Abstract
Performance on olfactory tests can be influenced by a number of stimulus characteristics including chemical structure, concentration, perceptual similarity, and previous experience with the test odorants. Few of these parameters have been extensively characterized in the Fischer 344 rat strain. To investigate how odor quality affects perception in this rat strain, we measured how graded perceptual similarity, created by varying carbon chain length across a series of homologous alcohol pairs, influenced odor discrimination using a liquid-motivated go/no-go task. We employed an automated, liquid-dilution olfactometer to train Fischer 344 rats (N = 8) on a 2-odor discrimination task. Six odorants (1-propanol, 1-butanol, 1-pentanol, 1-hexanol, 1-heptanol, and 1-octanol) were arranged to produce 15 novel odorant pairs differing between 1 and 5 carbon atoms; testing sessions included presentation of only 1 pseudorandomly assigned pair daily (200 trials). Results show that although rats can learn to discriminate between any 2 odorant pairs, performance declines systematically as the pairs become more structurally similar and, therefore, more perceptually confusing. As such, the easier discrimination pairs produced reliable ceiling effects across all rats, whereas performance for the difficult discrimination pairs was consistently worse, even after repeated testing. These data emphasize the importance of considering odorant stimulus dimensions in experimental designs employing olfactory stimuli. Moreover, establishing baseline olfactory performance in Fischer 344 rats may be particularly useful for predicting age-related cognitive decline in this model.
Key words: animal psychophysics, carbon chains, cognitive impairment, odor discrimination, olfaction
Introduction
Recent studies have used generalization gradients to evaluate olfactory performance across a continuum (cf., Boesveldt et al. 2010); analogous to measurement techniques adopted from other sensory systems (Shepard 1987), this approach integrates knowledge of olfactory structure–activity relationships to design tasks that systematically vary in difficulty. Guided by glomerular representations in the bulb (Johnson et al. 2010; Falasconi et al. 2012), homologous chemical series varying in carbon chain length have been used to assess graded perceptual similarity (Cleland et al. 2002; Ho et al. 2006). Using this approach, a number of investigators have shown a significant correlation between discrimination performance and structural similarity in several species, including mice (Laska et al. 2008; Güven and Laska 2012), rats (Linster and Hasselmo 1999), spider monkeys (Laska et al. 2006), elephants (Rizvanovic et al. 2013), and humans (Laska and Teubner 1999). Similarly, previous investigations have shown that similarities in odorant-evoked glomerular activity patterns predict rat behavioral performance on odor habituation tasks (Linster and Hasselmo 1999; Ho et al. 2006). In both experimental paradigms, generalization is defined as the degree to which any 2 odorants are perceptually confused with one another (Mandairon et al. 2011). Taken together, these studies suggest that carbon chain length may be one of several key determinants of olfactory perception. These stimuli therefore provide an opportunity to capture subtle behavioral alterations that could be overlooked using more conventional olfactory behavioral procedures.
Using a homologous series of aliphatic odorants has other advantages as well. A number of factors may affect behavioral performance on odor-guided tasks (Slotnick 2007). Across studies, differing methodologies may result in different behavioral responses and, consequently, varied interpretations of the same odor processing mechanisms. Such disparities may arise from use of different behavioral measures (Wesson et al. 2008; Cleland et al. 2009; Frederick et al. 2011), as well as choice of test odorants. For example, most behavioral olfactory studies have been performed using a myriad of complex odorant mixtures presented over a wide range of concentrations (Eichenbaum 1998; LaSarge et al. 2007), though it is well established that stimulus properties can significantly affect behavioral performance (Gamble and Smith 2009; Perry and Felsen 2012; Schaefer and Margrie 2012). Therefore, to better facilitate comparisons of findings and consistency of interpretations across studies, stimulus properties and sampling parameters must be carefully considered.
The present study sought to investigate the contribution of carbon chain length as a key molecular feature in odor discrimination by Fischer 344 rats. It has long been recognized that olfactory acuity can be affected by a variety of neurodegenerative and age-related processes (Duda 2010; Wesson et al. 2010). Because the olfactory pathway is uniquely vulnerable to pathological insult (Doty 2008), experimental paradigms using odor-guided behavioral tasks have become increasingly popular. As the primary sensory modality for rodents, olfaction is ideal for both investigating natural animal behaviors and as a means to investigate neurocognitive processes (Abraham et al. 2012). Discrimination pairs were comprised of homologous aliphatic alcohols varying from 3 to 8 carbon atoms. In addition, we evaluated performance across multiple behavioral parameters to determine which response measures would be most useful for future investigations of olfactory behavior in this rat strain. To behaviorally characterize odor generalization, rats were trained using an odorant conditioning paradigm in an automated olfactometer to discriminate structurally related odorants. This approach was based on the assumption that using carbon chain differences to vary the difficulty of discrimination pairs would provide a perceptual similarity gradient.
F344 rats were chosen for this study because they have been shown to be a useful model for the study of age-related cognitive decline, and there are no extant behavioral data describing their basic olfactory acuity (LaSarge et al. 2007). Unlike previous studies assessing odor discrimination using a “digging task” in this rat strain, the olfactometer enabled precise control of stimulus delivery and behavioral responses; this design enhanced psychophysical output by providing comprehensive analyses for comparison across odorant pairs. In addition, because olfactory performance and hippocampal-dependent, age-related cognitive deficits are linked in the F344 rat model, it will be important to evaluate baseline, odor discrimination performance using more sensitive olfactory assessments.
Materials and methods
Subjects
Eight male Fischer 344 rats were used in this study. Rats were obtained at 3 months of age from the National Institute on Aging colony (Taconic) but were 13 months old at initiation of behavioral training. Rats were individually housed in the central Animal Care Services vivarium in the McKnight Brain Institute. The rats were maintained on a 12:12h light/dark cycle, and behavioral testing was conducted during the light cycle. Rats had ad libitum access to dry LabDiet rat chow (Purina Mills) and restricted access to water. This regimen resulted in the rats stabilizing at 85–90% of their free-feeding body weight, which facilitated use of a nutritional liquid food reinforcer during training and testing procedures (Ensure, Abbott Laboratories). During a typical session, rats received ~10mL of Ensure per day, followed by 2h of unrestricted access to water after daily testing. Rats were tested once daily, 5–7 days per week.
All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication no. 86-23, revised 1985) and were approved by the University of Florida Institutional Animal Care and Use Committee.
Olfactometer
An 8-channel, custom liquid-dilution rodent olfactometer was employed in this study to assess odor discrimination. The behavioral apparatus (adapted for use with the rat) and methods employed in this study are comparable with those used in our previous work with this olfactometer in mice, and detailed discussions of the training and testing techniques can be found in those previous publications (Smith et al. 2008; Gamble and Smith 2009). The rat olfactometer consists of a 21-cm deep, 30.5-cm wide, and 24.1-cm tall, ventilated Plexiglas operant chamber. The chamber is fitted with a conductive stainless steel floor and a PVC sniffing port containing a metal licking tube. The ventilation system provides a steady stream of fresh room air in the chamber, maintaining positive pressure and ensuring that the odorant remains within the sniffing port air stream.
A photo beam was broken when the rat inserted its head into the sniffing port, initiating a trial sequence. Rats were required to keep their noses within the port and sample the stimulus air stream for a minimum of 200ms, at which time a stimulus, either the S+ (target stimulus) or S− (control stimulus), as defined below, was introduced through the bottom of the sampling port. The air stream and odorant were drawn through the sampling port in which the rat positioned its nose and were then exhausted out of the top by an in-line exhaust fan and fed into a central room evacuation system. Stimulus delivery and behavioral responses were controlled and monitored by a computer running custom-designed software.
Initial training
Training methods followed those described by Bodyak and Slotnick (1999). Briefly, rats were initially rewarded for contacting the lick tube with their tongue, followed by nose pokes into the sampling port, and finally for remaining in the sampling port for odorant presentation. During the last stage of training, the final valve was introduced, gradually requiring rats to sample the odorant for intervals up to 1 s. A 10% v/v solution of coconut extract (Gordon Food Service) served as the initial training stimulus. Reliable performance during this initial training stage was achieved within 2 sessions (45 min–1.5h) for all rats. Prior to the discrimination task, rats therefore acquired an association between the target odorant and delivery of liquid reinforcement. Once the rats successfully completed training, they were transferred to a 2-odorant discrimination program.
Discrimination training
Rats were trained to discriminate dilutions of the target (S+) odorant (coconut extract) in a diluent from the diluent alone (S−). The diluent was near-odorless diethyl phthalate. Reinforcement was contingent upon the rat reporting detection of the S+ odorant by licking on the metal tube (correct detection), which completed an electrical circuit with the metal floor and registered the response with the computer-based olfactometer control program. A correct detection was followed by presentation of ~5 μL of Ensure through the lick tube. Failure to report the presence of the S+ (a miss) or licking the response tube during presentation of the S− stimulus (false alarm) were recorded as incorrect responses and required rats to withdraw their nose from the sampling port for 5 s before reinserting their nose to initiate a new trial. Consistent with previous olfactometer studies in rodents (Bodyak and Slotnick 1999), rats were required to respond to the target (S+) odor, coconut extract, by maintaining contact with the lick tube for at least 7 of 10 time bins (each spanning 100ms) during a 1 s odor presentation. From these calculated lick intervals, the go/no-go criterion was set to 7. Hence, if the rat continuously licked for ~700ms (7×100ms bins) of the total ~1000ms (10×100ms bins) response time, the rat would receive the 5 μL liquid reinforcement. Conversely, if the rat refrained from licking or licked fewer than 7 bins on the control odorant (S−), the trial would be recorded as a correct rejection, thereby allowing the rat to initiate the next trial. Note that the rat was not required to lick during the control (S−) trials and therefore was free to leave the odor port once the decision was made.
Trials were presented in blocks of 20 (10 S+ and 10 S−). Within each block, the sequence of the 20 trials was quasirandom such that each stimulus was limited to 3 consecutive presentations. The percent correct was calculated (for both correct detection and correct rejection) individually for each block. Initial discrimination training consisted of 10 blocks (200 trials). Rats achieved criterion performance (85% or greater) within 2–4 blocks. The following training session consisted of a new target odorant (1% v/v vanilla extract). During this training session, rats were required to respond to the new target odorant, while ignoring the control odorant (1% v/v coconut). A final training session consisted of 1 parts per million (ppm) (10−4% v/v) orange extract as the target (S+) odorant and 1 ppm (10−4% v/v) vanilla extract as the control (S−) odorant. These additional sessions were incorporated to ensure that the rats would have sufficient experience with the behavioral paradigm to begin testing on homologous alcohol pairs, rather than to anticipate an odorless control. Further, the additional training ensured the rats could form reward–response associations with new target odorants daily, while simultaneously ignoring/inhibiting responses to a previously learned target odorant. Because the rats would encounter the alcohol test odorants under both conditions (target and control), it was important to acclimate them to potential shifts in reward associations between sessions.
Discrimination testing
There was a total of 15 separate discrimination test sessions. Table 1 shows the order of behavioral testing for the individual discrimination pairs. Only 1 odorant pair was tested daily. Order of presentation was randomized across rats. A total of 6 aliphatic alcohols were tested, ranging from 3 to 8 carbon atoms (C3–C8). The compounds were then combined to create 15 combinations (an additional 15 odorant pairings could be presented by reversing the valence [as S+ or S−] of the odorants, but the present study focused on initial generalizability and not reversals). These combinations were then subcategorized on the basis of the carbon atom difference between the odorants: Δ1 (n = 5 discrimination pairs), Δ2 (n = 4 discrimination pairs), Δ3 (n = 3 discrimination pairs), Δ4 (n = 2 discrimination pairs), and Δ5 (n = 1 discrimination pair). Odorant pairs were pseudorandomized such that no single odorant was repeated on consecutive days. This approach was designed to minimize formation of odor–reward associations with a given odorant. To further decrease possible biases, individual odorants were counterbalanced to approximate an even number of target (S+) and control (S−) pairings. Prior to each testing session, rats were exposed to 40 shaping trials. Similar to the training session described above, rats were reinforced for licking in the presence of the target odorant. This initiating procedure enabled sufficient time for the rats to acclimate to the expected target odorant, as well as confirm appropriate levels of motivation to perform. Were a rat to fail to achieve 85% correct responses during these introductory shaping trials, indicating problematic motivational state, potential olfactometer contamination, or other technical issues, testing would not proceed. However, no such instance was observed. During testing sessions, rats were required to complete a total of 200 trials (100 S+ trials and 100 S− trials). Accuracy, total errors (categorized as either misses or false alarms), and the percentage of 100ms intervals with a lick during S+ and S− trials were calculated for individual rats during each testing session. Similar to the training procedures described above, individual trials were partitioned by odor sampling time in 100ms bins. Contact with the lick tube for at least 7/10 of the time bins (response intervals) was considered the go/no-go criterion for defining a response.
Table 1.
Individual odorant pairs
| Discrimination pairs | Carbon length | Δ Carbons |
|---|---|---|
| Butanol–Propanol | 4 C vs. 3 C | 1 |
| Pentanol–Butanol | 5 C vs. 4 C | 1 |
| Hexanol–Pentanol | 6 C vs. 5 C | 1 |
| Heptanol–Hexanol | 7 C vs. 6 C | 1 |
| Octanol–Heptanol | 8 C vs. 7 C | 1 |
| Propanol–Pentanol | 3 C vs. 5 C | 2 |
| Butanol–Hexanol | 4 C vs. 6 C | 2 |
| Pentanol–Heptanol | 5 C vs. 7 C | 2 |
| Hexanol–Octanol | 6 C vs. 8 C | 2 |
| Hexanol–Propanol | 6 C vs. 3 C | 3 |
| Heptanol–Butanol | 7 C vs. 4 C | 3 |
| Octanol–Pentanol | 8 C vs. 5 C | 3 |
| Propanol–Heptanol | 3 C vs. 7 C | 4 |
| Butanol–Octanol | 4 C vs. 8 C | 4 |
| Octanol–Propanol | 8 C vs. 3 C | 5 |
Control procedures
To minimize possible detection of subtle airflow or auditory cues, unused odorant valves (i.e., those not controlling delivery of either S+ or S− stimuli) were randomly activated across conditions to provide a “masking” noise. Locations of saturation bottles were pseudorandomized across sessions. Control tests were conducted to determine whether inadvertent odorant or nonodorant cues were available to the rats as discriminative cues. These tests were administered by replacing the target (S+) odorant bottle with the diluent alone. In this case, both the S+ and S− saturation bottles contained identical volumes of the control (S−) stimulus. A second, quick control check was also conducted by simply pinching off the S+ saturator bottle tubes during an S+ trial. Under both control conditions, trained rats performed at chance levels, indicating a lack of reliable discrimination cues. Finally, to ensure that the rats were responding only to presence of airflow (odor), an additional control measure consisted of disconnecting the stimulus stream. Under this condition, rats would initiate a trial, but receive no airflow. In the absence of airflow, rats consistently refrained from responding.
Stimuli
Vanilla extract (35% ethanol), coconut extract (25% ethanol), and orange extract (25% ethanol), purchased in bulk (Gordon Food Service), served as the initial training mixtures. A series of 6 aliphatic alcohol odorants with carbon chain lengths C3–C8 were used as test odorants in this study: 1-propanol (CAS #71-23-8), 1-butanol (CAS #71-36-3), 1-pentanol (CAS #71-41-0), 1-hexanol (CAS #111-27-3), 1-heptanol (CAS #111-70-6), and 1-octanol (CAS #111-87-5). All compounds were obtained from Sigma-Aldrich and contained a nominal purity of at least 99%. Diethyl phthalate was used as the near-odorless diluent for all experiments (Güven and Laska 2012). As described in previous behavioral experiments (cf., Laska et al. 2006, 2008; Güven and Laska 2012), the rationale for choosing these odorants was to assess perceptual similarity using a homologous series of primary aliphatic alcohols sharing the same functional group, but differing in carbon chain length. Further, these compounds have been shown to activate molecular, feature-specific domains in the glomerular sheet, collectively clustering in the lateral domain of the rat olfactory bulb (Uchida et al. 2000). The primary aliphatic alcohols used here have therefore been well characterized both physiologically and behaviorally.
For all discrimination pairs, odorants were presented at 1 ppm liquid concentration (equivalent to 0.0001% v/v). Odorant concentrations are described in terms of liquid dilution, though the odor concentration experienced by the rats was ~2.5% of the liquid concentration prepared in the saturation bottles (Slotnick 2007). One parts per million concentration was selected on the basis of previous studies (Laska et al. 2008) indicating this concentration is low enough to cause discrimination errors but high enough to compensate for any variability in odor sensitivity between rats. Finally, threshold testing in mice suggests these stimuli are perceived with similar intensities at 1 ppm presentation (Laska et al. 2006, 2008).
The stock odorants, once opened, were stored under inert gas (nitrogen) in glass and refrigerated to prevent oxidation. Serial dilutions of the target and control odorants were prepared using diethyl phthalate as a diluent. Ten milliliters of the liquid phase (1-propanol, 1-butanol, 1-pentanol, 1-hexanol, 1-heptanol, 1-octanol) odorant, placed in a 500-mL glass saturation jar, served as either the target or control stimulus. The olfactometer functioned by use of digitally controlled solenoid pinch valves, which briefly bubbled the stimulus air stream through a tube submerged in the liquid phase odorant to produce a volatilized stimulus that filled the headspace before introduction into the carrier stream and presentation to the rat.
Statistical analyses
Correlations between discrimination performance and structural similarity of odorants in terms of differences in carbon chain length were evaluated using the Spearman rank correlation coefficient (SigmaPlot, Systat Software Inc.). Correlations were calculated for multiple behavioral parameters including percent correct, total misses, total false alarms, lick patterns (based on contact during individual time bins) on S+ trials, and lick patterns on S− trials. Data for all odorant pairs were analyzed across individual rats with 2-way analysis of variance (ANOVA; Factor 1: Individual Rat, Factor 2: ∆C). Following 2-way ANOVA testing, all pairwise multiple comparison post hoc tests (Tukey’s Honestly Significant Difference) were performed to determine significant differences. Level of significance was set to 0.05 and all tests were 2 tailed.
Results
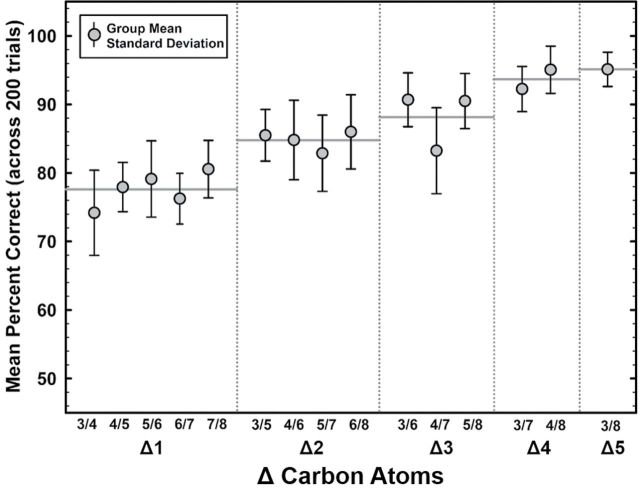
Eight rats were trained and tested on each of the aliphatic alcohol pairs, yielding a data set of 3000 trials per rat. Figure 1 shows group mean response accuracy across the 15 odorant pairs as a function of the difference in carbon atoms (ΔC) between the 2 odorants. Discrimination accuracy is displayed as the average percent correct across 200 total trials per rat. All 8 rats acquired discrimination performance levels above chance for the most difficult Δ1 odorant pairs, and accuracy increased systematically as a function of carbon chain length (ΔC). As the difference in ΔC between the pairs increased, consistently better performance was achieved, from a mean of 77.6% performance for Δ1, 84.8% for Δ2, 88.1% for Δ3, 93.7% for Δ4, and 95.1% for Δ5. Interindividual variability for discrimination accuracy was low across all tasks, though variability decreased as ΔC and odor quality differences between the pairs increased. There was a significant positive correlation between performance accuracy and ΔC for the aliphatic alcohols tested (Spearman, r s = 0.886, P < 0.001). Given this pattern, it can be inferred that carbon chain length is a determinant of stimulus receptor activation (Johnson and Leon 2000). Despite this strong correlation, however, variability between individual pairs differing by the same ΔC suggests that other stimulus properties must also play a role.
Figure 1.
Accuracy of 8 F344 rats in pairwise discriminations of a homologous series of aliphatic alcohols, expressed as the difference in carbon atoms. Each data point represents mean response accuracy from a total of 200 trials each for all 8 rats. Error bars indicate standard deviation. Horizontal solid gray lines represent mean of all pairwise comparisons for same ΔC: (Δ1, 5 pairs total, n = 40); (Δ2, 4 pairs total, n = 32); (Δ3, 3 pairs total, n = 24); (Δ4, 2 pairs total, n = 16); (Δ5, 1 pair total, n = 8). Numbered pairs refer to the names of aliphatic alcohols provided in Table 1.
Mean response accuracy as a function of ΔC was compared for each pairwise discrimination. Repeated measures ANOVA did not show significant differences between mean discrimination accuracy for any of the Δ3, Δ4, or Δ5 pairwise discriminations, suggesting these pairs were similarly grouped in terms of discrimination difficulty level. Overall, Δ1 pairwise comparisons displayed significant performance variability across pairs. ANOVA tests revealed a main effect of rat, such that certain rats consistently performed worse than others across all the discrimination pairs [F(7, 119 = 4.716), P < 0.001]. In addition, there was a main effect of ΔC, such that accuracy changed systematically as a function of ΔC [F(4, 119) = 61.589, P < 0.001]. There was no rat × ΔC interaction [F(28, 199) = 0.823, P = 0.713]. Pairwise multiple comparisons showed significant differences between accuracy on Δ1 discriminations and all other ΔC discriminations (P < 0.001). Δ2 discriminations were significantly different from all other ΔC discriminations (P < 0.001 for Δ1, Δ4, Δ5; P = 0.009 for Δ3). Likewise, Δ3 discriminations were significantly different from all other ΔC discriminations (P < 0.001 for Δ1, Δ4, Δ5; P = 0.009 for Δ2). Δ4 and Δ5 were not significantly different (P = 0.427).
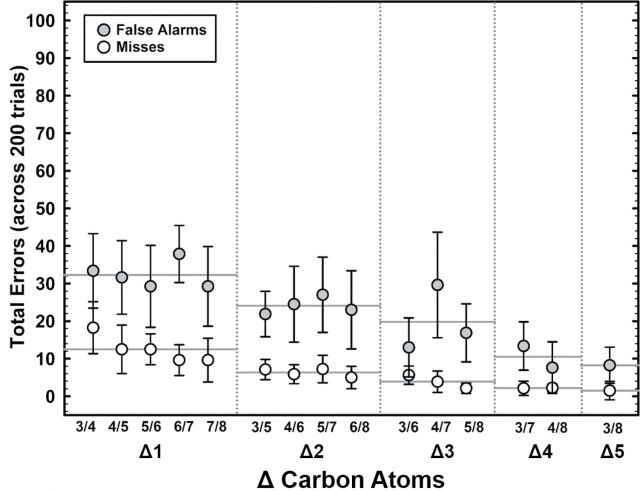
To facilitate analysis of the effects of ΔC on pairwise errors, errors were subsequently divided into misses and false alarms. Figure 2 shows the mean error rates for the 8 rats tested as a function of ΔC. In general, and not surprisingly, errors decreased with ΔC. Despite relatively few misses overall, a systematic decrease in misses was observed with increasing ΔC, as odorant pairs became easier to discriminate. Across all pairwise discriminations, rats made fewer misses than false alarms, suggesting the rats adopted the most advantageous reinforcement strategy regardless of discrimination difficulty, by responding most often to S+ stimuli. A significant negative correlation was found for misses (Spearman, r s = −0.74, P < 0.001) and false alarms (Spearman, r s = −0.79, P < 0.001).
Figure 2.
Discrimination errors for 8 F344 rats in pairwise discriminations of a homologous series of aliphatic alcohols, expressed as the ΔC. Filled circles represent mean false alarms, and open circles represent misses. Error bars indicate standard deviation. Horizontal solid gray lines represent group means collapsed across the columns: (Δ1, 5 pairs total, n = 40); (Δ2, 4 pairs total, n = 32); (Δ3, 3 pairs total, n = 24); (Δ4, 2 pairs total, n = 16); (Δ5, 1 pair total, n = 8). Numbered pairs refer to the names of aliphatic alcohols provided in Table 1.
Discrimination behaviors were also analyzed by monitoring the number of contact responses with the lick spout for each individual stimulus presentation. Licking behaviors for target (S+) and control (S−) trials were averaged across 200 trials per subject. A significant positive correlation was found between ΔC and percentage of time bins contacted during S+ trials (Spearman, r s = 0.727, P < 0.001). Licking to both false alarms and to misses (infrequent) decreased with increased ΔC. Although rats could theoretically lick up to 6/10 of the time bins on S− trials and still receive no penalty, these data show that none of the rats adopted such a strategy. Rather, when compared with Figure 2, lick patterns on (S−) trials suggest that rats generally only licked when they confused the control (S−) stimulus with the target odorant (S+). After initial training, the rats extracted their heads from the sniffing port fairly rapidly once they judged the stimulus presentation was not the target odor. Indeed, a significant positive correlation was found between false alarms and lick intervals on (S−) trials (Spearman, r s = 0.819, P < 0.001). Except in the case of infrequent misses, rats generally maintained contact with the lick tube for all (S+) trials. Even when presented with perceptually similar odor pairs (i.e., Δ1 or Δ2), rats typically made few misses, the strategy most advantageous for reinforcement. For this reason, both false alarms and licking on (S−) trials yielded comparatively steeper gradients of stimulus generalization. Nevertheless, a significant negative correlation was found between misses and (S+) lick intervals (Spearman, r s = −0.684, P < 0.001). A significant negative correlation was also found for the percentage of time bins contacted during S− trials (Spearman, r s = −0.851, P < 0.001).
Discussion
The present study evaluated performance on olfactory discriminations among structurally related odorants in F344 rats. A series of 6 homologous aliphatic alcohols sharing the same functional group, but varying from 3 to 8 carbon atoms in length, were used to test 15 pairwise discriminations for each rat. All rats learned to discriminate between all aliphatic alcohols, suggesting pairs were sufficiently different to allow discrimination at the concentration presented. Consistent with previous studies, performance on pairwise discriminations was an orderly function of relative difference in the number of carbon atoms between the 2 odorants presented (Linster and Hasselmo 1999; Laska et al. 2008). Overall, the poorest performance was indicated for Δ1 (C3 vs. C4; 1-propanol vs. 1-butanol), with rats displaying a mean accuracy of ~74%. Conversely, mean performance for Δ5 (C3 vs. C8; 1-propanol vs. 1-octanol) was ~95% (Figure 1). Also in line with previous work, the present study supports the hypothesis that perceptual similarity predicts performance on odor-guided measures (Youngentob et al. 2006). In accordance with results from previous studies in other mammals (Laska and Teubner 1999; Linster and Hasselmo 1999; Laska and Seibt 2002; Laska et al. 2006, 2008; Güven and Laska 2012; Rizvanovic et al. 2013), these data show a negative correlation between ΔC and discrimination performance. To provide for additional comparisons between odor stimuli and behavior beyond simple discrimination accuracy, data were analyzed for error response type and lick patterns. Both false alarms and misses progressively decreased as ΔC increased (Figure 2). The systematic shift in error rate was more pronounced for false alarms. Given that rodents have been shown to selectively avoid responding on overly difficult trials (Carandini and Churchland 2013), the observed error patterns suggest that the rats were nevertheless sufficiently motivated and responded to each trial during the challenging pairwise discrimination presentations.
Although many previous studies employing carbon chain discrimination paradigms have used a single exemplar as the standard target (S+) (cf., Laska et al. 2008), the present study examined all possible pairwise discriminations for each ΔC. Given that training can refine olfactory representations (Fletcher and Wilson 2002; Takiguchi et al. 2008), the current approach sought to minimize potential learning effects by arranging the target (S+) and control (S−) odorants in a pseudorandomized manner. Although Laska and colleagues (2008) did report an increase in discriminability with increasing ΔC in mice for 2-ketones and acid esters, they did not find a statistically significant correlation between ΔC and discrimination performance for aliphatic alcohols, n-aldehydes, or carboxylic acids. To date, their failure to find the negative correlation between ΔC and discrimination performance for aliphatic alcohols, n-aldehydes, or carboxylic acids in mice are the first of which we are aware for any species tested to show this relationship. The reasons for this discrepancy are unclear. Although the authors attributed this finding primarily to the odor concentrations used (1 ppm), they also suggested that noted similarities in glomerular activation patterns for related odorants, primarily from the rat, may fail to generalize to mice (Johnson and Leon 2000; Xu et al. 2003; Niimura and Nei 2006). Why these patterns might hold true for some odorants (2-ketones and acid esters), but not others (aliphatic alcohols, n-aldehydes, or carboxylic acids) is also unclear. Nevertheless, other studies have shown similar neural activation patterns in the olfactory bulbs of rats and mice (Soucy et al. 2009).
Conversely, several experimental distinctions between the mouse study and ours may account for the inconsistency. First, each test group from the previous study contained only 2 mice. Additionally, given the large number of odorants tested in their study, it was necessary to present odorants sequentially during a single test session to maximize efficiency. Under these conditions, only the S− (control) stimuli varied between conditions; the mice therefore had greater exposure to the reinforcing (S+) stimulus. Importantly, sequential presentation has been shown to elicit response biases (Doty et al. 2003). It is clear from their data (see Figure 2) that a significant learning effect was observed between the first 20 trials and the last 5 blocks of 20 trials (i.e., over 100 trials). We attempted to avoid this limitation by randomly testing all rats on all pairwise comparisons, in which both odorants served as the target (S+) and the control (S−). It should be noted, however, that a methodological strength of the mouse discrimination study was the use of automated olfactometers (Laska et al. 2008). The automated approach allowed for more precise control of stimulus delivery and measurement of behavioral responses. The present study also employed a computer-based olfactometer to strictly control behavioral parameters, stimulus timing, and delivery, while also providing computational advantages not previously assessed in the F344 rat model.
Overall, the behavioral results described above correspond well with previous reports of the relationships between chemical structure, behavioral responses, and glomerular activation patterns seen in the olfactory bulb (Linster and Hasselmo 1999). Numerous studies have shown behaviorally that perceptual odor quality is influenced by functional group and carbon chain length in both invertebrates (Guerrieri et al. 2005) and mammals (Laska et al. 1999; Linster et al. 2001; Cleland et al. 2002; Laska et al. 2008; Boesveldt et al. 2010). Such findings have been further strengthened by electrophysiological recordings of mitral cell responses (Imamura et al. 1992; Mori et al. 1992; Katoh et al. 1993), intrinsic signal imaging of the rat olfactory bulb (Uchida et al. 2000; Soucy et al. 2009; Matsumoto et al. 2010), and radiolabeled 2-deoxyglucose uptake in the rat olfactory bulb (Sharp et al. 1977; Johnson et al. 2009). Although the neurobiology of olfactory coding is not fully understood, it is generally accepted that aliphatic alcohols activate overlapping patterns in the rat olfactory bulb based on their chemical class and shared hydroxyl (–OH) group (Mori et al. 2006; Johnson and Leon 2007). Within functional groups, increasing the number of carbon atoms generates a spatial progression in glomerular activation patterns (Uchida et al. 2000), suggesting that carbon chain length may be a key determinant of odor coding.
Beyond confirming the influence of carbon chain length on odor discrimination shown in previous studies, the present study sought to establish baseline olfactory performance for the F344 rat strain. This strain has become increasingly recognized as a model of cognitive aging because subsets of these rats develop hippocampal-dependent cognitive impairments around 22 months of age. A correlation has been identified between these cognitive deficits and olfactory impairments (LaSarge et al. 2007). Nevertheless, the olfactory assessment previously used involved a digging task with complex mixtures and limited stimulus control. To investigate the relationship between olfactory performance and cognitive decline more thoroughly, it will be necessary to comprehensively assess olfactory performance in this strain. Importantly, because the present data support findings from previous studies utilizing similar techniques and stimuli, we can conclude that the F344 rat model displays olfactory behavior consistent with other rat strains.
Although there is growing interest surrounding the age-related, cognitive deficits displayed in this rat model, 1 potential limitation concerns stimulus novelty, particularly when longitudinal designs are incorporated. A key issue will involve identifying the most efficacious stimuli for producing reliable performance, while not repeating the stimulus sets. It should be noted that all of the compounds were repeated. Although stimulus pairs were never repeated, the rats did encounter the alcohols multiple times throughout the experiment. While presenting the alcohols several times throughout the course of the experiment still showed a ΔC trend, the differences might be refined by limiting presentations to 1 session and fewer trials. Given that the focus of this strain is on individual variability at later ages, it will be necessary to incorporate modifications that will maximize such differences. Nevertheless, because there were no baseline data in the F344 rat model using monomolecular odorants or precise stimulus control, we selected 6 aliphatic alcohols previously shown to be effective in odor discrimination tasks. Importantly, however, in the age group tested here (14 months), minimal variability was shown between rats, suggesting relatively stable performance at younger ages. In addition to broadening the scope of stimuli tested and minimizing learning effects, it may be useful to make the discriminations more difficult. Using vapor pressure to match the intensity of the stimuli could be 1 potential option. Given the 1 ppm concentration used here, however, the effect of vapor pressure would likely be marginal. Another option would be to obtain individual, odor detection thresholds for each rat on all compounds tested to match the perceived intensity. Incorporating such a protocol may be additionally necessary to account for potential age-related differences in odor sensitivity (rather than discrimination).
These generalization gradients are intended to provide an early framework for evaluating odor-guided behavioral patterns across structurally related odors in F344 rats. Incorporating olfactory paradigms with graded perceptual similarity tasks may reveal subtle behavioral differences overlooked using previous “digging” techniques (LaSarge et al. 2007). Notably, the ability to detect minor deviations between groups may have substantial implications for detecting subtle, underlying disruptions in odor processing. As a recent example, Hellier and colleagues (2010) investigated the contribution of α7-nicotinic acetylcholine receptors to olfactory dysfunction in mice. Using 1% concentrations of 1-heptanol versus 1-octanol (Δ1), differences in odor discrimination performance were found between α7 knockout mice and wild-type mice. Notably, in the same mice, substantial differences were not evident with more dissimilar odorants.
In the present study, our intention was to characterize the effects of carbon atom difference on odor discriminations in the F344 rat model, as a means of developing baseline measures against which subtle, early, age-related changes in olfaction can be assessed. Recent work by LaSarge et al. (2007) showed that changes in olfactory behavior correlate with cognitive decline in the F344 rat. By yielding sensitive performance differences, this automated technique may be useful for understanding how olfactory circuits operate and how they become disrupted in humans. Because odor-evoked patterns in the piriform cortex do not show chemotopic organization (Stettler and Axel 2009), this approach may also be useful for isolating olfactory bulb alterations from those farther downstream.
Funding
National Institutes of Health (R01 AG024671) and the McKnight Brain Research Foundation.
Acknowledgements
We wish to thank S. Srinivasan and S. Currlin for assistance in conducting these studies.
References
- Abraham NM, Guerin D, Bhaukaurally K, Carleton A. 2012. Similar odor discrimination behavior in head-restrained and freely moving mice. PLoS One. 7:e51789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodyak N, Slotnick B. 1999. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem Senses. 24:637–645 [DOI] [PubMed] [Google Scholar]
- Boesveldt S, Olsson MJ, Lundström JN. 2010. Carbon chain length and the stimulus problem in olfaction. Behav Brain Res. 215:110–113 [DOI] [PubMed] [Google Scholar]
- Carandini M, Churchland AK. 2013. Probing perceptual decisions in rodents. Nat Neurosci. 16:824–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. 2002. Behavioral models of odor similarity. Behav Neurosci. 116:222–231 [DOI] [PubMed] [Google Scholar]
- Cleland TA, Narla VA, Boudadi K. 2009. Multiple learning parameters differentially regulate olfactory generalization. Behav Neurosci. 123:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. 2008. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol. 63:7–15 [DOI] [PubMed] [Google Scholar]
- Doty RL, Diez JM, Turnacioglu S, McKeown DA, Gledhill J, Armstrong K, Lee WW. 2003. Influences of feedback and ascending and descending trial presentations on perithreshold odor detection performance. Chem Senses. 28:523–526 [DOI] [PubMed] [Google Scholar]
- Duda JE. 2010. Olfactory system pathology as a model of Lewy neurodegenerative disease. J Neurol Sci. 289:49–54 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. 1998. Using olfaction to study memory. Ann N Y Acad Sci. 855:657–669 [DOI] [PubMed] [Google Scholar]
- Falasconi M, Gutierrez-Galvez A, Leon M, Johnson BA, Marco S. 2012. Cluster analysis of rat olfactory bulb responses to diverse odorants. Chem Senses. 37:639–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M, Wilson D. 2002. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. J Neurosci. 22:RC201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick DE, Rojas-Líbano D, Scott M, Kay LM. 2011. Rat behavior in go/no-go and two-alternative choice odor discrimination: differences and similarities. Behav Neurosci. 125:588–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble KR, Smith DW. 2009. Discrimination of “odorless” mineral oils alone and as diluents by behaviorally trained mice. Chem Senses. 34:559–563 [DOI] [PubMed] [Google Scholar]
- Guerrieri F, Schubert M, Sandoz JC, Giurfa M. 2005. Perceptual and neural olfactory similarity in honeybees. PLoS Biol. 3:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güven S, Laska M. 2012. Olfactory sensitivity and odor structure-activity relationships for aliphatic carboxylic acids in CD-1 mice. PLoS One. 7:e34301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier JL, Arevalo NL, Blatner MJ, Dang AK, Clevenger AC, Adams CE, Restrepo D. 2010. Olfactory discrimination varies in mice with different levels of α7-nicotinic acetylcholine receptor expression. Brain Res. 1358:140–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SL, Johnson BA, Chen AL, Leon M. 2006. Differential responses to branched and unsaturated aliphatic hydrocarbons in the rat olfactory system. J Comp Neurol. 499:519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Mataga N, Mori K. 1992. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. I. Aliphatic compounds. J Neurophysiol. 68:1986–2002 [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. 2000. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 422:496–509 [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. 2007. Chemotopic odorant coding in a mammalian olfactory system. J Comp Neurol. 503:1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ong J, Leon M. 2010. Glomerular activity patterns evoked by natural odor objects in the rat olfactory bulb are related to patterns evoked by major odorant components. J Comp Neurol. 518:1542–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Xu Z, Ali SS, Leon M. 2009. Spatial representations of odorants in olfactory bulbs of rats and mice: similarities and differences in chemotopic organization. J Comp Neurol. 514:658–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Koshimoto H, Tani A, Mori K. 1993. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. II. Aromatic compounds. J Neurophysiol. 70:2161–2175 [DOI] [PubMed] [Google Scholar]
- LaSarge CL, Montgomery KS, Tucker C, Slaton GS, Griffith WH, Setlow B, Bizon JL. 2007. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiol Aging. 28:928–936 [DOI] [PubMed] [Google Scholar]
- Laska M, Galizia CG, Giurfa M, Menzel R. 1999. Olfactory discrimination ability and odor structure-activity relationships in honeybees. Chem Senses. 24:429–438 [DOI] [PubMed] [Google Scholar]
- Laska M, Rivas Bautista RM, Hernandez Salazar LT. 2006. Olfactory sensitivity for aliphatic alcohols and aldehydes in spider monkeys (Ateles geoffroyi). Am J Phys Anthropol. 129:112–120 [DOI] [PubMed] [Google Scholar]
- Laska M, Rosandher A, Hommen S. 2008. Olfactory discrimination of aliphatic odorants at 1 ppm: too easy for CD-1 mice to show odor structure-activity relationships? J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 194:971–980 [DOI] [PubMed] [Google Scholar]
- Laska M, Seibt A. 2002. Olfactory sensitivity for aliphatic alcohols in squirrel monkeys and pigtail macaques. J Exp Biol. 205:1633–1643 [DOI] [PubMed] [Google Scholar]
- Laska M, Teubner P. 1999. Olfactory discrimination ability for homologous series of aliphatic alcohols and aldehydes. Chem Senses. 24:263–270 [DOI] [PubMed] [Google Scholar]
- Linster C, Hasselmo ME. 1999. Behavioral responses to aliphatic aldehydes can be predicted from known electrophysiological responses of mitral cells in the olfactory bulb. Physiol Behav. 66:497–502 [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Yue E, Morse A, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. 2001. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 21:9837–9843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon N, Peace ST, Boudadi K, Boxhorn CE, Narla VA, Suffis SD, Cleland TA. 2011. Compensatory responses to age-related decline in odor quality acuity: cholinergic neuromodulation and olfactory enrichment. Neurobiol Aging. 32:2254–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Kobayakawa K, Kobayakawa R, Tashiro T, Mori K, Sakano H, Mori K. 2010. Spatial arrangement of glomerular molecular-feature clusters in the odorant-receptor class domains of the mouse olfactory bulb. J Neurophysiol. 103:3490–3500 [DOI] [PubMed] [Google Scholar]
- Mori K, Mataga N, Imamura K. 1992. Differential specificities of single mitral cells in rabbit olfactory bulb for a homologous series of fatty acid odor molecules. J Neurophysiol. 67:786–789 [DOI] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. 2006. Maps of odorant molecular features in the Mammalian olfactory bulb. Physiol Rev. 86:409–433 [DOI] [PubMed] [Google Scholar]
- Niimura Y, Nei M. 2006. Evolutionary dynamics of olfactory and other chemosensory receptor genes in vertebrates. J Hum Genet. 51:505–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C, Felsen G. 2012. Rats can make relative perceptual judgments about sequential stimuli. Anim Cogn. 15:473–481 [DOI] [PubMed] [Google Scholar]
- Rizvanovic A, Amundin M, Laska M. 2013. Olfactory discrimination ability of Asian elephants (Elephas maximus) for structurally related odorants. Chem Senses. 38:107–118 [DOI] [PubMed] [Google Scholar]
- Schaefer AT, Margrie TW. 2012. Psychophysical properties of odor processing can be quantitatively described by relative action potential latency patterns in mitral and tufted cells. Front Syst Neurosci. 6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Kauer JS, Shepherd GM. 1977. Laminar analysis of 2-deoxyglucose uptake in olfactory bulb and olfactory cortex of rabbit and rat. J Neurophysiol. 40:800–813 [DOI] [PubMed] [Google Scholar]
- Shepard RN. 1987. Toward a universal law of generalization for psychological science. Science. 237:1317–1323 [DOI] [PubMed] [Google Scholar]
- Slotnick B. 2007. Odor-sampling time of mice under different conditions. Chem Senses. 32:445–454 [DOI] [PubMed] [Google Scholar]
- Smith DW, Thach S, Marshall EL, Mendoza MG, Kleene SJ. 2008. Mice lacking NKCC1 have normal olfactory sensitivity. Physiol Behav. 93:44–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. 2009. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 12:210–220 [DOI] [PubMed] [Google Scholar]
- Stettler DD, Axel R. 2009. Representations of odor in the piriform cortex. Neuron. 63:854–864 [DOI] [PubMed] [Google Scholar]
- Takiguchi N, Okuhara K, Kuroda A, Kato J, Ohtake H. 2008. Performance of mice in discrimination of liquor odors: behavioral evidence for olfactory attention. Chem Senses. 33:283–290 [DOI] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. 2000. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 3:1035–1043 [DOI] [PubMed] [Google Scholar]
- Wesson DW, Donahou TN, Johnson MO, Wachowiak M. 2008. Sniffing behavior of mice during performance in odor-guided tasks. Chem Senses. 33:581–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Levy E, Nixon RA, Wilson DA. 2010. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J Neurosci. 30:505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Liu N, Kida I, Rothman DL, Hyder F, Shepherd GM. 2003. Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc Natl Acad Sci U S A. 100:11029–11034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Johnson BA, Leon M, Sheehe PR, Kent PF. 2006. Predicting odorant quality perceptions from multidimensional scaling of olfactory bulb glomerular activity patterns. Behav Neurosci. 120:1337–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]