Abstract
The prevalence of metabolic syndrome (MetS) in people with chronic obstructive pulmonary disease (COPD) varies in European and Asian countries and does not always mirror the prevalence of the general population in a given country. We compared the prevalence of MetS in people with COPD with a comparison group in the United States. The National Health and Nutrition Evaluation Survey data set (2007–2010) was used to identify 94 people with COPD (mean age = 62). Data for demographic and clinical characteristics were obtained by interview and physical examination. Descriptive and inferential statistics were used to analyze the data. The prevalence of MetS was 57.5% in the COPD group and 53.6% in the comparison group. In people with COPD, the factors most significantly associated with MetS were age, income level, marital status, and respiratory symptoms. People with COPD should be screened for MetS.
Keywords: National Health and Nutrition Evaluation Survey, metabolic syndrome, chronic obstructive pulmonary disease
Many people with chronic obstructive pulmonary disease (COPD) are at higher risk of cardiovascular disease and die from cardiovascular causes (Maclay, McAllister, & Macnee, 2007; Sin & Man, 2003). Metabolic syndrome (MetS) presents multiple risks for the development of cardiovascular disease (Grundy, Brewer, Cleeman, Smith, & Lenfant, 2004; Hu et al., 2004). How MetS develops in people with COPD has not been clearly elucidated, but it has been postulated that obesity, smoking, and systemic inflammation may play a role in its development (Clini, Crisafulli, Radaeli, & Malerba, 2013). The prevalence of MetS in people with COPD has been estimated to be between 21% and 53% (Clini et al., 2013; Minas et al., 2011). Because patients with COPD are known to have a sedentary lifestyle (Hernandes et al., 2009; Pitta et al., 2005), they may be at greater risk of developing MetS. We examined the prevalence of MetS and its associated factors in people with COPD in the United States who were selected from a nationally representative data set.
COPD is preventable and treatable. Once considered primarily a pulmonary disease, it is now associated with a variety of systemic manifestations (Nussbaumer-Ochsner & Rabe, 2011). MetS has been recognized to coexist with COPD (Clini et al., 2013). Although several definitions of MetS have been advanced (Alberti et al., 2009; Ford, Li, & Zhao, 2010), it is generally defined as a cluster of five components: high blood pressure (BP), a high triglyceride level (TG), low high-density lipoprotein cholesterol (HDL), abdominal obesity, and a high glucose level (Alberti et al., 2009). Several risk factors have been associated with MetS in people with COPD: smoking, systemic inflammation, obesity, and physical inactivity (Clini et al., 2013). Smoking, the principal risk factor for developing COPD, has been considered to be one of the main causes of increased systemic inflammation, which explains the connection between MetS and COPD (Lam et al., 2010). Systemic inflammation promotes insulin resistance, which contributes to the development of MetS in people with COPD (Bolton et al., 2007). Studies of COPD in people with and without MetS have reported a significant increase in the levels of some systemic inflammatory mediators in the former (Stanciu et al., 2009; Watz et al., 2009). Several studies have reported that body mass index (BMI), especially central obesity, was significantly associated with impaired lung function (Lam et al., 2010; Leone et al., 2009). Furthermore, central obesity has been known to be associated with the development of insulin resistance (Carr et al., 2004). In addition to the risk factors mentioned, physical inactivity increases the chance for people with COPD to develop MetS and complicates the condition, such as worsening obesity, and it has been associated with higher levels of systemic inflammation in people with COPD (Clini et al., 2013; Watz et al., 2009). Several studies have compared the level of physical activity in people with COPD with healthy controls or people with other chronic diseases and found that people with COPD were extremely sedentary and less active than people with other chronic diseases (Arne et al., 2009; S. K. Park, Richardson, Holleman, & Larson, 2013). All of the factors reviewed above have some influence in the development of MetS in people with COPD.
Depending on the definition used and the country studied, the prevalence of MetS in people with COPD varies. Furthermore, the prevalence of MetS in COPD does not consistently mirror the prevalence of MetS in the general population. In a Korean population-based study, the prevalence of MetS in people with COPD was 36.8%, and in people without COPD, it was 26.6% (B. H. Park et al., 2012). In a Canadian study, the prevalence in people with COPD was 47.7% and in age-matched comparison group without COPD was 20.6% (Marquis et al., 2005). In Turkish people with COPD, the prevalence was 44.6% compared with 17.1% in age- and sex-matched group with normal lung function (Akpinar, Akpinar, Ertek, Sayin, & Gülhan, 2012). The prevalence of MetS was reported to be 37% to 53% in German people with COPD (Watz et al., 2009), 21% in Greek people with COPD (Minas et al., 2011), and 42.9% in Spanish people with COPD (Díez-Manglano et al., 2013). From 2003 to 2006, 55% of people with self-reported COPD in the United States had MetS (S. K. Park & Larson, 2013). Few population-based studies have examined the current prevalence of MetS in people with COPD and compared it with the general population in the United States.
Several demographic and clinical factors have been associated with MetS in the general population and people with chronic diseases. Older age has been significantly associated with increasing the prevalence of MetS in the general population (Ford et al., 2010; Ortiz et al., 2010; Y. W. Park et al., 2003), in people with systemic lupus erythematosus (Negrón, Molina, Mayor, Rodríguez, & Vilá, 2008), and in people who have had liver transplantation (Laish et al., 2011). Research has shown that women are more likely to have MetS than men (Cankurtaran et al., 2006). There have been conflicting reports about the relationship between race and MetS. One study (Y. W. Park et al., 2003) reported a significant association between Mexican Americans and MetS, but another study (Ford et al., 2010) reported that African Americans and Mexican Americans were less likely to have MetS than Whites. People with a high level of education have been shown to be less likely to have MetS (Ford et al., 2010). Smoking has been associated with increasing the prevalence of MetS (Y. W. Park et al., 2003; Sun, Liu, & Ning, 2012). A positive association between average volume of alcohol and the prevalence of MetS has been found in Korean men but not in Korean women (Shin et al., 2013). Physical inactivity has been associated with MetS (Ford et al., 2010; Y. W. Park et al., 2003) as has BMI (Ortiz et al., 2010; Y. W. Park et al., 2003). And, several symptoms (i.e., pain and depression) have been related to MetS in people with rheumatoid arthritis (Zonana-Nacach, Santana-Sahagún, Jiménez-Balderas, & Camargo-Coronel, 2008), in women with suspected myocardial infarction (Vaccarino et al., 2008), and in Japanese people (Takeuchi et al., 2009).
Several factors have also been associated with MetS in people with COPD. Marquis and colleagues (2005) found that the prevalence of MetS was much higher in men than in women with COPD. Epidemiologic data have confirmed a link between obesity, MetS, and COPD (Poulain et al., 2008). In one study (Poulain et al., 2008), obese men with COPD (BMI ≥ 25) had more criteria for MetS than patients with normal weight (BMI < 25). Physical inactivity was also associated with MetS in people with COPD (S. K. Park & Larson, 2013; Watz et al., 2009). Studies (Díez-Manglano et al., 2013; Minas et al., 2011; Poulain et al., 2008) found that people with COPD and MetS had a greater percent predicted value for forced expiratory volume in 1 s (FEV1). Certain comorbidities (i.e., heart failure and osteoporosis) and dyspnea have been associated with MetS (Díez-Manglano et al., 2013). Overall, most studies have been conducted outside the United States and have not provided a comprehensive assessment of associated factors for MetS in people with COPD.
Purpose
The purposes of this study were two: to compare the prevalence of MetS in people with COPD with a comparison group and to examine the relationship between demographic and clinical characteristics and MetS in people with COPD in the United States.
Method
Design
This cross-sectional study used data (2007–2010) from the National Health and Nutrition Evaluation Survey (NHANES). The NHANES, which is an ongoing survey, is designed to assess the health and nutritional status of adults and children in the Unites States. It is conducted by the National Center for Health Statistics. The NHANES uses a complex sampling design to select representatives of civilian, noninstitutionalized populations. People of low income, older age, and Black and Hispanic ethnicity were oversampled to increase the reliability and precision of estimates of health status indicators in the NHANES (Centers for Disease Control and Prevention, 2013c).
Sample, Settings, and Procedures
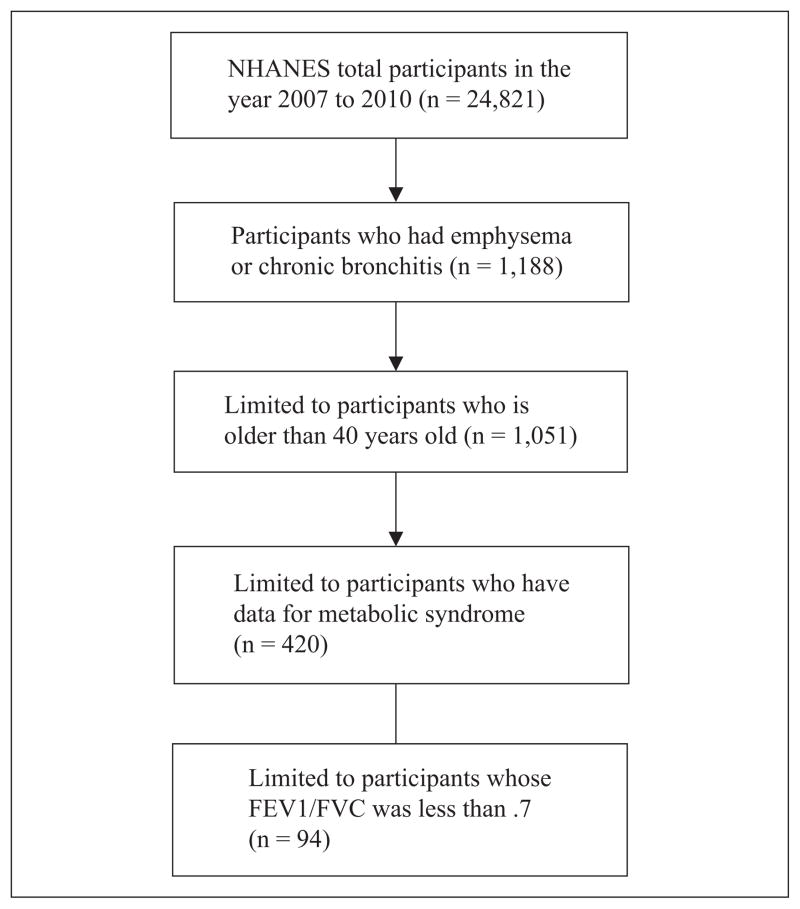
Between 2007 and 2010, 24,821 people completed an NHANES interview. Of those, we included only individuals aged 40 years or more who reported physician-diagnosed emphysema or chronic bronchitis. Those with an FEV1/FVC < .7 were included (see Figure 1). Individuals in the same NHANES data set who did not have COPD, were aged 40 years or more, and had data for all variables were included as a comparison group. In addition, those who had results from a spirometry test were also included in the comparison group. Because spirometry was only performed in people aged 6 to 79, those older than 80 were excluded from this analysis.
Figure 1.
Flowchart for study sample.
The NHANES survey uses interviews and physical examinations to collect sociodemographic and clinical information. The interviews were conducted in the participants’ home and included demographic, socioeconomic, and health-related questions. Physical examinations and measurements were performed in specially designed and equipped mobile centers that traveled to locations throughout the country. The examination components included medical, physiological measurements, and laboratory tests. The NHANES study team consisted of a physician, a dentist, medical and health technicians, and health interviewers. In each location, letters distributed to households and local media were used to introduce the survey. Transportation was provided to and from the mobile centers, if necessary. The NHANES survey was approved by the Centers for Disease Control and Prevention’s institutional review board.
Instruments
Demographic and clinical characteristics
We used data for age, gender, race, level of education, household income, marital status, smoking history, drinking, medication use, respiratory symptoms (e.g., shortness of breath, coughing, phlegm, and wheezing), depression, and comorbidity to describe sample characteristics. These data were obtained by interview, as were sedentary time and self-reported health.
Household income was assessed by asking one question about total income before taxes from all sources in the past calendar year including wages, Social Security or retirement benefits, and financial assistance from relatives. Participants were asked to report the actual amount.
Smoking status was ascertained by asking two questions: “Have you smoked at least 100 cigarettes in your entire life?” and “Do you now smoke cigarettes?” Participants could answer “yes” or “no” to the first question and “every day, some days, or not at all” to the second question. Those who answered “no” to the first question were designated as a nonsmoker. Those who answered “yes” to the first question and “not at all” to the second question were designated as a former smoker. Those who answered “every day” or “some days” to the second question were designated as a current smoker.
Drinking was assessed by asking, “In the past 12 months, how often did you drink any type of alcoholic beverages?” To determine frequency, participants were asked to indicate the actual number of beverages. Those who answered “0” to this question were designated as a nondrinker. Those who had “less than one drink per day” were designated as a moderate drinker. And those who had “more than one drink per day” were designated as a heavy drinker.
Shortness of breath, cough, phlegm, and wheezing were assessed by asking yes/no questions such as, “Have you had shortness of breath either when hurrying on the level or walking up a slight hill?” “Do you usually cough on most days for 3 consecutive months or more during the year?” “Do you bring up phlegm on most days for 3 consecutive months or more during the year?” and “In the past 12 months, have you had wheezing or whistling in your chest?”
Depression was evaluated by asking this question, “Over the last 2 weeks, how often have you been bothered by the following problems: feeling down, depressed or hopeless?” Participants could answer 0 (not at all), 1 (several days), 2 (more than half days), and 3 (nearly every day).
The total number of possible comorbidities was 12: angina, arthritis, asthma, cancer, congestive heart disease, coronary heart disease, heart attack, kidney disease, liver disease, osteoporosis, stroke, and thyroid problems. Participants were asked to answer yes/no to these comorbidities. Cardiovascular disease included angina, congestive heart disease, coronary heart disease, and heart attack.
Sedentary time was evaluated by asking how much time participants spent in sitting or reclining on a typical day (e.g., sitting with friends; traveling in a car, bus, or train; reading; playing cards; watching television; or using a computer) but excluding time spent sleeping. Participants were asked to indicate how much time they spent in these activities.
Self-reported health was evaluated by asking, “Would you say your health in general is?” Responses to this question were “poor, fair, good, very good, or excellent.”
BMI was calculated by measured weight and height. All body measurements were performed by trained health technicians using standardized examination methods and calibrated equipment (Centers for Disease Control and Prevention, 2013b).
Lung function test
Certain individuals were excluded from taking a spirometry test: those who had had a surgical operation on their eyes, a surgical operation on their chest or abdomen, a stroke, or a heart attack in the past 3 months and those who had a history of aneurysm, collapsed lung, detached retina, or hemoptysis. The test was conducted using the Ohio 822/827 dry-rolling seal volume spirometer. The testing procedures were based on guidelines issued by the American Thoracic Society; the goal was to perform three acceptable forced vital capacity maneuvers (Miller et al., 2005). The National Institute for Occupational Safety and Health’s (NIOSH’s) Division of Respiratory Disease served as the NHANES’s spirometry training and quality control consultant. All NHANES spirometry data were reviewed by expert reviewers at NIOSH’s quality center. Health technician performance was also monitored.
Before testing began, participants were asked to loosen any tight clothing. All testing was performed in a standing position. Values used for this analysis included FEV1 and the FEV1/FVC ratio. The predicted values for FEV1 were determined by using previously published prediction equations (Hankinson, Odencrantz, & Fedan, 1999). The participants were classified according to the criteria of the Global Initiative for Chronic Obstructive Lung Disease (2013) for FEV1% predicted value and FEV1/FVC ratio:
Stage I Mild COPD (FEV1% predicted ≥ 80% and FEV1/FVC < .7),
Stage II Moderate COPD (FEV1% predicted; 50%–79% and FEV1/FVC < .7),
Stage III Severe COPD (FEV1% predicted; 30%–49% and FEV1/FVC < .7), and
Stage IV Very severe COPD (FEV1% predicted < 30% and FEV1/FVC < .7).
Metabolic syndrome
Systolic and diastolic blood pressure (SBP/DBP), fasting glucose, HDL, TG levels, and waist circumference were used to determine whether participants had MetS. SBP and DBP were measured three or four times for each participant, and mean SBP and DBP were calculated for our analysis. Fasting glucose and TG levels were tested during the participants’ morning session after a 9-hr fast. Waist circumference was measured at the upper most lateral border of the right ileum at the end of a participant’s normal expiration of breath. All of this clinical information was collected by NHANES staff who were well trained to maintain quality control in measuring these data. Further details of these measurements are available on the NHANES website (Centers for Disease Control and Prevention, 2013a).
The criteria on which we based our definition of MetS are those promulgated by six major health organizations (Alberti et al., 2009). Three or more of the following criteria are required to document the presence of MetS: (a) abdominal obesity (waist circumference: men ≥ 102 cm, women ≥ 88 cm), (b) TG levels ≥ 150 mg/dL or drug treatment for elevated TG levels, (c) HDL levels of <40 mg/dL in men and 50 mg/dL in women or drug treatment for reduced HDL levels, (d) SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or antihypertensive treatment with a history of hypertension, and (e) fasting glucose level of ≥ 100 mg/dL or drug treatment for elevated glucose levels.
Analysis
We conducted data analysis with Stata version 12.0. All continuous variables were expressed as mean and standard deviation. Categorical variables were presented as percentage and frequency. We used the chi-square test and univariate linear regression and the “lincom” procedure in Stata to compare study variables between the COPD and comparison group and between participants who had MetS and those who did not. A univariate and multivariate logistic regression model was used to examine the relationship of demographic and clinical characteristics to MetS. Independent variables for the multivariate logistic regression model were selected from results of univariate logistic regression and Pearson’s correlation between independent variables and MetS. A p value of <.05 was considered statistically significant.
Results
Sample Characteristics of the COPD Group and the Comparison Group
The sample for the COPD group was 94; the sample for the comparison group was 3,661 (see Tables 1 and 2). In relation to the comparison group, the COPD group was older and had less education, less income, poorer self-reported health, a lower FEV1% predicted value and FEV1/FVC ratio, more respiratory symptoms, and more comorbidities.
Table 1.
Sample Characteristics and Characteristics of Metabolic Syndrome for People With COPD and the Comparison Group.
| COPD (n = 94)
|
Comparison Group (n = 3,661)
|
Test Statistics and p Value | |
|---|---|---|---|
| M (SD) | M (SD) | ||
| Age | 62.06 (9.8) | 56.64 (10.78) | t = 6.28; p = .0001 |
| FEV1 % pred. | 0.67 (0.21) | 0.95 (0.16) | t = −7.37; p = .0001 |
| FEV1/FVC | 0.58 (0.10) | 0.76 (0.08) | t = −17.78; p = .0001 |
| Number of respiratory symptoms | 1.59 (1.20) | 0.41 (0.75) | t = 5.78; p = .0001 |
| Number of comorbiditiesa | 2.20 (1.60) | 0.91 (1.11) | t = 6.11; p = .0001 |
| Body mass index, kg/m2 | 26.98 (6.42) | 29.30 (6.13) | t = −3.94; p = .0001 |
| Sedentary time (daily; minutes) | 355.85 (206.43) | 305.78 (192.89) | t = 0.53; p = .60 |
| Waist circumference, cm | 98.98 (15.55) | 100.80 (14.86) | t = −1.60; p = .12 |
| Triglycerides, mg/dL | 137.20 (70.70) | 139.55 (12.46) | t = 0.13; p = .90 |
| Mean SBP, mmHg | 125.22 (20.68) | 124.71 (17.96) | t = 0.45; p = .66 |
| Mean DBP, mmHg | 65.75 (17.57) | 70.65 (12.12) | t = −1.75; p = .09 |
| HDL cholesterol, mg/dL | 53.66 (17.22) | 54.56 (16.50) | t = 1.01; p = .32 |
| Fasting glucose, mg/dL | 112. 53 (28.20) | 111.50 (35.74) | t = 0.82; p = .42 |
Note. COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; SBP = systolic blood pressure; DBP = diastolic blood pressure; HDL = high-density lipoprotein.
Number of comorbidity does not include diabetes and hypertension.
Table 2.
Sample Characteristics and Components of Metabolic Syndrome for People With COPD and the Comparison Group.
| COPD (n = 94)
|
Comparison Group (n = 3,661)
|
Test Statistics and p Value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Gender (male) | 42 (44.7%) | 1,870 (51.1%) | χ2 = 2.35; p = .33 |
| Race | χ2 = 7.42; p = .0007 | ||
| Mexican American, other Hispanic, non-Hispanic Black | 26 (27.7%) | 1,899 (51.9%) | |
| Non-Hispanic White | 68 (72.3%) | 1,762 (48.1%) | |
| Education | χ2 = 28.28; p = .005 | ||
| High school or less | 77 (81.9%) | 1,896 (51.9%) | |
| Some college education or above | 17 (18.1%) | 1,759 (48.1%) | |
| Income | χ2 = 28.61; p = .008 | ||
| <$35,000 | 55 (67.9%) | 1,335 (40.5%) | |
| $35,000–$65,000 | 16 (19.8%) | 863 (26.2%) | |
| >$65,000 | 10 (12.4%) | 1,096 (33.3%) | |
| Marital status | χ2 = 4.75; p = .12 | ||
| Living with someone, married | 51 (54.3%) | 2,436 (66.5%) | |
| Separated, widowed, divorced | 43 (45.7%) | 1,225 (33.5%) | |
| Self-reported health | χ2 = 40.87; p = .00001 | ||
| Excellent, very good, and good | 42 (46.7%) | 2,669 (76.4%) | |
| Fair and poor | 48 (53.3%) | 824 (23.6%) | |
| Symptoms | |||
| Shortness of breath on stairs/inclines | 48 (80%) (n = 60) |
658 (26.0%) (n = 2,532) |
χ2 = 52.48; p = .003 |
| Coughing | 28 (29.8%) | 269 (7.4%) | χ2 = 46.18; p = .0002 |
| Phlegm | 34 (36.2%) | 238 (6.5%) | χ2 = 69.49; p = .00001 |
| Wheezing | 39 (41.5%) | 355 (9.7%) | χ2 = 41.70; p = .00001 |
| Depression (at least several days) | 39 (43.4%) | 798 (22.9%) | χ2 = 21.65; p = .001 |
| Smoking | |||
| Current smoker | 45 (47.9%) | 658 (18.0%) | χ2 = 14.10; p = .03 |
| Former smoker | 30 (31.9%) | 1,106 (30.2%) | χ2 = 0.28; p = .76 |
| Nonsmoker | 19 (20.2%) | 1,896 (51.8%) | χ2 = 29.14; p = .007 |
| Drinking | χ2 = 8.42; p = .15 | ||
| Non-drinker | 33 (38.4%) | 701 (22.8%) | |
| Moderate drinker | 20 (23.3%) | 599 (19.5%) | |
| Heavy drinker | 33 (38.4%) | 1,777 (57.8%) | |
| Diabetes | 19 (20.2%) | 506 (13.8%) | χ2 = 0.99; p = .32 |
| Hypertension | 56 (59.6%) | 1,527 (41.8%) | χ2 = 9.66; p = .05 |
| Cardiovascular disease (present) | 15 (26.6%) | 167 (4.6%) | χ2 = 34.59; p = .002 |
| Body mass index (BMI) | |||
| BMI < 25 | 41 (43.6%) | 876 (24.0%) | χ2 = 21.26; p = .007 |
| 25 ≤ BMI < 30 | 24 (25.5%) | 1,378 (37.7%) | |
| 30 ≤ BMI | 29 (30.1%) | 1,401 (38.3%) | |
| Large waist circumferencea | 50 (53.2%) | 2,257 (61.7%) | χ2 = 6.83; p = .05 |
| High triglyceridesa | 46 (48.9%) | 1,657 (45.3%) | χ2 = .30; p = .70 |
| High BPa | 62 (66.0%) | 2,052 (56.1%) | χ2 = 2.70; p = .35 |
| Low HDL cholesterola | 49 (52.1%) | 1,605 (43.8%) | χ2 = .01; p = .94 |
| High glucosea | 64 (68.1%) | 2,234 (61.0%) | χ2 = 2.45; p = .32 |
| Metabolic syndrome | 54 (57.5%) | 1,961 (53.6%) | χ2 = .06; p = .87 |
Note. COPD = chronic obstructive pulmonary disease; BP = blood pressure; HDL = high-density lipoprotein.
Criteria for metabolic syndrome; waist circumference of ≥ 102 cm in men and ≥ 88 cm in women, triglycerides levels ≥ 150 mg/dL or specific treatment for lipid abnormality; HDL cholesterol levels of <40 mg/dL in men and 50 mg/dL in women or specific treatment for the lipid abnormality; SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or treatment of previously diagnosed hypertension; fasting glucose level of ≥ 100 mg/dL or previously diagnosed type 2 diabetes.
Prevalence of Metabolic Syndrome in Study Groups
The prevalence of MetS and its components were not significantly different between the two groups (see Table 2). However, the comparison of the proportion of large waist circumference between the two groups was marginal (p = .052). The most prevalent component in participants with COPD was high BP, whereas the most prevalent component in the comparison group was waist circumference. The prevalence of MetS in the COPD group and the comparison group was 57.5% and 53.6%, respectively. The prevalence of MetS in the COPD group, based on the GOLD criteria, was as follows: 13 out of 26 (50%) in Stage 1, 26 out of 48 (54%) in Stage 2, 13 out of 18 (72%) in Stage 3, and 2 out of 2 (100%) in Stage 4.
Sample Characteristics of COPD Group With and Without MetS
Compared with COPD group who did not have MetS, COPD group with MetS were older and had less education, less income, poorer self-reported health, a lower FEV1% predicted value and FEV1/FVC ratio, more respiratory symptoms, more comorbidities, and higher BMIs (see Tables 3 and 4).
Table 3.
Sample Characteristics for COPD Patients With Metabolic Syndrome (MetS) and Without MetS (n = 94).
| COPD With MetS (n =54; 57.5%)
|
COPD Without MetS (n = 40; 42.5%)
|
|
|---|---|---|
| M (SD) | M (SD) | |
| Age | 64.57 (9.48) | 58.68 (9.31) |
| FEV1 % pred. | 0.65 (0.23) | 0.70 (0.21) |
| FEV1/FVC | 0.56 (0.25) | 0.60 (0.08) |
| Number of comorbiditiesa | 2.5 (1.66) | 1.8 (1.45) |
| Body mass index, kg/m2 | 29.11 (6.14) | 24.10 (5.68) |
| Sedentary time (daily; minutes) | 354.44 (194.08) | 357.8 (224.54) |
Note. COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
Number of comorbidity does not include diabetes and hypertension.
Table 4.
Sample Characteristics for COPD Patients With Metabolic Syndrome (MetS) and Without MetS (n = 94).
| COPD With MetS (n =54; 57.5%)
|
COPD Without MetS (n = 40; 42.5%)
|
|
|---|---|---|
| n (%) | n (%) | |
| Gender | ||
| male | 27 (50.0%) | 15 (37.5%) |
| female | 27 (50.0%) | 25 (62.5%) |
| Race | ||
| Mexican American, other Hispanic, non-Hispanic Black | 13 (24.1%) | 13 (32.5%) |
| Non-Hispanic White | 41 (75.9%) | 27 (67.5%) |
| Education | ||
| High school or less | 49 (90.7%) | 28 (70.7%) |
| Some college education or above | 5 (9.3%) | 12 (30.0%) |
| Income | ||
| <$35,000 | 37 (72.6%) | 18 (60.0%) |
| $35,000–$65,000 | 12 (23.5%) | 4 (13.3%) |
| >$65,000 | 2 (3.9%) | 8 (26.7%) |
| Marital status | ||
| Living with someone, married | 34 (63.0%) | 17 (42.5%) |
| Separated, widowed, divorced | 20 (37.0%) | 23 (57.5%) |
| Self-reported health | ||
| Excellent, very good, and good | 21 (39.6%) | 21 (56.8%) |
| Fair and poor | 32 (60.4%) | 16 (43.2%) |
| Respiratory symptoms | ||
| 0 | 9 (16.7%) | 10 (25.0%) |
| >0 | 45 (83.3%) | 30 (75.0%) |
| Depression | ||
| None | 32 (60.4%) | 19 (51.4%) |
| At least several days | 21 (39.6%) | 18 (48.7%) |
| Smoking | ||
| Current smoker | 24 (44.4%) | 21 (52.5%) |
| Former smoker | 22 (40.7%) | 8 (20.0%) |
| Nonsmoker | 8 (14.8%) | 11 (27.5%) |
| Drinking | ||
| Non-drinker | 21 (41.2%) | 12 (34.3%) |
| Moderate drinker | 12 (23.5%) | 8 (22.9%) |
| Heavy drinker | 18 (35.3%) | 15 (42.9%) |
| Cardiovascular disease | 16 (29.6%) | 9 (22.5%) |
| Body mass index (BMI) | ||
| BMI < 25 | 15 (27.8%) | 26 (65.0%) |
| 25 ≤ BMI < 30 | 16 (29.6%) | 8 (20.0%) |
| 30 ≤ BMI | 23 (42.6%) | 8 (15.0%) |
Note. COPD = chronic obstructive pulmonary disease.
Relationship Between Demographic and Clinical Characteristics and Metabolic Syndrome in People With COPD
Univariate logistic regression showed that COPD participants who were older and had respiratory symptoms, more comorbidities, and larger BMIs were more likely to have MetS (see Table 5). Participants who had more education and income were less likely to have MetS (see Table 5).
Table 5.
Odds Ratios for Association of Sample Characteristics With Metabolic Syndrome (MetS) From Univariate Logistic Regression in People With COPD (n = 94).
| OR for MetS [95% CI] | |
|---|---|
| Age | 1.08* [1.01, 1.15] |
| Gender | |
| male | 1 |
| female | 0.78 [0.20, 2.98] |
| Race | |
| Mexican American, other Hispanic, non-Hispanic Black | 1 |
| Non-Hispanic White | 1.57 [0.46, 5.39] |
| Education | |
| High school or less | 1 |
| Some college education or above | 0.08* [0.02, 0.39] |
| Income | |
| <$35,000 | 1 |
| $35,000–$65,000 | 0.86 [0.13, 5.47] |
| >$65,000 | 0.05* [0.01, 0.50] |
| Marital status | |
| Living with someone, married | 1 |
| Separated, widowed, divorced | 0.79 [0.18, 3.49] |
| Self-reported health | |
| Excellent, very good, and good | 1 |
| Fair and poor | 3.82 [0.85, 17.14] |
| FEV1 % pred. | 0.20 [0.01, 8.86] |
| FEV1/FVC | 0.02 [0.00, 8.99] |
| Respiratory symptoms | |
| 0 | 1 |
| >0 | 3.60* [1.00, 11.52] |
| Depression | |
| None | 1 |
| At least several days | 0.95 [0.21, 4.30] |
| Smoking | |
| Current smoker | 1 |
| Former smoker | 2.20 [0.46, 10.49] |
| Nonsmoker | 0.27 [0.06, 1.20] |
| Drinking | |
| Non-drinker | 1 |
| Moderate drinker | 1.91 [0.35, 10.53] |
| Heavy drinker | 0.69 [0.17, 2.77] |
| Number of comorbiditiesa | 1.39* [1.05, 1.85] |
| Cardiovascular disease | 0.87 [0.25, 3.06] |
| Body mass index (BMI), kg/m2 | 1.22* [1.05, 1.42] |
| BMI < 25 | 1 |
| 25 ≤ BMI < 30 | 4.97 [0.94, 26.26] |
| 30 ≤ BMI | 9.27* [2.40, 35.85] |
| Sedentary time (daily; minutes) | 1.00 [1.00, 1.00] |
Note. COPD = chronic obstructive pulmonary disease; CI = confidence interval; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
Number of comorbidity does not include diabetes and hypertension.
p < .05.
The multivariate logistic regression showed that the overall model was significant, F(8,16) = 4.24, p = .007, and that COPD participants who had more comorbidity (OR = 1.38, CI [0.79, 2.41], p = .24), were older (OR = 1.12, CI [1.00, 1.26], p = .04), and had more respiratory symptoms (OR = 2.40, CI [1.09, 5.29], p = .03) and drinking (OR = 1.28, CI [0.42, 3.87], p = .65) were more likely to have MetS. The multivariate logistic regression also showed that participants who were females (OR = 0.98, CI [0.16, 6.15], p = .97), lived alone (OR = 0.02, CI [0.00, 0.46], p = .02), had more education (OR = 0.72, CI [0.05, 9.93], p = .80), and had more income (OR = 0.10, CI [0.01, 0.94], p = .04) were less likely to have MetS.
Discussion
Ours is the first study in the United States to compare the prevalence of MetS in people with and without COPD and to examine the relationship between MetS and various demographic and clinical characteristics in people with COPD. We found that 57.5% people with COPD had MetS and 53.6% people without COPD had MetS. The most significantly associated factors to MetS in people with COPD were old age, income level, marital status, and respiratory symptoms.
We found no significant difference in the prevalence of MetS in people with and without COPD, although the former displayed more MetS than the latter. This insignificant finding can be attributed to the fact that many sample characteristics were significantly different between people with COPD and the comparison group. Mean BMI in particular was significantly higher in the comparison group than in the COPD group, and our comparison group was not age and gender matched. Furthermore, this finding reflects increasing obesity in the United States and a higher obesity rate than other countries (Ogden, Carroll, Kit, & Flegal, 2012; World Health Organization, 2013). Our previous study covering the years 2003–2006 (S. K. Park & Larson, 2013) showed that 55% of self-reported COPD patients had MetS. The current study, which covered the years 2007–2010, showed that 57.5% of COPD patients had MetS, which may reflect the current trend of increasing obesity in the United States. The higher prevalence of MetS in people with COPD than in the comparison group in this study is reasonable because people with COPD are known to be more physically inactive than the general population, which may contribute to MetS (S. K. Park et al., 2013; Pitta et al., 2005). The prevalence of MetS observed in people with COPD in the United States was higher than in many other populations with COPD including German (Watz et al., 2009), Korean (B. H. Park et al., 2012), Canadian (Marquis et al., 2005), and Turkish (Akpinar et al., 2012). Again, it is not surprising that a high prevalence of MetS exists in people with COPD in the United States because obesity is prevalent throughout the country (Ogden et al., 2012). However, differences in the prevalence of MetS may be attributed to the ethnicities of the study population, the definitions used, and mean age. In this study, we observed a high frequency of abdominal obesity, hyperglycemia, and high BP in people with COPD. This is consistent with findings from other studies that were conducted in various countries and reported a high frequency of abdominal obesity (Marquis et al., 2005; Watz et al., 2009) or high BP (Akpinar et al., 2012; Marquis et al., 2005; Watz et al., 2009). This finding also confirms García-Olmos and colleagues’ (2013) results, which revealed that the prevalent comorbidities in 3,183 people with COPD in Spain were diabetes, hypertension, lipid metabolism disorders, and obesity.
Multivariate logistic regression showed that several characteristics were significantly associated with MetS. In general, these factors were similar to those associated with MetS in people with COPD in other countries, in the general population, and in people with other chronic diseases. Aging people were more likely to have MetS, which is consistent with a study of the general population that found that the prevalence of MetS rose with age, reaching peak levels in the sixth or seventh decade (Y. W. Park et al., 2003). It has been postulated that increases in the prevalence of MetS with aging are associated with increased body fat and changes in lifestyles (Boden, Chen, DeSantis, & Kendrick, 1993). Participants who earned more income were less likely to have MetS, which is consistent with Y. W. Park et al.’s (2003) finding. The significant relationship between low socioeconomic status and MetS may be associated with unhealthy behaviors such as smoking, excessive alcohol consumption, or obesity (Loucks et al., 2007).
Respiratory symptoms were significantly associated with MetS in our study, which is consistent with Díez-Manglano and colleagues’ (2013) study, which found that people with MetS had more dyspnea than those without MetS. The relationship between respiratory symptoms and MetS may be mediated by other factors such as physical inactivity. Dyspnea, the main symptom experienced by people with COPD, leads to an inactive lifestyle that negatively affects health in several ways, such as deconditioning. Unexpectedly, sedentary time in this study was not associated with MetS. Because the NHANES did not use an objective measure of physical activity for the years 2007 to 2010 (unlike 2003–2006), we were unable to determine the true relationship between MetS and physical activity. In the past, a significant relationship between MetS and physical activity has been reported for people with COPD (S. K. Park & Larson, 2013; Watz et al., 2009). Thus, more effort to relieve symptoms is necessary to decrease cardiovascular risk factors in people with COPD.
We found a significant relationship between BMI and MetS in univariate logistic regression analysis, which is an expected finding. Previous studies have already established the connection between BMI and MetS in people with COPD (Marquis et al., 2005; Poulain et al., 2008). Obesity among men in the United States has increased from 27.5% in 1999–2000 to 35.5% in 2009–2010 (Ogden et al., 2012). Logically then, more effort should be expended to reduce obesity in people with COPD to decrease the rate of their developing MetS. A similar effort should be made to reduce obesity in the general population: The mean BMI in the comparison group was higher than that in the COPD group in this study.
Unexpectedly, we found no significant relationship between smoking status and MetS in people with COPD, but the people with COPD had more current smokers than the comparison group. Being a smoker has been associated with higher risk of having MetS. Studies have reported that smoking is considered to be one of the major causes of systemic inflammation in people with COPD and MetS (Clini et al., 2013). Consequently, more patient education on the benefits of stopping smoking should be encouraged. Another unexpected finding was that no significant relationship was found between FEV1 levels and MetS. Studies have reported that people with MetS were likely to have a better FEV1 and a mild stage of COPD (Díez-Manglano et al., 2013; Poulain et al., 2008; Watz et al., 2009). Researchers have suggested that this is because there is weight loss and loss of muscle mass in the later stages of the disease (Poulain et al., 2008; Watz et al., 2009). Thus, usually high prevalence of MetS was found in the early stages of COPD (Watz et al., 2009). Most of our participants had mild-to-moderate COPD, and disease severity was not well-distributed. Thus, we were unable to capture the real relationship between FEV1values and MetS. This relationship should be reexamined in people with well-distributed COPD.
One of this study’s strengths is that it used population-based data in the United States. And the prevalence of MetS in people with COPD in the United States is relatively current. However, a few limitations are noteworthy. Some of the variables were measured with single-item from the NHANES questionnaire. This is one of the disadvantages of using survey data. Further study using stronger measures is needed to confirm our findings. Because study participants had mostly mild-to-moderate COPD, our findings cannot be generalized to people with advanced COPD.
In their study, which covered the period 2003 to 2006, Ervin (2009) reported that 37.2% to 40.8% of Americans aged 40 to 59 and 51.5% to 54.4% of those aged 60 and older had MetS. In that same period, S. K. Park and Larson (2013) found 55% of people with self-reported COPD older than 55 years (mean age = 70.6) had MetS. This study for the period 2007 to 2010 confirms that, in the United States, a significant portion of people with and without COPD also had MetS, which may explain the increased cardiovascular mortality in people with COPD. The results of this study demonstrate the extent of the problem for people with COPD in the United States. To address this problem will require more attention to people with COPD for MetS and risk factors of MetS and it will require an emphasis on lifestyle modification to reduce the risk. Further study of people with more advanced COPD is required to confirm this study’s findings.
Acknowledgments
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Reprints and permissions: sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Akpinar EE, Akpinar S, Ertek S, Sayin E, Gülhan M. Systemic inflammation and metabolic syndrome in stable COPD patients. Tuberkuloz ve Toraks. 2012;60:230–237. [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Smith SC. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Arne M, Janson C, Janson S, Boman G, Lindqvist U, Berne C, Emtner M. Physical activity and quality of life in subjects with chronic disease: Chronic obstructive pulmonary disease compared with rheumatoid arthritis and diabetes mellitus. Scandinavian Journal of Primary Health Care. 2009;27:141–147. doi: 10.1080/02813430902808643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Chen X, DeSantis RA, Kendrick Z. Effects of age and body fat on insulin resistance in healthy men. Diabetes Care. 1993;16:728–733. doi: 10.2337/diacare.16.5.728. [DOI] [PubMed] [Google Scholar]
- Bolton CE, Evans M, Ionescu AA, Edwards SM, Morris RH, Dunseath G, Shale DJ. Insulin resistance and inflammation: A further systemic complication of COPD. Chronic Obstructive Pulmonary Disease. 2007;4:121–126. doi: 10.1080/15412550701341053. [DOI] [PubMed] [Google Scholar]
- Cankurtaran M, Halil M, Yavuz BB, Dagli N, Oyan B, Ariogul S. Prevalence and correlates of metabolic syndrome (MS) in older adults. Archives of Gerontology and Geriatrics. 2006;42:35–45. doi: 10.1016/j.archger.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, Kahn SE. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Laboratory components. 2013a Retrieved from http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/labcomp_e.pdf.
- Centers for Disease Control and Prevention. Mobile exam center components descriptions. 2013b Retrieved from http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/meccomp_e.pdf.
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey, 2011–2012. 2013c Retrieved from http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/2011-12_overview_brochure.pdf.
- Clini E, Crisafulli E, Radaeli A, Malerba M. COPD and the metabolic syndrome: An intriguing association. Internal and Emergency Medicine. 2013;8:283–289. doi: 10.1007/s11739-011-0700-x. [DOI] [PubMed] [Google Scholar]
- Díez-Manglano J, Barquero-Romero J, Almagro P, Cabrera FJ, López García F, Montero L, Soriano JB. COPD patients with and without metabolic syndrome: Clinical and functional differences. Internal and Emergency Medicine. 2013 doi: 10.1007/s11739-013-0945-7. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Ervin B. National Health Statistics No 13. Hyattsville, MD: National Center for Health Statistics; 2009. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race, and ethnicity, and body mass index: United States, 2003–2006. [PubMed] [Google Scholar]
- Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. Journal of Diabetes. 2010;2:180–193. doi: 10.1111/j.1753-0407.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- García-Olmos L, Alberquilla A, Ayala V, García-Sagredo P, Morales L, Carmona M, Monteagudo JL. Comorbidity in patients with chronic obstructive pulmonary disease in family practice: A cross sectional study. BMC Family Practice. 2013;14:11. doi: 10.1186/1471-2296-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease. Global initiative for chronic obstructive lung disease: Pocket guide to COPD diagnosis, management, and prevention. 2013 Retrieved from http://www.goldcopd.org/uploads/users/files/GOLD_Pocket_May2512.pdf.
- Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American Journal of Respiratory and Critical Care Medicine. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Hernandes NA, de Teixeira DC, Probst VS, Brunetto AF, Ramos EM, Pitta F. Profile of the level of physical activity in the daily lives of patients with COPD in Brazil. Jornal Brasileiro de Pneumologia. 2009;35:949–956. doi: 10.1590/s1806-37132009001000002. [DOI] [PubMed] [Google Scholar]
- Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Archives of Internal Medicine. 2004;164:1066–1076. doi: 10.1001/archinte.164.10.1066. [DOI] [PubMed] [Google Scholar]
- Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: Prevalence, risk factors, and association with cardiovascular events. Liver Transplantation. 2011;17:15–22. doi: 10.1002/lt.22198. [DOI] [PubMed] [Google Scholar]
- Lam KB, Jordan RE, Jiang CQ, Thomas GN, Miller MR, Zhang WS, Adab P. Airflow obstruction and metabolic syndrome: The Guangzhou Biobank Cohort Study. European Respiratory Journal. 2010;35:317–323. doi: 10.1183/09031936.00024709. [DOI] [PubMed] [Google Scholar]
- Leone N, Courbon D, Thomas F, Bean K, Jégo B, Leynaert B, Zureik M. Lung function impairment and metabolic syndrome: The critical role of abdominal obesity. American Journal of Respiratory and Critical Care Medicine. 2009;179:509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Magnusson KT, Cook S, Rehkopf DH, Ford ES, Berkman LF. Socioeconomic position and the metabolic syndrome in early, middle, and late life: Evidence from NHANES 1999–2002. Annals of Epidemiology. 2007;17:782–790. doi: 10.1016/j.annepidem.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Maclay JD, McAllister DA, Macnee W. Cardiovascular risk in chronic obstructive pulmonary disease. Respirology. 2007;12:634–641. doi: 10.1111/j.1440-1843.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- Marquis K, Maltais F, Duguay V, Bezeau AM, LeBlanc P, Jobin J, Poirier P. The metabolic syndrome in patients with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation. 2005;25:226–232. doi: 10.1097/00008483-200507000-00010. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Wanger J. Standardisation of spirometry. European Respiratory Journal. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Minas M, Kostikas K, Papaioannou AI, Mystridou P, Karetsi E, Georgoulias P, Gourgoulianis KI. The association of metabolic syndrome with adipose tissue hormones and insulin resistance in patients with COPD without co-morbidities. Chronic Obstructive Pulmonary Disease. 2011;8:414–420. doi: 10.3109/15412555.2011.619600. [DOI] [PubMed] [Google Scholar]
- Negrón AM, Molina MJ, Mayor AM, Rodríguez VE, Vilá LM. Factors associated with metabolic syndrome in patients with systemic lupus erythematosus from Puerto Rico. Lupus. 2008;17:348–354. doi: 10.1177/0961203307086645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaumer-Ochsner Y, Rabe KF. Systemic manifestations of COPD. Chest. 2011;139:165–173. doi: 10.1378/chest.10-1252. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. NCHS Data Brief No 82. Hyattsville, MD: National Center for Health Statistics; 2012. Prevalence of obesity in the United States, 2009–2010. [PubMed] [Google Scholar]
- Ortiz AP, Suárez E, Beauchamp G, Romaguera J, Soto-Salgado M, Pérez CM. Correlates of the metabolic syndrome among a sample of women in the San Juan Metropolitan area of Puerto Rico. Metabolic Syndrome and Related Disorder. 2010;8:235–242. doi: 10.1089/met.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BH, Park MS, Chang J, Kim SK, Kang YA, Jung JY, Kim C. Chronic obstructive pulmonary disease and metabolic syndrome: A nationwide survey in Korea. International Journal of Tuberculosis and Lung Disease. 2012;16:694–700. doi: 10.5588/ijtld.11.0180. [DOI] [PubMed] [Google Scholar]
- Park SK, Larson JL. The relationship between physical activity and metabolic syndrome in people with chronic obstructive pulmonary disease. Journal of Cardiovascular Nursing. 2013 doi: 10.1097/JCN.0000000000000096. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Richardson CR, Holleman RG, Larson JL. Physical activity in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003–2006) Heart & Lung. 2013;42:235–240. doi: 10.1016/j.hrtlng.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Archives of Internal Medicine. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2005;171:972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- Poulain M, Doucet M, Drapeau V, Fournier G, Tremblay A, Poirier P, Maltais F. Metabolic and inflammatory profile in obese patients with chronic obstructive pulmonary disease. Chronic Respiratory Disease. 2008;5:35–41. doi: 10.1177/1479972307087205. [DOI] [PubMed] [Google Scholar]
- Shin MH, Kweon SS, Choi JS, Rhee JA, Nam HS, Jeong SK, Lee YH. Average volume of alcohol consumed, drinking patterns, and metabolic syndrome in older Korean adults. Journal of Epidemiology. 2013;23:122–131. doi: 10.2188/jea.JE20120065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- Stanciu S, Marinescu R, Iordache M, Dumitrescu S, Mureşan M, Bogdan MA. Are systemic inflammatory profiles different in patients with COPD and metabolic syndrome as compared to those with COPD alone? Romanian Journal of Internal Medicine. 2009;47:381–386. [PubMed] [Google Scholar]
- Sun K, Liu J, Ning G. Active smoking and risk of metabolic syndrome: A meta-analysis of prospective studies. PLoS One. 2012;7:e47791. doi: 10.1371/journal.pone.0047791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Nakao M, Nomura K, Inoue M, Tsurugano S, Shinozaki Y, Yano E. Association of the metabolic syndrome with depression and anxiety in Japanese men: A 1-year cohort study. Diabetes/Metabolism Research and Reviews. 2009;25:762–767. doi: 10.1002/dmrr.1041. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, McClure C, Johnson BD, Sheps DS, Bittner V, Rutledge T, Merz CN. Depression, the metabolic syndrome and cardiovascular risk. Psychosomatic Medicine. 2008;70:40–48. doi: 10.1097/PSY.0b013e31815c1b85. [DOI] [PubMed] [Google Scholar]
- Watz H, Waschki B, Kirsten A, Müller KC, Kretschmar G, Meyer T, Magnussen H. The metabolic syndrome in patients with chronic bronchitis and COPD: Frequency and associated consequences for systemic inflammation and physical inactivity. Chest. 2009;136:1039–1046. doi: 10.1378/chest.09-0393. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global Health Observatory: Overweight and Obesity. 2013 Retrieved from http://www.who.int/gho/ncd/risk_factors/overweight/en/
- Zonana-Nacach A, Santana-Sahagún E, Jiménez-Balderas FJ, Camargo-Coronel A. Prevalence and factors associated with metabolic syndrome in patients with rheumatoid arthritis and systemic lupus erythematosus. Journal of Clinical Rheumatology. 2008;14:74–77. doi: 10.1097/RHU.0b013e31816b2faa. [DOI] [PubMed] [Google Scholar]