Highlights
-
•
New phleboviruses (family Bunyaviridae) have emerged in China and the US.
-
•
Tick borne phleboviruses do not encode an NSm protein.
-
•
Tick borne phleboviruses show differential pathogenicity in humans.
Abstract
The Bunyavidae family is the largest grouping of RNA viruses and arguably the most diverse. Bunyaviruses have a truly global distribution and can infect vertebrates, invertebrates and plants. The majority of bunyaviruses are vectored by arthropods and thus have the remarkable capability to replicate in hosts of disparate phylogeny. The family has provided many examples of emerging viruses including Sin Nombre and related viruses responsible for hantavirus cardiopulmonary syndrome in the Americas, first identified in 1993, and Schmallenberg virus which emerged in Europe in 2011, causing foetal malformations in ruminants. In addition, some well-known bunyaviruses like Rift Valley fever and Crimean-Congo haemorrhagic fever viruses continue to emerge in new geographical locations. In this short review we focus on newly identified viruses associated with severe haemorrhagic disease in humans in China and the US.
Current Opinion in Virology 2014, 5:50–57
This review comes from a themed issue on Emerging viruses
Edited by Christopher F Basler and Patrick CY Woo
For a complete overview see the Issue and the Editorial
Available online 28th February 2014
1879-6257/$ – see front matter, © 2014 The Authors. Published by Elsevier B.V. All rights reserved.
Introduction
Bunyaviruses are characterised by the possession of a tri-segmented negative-sense RNA genome. Viruses are enveloped, replicate in the cytoplasm and mature by budding at the Golgi. Virions are about 100 nm in diameter and are composed of just four proteins: two glycoproteins termed Gn and Gc that are embedded in the Golgi-derived viral membrane and two internal proteins, the nucleocapsid (N) protein that encapsidates each of the three genome segments in the form of ribonucleoprotein complexes, and the L protein (RNA dependent RNA polymerase). The largest RNA segment, L, encodes the L protein, the medium segment, M, the glycoproteins, and the smallest segment, S, the N protein. In addition some viruses encode up to three nonstructural proteins [1,2]. The more than 350 named bunyaviruses are subdivided into five genera (Orthobunyavirus, Hantavirus, Nairovirus, Phlebovirus and Tospovirus) on serological, morphological and biochemical features [3]. There are differences in the patterns of sizes of the viral RNAs and structural proteins between the different genera, and the expression strategy of non-structural proteins also differs between different genera. In addition, there are consensus terminal sequences of the genome segments that are conserved on a genus specific manner; for phleboviruses these are 5′-ACACAAAG… and …CUUUGUGU-3′.
The Phlebovirus genus
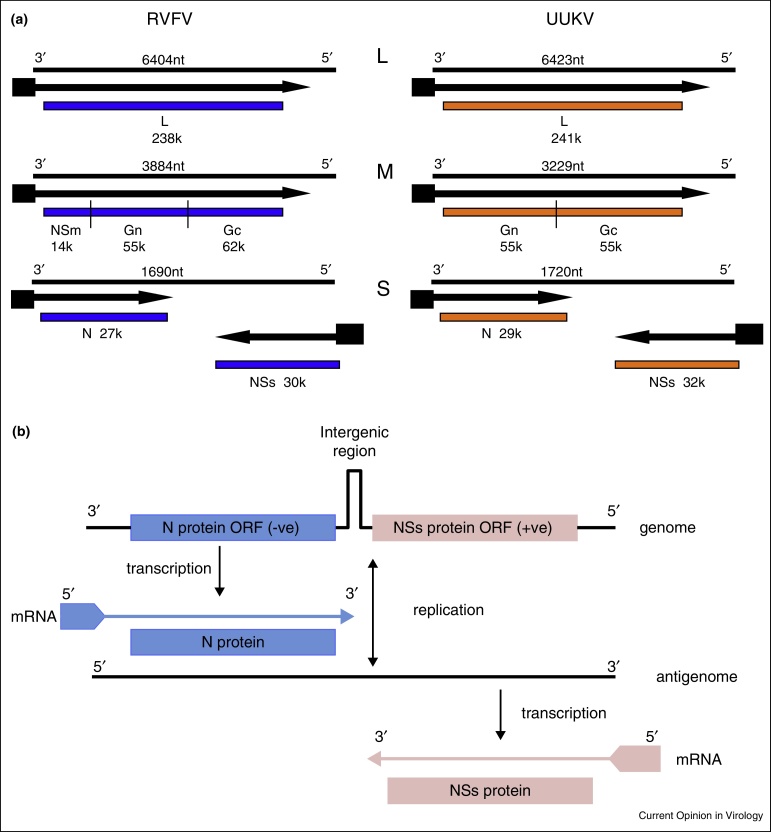
In the latest report of the International Committee on Taxonomy of Viruses (ICTV) [3] the Phlebovirus genus contains 70 viruses, which comprise 9 species and 33 tentative species, and can be divided into two groups, the sandfly fever virus group and the Uukuniemi-like virus group (Table 1). Before 1991, the Uukuniemi-like viruses were in a separate genus (Uukuvirus), but the similar molecular biological characteristics along with some weak serological cross reactivity shared with viruses in the Phlebovirus genus resulted in their reclassification [4]. There are some important differences between these viruses, notably that the Uukuniemi-like viruses do not encode a nonstructural (NSm) protein at the N-terminus of the glycoprotein precursor coding region (Figure 1a), and that the Uukuniemi-like viruses are all transmitted by ticks whereas the sandfly fever group are transmitted by dipterans (phlebotomines and mosquitoes) [5].
Table 1.
Species in the Phlebovirus genus according to current classification [3]
| Species | Notable virus | Geographic distribution | Principal vector | Disease |
|---|---|---|---|---|
| Bujaru virus | Bujaru virus | South America | N.D. | |
| Candiru virus | Alenquer virus | South America | N.D. | Human |
| Candiru virus | South America | N.D. | Human | |
| Chilibre virus | Chilibre virus | North America | Phlebotomines | |
| Frijoles virus | Frijoles virus | North America | Phlebotomines | |
| Punta Toro virus | Punta Toro virus | North America, South America | Phlebotomines | Human |
| Rift Valley fever virus | Rift Valley fever virus | Africa | Mosquitoes | Human, cattle |
| Salehabad virus | Salehabad virus | Asia | Phlebotomines | |
| Sandfly fever Naples virus | Sandfly fever Naples virus | Europe, Africa, Asia | Phlebotomines | Human |
| Sandfly fever Sicilian virus | Europe | Phlebotomines | Human | |
| Toscana virus | Europe | Phlebotomines | Human | |
| Uukuniemi virus | Uukuniemi virus | Europe | Ticks | |
Figure 1.
The phlebovirus genome. (a) Comparison of the coding strategy of the sandfly fever group (Rift Valley fever, RVFV) and Uukuniemi group (UUKV) genomes. RNAs are represented by thin lines (the length in nucleotides is given above each segment) and the mRNAs are shown as arrows (■ indicates host-derived sequences at 5′ end). Gene products, with their apparent Mr, are represented by coloured boxes. (b) Transcription and replication scheme of ambisense-sense phlebovirus S genome segment. The genome RNA encodes the N protein in the negative-sense and the NSs protein in positive-sense orientation, separated by an intergenic region that has the potential to form a hairpin structure. The proteins are translated from specific sub-genomic mRNAs, with the mRNA encoding NSs transcribed from the antigenome RNA.
A characteristic of all phleboviruses is the ambisense coding strategy of the S genome segment: the N protein is encoded in the negative-sense orientation on the S segment while the NSs protein is encoded in the positive-sense. However, both proteins are translated from separate subgenomic mRNAs that are transcribed from the genomic or antigenomic RNA as shown in Figure 1b [6].
The best-known phlebovirus is Rift Valley fever virus, a serious pathogen of ruminants but also capable of causing disease, sometimes fatal, in man [7,8]. Other human pathogens include sandfly fever Naples, sandfly fever Sicilian and Toscana viruses that cause febrile illness and occasionally encephalitis, and are found in countries of the Mediterranean basin. On the other hand, the tick-transmitted Uukuniemi and Uukuniemi-like viruses are non pathogenic for humans [5]. Recently, new tick-transmitted phleboviruses have been described, called severe fever with thrombocytopenia syndrome virus and Heartland virus, that can cause serious disease in humans.
Severe fever with thrombocytopenia syndrome virus
Between 2007 and 2010 cases of an unknown infectious disease were reported in Henan and Hubei Provinces, China, with patients presenting gastrointestinal symptoms, chills, joint pain, myalgia, thrombocytopenia, leukocytopenia and some haemorrhagic manifestations, resulting in a case fatality rate of 12–30% [9••]. The disease was originally suspected to be anaplasmosis, but some clinical symptoms were inconsistent with this diagnosis. Subsequently, studies by different groups in China involving virus isolation in cell culture, genome amplification and sequencing, and metagenomic analysis of patient material revealed the presence of a novel bunyavirus that was most closely related to phleboviruses. The virus has been variously called DaBie Mountain virus [9••,10], Henan fever virus [11], Huaiyangshan virus [12] and severe fever with thrombocytopenia syndrome virus (SFTSV) [9••]. SFTSV is the name preferred by the Chinese CDC and will used here, although the ICTV has yet to formally approve a name for this new phlebovirus.
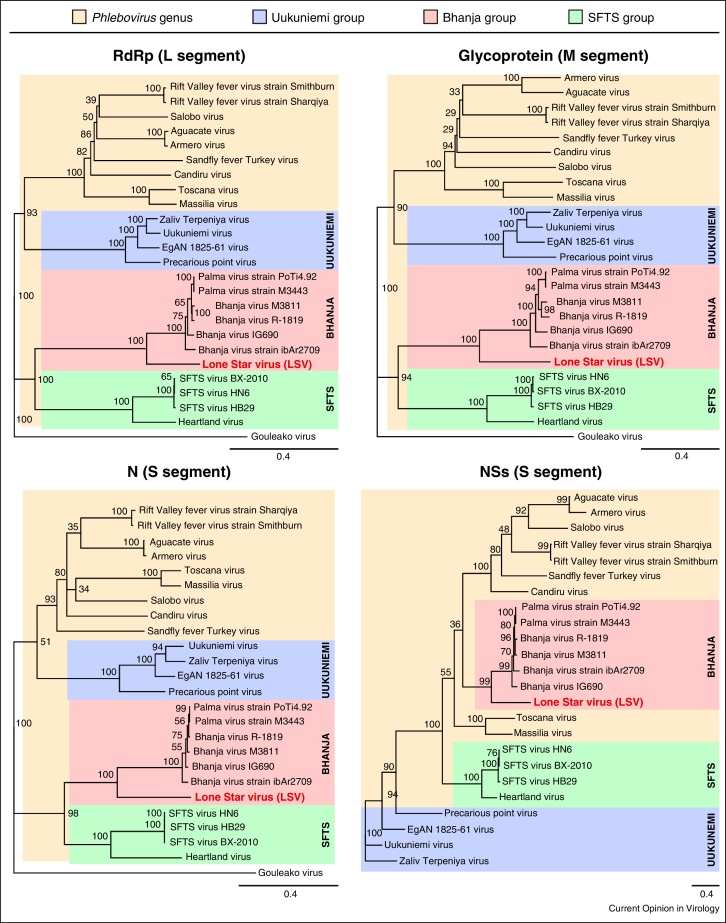
Comparison of SFTSV viral proteins showed 33%, 30–36%, 30–41% and 11–13% similarity to either RVFV or UUKV RNA dependent RNA polymerase, glycoprotein precursor, N or NSs proteins respectively [9••]. Notably the glycoprotein precursor lacked a putative NSm protein at its N-terminus. Phylogenetic anlaysis of SFTSV sequences indicated that it represents a new clade within the Phlebovirus genus, roughly equidistant from the already established sandfly fever and Uukuniemi groups [9••] (Figure 2).
Figure 2.
Phylogeny of representative phleovirus proteins. The analogous protein from Gouleako virus, a mosquito-associated phlebovirus-like virus, was used as the outgroup in each case. Taken from Swei et al. [38•].
Both SFTSV and viral RNA have been isolated from Haemaphysalis longicornis ticks, and viral RNA has been detected in Rhipicephalus microplus ticks gathered from domestic animals in China [9••,13]. Detection of SFTSV RNA was highest in H. longicornis, a species that has a widespread geographical distribution outside of China including Korea, Japan, Australia, New Zealand and Pacific Islands [14]. The subsequent reports of SFTSV positive ticks and confirmed cases of disease in South Korea [15•] and Japan [16•] indicate that SFTSV and SFTSV-like viruses may have a broad distribution and further surveillance is warranted.
Many SFTSV genome sequences have been determined, and a deviation from the consensus terminal nucleotide sequence was observed at the 5′ termini of the L and M segments (Table 2). A comprehensive analysis of available sequences suggested that they could be separated into three major lineages (I to III), with two sublineages in lineage I [11]. Significantly, the M segment of one of the sublineages showed evidence for recombination, with two break points, between the M segments of lineage I and lineage III [17•]. This resulted in lineage I having acquired almost the complete coding sequence for Gn from lineage III. Homologous recombination is regarded as a rare event among negative-sense RNA viruses [18] though has previously been reported in the bunyavirus family, specifically hantaviruses [19]. Thus, in addition to the more usual evolutionary mechanisms of genetic drift and genome segment reassortment [20], recombination could also play a role in the evolution of SFTSV [11].
Table 2.
Nucleotide sequences of the 5′ and 3′ termini of phlebovirus antigenomic sense RNA segments. Bases deviating from the consensus are highlighted
| Virus | Accession no. | Segment | 5′ UTR | 3′ UTR |
|---|---|---|---|---|
| RVFV | DQ375404 | L | ACACAAAG… | …CUUUGUGU |
| DQ380208 | M | ACACAAAG… | …CUUUGUGU | |
| DQ380154a | S | ACACAAAG… | …CUUUGUGU | |
| UUKV | D10759 | L | ACACAAAG… | …CUUUGUGU |
| M17417 | M | ACACAAAG… | …CUUUGUGU | |
| M33551 | S | ACACAAAG… | …CUUUGUGU | |
| SFTSV | HM745930 | L | ACACAGAG… | …CUUUGUGU |
| HM745931 | M | ACACAGAG… | …CUUUGUGU | |
| HM745932 | S | ACACAAAG… | …CUUUGUGU | |
| HRTV | JX005847 | L | ACACAAAG… | …CUUUGUGU |
| JX005845 | M | ACACAGAG… | …CUUUGUGU | |
| JX005843a | S | ACACAGAG… | …CUUUGUGU | |
| BHAV | JX961619 | L | ACACAGAG… | …CUUUGUGU |
| JX961620 | M | ACACAAAG… | …CUUUGUGU | |
| JX961621 | S | ACACAAAG… | …CUCUGUGU | |
| LSV | KC589005 | L | ACACAAAG… | …CUUUGUGU |
| KC589006 | M | ACACAAAG… | …CUUUGUGU | |
| KC589007 | S | ACACAAAG… | …CUCUGUGU | |
Sequence in database presented as genomic sense RNA.
Research efforts are now focusing on understanding the molecular biology of the virus lifecycle and its pathogenesis in animal hosts. Qu et al. [21] found SFTSV able to infect a human monocytic cell line THP-1 in the absence of any discernable cytopathic effect (CPE) evidenced by a lack of caspase-3 cleavage, in contrast to the extensive CPE associated with RVFV infection [22]. Infection resulted in an up-regulation of interferon induction through IRF and NF-κB activation, though some regulatory molecules such as TRAF3, TRAF6 or MAVS were down-regulated or unchanged. It was further shown through the use of reporter assays that the NSs protein of SFTSV was responsible for this effect, and able to inhibit the induction of IFN. The NSs proteins of other phleboviruses as well as of orthbunyaviruses have also been shown to act as IFN antagonists [23]. An interaction of SFTSV NSs with Tank-binding kinase 1 (TBK-1) was described, suggesting a potential mechanism for the deregulation of the interferon response during infection as TBK-1, a homolog of IKKɛ, activates both NF-κB and IRF-3. The authors also reported a novel role for the SFTSV nucleocapsid protein (N) in the suppression of the IFN-β promoter [21], a function not previously reported for the N proteins of other members of the Bunyaviridae.
The crystal structure of SFTSV N protein has been solved by two groups [24•,25•]. Despite having a distinct amino acid sequence to that of RVFV, the SFTSV nucleocapsid protein also oligomerised to form a hexameric ring [26,27] to accomplish encapsidation of the genomic RNAs. Three amino acids (R64, K67 and K74) were identified to be crucial for the ability of the protein to bind RNA in vitro [24•], while mutations at residues A8, F11, A25, and L28 were shown to significantly hinder the ability of the protein to form oligomeric conformations [25•]. The interaction of the N-terminal arm of one protein with a neighbouring N protomer is predicted to be the mechanism by which the hexameric oligomerisation of the SFTSV nucleocapsid occurs. Indeed, deletion of the N-terminal 34 amino acids of SFSTV N disrupts the functional interactions of the N-terminal arm, and resulted in only monomeric protein being detected [25•]. Suramin was reported to inhibit SFTSV-N RNA binding by locating in the RNA binding cavity on N, and also had functionality against other members of the Phlebovirus genus, but not viruses in other Bunyaviridae genera. Suramin inhibited SFTSV replication in infected Vero E6 cells. [24•].
Using rhabdovirus-based vectors for expression of SFTSV Gn and Gc proteins, Hofmann et al. [28] demonstrated that SFTSV entered a wide range of cell lines, including human macrophage and dendritic cells, in a pH-dependent manner. Infection by the SFTSV pseudotypes could be abolished using sera from convalescent SFTS patients. Similar to RVFV [29], SFTSV utilizes the C-type lectin DC-SIGN as a receptor for entry into the cells [28].
These insights have been valuable to the understanding of SFTSV biology at the cell and molecular level. On the organismal level, several animal models of infection have been described to begin to understand the underlying pathologies associated with SFTSV infection such as thrombocytopenia and leukocytopenia. Wistar rats, Kunming mice, BalB/C mice, C57/BL mice, C57/BL6 mice and hamsters have been explored [30,31]. Newborn animals were highly susceptible to SFTSV infection, with the exception of newborn hamsters (no deaths resulted from either an intracerebral or intraperitoneal inoculation), while no reduction of white blood cells or platelets could be detected during infection of BalB/C mice or hamsters [30,31]. The C57/BL6 mouse model shows particular promise as SFTSV infection in these animals mimics the major clinical manifestations observed in infected patients and identified the major cause of thrombocytopenia to be an enhanced clearance of virus-infected platelets promoted by splenic macrophages. The authors suggest that SFTSV could bind to platelets and cause their recognition and phagocytosis by macrophages in the red pulp of the spleen [30].
Heartland virus
Heartland virus was isolated from two patients in Missouri, USA [32••] who experienced fever, fatigue, anorexia, diarrhoea and thrombocytopenia. Both patients reported tick-bites prior to disease, and both recovered after extended hospitalisation. Deep-sequencing of total RNA from tissue culture cells inoculated with the patients’ blood revealed a novel phlebovirus that clustered phylogenetically with SFTSV (Figure 2). However HRTV is quite distinct from SFTSV, showing 27% and 38% difference in the RdRp and N protein sequences respectively. The two HRTV isolates showed 95-99% nucleotide identity to each other, depending on the segment, indicating that the patients were infected independently [32••]. The HRTV S and M segments showed a G residue rather than A at position 6 in the 5′ terminus (Table 2). HRTV RNA was subsequently detected in 10 pools of nymphs of Amblyomma americanum ticks (nine of which were found on the property of one of the patients) and virus has been isolated from 8 of the pools, implicating A. americanum as the likely primary vector for transmission of the virus within the USA [33].
Bhanja virus
Bhanja virus (BHAV) is not a newly emerged virus but recent molecular characterisation of this and related viruses is pertinent to the above discussion of SFTSV and HRTV. The Bhanja virus antigenic complex (Bhanja, Forecariah, Kismayo and Palma viruses) comprises tick-borne viruses that were assigned to the family Bunyaviridae but were not further classified into a genus. BHAV was isolated in India in 1954 from a tick on a paralysed goat, and causes fever and signs of central nervous system involvement in young ruminants but not in adult animals. A few cases of febrile illness in humans have also been described. BHAV has been reported in southern and central Asia, Africa, and southern Europe (Italy, Croatia, Bulgaria, Slovakia, and Romania) [34]. Two recent papers report nucleotide sequence determination of Bhanja group viruses [35•,36•] and show that they are related to SFTSV and HRTV (Figure 2). Variations from the consensus terminal sequences were noted at the 5′ terminus of the L segment and the 3′ end of the S segment (Table 2). The Bhanja group viruses can be divided into African and Eurasian lineages possibly due to association with different tick vectors: the Eurasian viruses have been isolated from Haemaphysalis ticks, like SFTSV, whereas the African strains have been isolated from a wider range of tick species.
Lone Star virus
Lone Star virus was originally isolated from A. americanum (the lone star tick) in Kentucky in 1967 [37], and like BHAV was an unclassified member of the Bunyaviridae. The sequence of the viral genome has recently been determined by deep sequencing and shown to be in the same clade as BHAV [38•] (Figure 2). The S segment 5′ terminus has a G residue at position 6 rather than A as found in the phlebovirus consensus sequence (Table 2). LSV can infect human (HeLa) and monkey (Vero) cells in culture but there is no evidence for human infection.
Conclusions
The number of known phleboviruses has increased markedly in recent years with the emergence of SFTSV and HRTV, and the genetic characterisation of the previously unassigned BHAV, LSV and related viruses. These viruses form clades (Figure 2) that are related to the Uukuniemi-like viruses, and all share the properties of not encoding an NSm protein and being transmitted by ticks. For Rift Valley fever virus, the NSm protein is important for infectivity in Aedes aegypti mosquitoes [39] as a deletion mutant was barely able to establish infection. In addition, although not essential for growth in mammalian cells or animals [40–42] NSm regulates the p38 mitogen-activated protein kinase stress response [43] and has an antiapoptotic role [22], mediated by its interaction with the outer mitochondrial membrane [44]. Whether the Uukuniemi-like viruses inhibit apoptosis, and if so, which viral proteins perform this task, awaits further investigation.
It is likely that more relatives of these tick-borne phleboviruses will be discovered in the future, aided by advances in metagenomic analysis and perhaps heightened surveillance, as has been implemented in China. Of great importance is to understand the molecular basis for the different pathogenicity of these viruses in man, a task that will keep bunyavirologists occupied for years to come.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgement
Work in RME's laboratory is supported by grants from the Wellcome Trust and Medical Research Council.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
References
- 1.Elliott R.M., Schmaljohn C.S. Bunyaviridae. In: Knipe D.M., Howley P.M., editors. Fields Virology. edn 6. Wolters Kluwer; 2013. pp. 1244–1282. [Google Scholar]
- 2.Plyusnin A., Elliott R.M., editors. Bunyaviridae. Molecular and Cellular Biology. Caister Academic Press; Norfolk: 2011. [Google Scholar]
- 3.Plyusnin A., Beaty B.J., Elliott R.M., Goldbach R., Kormelink R., Lundkvist Å., Schmaljohn C.S., Tesh R.B. Bunyaviridae. In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowits E.J., editors. Virus taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press; 2012. pp. 725–741. [Google Scholar]
- 4.Calisher C. Classification and nomenclature of viruses. Fifth report of the international committeee on taxonomy of viruses. In: Francki R.I.B., Fauquet C.M., Knudson D.L., Brown F., editors. vol Suppl 2. 1991. pp. 273–283. (Archives of Virology). [Google Scholar]
- 5.Bouloy M. Molecular biology of phleboviruses. In: Plyusnin A., Elliott R.M., editors. Bunyaviridae. Molecular and Cellular Biology. Caister Academic Press; 2011. [Google Scholar]
- 6.Giorgi C., Accardi L., Nicoletti L., Gro M.C., Takehara K., Hilditch C., Morikawa S., Bishop D.H. Sequences and coding strategies of the S RNAs of Toscana and Rift Valley fever viruses compared to those of Punta Toro, Sicilian Sandfly fever, and Uukuniemi viruses. Virology. 1991;180:738–753. doi: 10.1016/0042-6822(91)90087-r. [DOI] [PubMed] [Google Scholar]
- 7.Ikegami T. Molecular biology and genetic diversity of Rift Valley fever virus. Antiviral Res. 2012;95:293–310. doi: 10.1016/j.antiviral.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikegami T., Makino S. The pathogenesis of Rift Valley fever. Viruses. 2011;3:493–519. doi: 10.3390/v3050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Yu X.-J., Liang M.-F., Zhang S.-Y., Liu Y., Li J.-D., Sun Y.-L., Zhang L., Zhang Q.-F., Popov V.L., Li C. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]; Original description of a novel phlebovirus causing severe fever with thrombocytopenia syndrome.
- 10.Stone R. Rival teams identify a virus behind deaths in Central China. Science. 2010;330:20–21. doi: 10.1126/science.330.6000.20. [DOI] [PubMed] [Google Scholar]
- 11.Xu B., Liu L., Huang X., Ma H., Zhang Y., Du Y., Wang P., Tang X., Wang H., Kang K. Metagenomic analysis of fever, thrombocytopenia and leukopenia syndrome (FTLS) in Henan Province, China: discovery of a new bunyavirus. PLoS Pathog. 2011;7:e1002369. doi: 10.1371/journal.ppat.1002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y.-Z., He Y.-W., Dai Y.-A., Xiong Y., Zheng H., Zhou D.-J., Li J., Sun Q., Luo X.-L., Cheng Y.-L. Hemorrhagic fever caused by a novel Bunyavirus in China: pathogenesis and correlates of fatal outcome. Clin Infect Dis. 2012;54:527–533. doi: 10.1093/cid/cir804. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y.-Z., Zhou D.-J., Qin X.-C., Tian J.-H., Xiong Y., Wang J.-B., Chen X.-P., Gao D.-Y., He Y.-W., Jin D. The ecology, genetic diversity, and phylogeny of Huaiyangshan virus in China. J Virol. 2012;86:2864–2868. doi: 10.1128/JVI.06192-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohmeyer K.H., Pound J.M., May M.A., Kammlah D.M., Davey R.B. Distribution of Rhipicephalus (Boophilus) microplus and Rhipicephalus (Boophilus) annulatus (Acari: Ixodidae) infestations detected in the United States along the Texas/Mexico border. J Med Entomol. 2011;48:770–774. doi: 10.1603/me10209. [DOI] [PubMed] [Google Scholar]
- 15•.Kim K.-H., Yi J., Kim G., Choi S.J., Jun K.I., Kim N.-H., Choe P.G., Kim N.-J., Lee J.-K., Oh M-d. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerging Infect Dis. 2013;19 doi: 10.3201/eid1911.130792. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of SFTSV outside of China.
- 16•.Takahashi T., Maeda K., Suzuki T., Ishido A., Shigeoka T., Tominaga T., Kamei T., Honda M., Ninomiya D., Sakai T. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis. 2013:1603. doi: 10.1093/infdis/jit603. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report of SFTSV outside of China
- 17•.He C.-Q., Ding N.-Z. Discovery of severe fever with thrombocytopenia syndrome bunyavirus strains originating from intragenic recombination. J Virol. 2012;86:12426–12430. doi: 10.1128/JVI.01317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes intragenic recombination in SFTSV M genome segmeint, an unusual occurrence in a negative strand RNA virus.
- 18.Chare E.R., Gould E.A., Holmes E.C. Phylogenetic analysis reveals a low rate of homologous recombination in negative-sense RNA viruses. J Gen Virol. 2003;84:2691–2703. doi: 10.1099/vir.0.19277-0. [DOI] [PubMed] [Google Scholar]
- 19.Sibold C., Meisel H., Kruger D.H., Labuda M., Lysy J., Kozuch O., Pejcoch M., Vaheri A., Plyusnin A. Recombination in Tula hantavirus evolution: analysis of genetic lineages from Slovakia. J Virol. 1999;73:667–675. doi: 10.1128/jvi.73.1.667-675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding N.Z., Luo Z.F., Niu D.D., Ji W., Kang X.H., Cai S.S., Xu D.S., Wang Q.W., He C.Q. Identification of two severe fever with thrombocytopenia syndrome virus strains originating from reassortment. Virus Res. 2013;178:543–546. doi: 10.1016/j.virusres.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Qu B., Qi X., Wu X., Liang M., Li C., Cardona C.J., Xu W., Tang F., Li Z., Wu B. Suppression of the interferon and NF-κB responses by severe fever with thrombocytopenia syndrome virus. J Virol. 2012;86:8388–8401. doi: 10.1128/JVI.00612-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Won S., Ikegami T., Peters C.J., Makino S. NSm protein of Rift Valley fever virus suppresses virus-induced apoptosis. J Virol. 2007;81:13335–13345. doi: 10.1128/JVI.01238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber F., Elliott R.M. Bunyaviruses and innate immunity. In: Brasier A., Garcia-Sastre A., Lemon S., editors. Cellular signaling and innate immune responses to RNA virus infections. ASM Press; 2009. pp. 287–299. [Google Scholar]
- 24•.Jiao L., Ouyang S., Liang M., Niu F., Shaw N., Wu W., Ding W., Jin C., Peng Y., Zhu Y. Structure of severe fever with thrombocytopenia syndrome virus nucleocapsid protein in complex with Suramin reveals therapeutic potentials. J Virol. 2013;87:6829–6839. doi: 10.1128/JVI.00672-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; Atomic structure of SFTSV nucleoprotein and studeis on Suramin as a potential antiviral.
- 25•.Zhou H., Sun Y., Wang Y., Liu M., Liu C., Wang W., Liu X., Li L., Deng F., Wang H. The nucleoprotein of severe fever with thrombocytopenia syndrome virus processes a stable hexameric ring to facilitate RNA encapsidation. Protein Cell. 2013;4:445–455. doi: 10.1007/s13238-013-3901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Atomic structure of SFTSV nucleoprotein and its similarity to that of Rift Valley fever virus.
- 26.Ferron F., Li Z., Danek E.I., Luo D., Wong Y., Coutard B., Lantez V., Charrel R., Canard B., Walz T. The hexamer structure of Rift Valley fever virus nucleoprotein suggests a mechanism for its assembly into ribonucleoprotein complexes. PLoS Pathog. 2011;7:e1002030. doi: 10.1371/journal.ppat.1002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raymond D.D., Piper M.E., Gerrard S.R., Smith J.L. Structure of the Rift Valley fever virus nucleocapsid protein reveals another architecture for RNA encapsidation. Proc Natl Acad Sci USA. 2010;107:11769–11774. doi: 10.1073/pnas.1001760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann H., Li X., Zhang X., Liu W., Kühl A., Kaup F., Soldan S.S., González-Scarano F., Weber F., He Y. Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines. J Virol. 2013;87:4384–4394. doi: 10.1128/JVI.02628-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozach P.Y., Kuhbacher A., Meier R., Mancini R., Bitto D., Bouloy M., Helenius A. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe. 2011;10:75–88. doi: 10.1016/j.chom.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Jin C., Liang M., Ning J., Gu W., Jiang H., Wu W., Zhang F., Li C., Zhang Q., Zhu H. Pathogenesis of emerging severe fever with thrombocytopenia syndrome virus in C57/BL6 mouse model. Proc Natl Acad Sci USA. 2012;109:10053–10058. doi: 10.1073/pnas.1120246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X.P., Cong M.L., Li M.H., Kang Y.J., Feng Y.M., Plyusnin A., Xu J., Zhang Y.Z. Infection and pathogenesis of Huaiyangshan virus (a novel tick-born bunyavirus) in laboratory rodents. J Gen Virol. 2012;93:1288–1293. doi: 10.1099/vir.0.041053-0. [DOI] [PubMed] [Google Scholar]
- 32••.McMullan L.K., Folk S.M., Kelly A.J., MacNeil A., Goldsmith C.S., Metcalfe M.G., Batten B.C., Albarino C.G., Zaki S.R., Rollin P.E. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]; Original description of Heartland virus in the US.
- 33.Savage H.M., Godsey M.S., Jr., Lambert A., Panella N.A., Burkhalter K.L., Harmon J.R., Lash R.R., Ashley D.C., Nicholson W.L. First detection of Heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg. 2013;89:445–452. doi: 10.4269/ajtmh.13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hubalek Z. Biogeography of tick-borne Bhanja virus (Bunyaviridae) in Europe. Interdiscip Perspect Infect Dis 2009. 2009:372691. doi: 10.1155/2009/372691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Dilcher M., Alves M.J., Finkeisen D., Hufert F., Weidmann M. Genetic characterization of Bhanja virus and Palma virus, two tick-borne phleboviruses. Virus Genes. 2012;45:311–315. doi: 10.1007/s11262-012-0785-y. [DOI] [PubMed] [Google Scholar]; Genetic analysis of Bhanja virus that charcterises it as a phlebovirus.
- 36•.Matsuno K., Weisend C., Travassos da Rosa A.P., Anzick S.L., Dahlstrom E., Porcella S.F., Dorward D.W., Yu X.J., Tesh R.B., Ebihara H. Characterization of the Bhanja serogroup viruses (Bunyaviridae): a novel species of the genus Phlebovirus and its relationship with other emerging tick-borne phleboviruses. J Virol. 2013;87:3719–3728. doi: 10.1128/JVI.02845-12. [DOI] [PMC free article] [PubMed] [Google Scholar]; Another genetic study that shows Bhanja virus is a phlebovirus.
- 37.Kokernot R.H., Calisher C.H., Stannard L.J., Hayes J. Arbovirus studies in the Ohio–Mississippi Basin, 1964–1967. VII. Lone Star virus, a hitherto unknown agent isolated from the tick Amblyomma americanum (Linn) Am J Trop Med Hyg. 1969;18:789–795. [PubMed] [Google Scholar]
- 38•.Swei A., Russell B.J., Naccache S.N., Kabre B., Veeraraghavan N., Pilgard M.A., Johnson B.J.B., Chiu C.Y. The genome sequence of Lone Star Virus, a highly divergent Bunyavirus found in the Amblyomma americanum tick. PLoS ONE. 2013;8:e62083. doi: 10.1371/journal.pone.0062083. [DOI] [PMC free article] [PubMed] [Google Scholar]; Genetic analysis of Lone Star virius.
- 39.Crabtree M.B., Kent Crockett R.J., Bird B.H., Nichol S.T., Erickson B.R., Biggerstaff B.J., Horiuchi K., Miller B.R. Infection and transmission of Rift Valley Fever viruses lacking the NSs and/or nsm genes in mosquitoes: potential role for NSm in mosquito infection. PLoS Negl Trop Dis. 2012;6:e1639. doi: 10.1371/journal.pntd.0001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bird B.H., Albarino C.G., Hartman A.L., Erickson B.R., Ksiazek T.G., Nichol S.T. Rift Valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J Virol. 2008;82:2681–2691. doi: 10.1128/JVI.02501-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bird B.H., Albarino C.G., Nichol S.T. Rift Valley fever virus lacking NSm proteins retains high virulence in vivo and may provide a model of human delayed onset neurologic disease. Virology. 2007;362:10–15. doi: 10.1016/j.virol.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 42.Won S., Ikegami T., Peters C.J., Makino S. NSm and 78-Kilodalton proteins of Rift Valley fever virus are nonessential for viral replication in cell culture. J Virol. 2006;80:8274–8278. doi: 10.1128/JVI.00476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narayanan A., Popova T., Turell M., Kidd J., Chertow J., Popov S.G., Bailey C., Kashanchi F., Kehn-Hall K. Alteration in superoxide dismutase 1 causes oxidative stress and p38 MAPK activation following RVFV infection. PLoS ONE. 2011;6:e20354. doi: 10.1371/journal.pone.0020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terasaki K., Won S., Makino S. The C-terminal region of Rift Valley fever virus NSm protein targets the protein to the mitochondrial outer membrane and exerts antiapoptotic function. J Virol. 2013;87:676–682. doi: 10.1128/JVI.02192-12. [DOI] [PMC free article] [PubMed] [Google Scholar]