Abstract
Objective
We have previously described thermoregulatory responses of severely burned children during submaximal exercise in a thermoneutral environment. However, the thermoregulatory response of burned children to exercise in the heat is not well understood and could have important safety implications for rehabilitation.
Study design
Children (n=10) with >40% total body surface area burns and non-burned children (n=10) performed a 30 minute bout of treadmill exercise at 75% of their peak aerobic power in a heated environment. Intestinal temperature, burned and unburned skin temperature, and heart rate were recorded pre-exercise, every 2 minutes during exercise, and during recovery.
Results
Three of the 10 burned children completed the exercise bout in the heat; however, all of the non-burned children completed the 30 minute bout. One burned child reached a core body temperature > 39°C at minute 23. Burned children had a significantly higher core body temperature thru the first 12 minutes of exercise compared to non-burned children. However, 9 of 10 (90%) burned children did not become hyperthermic during exercise in the heat.
Conclusion
Specific to this study, hyperthermia did not typically occur in burned children, relative to non-burned children. Whether this is due to an intolerance to exercise in the heat or to an inability to generate sufficient heat during exercise needs to be explored further.
Keywords: exertional hyperthermia, heat illness, burns, exercise
INTRODUCTION
During exercise or periods of thermal stress, sweat production and blood flow to the skin increase. These responses are critical for normal heat dissipation to occur. For these responses to occur, the functional integrity of sweat glands, skin circulation and neural control of the skin circulation must be present [1]. However, in individuals with severe third degree burns, normal skin along with its appendages (sweat glands, nerves, and blood vessels) are destroyed and replaced with scar tissue, skin grafts, and reepithelialized areas. This results in healed burn wounds that are unable to sweat or have altered circulatory function [2, 3].
In burn rehabilitation, exercise training is essential to improve physical performance and counteract low work capacity, both of which are major determinants of a severely burned patient's ability to return to school or work [4]. Our hospital has implemented a program of aerobic and resistive exercise training, along with standard physical therapy; to counteract the skeletal muscle catabolism and weakness that occurs post-burn [5]. In addition, we have previously reported that hyperthermia (defined as core body temperature greater than 40°C) [6] does not occur with submaximal exercise in a thermoneutral environment and is therefore safe for children with <75% total body surface area (TBSA) burns [7]. However, this study was done in a thermoneutral environment and was of a relatively short duration (20 minutes).
In children with severe burns, the thermoregulatory response to exercise in the heat is not well understood and whether an abnormal or detrimental response occurs during exercise in the heat in these children is presently unknown. Therefore, we designed a study in burned children and non-burned children to assess temperature responses during thirty minutes of submaximal exercise in a heated environment. We hypothesize that children with severe burns will have significantly higher absolute values of core temperature (Tcore) during exercise and also have a higher change in Tcore during exercise relative to rest.
METHODS
Subjects
Ten children with greater than 40% TBSA burns and ten non-burned children participated in this study. Informed consent was given by a parent or legal guardian and each child gave their assent prior to participation in the study. The study was approved by the Institutional Review Board of the University of Texas Medical Branch.
For all exercise tests, burned children wore comfortable, loose clothing (shorts, t-shirt and running shoes). In addition, they wore pressure garments, issued as standard of care, to reduce scar formation [8]. This was done to best simulate the conditions that these burned children would be exposed to outside of laboratory conditions. Non-burned children wore similar clothing to the burned children, but did not wear pressure garments.
Exercise Testing
All participants completed a modified Bruce protocol maximal treadmill exercise test to determine peak aerobic power. Heart rate and oxygen consumption (V̇O2) were assessed using methods previously described [5]. Briefly, breath-by-breath data was recorded using an automated MedGraphics (St Paul, MN) CardiO2 metabolic cart. Speed and angle of elevation started at 1.7 miles per hour and 0%, respectively. Thereafter, the speed and level of incline were increased every three minutes. Subjects were constantly encouraged to complete 3-minute stages and the test was terminated once peak volitional effort was achieved. The peak oxygen consumption (V̇O2peak) and peak heart rate were used to establish the intensity (approximately 75% of their peak aerobic power) for each subject during the submaximal exercise session.
On a subsequent day (within the same week), subjects completed a 30 minute treadmill submaximal exercise test in a heated environment. A workload of approximately 75% of their V̇O2peak, as determined from the previous V̇O2peak test, was used to determine the treadmill speed and grades during the submaximal exercise test. Treadmill speed and angle of elevation remained constant throughout the submaximal exercise test. Heart rate was continuously monitored pre, during, and post-exercise. The selection of the relative V̇O2 intensity of approximately 75% of V̇O2peak is based on the intensity used during indoor sessions of our exercise rehabilitation program. This exercise rehabilitation program has been demonstrated to be beneficial in terms of increasing lean mass, muscle function, and peak aerobic capacity in children with severe burns [5, 9]. Criteria for early test termination included core body temperature greater than 39°C, heat illness symptoms (dizziness, severe fatigue, headache, nausea), and the inability to safely maintain the set treadmill speed and grade.
Temperature Measurements
Intestinal temperature (Tcore) was measured via telemetry using an ingestible temperature capsule (MiniMitter, Seattle, WA) swallowed a minimum of 5 hours prior to exercise testing. No food or drinks were allowed within 1 hour of testing. A Tcore measurement greater than 39.0°C was chosen for test termination to ensure the safety of all children. This value was chosen based on temperature cut-off points used in previous reports of burn patients during exercise in the heat [2, 10-12].
Skin temperature was assessed using a Mon-A-Therm Model 6510 temperature monitoring system (Mallinckrodt Co., Mexico). In each burned child, the temperature of one area of burned, fascially excised skin and one area of unburned skin was measured. Sites were chosen based on review of the child's medical record. In the non-burned children, the temperature probe was placed on the right rear shoulder, (mid-Trapezius area). Measurement of room temperature, relative humidity and barometric pressure were recorded for 6 minutes pre-exercise, every two minutes during the 30 minute exercise bout, and for 6 minutes during recovery. Tcore and skin temperatures were also recorded at similar intervals as room temperature.
All fans and ventilation outlets were turned off during each exercise session to diminish any fluctuations in room temperature during the test, as we do not have access to an environmental chamber at this time. The size of the room in which the exercise sessions were carried out was 42 × 20 feet. During the testing, there was one child, an investigator, and a parent/guardian present in the room. Room temperature, humidity, and barometric pressure were measured using the Davis Instruments Perception II temperature monitor (Hayward, CA).
Statistics
All values are reported as means±standard deviation. Student's two tailed t-tests and two-way repeated measures ANOVA for comparisons within and between groups (Burned Children and Non-burned Children) were used for data analysis. A Kaplan-Meier curve was constructed for exercise duration with differences determined by a log-rank test. Intestinal temperature (Tcore) was set as the primary outcome variable. Statistical significance was accepted at p<0.05.
It is possible that a type II error was committed. However, sample size was determined in advance by power analysis. We determined the number of subjects required for our current study based on results from our previous study [7], in which we found that the standard deviation of Tcore in burned children was 0.5°C. We felt that differences of less than 0.8°C were not physiologically meaningful, so we used that difference as the minimum difference to be detected. Our power calculation, assuming a difference and standard deviation of 0.8 °C ± 0.5°C and an alpha of 0.05, determined that 8 patients per group were required to achieve a power of 80%, so we believe we have sufficient power to detect meaningful differences in this study.
RESULTS
There were no significant differences between burned and non-burned children in age, height, or weight. As expected, V̇O2peak was significantly higher in non-burned children compared to burned children (36.1 vs 24.1 ml/kg/min, respectively) (Table 1). TBSA burned ranged from 42-77%, with an average of 59±13% TBSA burned. Mean TBSA 3rd degree burned was 46±22% of total TBSA (range 7-77%). As expected, the unburned TBSA available for heat dissipation was significantly less in burned children (0.55±0.31 m2, based on an average BSA of 1.31±0.45 m2) than non-burned children (1.46±0.33 m2). Mean time post-burn was 5.4±3.0 months (range 1-9 months).
Table 1.
Subject characteristics.
| Age (years) | Height (cm) | Weight (kg) | VO2peak (ml/kg/min) | |
|---|---|---|---|---|
| Burn | 11.9±4.1 | 145.2±22.4 | 44.2±26.3 | 24.1±6.5 |
| Non-burn | 11.3±3.5 | 153.6±17.6 | 50.9±18.3 | 36.1±9.8* (p=0.004) |
VO2peak; peak aerobic capacity.
Significantly different from burned children. All values are means±standard deviation.
All of the non-burned children completed the 30 minutes of exercise in the heat. However, the burned children were able to complete 20.2±8.5 minutes (range 6-30 min) in the heat. Only three of the burned children were able to complete the 30 minutes of exercise in the heat. Interestingly, one burned child had to be stopped at minute 23 due to a core body temperature of 39.04°C. To best demonstrate the significant differences in completion times (p=0.001, log rank test) between burned and non-burned children, we have used a Kaplan-Meier curve (Figure 1).
Figure 1.
Kaplan-Meier curve representing the ability of burned children (n=10) and non-burned children (n=10) to complete the 30 minute exercise test in a heated environment (p=0.001).
Heart rate (HR) measurements
Room temperature, relative humidity, and barometric pressure values are reported in Table 2 for the submaximal exercise test. Resting HR was significantly higher in burned children compared to non-burned children (126.9±21.6 vs 101.8±10.6 bpm; p=0.004, respectively). However, during the submaximal exercise bout, the maximal HR achieved was similar between groups. The maximal change in HR from baseline was significantly less in burned children compared to non-burned children (42.1±17.2 vs 62.9±23.7 bpm; p=0.04, respectively) (Table 3).
Table 2.
Submaximal exercise test characteristics.
| Room Temperature (°C) | Relative Humidity (%) | Barometric Pressure (mmHg) | |
|---|---|---|---|
| Burn | 34.4±2.1 | 24.4±4.6 | 763.8±3.5 |
| Non-burn | 35.1±0.4 | 25.9±2.0 | 761.9±1.4 |
All values are means±standard deviation.
Table 3.
Heart rate measurements.
| HRrest (bpm) | HRmax (bpm) | Change in HR from baseline (bpm) | |
|---|---|---|---|
| Burn | 126.9±21.6 | 169.2±22.3 | 42.1±17.2 |
| Non-burn | 101.8±10.6* (p=0.004) | 164.7±19.3 | 62.9±23.7* (p=0.04) |
HRrest; resting heart rate, HRmax; maximal heart rate during submaximal exercise test.
Significantly different from burned children. All values are means±standard deviation.
Core body temperature measurements
Core temperature at baseline was higher in burned children compared to non-burned children (37.68±0.36 vs 37.28±0.31 °C, p=0.02). The maximal change in Tcore from baseline was significantly less in burned children (0.34±0.24°C) than in non-burned children (0.85±0.55°C, p=0.02). However, maximal Tcore was not significantly different between groups (Table 4). Tcore was significantly warmer during the first 12 minutes of exercise in burned children (p<0.001); however, the slope of the change in Tcore was significantly less steep in burned children during this time period (0.02±0.01 vs 0.04±0.02°C/min, p=0.03) (Figure 2).
Table 4.
Core temperature measurements.
| Tcore at rest (°C) | Maximal Tcore (°C) | Change in Tcore from baseline (°C) | |
|---|---|---|---|
| Burn | 37.68±0.36 | 38.03±0.52 | 0.34±0.24 |
| Non-burn | 37.28±0.31* (p=0.02) | 38.14±0.41 | 0.85±0.55* (p=0.02) |
Tcore; intestinal (core) temperature.
Significantly different than burned children. All values are means±standard deviation.
Figure 2.
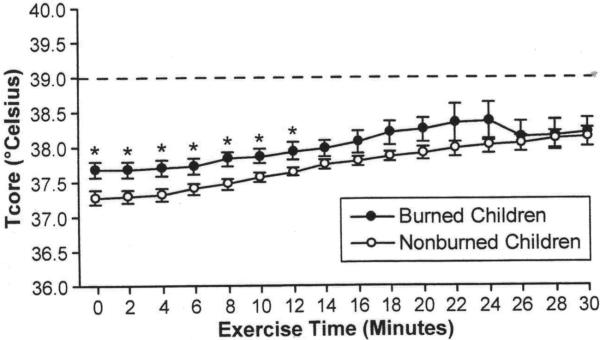
Intestinal (Tcore) temperature during submaximal exercise. *Tcore of burned children was significantly hotter during exercise than non-burned children (p<0.05). Dashed line represents the temperature chosen as a safety cut-off point.
Skin temperature measurements
Skin temperature data from one burned child was not included in the statistical analysis due to equipment malfunction. There were no significant differences between the temperature of burned children's burned and unburned skin and non-burned children's skin at baseline. Non-burned children's skin temperature rose 1.52±0.69°C from baseline, which was significantly greater (p=0.03) than the maximal change in burned skin temperature (0.70±0.66°C). The maximal temperature change in burned children's unburned skin (1.03±0.60°C) was not different from their burned skin or from non-burned children's skin temperature (Table 5). There were no significant interactions between time and burned children's and non-burned children's skin temperature (p=0.14) and the slope of the absolute temperature was not different between burned, unburned, or non-burned skin (p=0.30) (Figure 3).
Table 5.
Skin temperature measurements.
| Temperature at rest (°C) | Maximal temperature (°C) | Change in temperature from baseline (°C) | |
|---|---|---|---|
| Burned skin of burned children | 36.02±0.78 | 36.72±0.71 | 0.70±0.66 |
| Unburned skin of burned children | 36.04±0.63 | 37.08±0.49 | 1.03±0.60 |
| Non-burned children's skin | 35.42±0.68 | 36.94±0.30 | 1.52±0.69* (p=0.03) |
Significantly different than the burned skin of burned children. All values are means±standard deviation.
Figure 3.
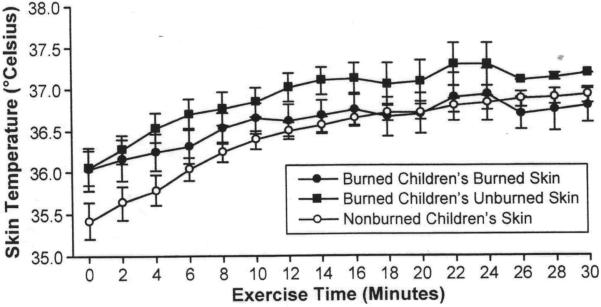
Skin temperature during submaximal exercise.
DISCUSSION
Our results demonstrate that children with 42-77% TBSA burns do have different temperature responses compared to non-burned children during submaximal treadmill exercise in a heated environment. Resting core body temperature was elevated in burned children, but the maximal temperature change from rest to the end of exercise was significantly less in burned children compared to non-burned children. Furthermore, only one of the burned children achieved a Tcore > 39°C, indicating that hyperthermia does not typically occur in burned children during exercise. As expected, non-burned children's skin temperature increased significantly more than the burned skin temperature of burned children. Interestingly, there were no differences between the burned children's burned and unburned skin temperatures or between unburned skin of burned children and non-burned children's skin.
Seven of the burned children were unable to complete the 30 minute exercise bout in the heat and did not present with a hyperthermic state. Based on this, we believe that these burned children demonstrate an “intolerance” to exercise in the heat. On average, the burned children were able to complete 20 minutes (67%) of exercise in the heat. Regardless of time completed (with the exception of the child with an elevated core temperature), the burned children stated that they felt “too hot” to continue and many complained of symptoms of severe fatigue, headaches, and dizziness. This is important as these symptoms occurred when Tcore was less than or equal to 39°C, which is 1°C below the definition of hyperthermia [6, 13]. Burned children seem to have a greater intolerance to exercise in the heat. However, whether this is due to an abnormal thermoregulatory response or to a debilitated state is unknown.
We utilized heart rate to reflect the relative intensity of exercise rather than the measured V̇O2. The children did not want to exercise in the heat while breathing through a mouthpiece, with the nose clipped or pinched off. We acknowledge that we cannot make statements on submaximal V̇O2 during the 30 minute exercise bout. However, the burned children's heart rate response was similar in linearity with non-burned children's heart rate. Therefore, the heart rate response together with the accompanying work load during the 30 minute exercise bout provides valid indirect support that the absolute workload was lower in burned children, but the relative workload was similar between burned and non-burned children.
The V̇O2peak achieved by burned children during the maximal exercise test and the workload that these children exercised at during the submaximal 30 minute challenge, was lower (albeit not significantly lower) than that of the non-burned group. Therefore, it cannot be ruled out that the burned children's muscles or metabolism during exercise did not generate enough heat to produce hyperthermia. Stated another way, the absolute exercise workload during the 30 minute challenge could have reflected physical debilitation in most burned children, rather than abnormal temperature responses.
On the other hand, we present the case of a burned child with a V̇O2peak of ~2570 ml/min, a value much higher than the mean V̇O2peak of 1889 ml/min of non-burned children. At 75% of this V̇O2peak, the heat generation should have been greater in the burned child, yet, the maximal Tcore was 37.98°C and the change in Tcore was 0.40°C compared with the maximal Tcore of 38.14±0.41°C and a change in Tcore of 0.85±0.55 °C in non-burned children. We present yet another case of a burned child with an absolute V̇O2peak of ~1090 ml/min, a value much lower than the mean V̇O2peak of 1889 ml/min of non-burned children. At 75% of this V̇O2peak, the heat generation should have been much lower than that of non-burned children or of the burned child just described. However, the maximal Tcore was 38.60°C and the change in Tcore was 0.44°C, values greater than those of the burned child previously mentioned, who exercised at a much higher absolute V̇O2. The conundrum of trying to determine the magnitude of the influence of absolute or relative V̇O2 on heat generation and their importance when designing studies on temperature regulation during exercise needs to be further explored.
Core body temperature rises during exercise and demonstrates a linear relationship with the relative intensity of the workload. Following 10-20 minutes of steady state exercise, muscle temperature reaches a state of equilibrium [14]. Rectal temperature in healthy individuals is reported to be determined by the percentage of maximal aerobic capacity rather than the metabolic rate [15]. However, Wyndham et al have reported that during hot and very humid conditions, body temperature is determined by the aerobic capacity in relation to total body surface area available for heat dissipation. Thus, the limiting factor for heat dissipation is thought to be the surface area available rather than the amount of heat produced relative to body weight [16]. So, how does this relate to our findings? Post-burn, there is a loss of muscle mass and the healthy skin available for heat dissipation is significantly less than their non-burned counterparts [2, 3, 17, 18]. In this study, Tcore was elevated at rest and during the first 12 minutes of exercise in the burned children. However, their maximal Tcore was not different from that of non-burned children. Burned children also demonstrated a smaller increase in Tcore from rest compared to non-burned children. The temperature of non-burned children's skin increased significantly more than burned children's skin. In addition, burned children's burned skin demonstrated a slower rise in temperature compared to non-burned children. These differences could potentially be a compensatory mechanism that prevents an abnormal rise in Tcore in burned children. Although, until all burned children are able to complete the entire exercise bout, we can only speculate that a compensatory mechanism exists. However, hyperthermia does not typically occur during submaximal exercise in the current conditions.
Previous research involving burned individuals during exercise in the heat have been of a greater duration than the present study. We chose the 30 minute time period to mirror the cardiovascular component of our in-hospital exercise program. Previous studies (all in adults) performed 60 minutes or more of exercise in the heat [3, 11, 12, 19]. However, as evidenced in this study, burned children within 9 months of their burn injury are still physically decompensated and an exercise bout of long duration in the heat would not be possible. The 30 minute time period chosen seems to be indicative of a realistic time that burned children can complete an exercise bout in the heat. It may also be reflective of how children exercise and participate in sports (i.e. shorter durations of higher intensity exercise followed by rest periods).
Temperatures above 40°C are defined as exertional hyperthermia [6], and our results show that only one child with burns presented with a Tcore above 39.0°C (a value chosen as a safety cut-off point). The fact that 90 percent of the burned children did not approach a Tcore value indicative of hyperthermia is interesting and may have potential safety implications. To state that hyperthermia does not typically occur in burned children during exercise in the heat under these laboratory conditions and is therefore safe is tempting, but requires further scrutiny. This one child did not complain of any symptoms of heat related illnesses. He would have been able to complete the 30 minute exercise bout, if we had not stopped him due to the elevated core temperature. Core body temperature prior to exercise was 38.35°C in this child. Exercise was terminated at 39.04°C, which is an increase of 0.69°C. He also stated a willingness to complete the exercise bout even as his core temperature was rising. This is an important issue to consider during exercise because these individuals may be “heat tolerant” thereby not becoming symptomatic until their Tcore is very high, leading to a potentially serious heat related illness [20, 21]. Again, our results only apply to submaximal exercise in the heat under our conditions and for children with 42-77% TBSA burns.
LIMITATIONS
It is still unclear as to whether it is the relative or the absolute intensity of exercise that contributes more to core body temperature regulation [15, 22-24]. In our study, we set all sessions of exercise to be the same relative intensity, so we cannot ascertain the influence of an absolute work intensity or V̇O2 on core body temperature. However, because of the wide range of absolute peak oxygen consumption values, even in healthy non-burned children, the matching of work intensities or V̇O2 using relative intensities (as a percent of V̇O2peak) would be desirable and more practical than using absolute workloads. In addition, the exercise prescription offered to our burned children, as part of their rehabilitation program, is based on relative intensity [5].
The burned children in this study wore pressure garments during the exercise testing. The garments are issued as part of standard of care for rehabilitation. We chose to have them wear the garments during testing as we wanted to simulate the conditions that they would be under outside our hospital care. However, we did not include a control group that wore pressure garments, which makes it difficult to know whether the increases in core body and skin temperature were the result of the burn itself or the pressure garments increasing the amount of heat that was retained in the skin. Future studies will include exercise testing without pressure garments, specifically at 2-4 years post-burn when these garments are no longer prescribed.
In summary, 90 percent of the burned children in our study presented with Tcore values that were not hyperthermic by definition. Whether our results translate into the safety of participation in physical activities in the heat requires further study. Our study was done under laboratory conditions and future studies under more severe conditions (ie outdoor, high humidity) may result in the occurrence of hyperthermia. As such, the use of common sense and monitoring when exercising burned children in the heat is mandated.
Acknowledgement
This study was partially supported by grants from the National Institute for Disabilities and Rehabilitation Research H133A70019 and H133A070026; the National Institutes of Health K01-HL70451 and RO1-HD049471; and Shriners Hospitals for Children grant 8741 and 8760. There are no potential conflicts of interest pertaining to this manuscript for any authors. This first draft of this manuscript was written by the corresponding author.
Footnotes
In addition, no authors have any financial disclosures related to this manuscript.
REFERENCES
- 1.Williams WG. Pathophysiology of the burn wound. In: Herndon DN, editor. Total Burn Care. WB Saunders; London: 2002. pp. 519–520. [Google Scholar]
- 2.McGibbon B, Beaumont WV, Strand J, Paletta FX. Thermal regulation in patients after the healing of large deep burns. Plast Reconstr Surg. 1973;52(2):164–70. doi: 10.1097/00006534-197308000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro Y, Epstein Y, Ben-Simchon C, Tsur H. Thermoregulatory responses of patients with extensive healed burns. J Appl Physiol. 1982;53(4):1019–22. doi: 10.1152/jappl.1982.53.4.1019. [DOI] [PubMed] [Google Scholar]
- 4.Xiao J, Cai BR. Functional and occupational outcome in patients surviving massive burns. Burns. 1995;21(6):415–21. doi: 10.1016/0305-4179(95)00010-9. [DOI] [PubMed] [Google Scholar]
- 5.Suman OE, Spies RJ, Celis MM, Mlcak RP, Herndon DN. Effects of a 12-wk resistance exercise program on skeletal muscle strength in children with burn injuries. J Appl Physiol. 2001;91(3):1168–75. doi: 10.1152/jappl.2001.91.3.1168. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong LE, Casa DJ, Millard-Stafford M, Moran DS, Pyne SW, Roberts WO. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556–72. doi: 10.1249/MSS.0b013e31802fa199. [DOI] [PubMed] [Google Scholar]
- 7.McEntire SJ, Herndon DN, Sanford AP, Suman OE. Thermoregulation during exercise in severely burned children. Pediatr Rehabil. 2006;9(1):57–64. doi: 10.1080/13638490500074576. [DOI] [PubMed] [Google Scholar]
- 8.Burke Evans E, Alvarado MI, Ott S, McElroy K. Prevention and treatment of deformity in burned patients. In: Herndon DN, editor. Total Burn Care. WB Saunders Co.; London: 2000. pp. 449–451. [Google Scholar]
- 9.Suman OE, Herndon DN. Effects of cessation of a structured and supervised exercise conditioning program on lean mass and muscle strength in severely burned children. Arch Phys Med Rehabil. 2007;88(12 Suppl 2):S24–9. doi: 10.1016/j.apmr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong LE, De Luca JP, Hubbard RW. Time course of recovery and heat acclimation ability of prior exertional heatstroke patients. Med Sci Sports Exerc. 1990;22(1):36–48. [PubMed] [Google Scholar]
- 11.Austin KG, Hansbrough JF, Dore C, Noordenbos J, Buono MJ. Thermoregulation in burn patients during exercise. J Burn Care Rehabil. 2003;24(1):9–14. doi: 10.1097/00004630-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Simchon C, Tsur H, Keren G, Epstein Y, Shapiro Y. Heat tolerance in patients with extensive healed burns. Plast Reconstr Surg. 1981;67(4):499–504. doi: 10.1097/00006534-198104000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong LE, Epstein Y, Greenleaf JE, Haymes EM, Hubbard RW, Roberts WO, Thompson PD. American College of Sports Medicine position stand. Heat and cold illnesses during distance running. Med Sci Sports Exerc. 1996;28(12):i–x. [PubMed] [Google Scholar]
- 14.Saltin B, Gagge AP, Stolwijk JA. Muscle temperature during submaximal exercise in man. J Appl Physiol. 1968;25(6):679–88. doi: 10.1152/jappl.1968.25.6.679. [DOI] [PubMed] [Google Scholar]
- 15.Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol. 1966;21(6):1757–62. doi: 10.1152/jappl.1966.21.6.1757. [DOI] [PubMed] [Google Scholar]
- 16.Wyndham CH, Strydom NB, Van Rensburg AJ, Benade AJ, Heyns AJ. Relation between VO2 max and body temperature in hot humid air conditions. J Appl Physiol. 1970;29(1):45–50. doi: 10.1152/jappl.1970.29.1.45. [DOI] [PubMed] [Google Scholar]
- 17.Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, Obeng MK, Lal S, Gold WF, Wolfe RR, Herndon DN. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232(4):455–65. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, Ferrando AA, Wolfe RR, Herndon DN. Persistence of muscle catabolism after severe burn. Surgery. 2000;128(2):312–9. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 19.Roskind JL, Petrofsky J, Lind AR, Paletta FX. Quantitation of Thermoregulatory Impairment in Patients with Healed Burns. Annals of Plastic Surgery. 1978;1(2):172–176. doi: 10.1097/00000637-197803000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Docherty D, Eckerson JD, Hayward JS. Physique and thermoregulation in prepubertal males during exercise in a warm, humid environment. Am J Phys Anthropol. 1986;70(1):19–23. doi: 10.1002/ajpa.1330700105. [DOI] [PubMed] [Google Scholar]
- 21.Hayward JS, Eckerson JD, Dawson BT. Effect of mesomorphy on hyperthermia during exercise in a warm, humid environment. Am J Phys Anthropol. 1986;70(1):11–7. doi: 10.1002/ajpa.1330700104. [DOI] [PubMed] [Google Scholar]
- 22.Freund BJ, Joyner MJ, Jilka SM, Kalis J, Nittolo JM, Taylor JA, Peters H, Feese G, Wilmore JH. Thermoregulation during prolonged exercise in heat: alterations with beta-adrenergic blockade. J Appl Physiol. 1987;63(3):930–6. doi: 10.1152/jappl.1987.63.3.930. [DOI] [PubMed] [Google Scholar]
- 23.Gant N, Williams C, King J, Hodge BJ. Thermoregulatory responses to exercise: relative versus absolute intensity. J Sports Sci. 2004;22(11-12):1083–90. doi: 10.1080/02640410410001730025. [DOI] [PubMed] [Google Scholar]
- 24.Taylor WF, Johnson JM, Kosiba WA. Roles of absolute and relative load in skin vasoconstrictor responses to exercise. J Appl Physiol. 1990;69(3):1131–6. doi: 10.1152/jappl.1990.69.3.1131. [DOI] [PubMed] [Google Scholar]



