Abstract
The incidence of testicular cancer has been increasing over the past several decades in many developed countries. The reasons for the increases are unknown because risk factors for the disease are poorly understood. Some research suggests that exposures in utero or in early childhood are likely to be important in determining an individual's level of risk. However, other research suggests that exposure to various factors in adolecence and adulthood are also linked to the development of testicular cancer. Of these, two occupational exposures—firefighting and aircraft maintenance—and one environmental exposure (to organochloride pesticides) are likely to be associated with increased risk of developing testicular cancer. By contrast, six of the identified factors—diet, types of physical activity, military service as well as exposure to ionizing radiation, electricity and acrylamide—are unlikely to increase the risk of developing testicular cancer. Finally, seven further exposures—to heat, polyvinylchloride, nonionizing radiation, heavy metals, agricultural work, pesticides and polychlorinated biphenyls as well as marijuana use—require further study to determine their association with testicular cancer.
Introduction
Testicular cancer is the most common neoplasm among young men (aged 15–40 years) in many parts of the world.1 The majority (98%) of testicular cancers are germ cell tumors;2 for this reason the terms testicular germ cell tumor (TGCT) and testicular cancer are often used interchangeably. Germ cell tumors can be grouped histologically into seminomas, nonseminomas and spermatocytic seminomas.3 Unlike most cancers that occur in adulthood, the incidence of testicular cancer does not increase with age. The peak ages of occurrence are 25-29 years for nonseminomas and 35-39 years for seminomas.4 In contrast to other germ cell tumors, spermatocytic seminomas are less aggressive, do not appear to share common risk factors with seminomas and nonseminomas and have an older peak age of occurrence (50-54 years). Spermatocytic seminomas are also much less common, comprising only 0.6% of all germ cell tumors, while seminomas comprise 56% and nonseminomas, 43%.2 The small percentage (2%) of testicular cancers that are not germ cell tumors include stromal tumors, such as Leydig cell and Sertoli cell tumors, as well other rare or poorly defined histologic types.2
Men in Scandinavian countries, in particular Norway and Denmark, have the highest incidence of testicular cancer in the world (Figure 1).1 By comparison, incidence rates are very low in Asian and African countries. Although the incidence of testicular cancer has been increasing in the developed world for at least four decades1, mortality rates have declined since the 1970s owing to major improvements in chemotherapeutic regimes.5 The 5-year relative survival rate for testicular cancer diagnosed in the U.S. between 2001 and 2007 is 96.4%. Regardless of the overall risk in a particular country, incidence rates vary by ethnic group. For example, white men of European descent, wherever they live, are more likely to develop testicular cancer than black or Asian men living in the same geographic region.6 In addition, white men have experienced the greatest increases in incidence throughout the late twentieth century than any other ethnic group. In a number of countries, analyses of testicular cancer incidence trends have found them to be more consistent with a birth-cohort effect than with a calendar-period effect. These observations suggest that any risk factor behind the increasing incidence of this cancer would probably be an exposure that varied by birth year.4, 7-8 For example, men born during World War II in Denmark had a lower risk of testicular cancer at all ages than men born in the preceding or succeeding periods.9
Figure 1.
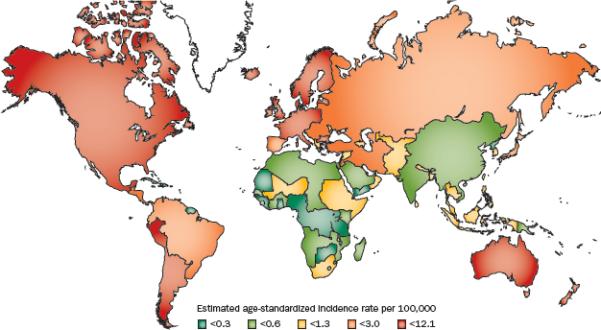
Incidence rates of testicular cancer (per 100,000 man-years) age-standardized to the world population in 2008. [Globocan 2008]
In line with these observations, the prevailing hypothesis of the etiology of testicular cancer is that risk is largely or solely determined in utero. Certainly, a strong relationship between a congenital anomaly (cryptorchidism) and testicular cancer is evident10 however, the risk factors for testicular cancer are not well characterized. The only other factors clearly associated with risk are prior unilateral testicular cancer, a family history of testicular cancer and increased adult height (Table 1). Among these factors, the greatest relative risk is conferred by having a brother with testicular cancer, which increases an individual's risk by approximately 10-fold,11 suggesting that there might be a strong heritable component to risk. Several genome-wide association studies have been conducted with the aim of identifying genetic markers that are likely to be related to the risk of testicular cancer.12-15 These studies have identified six loci on four chromosomes that seem to be related to testicular cancer: 5p15 (TERT, CLPTM1L), 5q31 (SPRY4), 6p21 (BAK1), 9q24 (DMRT1), 12p13 (ATF7IP), and 12q21 (KITLG). The strongest association has been observed for single-nucleotide polymorphisms in the 12q21 locus, which confer an approximately threefold increase in risk per affected allele. However, even among first-degree relatives of men with testicular cancer, these risk loci are estimated to account for only 11% of the risk of developing testicular cancer in brothers and 16% of the risk in sons.16 Thus, other environmental factors and as yet unidentified genetic loci probably also have a role. Many investigations have, therefore, looked for other possible perinatal risk factors. For example, two meta-analyses of perinatal factors found that, in addition to cryptorchidism, the factors most consistently associated with increased risk of testicular cancer are prior inguinal hernia, low birth order, maternal bleeding, small sibship size, and being a twin.10, 17 These meta- analyses also provided tentative support for links between testicular cancer and low birth weight as well as shorter gestational age. The proposed relationship between early-life risk factors, congenital anomalies and testicular cancer was further expanded by the testicular dysgenesis syndrome (TDS) hypothesis.18 TDS is thought to include four conditions—testicular cancer, cryptorchidism, hypospadias and impaired spermatogenesis—that are linked by a common in utero etiology.
Table 1.
Known Risk factors for testicular cancer
| Risk factor | Risk estimate or range OR, (95% CI) | Reference |
|---|---|---|
| Known risk factors | ||
| Cryptorchidism* | 4.30 (3.62–5.11) | 10 |
| Prior TGCT‡ | 12.4 (11.0–13.9) | 154 |
| Father with TGCT§∥¶ | 1.75 (0.64–3.81) to 3.78 (1.94–6.63) | 11, 155-156 |
| Brother with TGCT§∥¶ | 7.55 (5.13–10.73) to 12.74 (6.38–22.64) | 11, 155-156 |
| Height (per 5 cm increase)# | 1.13 (1.07–1.19) | 157 |
Cook MB, Int J Epidemiol 2010;39:1605-18.
Fossa S, et al., J Natl Cancer Inst 2005;97:1056-66.
Westergaard T, et al., Int J Cancer 1996;66:627-31.
Sonneveld DJ, et al., Eur J Cancer 1999;35:1368-73.
Hemminki K, et al., Int J Androl 2006;29:205-10
Lerro CC, et al., Br J Cancer 2010;103:1467-74.
Abbreviation: TGCT, testicular germ cell tumor.
However, the existence of an initiating in utero event does not preclude the possibility that other factors contribute to the development of testicular cancer. As seminomas and nonseminomas in young men seem to arise from testicular carcinoma in situ,19 these lesions could conceivably develop into testicular cancers once exposed to as-yet-undetermined risk factors. Possible nonperinatal risk factors studied by a number of groups include diet, involvement in specific types of physical activity, occupation, and exposures to pesticides, metals, heat and drugs.
In this Review, we summarize the emerging evidence in support of these risk factors. Perinatal factors will not be included herein as these factors have been discussed in detail elsewhere.10, 17, 20-21
Diet
An epidemiological investigation conducted in 1975 was one of the first studies to examine a possible nutritional etiology of testicular cancer.22 The study reported that total dietary fat levels were highly correlated with the incidence of this neoplasm (r = 0.76). A subsequent study, published in 2002, replicated the finding of a correlation with high fat intake (r = 0.77) and also identified correlations with increased consumption of cheese (r = 0.80) and milk (r = 0.74).23 The correlations with increased consumption of dairy products are not surprising, especially given that the intake of milk and cheese is highest in the Scandinavian countries, which also have the highest risks of testicular cancer; diary consumption is the lowest in Asian and African countries, which have the lowest incidence of testicular cancer.24 Persons of Asian and African descent individuals tend to become lactose-intolerant with age, whereas Northern European populations do not and, therefore, continue to consume milk throughout their life.24 However, whether continued consumption of dairy products (or the genetic ability to keep on producing lactase) is associated with testicular cancer cannot be definitively determined from ecologic studies.
Several case–control studies have examined milk, cheese and dietary fat intake as risk factors for the development of testicular cancer. Although one small (n = 160 testicular cancer cases) study conducted in a North American population reported a significant association with dietary fat intake,25 another North American study of similar size reported no association.26 Similarly, a high cheese intake was reported to be significantly associated with the risk of developing testicular cancer in a North American study,27 but this association was not supported in a study from the UK.28 A high milk intake was significantly associated with the risk of developing TGCT in studies from the UK29 and Germany,30 but these results were not reproduced in five other studies.25-27, 31-32 In addition, no association between total dairy food consumption and the risk of TGCT was found in three studies.27-28, 32 Taken as a whole, these studies do not provide conclusive evidence of a relationship between the risk of TGCT and increased consumption of dairy product or fat.
Other dietary factors have also been investigated, albeit to a lesser extent. For example, high intakes of cholesterol, meat, calcium, fiber and fruit were all significantly associated with the risk of developing testicular cancer in one study,25 but these associations have yet to be replicated. Two studies reported no association with cholesterol intake,26, 33 whereas another two studies found no support for an association with either meat31 or fiber26 intake.
The observed inconsistency between the findings of dietary studies might possibly be explained by their primary focus on the adult diet. Given the relationship between testicular cancer and adult height, which is an anthropometric feature related to childhood diet, examinations of early-life diet could potentially be more illuminating than those of adult dietary factors. Unfortunately, the studies that have focused on early-life diet have also reported inconsistent results.29-30, 32 In recognition of the scant evidence that dietary factors are associated with TGCT, a joint review conducted by the World Cancer Research Fund and American Institute of Cancer Research in 2007 concluded that the current evidence did not warrant a thorough investigation of the potential links between food, nutrition and testicular cancer.34
Physical activity
The relationship between types of physical activity and risk of testicular cancer has been examined from both recreational and occupational perspectives, as noted below. The recreational sports most frequently studied have been riding sports, including motorcycle riding, bicycle riding and horseback riding. An early recreational activity study conducted in Canada found that bicycle riding and horseback riding were both associated with significantly increased risks of testicular cancer, but motorcycle riding was not.35 By contrast, a second study by the same group found that bicycle riding was associated with a significantly decreased risk of testicular cancer.36 However, two US studies found no association between bicycle riding and testicular cancer.37-38 One of these studies also found no statistically significant association with motorcycle riding ,37 whereas the other study found that horseback riding was associated with a decreased risk.38 Overall, the results of studies on riding sports do not support an association with testicular cancer.
Similarly little evidence suggests that total recreational physical activity levels are associated with the risk of testicular cancer. Of the five studies that examined this factor, one reported an increased risk,39 two reported a decreased risk,36, 40 and two reported no association.41-42 Studies of occupational physical activity levels have been similarly inconsistent. One study reported a decreased risk of testicular cancer in men with increased occupational activity,43 whereas another reported an increased risk39 and three reported no association.36, 42, 44
In summary, the bulk of the available evidence finds little support for an association between physical activity and testicular cancer. Physical activity affects various physiological factors, such as hormone levels, body fat percentage, adipokine levels, insulin resistance, insulin-like growth factor levels, pulmonary function, vitamin D levels, inflammation and oxidative stress;45 any or all of these might be directly or indirectly associated with cancer of any organ. With respect to testicular cancer, a postulated effect on hormone levels might be the most likely mechanism mediating an influence on risk. However, whether physical activity has a strong effect on hormone levels in men remains unclear.46 Other mechanisms that have been postulated to explain a possible link between physical activity and testicular cancer are testicular trauma and/or heat.
Heat
Evidence gathered in the 1960s that high temperatures adversely affect spermatogenesis and cause changes to the germinal epithelium47-50 spurred an interest in heat as a potential risk factor for testicular cancer. To examine this hypothesis, several case–control studies have examined the relationships between underwear type and bathing and risk of TGCT.37, 51-53 No relationship between testicular cancer risk and tight-fitting underwear has been reported to date.51-53 Bathing, rather than showering, was significantly associated with risk in one study,37 but not in two others.51-52 Interestingly, a study of occupational exposure to heat found an increased risk of testicular cancer in men exposed to both elevated temperatures (≥80°F) and reduced temperatures(≤60°F).54 Several other occupational studies suggested that men working in maintenance at paper and pulp mills55 and ferrosilicon plants 56 are at increased risk of testicular cancer, possibly because of high working temperatures. However, separating the effects of heat from those of other exposures in these settings is difficult. In addition, some researchers have suggested that the observed increased risk of TGCT among firefighters (discussed below) could be related to heat exposure.57 However, this association has only been observed in studies published after 1995,50–54 suggesting that firefighters’ increase in risk is attributable to use of new building materials58 rather than exposure to heat. Taken as a whole, the evidence that heat is a risk factor for testicular cancer is unconvincing. Several additional occupational associations indicate that heat exposure might be related to the risk of TGCT, suggesting that further investigation of this hypothesis is warranted.
Occupational risk factors
Many occupational studies are not well-suited to examine testicular cancer because they are designed to evaluate mortality rather than the incidence of this malignancy. Testicular cancer mortality rates have declined sharply in developed countries over the past four decades,59 such that the use of mortality as an end point has become suboptimal. Nevertheless, occupational associations with testicular cancer have been suggested by a number of incidence studies, as discussed below. Although some occupational exposures can also involve environmental factors, the most extreme exposures to these factors tend to occur in occupational settings.
Firefighting
The association of testicular cancer with firefighting originates from reports of a significantly increased risk of this neoplasm among firefighters in New Zealand.58, 60 This association was supported by subsequent studies in California61 and Florida,62 which also found significantly increased risks of testicular cancer, as well as a study in Germany that reported a trend towards an increased risk.63 By contrast, a number of studies conducted before 1995 found that firefighters were not at an increased risk of developing testicular cancer.64-68 These differences between the findings of early (negative) versus later (positive) studies might be related to changes in building materials commonly used at the time.58 Two subsequent reviews of the risk of testicular cancer among firefighters concluded that there was a “probable association”69 and a “preponderance of evidence”70 that supported the presumption of causation. An association of this occupation with the risk of testicular cancer might be related to exposure to a wide variety of carcinogenic substances, including benzene and polycyclic aromatic hydrocarbons.71
Military service and aircraft maintenance
The risk factors associated with military service, mainly in the USA, have been examined in a number of studies. Among 12 US-based studies, five reported that servicemen were at a significantly increased risk of developing testicular cancer,72-76 whereas two reported a significantly decreased risk,77-78 and five reported no association.79-83 Of the five studies that found a positive correlation, three noted that the risk was increased primarily among pilots or aircrew.72-73, 75 One of the studies that found no overall association did report an increased risk among aviation mechanics and aircraft maintenance technicians.80 Although speculative, exposure to hydrocarbon carcinogens via degreasing agents and lubricating oils may be a common exposure among those with increased risk.80 Two of the other positive studies linked the increased risk to military service in either the Vietnam War74 or the Gulf War of 1990-91.76 However, other studies of Vietnam veterans79 and Gulf War veterans,81-82 reported no such association. What exactly these men had been exposed to and how that might increase their risk of testicular cancer is unclear. Indeed, although Agent Orange has been linked to other cancers among Vietnam veterans,84 studies of men likely exposed to Agent Orange in Vietnam have not supported an association with testicular cancer.85
Among the five studies of non-US military servicemen two found a significantly increased risk of developing TGCT,86-87 two studies88-89 reported trends toward increased risks and one study90 reported a decreased risk. Compared to the general population, an increased risk of testicular cancer was reported among men in the British Royal Air Force, with the highest risk was observed in men who worked on aircraft.86 Similar results were found in a case-control study that examined the risk of testicular cancer among British Royal Navy personnel by occupational title.87 Men in aviation-related occupations had double the risk of testicular cancer compared to controls, with aircraft handlers having a sevenfold increase in risk of this malignancy.
The increased risk of testicular cancer among military personnel involved in aircraft maintenance or operation prompted several studies of civilian aircrew and aircraft-manufacturing workers for comparison. A study of Canadian commercial pilots found these men had an increased risk, albeit not statistically significant, of developing testicular cancer.91 A similar association was found in a study of Bulgarian aircraft workers89 and a US study of F-4 Phantom jet repairmen.92 Finally, a study of mortality in aircraft workers at a manufacturing facility in California93 reported a significant increase in risk of death from testicular cancer among men exposed to mixed solvents, which was confirmed upon additional follow-up of the same cohort.94
Overall, the evidence suggests that either working on or operating aircraft is associated with an increased risk of testicular cancer. What the specific exposure might be is uncertain, but has been speculated to be hydrocarbon carcinogens, such as methylcholanthene, which induces testicular tumors in animals.80 Alternatively, exposure to glycol ethers in aviation fuel has also been suggested.87
Electrical work
A 1991 population-based case–control study reported an increased risk of testicular cancer among electricians in Washington State, USA.95 By contrast, other studies of electricians in Norway96 and New Zealand97 did not support this finding. However, in a study of nonoccupational exposure to electrical sources, electric blanket use was associated with a marginal increase in risk of nonseminoma.98 Despite this finding, overall little evidence suggests that electricians, or persons exposed to electrical sources, are at an increased risk of testicular cancer.
Law enforcement
Concern about testicular cancer among policemen arose in the early 1990s when clusters of cases were reported in two police departments in the USA.99 These officers shared the occupational practice of resting speed radar guns (which emit microwave frequency radiation) in their laps while in the “on” position. The incidence of testicular cancer among these policemen was significantly increased (ratio of observed:expected cases 6.9). An additional study of police officers in ON, Canada, reported only a trend towards an elevation in risk of developing testicular cancer.100 Other studies of police officers have reported some differences from the general population in mortality for cancers at specific sites; however, these studies have not reported any increased risk of mortality related to testicular cancer.101-105 Concerns over police exposure to microwave radiation form radar devices led the US National Institute for Occupational Safely and Health to review these data; they concluded that the evidence was insufficient to determine whether these devices caused an increased cancer risk.106
Heavy metal extraction and industries
Metals extracted from the earth and used in industry are globally distributed pollutants that tend to accumulate in human tissues. Importantly, many heavy metals have the potential to cause toxic effects at low concentrations. Indeed, mining and manufacturing activities involving zinc and cadmium might be etiologically relevant to testicular cancer given that these metals in particular have special affinity for the testes.107 Cadmium is classified as a group 1 carcinogen by the International Agency for Research on Cancer and, in animal studies, has deleterious effects on the testes.108 However, direct evidence of a link between either zinc or cadmium exposure and testicular cancer has not been ascertained. An ecologic study in the Netherlands and Belgium of various malignancies (including testicular cancer) in municipalities either close to or distant from a cadmium smelter found no difference in their incidence.109 Similarly, a case–control study from the Netherlands reported no testicular cancers among men with occupational exposure to cadmium.110
In other studies of heavy metal exposure, an excess incidence of testicular cancer was reported among 8,530 ferrosilicon and silicon metal furnace workers in Norway (standardized incidence ratio [SIR] 2.30) The carcinogenicity of ferrosilicon is not well defined, but the manufacturing process can include exposure to asbestos, crystalline silica, nonionizing radiation and heat.56 A case–control study of metal workers in Germany supported a link between heavy metal exposure and testicular cancer, although the exposure definition in the study was rather broad.107 Metal workers were defined as skilled individuals directly exposed to metals or metal dust for at least 3 years, including locksmiths, car mechanics, electricians, turners, mold makers, installers and technicians in the radio and television industry. Heat and other agents used in metalwork might also have a role in their association with testicular cancer and cannot be ruled out.
Although some studies support an increased risk of testicular cancer in men exposed to heavy metals, adequate data on which to base a conclusion are lacking. The mechanisms by which exposure to heavy metals could influence testicular cancer risk likely depends on the metal being processed. Furthermore, the exposure to gases, fumes, dust, manual labor and heat, which coincide with metal work, cannot be ruled out as potentially carcinogenic.
Industrial plastic manufacturing
Exposure to polyvinyl chloride (PVC) plastics is commonplace for many industrial workers. One population-based case-control study utilizing Swedish Cancer Registry data reported an increased risk of testicular cancer among workers in plastics, and a more than sixfold risk among workers specifically exposed to PVCs.111 In another population-based case-control study from Sweden, which defined exposure based on a job-exposure matrix, probable exposure to PVC was associated with a modestly increased risk of testicular cancer (OR=1.35)112 but, surprisingly, unexpectedly, the greatest increases in risk (OR = 2.50) were observed for categories of exposure relating to less than daily handling of PVC. In contrast to the Swedish studies, a Norwegian occupational cohort study of 428 vinyl chloride workers followed-up for 23 years, recorded only one case of testicular cancer.113 However, the small size of the cohort might have hampered detection of an association. Although the data are limited, the available studies do suggest that an increased risk of testicular cancer is associated with occupational exposure to PVCs. The mechanism underlying this association is unclear; however, phthalates (plasticizers with documented estrogenic effects)114 are a component of PVCs and are used in their production. These compounds might be involved in carcinogenesis.
Exposure to acrylamide has been evaluated in two studies by the same group. An initial report included workers at three US plants,115 whereas a second report extended the follow-up of these US workers and added workers from a plant in The Netherlands.116 Mortality from testicular cancer was not associated with occupational exposure to acrylamide in either study.115-116
Radiation
Electromagnetic radiation is classified as either nonionizing or ionizing, based on its capability to ionize atoms and break chemical bonds. Electromagnetic waves with frequencies in or above the ultraviolet range are classified as ionizing radiation. The majority of studies on testicular cancer have focused on occupational exposure to this type of radiation. A systematic review of these studies concluded that the available data offer little support for an association between occupational exposure to ionizing radiation and either the incidence of or mortality from testicular cancer (31 studies in total).117 In agreement, researchers who assessed data from the US Radiologic Technologists Health Study Cohort reported that the incidence of testicular cancer was not significantly elevated among technologists followed-up for 15 years (SIR =1.32, 95% CI 0.76–2.13).118 The current evidence, therefore, does not support an association between testicular cancer and ionizing radiation.
Comparatively few studies have evaluated the effects of nonionizing radiation. Although the same systematic review also identified an association between occupational nonionizing radiation exposure and an increased risk of testicular cancer, this conclusion was based on data from only nine studies (5 case-control and 4 cohorts).117 The number of cases ranged from 5-120 in 8 of the 9 studies; while one study included 607 cases.119 Some of these studies evaluated very-low-intensity exposure, such as work in front of a visual display unit or in complex electrical environments (for example, computer rooms or telephone switchboards), whereas other studies examined high-intensity sources, such as exposure to police radar units, airplanes, airports and ship or military radar. The study with the largest number of cases reported increased risk with both moderate (0.084-0.115 microTesla) and high exposure categories (>0.116 microTesla), but no dose-response association.119 The largest risk was observed in the previously mentioned study of police officers in Washington State that included high-intensity exposure that was most likely proximal to the testis.99 The sum of the epidemiological data suggests that nonionizing radiation might be associated with a slightly increased risk of testicular cancer, although these results require validation in future studies.
Agricultural work and pesticide application
Several,120-123 although not all,124-127 studies conducted prior to 1990 suggested a link between employment in the agricultural sector and an increased risk of testicular cancer. A meta-analysis of 11 studies of cancer risk among farmers, published in 1992, found no evidence of increased risk of testicular cancer.128 An expanded meta-analysis of 14 studies, conducted 6 years later, reached the same conclusion.129 Studies of testicular cancer among sons of farmers have, however, provided inconsistent results. A study from Norway130 reported an increased incidence of testicular cancer in farmers’ sons, whereas studies from Denmark131 and the USA132 have not replicated this finding.
The null results of the meta-analyses of testicular cancer risk among farmers does not preclude the possibility that exposure to certain agricultural chemicals, such as fertilizers and pesticides, might be associated with an increased risk of this malignancy. Attempts to examine a possible association with work-related exposure to pesticides have been made in both general occupational studies and in studies focused on pesticide applicators, as noted below. At least one general occupational study of testicular cancer in Finland reported a significantly increased risk among workers exposed to pesticides, although the results were based on a small number of testicular cancers.133 Among the studies of pesticide applicators, an early report of an increased risk of testicular cancer among Swedish workers127 was not confirmed upon further follow-up of the cohort.134 However, excess risk was found among pesticide applicators in the USA135 and the UK.136 Indeed, geographical differences in risk are not entirely unexpected because pesticide applicators are likely to have different exposures in various regions. The results among pesticide applicators suggest that in-depth study of this occupational group might be useful.
Environmental exposure
Organochlorine compounds
Molecular epidemiological examinations of serum levels of pesticides, which began to be reported in the early 2000s, are a somewhat better indication of environmental exposure to these agents. The pesticides that have been most commonly examined in this type of study to date have been the organochlorine compounds. The organochlorines are among the oldest pesticides used and are persistent, both in the environment and in human adipose tissue.137 In addition, organochlorine compounds can mimic sex steroid hormones and, therefore, might alter gene expression patterns that are important in urogenital development and homeostasis.138-139 The endocrine-disrupting properties of organochlorines have made them primary compounds of interest in studying the etiology of testicular cancer. Of note, all the molecular epidemiology studies of organochlorine exposure, to the best of our knowledge, are general population, case– control studies that lack information on the settings in which the participants were exposed; nonetheless, the majority of participants in these studies were unlikely to be ever employed as pesticide applicators.
Organochlorine pesticides include dichlorodiphenyltrichloroethane (DDT) and its most persistent metabolite dichlorodiphenyldichloroethylene (DDE), as well as chlordane, mirex and β-hexachlorocyclohexane (HCH) and many others. To date, eight reports have examined whether serum levels of organochlorine pesticides and their metabolites are associated with testicular cancer.53, 140-145 Six reports included men who had been, or subsequently were, diagnosed with testicular cancer,53, 140-143, 145 whereas three reports included mothers of sons who developed testicular cancer.140-141, 144 The results of these studies have been recently reviewed.146 The review noted that all four studies that examined DDT and DDE140, 142-143, 145 reported null associations with DDT. However, three of the four studies reported DDE results that were consistent with a positive association with testicular cancer;140, 143, 145 the two studies that included prediagnostic serum samples provided the strongest evidence.143, 145 A subsequent study also supported an association with DDE.53
Three molecular epidemiological studies have assessed polychlorinated biphenyl (PCB) exposure, both as individual congeners and as functional groups included in other compounds, in relation to testicular cancer.141, 145, 147 A US study reported consistent inverse associations of testicular cancer with both PCB congeners and functional groups.147 However, these findings were not supported by the results of studies conducted in Sweden141 or Norway,145 although the European studies had much smaller sample sizes and only the Norwegian study included prediagnostic samples, which makes strict comparisons difficult. Regardless of these differences, little evidence currently supports the premise that PCB exposure increases the risk of testicular cancer, and some evidence suggests that exposure to these agents might actually decrease the risk of testicular cancer. 147
Cyclodienes derived from hexachlorocyclopentadiene, including chlordane, heptachlor, dieldrin and mirex, have been assessed in relation to testicular cancer. Chlordane and its derivatives (oxychlordane, cis-nonachlor, trans-nonachlor and MC6) have been assessed in at least four molecular epidemiological studies,140, 142-143, 145 of which all save one142 supported associations between both cis-nonachlor and trans-nonachlor and increased risk of testicular cancer. Conversely, little evidence indicates that oxychlordane and MC6 are associated with TGCT, as is also the case for other important hexachlorocyclopentadiene derivatives (heptachlor, dieldrin and mirex).
γ-HCH and a byproduct of its production, β-HCH, have been examined in two molecular epidemiological studies.126,145 Only one of these studies found evidence of an association between an increased risk of TGCT and β-HCH.142 Neither study found evidence of an association with γ-HCH. The fungicide hexachlorobenzene has been assessed in four studies,53, 140, 142, 145 although only one53 suggested a possible association with testicular cancer, albeit based on a small sample size.
Current evidence suggests that of the organochlorine pesticides examined, only DDE and chlordanes, particularly cis-nonachlor and trans-nonachlor, are associated with an increased risk of testicular cancer. The collective evidence does not support an association between testicular cancer and DDT, oxychlordane, MC6, heptachlor, dieldrin, mirex, HCB or HCH. Interestingly, PCBs have been both inversely and positively associated with TGCT and, therefore, require further investigation.
Drug use
Cocaine and cannabinoids both impair spermatogenesis in experimental animals.148-149 Few studies of their corresponding effects on humans have been conducted, although men who are chronic users of marijuana have lower testosterone levels than nonusers.150 In addition, an autopsy study showed that men addicted to a variety of drugs and alcohol were more likely than the general population to have testicular pathology.151 In line with these observations, two case– control studies from the USA found an increased risk of testicular cancer, in particular nonseminoma, among individuals with frequent and long-term marijuana use.152-153 Although these findings are suggestive of an association with testicular carcinogenesis, further studies are required to confirm any association.
Conclusions
Testicular cancer incidence has been increasing for the past four decades, yet the associated risk factors remain poorly defined. Although a large body of evidence suggests that most testicular cancers are initiated in utero, studies of nonperinatal risk factors also indicate a role for nonperinatal exposures many (Box 1). Factors unlikely to be associated with the risk of testicular cancer include adult dietary components and some forms of physical activity, as well as occupational exposure to ionizing radiation, electrical sources and acrylamide. Factors that require additional study include occupational exposures to PVC, nonionizing radiation, heavy metals and heat, as well as environmental exposure to PCBs; the risks associated with agricultural work and long-term marijuana use also require further study. Two occupations— firefighting and aircraft maintenance—are likely to be associated with testicular cancer, as is environmental exposure to specific organochlorine compounds (Box 1). Of course, organochlorine pesticide exposure could also occur during in utero, andcould act as both a perinatal and nonperinatal risk factor.
In terms of attributable risk, firefighting and aircraft maintenance are unlikely to contribute greatly to the overall risk of testicular cancer in a given population. Additionally, exposure to burning building materials can affect persons other than firefighters. Pesticide exposure, by contrast, is very widely dispersed in populations and therefore might result in an increased proportion of the attributable risk. Further study into these as well as other as yet unidentified exposures will certainly be required to fully understand the etiology of testicular cancer. Given the low incidence of testicular cancer in all countries, collaborative investigations that pool data from a number of countries are strongly encouraged.
Key points.
The incidence of testicular cancer has risen globally, particularly in the developed world, over the past several decades
The risk factors for testicular cancer are not well understood, but include prior cryptorchidism, prior unilateral testicular cancer and a family history of testicular cancer
The prevailing hypothesis in the etiology of testicular cancer is that risk is largely determined in utero
Emerging evidence suggests that exposure to risk factors in adolescence and adulthood might also promote testicular cancer, including exposure to certain pesticides, or employment in occupations such as firefighting or aircraft maintenance.
Seven exposures—to heat, polyvinylchloride, nonionizing radiation, heavy metals, agricultural work, pesticides and polychlorinated biphenyls as well as marijuana use— might have an association with testicular cancer
Box 1.
Nonperinatal risk factors that may be linked to testicular cancer
Likely to be associated
Some organochlorine compounds53,140-145
Unlikely to be associated
Specific forms of physical activity35-44
In need of additional study
Nonionizing radiation99,117,119
Heavy metal exposure56,107-110
Agricultural employment120-132
Acknowledgments
The authors would like to thank our colleague, Dr. Michael B. Cook, for his suggestions and comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing interests
References
- 1.Chia VM, et al. International trends in the incidence of testicular cancer, 1973-2002. Cancer Epidemiol. Biomarkers Prev. 2010;19:1151–9. doi: 10.1158/1055-9965.EPI-10-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 9 Regs Research Data. 2011.
- 3.Sesterhenn IA, Davis CJ., Jr. Pathology of germ cell tumors of the testis. Cancer Control. 2004;11:374–87. doi: 10.1177/107327480401100605. [DOI] [PubMed] [Google Scholar]
- 4.McGlynn KA, et al. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003;97:63–70. doi: 10.1002/cncr.11054. [DOI] [PubMed] [Google Scholar]
- 5.Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N. Engl. J. Med. 1997;337:242–53. doi: 10.1056/NEJM199707243370406. [DOI] [PubMed] [Google Scholar]
- 6.McGlynn KA, Devesa SS, Graubard BI, Castle PE. Increasing incidence of testicular germ cell tumors among black men in the United States. J. Clin. Oncol. 2005;23:5757–61. doi: 10.1200/JCO.2005.08.227. [DOI] [PubMed] [Google Scholar]
- 7.Bergstrom R, et al. Increase in testicular cancer incidence in six European countries: a birth cohort phenomenon. J. Natl. Cancer Inst. 1996;88:727–33. doi: 10.1093/jnci/88.11.727. [DOI] [PubMed] [Google Scholar]
- 8.Ekbom A, Akre O. Increasing incidence of testicular cancer--birth cohort effects. APMIS. 1998;106:225–9. doi: 10.1111/j.1699-0463.1998.tb01340.x. discussion 229-31. [DOI] [PubMed] [Google Scholar]
- 9.Moller H. Decreased testicular cancer risk in men born in wartime. J. Natl. Cancer Inst. 1989;81:1668–9. doi: 10.1093/jnci/81.21.1668-a. [DOI] [PubMed] [Google Scholar]
- 10.Cook MB, et al. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer--experiences of the son. Int. J. Epidemiol. 2010;39:1605–18. doi: 10.1093/ije/dyq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemminki K, Chen B. Familial risks in testicular cancer as aetiological clues. Int. J. Androl. 2006;29:205–10. doi: 10.1111/j.1365-2605.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 12.Rapley EA, et al. A genome-wide association study of testicular germ cell tumor. Nat. Genet. 2009;41:807–10. doi: 10.1038/ng.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanetsky PA, et al. Common variation in KITLG and at 5q31.3 predisposes to testicular germ cell cancer. Nat. Genet. 2009;41:811–5. doi: 10.1038/ng.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanetsky PA, et al. A second independent locus within DMRT1 is associated with testicular germ cell tumor susceptibility. Hum. Mol. Genet. 2011;20:3109–17. doi: 10.1093/hmg/ddr207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turnbull C, et al. Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat. Genet. 2010;42:604–7. doi: 10.1038/ng.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbull C, Rahman N. Genome-wide association studies provide new insights into the genetic basis of testicular germ-cell tumour. Int. J. Androl. 2011;34:e86–96. doi: 10.1111/j.1365-2605.2011.01162.x. discussion e96-7. [DOI] [PubMed] [Google Scholar]
- 17.Cook MB, et al. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer--experiences of the mother. Int. J. Epidemiol. 2009;38:1532–42. doi: 10.1093/ije/dyp287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum. Reprod. 2001;16:972–8. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- 19.Rajpert-De Meyts E. Developmental model for the pathogenesis of testicular carcinoma in situ: genetic and environmental aspects. Hum. Reprod. Update. 2006;12:303–23. doi: 10.1093/humupd/dmk006. [DOI] [PubMed] [Google Scholar]
- 20.Tuomisto J, et al. Maternal smoking during pregnancy and testicular cancer in the sons: a nested case-control study and a meta-analysis. Eur. J. Cancer. 2009;45:1640–8. doi: 10.1016/j.ejca.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 21.Alam SS, Cantwell MM, Cardwell CR, Cook MB, Murray LJ. Maternal body mass index and risk of testicular cancer in male offspring: a systematic review and meta-analysis. Cancer Epidemiol. 2010;34:509–15. doi: 10.1016/j.canep.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int. J. Cancer. 1975;15:617–31. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 23.Ganmaa D, et al. Incidence and mortality of testicular and prostatic cancers in relation to world dietary practices. Int. J. Cancer. 2002;98:262–7. doi: 10.1002/ijc.10185. [DOI] [PubMed] [Google Scholar]
- 24.Beja-Pereira A, et al. Gene-culture coevolution between cattle milk protein genes and human lactase genes. Nat. Genet. 2003;35:311–3. doi: 10.1038/ng1263. [DOI] [PubMed] [Google Scholar]
- 25.Sigurdson AJ, et al. A case-control study of diet and testicular carcinoma. Nutr. Cancer. 1999;34:20–6. doi: 10.1207/S15327914NC340103. [DOI] [PubMed] [Google Scholar]
- 26.Bonner MR, McCann SE, Moysich KB. Dietary factors and the risk of testicular cancer. Nutr. Cancer. 2002;44:35–43. doi: 10.1207/S15327914NC441_5. [DOI] [PubMed] [Google Scholar]
- 27.Garner MJ, et al. Dietary risk factors for testicular carcinoma. Int. J. Cancer. 2003;106:934–41. doi: 10.1002/ijc.11327. [DOI] [PubMed] [Google Scholar]
- 28.Swerdlow AJ, De Stavola BL, Swanwick MA, Mangtani P, Maconochie NE. Risk factors for testicular cancer: a case-control study in twins. Br. J. Cancer. 1999;80:1098–102. doi: 10.1038/sj.bjc.6690470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies TW, Palmer CR, Ruja E, Lipscombe JM. Adolescent milk, dairy product and fruit consumption and testicular cancer. Br. J. Cancer. 1996;74:657–60. doi: 10.1038/bjc.1996.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stang A, et al. Adolescent milk fat and galactose consumption and testicular germ cell cancer. Cancer Epidemiol. Biomarkers Prev. 2006;15:2189–95. doi: 10.1158/1055-9965.EPI-06-0372. [DOI] [PubMed] [Google Scholar]
- 31.Swerdlow AJ, Huttly SR, Smith PG. Testis cancer: post-natal hormonal factors, sexual behaviour and fertility. Int. J. Cancer. 1989;43:549–53. doi: 10.1002/ijc.2910430403. [DOI] [PubMed] [Google Scholar]
- 32.McGlynn KA, et al. Body size, dairy consumption, puberty, and risk of testicular germ cell tumors. Am. J. Epidemiol. 2007;165:355–63. doi: 10.1093/aje/kwk019. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, et al. Dietary cholesterol intake and cancer. Ann. Oncol. 2011 doi: 10.1093/annonc/mdr155. [DOI] [PubMed] [Google Scholar]
- 34.Food, nutrition, physical activity and the prevention of cancer : a global perspective : a project of World Cancer Research Fund International. American Institute for Cancer Research; Washington, D.C.: 2007. [Google Scholar]
- 35.Coldman AJ, Elwood JM, Gallagher RP. Sports activities and risk of testicular cancer. Br. J. Cancer. 1982;46:749–56. doi: 10.1038/bjc.1982.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallagher RP, et al. Physical activity, medical history, and risk of testicular cancer (Alberta and British Columbia, Canada). Cancer Causes Control. 1995;6:398–406. doi: 10.1007/BF00052179. [DOI] [PubMed] [Google Scholar]
- 37.Haughey BP, et al. The epidemiology of testicular cancer in upstate New York. Am. J. Epidemiol. 1989;130:25–36. doi: 10.1093/oxfordjournals.aje.a115319. [DOI] [PubMed] [Google Scholar]
- 38.Littman AJ, et al. Physical activity in adolescence and testicular germ cell cancer risk. Cancer Causes Control. 2009;20:1281–90. doi: 10.1007/s10552-009-9347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srivastava A, Kreiger N. Relation of physical activity to risk of testicular cancer. Am. J. Epidemiol. 2000;151:78–87. doi: 10.1093/oxfordjournals.aje.a010126. [DOI] [PubMed] [Google Scholar]
- 40.United Kingdom Testicular Cancer Study Group Aetiology of testicular cancer: association with congenital abnormalities, age at puberty, infertility, and exercise. BMJ. 1994;308:1393–9. [PMC free article] [PubMed] [Google Scholar]
- 41.Paffenbarger RS, Jr., Hyde RT, Wing AL. Physical activity and incidence of cancer in diverse populations: a preliminary report. Am. J. Clin. Nutr. 1987;45:312–7. doi: 10.1093/ajcn/45.1.312. [DOI] [PubMed] [Google Scholar]
- 42.Thune I, Lund E. Physical activity and the risk of prostate and testicular cancer: a cohort study of 53,000 Norwegian men. Cancer Causes Control. 1994;5:549–56. doi: 10.1007/BF01831383. [DOI] [PubMed] [Google Scholar]
- 43.Brownson RC, Chang JC, Davis JR, Smith CA. Physical activity on the job and cancer in Missouri. Am. J. Public Health. 1991;81:639–42. doi: 10.2105/ajph.81.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dosemeci M, et al. Occupational physical activity, socioeconomic status, and risks of 15 cancer sites in Turkey. Cancer Causes Control. 1993;4:313–21. doi: 10.1007/BF00051333. [DOI] [PubMed] [Google Scholar]
- 45.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur. J. Cancer. 2010;46:2593–604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 46.Leitzmann MF. In: Physical activity and cancer. Courneya KS, Friedenreich CM, editors. Springer, Heidelberg; London: 2011. [Google Scholar]
- 47.Robinson D, Rock J. Intrascrotal hyperthermia induced by scrotal insulation: effect on spermatogenesis. Obstet. Gynecol. 1967;29:217–23. [PubMed] [Google Scholar]
- 48.Robinson D, Rock J, Menkin MF. Control of human spermatogenesis by induced changes of intrascrotal temperature. JAMA. 1968;204:290–7. [PubMed] [Google Scholar]
- 49.Rock J, Robinson D. Effect of induced intrascrotal hyperthermia on testicular function in man. Am. J. Obstet. Gynecol. 1965;93:793–801. doi: 10.1016/0002-9378(65)90080-3. [DOI] [PubMed] [Google Scholar]
- 50.Procope BJ. Effect of repeated increase of body temperature on human sperm cells. Int. J. Fertil. 1965;10:333–9. [PubMed] [Google Scholar]
- 51.UK Testicular Cancer Study Group Social, behavioural and medical factors in the aetiology of testicular cancer: results from the UK study. Br. J. Cancer. 1994;70:513–20. doi: 10.1038/bjc.1994.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karagas MR, Weiss NS, Strader CH, Daling JR. Elevated intrascrotal temperature and the incidence of testicular cancer in noncryptorchid men. Am. J. Epidemiol. 1989;129:1104–9. doi: 10.1093/oxfordjournals.aje.a115232. [DOI] [PubMed] [Google Scholar]
- 53.Giannandrea F, Gandini L, Paoli D, Turci R, Figa-Talamanca I. Pesticide exposure and serum organochlorine residuals among testicular cancer patients and healthy controls. J. Environ. Sci. Health. B. 2011;46:780–7. doi: 10.1080/03601234.2012.597704. [DOI] [PubMed] [Google Scholar]
- 54.Zhang ZF, et al. Occupational exposure to extreme temperature and risk of testicular cancer. Arch. Environ. Health. 1995;50:13–8. doi: 10.1080/00039896.1995.9955007. [DOI] [PubMed] [Google Scholar]
- 55.Andersson E, Nilsson R, Toren K. Testicular cancer among Swedish pulp and paper workers. Am. J. Ind. Med. 2003;43:642–6. doi: 10.1002/ajim.10223. [DOI] [PubMed] [Google Scholar]
- 56.Hobbesland A, Kjuus H, Thelle DS. Study of cancer incidence among 8530 male workers in eight Norwegian plants producing ferrosilicon and silicon metal. Occup. Environ. Med. 1999;56:625–31. doi: 10.1136/oem.56.9.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.James WH. Further grounds for abandoning the concept of testicular dysgenesis syndrome: a response to the paper of Akre and Richiardi (2009). Hum. Reprod. 2010;25:1084–6. doi: 10.1093/humrep/dep461. [DOI] [PubMed] [Google Scholar]
- 58.Bates MN, et al. Is testicular cancer an occupational disease of fire fighters? Am. J. Ind. Med. 2001;40:263–70. doi: 10.1002/ajim.1097. [DOI] [PubMed] [Google Scholar]
- 59.Rosen A, Jayram G, Drazer M, Eggener SE. Global trends in testicular cancer incidence and mortality. Eur. Urol. 2011;60:374–9. doi: 10.1016/j.eururo.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Bates MN, Lane L. Testicular cancer in fire fighters: a cluster investigation. N. Z. Med. J. 1995;108:334–7. [PubMed] [Google Scholar]
- 61.Bates MN. Registry-based case-control study of cancer in California firefighters. Am. J. Ind. Med. 2007;50:339–44. doi: 10.1002/ajim.20446. [DOI] [PubMed] [Google Scholar]
- 62.Ma F, Fleming LE, Lee DJ, Trapido E, Gerace TA. Cancer incidence in Florida professional firefighters, 1981 to 1999. J. Occup. Environ. Med. 2006;48:883–8. doi: 10.1097/01.jom.0000235862.12518.04. [DOI] [PubMed] [Google Scholar]
- 63.Stang A, Jockel KH, Baumgardt-Elms C, Ahrens W. Firefighting and risk of testicular cancer: results from a German population-based case-control study. Am. J. Ind. Med. 2003;43:291–4. doi: 10.1002/ajim.10178. [DOI] [PubMed] [Google Scholar]
- 64.Pearce N, Sheppard RA, Howard JK, Fraser J, Lilley BM. Time trends and occupational differences in cancer of the testis in New Zealand. Cancer. 1987;59:1677–82. doi: 10.1002/1097-0142(19870501)59:9<1677::aid-cncr2820590926>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 65.Sama SR, Martin TR, Davis LK, Kriebel D. Cancer incidence among Massachusetts firefighters, 1982-1986. Am. J. Ind. Med. 1990;18:47–54. doi: 10.1002/ajim.4700180106. [DOI] [PubMed] [Google Scholar]
- 66.Giles G, Staples M, Berry J. Cancer incidence in Melbourne Metropolitan Fire Brigade members, 1980-1989. Health Rep. 1993;5:33–8. [PubMed] [Google Scholar]
- 67.Demers PA, et al. Cancer incidence among firefighters in Seattle and Tacoma, Washington (United States). Cancer Causes Control. 1994;5:129–35. doi: 10.1007/BF01830258. [DOI] [PubMed] [Google Scholar]
- 68.Tornling G, Gustavsson P, Hogstedt C. Mortality and cancer incidence in Stockholm fire fighters. Am. J. Ind. Med. 1994;25:219–28. doi: 10.1002/ajim.4700250208. [DOI] [PubMed] [Google Scholar]
- 69.LeMasters GK, et al. Cancer risk among firefighters: a review and meta-analysis of 32 studies. J. Occup. Environ. Med. 2006;48:1189–202. doi: 10.1097/01.jom.0000246229.68697.90. [DOI] [PubMed] [Google Scholar]
- 70.Guidotti TL. Evaluating causality for occupational cancers: the example of firefighters. Occup Med (Lond) 2007;57:466–71. doi: 10.1093/occmed/kqm031. [DOI] [PubMed] [Google Scholar]
- 71.Caux C, O'Brien C, Viau C. Determination of firefighter exposure to polycyclic aromatic hydrocarbons and benzene during fire fighting using measurement of biological indicators. Appl Occup Environ Hyg. 2002;17:379–86. doi: 10.1080/10473220252864987. [DOI] [PubMed] [Google Scholar]
- 72.Grayson JK, Lyons TJ. Cancer incidence in United States Air Force aircrew, 1975-89. Aviat. Space Environ. Med. 1996;67:101–4. [PubMed] [Google Scholar]
- 73.Hoiberg A, Blood C. Age-specific morbidity among Navy pilots. Aviat. Space Environ. Med. 1983;54:912–8. [PubMed] [Google Scholar]
- 74.Tarone RE, et al. Service in Vietnam and risk of testicular cancer. J. Natl. Cancer Inst. 1991;83:1497–9. doi: 10.1093/jnci/83.20.1497. [DOI] [PubMed] [Google Scholar]
- 75.Yamane GK, Johnson R. Testicular carcinoma in U.S. Air Force aviators: a case-control study. Aviat. Space Environ. Med. 2003;74:846–50. [PubMed] [Google Scholar]
- 76.Levine PH, et al. Is testicular cancer related to Gulf War deployment? Evidence from a pilot population-based study of Gulf War era veterans and cancer registries. Mil. Med. 2005;170:149–53. [PubMed] [Google Scholar]
- 77.Yamane GK. Cancer incidence in the U.S. Air Force: 1989-2002. Aviat. Space Environ. Med. 2006;77:789–94. [PubMed] [Google Scholar]
- 78.Enewold L, et al. Trends in testicular germ cell tumors among U.S. military servicemen, 1990-2003. Mil. Med. 2011;176:1184–7. doi: 10.7205/milmed-d-10-00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Breslin P, Kang HK, Lee Y, Burt V, Shepard BM. Proportionate mortality study of US Army and US Marine Corps veterans of the Vietnam War. J. Occup. Med. 1988;30:412–9. doi: 10.1097/00043764-198805000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Garland FC, Gorham ED, Garland CF, Ducatman AM. Testicular cancer in US Navy personnel. Am. J. Epidemiol. 1988;127:411–4. doi: 10.1093/oxfordjournals.aje.a114815. [DOI] [PubMed] [Google Scholar]
- 81.Gray GC, et al. The postwar hospitalization experience of U.S. veterans of the Persian Gulf War. N. Engl. J. Med. 1996;335:1505–13. doi: 10.1056/NEJM199611143352007. [DOI] [PubMed] [Google Scholar]
- 82.Knoke JD, Gray GC, Garland FC. Testicular cancer and Persian Gulf War service. Epidemiology. 1998;9:648–53. [PubMed] [Google Scholar]
- 83.Zhu K, et al. Cancer incidence in the U.S. military population: comparison with rates from the SEER program. Cancer Epidemiol. Biomarkers Prev. 2009;18:1740–5. doi: 10.1158/1055-9965.EPI-09-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frumkin H. Agent Orange and cancer: an overview for clinicians. CA. Cancer J. Clin. 2003;53:245–55. doi: 10.3322/canjclin.53.4.245. [DOI] [PubMed] [Google Scholar]
- 85.Bullman TA, Watanabe KK, Kang HK. Risk of testicular cancer associated with surrogate measures of Agent Orange exposure among Vietnam veterans on the Agent Orange Registry. Ann. Epidemiol. 1994;4:11–6. doi: 10.1016/1047-2797(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 86.Foley S, Middleton S, Stitson D, Mahoney M. The incidence of testicular cancer in Royal Air Force personnel. Br. J. Urol. 1995;76:495–6. doi: 10.1111/j.1464-410x.1995.tb07755.x. [DOI] [PubMed] [Google Scholar]
- 87.Ryder SJ, Crawford PI, Pethybridge RJ. Is testicular cancer an occupational disease? A case-control study of Royal Naval personnel. J. R. Nav. Med. Serv. 1997;83:130–46. [PubMed] [Google Scholar]
- 88.Gustavsson P, et al. Incidence of cancer among Swedish military and civil personnel involved in UN missions in the Balkans 1989-99. Occup. Environ. Med. 2004;61:171–3. doi: 10.1136/oem.2002.005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Milanov L, Dimitrov D, Danon S. Cancer incidence in Republic of Bulgaria aircrew, 1964-1994. Aviat. Space Environ. Med. 1999;70:681–5. [PubMed] [Google Scholar]
- 90.Sulem P, Rafnsson V. Cancer incidence among Icelandic deck officers in a population-based study. Scand. J. Work. Environ. Health. 2003;29:100–5. doi: 10.5271/sjweh.711. [DOI] [PubMed] [Google Scholar]
- 91.Band PR, Spinelli JJ, Ng VT, Moody J, Gallagher RP. Mortality and cancer incidence in a cohort of commercial airline pilots. Aviat. Space Environ. Med. 1990;61:299–302. [PubMed] [Google Scholar]
- 92.Ducatman AM, Conwill DE, Crawl J. Germ cell tumors of the testicle among aircraft repairmen. J. Urol. 1986;136:834–6. doi: 10.1016/s0022-5347(17)45096-8. [DOI] [PubMed] [Google Scholar]
- 93.Boice JD, Jr., Marano DE, Fryzek JP, Sadler CJ, McLaughlin JK. Mortality among aircraft manufacturing workers. Occup. Environ. Med. 1999;56:581–97. doi: 10.1136/oem.56.9.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lipworth L, et al. Cancer mortality among aircraft manufacturing workers: an extended follow-up. J. Occup. Environ. Med. 2011;53:992–1007. doi: 10.1097/JOM.0b013e31822e0940. [DOI] [PubMed] [Google Scholar]
- 95.Van den Eeden SK, Weiss NS, Strader CH, Daling JR. Occupation and the occurrence of testicular cancer. Am. J. Ind. Med. 1991;19:327–37. doi: 10.1002/ajim.4700190307. [DOI] [PubMed] [Google Scholar]
- 96.Tynes T, Andersen A, Langmark F. Incidence of cancer in Norwegian workers potentially exposed to electromagnetic fields. Am. J. Epidemiol. 1992;136:81–8. doi: 10.1093/oxfordjournals.aje.a116423. [DOI] [PubMed] [Google Scholar]
- 97.Pearce N, Reif J, Fraser J. Case-control studies of cancer in New Zealand electrical workers. Int. J. Epidemiol. 1989;18:55–9. doi: 10.1093/ije/18.1.55. [DOI] [PubMed] [Google Scholar]
- 98.Verreault R, Weiss NS, Hollenbach KA, Strader CH, Daling JR. Use of electric blankets and risk of testicular cancer. Am. J. Epidemiol. 1990;131:759–62. doi: 10.1093/oxfordjournals.aje.a115565. [DOI] [PubMed] [Google Scholar]
- 99.Davis RL, Mostofi FK. Cluster of testicular cancer in police officers exposed to hand-held radar. Am. J. Ind. Med. 1993;24:231–3. doi: 10.1002/ajim.4700240209. [DOI] [PubMed] [Google Scholar]
- 100.Finkelstein MM. Cancer incidence among Ontario police officers. Am. J. Ind. Med. 1998;34:157–62. doi: 10.1002/(sici)1097-0274(199808)34:2<157::aid-ajim8>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 101.Violanti JM, Vena JE, Petralia S. Mortality of a police cohort: 1950-1990. Am. J. Ind. Med. 1998;33:366–73. doi: 10.1002/(sici)1097-0274(199804)33:4<366::aid-ajim6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 102.Vena JE, Violanti JM, Marshall J, Fiedler RC. Mortality of a municipal worker cohort: III. Police officers. Am. J. Ind. Med. 1986;10:383–97. doi: 10.1002/ajim.4700100406. [DOI] [PubMed] [Google Scholar]
- 103.Feuer E, Rosenman K. Mortality in police and firefighters in New Jersey. Am. J. Ind. Med. 1986;9:517–27. doi: 10.1002/ajim.4700090603. [DOI] [PubMed] [Google Scholar]
- 104.Demers PA, et al. Cancer identification using a tumor registry versus death certificates in occupational cohort studies in the United States. Am. J. Epidemiol. 1992;136:1232–40. doi: 10.1093/oxfordjournals.aje.a116431. [DOI] [PubMed] [Google Scholar]
- 105.Forastiere F, et al. Mortality among urban policemen in Rome. Am. J. Ind. Med. 1994;26:785–98. doi: 10.1002/ajim.4700260607. [DOI] [PubMed] [Google Scholar]
- 106.Hardin BD. Washington, D.C.: 1993. pp. 55–60. [Google Scholar]
- 107.Rhomberg W, Schmoll HJ, Schneider B. High frequency of metalworkers among patients with seminomatous tumors of the testis: a case-control study. Am. J. Ind. Med. 1995;28:79–87. doi: 10.1002/ajim.4700280107. [DOI] [PubMed] [Google Scholar]
- 108.Xu C, et al. In vivo studies of cadmium-induced apoptosis in testicular tissue of the rat and its modulation by a chelating agent. Toxicology. 1996;107:1–8. doi: 10.1016/0300-483x(95)03195-l. [DOI] [PubMed] [Google Scholar]
- 109.Verhoeven RH, et al. Variation in cancer incidence in northeastern Belgium and southeastern Netherlands seems unrelated to cadmium emission of zinc smelters. Eur. J. Cancer Prev. 2011;20:549–55. doi: 10.1097/CEJ.0b013e3283498e9c. [DOI] [PubMed] [Google Scholar]
- 110.Nawrot T, et al. Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol. 2006;7:119–26. doi: 10.1016/S1470-2045(06)70545-9. [DOI] [PubMed] [Google Scholar]
- 111.Ohlson CG, Hardell L. Testicular cancer and occupational exposures with a focus on xenoestrogens in polyvinyl chloride plastics. Chemosphere. 2000;40:1277–82. doi: 10.1016/s0045-6535(99)00380-x. [DOI] [PubMed] [Google Scholar]
- 112.Hardell L, Malmqvist N, Ohlson CG, Westberg H, Eriksson M. Testicular cancer and occupational exposure to polyvinyl chloride plastics: a case-control study. Int. J. Cancer. 2004;109:425–9. doi: 10.1002/ijc.11709. [DOI] [PubMed] [Google Scholar]
- 113.Langard S, Rosenberg J, Andersen A, Heldaas SS. Incidence of cancer among workers exposed to vinyl chloride in polyvinyl chloride manufacture. Occup. Environ. Med. 2000;57:65–8. doi: 10.1136/oem.57.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harris CA, Henttu P, Parker MG, Sumpter JP. The estrogenic activity of phthalate esters in vitro. Environ. Health Perspect. 1997;105:802–11. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marsh GM, Lucas LJ, Youk AO, Schall LC. Mortality patterns among workers exposed to acrylamide: 1994 follow up. Occup. Environ. Med. 1999;56:181–90. doi: 10.1136/oem.56.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marsh GM, Youk AO, Buchanich JM, Kant IJ, Swaen G. Mortality patterns among workers exposed to acrylamide: updated follow up. J. Occup. Environ. Med. 2007;49:82–95. doi: 10.1097/JOM.0b013e31802db536. [DOI] [PubMed] [Google Scholar]
- 117.Yousif L, Blettner M, Hammer GP, Zeeb H. Testicular cancer risk associated with occupational radiation exposure: a systematic literature review. J. Radiol. Prot. 2010;30:389–406. doi: 10.1088/0952-4746/30/3/R01. [DOI] [PubMed] [Google Scholar]
- 118.Sigurdson AJ, et al. Cancer incidence in the US radiologic technologists health study, 1983-1998. Cancer. 2003;97:3080–9. doi: 10.1002/cncr.11444. [DOI] [PubMed] [Google Scholar]
- 119.Floderus B, Stenlund C, Persson T. Occupational magnetic field exposure and site-specific cancer incidence: a Swedish cohort study. Cancer Causes Control. 1999;10:323–32. doi: 10.1023/a:1008953920877. [DOI] [PubMed] [Google Scholar]
- 120.Mills PK, Newell GR, Johnson DE. Testicular cancer associated with employment in agriculture and oil and natural gas extraction. Lancet. 1984;1:207–10. doi: 10.1016/s0140-6736(84)92125-1. [DOI] [PubMed] [Google Scholar]
- 121.McDowall ME, Balarajan R. Testicular cancer mortality in England and Wales 1971-80: variations by occupation. J. Epidemiol. Community Health. 1986;40:26–9. doi: 10.1136/jech.40.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mills PK, Newell GR. Testicular cancer risk in agricultural occupations. J. Occup. Med. 1984;26:798–9. doi: 10.1097/00043764-198411000-00003. [DOI] [PubMed] [Google Scholar]
- 123.Graham S, Gibson RW. Social epidemiology of cancer of the testis. Cancer. 1972;29:1242–9. doi: 10.1002/1097-0142(197205)29:5<1242::aid-cncr2820290517>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 124.Brown LM, Pottern LM. Testicular cancer and farming. Lancet. 1984;1:1356. doi: 10.1016/s0140-6736(84)91853-1. [DOI] [PubMed] [Google Scholar]
- 125.Sewell CM, Castle SP, Hull HF, Wiggins C. Testicular cancer and employment in agriculture and oil and natural gas extraction. Lancet. 1986;1:553. doi: 10.1016/s0140-6736(86)90902-5. [DOI] [PubMed] [Google Scholar]
- 126.Jensen OM, Olsen JH, Osterlind A. Testis cancer risk among farmers in Denmark. Lancet. 1984;1:794. doi: 10.1016/s0140-6736(84)91305-9. [DOI] [PubMed] [Google Scholar]
- 127.Wiklund K, Dich J, Holm LE. Testicular cancer among agricultural workers and licensed pesticide applicators in Sweden. Scand. J. Work. Environ. Health. 1986;12:630–1. doi: 10.5271/sjweh.2091. [DOI] [PubMed] [Google Scholar]
- 128.Blair A, Zahm SH, Pearce NE, Heineman EF, Fraumeni JF., Jr. Clues to cancer etiology from studies of farmers. Scand. J. Work. Environ. Health. 1992;18:209–15. doi: 10.5271/sjweh.1578. [DOI] [PubMed] [Google Scholar]
- 129.Acquavella J, et al. Cancer among farmers: a meta-analysis. Ann. Epidemiol. 1998;8:64–74. doi: 10.1016/s1047-2797(97)00120-8. [DOI] [PubMed] [Google Scholar]
- 130.Kristensen P, Andersen A, Irgens LM, Bye AS, Sundheim L. Cancer in offspring of parents engaged in agricultural activities in Norway: incidence and risk factors in the farm environment. Int. J. Cancer. 1996;65:39–50. doi: 10.1002/(SICI)1097-0215(19960103)65:1<39::AID-IJC8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 131.Moller H. Work in agriculture, childhood residence, nitrate exposure, and testicular cancer risk: a case-control study in Denmark. Cancer Epidemiol. Biomarkers Prev. 1997;6:141–4. [PubMed] [Google Scholar]
- 132.Kardaun JW, Hayes RB, Pottern LM, Brown LM, Hoover RN. Testicular cancer in young men and parental occupational exposure. Am. J. Ind. Med. 1991;20:219–27. doi: 10.1002/ajim.4700200208. [DOI] [PubMed] [Google Scholar]
- 133.Guo J, et al. Testicular cancer, occupation and exposure to chemical agents among Finnish men in 1971-1995. Cancer Causes Control. 2005;16:97–103. doi: 10.1007/s10552-004-2236-0. [DOI] [PubMed] [Google Scholar]
- 134.Dich J, Wiklund K, Holm LE. Testicular cancer in pesticide applicators in Swedish agriculture. Scand. J. Work. Environ. Health. 1996;22:66. doi: 10.5271/sjweh.112. [DOI] [PubMed] [Google Scholar]
- 135.Fleming LE, Bean JA, Rudolph M, Hamilton K. Cancer incidence in a cohort of licensed pesticide applicators in Florida. J. Occup. Environ. Med. 1999;41:279–88. doi: 10.1097/00043764-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 136.Frost G, Brown T, Harding AH. Mortality and cancer incidence among British agricultural pesticide users. Occup Med (Lond) 2011;61:303–10. doi: 10.1093/occmed/kqr067. [DOI] [PubMed] [Google Scholar]
- 137.Jaga K, Dharmani C. Global surveillance of DDT and DDE levels in human tissues. Int. J. Occup. Med. Environ. Health. 2003;16:7–20. [PubMed] [Google Scholar]
- 138.Roy JR, Chakraborty S, Chakraborty TR. Estrogen-like endocrine disrupting chemicals affecting puberty in humans--a review. Med Sci Monit. 2009;15:RA137–45. [PubMed] [Google Scholar]
- 139.Toppari J. Environmental endocrine disrupters. Sex Dev. 2008;2:260–7. doi: 10.1159/000152042. [DOI] [PubMed] [Google Scholar]
- 140.Hardell L, et al. Increased concentrations of polychlorinated biphenyls, hexachlorobenzene, and chlordanes in mothers of men with testicular cancer. Environ. Health Perspect. 2003;111:930–4. doi: 10.1289/ehp.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hardell L, et al. Concentrations of polychlorinated biphenyls in blood and the risk for testicular cancer. Int. J. Androl. 2004;27:282–90. doi: 10.1111/j.1365-2605.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 142.Biggs ML, et al. Serum organochlorine pesticide residues and risk of testicular germ cell carcinoma: a population-based case-control study. Cancer Epidemiol. Biomarkers Prev. 2008;17:2012–8. doi: 10.1158/1055-9965.EPI-08-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McGlynn KA, et al. Persistent organochlorine pesticides and risk of testicular germ cell tumors. J. Natl. Cancer Inst. 2008;100:663–71. doi: 10.1093/jnci/djn101. [DOI] [PubMed] [Google Scholar]
- 144.Cohn BA, Cirillo PM, Christianson RE. Prenatal DDT exposure and testicular cancer: a nested case-control study. Arch Environ Occup Health. 2010;65:127–34. doi: 10.1080/19338241003730887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Purdue MP, et al. Prediagnostic serum concentrations of organochlorine compounds and risk of testicular germ cell tumors. Environ. Health Perspect. 2009;117:1514–9. doi: 10.1289/ehp.0800359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cook MB, Trabert B, McGlynn KA. Organochlorine compounds and testicular dysgenesis syndrome: human data. Int. J. Androl. 2011;34:e68–84. doi: 10.1111/j.1365-2605.2011.01171.x. discussion e84-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McGlynn KA, et al. Polychlorinated biphenyls and risk of testicular germ cell tumors. Cancer Res. 2009;69:1901–9. doi: 10.1158/0008-5472.CAN-08-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bracken MB, et al. Association of cocaine use with sperm concentration, motility, and morphology. Fertil. Steril. 1990;53:315–22. doi: 10.1016/s0015-0282(16)53288-9. [DOI] [PubMed] [Google Scholar]
- 149.Brown TT, Dobs AS. Endocrine effects of marijuana. J. Clin. Pharmacol. 2002;42:90S–96S. doi: 10.1002/j.1552-4604.2002.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 150.Barnett G, Chiang CW, Licko V. Effects of marijuana on testosterone in male subjects. J. Theor. Biol. 1983;104:685–92. doi: 10.1016/0022-5193(83)90255-2. [DOI] [PubMed] [Google Scholar]
- 151.Reuhl J, Bachl M, Schneider M, Lutz F, Bratzke H. Morphometric assessment of testicular changes in drug-related fatalities. Forensic Sci. Int. 2001;115:171–81. doi: 10.1016/s0379-0738(00)00327-3. [DOI] [PubMed] [Google Scholar]
- 152.Daling JR, et al. Association of marijuana use and the incidence of testicular germ cell tumors. Cancer. 2009;115:1215–23. doi: 10.1002/cncr.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Trabert B, Sigurdson AJ, Sweeney AM, Strom SS, McGlynn KA. Marijuana use and testicular germ cell tumors. Cancer. 2010 doi: 10.1002/cncr.25499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fossa SD, et al. Risk of contralateral testicular cancer: a population-based study of 29,515 U.S. men. J. Natl. Cancer Inst. 2005;97:1056–66. doi: 10.1093/jnci/dji185. [DOI] [PubMed] [Google Scholar]
- 155.Westergaard T, et al. Cancer risk in fathers and brothers of testicular cancer patients in Denmark. A population-based study. Int. J. Cancer. 1996;66:627–31. doi: 10.1002/(SICI)1097-0215(19960529)66:5<627::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 156.Sonneveld DJ, et al. Familial testicular cancer in a single-centre population. Eur. J. Cancer. 1999;35:1368–73. doi: 10.1016/s0959-8049(99)00140-9. [DOI] [PubMed] [Google Scholar]
- 157.Lerro CC, McGlynn KA, Cook MB. A systematic review and meta-analysis of the relationship between body size and testicular cancer. Br. J. Cancer. 2010;103:1467–74. doi: 10.1038/sj.bjc.6605934. [DOI] [PMC free article] [PubMed] [Google Scholar]


