Highlights
-
•
Few studies have examined associations between laboratory and everyday cortisol.
-
•
Cortisol responses to laboratory stress were associated with cortisol AUCday.
-
•
This provides evidence of the ecological validity of laboratory stress testing.
Keywords: Stress reactivity, Laboratory–field, HPA axis, Cortisol
Abstract
Relationships between cortisol responses to laboratory stress and cortisol output over the day have not been studied extensively. We tested associations between cortisol responses to a set of laboratory challenges (colour/word interference and mirror tracing) and three aspects of cortisol output over the day, namely total area under the curve (AUCday), the cortisol awakening response (CAR) and the slope of cortisol decline over the day. Participants were 466 men and women aged 54–76 years. We found that cortisol responses to laboratory stress were positively associated with cortisol AUCday independently of sex, age, socioeconomic status, smoking, body mass index, and time of laboratory testing (B = 0.212, 95% C.I. 0.143–0.282, p < 0.001). No associations between laboratory responses and the CAR or cortisol slope were observed. The laboratory–field association was not moderated by demographic or psychosocial factors. The study provides evidence for the ecological validity of acute laboratory stress testing.
1. Introduction
The investigation of cortisol responses to acute mental stress in the laboratory is an important technique in psychoneuroendocrinology (Dickerson & Kemeny, 2004; Kudielka & Wust, 2010). Procedures such as the Trier Social Stress Test (TSST, Kirschbaum, Pirke, & Hellhammer, 1993) and other behavioural challenges have been used to study the impact of stress on hypothalamic-pituitary-adrenocortical (HPA) axis function in relation to demographic factors, background stress, psychological characteristics, cognitive function, early life experience, and physical and mental health conditions (Burke, Davis, Otte, & Mohr, 2005; Chida & Hamer, 2008; Kajantie & Raikkonen, 2010). This work primarily involves examining individual or group differences in the magnitude or duration of cortisol responses and other markers of HPA function. Laboratory mental stress testing has several methodological strengths, including the precise delineation of the profile of responses to standardised stimuli under controlled conditions in which the confounding effects of concurrent activities and exposures are eliminated (Steptoe, 2007).
Studying cortisol responses to laboratory stress suffers from the same limitations as those of psychophysiological mental stress testing more generally: namely, it involves assessing acute responses to arbitrary short-term behavioural stimuli under artificial conditions that are seldom encountered in everyday life. Since most research is cross-sectional, it is not clear whether variations in response to stress are relevant to the development of physical or mental health problems or are consequences of these conditions. The notion underlying the strategy of studying biological responses to laboratory stress is that individual differences in response magnitude or duration reflect the way people react biologically in everyday life. A person who is highly reactive in the laboratory will experience repeated episodes of heightened biological activity in their lives. These effects will lead to sustained differences in biological activity in everyday life, and will over months and years subsequently impact on health risk (Steptoe, 2007). The validity of mental stress testing is therefore typically assessed in two ways. The first is to evaluate whether variations in biological responses to laboratory stress predict future health outcomes or the development of clinical conditions. This issue has been examined extensively in relation to cardiovascular stress responses (Chida & Steptoe, 2010), but evidence related to cortisol stress responses is limited. Our group has shown that individuals with larger cortisol responses to laboratory stress are at increased risk of developing hypertension (Hamer & Steptoe, 2012), and of showing accelerated progression of subclinical coronary atherosclerosis as indexed by coronary artery calcification (Hamer, Endrighi, Venuraju, Lahiri, & Steptoe, 2012). Another study showed no association between cortisol responses and changes in self-reported body mass 4–7 years later (Phillips, Roseboom, Carroll, & de Rooij, 2012). No studies relating variations in cortisol responses to laboratory stress prospectively with depression, atopic conditions, or other health outcomes have yet been reported, so the relevance of individual differences in responsivity to future health risk remains untested in these conditions.
The second approach to evaluating the validity of mental stress testing is to test relationships with function in everyday life. The ‘laboratory–field’ problem has been studied intensively with respect to blood pressure and heart rate responses, comparing individual differences in acute responses to laboratory stress with values recorded over the day using ambulatory monitoring (Kamarck, Schwartz, Janicki, Shiffman, & Raynor, 2003; Turner et al., 1994). The consensus is that individual differences in levels of blood pressure and heart rate correlate well across the two situations (so people with higher blood pressure during stress tasks in the laboratory display higher ambulatory blood pressure over the day), but that the relationship between responses to acute laboratory stress (measured as change from baseline) and values recorded in daily life are less robust. A number of explanations have been put forward, including the possibility that the strength of associations depends on the type of laboratory task, the level of stress experienced in everyday life, and the reliability of the estimates of acute stress responses (Manuck, 1994; Steptoe, Cropley, & Joekes, 2000).
A few studies have assessed the relationship between cortisol responses to laboratory stress with the cortisol awakening response (CAR) in daily life, with largely negative results. For example, Schmidt-Reinwald et al. (1999) found no association between cortisol responses to the TSST and the CAR in a study of 22 young adults, a result that was replicated in a study of 21 student teachers (Wolfram, Bellingrath, & Kudielka, 2011). But a negative correlation was observed by Quirin, Pruessner, and Kuhl (2008), with smaller CARs in individuals who were more stress responsive in the laboratory. Other groups have reported between-group differences in cortisol responses to laboratory stress but not the CAR, or vice versa, further implying that the two phenomena are not closely related (Buske-Kirschbaum, Ebrecht, & Hellhammer, 2010; Petrowski, Herold, Joraschky, Wittchen, & Kirschbaum, 2010). To the best of our knowledge, the only study to investigate associations between cortisol responses to laboratory stress and cortisol over the day was an investigation of 87 employed men (van Eck, Nicolson, Berkhof, & Sulon, 1996); this showed a positive relationship between pre-stress baseline cortisol in the laboratory and measures taken at a similar time in daily life, but no correlation between laboratory stress responses and assessments at other times. The first aim of the present study was therefore to test in a large sample of older men and women the association between cortisol responses to laboratory stress and salivary cortisol over the day. We explored three aspects of cortisol dynamics: the CAR, cortisol output over the whole day computed as area under the curve (AUCday), and the cortisol slope over the day, and tested whether associations with cortisol responses to laboratory stress were independent of baseline (pre-stress) cortisol levels.
The second aim of the study was to discover whether associations between cortisol responses to laboratory stress and cortisol in daily life were moderated by other factors. If the relationship between the magnitude of responses to acute stress and values recorded in everyday life varies with demographic or other factors, then comparisons involving different groups may be compromised. There is evidence that cortisol responses to laboratory stress vary with sex, age, depression and ongoing stress (Burke et al., 2005; Chida & Hamer, 2008; Kajantie & Phillips, 2006), while cortisol output over the day varies with socioeconomic status (SES) and affect in daily life (Adam, Hawkley, Kudielka, & Cacioppo, 2006; Kumari et al., 2010). It is conceivable therefore that the correlation between measures in the laboratory and over the day differs with the levels of these factors. Such associations would limit the generalizability of cortisol responses to laboratory stress. We therefore carried out moderator analyses of the laboratory–field relationship, testing differences in relation to sex, age, SES (defined by grade of employment), chronic stress (operationalised as financial strain, lack of social cohesion, social isolation and loneliness), subjective stress response to the task, depressive symptoms, and affect balance over the day evaluated using ecological momentary assessments (EMA).
2. Methods
2.1. Participants
We analysed data from the Heart Scan Study, which involved a sample of healthy older adults (n = 543) drawn from the Whitehall II cohort in 2006–2008 to investigate the association between physiological reactivity to behavioural stressors and subclinical coronary artery calcification (Hamer, O’Donnell, Lahiri, & Steptoe, 2010; Kidd, Hamer, & Steptoe, 2011). The Whitehall II study is a well-established epidemiological study of socioeconomic, psychosocial and biological risk factors for coronary heart disease and other disorders of ageing, involving men and women in the British civil service (Marmot & Brunner, 2005). Criteria for inclusion in the Heart Scan Study were white European origin, no history or objective signs of coronary heart disease (CHD), hypertension, or inflammatory disease, no history of mental illness, or any medication that might affect cortisol levels, including hormone treatment. All female participants reported postmenopausal status. All participants who had complete cortisol data from both the laboratory stress session and samples over the day were included in the analyses. Complete cortisol over the day was missing from 69 cases, and a further eight were eliminated because cortisol assays from the laboratory stress session were unsatisfactory. The final sample therefore consisted of 466 individuals, of whom 47% were women, and 53% men. There was no difference in demographic characteristics between those who did and did not provide complete cortisol data. Ethical approval for the study was given by the Research Ethics Committee for University College London/UCL Hospitals.
2.2. Laboratory stress procedures
Laboratory stress testing took place either in the morning (starting at 9:15 am) or afternoon (starting at 1:30 pm). Multiple physiological markers were measured during the acute stress testing in the laboratory; however, for the purposes of this paper we report the cortisol response only. After anthropometric measurements, a cannula was inserted for drawing blood. Blood pressure and heart rate were recorded continuously using a Finometer, a device that monitors blood pressure from the finger using the vascular unloading method (Guelen et al., 2008). The acute stress protocol was as follows: after a 30-min rest period following cannulation, a 5 min period of baseline blood pressure was carried out, together with a resting saliva sample, and blood sample. Two behavioural tasks were administered in a random order. The first task was a mirror tracing task, and the second was a computerised colour/word interference task (Kidd et al., 2011; Steptoe et al., 2002). Each task lasted 5 min, and task order was randomised across participants. Saliva samples were collected immediately after the tasks were completed, and then at 20, 45, and 75 min after the tasks for the assessment of salivary cortisol. Monitoring of cardiovascular activity continued throughout the study, with further blood draws at 45 min and 75 min post task. Participants were asked to rate their level of stress at baseline, immediately after each task, and during recovery on a seven point Likert scale ranging from 1 (no stress) to 7 (feeling very stressed). Scores from both stress tasks were aggregated to produce one average stress task score.
2.3. Cortisol sampling over the day
Saliva was collected over the course of a single day during the seven days following the laboratory testing. Participants were asked to provide five samples in salivettes over the course of a normal weekday at waking, at waking plus 30 min, 10–10.30 am, 4–4.30 pm, and 8–8.30 pm. They were instructed not to brush their teeth or eat or drink anything for 15 min prior to sample collection. Information on the day of sampling was recorded in a booklet, which included information on time of waking, and the time each sample was taken. The salivettes and booklet were posted back in a freepost envelope. Participants also rated positive affect and stress for the 20 min before each of the saliva samples on a five-point rating scale (ranging from 1, low stress/happiness to 5, high stress/happiness). Positive affect and stress scores were each averaged to give one total score for the day, and affect balance was computed as recommended by Kahneman, Krueger, Schkade, Schwarz, and Stone (2004) by subtracting stress from positive affect. High scores indicate higher levels of positive well-being over the day.
2.4. Covariates and potential effect modifiers
We recorded height and weight for the calculation of body mass index (BMI). Grade of employment at time of testing, or most recent grade if the person had retired or moved to another occupation, was used as the indicator of SES, and participants were divided into higher, intermediate and lower SES. Since some individuals had retired, we were not able to use work stress as an indicator of chronic stress. We therefore analysed a number of other indicators of economic, neighbourhood and interpersonal stress. We measured financial strain with an adaptation of the economic strain measure of Pearlin, Menaghan, Lieberman, and Mullan (1981). This assesses difficulty paying bills, being able to replace items such as furniture or a car when needed, and being able to provide for one's family in terms of food, clothing, and medical care. Scores were skewed towards low values, so the sample was divided into those who reported high or little/no financial strain. Neighbourhood stress was measured with the social cohesion inventory developed for the Project on Human Development in Chicago Neighbourhoods (Sampson, Raudenbush, & Earls, 1997). This assesses perceived interpersonal trust and solidarity among neighbours; scores could range from 0 to 20 with higher scores indicating greater social cohesion. Social isolation was measured with the 12-item social network inventory described by Cohen, Doyle, Skoner, Rabin, and Gwaltney (1997). Scores could range from 0 to 11, with lower scores indicating greater social isolation. Loneliness was assessed with the revised UCLA loneliness scale, a self-report questionnaire consisting of 20 items which are rated on 4-point scale from 1 = never to 4 = often (Peplau & Cutrona, 1980). Total scores could range from 20 to 80, with higher scores indicating greater loneliness. Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression (CESD) scale; scores can range from 0 to 60, with higher values indicating greater depressive symptom intensity.
2.5. Cortisol assays and data reduction
The salivettes were centrifuged at 3000 rpm for 5 min, resulting in a clear supernatant of low viscosity. Salivary cortisol levels were measured using a commercial immunoassay with chemiluminescence detection (CLIA; IBL-Hamburg, Hamburg, Germany). The lower concentration limit of this assay is 0.44 nmol/L; intraassay and interassay coefficients of variance were <8%. Any sample over 50 nmol/L was repeated.
Cortisol responses to laboratory stress were assessed by computing cortisol AUC for all samples using the method described by Pruessner, Kirschbaum, Meinlschmidt, and Hellhammer (2003). We then calculated an estimated cortisol output based on the baseline value multiplied by the total time between baseline and 75 min sample, and subtracted this from the actual AUC. The result was the area under the curve with respect to baseline (AUCi), reflecting the changes in cortisol associated with the behavioural tasks. The AUCi was not normally distributed so was log transformed for analysis, with a constant of 500 added to ensure that all values were positive. The laboratory cortisol baseline was also log transformed before analysis.
Overall output of cortisol over the day was quantified by computing cortisol area under the curve with respect to ground (AUCday) for all data points. Values were log transformed before analysis. The CAR was computed by subtracting cortisol measured at time 1 (waking) from cortisol measured at time 2 (waking + 30 min). In view of the evidence that delays between waking up and taking the waking sample distort the CAR, participants who reported delays >15 min were excluded from these analyses (Dockray, Bhattacharyya, Molloy, & Steptoe, 2008); 413 of the 466 participants were included. The slope of cortisol decline over the day was computed by regressing raw values on the time they were collected, excluding the waking + 30 sample. The CAR and cortisol slope were normally distributed, so were not transformed before analysis.
2.6. Statistical analysis
Cortisol and self-reported stress responses during the laboratory protocol were analysed using repeated measures analysis of variance, with trial as the within-person factor. Associations between cortisol responses to laboratory stress and cortisol over the day were analysed using standard multiple linear regression, regressing laboratory cortisol AUCi on the three diurnal cortisol parameters (AUCday, CAR, and slope) in separate models. Age, sex, employment grade (higher, intermediate, lower), current employment status, BMI, smoking status, and time of laboratory testing (morning/afternoon) were entered simultaneously as covariates alongside AUCi, since these factors are known to be associated with cortisol (Adam & Kumari, 2009). Bootstrapping with 500 replications was used to generate more precise standard errors than conventional OLS regression. Results are presented as regression coefficients with 95% confidence intervals (C.I.) and standard errors. Moderation of associations between cortisol responses to laboratory stress and cortisol over the day was tested using the PROCESS computational method for path analysis-based moderation (Hayes, 2013). Multiple moderation models were tested, so we applied a Bonferroni correction to control for the familywise error rate. We carried out 12 comparisons, so the p value was set at <0.004. Analyses were carried out in STATA 12 and SPSS 21.
3. Results
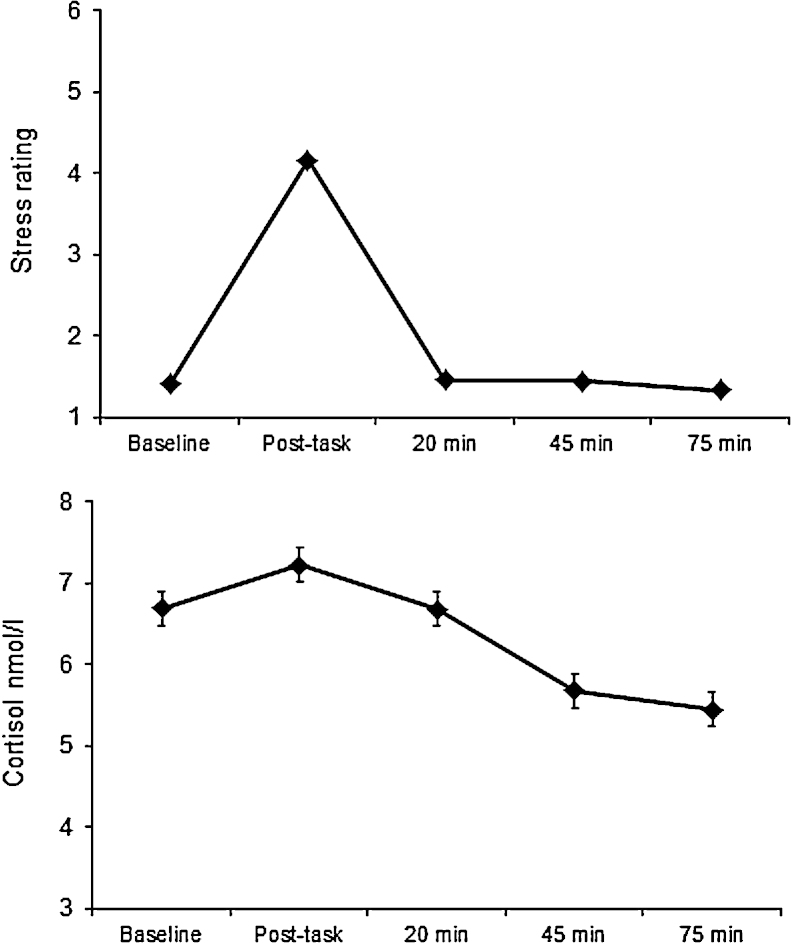
The characteristics of the study sample are detailed in Table 1. There were slightly more men than women in the analysis, with ages ranging from 54 to 76 years. Participants of higher and intermediate grade of employment predominated, and only a minority were current smokers. The majority of testing sessions took place in the afternoon; 31% of males and 37% of females were tested in the morning session. There were no sex differences on time of testing (p > 0.05). Fig. 1 summarises the cortisol and subjective stress responses during the laboratory session. The mean cortisol responses were modest but highly significant, with a significant rise in cortisol between baseline and post-task values (p < 0.001). Post hoc tests confirmed that all trials were different from one another (p < 0.001). Subjective stress increased markedly with tasks, returning to baseline during the recovery period (p < 0.001). Participants showed a typical profile of cortisol over the day, with a relatively high level on waking (mean 15.52, SD 7.76 nmol/L), a CAR averaging 8.83 (SD 10.03 nmol/L, and a decline over the remainder of the day to an average of 2.82 (SD 3.58) nmol/L in the evening. Fifty-three participants were excluded from the analyses of the CAR because of delay in the interval between waking and taking the first saliva sample; the CAR in these individuals averaged only 1.99 (SD 11.23), suggesting that the mistiming had resulted in failures to measure the CAR accurately. Cortisol output over the day (AUCday) averaged 4.69 (SD 0.38), and the decrease in cortisol over the day averaged 1.06 nmol/L/hr. There were wide variations in laboratory cortisol AUCi, and in cortisol AUCday, as indicated in Table 1.
Table 1.
Characteristics of participants. Mean (standard deviation) or N (percent) and range.
| Mean/N | Standard deviation/percent | Range | |
|---|---|---|---|
| Sex | |||
| Men | 249 | 53.4% | |
| Women | 217 | 46.6% | |
| Age (average) | 63.0 | 5.72 | 54–76 |
| Male | 62.1 | 5.80 | |
| Female | 64.0 | 5.41 | |
| Grade of employment | |||
| Higher | 181 | 38.8% | |
| Intermediate | 178 | 38.2% | |
| Lower | 107 | 23.0% | |
| Current paid employment | 173 | 37.1% | |
| Current smoker | 23 | 4.9% | |
| Body mass index | 25.63 | 3.73 | 15.0–41.8 |
| Time of testing | |||
| Morning | 184 | 39.5% | |
| Afternoon | 282 | 60.5% | |
| Laboratory cortisol baseline (nmol/L, log transformed) | 1.92 | 0.46 | 0.36–3.70 |
| Laboratory cortisol AUCi (log transformed) | 5.95 | 0.64 | 3.17–8.05 |
| Day cortisol AUC (log transformed) | 4.69 | 0.38 | 2.91–6.07 |
| Cortisol awakening response (nmol/L) | 8.83 | 10.03 | −22.22 to 50.70 |
| Cortisol slope over day (nmol/L/hr) | 1.06 | 0.91 | −3.44 to 6.34 |
| High financial strain | 190 | 40.9% | |
| CESD depression | 6.51 | 6.26 | 0–36 |
| Affect balance over the day | 2.07 | 1.27 | −2.50 to 4.00 |
CAR calculation excludes 53 participants with 15 minutes or more delay between waking and taking the first cortisol sample.
Fig. 1.
Mean subjective stress rating (top panel) and salivary cortisol (bottom panel) during laboratory stress testing. Error bars are standard error of the mean; these are very small in the case of stress ratings. Although cortisol data were log transformed before analysis, the figure presents untransformed values (N = 466).
All trials were significantly different from one another (p < 0.001).
3.1. Laboratory cortisol AUCi and cortisol over the day
The regression of laboratory cortisol AUCi on cortisol AUCday is summarised in Table 2. There was a positive association between the magnitude of the cortisol response to stress in the laboratory and cortisol output over the day (p < 0.001) after sex, age, SES (grade of employment), current employment status, smoking, BMI and time of testing had been taken into account. The laboratory cortisol AUCi accounted for 7.2%of the variance in cortisol output over the day, with overall R2 = 0.116. Other independent predictors of cortisol output were SES and smoking, since cortisol output over the day was greater in lower SES individuals and in smokers. There was a marginal association with time of laboratory testing, with higher cortisol AUCday in participants tested in the afternoon. Additional statistical adjustment for baseline cortisol levels in the laboratory had only a modest impact on the association between laboratory AUCi and cortisol AUCday (B = 0.183, 95% C.I. .095–.271, p < 0.001). By contrast, there was no association between laboratory cortisol AUCi and the CAR (B = 0.707, C.I. −.938 to 2.353, p = 0.40) or the cortisol slope over the day (B = 0.090, C.I. 0.046–.226, p = 0.19).
Table 2.
Regression of laboratory cortisol AUCi on cortisol AUC over the day.
| Unstandardised regression coefficient B | 95% C.I. | Standard error (bootstrap) | p | |
|---|---|---|---|---|
| Sexa | 0.003 | −.077 to .082 | .041 | 0.95 |
| Age | 0.005 | −.003 to .012 | .004 | 0.26 |
| Grade dummy High/intermediate vs lowb |
0.148 | .046–.229 | .051 | 0.004 |
| Grade dummy High vs intermediate/lowc |
−.026 | −.117 to .064 | .046 | 0.57 |
| Paid employmentd | −.007 | −.118 to .104 | .057 | 0.89 |
| Smoking statuse | 0.237 | .057–.417 | .092 | 0.010 |
| BMI | 0.010 | −.002 to .022 | .006 | 0.098 |
| Time of testingf | 0.086 | .004–.168 | .042 | .041 |
| Laboratory AUCi | 0.212 | .143–.282 | .035 | 0.001 |
Men are the reference group.
High/intermediate grade is the reference category.
High is the reference category.
Not being in work is the reference category.
Not being a smoker is the reference category.
Morning testing is the reference category.
3.2. Moderators of the relationship between laboratory AUCi and cortisol over the day
We tested several potential moderators of the relationship between laboratory cortisol AUCi and AUCday using PROCESS methods. There was no evidence of moderation by sex, SES (grade of employment), current employment status, chronic stress (operationalised as financial strain, lack of social cohesion, social isolation and loneliness), symptoms of depression, subjective stress responses to the task, or affect balance over the day. A moderation effect was identified for age, with the R2 increase due to the interaction of age and laboratory cortisol AUCi being 0.125 (p = 0.020); however, this was not significant following Bonferroni correction for multiple comparisons. The sex by age interaction was not significant either.
4. Discussion
The aim of this study was to evaluate the ecological validity of standardised mental stress testing of cortisol responses by assessing the association between cortisol responses to laboratory stress and salivary cortisol over the day, hypothesising that individuals with larger cortisol responses to laboratory stress would show high cortisol output over the day. We found that variation in cortisol responses to laboratory stress predicted cortisol AUCday, with greater output in participants with larger laboratory stress responses. This association was independent of sex, age, SES, smoking status and BMI, and also remained significant once baseline cortisol in the laboratory had been taken into account. Our exploration of moderators of this relationship indicated that the association between laboratory and daily life was robust to differences in sex, SES, age, stress (operationalised as financial strain, loneliness, social isolation, and social cohesion), and mood. Cortisol responses to laboratory stress were not associated with the CAR or with cortisol slope over the day.
The strong association between cortisol responses to laboratory stress and cortisol AUCday provides evidence that despite laboratory stress testing involving short-term stimuli of limited real-life relevance, variations in cortisol responses nevertheless predict output over the day. Importantly, this association was independent of baseline cortisol levels in the laboratory, and reflects variations in stress responses per se. In this respect, the findings are stronger than many investigations of laboratory–field effects for cardiovascular parameters, where associations between baseline levels of blood pressure or heart rate and values over the day have often been stronger than those between cardiovascular reactivity and daytime values (Turner et al., 1994). The large sample tested in this investigation in comparison with most laboratory stress experiments may have allowed us to generate more reliable findings than in previous studies. The results therefore add to the literature endorsing the importance of individual differences in cortisol responses to standardised stress, in showing that these variations not only predict future health outcomes (Hamer et al., 2012; Hamer & Steptoe, 2012) but also values recorded in everyday life.
We did not find any correlation between cortisol responses to laboratory stress and the CAR. This result replicates previous studies with smaller samples (Schmidt-Reinwald et al., 1999; Wolfram et al., 2011). It suggests that even though variations in the CAR in part reflect stress-related phenomena, these appear to be distinct from stress responses elicited by acute exposure to challenge. We also investigated associations with the cortisol slope over the day. Flatter cortisol slopes have been related to all-cause and cardiovascular mortality (Kumari, Shipley, Stafford, & Kivimaki, 2011), coronary artery calcification (Matthews, Schwartz, Cohen, & Seeman, 2006), breast cancer survival (Sephton, Sapolsky, Kraemer, & Spiegel, 2000), early life adversity (Kumari, Head, Bartley, Stansfeld, & Kivimaki, 2012), and with stress-related processes (Adam et al., 2006). However, no association with cortisol responses to laboratory stress was observed.
The second aim of this study was to assess potential modifiers of the laboratory–field association. In the cardiovascular domain, it has been proposed that associations might be stronger among people who experience greater stress in their lives (Kamarck et al., 2003; Steptoe et al., 2000). Such associations would diminish the power of laboratory testing to predict cortisol values in daily life. However, moderation analysis indicated that the laboratory–field association did not vary with sex, age, SES, background stress (assessed with measures of financial, neighbourhood and interpersonal strain), depressive symptoms and mood over the monitoring day. So it was not the case that the strength of correlations between cortisol responses to laboratory stress and cortisol over the day was affected by these factors.
The magnitude of cortisol responses to laboratory stress was small compared with those elicited by more socially evaluative tasks such as the TSST. The tasks were nevertheless perceived as stressful as is apparent from the 3-fold increase in self-reported stress. Furthermore, individual differences in cortisol responses to these tasks have been shown to be clinically significant in terms of future disease risk (Hamer et al., 2012; Hamer & Steptoe, 2012). There were several reasons for choosing tasks that elicited moderate rather than intense responses. First, intense biological response such as those stimulated by the TSST represent conditions that are very rarely encountered in everyday life, so may have limited ecological validity. Since the challenges were moderate, they were more likely to correspond with the demands of everyday life. Second, we pretested these tasks along with others to ensure that they were appraised as similarly challenging and demanding by people across the socioeconomic spectrum (Steptoe & Marmot, 2002). Tasks involving public speaking are perceived differently by people who are accustomed to speaking in front of others compared with those who are not, so are less satisfactory for population-level investigations. Additionally, very intense tasks are likely to produce responses at or near the maximum capacity of the individual. This is valuable for some types of investigation, but means that variance is typically reduced and the role of individual differences such as coping capacity may be attenuated. In the same way, an earlier study of blood pressure showed that levels in everyday life corresponded more closely to responses to low rather than high demand tasks (Steptoe et al., 2000).
This study had a number of limitations that should be acknowledged when interpreting the results. The study involved white, middle-aged, healthy, older participants, so we cannot generalise the results to other age, ethnic or clinical groups. We included several measures of chronic stress, including exposure to financial, neighbourhood and interpersonal stress, but there are dimensions of chronic stress that were not included. Cortisol was sampled over a single day with only five samples, and it has been recommended that cortisol profiles should be recorded over several days to establish reliable profiles (Hellhammer et al., 2007). Timing of cortisol sampling over the day was self-reported, and objective measures of sample times are desirable (Kudielka, Gierens, Hellhammer, Wüst, & Schlotz, 2012). Objective measures of waking time using accelerometers or other devices are also helpful in ensuring the accuracy of sample timing (Dockray et al., 2008). Our tasks elicited modest cortisol responses in comparison with more emotionally involving conditions, so it is possible that different associations would have emerged with more provocative stimuli.
Nonetheless, the study had significant strengths as it was carried out with a large, well characterised sample with careful standardisation of laboratory conditions. The observation that individual differences in cortisol responses to standardised laboratory challenge predict values over the day is encouraging and helps strengthen the ecological relevance of mental stress testing. Understanding the processes underlying these differences in responsivity may therefore provide valuable insights into the pathways through which cortisol contributes to health outcomes and impairment in everyday function.
Role of the funding source
This study was supported by the British Heart Foundation (grant RG/10/005/28296).
Conflict of interest statement
None declared.
References
- Adam E.K., Hawkley L.C., Kudielka B.M., Cacioppo J.T. Day-to-day dynamics of experience – Cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam E.K., Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Burke H.M., Davis M.C., Otte C., Mohr D.C. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A., Ebrecht M., Hellhammer D.H. Blunted HPA axis responsiveness to stress in atopic patients is associated with the acuity and severeness of allergic inflammation. Brain, Behavior and Immunity. 2010;24:1347–1353. doi: 10.1016/j.bbi.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Chida Y., Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychological Bulletin. 2008;134:829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Chida Y., Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: A meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cohen S., Doyle W.J., Skoner D.P., Rabin B.S., Gwaltney J.M., Jr. Social ties and susceptibility to the common cold. JAMA: The Journal of the American Medical Association. 1997;277:1940–1944. [PubMed] [Google Scholar]
- Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dockray S., Bhattacharyya M.R., Molloy G.J., Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33:77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Guelen I., Westerhof B.E., van der Sar G.L., van Montfrans G.A., Kiemeneij F., Wesseling K.H. Validation of brachial artery pressure reconstruction from finger arterial pressure. Journal of Hypertension. 2008;26:1321–1327. doi: 10.1097/HJH.0b013e3282fe1d28. [DOI] [PubMed] [Google Scholar]
- Hamer M., Endrighi R., Venuraju S.M., Lahiri A., Steptoe A. Cortisol responses to mental stress and the progression of coronary artery calcification in healthy men and women. PLoS ONE. 2012;7:e31356. doi: 10.1371/journal.pone.0031356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M., O’Donnell K., Lahiri A., Steptoe A. Salivary cortisol responses to mental stress are associated with coronary artery calcification in healthy men and women. European Heart Journal. 2010;31:424–429. doi: 10.1093/eurheartj/ehp386. [DOI] [PubMed] [Google Scholar]
- Hamer M., Steptoe A. Cortisol responses to mental stress and incident hypertension in healthy men and women. Journal of Clinical Endocrinology & Metabolism. 2012;97:E29–E34. doi: 10.1210/jc.2011-2132. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. Guilford Press; New York: 2013. Mediation, moderation, and conditional process analysis. [DOI] [PubMed] [Google Scholar]
- Hellhammer J., Fries E., Schweisthal O.W., Schlotz W., Stone A.A., Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Kahneman D., Krueger A.B., Schkade D.A., Schwarz N., Stone A.A. A survey method for characterizing daily life experience: The day reconstruction method. Science. 2004;306:1776–1780. doi: 10.1126/science.1103572. [DOI] [PubMed] [Google Scholar]
- Kajantie E., Phillips D.I. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kajantie E., Raikkonen K. Early life predictors of the physiological stress response later in life. Neuroscience & Biobehavioral Review. 2010;35:23–32. doi: 10.1016/j.neubiorev.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Kamarck T.W., Schwartz J.E., Janicki D.L., Shiffman S., Raynor D.A. Correspondence between laboratory and ambulatory measures of cardiovascular reactivity: A multilevel modeling approach. Psychophysiology. 2003;40:675–683. doi: 10.1111/1469-8986.00069. [DOI] [PubMed] [Google Scholar]
- Kidd T., Hamer M., Steptoe A. Examining the association between adult attachment style and cortisol responses to acute stress. Psychoneuroendocrinology. 2011;36:771–779. doi: 10.1016/j.psyneuen.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. The ‘Trier Social Stress Test’ – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Gierens A., Hellhammer D.H., Wüst S., Schlotz W. Salivary cortisol in ambulatory assessment – Some dos, some don’ts, and some open questions. Psychosomatic Medicine. 2012;74:418–431. doi: 10.1097/PSY.0b013e31825434c7. [DOI] [PubMed] [Google Scholar]
- Kudielka B.M., Wust S. Human models in acute and chronic stress: Assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress. 2010;13:1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- Kumari M., Badrick E., Chandola T., Adler N.E., Epel E., Seeman T. Measures of social position and cortisol secretion in an aging population: Findings from the Whitehall II study. Psychosomatic Medicine. 2010;72:27–34. doi: 10.1097/PSY.0b013e3181c85712. [DOI] [PubMed] [Google Scholar]
- Kumari M., Head J., Bartley M., Stansfeld S., Kivimaki M. Maternal separation in childhood and diurnal cortisol patterns in mid-life: Findings from the Whitehall II study. Psychological Medicine. 2012;43:633–643. doi: 10.1017/S0033291712001353. [DOI] [PubMed] [Google Scholar]
- Kumari M., Shipley M., Stafford M., Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: Findings from the Whitehall II study. Journal of Clinical Endocrinology & Metabolism. 2011;96:1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuck S.B. Cardiovascular reactivity in cardiovascular disease: Once more unto the breach. International Journal of Behavioral Medicine. 1994;1:4–31. doi: 10.1207/s15327558ijbm0101_2. [DOI] [PubMed] [Google Scholar]
- Marmot M., Brunner E. Cohort profile: The Whitehall II study. International Journal of Epidemiology. 2005;34:251–256. doi: 10.1093/ije/dyh372. [DOI] [PubMed] [Google Scholar]
- Matthews K., Schwartz J., Cohen S., Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosomatic Medicine. 2006;68:657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- Pearlin L.I., Menaghan E.G., Lieberman M.A., Mullan J.T. The stress process. Journal of Health & Social Behavior. 1981;22:337–356. [PubMed] [Google Scholar]
- Peplau L.A., Cutrona C.E. The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. Journal of Personality and Social Psychology. 1980;39:472–480. doi: 10.1037//0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- Petrowski K., Herold U., Joraschky P., Wittchen H.U., Kirschbaum C. A striking pattern of cortisol non-responsiveness to psychosocial stress in patients with panic disorder with concurrent normal cortisol awakening responses. Psychoneuroendocrinology. 2010;35:414–421. doi: 10.1016/j.psyneuen.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Phillips A.C., Roseboom T.J., Carroll D., de Rooij S.R. Cardiovascular and cortisol reactions to acute psychological stress and adiposity: Cross-sectional and prospective associations in the Dutch Famine Birth Cohort Study. Psychosomatic Medicine. 2012;74:699–710. doi: 10.1097/PSY.0b013e31825e3b91. [DOI] [PubMed] [Google Scholar]
- Pruessner J.C., Kirschbaum C., Meinlschmidt G., Hellhammer D. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Quirin M., Pruessner J.C., Kuhl J. HPA system regulation and adult attachment anxiety: Individual differences in reactive and awakening cortisol. Psychoneuroendocrinology. 2008;33:581–590. doi: 10.1016/j.psyneuen.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Sampson R.J., Raudenbush S.W., Earls F. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A., Pruessner J.C., Hellhammer D.H., Federenko I., Rohleder N., Schurmeyer T.H. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sciences. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Sephton S.E., Sapolsky R.M., Kraemer H.C., Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Steptoe A. Psychophysiological contributions to behavioral medicine and psychosomatics. In: Cacioppo J.T., Tassinary L.G., Bernston G., editors. Handbook of psychophysiology. 3rd ed. Cambridge University Press; New York: 2007. pp. 723–751. [Google Scholar]
- Steptoe A., Cropley M., Joekes K. Task demands and the pressures of everyday life: Associations between cardiovascular reactivity and work blood pressure. Health Psychology. 2000;19:46–54. doi: 10.1037//0278-6133.19.1.46. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Feldman P.M., Kunz S., Owen N., Willemsen G., Marmot M. Stress responsivity and socioeconomic status: A mechanism for increased cardiovascular disease risk? European Heart Journal. 2002;23:1757–1763. doi: 10.1053/euhj.2001.3233. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Marmot M. The role of psychobiological pathways in socio-economic inequalities in cardiovascular disease risk. European Heart Journal. 2002;23:13–25. doi: 10.1053/euhj.2001.2611. [DOI] [PubMed] [Google Scholar]
- Turner J., Ward M., Gellman M., Johnston D., Light K., van Doornen L. The relationship between laboratory and ambulatory cardiovascular activity: Current evidence and future directions. Annals of Behavioral Medicine. 1994;16:12–23. [Google Scholar]
- van Eck M.M., Nicolson N.A., Berkhof H., Sulon J. Individual differences in cortisol responses to a laboratory speech task and their relationship to responses to stressful daily events. Biological Psychology. 1996;43:69–84. doi: 10.1016/0301-0511(95)05159-7. [DOI] [PubMed] [Google Scholar]
- Wolfram M., Bellingrath S., Kudielka B.M. The cortisol awakening response (CAR) across the female menstrual cycle. Psychoneuroendocrinology. 2011;36:905–912. doi: 10.1016/j.psyneuen.2010.12.006. [DOI] [PubMed] [Google Scholar]