Abstract
Circadian changes in visual sensitivity have been observed in a wide range of species, vertebrates, and invertebrates, but the processes impacted and the underlying mechanisms largely are unexplored. Among arthropods, effects of circadian signals on vision have been examined in most detail in the lateral compound eye (LE) of the American horseshoe crab, Limulus polyphemus, a chelicerate arthropod. As a consequence of processes influenced by a central circadian clock, Limulus can see at night nearly as well as they do during the day. The effects of the clock on horseshoe crab LE retinas are diverse and include changes in structure, gene expression, and rhabdom biochemistry. An examination of the known effects of circadian rhythms on LEs shows that the effects have three important outcomes: an increase in visual sensitivity at night, a rapid decrease in visual sensitivity at dawn, and maintenance of eyes in a relatively low state of sensitivity during the day, even in the dark. All three outcomes may be critically important for species’ survival. Specific effects of circadian rhythms on vision will certainly vary with species and according to life styles. Studies of the circadian regulation of Limulus vision have revealed that these effects can be extremely diverse and profound and suggest that circadian clocks can play a critical role in the ability of animals to adapt to the dramatic daily changes in ambient illumination.
Introduction
Sustained, daily changes in retinal structure and function are typically observed in animals that live in diurnal environments. These changes underlie the ability of visual systems to function optimally over large daily fluctuations in ambient illumination; many of these changes are regulated by signals from circadian clocks. Studies of the circadian regulation of vision are relatively limited. However, among invertebrates, effects of circadian signals on vision have been examined in most detail in the lateral compound eye (LE) of the American horseshoe crab Limulus polyphemus, a chelicerate arthropod. As a consequence of processes influenced by a central circadian clock, this animal sees at night nearly as well as it does during the day (Powers et al. 1991), and in Limulus LEs, the clock impacts almost every aspect of retinal function. It drives coordinated changes in retinal structure and in the physiology and biochemistry of photoreceptors in ways that are predicted to increase visual sensitivity at night. It also primes light-dependent processes predicted to produce a rapid down-regulation of visual sensitivity at first light.
This contribution describes the circadian organization of the Limulus visual system and summarizes known effects of clock input on the LE, with an emphasis on the clock’s effects on concentrations of two proteins at photosensitive membranes (rhabdoms) that are critical for the photoresponse: opsin, an integral membrane protein that is the protein component of visual pigment, and the alpha subunit of the G-protein activated by visual pigment (Gqα), a soluble protein. Data summarized here show that signals from the circadian clock drive increase in the concentrations of both proteins at the rhabdom at night, that the clock primes a rapid loss of opsin from the rhabdom at first light, and that these effects of the clock are mediated by cAMP. Also described are results that indicate the clock may produce a day-to-night change in the spectral sensitivity of photoreceptors.
Circadian organization of the Limulus visual system
Limulus has three different types of eyes. A pair of LEs and a pair of median ocelli are evident on the dorsal carapace. In addition, it has three pairs of what are referred to in the literature as “rudimentary eyes” (lateral, median, and ventral). These are larval eyes that develop before the compound eyes and median ocelli (Harzsch et al. 2006), and they persist in the adult (Fig. 1A). Each type of eye shows circadian changes in sensitivity to light (Barlow 1983; Kass and Renninger 1988).
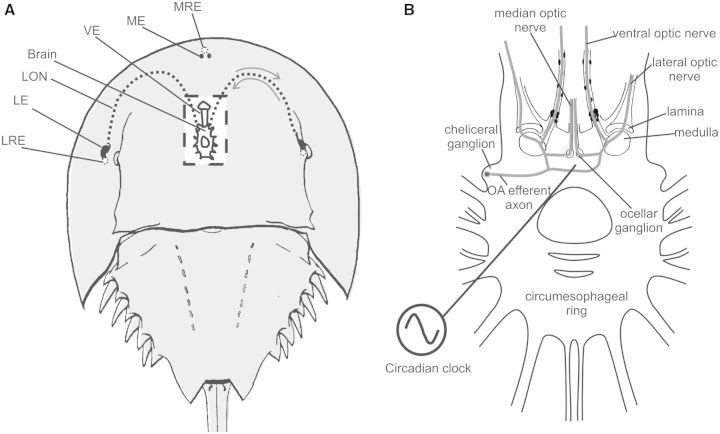
Fig. 1.
(A) Dorsal view of Limulus showing the locations of its eyes. Rectangle in center: cut-away to show the locations of the brain and the ventral eyes lying just under the ventral cuticle. The dashed lines show the projections of the lateral optic nerves. The arrows indicate that information in the optic nerve travels in two directions, from the eye to the brain and from the brain to the eye. LE, lateral eye; LON, lateral optic nerve; LRE, lateral rudimentary eye; ME, median eye; MRE, median rudimentary eye; VE, ventral eye. (B) The brain and circumesophageal ring of Limulus. The circadian clock(s) regulating the eyes is(are) in the brain. The location of one clock-driven efferent neuron is diagramed along with its proposed projections. Bilateral clusters of these efferent neurons are in the cheliceral ganglia, and each is thought to project to all of the eyes through the optic nerves. Based on Calman and Battelle (1991).
The circadian clock that influences the sensitivity of Limulus eyes is located in the animal’s brain (protocerebrum), and circadian signals reach the eyes via well-characterized clock-driven efferent neurons that project from the brain to the eyes (reviewed by Battelle 2006). The nature and location of the central circadian clock is not known, and some evidence suggests that there are two interconnected central clocks, one on each side of the brain (Kass and Barlow 1992). The central clock(s) can be entrained by illuminating any of the eyes mentioned above, as well as by illuminating extraocular photoreceptors in the tail (Hanna et al. 1988; Horne and Renninger 1988).
Although little is known about the clock itself, the clock-driven efferent neurons innervating the eyes are well characterized. Their cell bodies are located in bilateral clusters within the cheliceral ganglia deep in the protocerebrum, and anatomical and physiological evidence (Calman and Battelle 1991; Kass and Barlow 1992) suggests that each efferent neuron projects to all of the eyes (Fig. 1B). A critical feature of the clock-driven efferent input to the eyes is that it is active only at night and is silent during the day (Barlow 1983). In natural illumination, the efferents begin firing action potentials about 45 min before sunset and stop firing at about sunrise (Pieprzyk et al. 2003; Liu and Passaglia 2011). All cell types in the LE are innervated by the clock-driven efferents; thus, all cell types are potential targets for regulation by the circadian clock.
The clock-driven retinal efferents are octopaminergic, meaning they synthesize, store, and release the biogenic amine octopamine (Battelle et al. 1982). Limulus photoreceptors, and very likely other cell types in the LE, have receptors for octopamine that are coupled to adenylyl cyclase; thus, when octopamine is applied to Limulus photoreceptors, there is an increase in intracellular cAMP (Kaupp et al. 1982; Dalal and Battelle 2010). This leads to the prediction that the effects of clock input to Limulus eyes are mediated by an increase in cAMP levels in target cells, and, as is summarized in Table 1, for many known effects of clock input to Limulus eyes, this has been shown to be the case.
Table 1.
LE responses to clock input mimicked by OA and cAMP
However, our understanding of the transmitter chemistry of the clock-driven efferent neurons is incomplete. Postsynaptic targets of clock input probably express more than one type of octopamine receptor, and some receptors may produce effects other than an increase in cAMP. Furthermore, in addition to octopamine, these efferents probably release one or more neuroactive peptides that may influence retinal functions. Terminals of the clock-driven efferents contain dense granules that are characteristic of peptidergic neurons in arthropods (Fahrenbach 1981), but the peptides have not yet been identified.
The clock-driven, octopaminergic efferents are not the only efferent inputs to LEs. There is an entirely separate Substance P/FMRFamide-containing efferent projection to LEs, that also has been implicated in the regulation of LE function (Chamberlain and Engbretson 1982; Mancillas and Brown 1984; Mancillas and Selverston 1984, 1985; Lewandowski et al. 1989; Bolbecker et al. 2009). However, since this projection has not yet been shown to be regulated by a circadian clock, a discussion of this projection is outside the scope of the current contribution.
Effects of the clock on Limulus eyes
The known effects of the clock on Limulus LE retinas are diverse, ranging from changes in structure to gene expression (Table 1). Some effects result from processes directly driven by clock input (points 1–12 in Table 1). Each of these is predicted to increase sensitivity and responsiveness of LEs to light at night. Other effects involve processes triggered or driven by light but which must be “primed” by clock input. This means that if clock input is eliminated, these light-dependent processes do not occur. Among processes primed by clock input are light-dependent movements of screening pigment granules within photoreceptors and light-triggered transient rhabdom shedding (13 and 14 in Table 1). These “clock-primed” processes are predicted to decrease the sensitivity of LEs at first light.
Clock-dependent changes in retinal structure
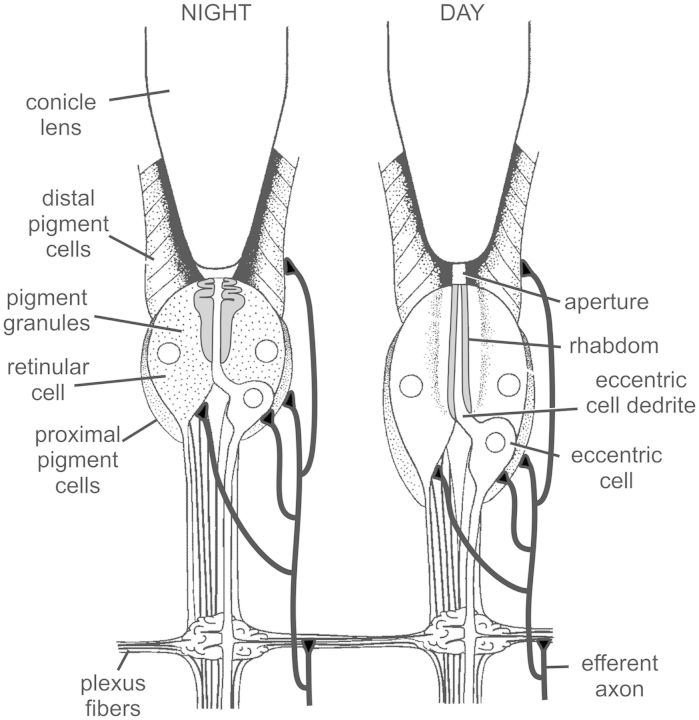
Structural changes are among the most dramatic effects of clock input to LEs (Fig. 2). During the day in the light, the aperture at the base of the lens is long and narrow, the rhabdom is extended below the narrow aperture and photoreceptor screening pigment is clustered near the rhabdom. At night, pigment cells move away from the base of the lens, thereby widening and shortening the aperture. Photoreceptors move closer to the base of the lens, and the distal half of the rhabdom close to the base of the lens is thrown into extensive folds, increasing the probability that entering photons strike the rhabdom. Screening pigment also disperses away from the rhabdom, decreasing the probability of absorbing incoming photons. Structural changes continue in constant darkness, indicating they are driven by the clock, but their amplitude is reduced. Thus, the effects of the clock on structure are amplified by diurnal light. However, in the absence of clock input, no structural changes are observed, even in diurnal light, and the structure of the ommatidia becomes frozen in a configuration not seen in a normal eye (Chamberlain and Barlow 1987).
Fig. 2.
Longitudinal sections through LE ommatidia in their daytime and nighttime states. Based on Barlow et al. (1980) and Chamberlain and Barlow (1987). See text for details. The schematic also shows projections of the clock-driven efferent neurons innervating all cell-types in the LE. Based on Fahrenbach (1981).
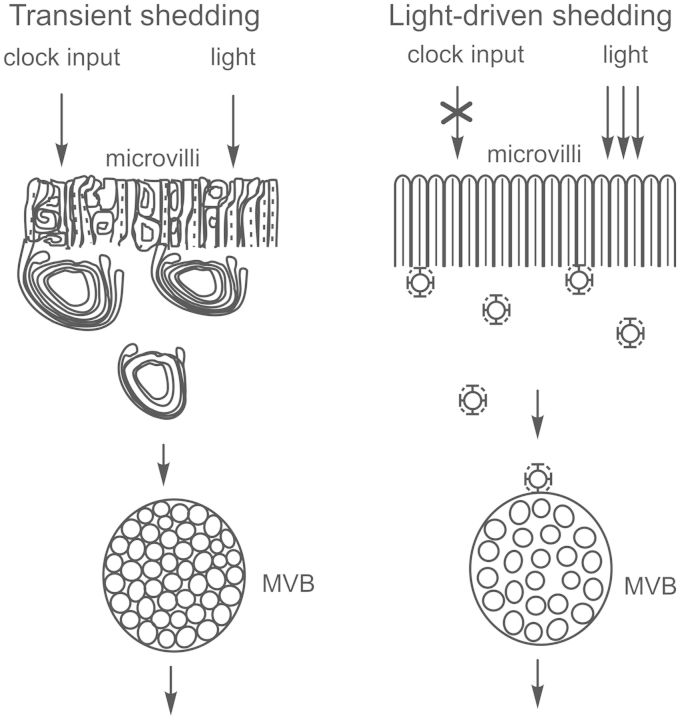
Another clock-dependent structural change is transient rhabdom shedding, a process triggered by the dim light of dawn and which must be primed by clock input the night before. During transient shedding, microvilli in the rhabdomeral rays rapidly and transiently become disorganized and membranes containing opsin are shed (Chamberlain and Barlow 1984). This process is distinct from a second membrane-shedding process called light-driven shedding that is not dependent on clock input, continues throughout the day in the light, and is a clathrin-mediated endocytosis of membranes containing opsin (Sacunas et al. 2002) (Fig. 3).
Fig. 3.
Schematic differentiating clock-primed, light-triggered transient rhabdom-shedding from light-driven shedding. Transient shedding is primed by clock input during the night and triggered by the dim light of dawn. It is characterized by a rapid, transient disorganization of microvilli in the rays of the rhabdom and a breakdown of the actin cytoskeleton, accompanied by the formation of large whorls of opsin-containing membranes that accumulate between the rays of the rhabdom as densely packed multivesicular bodies (MVBs). Light-driven shedding is a progressive process driven by brighter light that does not require clock input. It is characterized by the clathrin-mediated endocytosis of opsin-containing membranes from the base of the microvilli that then accumulates in loosely packed MVBs. Transient shedding begins at about sunrise and is largely complete by 1 h after sunrise; light-driven shedding continues throughout the remaining daylight hours. Based on Chamberlain and Barlow (1979), Sacunas et al. (2002), and Battelle (2013).
Clock-dependent changes in rhabdom biochemistry
As described above, clock-driven structural changes in ommatidia that begin at dusk are predicted to increase the capture of photons by rhabdoms. Recent studies, summarized below and described in detail by Battelle et al. (2013), indicate that as the clock drives changes in rhabdom structure, it also drives changes in rhabdom biochemistry that are predicted to increase the probability that an incoming photon initiates phototransduction. In these studies, we examined diurnal changes in the concentrations at rhabdoms of proteins that drive phototransduction, opsins, and Gqα. There is good evidence that the concentrations of these proteins in rhabdoms influence the sensitivity of photoreceptors to light (Stephenson et al. 1983; Frechter et al. 2007). The concentrations of these proteins at Limulus rhabdoms were quantified by immunocytochemistry and confocal microscopy using procedures described in detail elsewhere (Katti et al. 2010; Battelle et al. 2013). Briefly, the total immunoreactive intensity present over rhabdoms was divided by the total area of rhabdoms to produce a measure of protein concentration: average immunoreactive intensity per µm2 of rhabdom.
The opsins on which we focused are Limulus opsins 1 and 2. These are encoded by two closely related genes (Smith et al. 1993; Dalal et al. 2003) and quantitatively are the major opsins expressed in LE rhabdoms (Katti et al. 2010). The antibody used in this study was generated against the C-terminus of opsin 1. Since opsins 1 and 2 differ from one another by only one amino acid in this region, the two opsins cannot be distinguished immunocytochemically (Battelle et al. 2001). Therefore, the opsin immunoreactivity described here is the combined immunoreactivity of opsins 1 and 2 (Ops1-2).
The intensities of Ops1-2- and Gqα-immunoreactivity (ir) at rhabdoms (RhOps1-2 and RhGqα) change significantly with a diurnal rhythm; the immunoreactive intensities of both proteins at rhabdoms was greater during the night compared with daytime (Fig. 4). The dynamics of these diurnal changes in natural illumination were quantified, and the circadian clock’s role in producing these changes was tested by comparing RhOps1-2 and RhGqα concentrations in LEs with and without clock input. Clock input to LEs was eliminated by cutting the lateral optic nerve (Fig. 1) (Battelle et al. 2013).
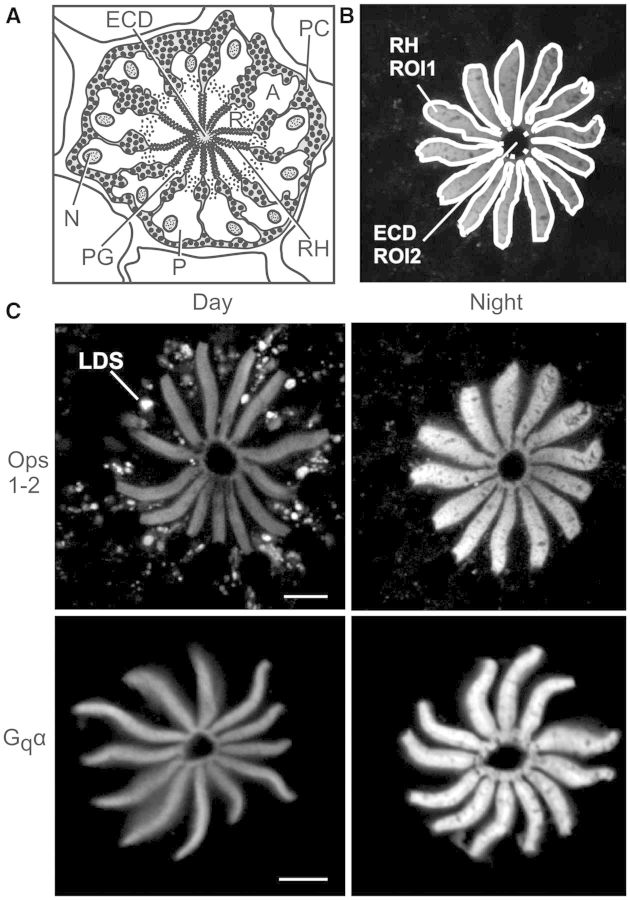
Fig. 4.
Immunoreactive intensities of Ops1-2 and Gqα over rhabdoms changes significantly from day to night. (A) Cross-section of a LE ommatidium. The following are labeled: A, arhabdomeral segment; ECD, eccentric cell dendrite; N, nucleus; PC pigment cells; PG, pigment granules in photoreceptors; R, rhabdomeral segment; and RH, rhabdom. (B) Confocal image of Ops1-2-ir in the R-segment and proximal A-segment of an ommatidium from a LE fixed during the night. Shown are the regions of interest (ROIs) drawn to quantify average immunoreactive intensities over rhabdoms. Total intensity of ROI1 minus ROI2 was divided by total area of ROI1 minus ROI2 to calculate the average intensity over rhabdoms/µm2. (C) Representative images of single confocal optical sections showing Ops1-2- and Gqα-ir in the R-segment and proximal A-segment of LEs fixed at midday (Day) and during the night (Night), between 4 and 6 h after sunset. Day-images and night-images of each antigen were immunostained and imaged together during the same confocal session, using identical confocal settings. Images were intensified in Photoshop to exactly the same extent and then assembled in CorelDraw. LDS, opsin-containing membranes shed during the day by light-driven shedding. Scale bar = 10 µm.
In eyes with intact optic nerves that receive natural clock input, the concentration of RhOps1-2 begins to increase gradually before dark and continues to increase gradually after dark until it reaches a maximum concentration at about 4 h after sunset (Fig. 5A, + Clock). The concentration of RhOps1-2 remains high throughout the night and into dawn, then falls sharply between sunrise and 1 h after sunrise (SR + 1). This sharp decline correlates with clock-primed, transient rhabdom shedding. From previous studies of transient rhabdom shedding, it was not clear whether it was a mechanism for opsin turnover or a mechanism that reduces the opsin concentration at rhabdoms. Figure 5 shows clearly that transient shedding rapidly decreases the concentration of Ops1-2 in rhabdoms. After SR + 1, RhOps1-2 falls more slowly until at 3 h after sunrise it is at the same level observed at midday. The more gradual decline is attributed to light-driven shedding because it also occurs in LEs without clock input and thus is driven solely by light.
Fig. 5.
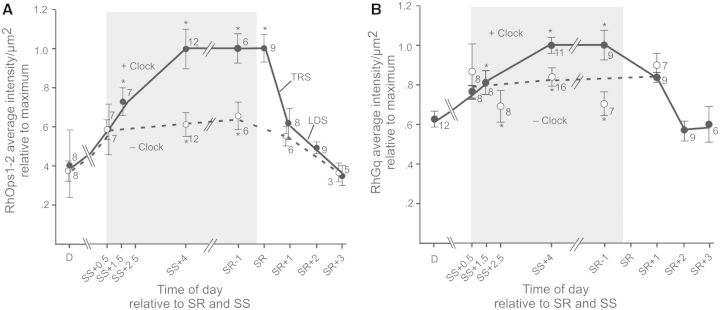
Clock input drives increases in concentrations of (A) RhOps1-2 and of (B) Rh Gqα. The average intensity of RhOps1-2- and RhGqα-ir per µm2 of rhabdom is plotted versus time of day in hours relative to sunset (SS) and sunrise (SR). All animals were exposed to natural illumination. The shaded area indicates when it was dark in the room. The figure summarizes three series of studies: (1) A Dusk Series that included LEs fixed between midday (D) and SS + 4. (2) A Dawn Series that included LEs fixed from SR-1 to SR + 3. Concentrations of RhOps1-2 and RhGqα did not change significantly between SS + 4 and SR-1; therefore, these series could be combined. The data are normalized relative to the maximum concentration. All eyes assayed in Series 1 and 2 had intact optic nerves and thus received normal clock input (+Clock) (filled circles and dotted line). The asterisk in the dusk series indicates when concentrations of rhabdomeral protein were significantly higher than at midday (D). In the dawn series, the asterisk indicates when RhOps1-2 concentrations were significantly higher than at SR + 1 and RhGqα concentrations were significantly higher than at SR + 2 (P < 0.05). (3) In series 3, direct comparisons were made between RhOps1-2 or RhGqα concentrations in LEs from the same animal, one with, and the other without, clock input. The open circles and dashed line shows the average concentrations of RhOps1-2 or RhGqα in LEs lacking clock input (− Clock) relative to that in LEs with clock input (+ Clock). The asterisks (*) associated with these points indicate when there was a significant difference between RhOps1-2 or RhGqα concentrations in LEs with, and without, clock input. Means are plotted ± SEM. The number at each time-point indicates the number of animals assayed. The fall in concentration of RhOps1-2 during the morning is attributed to transient rhabdom shedding (TRS), and light-driven shedding (LDS), as indicated. For details, see Battelle (2013).
In eyes with severed optic nerves (− Clock), the RhOps1-2 concentration also increases gradually before dark, until it is about 20% above the midday level, but then the increase stalls, and throughout the night, in LEs without clock input, RhOps1-2 remains about 40% below the concentration in eyes with clock input. However, by 1 h after sunrise, after transient rhabdom shedding in LEs with clock input has occurred, RhOps1-2 concentrations in LEs with and without clock input are the same, and they remain the same throughout the day.
Similar results were obtained when RhGqα concentrations were examined (Fig. 5B). In eyes with intact optic nerves and natural clock input (+ Clock), the RhGqα concentration increases gradually through dusk and into the night, but at night, in eyes with severed optic nerves and no clock input, the RhGqα concentration remains significantly below that observed in eyes with clock input.
These results indicate that clock input is required for maximum dark-adaptive increases in Ops1-2 and in Gqα concentrations at rhabdoms. This was tested further by examining changes in RhOps1-2 and in RhGqα concentrations after 4 h of daytime dark-adaptation in vivo. Because clock input to LEs is naturally silent during the day, we predicted that during daytime dark-adaptation, changes in RhOps1-2 and in RhGqα concentrations would be similar to those observed during nighttime dark-adaptation in LEs without clock input. This is what we found. With 4 h of daytime dark-adaptation, RhOps1-2 concentrations increase significantly (approximately 20%) but also remain significantly below the concentration in normal nighttime dark-adapted eyes. The RhGqα concentration does not increase at all during 4 h of daytime dark-adaptation and remains significantly below that in normal eyes dark-adapted at night.
Many effects of clock input on LEs are mediated by an increase in cAMP. We therefore tested whether the same might be true for the clock-driven increases in RhOps1-2 and RhGqα concentrations. This was tested with in vitro experiments in which, during daytime dark-adaptation, slices of LEs were incubated without or with treatments known to increase intracellular cAMP: octopamine, the neurotransmitter released from the clock-driven efferent neurons that activates postsynaptic receptors coupled to adenylyl cyclase (Kaupp et al. 1982; Dalal and Battelle 2010) or forskolin, a direct activator of adenylyl cyclase (Beavo et al. 1970) (Fig. 6). These treatments significantly increased the dark-adaptive rise of RhOps 1-2 and RhGqα concentrations by an average of 35% (P < 0.05, N = 13) and 65% (P < 0.01, N = 10), respectively (Battelle et al. 2013). These results provide evidence that, as is the case for many other effects of clock input to Limulus eyes (Table 1), the clock-dependent rise in RhOps1-2 and RhGqα is mediated by a rise in cAMP.
Fig. 6.
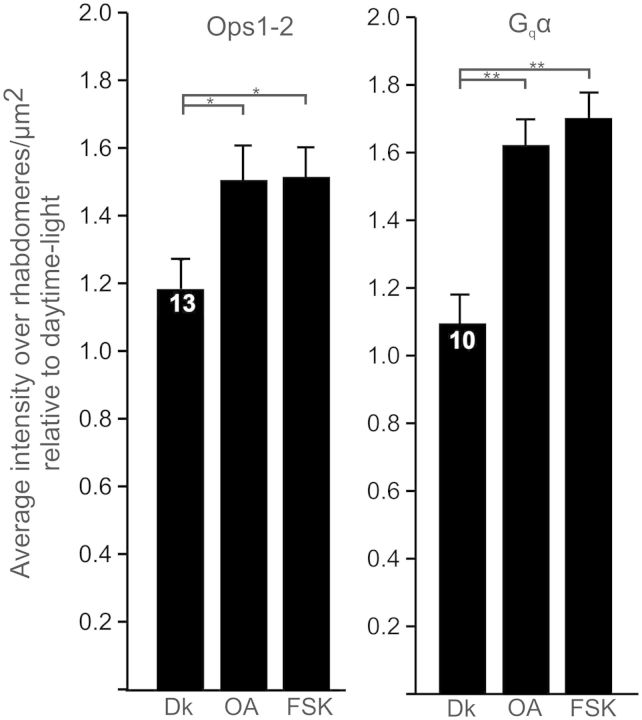
Octopamine and forskolin increase concentrations of RhOps1-2 and RhGqα during daytime dark-adaptation in vitro. LEs were dissected from animals at midday. Each LE was cut in half to yield four slices from each animal. One slice from each animal was fixed immediately in the light. The other three slices were incubated 4 h at room temperature in the dark in an organ-culture medium (Katti et al. 2010) +0.08% dimethyl sulfoxide and one of the following: Dk, no further additions; OA, octopamine (40 µM)+ IBMX (1 mM); FSK, forskolin (10 µM) + IBMX (1 mM). RhOps1-2 and RhGqα were quantified in each slice as described in Fig. 5. Data are expressed as mean immunoreactive intensities/µm2 of rhabdom ± SEM. Rhabdomeral protein concentrations in each dark-adapted slice were normalized to those in the light-adapted slice of the same animal. The light-adapted concentrations are expressed as 1. The number of animals assayed is shown in the first bar of each dataset. The significances of differences among the dark-adapted treatments are as follows: *P < 0.05; **P < 0.01. From Battelle et al. (2013).
The clock’s effects on concentrations of RhOps1-2 may change the spectral sensitivity of LE photoreceptors
It is clear from the experiments described above that in LEs with intact optic nerves and endogenous clock input, the concentration of RhOps1-2 roughly doubles from day to night. Ops1-2, however, is not the only opsin present in rhabdoms of LE photoreceptors. The receptors contain at least one additional opsin, opsin 5 (Ops5) that belongs to a clade of opsins predicted to have a spectral sensitivity that is blue-shifted compared with Ops1-2 (Katti et al. 2010). Unlike RhOps1-2, RhOps5 concentrations do not change significantly from day to night and are not influenced by the clock (Katti et al. 2010). As a result, the relative levels of Ops1-2 and 5 in rhabdoms change significantly from day to night (Fig. 7). Quantification of relative concentrations of Ops1-2 and 5 in rhabdoms indicates that at night the mean molar ratio of Ops5 in rhabdoms is 20% (N = 6) that of Ops1-2. This ratio is estimated to increase to 40% during the day because of the dramatic decrease in RhOps1-2 accompanied by no decrease in Ops5 (Fig. 6). Thus, although Ops1-2 remains the dominant opsins in rhabdoms both by day and by night, Ops5 should contribute more to the photoreceptor’s response during the day. If the spectral sensitivity of Ops5 is significantly different from that of Ops1-2, this could produce a day-to-night shift in the LE’s spectral tuning. Experiments designed to test this hypothesis are in progress.
Fig. 7.
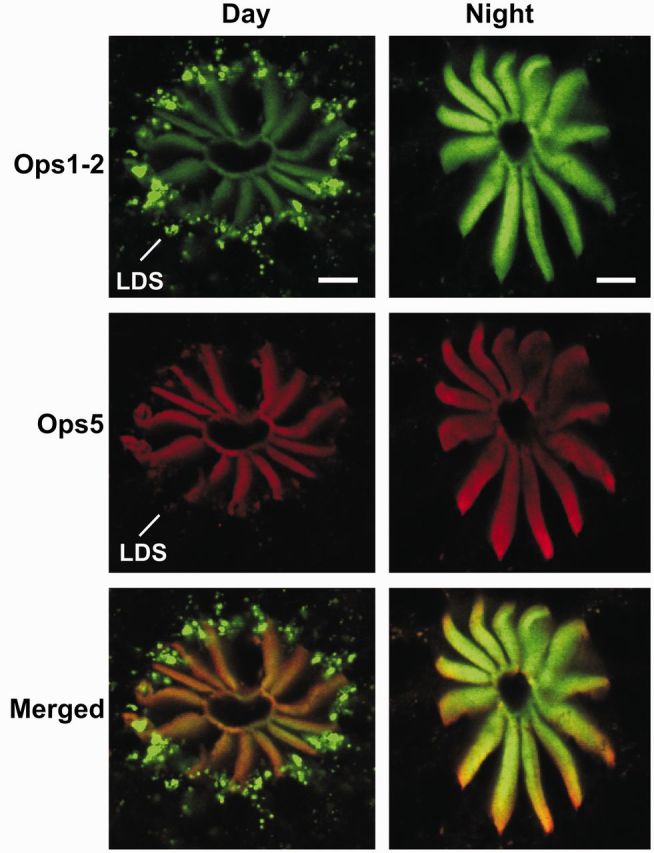
The relative level of Ops1-2 and Ops5 in rhabdoms changes from day to night. Shown for each time-point are confocal images of sequential scans of single optical sections and their merged images (Ops1-2-ir, green; Ops5-ir, red). Sections were immunostained at the same time and imaged during a single confocal session using identical settings. All images were intensified in Photoshop to exactly the same extent. During the night, the intensity of RhOps1-2-ir is considerably higher than during the day, whereas the intensity of RhOps5-ir does not change significantly from day to night. Rhabdomeral membrane shed during the day by LDS contains both Ops1-2- and Ops5-ir, although Ops1-2-ir in debris is clearly more intense. Scale bar = 10 µm.
Conclusions
Considering together all the known influences of the circadian clock on Limulus vision (Table 1), three important outcomes can be identified: an increase in visual sensitivity at night that is driven by the clock, a rapid decrease in visual sensitivity at dawn that is primed by the clock, and because clock input is silent during the day, maintenance of eyes in a relatively low state of sensitivity during the day, even when the eyes are in the dark. Increased visual sensitivity at night and a rapid decline in sensitivity at dawn may be critical for allowing the eyes to function optimally in very low light and in extremely bright light. Optimal visual function during the day and night may be critical for survival since Limulus use their eyes to find mates during spawning, and they spawn both during the day and at night (Barlow et al. 1982; Schwab and Brockmann 2007). Furthermore, Limulus often bury in the mud for long periods. Therefore, it may be particularly important for Limulus to maintain their eyes in a state of low sensitivity during the day, even in the dark, to prevent retinal damage should they emerge into the bright light of day.
Another potentially important consequence of the clock’s influence on Limulus photoreceptors is a day-to-night shift in relative levels of co-expressed opsins in photosensitive membranes that may produce daily changes in the photoreceptor’s spectral tuning. Limulus photoreceptors provide the first example of photoreceptors in which the levels of co-expressed opsin proteins in rhabdoms are regulated differently by light and a circadian clock, but evidence is accumulating that the photoreceptors of many invertebrates express multiple opsins. Limulus photoreceptors provide the first example for the differential regulation of opsin protein co-expression at rhabdoms by light and a circadian clock, but as more species are investigated the phenomenon may be discovered to be wide-spread. Studies with Limulus also provide the first evidence that the clock’s influence on levels of rhabdomeral opsin is exerted down-stream of transcription (Dalal et al. 2003; Katti et al. 2010; Battelle et al. 2013) and involves processes regulated by cAMP.
Light-dependent and dark-dependent changes in the concentration of G-proteins activated by photopigments are observed in photosensitive membranes in both ciliated and rhabdomeral photoreceptors, and across species these changes are attributed to the translocation of the G-protein between the cytoplasmic and photosensitive compartments (Frechter et al. 2007; Arshavsky and Burns 2012). In no system are the mechanisms regulating these translocations understood. G-protein translocations in rhabdomeral photoreceptors have been examined in detail only in retinas of Drosophila, a preparation only marginally influenced by circadian rhythms. Studies of Limulus eyes provide the first evidence that Gqα translocation to photosensitive membranes in the dark can be regulated by a circadian clock and that this process involves regulation by cAMP.
Circadian changes in visual sensitivity have been observed in a wide range of arthropod species (reviewed by Fleissner and Fleissner 1988) but the processes impacted and the underlying mechanisms are largely unexplored. Mechanisms similar to those described in Limulus may be typical among chelicerates. For example, the eyes of scorpions and spiders show circadian changes in sensitivity and, like Limulus, are innervated by clock-driven, octopaminergic efferent neurons that are active at night and silent during the day (Fleissner and Fleissner 1985; Yamashita 2002). Specific effects of circadian rhythms on vision will certainly vary with species and according to life styles. Studies of the circadian regulation of vision in Limulus have revealed that these effects can be extremely diverse and profound. Clearly, an understanding of the circadian clock’s effects on vision is required for a full understanding of how animals adapt to the dramatic daily changes in ambient illumination.
Funding
This work was funded by the National Science Foundation (IOS 0517273, IOS 1146175, DBI 0648969 to B.A.B.) and internal funds of the Whitney Laboratory.
References
- Arshavsky VY, Burns ME. Photoreceptor signaling: supporting vision across a wide range of sight sensitivities. J Biol Chem. 2012;287:1620–6. doi: 10.1074/jbc.R111.305243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RB, Chamberlain SC, Levinson J. Limulus brain modulates the structure and function of the lateral eyes. Science. 1980;210:1037–9. doi: 10.1126/science.7434015. [DOI] [PubMed] [Google Scholar]
- Barlow RB. Circadian-rhythms in the Limulus visual-system. J Neurosci. 1983;3:856–70. doi: 10.1523/JNEUROSCI.03-04-00856.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RB, Bolanowski SJ, Brachman ML. Efferent optic neve fibers mediate circadian rhythms in the Limulus eye. Science. 1977;197:86–9. doi: 10.1126/science.867057. [DOI] [PubMed] [Google Scholar]
- Barlow RB, Kaplan E, Renninger GH, Saito T. Circadian-rhythms in Limulus photoreceptors. 1. Intracellular studies. J Gen Physiol. 1987;89:353–78. doi: 10.1085/jgp.89.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow RB, Ireland L, Kass L. Vision has a role in Limulus mating behavior. Nature. 1982;296:65–6. doi: 10.1038/296065a0. [DOI] [PubMed] [Google Scholar]
- Battelle BA. The eyes of Limulus polyphemus (Xiphosura, Chelicerata) and their afferent and efferent projections. Arthropod Struct Dev. 2006;35:261–74. doi: 10.1016/j.asd.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Battelle BA, Evans JA, Chamberlain SC. Efferent fibers to Limulus eyes synthesize and release octopamine. Science. 1982;216:1250–2. doi: 10.1126/science.6123151. [DOI] [PubMed] [Google Scholar]
- Battelle BA, Andrews A, Calman B, Sellers J, Greenberg R, Smith WC. A myosin III from Limulus eyes is a clock-regulated phosphoprotein. J Neurosci. 1998;18:4548–59. doi: 10.1523/JNEUROSCI.18-12-04548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelle BA, Williams CD, Schremser-Berlin JL, Cacciatore C. Regulation of arrestin mRNA levels in Limulus lateral eye: separate and combined influences of circadian efferent input and light. Vis Neurosci. 2000;17:217–27. doi: 10.1017/s0952523800172049. [DOI] [PubMed] [Google Scholar]
- Battelle BA, Dabdoub A, Malone MA, Andrews AW, Cacciatore C, Calman BG, Smith WC, Payne R. Immunocytochemical localization of opsin, visual arrestin, myosin III, and calmodulin in Limulus lateral eye retinular cells and ventral photoreceptors. J Comp Neurol. 2001;435:211–25. doi: 10.1002/cne.1203. [DOI] [PubMed] [Google Scholar]
- Battelle BA, Kempler KE, Parker AK, Gaddie CD. Opsin 1-2, Gqα and arrestin levels at Limulus rhabdoms are controlled by diurnal light and a circadian clock. J Exp Biol. 2013 doi: 10.1242/jeb.083519. published online (PMID: 23393287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA, Rogers NL, Crofford OB, Hardman JG, Sutherland EW, Newman E. Effects of xanthine derivatives on lipolysis and on adenosine 3′,5′-monophosphate phosphodiesterase activity. Mol Pharmacol. 1970;6:597–603. [PubMed] [Google Scholar]
- Bolbecker AR, Lim-Kessler CCM, Li J, Swan A, Lewis A, Fleets J, Wasserman GS. Visual efference neuromodulates retinal timing: in vivo roles of octopamine, Substance P, circadian phase, and efferent activation in Limulus. J Neurophysiol. 2009;102:1132–8. doi: 10.1152/jn.91167.2008. [DOI] [PubMed] [Google Scholar]
- Calman BG, Battelle BA. Central origin of the efferent neurons projecting to the eyes of Limulus polyphemus. Vis Neurosci. 1991;6:481–95. doi: 10.1017/s0952523800001334. [DOI] [PubMed] [Google Scholar]
- Cardasis HL, Stevens SM, McClung S, Kempler KE, Powell DH, Eyler JR, Battelle BA. The actin-binding interface of a myosin III is phosphorylated in vivo in response to signals from a circadian clock. Biochemistry. 2007;46:13907–19. doi: 10.1021/bi701409f. [DOI] [PubMed] [Google Scholar]
- Chamberlain SC, Barlow RB. Light and efferent activity control rhabdom turnover in Limulus photoreceptors. Science. 1979;206:361–3. doi: 10.1126/science.482946. [DOI] [PubMed] [Google Scholar]
- Chamberlain SC, Barlow RB. Transient membrane shedding in Limulus photoreceptors—control mechanisms under natural lighting. J Neurosci. 1984;4:2792–810. doi: 10.1523/JNEUROSCI.04-11-02792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SC, Barlow RB. Control of structural rhythms in the lateral eye of Limulus: interactions of natural lighting and circadian efferent activity. J Neurosci. 1987;7:2135–44. doi: 10.1523/JNEUROSCI.07-07-02135.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SC, Engbretson GA. Neuropeptide immunoreactivity in Limulus: Substance-P-like immunoreactivity in the lateral eye and protocerebrum. J Comp Neurol. 1982;208:304–15. doi: 10.1002/cne.902080307. [DOI] [PubMed] [Google Scholar]
- Dalal JS, Battelle BA. Circadian regulation of Limulus visual functions: a role for octopamine and cAMP. Curr Zool. 2010;56:518–36. [Google Scholar]
- Dalal JS, Jinks RN, Cacciatore C, Greenberg RM, Battelle BA. Limulus opsins: diurnal regulation of expression. Vis Neurosci. 2003;20:523–34. doi: 10.1017/s095252380320506x. [DOI] [PubMed] [Google Scholar]
- Edwards SC, Battelle BA. Octopamine-stimulated and cyclic AMP-stimulated phosphorylation of a protein in Limulus ventral and lateral eyes. J Neurosci. 1987;7:2811–20. doi: 10.1523/JNEUROSCI.07-09-02811.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SC, Andrews A, Renninger G, Wiebe E, Battelle BA. Efferent innervation to Limulus eyes in vivo phosphorylates a 122 kd protein. Bio Bull. 1990;178:267–78. doi: 10.2307/1541828. [DOI] [PubMed] [Google Scholar]
- Fahrenbach WH. The morphology of the horseshoe-crab (Limulus-polyphemus) visual-system. 7. Innervation of photoreceptor neurons by neurosecretory efferents. Cell Tissue Res. 1981;216:655–9. doi: 10.1007/BF00238660. [DOI] [PubMed] [Google Scholar]
- Fleissner G, Fleissner G. Neurobiology of a circadian clock in the visual system of scorpions. In: Barth FG, editor. Neurobiology of arachnids. Berlin: Springer Verlag; 1985. pp. 351–75. [Google Scholar]
- Fleissner G, Fleissner G. Information processing in animals. 1988;Vol. 5 Efferent control of visual sensitivity in arthropode: with emphasis on circadian rhythms, Vol. 5. Mainz, West Germany: Akademie Der Wissenschaften und Der Literatur; New York (NY): Gustav Fischer Verlag; West Germany: Stuttgart. [Google Scholar]
- Frechter S, Elia N, Tzarfaty V, Selinger Z, Minke B. Translocation of G(q)alpha mediates long-term adaptation in Drosophila photoreceptors. J Neurosci. 2007;27:5571–83. doi: 10.1523/JNEUROSCI.0310-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna WJB, Horne JA, Renninger GH. Circadian photoreceptor organs in Limulus. 2. The telson. J Comp Physiol A. 1988;162:133–40. [Google Scholar]
- Harzsch S, Vilpoux K, Blackburn DC, Platchetzki D, Brown NL, Melzer RR, Kempler KE, Battelle BA. Evolution of arthropod visual systems: development of the eyes and central visual pathways in the horseshoe crab Limulus polyphemus Linnaeus, 1758 (Chelicerata, Xiphosura) Dev Dynam. 2006;235:2641–55. doi: 10.1002/dvdy.20866. [DOI] [PubMed] [Google Scholar]
- Horne JA, Renninger GH. Photoreceptor organs in Limulus, I. Ventral, median and lateral eyes. J Comp Physiol A. 1988;162:127–32. [Google Scholar]
- Kaplan E, Barlow RB. Circadian clock in Limulus brain increases response and decreases noise of retinal photoreceptors. Nature. 1980;286:393–5. doi: 10.1038/286393a0. [DOI] [PubMed] [Google Scholar]
- Kass L, Barlow RB. A circadian clock in the Limulus brain transmits synchronous efferent signals to all eyes. Vis Neurosci. 1992;9:493–504. doi: 10.1017/s0952523800011299. [DOI] [PubMed] [Google Scholar]
- Kass L, Barlow RB. Efferent neurotransmission of circadian rhythms in Limulus lateral eye. I. Octopamine-induced increases in retinal sensitivity. J Neurosci. 1984;4:908–17. doi: 10.1523/JNEUROSCI.04-04-00908.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass L, Renninger GH. Circadian change in function of Limulus ventral photoreceptors. Vis Neurosci. 1988;1:3–11. doi: 10.1017/s0952523800000985. [DOI] [PubMed] [Google Scholar]
- Katti C, Kempler K, Porter ML, Legg A, Gonzalez R, Garcia-Rivera E, Dugger D, Battelle BA. Opsin co-expression in Limulus photoreceptors: differential regulation by light and a circadian clock. J Exp Biol. 2010;213:2589–601. doi: 10.1242/jeb.043869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp UB, Malbon CC, Battelle BA, Brown JE. Octopamine stimulated rise of cAMP in Limulus ventral photoreceptors. Vis Res. 1982;22:1503–6. doi: 10.1016/0042-6989(82)90216-4. [DOI] [PubMed] [Google Scholar]
- Kempler K, Toth J, Yamashita R, Mapel G, Robinson K, Cardasis H, Stevens S, Sellers JR, Battelle BA. Loop 2 of Limulus myosin III is phosphorylated by protein kinase A and autophosphorylation. Biochemistry. 2007;46:4280–93. doi: 10.1021/bi062112u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadilkar RV, Mytinger JR, Thomason LE, Runyon SL, Washicosky KJ, Jinks RN. Central regulation of photosensitive membrane turnover in the lateral eye of Limulus. I. Octopamine primes the retina for daily transient rhabdom shedding. Vis Neurosci. 2002;19:283–97. doi: 10.1017/s0952523802192066. [DOI] [PubMed] [Google Scholar]
- Kier C, Chamberlain SC. Dual controls for screening pigment movement in photoreceptors of the Limulus lateral eye: circadian efferent input and light. Vis Neurosci. 1990;4:237–55. doi: 10.1017/s0952523800003382. [DOI] [PubMed] [Google Scholar]
- Lewandowski TJ, Lehman HK, Chamberlain SC. Immunoreactivity in Limulus. 3. Morphological and biochemical studies of FMRFamide-like immunoreactivity and colocalized substanceP-like immunoreactivity in the brain and lateral eye. J Comp Neurol. 1989;288:136–53. doi: 10.1002/cne.902880111. [DOI] [PubMed] [Google Scholar]
- Liu JS, Passaglia CL. Spike firing pattern of output neurons of the Limulus circadian clock. J Biol Rhythms. 2011;26:335–44. doi: 10.1177/0748730411409712. [DOI] [PubMed] [Google Scholar]
- Mancillas JR, Brown MR. Neuropeptide modulation of photoreceptor sensitivity. 1. Presende, distribution, and characterization of a substance-p-like peptide in the lateral eye of Limulus. J Neurosci. 1984;4:832–46. doi: 10.1523/JNEUROSCI.04-03-00832.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancillas JR, Selverston AI. Neuropeptide modulation of photoreceptor sensitivity. 2. Physiological and anatomical effects of substance-p on the lateral eye of Limulus. J Neurosci. 1984;4:847–59. doi: 10.1523/JNEUROSCI.04-03-00847.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancillas JR, Selverston AI. Substance P-like immunoreactivity is present in the central nervous system of Limulus polyphemus. J Comp Neurol. 1985;238:38–52. doi: 10.1002/cne.902380104. [DOI] [PubMed] [Google Scholar]
- Pieprzyk A, Weiner W, Chamberlain S. Mechanisms controlling the sensitivity of the Limulus lateral eye in natural lighting. J Comp Physiol A. 2003;189:643–53. doi: 10.1007/s00359-003-0437-8. [DOI] [PubMed] [Google Scholar]
- Powers M, Barlow RJ, Kass L. Visual performance of horseshoe crabs day and night. Vis Neurosci. 1991;7:179–89. doi: 10.1017/s0952523800004016. [DOI] [PubMed] [Google Scholar]
- Renninger GH, Schimmel R, Farrell CA. Octopamine modulates photoreceptor function in the Limulus lateral eye. Vis Neurosci. 1989;3:83–94. doi: 10.1017/s0952523800004405. [DOI] [PubMed] [Google Scholar]
- Runyon SL, Washicosky KJ, Brenneman RJ, Kelly JR, Khadilkar RV, Heacock KF, McCormick SM, Williams KE, Jinks RN. Central regulation of photosensitive membrane turnover in the lateral eye of Limulus. II. Octopamine acts via adenylate cyclase/cAMP-dependent protein kinase to prime the retina for transient rhabdom shedding. Vis Neurosci. 2004;21:749–63. doi: 10.1017/s0952523804215097. [DOI] [PubMed] [Google Scholar]
- Sacunas RB, Papuga MO, Malone MA, Pearson AC, Marjanovic M, Stroope DG, Weiner WW, Chamberlain SC, Battelle BA. Multiple mechanisms of rhabdom shedding in the lateral eye of Limulus polyphemus. J Com Neurol. 2002;449:26–42. doi: 10.1002/cne.10263. [DOI] [PubMed] [Google Scholar]
- Schwab RL, Brockmann HJ. The role of visual and chemical cues in the mating decisions of satellite male horseshoe crabs, Limulus polyphemus. Anim Behav. 2007;74:837–46. [Google Scholar]
- Smith WC, Price DA, Greenberg RM, Battelle BA. Opsins from the lateral eyes and ocelli of the horseshoe-crab, Limulus-polyphemus. Proc Natl Acad Sci U S A. 1993;90:6150–4. doi: 10.1073/pnas.90.13.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson RS, O’Tousa J, Scavarda NJ, Randall LL, Pak WL. Drosophila mutants with reduced rhodopsin content. Symp Soc Exp Biol. 1983;36:477–501. [PubMed] [Google Scholar]
- Yamashita S. Efferent innervation of photoreceptors in spiders. Microsc Res Tech. 2002;58:356–64. doi: 10.1002/jemt.10143. [DOI] [PubMed] [Google Scholar]