Abstract
MDS are myeloid clonal hematologic disorders that are most commonly diagnosed in the seventh decade of life. Several treatment options are currently available. However, allo HSCT remains the only curative therapy. Unfortunately, despite the higher incidence of MDS in the older population, less than 10 % of patients undergoing allo HSCT for MDS are > 65 years old. In this paper we discuss the various treatment options in older patients with high-risk MDS with particular emphasis on the role of allo HSCT in older MDS patients.
Keywords: MDS, Allogeneic hematopoietic stem cell transplantation, Age, Comprehensive geriatric assessment, Hypomethylating agents
Introduction
MDS are a group of clonal hematologic disorders diagnosed most often in older white males and characterized by progressive cytopenias with risk of leukemic transformation [1]. Estimates of MDS incidence from population-based cancer registries suggest there are 10,000 new cases of MDS in the US each year, with approximately 70 % of these diagnosed in persons age 70 years or older [2]. However, accurate registry-based estimates of MDS incidence are difficult to obtain due to under-appreciation of MDS as a possible cause of unexplained anemia in older patients [3], and incomplete reporting of cases that are managed outside the tertiary care environment or that rapidly transform into frank leukemias [4]. Despite difficulty estimating the true incidence of MDS [4, 5], it is expected that MDS is likely to become more common as life expectancies increase [6]. Therefore, there is an increasing emphasis on developing MDS therapies [1].
Risk of all-cause mortality as well as progression to acute myelogenous leukemia (AML) is most commonly assigned by applying the International Prognostic Scoring System (IPSS), which takes into account the bone marrow blasts percent, the number of peripheral blood cytopenias, and karyotype findings [7]. The IPSS separates patients into four different prognostic groups: Low, intermediate-1, intermediate-2 and high-risk. Median survival for these groups is 5.7, 3.5, 1.2 and 0.4 years, respectively [7]. Recently the IPSS was revised to incorporate a newer cytogenetic classification, depth of cytopenias and a new bone marrow blast percentage classification [8•].
At present, there are a wide range of therapeutic approaches for MDS, which are selected based on the patient’s risk stratification at diagnosis [7-14]. However, to date, only one potentially curative therapy exists: allo HSCT. Despite its curative potential, the perceived risks associated with allo HSCT limit its availability to the majority of the patient population, who are older patients with a high likelihood of presenting with comorbidities [15]. In a recent query of transplant activity reported to the Center for International Blood & Marrow Transplant Research (CIBMTR), for patients with MDS, only 232 (7.5 %) out of a total of 3,101 allo HSCTs performed in the US between 2000 and 2010 were among patients 65 years and older (unpublished data; W.S., personal communication). Furthermore, current treatment guidelines recommend proceeding immediately to allo HSCT, but only for persons with high-risk disease, who are felt to be good candidates for allo HSCT (based on age and performance status among other factors), and for whom a suitable donor is available [16].
However, a changing perception of the importance of age in referral for allo HSCT [17•, 18•, 19], revisions in the approach to allo HSCT [e.g. introducing reduced intensity conditioning regimens (RIC)] [20-23], increased coverage for the procedure by Medicare [24], and newly launched research into the efficacy of allo HSCT in older patients [19, 25] has the potential to expand the availability of this potentially curative therapy to more patients in the future.
Here we review emerging tools to quantify the impact of age and comorbidity on non-transplant as well as allo HSCT outcomes. We then discuss contemporary non-transplant treatment options for older MDS patients, and finally we focus on the emerging role of allo HSCT among this patient population.
The Impact of Age and Aging on Clinical Outcomes
Two recent retrospective studies addressed whether age is an important predictor of outcome among patients with MDS who are referred for allo HSCT [17, 18]. Lim et al. analyzed outcomes of 1,333 MDS patients that were older than 50 years, and underwent allo HSCT (62% received RIC) between 1998 and 2006 and were reported to the European Group for Blood and Marrow Transplantation (EBMT). In multivariate analysis, age [> 60 years (n=449) vs. 50–60 (n=884)] was not a significant predictor of survival post allo HSCT [relative risk (RR) 1.0; 95 % confidence intervals (CI) 0.90–1.27] [17]. McClune et al. analyzed data reported to the CIBMTR on 535 MDS patients older than 40 years, who underwent RIC allo HSCT between 1995 and 2005. Four age groups were compared [40–54 (n=208), 55–59 (n=146), 60–64 (n=126), and ≥ 65 years (n=55)], and in multivariate analysis 2-year survival was not significantly different among the four age groups (p=0.74) [18]. Furthermore, a recent interim analysis of an ongoing prospective observational study of outcomes after allo HSCT showed no difference in 100-day mortality among patients aged 55–65 compared to those > 65 years old supporting the safety of this procedure among older MDS patients [19]. However, it is important to note that these studies were conducted among patients who were referred and subsequently underwent allo HSCT, a highly select group of patients. Therefore, whether these results can be extended to all MDS patients (at the time of their initial diagnosis and not referral) is currently unknown.
The specific factors of aging that determine prognosis and tolerance to treatment are not well defined. The process of aging is heterogeneous. It involves physiologic changes as well as changes in physical, functional, social, psychiatric and cognitive domains. The Comprehensive Geriatric Assessment (CGA), a tool developed by geriatricians to help identify areas of vulnerability in these domains, can be used to “stage the age” of older patients [26]. Specifically, in older oncology patients, it can be used to identify those who appear healthy but may be susceptible to severe complications in response to aggressive treatments [27]. The CGA is often used to stratify patients into three categories: fit, vulnerable and frail. In evaluation of older patients with multiple different types of cancer, the CGA has been found to affect treatment decisions [28], as well as predict chemotherapy toxicities [29], early death [30] and overall survival [31].
While less is known about the use of the CGA in MDS, we do know that comorbidity, one of the standard domains of the CGA, plays an important role in determining outcomes of older patients with MDS. In a retrospective study of 600 MDS patients with a median age of 65 years who mostly received non-transplant therapies (only 8.5 % underwent allo HSCT), comorbidity was measured using the Adult Comorbidity Evaluation-27 (ACE-27) [32]. Using this measure, patients were stratified into four comorbidity groups: none, mild, moderate and severe. Dramatic differences in median survival were observed between these groups. According to ACE-27 categories, median survival was 31.8, 16.8, 15.2 and 9.7 months for those with none, mild, moderate and severe comorbidities, respectively (p<0.001). Patients with severe comorbidity had a 50 % decrease in survival, independent of age and IPSS risk group.
CGA was also evaluated in a prospective cohort study of 195 patients age ≥ 60 years (median 71 years) with MDS or acute myelogenous leukemia (AML) [33]. Patients were grouped according to treatment intensity: best supportive care, hypomethylating agents, or intensive chemotherapy/hematopoietic cell transplantation. Abnormal activities of daily living (ADL) and increased fatigue were highly predictive of overall survival across all of the patient groups [33]. Another prospective pilot study of the CGA in 166 older allo HSCT recipients (median age 58 years, 57 % AML/MDS) was also recently reported [34]. This study demonstrated that the CGA could uncover significant disability that is not identified by traditional oncologic measures. Applying the CGA to those with Zubrod Performance Status of 0, 28 % reported disability, 58 % were pre-frail, 15 % were frail, 35 % reported low physical function and 55 % reported low mental function [34].
Thus, the CGA has the potential to be quite useful in guiding treatment decisions in older patients with MDS, and further study of this important tool is warranted.
Non-Transplant Based Therapies
In the last decade, the Food and Drug Administration approved three new drugs for therapy of MDS: azacitidine (5-azacytidine), decitabine (5-aza-2′-deoxycytidine) and lenalidomide. Both azacitidine and decitabine are hypomethylating agents (HMA) while lenalidomide is a thalidomide analogue [35]. Both HMAs were developed as nucleoside analogs and were synthesized in the early 1960s.
Azacitidine has been evaluated in multiple phase II trials, and two phase III trials (Table 1). In the first randomized phase III trial by Silverman et al., 191 patients were randomized to azacitidine 75 mg/m2 daily for 7 days SQ vs. best supportive care (BSC) in a cross-over design [36]. The median age of the patients was 68 years, with 37 % of patients having either intermediate-2 or high-risk disease by the IPSS. The overall response rate was 60% vs. 5% in the azacitidine arm vs. BSC. Because of the cross-over design, there was no difference in the overall survival; however, the time to AML transformation or death was significantly longer in the azacitidine arm vs. BSC (21 months vs. 13 months, p=0.007). In addition, patients receiving azacitidine had significant improvement in fatigue, physical functioning, dyspnea, and psychosocial distress when compared to those receiving BSC only. Based on that study, the FDA approved azacitidine for the treatment of patients with all subtypes of MDS in 2004.
Table 1.
Phase III trials with hypomethylating agents
| Study (ref). | Median age, years | Hypomethylating agent vs. Comparator | Median survival, months | Crossover allowed | P value |
|---|---|---|---|---|---|
| Silverman et al. [36] | 68 | Azacitidine SQ at 75 mg/m2 ×7d vs. BSC | 20 vs. 14 | Yes | P=0.1 |
| Fenaux et al. [37] | 69 | Azacitidine SQ at 75 mg/m2 ×7d vs. CCR | 24.4 vs. 15 | No | P<0.001 |
| Lubbert et al. [41] | 70 | Decitabine 15 mg/m2 IV over 4 hours every 8 hours for 3 days vs. BSC | 10.1 vs. 8.5 | No | P=0.38 |
| Kantarjian et al. [40] | 70 | Decitabine 15 mg/m2 IV over 3 hours every 8 hours for 3 days vs. BSC | 14 vs. 14.9 | No | P=0.63 |
BSC: best supportive care
CCR: conventional care regimens (best supportive care, low-dose cytarabine, or intensive chemotherapy as selected by investigators before randomisation)
Another study performed in Europe randomized patients with intermediate-2/high-risk MDS to azacitidine 75 mg/m2 daily for 7 days SQ or standard of care regimens [37•]. The standard of care regimens included SQ cytarabine, BSC only or induction chemotherapy. Prior to randomization, the treating physician had to decide on the treatment comparator. A total of 358 patients were randomized and 340 were treated with 175 on the azacitidine arm and 165 on the conventional care regimens (CCR). The median age was 69 years, and 72% of the patients were ≥65 years old. With a median follow up of 21 months, the overall survival was 24 months vs. 15 months in the azacitidine arm vs. CCR (p<0.001). The median time to AML transformation was also significantly prolonged on the azacitidine arm compared to CCR (18 months vs. 11 months, p<0.001). In a further analysis of patients treated with azacitidine treated on the previously mentioned study, patients were categorized into three risk groups (low, intermediate and high) based on performance status, cytogenetic findings, presence of circulating blasts and red blood cell transfusion dependency [38]. After a median follow up of 41.3 months, the median survival of the low, intermediate and high-risk group was 32.1, 15.0 and 6.1 months, respectively. Thirty-four of the 282 patients were still alive at 3 years, but only six patients were still on azacitidine [39].
Decitabine, the other FDA approved drug, was also evaluated in multiple phase II trials and in two phase III trials (Table 1). The first reported phase III trial randomized patients with MDS to decitabine 15 mg/m2 administered three times daily for 3 days or BSC[40]. In that study, a total of 170 patients were enrolled. The median age was 70 years old, and approximately 70 % had intermediate-2/high-risk MDS by IPSS. The overall improvement rates were 30% and 7% in the decitabine and BSC groups, respectively. The median time to AML transformation or death in the decitabine arm vs. the BSC was 12 vs. 8 months, respectively (p=0.16). The median overall survival for responders vs. non-responders was 23 vs. 13 months respectively.
More recently, the same decitabine schedule was compared in patients with intermediate/high-risk MDS by IPSS above the age of 60 years. Patients were randomized to decitabine 15 mg/m2 given three times daily for 3 days in 6 week cycles vs. BSC [41]. The median age of the 233 patients enrolled in the study was 70 years. The median overall survival was 10 vs. 8 months (p=0.38). However, decitabine treatment was associated with improvement in quality of life. Several other schedules of decitabine have been evaluated in patients with MDS and have shown higher response rates when compared to the schedule used in the two previously mentioned phase II trials. In a large multicenter phase II trial, decitabine 20 mg/m2 over 5 days intravenously every 4 weeks was administered to 99 patients with MDS. The median age was 72 years old. The overall improvement rate was 51% [17% complete remission (CR), 15 % marrow CR and 18 % hematologic improvement (HI)]. The 1-year survival rate was 66 % with a median survival of 19.4 months. Of the 66 patients who were transfusion dependent, 33 % became transfusion independent [42].
Lenalidomide is currently FDA approved for the therapy of patients with MDS 5q- syndrome. In a multi-institutional phase II trial, lenalidomide 10 mg orally daily was administered to patients with MDS 5q- syndrome. Of the 146 patients enrolled, 112 had an erythroid response and 99 (67 %) became transfusion independent [43]. The median duration of transfusion independence was 2 years. Treatment was well tolerated with grade 3/4 neutropenia and thrombocytopenia occurring in 54.7 % and 43.9 %, respectively. Lenalidomide has activity in patients with low-risk MDS without 5q-, albeit response rates are lower. In a phase II trial lenalidomide 10 mg daily was administered to 214 patients with non 5q-low-risk MDS [44]. The median age was 72 years and 20% of patients had a platelet count <100×109/L. Of the patients on the study, 43 % responded and 26 % became transfusion independent. The median duration of transfusion independence was 41 weeks. Lenalidomide was also evaluated in patients with intermediate-2 and high-risk MDS. In that study lenaliodmide 10 mg daily for 21 days every 28 days was administered to 47 evaluable patients. The median age was 69 years and 40 % had high-risk MDS. The overall response rate, CR rate, marrow CR and HI was 26 %, 15 %, 4 % and 8 %, respectively. With a median follow up of 330 days, the median overall survival was 272 days [45].
An area of active investigation is identifying molecular biomarkers that can guide therapy. Itzykson et al. have shown that TET2 mutations were independent predictors of response to azacitidine therapy [46]. Similarly, Blum et al. have shown that higher levels of miR-29b were associated with response to decitabine therapy [47]. By identifying predictors of response, risk-adapted personalized therapy can be provided, further enhancing the benefits already observed with these novel drugs.
The Role of Allo HSCT Compared to Non-Transplant Therapies
Despite the progression-free survival [41] and overall survival advantages [36, 37] these drugs offer compared to supportive care only, allo HSCT remains the only curative therapy available. With the advent of reduced intensity conditioning regimens (RIC), allo HSCT can now be offered to older and less firm patients with a wide variety of hematologic malignancies, including MDS [20]. A number of observational studies and phase II trials of RIC allo HSCT for MDS patients have demonstrated the curative potential of this approach [17-19, 21-23].
Two recent retrospective analyses were conducted to define the optimal role of allo HSCT for older MDS patients [14, 48]. The first study, a cohort analysis of outcomes after allo HSCT vs. HMA among patients with high-risk [defined as refractory anemia with excess blasts (RAEB), refractory anemia with excess blasts in transformation (RAEB-T), chronic myelomonocytic leukemia (CMML) according to the FAB classification system [49], or as int-2/high-risk by the IPSS system [7]] de novo MDS, aged 60–70 years, who had an ECOG score <3. Seventy-five patients who only received HMA were compared to 103 patients who proceeded to allo HSCT (59 % received a RIC and 41 % received a high intensity conditioning regimen). Patients in the HMA cohort were diagnosed between 2004 and 2009, while patients in the allo HSCT cohort underwent the transplant procedure between 1995 and 2008. The allo HSCT cohort mostly received peripheral blood stem cells (n=94). Sixty-one percent received a graft from an unrelated donor and the rest were related. Single allele human leukocyte antigen (HLA) mismatched donors were used in 24 % of patients, and the rest were HLA-matched. Patients in the HMA cohort received a median of six cycles of HMA. In a multivariate Cox regression analysis, there was no difference in survival between the two groups during the first year after initiation of treatment [allo HSCT vs. HMA relative risk (RR) 1.3; 95% CI 0.8–2.3]; however, beyond the first year after the initiation of therapy, there was a significant improvement in survival among the allo HSCT cohort compared to the HMA cohort (RR 0.3; 95 % CI 0.1–0.7) [48].
The second study, a Markov model decision analysis of outcomes among de novo MDS patients (n=514), aged 60–70 years, compared quality-adjusted life expectancy after RIC allo HSCT from an HLA-matched donor vs. non-transplant therapeutic approaches [14]. The results indicated that among patients with low risk MDS [defined as low-risk/int-1 IPSS [7]] that non-transplant therapeutic approaches were associated with higher Quality-Adjusted Life Expectancy (QALE) than RIC allo HSCT. However, among patients with high-risk MDS [int-2/high-risk IPSS [7]], RIC allo HSCT was associated with superior QALE than non-transplant approaches (HMA). The authors conducted multiple sensitivity analyses among patients with low-risk disease, by comparing: RIC allo HSCT to patients receiving best supportive care only, RIC allo HSCT to patients receiving erythropoiesis-stimulating agents, RIC allo HSCT performed within 12 months from MDS diagnosis to all non-transplant therapies. None of these sensitivity analyses changed the results. Similarly, among high-risk MDS patients, the authors conducted a sensitivity analysis comparing RIC allo HSCT performed within 12 months of MDS diagnosis to non-transplant therapies (HMA), and the results remained consistent [14].
The Future of Allo HSCT in Older MDS Patients
Among patients who are referred for transplant evaluation, and for whom a HLA matched donor has been identified, widely held beliefs among patients and physicians regarding the value of allo HSCT in this setting renders the conduct of a true randomized clinical trial (i.e. randomize patients with HLA matched donors to allo HSCT vs. non transplant) not practical. And indeed, this was demonstrated previously [50, 51]. Biologic assignment trials have been used successfully to evaluate the role of allo HSCT [52-55]. However, the biggest threat to the internal validity of such design is enrollment bias. A detailed discussion of enrollment bias can be found elsewhere [56].
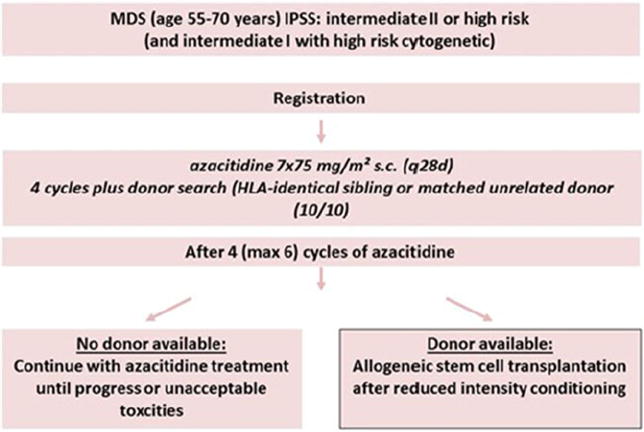
Given the lack of prospective data comparing allo HSCT to HMA, a phase II biologic assignment trial of 254 patients aged 55–70 years has been launched in Europe [25]. This trial will enroll patients with either de novo high risk MDS [int-2/high-risk IPSS [7]], therapy-related MDS or CMML. All patients will initially receive four cycles of azacitidine therapy, then patients will be assigned to the transplant arm if a suitable HLA-matched donor is found [either an HLA-matched related donor or a 10/10 (HLA-A, -B, –C, -DRB1, -DQ)-matched unrelated donor]. Those who do not have such a donor will remain on azacitidine therapy until progression or the occurrence of unacceptable toxicity. The primary outcome is overall survival at 3 years (Fig. 1).
Fig. 1.
Prospective phase II azacitidine-allo HSCT Study Schema. Figure provided by Prof. Nicolaus Kroeger; December 12, 2013
In the US, the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) recently introduced protocol 1102, a prospective comparative biologic assignment cohort analysis of outcomes after RIC allo HSCT from suitable related and unrelated donors vs. non-transplant therapies (W.S., personal communication). This protocol, which is expected to open enrollment in the last quarter of 2013, will determine whether RIC allo HSCT improves survival compared to non-transplant therapies, among patients with de novo high risk MDS [int-2/high-risk IPSS [7]], aged 50–75 years, and who are felt to be transplant eligible.
In BMT CTN 1102, a biological assignment trial, patients with de novo high-risk MDS aged 50–75 years who have a suitable HLA-matched donor will be assigned to the allo HSCT arm, while patients who do not have such a donor will be assigned to the non-transplantation arm (Fig. 2). Given the published [45] and unpublished data (Table 2) regarding inferior outcomes with donors other than HLA-identical sibling and 8/8 (HLA-A, -B, –C, -DRB1) well-matched unrelated donors, the BMT CTN 1102 study team restricted the donors to either an HLA-matched related donor or an 8/8 HLA well-matched unrelated donor. Patients will be analyzed in their respective arms whether or not they received the assigned treatment according to intention-to-treat principle (i.e. if a patient with a suitable donor identified in the 90-day window ultimately does not proceed to RIC allo HSCT, they will still be analyzed in the RIC allo HSCT arm). Outcomes include 3-year survival, 3-year leukemia-free survival, and quality of life. The study is designed to have at least 80 % statistical power to detect an absolute difference of 15 % in survival at 3 years post enrollment. A parallel cost-effectiveness analysis will also be conducted to determine the incremental cost-effectiveness ratio of RIC allo HSCT to non-transplant therapies (Table 1).
Fig. 2.
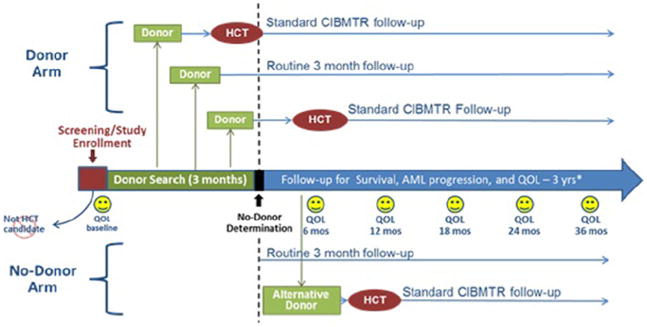
BMT CTN 1102 Study Schema. Provided by W.S. Abbreviations: HCT, hematopoietic cell transplantation; AML, acute myelogenous leukemia; QOL, quality of life; CIBMTR, Center for International Blood and Marrow Transplant Research
Table 2.
Survival of MDS Patients, Aged 21 Years and Older, Post Allo HSCT Performed in the US, From 2000–2010, by Donor Type
Conclusion
In conclusion, the decision to refer older patients with high-risk MDS for transplant evaluation should not be influenced by age alone [17, 18]. Tools such as the CGA hold the promise of more accurately assessing HSCT eligibility [33, 34]. Among patients with high-risk MDS with suitable donors, retrospective data suggest that a strategy of proceeding immediately to RIC allo HSCT is associated with an increase in QALE [14]. However, randomized studies comparing allo HSCT to non-transplant approaches are only now being undertaken. Two biologic assignment trials, one in Europe [25] and one in the US (BMT CTN 1102), will address this knowledge gap. Together, these studies have the potential to change practice. If survival advantage can be demonstrated with allo HSCT, the number of patients with MDS that will be referred for transplant evaluation will increase greatly, which will facilitate the conduct of larger clinical trials that will help further define patient characteristics [57, 58], disease characteristics [59-61], and transplant-related factors that should be considered during therapeutic decision making [62-64]. Collectively, these efforts should enhance the delivery of risk-adapted therapies, leading to improved outcomes post allo HSCT.
Footnotes
Conflict of Interest Dr. Ehab Atallah, Dr. Kathryn Bylow, Dr. Jesse Troy, and Dr. Wael Saber each declare no potential conflict of interest relevant to this article.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Ehab Atallah, Medical College of Wisconsin, Milwaukee, WI, USA.
Kathryn Bylow, Medical College of Wisconsin, Milwaukee, WI, USA.
Jesse Troy, The EMMES Corporation, 401 N. Washington Street, Suite 700, Rockville, MD 20850, USA.
Wael Saber, Email: wsaber@mcw.edu, Medical College of Wisconsin, Milwaukee, WI, USA; Center for International Blood and Marrow Transplant Research, Medical College of Wisconsin, 9200 W. Wisconsin Avenue, Suite C5500, Milwaukee, WI 53226, USA.
References
Papers of particular interest, published recently, have been highlighted as:
-
•
Of importance
- 1.Raza A, Galili N. The genetic basis of phenotypic heterogeneity in myelodysplastic syndromes. Nat Rev Cancer. 2012;12(12):849–59. doi: 10.1038/nrc3321. [DOI] [PubMed] [Google Scholar]
- 2.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263–8. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 4.Cogle CR, Craig BM, Rollison DE, List AF. Incidence of the myelodysplastic syndromes using a novel claims-based algorithm: high number of uncaptured cases by cancer registries. Blood. 2011;117(26):7121–5. doi: 10.1182/blood-2011-02-337964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg SL, Chen E, Corral M, Guo A, Mody-Patel N, Pecora AL, et al. Incidence and clinical complications of myelodysplastic syndromes among United States Medicare beneficiaries. J Clin Oncol. 2010;28(17):2847–52. doi: 10.1200/JCO.2009.25.2395. [DOI] [PubMed] [Google Scholar]
- 6.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109(8):1536–42. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 8•.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–65. doi: 10.1182/blood-2012-03-420489. The revised IPSS score refined the prognostication of patients with MDS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Perez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104(2):579–85. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Manero G, Shan J, Faderl S, Cortes J, Ravandi F, Borthakur G, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22(3):538–43. doi: 10.1038/sj.leu.2405070. [DOI] [PubMed] [Google Scholar]
- 11.Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23(30):7594–603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 12.Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25(23):3503–10. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Manero G. Myelodysplastic syndromes: 2012 update on diagnosis, risk-stratification, and management. Am J Hematol. 2012;87(7):692–701. doi: 10.1002/ajh.23264. [DOI] [PubMed] [Google Scholar]
- 14.Koreth J, Pidala J, Perez WS, Deeg HJ, Garcia-Manero G, Malcovati L, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol. 2013;31(21):2662–70. doi: 10.1200/JCO.2012.46.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–70. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg PL, Attar E, Bennett JM, Bloomfield CD, Borate U, De Castro CM, et al. Myelodysplastic syndromes. J Natl Compr Canc Netw. 2013;11(7):838–74. doi: 10.6004/jnccn.2013.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Lim Z, Brand R, Martino R, van Biezen A, Finke J, Bacigalupo A, et al. Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28(3):405–11. doi: 10.1200/JCO.2009.21.8073. EBMTR data showing no difference in post allo-HSCT survival based on age alone. [DOI] [PubMed] [Google Scholar]
- 18•.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878–87. doi: 10.1200/JCO.2009.25.4821. CIBMTR data showing no difference in post allo-HSCT survival based on age alone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atallah E, Pedersen TL, Warlick ED, Dircks A, Weisdorf D, Horowitz MM, et al. The outcome of Hematopoietic Cell Transplantation (HCT) for Myelodysplastic Syndrome (MDS) in adults >=65 years of age: first report of the Coverage with Evidence Development (CED) in medicare beneficiaries. ASH Annual Meeting Abstracts; 2012. p. 1983. [Google Scholar]
- 20.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2010. 2010 [updated 2010; cited 2011 June 16]; Available from: http://www.cibmtr.org.
- 21.Nakamura R, Rodriguez R, Palmer J, Stein A, Naing A, Tsai N, et al. Reduced-intensity conditioning for allogeneic hematopoietic stem cell transplantation with fludarabine and melphalan is associated with durable disease control in myelodysplastic syndrome. Bone Marrow Transplant. 2007;40(9):843–50. doi: 10.1038/sj.bmt.1705801. [DOI] [PubMed] [Google Scholar]
- 22.Oran B, Giralt S, Saliba R, Hosing C, Popat U, Khouri I, et al. Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol Blood Marrow Transplant. 2007;13(4):454–62. doi: 10.1016/j.bbmt.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laport GG, Sandmaier BM, Storer BE, Scott BL, Stuart MJ, Lange T, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14(2):246–55. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allogeneic hematopoietic stem cell transplantation for the treatment of myelodysplastic syndromes. [2013 August 8]; Available from: http://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/Allogeneic-Hematopoietic-Stem-Cell-Transplantation-for-the-treatment-of-Myelodysplastic-Syndromes-.html.
- 25.ClinicalTrials.gov. 5-azacytidine treatment versus 5-azacytidine followed by allogeneic stem cell transplantation in elderly patients with Myelodysplastic Syndrome (MDS) 2011 [updated 2011; cited 2012 December 19]; Available from: http://clinicaltrials.gov/show/NCT01404741.
- 26.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25(14):1936–44. doi: 10.1200/JCO.2006.10.2954. [DOI] [PubMed] [Google Scholar]
- 27.Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377–86. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 28.Caillet P, Canoui-Poitrine F, Vouriot J, Berle M, Reinald N, Krypciak S, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29(27):3636–42. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- 29.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457–65. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soubeyran P, Fonck M, Blanc-Bisson C, Blanc JF, Ceccaldi J, Mertens C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30(15):1829–34. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- 31.Kanesvaran R, Li H, Koo KN, Poon D. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. J Clin Oncol. 2011;29(27):3620–7. doi: 10.1200/JCO.2010.32.0796. [DOI] [PubMed] [Google Scholar]
- 32.Naqvi K, Garcia-Manero G, Sardesai S, Oh J, Vigil CE, Pierce S, et al. Association of comorbidities with overall survival in myelodysplastic syndrome: development of a prognostic model. J Clin Oncol. 2011;29(16):2240–6. doi: 10.1200/JCO.2010.31.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deschler B, Ihorst G, Platzbecker U, Germing U, Marz E, de Figuerido M, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98(2):208–16. doi: 10.3324/haematol.2012.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muffly LS, Boulukos M, Swanson K, Kocherginsky M, Cerro PD, Schroeder L, et al. Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol Blood Marrow Transplant. 2013;19(3):429–34. doi: 10.1016/j.bbmt.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Scott BL, Deeg HJ. Myelodysplastic syndromes. Annu Rev Med. 2010;61:345–58. doi: 10.1146/annurev.med.051308.132852. [DOI] [PubMed] [Google Scholar]
- 36.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–40. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 37•.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32. doi: 10.1016/S1470-2045(09)70003-8. First study to show definitive improvement in overall survival with hypomethylating agents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itzykson R, Thepot S, Quesnel B, Dreyfus F, Beyne-Rauzy O, Turlure P, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117(2):403–11. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- 39.Itzykson R, Thepot S, Quesnel B, Dreyfus F, Recher C, Wattel E, et al. Long-term outcome of higher-risk MDS patients treated with azacitidine: an update of the GFM compassionate program cohort. Blood. 2012;119(25):6172–3. doi: 10.1182/blood-2012-04-422204. [DOI] [PubMed] [Google Scholar]
- 40.Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794–803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 41.Lubbert M, Suciu S, Baila L, Ruter BH, Platzbecker U, Giagounidis A, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29(15):1987–96. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 42.Steensma DP, Baer MR, Slack JL, Buckstein R, Godley LA, Garcia-Manero G, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol. 2009;27(23):3842–8. doi: 10.1200/JCO.2008.19.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–65. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 44.Raza A, Reeves JA, Feldman EJ, Dewald GW, Bennett JM, Deeg HJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111(1):86–93. doi: 10.1182/blood-2007-01-068833. [DOI] [PubMed] [Google Scholar]
- 45.Ades L, Boehrer S, Prebet T, Beyne-Rauzy O, Legros L, Ravoet C, et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood. 2009;113(17):3947–52. doi: 10.1182/blood-2008-08-175778. [DOI] [PubMed] [Google Scholar]
- 46.Itzykson R, Kosmider O, Cluzeau T, Mansat-De Mas V, Dreyfus F, Beyne-Rauzy O, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia. 2011;25(7):1147–52. doi: 10.1038/leu.2011.71. [DOI] [PubMed] [Google Scholar]
- 47.Blum W, Garzon R, Klisovic RB, Schwind S, Walker A, Geyer S, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A. 2010;107(16):7473–8. doi: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Platzbecker U, Schetelig J, Finke J, Trenschel R, Scott BL, Kobbe G, et al. Allogeneic hematopoietic cell transplantation in patients age 60–70 years with de novo high-risk myelodysplastic syndrome or secondary acute myelogenous leukemia: comparison with patients lacking donors who received azacitidine. Biol Blood Marrow Transplant. 2012;18(9):1415–21. doi: 10.1016/j.bbmt.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51(2):189–99. [PubMed] [Google Scholar]
- 50.Wheatley K, Gray R. Commentary: Mendelian randomization-an update on its use to evaluate allogeneic stem cell transplantation in leukaemia. Int J Epidemiol. 2004;33(1):15–7. doi: 10.1093/ije/dyg313. [DOI] [PubMed] [Google Scholar]
- 51.Burnett AK, Hills RK, Milligan DW, Goldstone AH, Prentice AG, McMullin MF, et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol. 2010;28(4):586–95. doi: 10.1200/JCO.2009.22.9088. [DOI] [PubMed] [Google Scholar]
- 52.Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E, 3rd, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12(13):1195–203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cassileth PA, Harrington DP, Appelbaum FR, Lazarus HM, Rowe JM, Paietta E, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med. 1998;339(23):1649–56. doi: 10.1056/NEJM199812033392301. [DOI] [PubMed] [Google Scholar]
- 54.Reiffers J, Stoppa AM, Attal M, Michallet M, Marit G, Blaise D, et al. Allogeneic vs autologous stem cell transplantation vs chemotherapy in patients with acute myeloid leukemia in first remission: the BGMT 87 study. Leukemia. 1996;10(12):1874–82. [PubMed] [Google Scholar]
- 55.Mohty M, de Lavallade H, Ladaique P, Faucher C, Vey N, Coso D, et al. The role of reduced intensity conditioning allogeneic stem cell transplantation in patients with acute myeloid leukemia: a donor vs no donor comparison. Leukemia. 2005;19(6):916–20. doi: 10.1038/sj.leu.2403770. [DOI] [PubMed] [Google Scholar]
- 56.Logan B, Leifer E, Bredeson C, Horowitz M, Ewell M, Carter S, et al. Use of biological assignment in hematopoietic stem cell transplantation clinical trials. Clin Trials. 2008;5(6):607–16. doi: 10.1177/1740774508098326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lim ZY, Fiaccadori V, Gandhi S, Hayden J, Kenyon M, Ireland R, et al. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res. 2010;34(6):723–7. doi: 10.1016/j.leukres.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 58.Armand P, Kim HT, Rhodes J, Sainvil MM, Cutler C, Ho VT, et al. Iron overload in patients with acute leukemia or MDS undergoing myeloablative stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(6):852–60. doi: 10.1016/j.bbmt.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364(26):2496–506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bejar R, Stevenson KE, Stojanov P, Zaneveld JE, Bar-Natan M, Caughey B, et al. Detection of recurrent mutations by pooled targeted next-generation sequencing in MDS patients prior to treatment with hypomethylating agents or stem cell transplantation. ASH Annual Meeting Abstracts; 2012. p. 311. [Google Scholar]
- 61.Walter MJ, Shen D, Shao J, Ding L, White BS, Kandoth C, et al. Clonal diversity of recurrently mutated genes in myelodysplastic syndromes. Leukemia. 2013 Feb 27; doi: 10.1038/leu.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Field T, Perkins J, Huang Y, Kharfan-Dabaja MA, Alsina M, Ayala E, et al. 5-Azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2010;45(2):255–60. doi: 10.1038/bmt.2009.134. [DOI] [PubMed] [Google Scholar]
- 63.Scott BL, Storer B, Loken MR, Storb R, Appelbaum FR, Deeg HJ. Pretransplantation induction chemotherapy and posttransplantation relapse in patients with advanced myelodysplastic syndrome. Biol Blood Marrow Transplant. 2005;11(1):65–73. doi: 10.1016/j.bbmt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26(3):381–9. doi: 10.1038/leu.2011.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748–58. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]