a consequence of late temporal lobe maturation during the preterm period (Constable et al., 2008).
Methodological considerations
It is important to acknowledge the potential limitations of this study. One consideration is the possibility that the prevalence of LI might have been overestimated because we recruited a larger proportion of children with positive cranial ultrasound findings at birth than are present in the total birth cohort. However, this proved to be beneficial, as we were able to assess the impact of a very wide spectrum of injury to the periventricular and interhemispheric white matter.
It is also important to bear in mind that the impairments in the LI group were not confined to the language domain, since the non-verbal IQ of the group was also lower. To overcome this potential confound, we used performance IQ as a covariate [but see Dennis et al. (2009) for a critical discussion] in our analyses and showed that the contribution of interhemispheric connectivity was still evident. We also confirmed our findings in a subgroup analysis of participants matched for non-verbal IQ.
There are also limitations for each of the neuroimaging methods used in this study, in terms of spatial resolution and susceptibility to artefacts, particularly when applied to abnormal brains. To overcome this problem, we provided converging evidence from a number of imaging modalities. For this reason, the size reductions in the anterior portions (I and II) of the corpus callosum were not investigated further here (as this was not confirmed on whole-brain analysis). Nevertheless, it is not implausible that reductions in the interhemispheric connections between inferior frontal gyri are also functionally relevant; however, these are difficult to quantify owing to the high density of crossing fibres in this region (Jones, 2010; Tournier et al., 2011).
The importance of interhemispheric connections for language in the developing brain
Our data are entirely in keeping with recent theories regarding the importance of interhemispheric connections for specific elements of language in the adult brain, from single-word comprehension (Bozic et al., 2010) to the integration of syntax and prosody at the sentence level, which should also have implications for language development. There is indeed evidence for a critical role of prosodic cues in early language acquisition (Shukla et al., 2011) and in predicting later language ability (Weber et al., 2005), whereas children with specific LI show deficits in integrating prosody with syntax (Marshall et al., 2009). Given the early vulnerability of the splenium of the corpus callosum (Thompson et al., 2011), it is therefore not surprising that preterm infants are deficient in discriminating prosodic cues in speech (Herold et al., 2008). Although information from prospective studies is not yet available, retrospective review of our cohort shows that parental report of delayed language development was associated with reductions in interhemispheric connections. Future studies should test the proposed relationship between vulnerability of interhemispheric connections and early language development in preterm infants and evaluate whether early targeted interventions can be effective.
Finally, further investigation of the relationship between structural and functional connectivity (Schafer et al., 2009) in the developing brain is also warranted. Recent evidence from resting state functional connectivity analyses suggests a maturational shift between childhood and adulthood from predominantly interhemispheric to predominantly intrahemispheric connectivity (Power et al., 2011). More specifically, functional cortical connectivity during language tasks in adults shows predominantly fronto-temporal functional connectivity in the left hemisphere, while children show stronger interhemispheric connectivity, especially involving the posterior temporal regions (Friederici et al., 2011). It is therefore conceivable that damage to interhemispheric connections in preterm infants may affect the normal maturation of left-hemisphere language networks.
Conclusion
Our findings suggest that localized damage to interhemispheric white matter connections (specifically those interconnecting the temporal lobes) is associated with LI in the preterm population and that the risk of language difficulties increases with degree of prematurity. Importantly, this can occur in addition to the global reduction in brain white matter that has previously been found to be associated with reduced general intelligence in this group. The early identification of children at risk of LI—and ultimately, the prevention of white matter injury in the preterm brain—will offer significant benefit for the educational progress of this expanding population.
This study also supports the concept of a more general role for interhemispheric connections in the acquisition of normal language abilities, underscored by the robust relationship that we have observed between measures of temporal lobe connectivity and language scores in term-born controls. This calls for a systematic investigation of interhemispheric connections in other developmental populations where LI is a predominant feature, including developmental language disorders, autism and temporal lobe epilepsy.
Funding
Action Medical Research UK [SN4051], the Garfield Weston Foundation and Great Ormond Street Hospital Children’s Charity.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors thank Tina Banks for MRI scanning, Frances Cowan and Sharon Geva for helpful comments and discussion.
Glossary
Abbreviations
- CELF-3UK
Clinical Evaluation of Language Fundamentals
- CC
corpus callosum
- DWI
diffusion-weighted imaging
- ECFS
extreme capsule fibre system
- FA
fractional anisotropy
- LI
language-impaired
- ROI
region of interest
- UF
uncinate fasciculus
- VBM
voxel-based morphometry
References
- Allin M, Nosarti C, Narberhaus A, Walshe M, Frearson S, Kalpakidou A, et al. Growth of the corpus callosum in adolescents born preterm. Arch Pediatr Adolesc Med. 2007;161:1183–9. doi: 10.1001/archpedi.161.12.1183. [DOI] [PubMed] [Google Scholar]
- Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knosche TR. Connectivity-based parcellation of Broca’s area. Cereb Cortex. 2007;17:816–25. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- Baldeweg T, Liegeois F. Functional MRI in pediatric epilepsy surgery. In: Cataltepe O, Jallo G, editors. Pediatric epilepsy surgery: preoperative assessment and surgical treatment. New York: Thieme Medical Publishers; 2010. pp. 74–81. [Google Scholar]
- Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N, et al. The effect of preterm birth on thalamic and cortical development. Cereb Cortex. 2012;22:1016–24. doi: 10.1093/cercor/bhr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: a meta-analysis. J Pediatr. 2011;158:766–74. doi: 10.1016/j.jpeds.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–7. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M, Dougherty RF, Wandell BA. White matter pathways in reading. Curr Opin Neurobiol. 2007;17:258–70. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bishop D. Test for reception of grammar. London: Psychological Corporation; 2003. [Google Scholar]
- Bossy JG. Morphological study of a case of complete, isolated, and asymptomatic agenesis of the corpus callosum. Arch Anat Histol Embryol. 1970;53:289–340. [PubMed] [Google Scholar]
- Bozic M, Tyler LK, Ives DT, Randall B, Marslen-Wilson WD. Bihemispheric foundations for human speech comprehension. Proc Natl Acad Sci USA. 2010;107:17439–44. doi: 10.1073/pnas.1000531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Gathercole SE, Marlow N. Short-term memory and language outcomes after extreme prematurity at birth. J Speech Lang Hear Res. 1998;41:654–66. doi: 10.1044/jslhr.4103.654. [DOI] [PubMed] [Google Scholar]
- Brownell R. Novato, CA: Academic Therapy Publications Inc; 2000. Expressive one-word picture vocabulary test. [Google Scholar]
- Brownell R. Novato, CA: Academic Therapy Publications Inc; 2000. Receptive one-word picture vocabulary test. [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldu X, Narberhaus A, Junque C, Gimenez M, Vendrell P, Bargallo N, et al. Corpus callosum size and neuropsychologic impairment in adolescents who were born preterm. J Child Neurol. 2006;21:406–10. doi: 10.1177/08830738060210050801. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estevez A, Lozano A, Devlin JT, et al. An anatomical signature for literacy. Nature. 2009;461:983–6. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, et al. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA. 2007;104:17163–8. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–32. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Constable RT, Ment LR, Vohr BR, Kesler SR, Fulbright RK, Lacadie C, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121:306–16. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- Counsell SJ, Edwards AD, Chew AT, Anjari M, Dyet LE, Srinivasan L, et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain. 2008;131:3201–8. doi: 10.1093/brain/awn268. [DOI] [PubMed] [Google Scholar]
- de Guibert C, Maumet C, Jannin P, Ferre JC, Treguier C, Barillot C, et al. Abnormal functional lateralization and activity of language brain areas in typical specific LI (developmental dysphasia) Brain. 2011;134:3044–58. doi: 10.1093/brain/awr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human CC. J Neuropathol Exp Neurol. 1985;44:578–91. doi: 10.1097/00005072-198511000-00004. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR. Cell proliferation in human ganglionic eminence and suppression after prematurity-associated haemorrhage. Brain. 2011;134:1344–61. doi: 10.1093/brain/awr052. [DOI] [PubMed] [Google Scholar]
- Demeter S, Rosene DL, Van Hoesen GW. Fields of origin and pathways of the interhemispheric commissures in the temporal lobe of macaques. J Comp Neurol. 1990;302:29–53. doi: 10.1002/cne.903020104. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 2009;15:331–43. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio G, Clarke S, Pizzolato G, Schaffner T. Cortical regions contributing to the anterior commissure in man. Exp Brain Res. 1999;124:1–7. doi: 10.1007/s002210050593. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc Natl Acad Sci USA. 2007;104:8556–61. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbro F, Libera L, Tavano A. A callosal transfer deficit in children with developmental language disorder. Neuropsychologia. 2002;40:1541–6. doi: 10.1016/s0028-3932(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Field A. London: Sage Publications; 2005. Discovering Statistics Using SPSS - 2nd Edition. [Google Scholar]
- Foster-Cohen SH, Friesen MD, Champion PR, Woodward LJ. High prevalence/low severity language delay in preschool children born very preterm. J Dev Behav Pediatr. 2010;31:658–67. doi: 10.1097/DBP.0b013e3181e5ab7e. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28:11435–44. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Pathways to language: fiber tracts in the human brain. Trends Cogn Sci. 2009;13:175–81. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Friederici AD. The cortical language circuit: from auditory perception to sentence comprehension. Trends Cogn Sci. 2012;16:262–8. doi: 10.1016/j.tics.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Alter K. Lateralization of auditory language functions: a dynamic dual pathway model. Brain Lang. 2004;89:267–76. doi: 10.1016/S0093-934X(03)00351-1. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Brauer J, Lohmann G. Maturation of the language network: from inter- to intrahemispheric connectivities. PLoS One. 2011;6:e20726. doi: 10.1371/journal.pone.0020726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, von Cramon DY, Kotz SA. Role of the corpus callosum in speech comprehension: interfacing syntax and prosody. Neuron. 2007;53:135–45. doi: 10.1016/j.neuron.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Frye RE, Hasan K, Malmberg B, Desouza L, Swank P, Smith K, et al. Superior longitudinal fasciculus and cognitive dysfunction in adolescents born preterm and at term. Dev Med Child Neurol. 2010;52:760–6. doi: 10.1111/j.1469-8749.2010.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, et al. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:571–88. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Goodman R, Meltzer H, Bailey V. The Strengths and Difficulties Questionnaire: a pilot study on the validity of the self-report version. Eur Child Adolesc Psychiatry. 1998;7:125–30. doi: 10.1007/s007870050057. [DOI] [PubMed] [Google Scholar]
- Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J, et al. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009;48:458–63. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffths R. London: University of London Press; 1954. The Abilities of babies. [Google Scholar]
- Guarini A, Sansavini A, Fabbri C, Alessandroni R, Faldella G, Karmiloff-Smith A. Reconsidering the impact of preterm birth on language outcome. Early Hum Dev. 2009;85:639–45. doi: 10.1016/j.earlhumdev.2009.08.061. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Yamada K, Morimoto M, Morioka S, Tozawa T, Isoda K, et al. Development of corpus callosum in preterm infants is affected by the prematurity: in vivo assessment of diffusion tensor imaging at term-equivalent age. Pediatr Res. 2011;69:249–54. doi: 10.1203/PDR.0b013e3182084e54. [DOI] [PubMed] [Google Scholar]
- Herold B, Hohle B, Walch E, Weber T, Obladen M. Impaired word stress pattern discrimination in very-low-birthweight infants during the first 6 months of life. Dev Med Child Neurol. 2008;50:678–83. doi: 10.1111/j.1469-8749.2008.03055.x. [DOI] [PubMed] [Google Scholar]
- Herweh C, Akbar M, Wengenroth M, Blatow M, Mair-Walther J, Rehbein N, et al. DTI of commissural fibers in patients with Chiari II-malformation. Neuroimage. 2009;44:306–11. doi: 10.1016/j.neuroimage.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–94. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Jancke L, Siegenthaler T, Preis S, Steinmetz H. Decreased white-matter density in a left-sided fronto-temporal network in children with developmental language disorder: evidence for anatomical anomalies in a motor-language network. Brain Lang. 2007;102:91–8. doi: 10.1016/j.bandl.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007;36(Suppl 2):T16–21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Wolke D, Hennessy E, Marlow N. Educational outcomes in extremely preterm children: neuropsychological correlates and predictors of attainment. Dev Neuropsychol. 2011;36:74–95. doi: 10.1080/87565641.2011.540541. [DOI] [PubMed] [Google Scholar]
- Jones DK. Precision and accuracy in diffusion tensor magnetic resonance imaging. Top Magn Reson Imaging. 2010;21:87–99. doi: 10.1097/RMR.0b013e31821e56ac. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends Cogn Sci. 2005;9:512–18. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW. How infants begin to extract words from speech. Trends Cogn Sci. 1999;3:323–8. doi: 10.1016/s1364-6613(99)01363-7. [DOI] [PubMed] [Google Scholar]
- Keith RW. San Antonio, TX: The Psychological Corporation; 1994. Scan-A: a test for auditory processing disorders in adolescents and adults. [Google Scholar]
- Lopez-Barroso D, de Diego-Balaguer R, Cunillera T, Camara E, Munte TF, Rodriguez-Fornells A. Language learning under working memory constraints correlates with microstructural differences in the ventral language pathway. Cereb Cortex. 2011;21:2742–50. doi: 10.1093/cercor/bhr064. [DOI] [PubMed] [Google Scholar]
- Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics. 2009;123:1037–44. doi: 10.1542/peds.2008-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu TM, Vohr BR, Allan W, Schneider KC, Ment LR. Evidence for catch-up in cognition and receptive vocabulary among adolescents born very preterm. Pediatrics. 2011;128:313–22. doi: 10.1542/peds.2010-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Harcourt-Brown S, Ramus F, van der Lely HK. The link between prosody and language skills in children with specific LI (SLI) and/or dyslexia. Int J Lang Commun Disord. 2009;44:466–88. doi: 10.1080/13682820802591643. [DOI] [PubMed] [Google Scholar]
- Ment LR, Hirtz D, Huppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8:1042–55. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- Moses P, Courchesne E, Stiles J, Trauner D, Egaas B, Edwards E. Regional size reduction in the human corpus callosum following pre- and perinatal brain injury. Cereb Cortex. 2000;10:1200–10. doi: 10.1093/cercor/10.12.1200. [DOI] [PubMed] [Google Scholar]
- Mullen KM, Vohr BR, Katz KH, Schneider KC, Lacadie C, Hampson M, et al. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage. 2011;54:2563–70. doi: 10.1016/j.neuroimage.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers EH, Hampson M, Vohr B, Lacadie C, Frost SJ, Pugh KR, et al. Functional connectivity to a right hemisphere language center in prematurely born adolescents. Neuroimage. 2010;51:1445–52. doi: 10.1016/j.neuroimage.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Ashburner J, Andersson J, Jbabdi S, Draganski B, Skare S, et al. Structural correlates of preterm birth in the adolescent brain. Pediatrics. 2009;124:e964–72. doi: 10.1542/peds.2008-3801. [DOI] [PubMed] [Google Scholar]
- Narberhaus A, Segarra D, Caldu X, Gimenez M, Junque C, Pueyo R, et al. Gestational age at preterm birth in relation to corpus callosum and general cognitive outcome in adolescents. J Child Neurol. 2007;22:761–5. doi: 10.1177/0883073807304006. [DOI] [PubMed] [Google Scholar]
- Njiokiktjien C, de SL, Vaal J. Callosal size in children with learning disabilities. Behav Brain Res. 1994;64:213–18. doi: 10.1016/0166-4328(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Northam GB, Liegeois F, Chong WK, Wyatt JS, Baldeweg T. Total brain white matter is a major determinant of IQ in adolescents born preterm. Ann Neurol. 2011;69:702–11. doi: 10.1002/ana.22263. [DOI] [PubMed] [Google Scholar]
- Northam GB, Liegeois F, Chong WK, Baker K, Tournier JD, Wyatt JS, et al. Speech and oromotor outcome in adolescents born preterm: relationship to motor tract integrity. J Pediatr. 2012;160:402–8. doi: 10.1016/j.jpeds.2011.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2008;131:205–17. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Rushe TM, Woodruff PW, Stewart AL, Rifkin L, Murray RM. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain. 2004;127:2080–9. doi: 10.1093/brain/awh230. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria A, van Zijl P, Mori S. 2nd edn. London, UK: Academic press; 2010. MRI atlas of human white matter. [Google Scholar]
- Patel MD, Toussaint N, Charles-Edwards GD, Lin JP, Batchelor PG. Distribution and fibre field similarity mapping of the human anterior commissure fibres by diffusion tensor imaging. MAGMA. 2010;23:399–408. doi: 10.1007/s10334-010-0201-3. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Paul LK. Developmental malformation of the corpus callosum: a review of typical callosal development and examples of developmental disorders with callosal involvement. J Neurodev Disord. 2011;3:3–27. doi: 10.1007/s11689-010-9059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier J, Verclytte S, Delmaire C, Pruvo JP, Havet E, Le GD. Microsurgical anatomy of the anterior commissure: correlations with diffusion tensor imaging fiber tracking and clinical relevance. Neurosurgery. 2011;69:ons241–6. doi: 10.1227/NEU.0b013e31821bc822. [DOI] [PubMed] [Google Scholar]
- Perlman JM, Volpe JJ. Intraventricular hemorrhage in extremely small premature infants. Am J Dis Child. 1986;140:1122–4. doi: 10.1001/archpedi.1986.02140250048034. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–47. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlagger BL, Peterson SE. The development of human functional brain networks. Neuron. 2010;67:735–48. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis S, Steinmetz H, Knorr U, Jancke L. Corpus callosum size in children with developmental language disorder. Brain Res Cogn Brain Res. 2000;10:37–44. doi: 10.1016/s0926-6410(00)00020-3. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Rademaker KJ, Lam JN, van Haastert I, Uiterwaal CS, Lieftink AF, Groenendaal F, et al. Larger corpus callosum size with better motor performance in prematurely born children. Semin Perinatol. 2004;28:279–87. doi: 10.1053/j.semperi.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Raybaud C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology. 2010;52:447–77. doi: 10.1007/s00234-010-0696-3. [DOI] [PubMed] [Google Scholar]
- Redmond SM, Rice ML. The socioemotional behaviors of children with SLI: social adaptation or social deviance? J Speech Lang Hear Res. 1998;41:688–700. doi: 10.1044/jslhr.4103.688. [DOI] [PubMed] [Google Scholar]
- Rutherford MA, Supramaniam V, Ederies A, Chew A, Bassi L, Groppo M, et al. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology. 2010;52:505–21. doi: 10.1007/s00234-010-0700-y. [DOI] [PubMed] [Google Scholar]
- Sammler D, Kotz SA, Eckstein K, Ott DV, Friederici AD. Prosody meets syntax: the role of the corpus callosum. Brain. 2010;133:2643–55. doi: 10.1093/brain/awq231. [DOI] [PubMed] [Google Scholar]
- Sansavini A, Guarini A, Justice LM, Savini S, Broccoli S, Alessandroni R, et al. Does preterm birth increase a child’s risk for LI? Early Hum Dev. 2010;86:765–72. doi: 10.1016/j.earlhumdev.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105:18035–40. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer RJ, Lacadie C, Vohr B, Kesler SR, Katz KH, Schneider KC, et al. Alterations in functional connectivity for language in prematurely born adolescents. Brain. 2009;132:661–70. doi: 10.1093/brain/awn353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–53. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Scott SK, Wise RJ. The functional neuroanatomy of prelexical processing in speech perception. Cognition. 2004;92:13–45. doi: 10.1016/j.cognition.2002.12.002. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig E, Secord W. Clinical evaluation of langauge fundamentals. 3rd UK edn. London: Psyhcological Corporation; 2000. [Google Scholar]
- Shukla M, White KS, Aslin RN. Prosody guides the rapid mapping of auditory word forms onto visual objects in 6-mo-old infants. Proc Natl Acad Sci USA. 2011;108:6038–43. doi: 10.1073/pnas.1017617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skranes JS, Martinussen M, Smevik O, Myhr G, Indredavik M, Vik T, et al. Cerebral MRI findings in very-low-birth-weight and small-for-gestational-age children at 15 years of age. Pediatr Radiol. 2005;35:758–65. doi: 10.1007/s00247-005-1446-2. [DOI] [PubMed] [Google Scholar]
- Soria-Pastor S, Gimenez M, Narberhaus A, Falcon C, Botet F, Bargallo N, et al. Patterns of cerebral white matter damage and cognitive impairment in adolescents born very preterm. Int J Dev Neurosci. 2008;26:647–54. doi: 10.1016/j.ijdevneu.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Soria-Pastor S, Padilla N, Zubiaurre-Elorza L, Ibarretxe-Bilbao N, Botet F, Costas-Moragas C, et al. Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics. 2009;124:e1161–70. doi: 10.1542/peds.2009-0244. [DOI] [PubMed] [Google Scholar]
- Stolt S, Klippi A, Launonen K, Munck P, Lehtonen L, Lapinleimu H, et al. Size and composition of the lexicon in prematurely born very-low-birth-weight and full-term Finnish children at two years of age. J Child Lang. 2007;34:283–310. doi: 10.1017/s0305000906007902. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Inder TE, Faggian N, Johnston L, Warfield SK, Anderson PJ, et al. Characterization of the corpus callosum in very preterm and full-term infants utilizing MRI. Neuroimage. 2011;55:479–90. doi: 10.1016/j.neuroimage.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007;35:1459–72. doi: 10.1016/j.neuroimage.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Connelly A. MRtrix: diffusion tractography in crossing fibre regions. Int J Imag Sci Technol. 2012;22:53–66. [Google Scholar]
- Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;65:1532–56. doi: 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noort-van der Spek IL, Franken MC, Weisglas-Kuperus N. Language functions in preterm-born children: a systematic review and meta-analysis. Pediatrics. 2012;129:745–54. doi: 10.1542/peds.2011-1728. [DOI] [PubMed] [Google Scholar]
- Vasung L, Jovanov-Milosevic N, Pletikos M, Mori S, Judas M, Kostovic I. Prominent periventricular fiber system related to ganglionic eminence and striatum in the human fetal cerebrum. Brain Struct Funct. 2011;215:237–53. doi: 10.1007/s00429-010-0279-4. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–24. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Torgesen J, Rashotte C. Austin, Texas: PRO-ED; 1999. Comprehensive Test of Phonological Processing (CTOPP) [Google Scholar]
- Warren JE, Crinion JT, Lambon Ralph MA, Wise RJ. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain. 2009;132:3428–42. doi: 10.1093/brain/awp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Hahne A, Friedrich M, Friederici AD. Reduced stress pattern discrimination in 5-month-olds as a marker of risk for later LI: neurophysiologial evidence. Brain Res Cogn Brain Res. 2005;25:180–7. doi: 10.1016/j.cogbrainres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd UK edn. Sidcup, UK: Psychological Corporation; 1992. [Google Scholar]
- Wechsler D. Sidcup, UK: Psychological Corporation; 1993. Wechsler Objective Reading Dimensions (WORD) [Google Scholar]
- Westerhausen R, Gruner R, Specht K, Hugdahl K. Functional relevance of interindividual differences in temporal lobe callosal pathways: a DTI tractography study. Cereb Cortex. 2009;19:1322–9. doi: 10.1093/cercor/bhn173. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJ, Bishop DV. Cerebral dominance for language function in adults with specific LI or autism. Brain. 2008;131:3193–200. doi: 10.1093/brain/awn266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage. 2008;41:903–13. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- Wolke D, Meyer R. Cognitive status, language attainment, and prereading skills of 6-year-old very preterm children and their peers: the Bavarian longitudinal study. Dev Med Child Neurol. 1999;41:94–109. doi: 10.1017/s0012162299000201. [DOI] [PubMed] [Google Scholar]
- Wolke D, Samara M, Bracewell M, Marlow N. Specific language difficulties and school achievement in children born at 25 weeks of gestation or less. J Pediatr. 2008;152:256–62. doi: 10.1016/j.jpeds.2007.06.043. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Moor S, Hood KM, Champion PR, Foster-Cohen S, Inder TE, et al. Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Arch Dis Child Fetal Neonatal Ed. 2009;94:F339–44. doi: 10.1136/adc.2008.146282. [DOI] [PubMed] [Google Scholar]
- Yogarajah M, Focke NK, Bonelli SB, Thompson P, Vollmar C, McEvoy AW, et al. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain. 2010;133:2348–64. doi: 10.1093/brain/awq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel E, Iacoboni M, editors. The parallel brain: the cognitive neuroscience of the CC. Cambridge, MA: MIT Press; 2003.
- Zubiaurre-Elorza L, Soria-Pastor S, Junque C, Vendrell P, Padilla N, Rametti G, et al. Magnetic resonance imaging study of cerebral sulci in low-risk preterm children. Int J Dev Neurosci. 2009;27:559–65. doi: 10.1016/j.ijdevneu.2009.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.