Abstract
Bradykinin increases during cardiopulmonary bypass (CPB) and stimulates the release of nitric oxide, inflammatory cytokines, and tissue-type plasminogen activator (t-PA), acting through its B2 receptor. This study tested the hypothesis that endogenous bradykinin contributes to the fibrinolytic and inflammatory response to CPB and that bradykinin B2 receptor antagonism reduces fibrinolysis, inflammation, and subsequent transfusion requirements. Patients (N = 115) were prospectively randomized to placebo, ε-aminocaproic acid (EACA), or HOE 140, a bradykinin B2 receptor antagonist. Bradykinin B2 receptor antagonism decreased intraoperative fibrinolytic capacity as much as EACA, but only EACA decreased D-dimer formation and tended to decrease postoperative bleeding. Although EACA and HOE 140 decreased fibrinolysis and EACA attenuated blood loss, these treatments did not reduce the proportion of patients transfused. These data suggest that endogenous bradykinin contributes to t-PA generation in patients undergoing CPB, but that additional effects on plasmin generation contribute to decreased D-dimer concentrations during EACA treatment.
Cardiac surgery requiring cardiopulmonary bypass (CPB) is associated with an increased risk of blood product transfusion. Estimates of red cell transfusion risk range from 8% to more than 90% and vary dramatically across institutions.1 The etiology of postoperative bleeding and blood product transfusion is multifactorial and includes platelet dysfunction, hemodilution and consumption of coagulation factors, fibrinolysis, and surgical bleeding. Enhanced intraoperative fibrinolysis appears to play an important role in postoperative bleeding as suggested by the blood transfusion sparing effects of antifibrinolytic drugs such as ε-aminocaproic acid (EACA) and tranexamic acid.2–5
CPB activates the kallikrein–kinin system, leading to increased bradykinin concentrations.6–8 Bradykinin, acting through its B2 receptor, stimulates the release of nitric oxide, inflammatory cytokines, and tissue-type plasminogen activator (t-PA).9–14 Specifically, we have found that during CPB, bradykinin concentrations correlate inversely with mean arterial pressure (MAP) and directly with t-PA.6 Studies in animals suggest that bradykinin B2 receptor antagonism inhibits reperfusion-induced increases in vascular permeability and neutrophil recruitment.15,16 A randomized, placebo-controlled clinical trial of a bradykinin B2 receptor antagonist demonstrated some effect on survival in patients with systemic inflammatory response syndrome and Gram-negative sepsis.17 In addition, we and others have shown that bradykinin B2 receptor antagonism reduces vascular t-PA release during angiotensin-converting enzyme (ACE) inhibition.14,18 Taken together, these findings suggest that blocking the effects of bradykinin with a specific receptor antagonist could potentially reduce bradykinin-mediated inflammation, fibrinolysis, and ensuing transfusion requirements in patients undergoing CPB.
This study tested the hypothesis that endogenous bradykinin contributes to the fibrinolytic and inflammatory response to CPB and that bradykinin B2 receptor antagonism will reduce fibrinolysis, inflammation, and subsequent transfusion requirements.
RESULTS
Subject characteristics
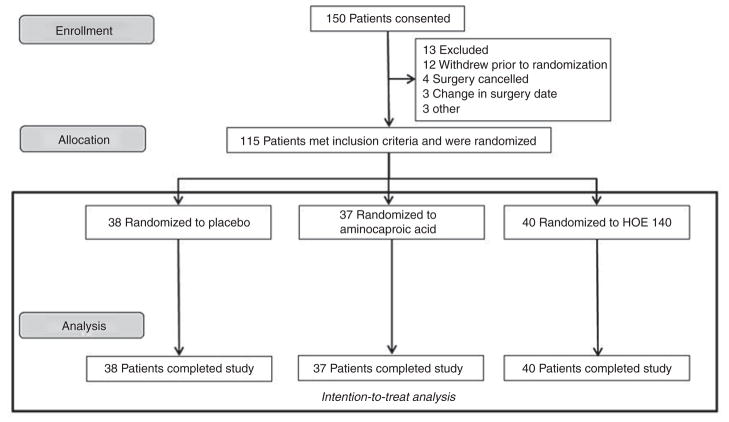
One hundred and fifty patients consented to participate in the study (Figure 1). Of these patients, 13 were excluded, 12 withdrew before randomization, 4 had their surgeries canceled, 3 had their surgery dates changed, and 3 patients did not proceed with the study for other reasons. The remaining 115 patients were randomly assigned to one of three groups and were included in the intention-to-treat analysis. There were no protocol violations for study drug administration. Table 1 provides baseline characteristics for the three treatment groups. The groups were similar in all baseline characteristics before treatment except that preoperative statin use was higher in the HOE 140 group. Intraoperative characteristics are provided in Table 2. The three treatment groups were similar with regard to the type of surgical procedure, CPB duration, use of aortic cross-clamp, use of hemoconcentration, use of steroids in pump prime, use of intropes/vasopressor drugs, and fluids given. Blood loss as measured by 24-h chest tube output tended to be lower in the EACA group as compared with the placebo group (424 ± 44 vs. 568 ± 68 ml, P = 0.09 by Mann–Whitney U test).
Figure 1.
Enrollment. HOE 140 is a specific bradykinin B2 receptor antagonist.
Table 1.
Preoperative subject characteristics
| Characteristic | Placebo (n = 38) | EACA (n = 37) | HOE 140 (n = 40) | P value |
|---|---|---|---|---|
| Age, years | 60.1 ± 1.8 | 58.5 ± 2.0 | 61.0 ± 2.0 | 0.61a |
| Gender, women n (%) | 12 (31.6) | 18 (48.6) | 18 (45.0) | 0.28b |
| Race, Caucasian n (%) | 36 (94.7) | 33 (89.2) | 39 (97.5) | 0.49b |
| Body mass index (kg/m2) | 27.5 ± 0.8 | 27.2 ± 0.8 | 28.3 ± 0.9 | 0.55a |
| Mean arterial pressure (mm Hg) | 96.2 ± 2.0 | 91.1 ± 2.1 | 92.3 ± 1.5 | 0.15a |
| Medical history, n (%) | ||||
| Hypertension | 27 (71.1) | 22 (59.5) | 27 (67.5) | 0.55b |
| Atrial fibrillation | 11 (28.9) | 7 (18.9) | 10 (25.0) | 0.60b |
| Diabetes | 6 (15.8) | 5 (13.5) | 8 (20.0) | 0.74b |
| Smoking history | 16 (42.1) | 17 (45.9) | 17 (42.5) | 0.93b |
| Preoperative medications, n (%) | ||||
| β-Blockers | 18 (47.4) | 14 (37.8) | 23 (57.5) | 0.23b |
| ACE inhibitors | 15 (39.5) | 14 (37.8) | 13 (32.5) | 0.80b |
| ARB | 10 (26.3) | 3 (8.3) | 9 (22.5) | 0.12b |
| Statins | 13 (34.2)* | 10 (27.8)* | 24 (60.0) | 0.01b |
| Diuretic | 19 (50.0) | 12 (32.4) | 16 (40.0) | 0.30b |
| Aspirin | 12 (31.6) | 13 (35.1) | 20 (50.0) | 0.21b |
| Clopidogrel | 2 (5.3) | 3 (8.1) | 3 (7.5) | 0.88b |
| Preoperative | ||||
| Hematocrit, % | 41.7 ± 0.7 | 41.4 ± 0.6 | 41.4 ± 0.6 | 0.99a |
| Platelet count, (109 ml−1) | 209.4 ± 8.9 | 217.1 ± 7.1 | 216.5 ± 7.5 | 0.66a |
| Creatinine, mg/dl | 1.03 ± 0.04 | 0.95 ± 0.03 | 0.95 ± 0.03 | 0.09a |
| Potassium, mmol/l | 4.1 ± 0.1 | 4.2 ± 0.1 | 4.1 ± 0.1 | 0.51a |
| Ejection fraction, % | 58.6 ± 1.5 | 61.0 ± 1.2 | 58.7 ± 1.4 | 0.46a |
HOE 140 is a specific bradykinin B2 receptor antagonist.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; EACA, ε-aminocaproic acid.
Kruskal–Wallis test.
χ2 test.
P < 0.05 vs. HOE 140.
Table 2.
Intra- and postoperative subject characteristics
| Characteristic | Placebo (n = 38) | EACA (n = 37) | HOE 140 (n = 40) | P value |
|---|---|---|---|---|
| Surgery type | 0.93a | |||
| CABG surgery, n (%) | 4 (10.5) | 4 (10.8) | 4 (10.0) | |
| Valvular surgery, n (%) | 33 (86.8) | 30 (81.1) | 34 (85.0) | |
| Other surgery, n (%) | 1 (2.6) | 3 (8.1) | 2 (5.0) | |
| Cardiopulmonary bypass time, min | 111.7 ± 6.0 | 122.2 ± 6.3 | 115.3 ± 5.9 | 0.56b |
| Cardioplegia, n (%) | 17 (44.7) | 14 (37.8) | 18 (45.0) | 0.78a |
| Cross-clamp time, min | 80.6 ± 6.4 | 82.4 ± 6.7 | 76.7 ± 3.4 | 0.70b |
| Lowest hematocrit on pump, % | 27.0 ± 0.7 | 25.8 ± 0.7 | 27.0 ± 0.6 | 0.40b |
| Hemoconcentration used, n (%) | 7 (18.4) | 12 (32.4) | 11 (27.5) | 0.37a |
| Steroids in pump prime, n (%) | 26 (68.4) | 28 (75.7) | 34 (85.0) | 0.22a |
| Inotropes in OR, n (%) | ||||
| Dobutamine | 12 (31.6) | 6 (16.2) | 8 (20.0) | 0.25a |
| Milrinone | 8 (21.1) | 7 (18.9) | 4 (10.0) | 0.38a |
| Epinephrine | 4 (10.5) | 5 (13.5) | 3 (7.5) | 0.69a |
| Norepinephrine | 36 (94.7) | 33 (89.2) | 35 (87.5) | 0.53a |
| Cell saver blood, ml | 645.1 ± 51.8 | 715.3 ± 49.0 | 651.2 ± 36.1 | 0.37b |
| Total crystalloid given in OR, ml | 3,297 ± 165 | 3,054 ± 121 | 3,206 ± 140 | 0.56b |
| Total colloid given in OR, ml | 97 ± 42 | 95 ± 43 | 75 ± 29 | 0.97b |
| Chest tube output in 24 h (ml) | 568 ± 68 | 424 ± 44 | 508 ± 62 | 0.22b |
HOE 140 is a specific bradykinin B2 receptor antagonist.
CABG, coronary artery bypass graft; EACA, ε-aminocaproic acid; OR, operating room.
χ2 test;
Kruskal–Wallis test.
Blood product transfusion
Blood product transfusion data are presented in Table 3. The overall transfusion exposure for all patients was 44% (51/115) for packed red blood cells, 24% (28/115) for fresh frozen plasma, 19% (22/115) for platelets, and 6% (7/115) for cryoprecipitate. The primary outcome of any blood product transfusion, as well as the individual blood component exposure, was not significantly different among the three treatment groups. As compared with the placebo group, the odds ratio of any transfusion was 1.20 (95% confidence interval 0.77–1.86) for the EACA group and 1.11 (95% confidence interval 0.71–1.73) for the HOE 140 group. In addition, the number of units of packed red blood cells, fresh frozen plasma, and cryoprecipitate, and the volume of platelets transfused were not significantly different among the treatment groups. Excluding patients who were taken back to the operating room for postoperative bleeding did not change the transfusion exposure or number of units transfused results among the three treatment groups.
Table 3.
Blood product transfusion during hospitalization
| Placebo (n = 38) | EACA (n = 37) | HOE 140 (n = 40) | P value | EACA vs. placebo OR (95% CI) |
HOE 140 vs. placebo OR (95% CI) |
|
|---|---|---|---|---|---|---|
| Received any transfusion, n (%) | 18 (47.4) | 21 (56.8) | 21 (52.5) | 0.72a | 1.20 (0.77–1.86) | 1.11 (0.71–1.73) |
| Received packed red blood cells, n (%) | 14 (36.8) | 18 (48.6) | 19 (47.5) | 0.52a | 1.28 (0.79–2.05) | 1.26 (0.78–2.04) |
| Received plasma, n (%) | 12 (31.6) | 9 (24.3) | 7 (17.5) | 0.35a | 0.84 (0.53–1.34) | 0.70 (0.45–1.09) |
| Received platelets, n (%) | 9 (23.7) | 6 (16.2) | 7 (17.5) | 0.68a | 0.81 (0.49–1.31) | 0.83 (0.50–1.38) |
| Received cryoprecipitate, n (%) | 4 (10.5) | 1 (2.7) | 2 (5.0) | 0.39a | 0.61 (0.37–1.001) | 0.71 (0.38–1.31) |
| Number of units or volume | ||||||
| Packed red blood cells (units) | 1.97 ± 0.61 | 1.41 ± 0.30 | 1.45 ± 0.34 | 0.88b | ||
| Plasma (units) | 1.08 ± 0.37 | 0.68 ± 0.25 | 0.82 ± 0.36 | 0.43b | ||
| Platelets (ml) | 60.53 ± 19.79 | 55.41 ± 22.07 | 71.25 ± 36.42 | 0.76b | ||
| Cryoprecipitate (units) | 0.63 ± 0.30 | 0.16 ± 0.16 | 0.40 ± 0.29 | 0.36b | ||
HOE 140 is a specific bradykinin B2 receptor antagonist.
CI, confidence interval; EACA, ε-aminocaproic acid; OR, odds ratio.
χ2 test;
Kruskal–Wallis test.
Secondary outcomes
MAP decreased significantly from a baseline of 84.3 ± 1.3 mm Hg to a nadir of 60.7 ± 1.2 mm Hg at 30 min of bypass (P < 0.001) in all patients, with no significant effect of study drug on intraoperative MAP (P = 0.16 for effect of study drug in mixed-effects model). Other secondary outcomes are presented in Table 4. There were no significant differences in rate of re-exploration for bleeding, prolonged ventilation, new-onset postoperative atrial fibrillation, placement of a permanent pacemaker, acute kidney injury (AKI), length of hospital stay, or rate of readmission among the three treatment groups. The two active treatment groups, however, compared favorably with the placebo group in prolonged ventilation, AKI, length of hospital stay, or rate of readmission.
Table 4.
Secondary outcomes
| Characteristic | Placebo (n = 38) | EACA (n = 37) | HOE 140 (n = 40) | P value | EACA vs. placebo OR (95% CI) |
HOE 140 vs. placebo OR (95% CI) |
|---|---|---|---|---|---|---|
| Re-exploration for bleeding, n (%) | 1 (2.6) | 1 (2.7) | 1 (2.5) | 1.0a | 1.01 (0.25–4.13) | 0.97 (0.24–3.97) |
| Prolonged ventilation (>24 h), n (%) | 4 (10.5) | 1 (2.7) | 3 (7.5) | 0.48a | 0.61 (0.37–1.001) | 0.84 (0.42–1.66) |
| New postoperative AF, n (%) | 5 (13.2) | 5 (13.5) | 9 (22.5) | 0.45a | 1.02 (0.52–1.97) | 1.44 (0.69–3.03) |
| Permanent pacemaker placement, n (%) | 2 (5.3) | 2 (5.4) | 3 (7.5) | 1.0a | 1.01 (0.37–2.77) | 1.23 (0.41–3.70) |
| Acute kidney injuryb, n (%) | 7 (18.4) | 4 (10.8) | 6 (15.0) | 0.65a | 0.76 (0.46–1.27) | 0.89 (0.50–1.56) |
| Readmitted within 30 days, n (%) | 7 (18.9) | 2 (5.9) | 3 (7.7) | 0.20a | 0.62 (0.40–0.96) | 0.65 (0.40–1.05) |
| Length of hospital stay (days) | 7.5 ± 1.1 | 5.8 ± 0.3 | 6.6 ± 0.6 | 0.84c |
HOE 140 is a specific bradykinin B2 receptor antagonist.
AF, atrial fibrillation; CI, confidence interval; EACA, ε-aminocaproic acid; OR, odds ratio.
χ2 test;
Acute kidney injury was defined according to Acute Kidney Injury Network criteria as an increase in subject serum creatinine concentration of 50% or 0.3 mg/dl within 48 h of surgery;
Kruskal–Wallis test.
Fibrinolytic response
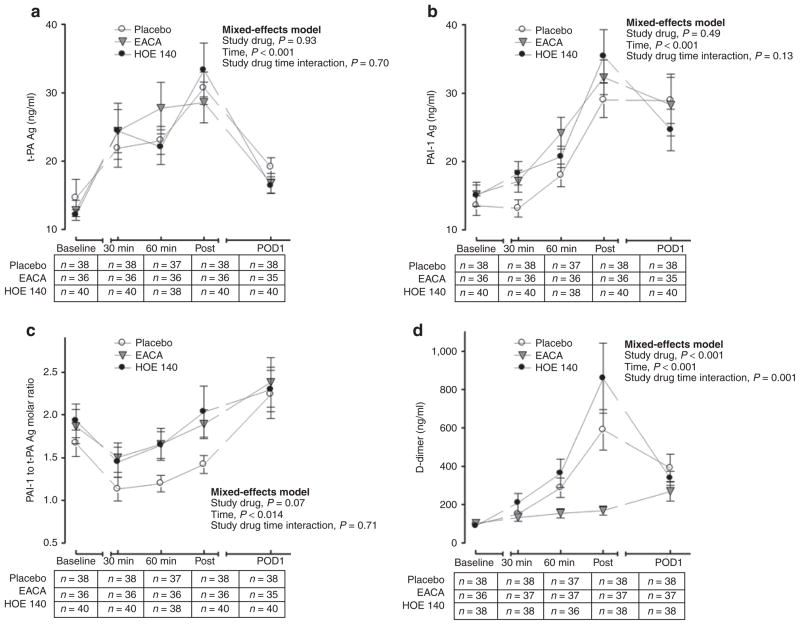
CPB activates the kallikrein–kinin system, causing a subsequent increase in bradykinin concentrations. Because bradykinin stimulates t-PA release through the bradykinin B2 receptor, we evaluated the effect of HOE 140 (bradykinin B2 receptor antagonist) on fibrinolysis as quantified by the t-PA antigen, plasminogen activator inhibitor-1 (PAI-1) antigen, PAI-1 to t-PA molar ratio, and D-dimer response (Figure 2a–d). CPB was associated with a significant increase in t-PA antigen (from a baseline of 13.1 ± 1.1 to a peak of 30.9 ± 1.8 ng/ ml postbypass, P < 0.001), PAI-1 antigen (from a baseline of 14.6 ± 0.9 to a peak of 32.3 ± 1.8 ng/ml postbypass, P < 0.001), and D-dimer concentrations (from a baseline of 95.4 ± 7.9 to a peak of 542.3 ± 75.4 ng/ml postbypass, P < 0.001) and a significant decrease in PAI-1 to t-PA molar ratio (from a baseline of 1.8 ± 0.1 to 1.35 ± 0.1 at 30 min of bypass, P < 0.001). Baseline t-PA antigen (P = 0.85), PAI-1 antigen (P = 0.77), and D-dimer (P = 0.86) concentrations and PAI to t-PA molar ratio (P = 0.75) were not significantly different among the three treatment groups. Although there was no significant effect of study drug on t-PA antigen (Figure 2a) or PAI-1 antigen (Figure 2b) concentrations over time, active study drug tended to decrease fibrinolytic capacity as indicated by an increased PAI-1 to t-PA molar ratio (Figure 2c). Because of this trend and the fact that the fibrinolytic response following CPB surgery is characterized by an initial hyperfibrinolytic intraoperative phase and a subsequent hypofibrinolytic postoperative phase,19 we investigated the effect of study drug on intraoperative PAI-1 to t-PA molar ratio separately. The intraoperative PAI-1 to t-PA molar ratio for both EACA (P = 0.05) and HOE 140 (P = 0.04) was increased as compared with placebo, indicating decreased intraoperative fibrinolytic capacity. D-dimer concentrations were significantly lower in the EACA group as compared with either the placebo or HOE 140 group (Figure 2d).
Figure 2.
Fibrinolytic response to surgery as measured by (a) tissue-type plasminogen activator (t-PA) antigen concentrations, (b) plasminogen activator inhibitor-1 (PAI-1) antigen concentrations, (c) PAI-1 to t-PA antigen molar ratio, and (d) D-dimer concentrations in the three study groups. “30 min” indicates 30 min of cardiopulmonary bypass (CPB), “60 min” indicates 60 min of CPB, and “post” indicates postbypass. The intraoperative PAI-1 to t-PA molar ratio was decreased in the placebo group as compared with either active treatment group (P = 0.05 as compared with EACA and P = 0.04 as compared with HOE 140, a specific bradykinin B2 receptor antagonist), indicating enhanced fibrinolysis. Data are presented as mean ± SEM. EACA, ε-aminocaproic acid; POD, postoperative day.
Inflammatory response
Because bradykinin also contributes to inflammation, we evaluated the effect of HOE 140 (bradykinin B2 receptor antagonist) on the inflammatory response as quantified by the proinflammatory interleukin (IL)-6 and IL-8 and the anti-inflammatory IL-10. CPB was associated with a significant increase in IL-6 (from a baseline of 5.4 ± 0.6 pg/ml to a peak of 148.6 ± 20.1 pg/ml on postoperative day 1, P < 0.001), IL-8 (from a baseline of 14.0 ± 2.1 pg/ml to a peak of 59.0 ± 18.6 pg/ml postsurgery, P < 0.001), and IL-10 (from a baseline of 2.9 ± 0.5 pg/ml to a peak of 431.6 ± 55.4 pg/ml postsurgery, P < 0.001) concentrations. Baseline IL-6 (P = 0.99), IL-8 (P = 0.45), and IL-10 (P = 0.89) concentrations were not significantly different among the three treatment groups. There was no significant effect of study drug on IL-6 (Figure 3a, P = 0.92), IL-8 (Figure 3b, P = 0.58), or IL-10 (Figure 3c, P = 0.77) concentrations over time. Adjusting for the effect of preoperative statin use did not change the effect of study drug on any of the inflammatory markers measured.
Figure 3.
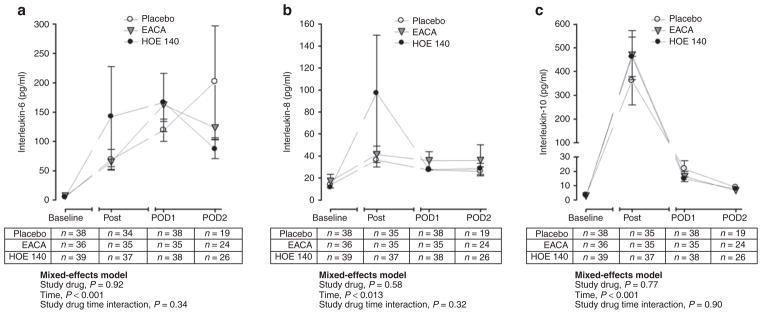
Inflammatory response as quantified by (a) interleukin (IL)-6, (b) IL-8, and (c) IL-10 concentrations in the three study groups. Data are presented as mean ± SEM. “Post” indicates postbypass. HOE 140 is a specific bradykinin B2 receptor antagonist. EACA, ε-aminocaproic acid; POD, postoperative day.
DISCUSSION
This prospective randomized clinical trial demonstrated that bradykinin B2 receptor antagonism decreased intraoperative fibrinolytic capacity to the same extent as EACA, but only EACA, not surprisingly, decreased D-dimer formation or bleeding via the chest tube. Although EACA and HOE 140 decreased fibrinolysis, and EACA tended to decrease blood loss, these treatments did not reduce the proportion of patients transfused. These data suggest that endogenous bradykinin contributes to t-PA generation in patients undergoing CPB, but that additional effects on plasmin generation contribute to decreased D-dimer concentrations during treatment with EACA.
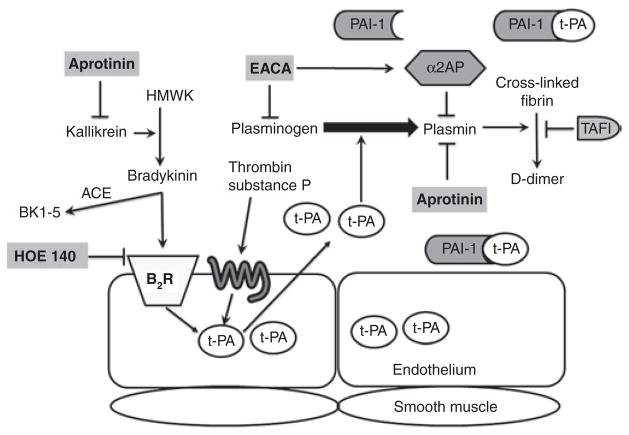
Bradykinin exerts both detrimental and beneficial effects mainly through its constitutively expressed bradykinin B2 receptor. 20 For example, we and others have demonstrated that during ACE inhibition, endogenous bradykinin enhances fibrinolysis, potentially contributing to the cardioprotective effects of ACE inhibitors.14,21 On the other hand, bradykinin promotes vasodilation and increases vascular permeability and inflammation.20 Whether or not bradykinin produces detrimental effects may depend on the underlying activity of the kallikrein–kinin system. During CPB, the kallikrein–kinin system is activated6,7 and increased bradykinin may cause detrimental effects such as enhanced stimulation of t-PA release, with resulting fibrinolysis and bleeding. The endothelium is the principal site of t-PA synthesis and storage, whereas PAI-1 is synthesized and secreted from several sources including endothelium, adipose tissue, liver, and platelets.22,23 Various agonists, including bradykinin and factors related to the coagulation cascade (for example, thrombin), stimulate the release of t-PA through G protein–coupled receptors (Figure 4). Once released, active t-PA catalyzes the conversion of plasminogen to plasmin that in turn degrades fibrin to fibrin degradation products. t-PA is inactivated mainly by circulating PAI-1, thereby inhibiting the fibrinolytic cascade.
Figure 4.
Antifibrinolytic drugs’ mechanisms of action. Activation of the kallikrein–kinin system results in the release of bradykinin from HMWK through the action of kallikrein. Bradykinin activates the B2R and stimulates the release of tissue-type plasminogen activator (t-PA) from the endothelium. Bradykinin is inactivated by ACE to its inactive metabolite, BK1-5. Other substances such as thrombin and substance P can also stimulate t-PA release through specific membrane-bound receptors. Free active t-PA converts plasminogen to plasmin, which subsequently degrades fibrinogen and fibrin to fibrin degradation products that include D-dimer. Endogenous antifibrinolytics include PAI-1, α2AP, and TAFI. The antifibrinolytic drug aprotinin, a nonspecific serine protease inhibitor, inhibits kallikrein, plasmin, and thrombin. EACA inhibits plasmin and increases α2-antiplasmin. HOE 140 is a specific bradykinin B2 receptor antagonist. α2AP, α2-antiplasmin; ACE, angiotensin-converting enzyme; B2R, bradykinin B2 receptor; EACA, ε-aminocaproic acid; HMWK, high-molecular-weight kininogen; PAI-1, plasminogen activator inhibitor-1; TAFI, thrombin activatable fibrinolysis inhibitor.
Bradykinin B2 receptor antagonism did not alter the t-PA antigen or PAI-1 antigen response. The intraoperative PAI-1 to t-PA molar ratio, however, was higher in the bradykinin B2 receptor antagonist group as compared with the placebo group, indicating decreased intraoperative fibrinolytic capacity. Although bradykinin B2 receptor antagonism decreased intraoperative fibrinolytic capacity, it did not alter the activation of plasminogen by active t-PA and subsequent degradation of fibrin to D-dimers. Although bradykinin is a potent stimulus of endothelial t-PA release through its B2 receptor, bradykinin B2 receptor antagonism is not expected to affect alternative pathways of endothelial t-PA release by agonists such as thrombin, which is also increased during CPB. In addition, HOE 140 is a weak thrombin inhibitor and at the micromolar concentrations used may have had an anticoagulant effect that contributed to bleeding and masked its effect as an antifibrinolytic.24 Therefore, bradykinin B2 receptor antagonism does not appear to be an effective strategy in reducing fibrinolysis as measured by D-dimer formation, blood loss, or blood product transfusions after CPB.
Bradykinin B2 receptor antagonism did not attenuate the inflammatory response. No prior studies have evaluated the anti-inflammatory effect of bradykinin B2 receptor antagonism in patients undergoing CPB. In other models in which activation of the kinin system is thought to contribute to the inflammatory response, bradykinin B2 receptor antagonism has conflicting results. In patients undergoing maintenance hemodialysis, bradykinin B2 receptor antagonism reduces monocyte chemoattractant protein-1 concentrations but enhances IL-8 and IL-10 concentrations and has no effect on IL-6 concentrations.25 In an animal model of Gram-negative sepsis, however, bradykinin B2 receptor antagonism had no effect on edema, shock, or inflammatory markers.26 Our results suggest that endogenous bradykinin does not significantly contribute to the inflammatory response following CPB.
Antifibrinolytic drugs are used to inhibit fibrinolysis during CPB (Figure 4). EACA and tranexamic acid are synthetic lysine analogs that competitively inhibit plasmin. They adhere to the lysine-binding sites of plasminogen and plasmin and interfere with plasmin’s ability to digest fibrinogen, fibrin, and platelet glycoprotein receptors.5 EACA also promotes the release of α2-antiplasmin, further inhibiting fibrinolysis.27 The antifibrinolytic effect of EACA, as indicated by decreased D-dimer formation, is consistent with previous studies.28–30 In addition, EACA treatment also had an antifibrinolytic effect as compared with placebo, as indicated by higher intraoperative PAI-1 to t-PA molar ratios. This observation is in agreement with the study by Eisses et al.30 in which EACA significantly reduced active t-PA during CPB.
Although EACA tended to reduce postoperative chest tube drainage, the efficacy of EACA in reducing blood product transfusion is supported by some4,31 but not all studies.32–34 Our results support a reduction in blood loss with the use of EACA, however, as in previous studies,32–34 we did not observe a reduction in blood product transfusion. In the current study, the lack of efficacy of EACA in reducing blood product transfusions may be attributable to differences in study population and/or drug dosing. Most studies demonstrating a reduction in blood product transfusion with EACA were in patients undergoing coronary artery bypass graft (CABG) surgery or combined CABG plus valve surgery, whereas our population was predominantly valve surgery patients.31,35 The efficacy of EACA in reducing blood product transfusion in low risk valve-only surgery patients is not known.35 The dosing regimen for EACA in cardiac surgery varies widely.36–38 Although dose-ranging outcome studies for EACA are lacking, the effective plasma concentration has been suggested to be two to four times the half-maximal effective concentration (65–70 μg/ml).37,39 We used a dosing regimen similar to that of Greilich et al., who achieved plasma concentrations approximately four times the half-maximal effective concentration.37 Although we did not measure EACA plasma concentrations, the inhibition of fibrinolysis as demonstrated by a reduction in D-dimer formation suggests that an adequate dose of EACA was achieved during CPB.
Previous studies suggest that EACA reduces IL-8 concentrations with no significant effect on IL-6 or IL-10 concentrations. 28,40 Our results are in agreement with these studies except that we did not observe an effect of EACA on IL-8 concentrations. Differences in study population may in part explain these findings because the type of surgery affects IL concentrations in some but not in other studies;41,42 our study population was predominantly valve surgery patients whereas the prior studies were exclusively CABG surgery patients.28,40
One limitation warrants mention. This study was conducted largely in a single center in a population that predominantly underwent valve-only surgery. EACA reduces the rate of red cell transfusion by 40% in CABG surgery and by 23% in CABG plus valve surgery, with no estimates for valve-only surgery. 35 Moreover, tranexamic acid use is associated with a 40% increased risk of red cell transfusion in valve-only surgery.35 We cannot exclude the possibility that our results may have been different if we studied only CABG surgery patients.
In conclusion, bradykinin B2 receptor antagonism decreased intraoperative fibrinolytic capacity (higher PAI-1 to t-PA molar ratio) to the same extent as EACA, but only EACA decreased D-dimer formation or bleeding via the chest tube. Although EACA and HOE 140 decreased fibrinolysis and EACA tended to decrease blood loss, these treatments did not reduce the proportion of patients transfused. These data suggest that endogenous bradykinin contributes to t-PA generation in patients undergoing CPB, but that additional effects on plasmin generation contribute to decreased D-dimer concentrations during treatment with EACA. Future studies are needed to determine if lysine analogs are effective in reducing blood product transfusion in a valve-only surgery population.
METHODS
The Bradykinin Receptor Antagonism during CPB study (ClinicalTrials.gov identifier: NCT00223704) was approved by the Vanderbilt University Institutional Review Board for Research on Human Subjects and the TN Valley Healthcare System Institutional Review Board and conducted according to the Declaration of Helsinki. Patients were enrolled by the research nurse at the time of the preoperative evaluation for surgery. All patients provided written informed consent. The study period was from June 2007 until June 2012. Patients were eligible for the study if they were undergoing primary elective on-pump cardiac surgery including CABG or valvular surgery. Exclusion criteria included impaired renal function (creatinine >1.6 mg/dl), anemia (hematocrit <30%), evidence of coagulopathy (international normalized ratio >1.7), platelet count of <100 × 109 ml−1, and taking a glycoprotein IIb/IIIa antagonist within 48 h of surgery. Preoperative medications were continued until the day of surgery. C.Y. generated the randomization schedule using an R program to generate blocks of three or six for three treatment arms. Randomization was stratified by prior ACE inhibitor use because ACE inhibition affects kinin concentrations6 and valve surgery status. Patients were randomized by the investigational pharmacy to receive HOE 140 (also known as Icatibant; bradykinin B2 receptor antagonist; Clinalfa, Switzerland, and Anaspec, Freemont CA), EACA (lysine analog; Hospira, Lake Forest, IL), or placebo (normal saline) in a 1:1:1 ratio. Drug administration was double blind. No patient, research nurse, investigator, or other medical or nursing staff was aware of the treatment assignments for the duration of the study. Unblinding of study drug occurred only after closure of the study protocol. The study drugs were all prepared and labeled in an identical fashion with the bolus provided in a 50 ml bag and the infusion in a 250 ml bag. Drug concentrations were calculated for each patient so that a constant infusion rate of 50 ml/h was used. The study drug was started in the operating room after induction of anesthesia and before heparinization, continued throughout the bypass period, and discontinued at the end of surgery. HOE 140 was given as an intravenous bolus of 22 μg/kg over one-half hour followed by an infusion of 18 μg/kg/hr. This dose of HOE 140 has been shown to suppress bradykinin-stimulated t-PA release in the forearm (data not shown). In addition, HOE 140 plasma concentrations measured at 60 min of CPB in five pilot subjects were 1,672 ± 138 ng/ ml (1.28 ± 0.11 μmol/l). These HOE 140 plasma concentrations compare favorably to that achieved after the subcutaneous administration of 30 mg HOE 140 (a peak plasma concentration of 974 ± 29 ng/ml; manufacturer prescriber information) used for the treatment of hereditary angioedema. EACA was given as an intravenous bolus of 100 mg/kg over one-half hour followed by an infusion of 30 mg/kg/h. This dose of EACA suppresses proinflammatory cytokine concentrations.28 No pump prime dose was given because of the routine practice of retrograde autologous priming, which removes crystalloid pump prime and replaces it with autologous blood. Patients randomized to placebo received an equivalent volume of normal saline throughout the study period.
Primary outcome
The primary end point of the study was the occurrence (yes or no) of any blood product transfusion. Blood product transfusion was recorded from the start of surgery until discharge from the hospital.
Secondary outcomes
Secondary end points measured were the proportion as well as the number of blood product units transfused, blood loss as measured by 24-h chest tube output, reexploration for bleeding, intraoperative MAP, prolonged ventilation (>24 h), new-onset postoperative atrial fibrillation, placement of permanent pacemaker, AKI, length of hospital stay, and 30-day readmission rate. AKI was defined according to Acute Kidney Injury Network criteria, 43 specifically any increase in subject serum creatinine concentration of 50% or 0.3 mg/dl (26.5 μmol/l) within 48 h of surgery. The Acute Kidney Injury Network urine output criteria for AKI diagnosis were not used due to confounding by intravascular hypovolemia and diuretic use,44 both of which are common among cardiac surgery patients. Predefined biomarkers included t-PA antigen, PAI-1 antigen, D-dimer, IL-6, IL-8, and IL-10 to quantify the fibrinolytic and inflammatory response.
Standardized patient treatment
Anesthesia management and CPB were conducted according to institutional protocols. Patients received general endotracheal anesthesia. Induction of anesthesia was achieved with either etomidate or propofol and maintained with isoflurane, fentanyl, air, and oxygen. Muscle relaxation was achieved and maintained with rocuronium or vecuronium. CPB was achieved with a nonpulsatile roller pump (Medtronic, Minneapolis, MN) and a trillium-coated circuit (Medtronic, Minneapolis, MN). Heparin was used for anticoagulation during CPB at an initial dose of 400 U/kg supplemented with additional heparin to achieve and maintain an activated clotting time of >400 s. Heparin was neutralized with protamine sulfate, after separation from CPB, at an initial dose of 250 mg and an additional 50 mg if activated clotting time remained >140 s. Retrograde autologous priming was performed in all patients if blood pressure permitted; only two patients, both in the placebo group, did not undergo retrograde priming. Moderate systemic hypothermia and cold retrograde and antegrade cardioplegia solution were applied to all patients if an aortic cross-clamp was used. Cardiotomy suction was used to retrieve blood from the surgical field and return it to the reservoir during the period of heparinization. After the administration of protamine, excess blood was collected in a Cell Saver (Haemonetics, Braintree, MA) before being washed and returned to the patient. During the period of CPB, the MAP was to be maintained between 50 and 70 mm Hg. If MAP decreased to <50 mm Hg, then pump flow was increased. If increasing pump flow did not restore MAP to >50 mm Hg, then a vasoconstrictor was administered. Inotropes were used for separation from CPB according to the following criteria: left-ventricle ejection fraction <40%, CPB time longer than 120 min, cardiac index <2 l/min/m2, or evidence of new-onset left-ventricular dysfunction by transesophageal echocardiography.
Patients were transfused according to the following guidelines: packed red blood cells were transfused for a hematocrit <20% during CPB and for a hematocrit <27% after CPB, CPB time >120 min, or evidence of end-organ dysfunction. Platelets were transfused for ongoing microvascular bleeding despite a normalized activated clotting time and a platelet count <100 × 109 ml−1. Fresh frozen plasma was given for continued bleeding and an international normalized ratio >1.5. Cryoprecipitate was given in six-pack units if fibrinogen concentrations were <200 mg/dl.
Safety and efficacy analyses
Before initiation of the trial, a Data Safety Monitoring Board (DSMB) was established, and the DSMB charter was approved by the DSMB. The DSMB consisted of Matt B. Weinger (chairman), Lorraine B. Ware, David Gailani, Brian S. Donahue, and Daniel W. Byrne (biostatistician), all at Vanderbilt University. Briefly, the DSMB charter stated that the study planned to have two interim analyses. The guidelines for decision making by the DSMB followed O’Brien and Fleming’s boundary for group sequential testing for the primary end point; specifically, if the two-sided test is significant at the levels of 0.00052 and 0.014 at the first and second interim analyses, respectively, the DSMB would recommend terminating the trial for efficacy. Consequently, the final analysis would need a two-sided P value <0.045 for declaring efficacy to ensure an overall type I error rate of 0.05. The DSMB could terminate the trial due to safety concerns at any time. In conducting the trial, the study team amended the trial by turning the first interim analysis into a safety assessment only. The stopping rule, however, remained in place. The DSMB completed a safety assessment after the first 30 subjects were enrolled and one interim analysis after 90 subjects were enrolled. Mock unblinded data were presented to the DSMB. The trial was completed as planned.
Assays
Blood samples were obtained to measure t-PA antigen, PAI-1 antigen, D-dimer, and inflammatory markers at six time points: after induction of anesthesia and before surgical incision (baseline); at 30 min of CPB; at 60 min of CPB; after separation from CPB and administration of protamine (postbypass); on postoperative day 1; and on postoperative day 2. All blood samples were taken from the indwelling arterial line. Not all markers were assayed at each time point. Blood for measurement of PAI-1 and t-PA was collected in vacutainer tubes containing 0.5 ml 0.5 mol/l citrate buffer (Tcoag Ireland, Wicklow, Ireland). Blood for measurement of D-dimer was collected in a citrate collection tube. All blood samples were collected on ice and centrifuged immediately at 4 °C for 20 min. Plasma samples were stored at −80 °C until the time of assay. PAI-1 antigen (TriniLIZE PAI-1 Antigen, Tcoag Ireland), t-PA antigen (TriniLIZE t-PA Antigen, Tcoag Ireland), and D-dimer (TintElize D-dimer, Tcoag Ireland) levels were determined using commercially available two-site enzyme-linked immunosorbent assays. The PAI-1 and t-PA molar ratio was determined by dividing plasma concentrations (ng/ml) by the molecular weights of the two proteins, with a value of 70,000 g/mol used for t-PA and a value of 50,000 g/mol used for PAI-1.45 The PAI-1 to t-PA molar ratio is indicative of the fibrinolytic balance, with a decrease in the ratio signifying a profibrinolytic state. A panel of human inflammatory cytokines consisting of IL-6, IL-8, and IL-10 were simultaneously measured by the Vanderbilt University Immunology Core laboratory using the Human Inflammation Cytokine Cytometric Bead array kit (BD Biosciences Pharmingen, San Diego, CA).
Statistical considerations
Data are presented as mean ± SEM unless otherwise indicated. Preliminary data from the Vanderbilt Cardiac Registry indicated the proportion of patients receiving any transfusion in cardiac surgery requiring CPB and not receiving an antifibrinolytic to be 71%. We powered the study to detect a decrease in transfusion of 30% in both the EACA and HOE 140 group. Forty-two subjects per group provide 80% power with a 0.05 two-sided significance level. Discrete variables were compared among treatment groups using χ2 test or Fisher’s exact test depending on the number of events for three-group or pair-wise comparisons. Continuous data were compared among treatment groups using a Kruskal–Wallis test. The time courses of PAI-1, t-PA, D-dimer, and IL concentrations were analyzed using mixed-effects models with fixed effects of drug treatment (placebo, EACA, HOE 140) and time since randomization. We included a random subject effect and a first-order autoregressive process1 to account for the correlation in the response variable in the mixed-effects model. Because smoking affects endothelial t-PA release,46 we included smoking status as a covariate in the mixed-effects model to assess t-PA and the PAI-1 to t-PA molar ratio. In separate mixed-effects models, preoperative statin use was included to adjust for its potential effect on the inflammatory response. A two-tailed P value <0.05 was considered statistically significant. Statistical analyses were performed with the statistical package IBM SPSS for Windows (version 20.0; IBM, New York, NY) and SAS for Windows (version 9; SAS Institute, Cary, NC).
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Fibrinolysis contributes to postoperative bleeding and blood product transfusion in patients undergoing cardiac surgery requiring CPB. Bradykinin increases during CPB and stimulates the release of inflammatory cytokines and t-PA acting through its B2 receptor.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study tested the hypothesis that endogenous bradykinin contributes to the fibrinolytic and inflammatory response to CPB and that bradykinin B2 receptor antagonism would reduce fibrinolysis, inflammation, and subsequent transfusion requirements.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
Bradykinin B2 receptor antagonism decreased intraoperative fibrinolytic capacity but not inflammation, bleeding via the chest tube, or blood product transfusion requirements. Aminocaproic acid decreased fibrinolysis and tended to decrease blood loss but also failed to reduce transfusion requirements.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY AND THERAPEUTICS
Bradykinin B2 receptor antagonism reduces intraoperative fibrinolytic capacity but is not an effective strategy to decrease blood loss or transfusion requirements in patients undergoing CPB.
Acknowledgments
We thank Patricia Wright for her nursing assistance and Jeff Petro for his technical assistance. This research was funded by the National Institutes of Health (HL085740 and HL065193) and supported in part by the National Center for Research Resources, grant UL1 RR024975, and is now at the National Center for Advancing Translational Sciences, grants 2 UL1TR000445 and UL1TR000011. Clinical Trial Registration Information: NCT00223704.
Footnotes
AUTHOR CONTRIBUTIONS
J.M.B., C.Y., J.G.B., S.K.B., M.R.P., N.J.B., and M.P. wrote the manuscript. C.Y., N.J.B., and M.P. designed the research. J.M.B., J.G.B., S.K.B., M.R.P., and M.P. performed the research. C.Y. and M.P. analyzed the data.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
References
- 1.Bennett-Guerrero E, et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304:1568–1575. doi: 10.1001/jama.2010.1406. [DOI] [PubMed] [Google Scholar]
- 2.Khuri SF, et al. Hematologic changes during and after cardiopulmonary bypass and their relationship to the bleeding time and nonsurgical blood loss. J Thorac Cardiovasc Surg. 1992;104:94–107. [PubMed] [Google Scholar]
- 3.Páramo JA, Rifón J, Llorens R, Casares J, Paloma MJ, Rocha E. Intra- and postoperative fibrinolysis in patients undergoing cardiopulmonary bypass surgery. Haemostasis. 1991;21:58–64. doi: 10.1159/000216203. [DOI] [PubMed] [Google Scholar]
- 4.Levi M, et al. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet. 1999;354:1940–1947. doi: 10.1016/S0140-6736(99)01264-7. [DOI] [PubMed] [Google Scholar]
- 5.Despotis GJ, Avidan MS, Hogue CW., Jr Mechanisms and attenuation of hemostatic activation during extracorporeal circulation. Ann Thorac Surg. 2001;72:S1821–S1831. doi: 10.1016/s0003-4975(01)03211-8. [DOI] [PubMed] [Google Scholar]
- 6.Pretorius M, McFarlane JA, Vaughan DE, Brown NJ, Murphey LJ. Angiotensin-converting enzyme inhibition and smoking potentiate the kinin response to cardiopulmonary bypass. Clin Pharmacol Ther. 2004;76:379–387. doi: 10.1016/j.clpt.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Campbell DJ, Dixon B, Kladis A, Kemme M, Santamaria JD. Activation of the kallikrein-kinin system by cardiopulmonary bypass in humans. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1059–R1070. doi: 10.1152/ajpregu.2001.281.4.R1059. [DOI] [PubMed] [Google Scholar]
- 8.Cugno M, Nussberger J, Biglioli P, Alamanni F, Coppola R, Agostoni A. Increase of bradykinin in plasma of patients undergoing cardiopulmonary bypass: the importance of lung exclusion. Chest. 2001;120:1776–1782. doi: 10.1378/chest.120.6.1776. [DOI] [PubMed] [Google Scholar]
- 9.Vanhoutte PM. Endothelium and control of vascular function. State of the Art lecture. Hypertension. 1989;13:658–667. doi: 10.1161/01.hyp.13.6.658. [DOI] [PubMed] [Google Scholar]
- 10.Brown NJ, Gainer JV, Murphey LJ, Vaughan DE. Bradykinin stimulates tissue plasminogen activator release from human forearm vasculature through B(2) receptor-dependent, NO synthase-independent, and cyclooxygenase-independent pathway. Circulation. 2000;102:2190–2196. doi: 10.1161/01.cir.102.18.2190. [DOI] [PubMed] [Google Scholar]
- 11.Pan ZK, Zuraw BL, Lung CC, Prossnitz ER, Browning DD, Ye RD. Bradykinin stimulates NF-kappaB activation and interleukin 1beta gene expression in cultured human fibroblasts. J Clin Invest. 1996;98:2042–2049. doi: 10.1172/JCI119009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunius G, Domeij H, Gustavsson A, Yucel-Lindberg T. Bradykinin upregulates IL-8 production in human gingival fibroblasts stimulated by interleukin-1beta and tumor necrosis factor alpha. Regul Pept. 2005;126:183–188. doi: 10.1016/j.regpep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Santos DR, Calixto JB, Souza GE. Effect of a kinin B2 receptor antagonist on LPS- and cytokine-induced neutrophil migration in rats. Br J Pharmacol. 2003;139:271–278. doi: 10.1038/sj.bjp.0705236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pretorius M, Rosenbaum D, Vaughan DE, Brown NJ. Angiotensin-converting enzyme inhibition increases human vascular tissue-type plasminogen activator release through endogenous bradykinin. Circulation. 2003;107:579–585. doi: 10.1161/01.cir.0000046268.59922.a4. [DOI] [PubMed] [Google Scholar]
- 15.Souza DG, et al. Role of bradykinin B2 and B1 receptors in the local, remote, and systemic inflammatory responses that follow intestinal ischemia and reperfusion injury. J Immunol. 2004;172:2542–2548. doi: 10.4049/jimmunol.172.4.2542. [DOI] [PubMed] [Google Scholar]
- 16.Souza DG, et al. Role of the bradykinin B2 receptor for the local and systemic inflammatory response that follows severe reperfusion injury. Br J Pharmacol. 2003;139:129–139. doi: 10.1038/sj.bjp.0705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fein AM, et al. Treatment of severe systemic inflammatory response syndrome and sepsis with a novel bradykinin antagonist, deltibant (CP-0127). Results of a randomized, double-blind, placebo-controlled trial CP-0127 SIRS and Sepsis Study Group. JAMA. 1997;277:482–487. [PubMed] [Google Scholar]
- 18.Witherow FN, Dawson P, Ludlam CA, Webb DJ, Fox KA, Newby DE. Bradykinin receptor antagonism and endothelial tissue plasminogen activator release in humans. Arterioscler Thromb Vasc Biol. 2003;23:1667–1670. doi: 10.1161/01.ATV.0000087142.99472.F6. [DOI] [PubMed] [Google Scholar]
- 19.Pretorius M, Murphey LJ, McFarlane JA, Vaughan DE, Brown NJ. Angiotensin-converting enzyme inhibition alters the fibrinolytic response to cardiopulmonary bypass. Circulation. 2003;108:3079–3083. doi: 10.1161/01.CIR.0000105765.54573.60. [DOI] [PubMed] [Google Scholar]
- 20.Maurer M, et al. New topics in bradykinin research. Allergy. 2011;66:1397–1406. doi: 10.1111/j.1398-9995.2011.02686.x. [DOI] [PubMed] [Google Scholar]
- 21.Witherow FN, Dawson P, Ludlam CA, Fox KA, Newby DE. Marked bradykinin-induced tissue plasminogen activator release in patients with heart failure maintained on long-term angiotensin-converting enzyme inhibitor therapy. J Am Coll Cardiol. 2002;40:961–966. doi: 10.1016/s0735-1097(02)02061-2. [DOI] [PubMed] [Google Scholar]
- 22.Oliver JJ, Webb DJ, Newby DE. Stimulated tissue plasminogen activator release as a marker of endothelial function in humans. Arterioscler Thromb Vasc Biol. 2005;25:2470–2479. doi: 10.1161/01.ATV.0000189309.05924.88. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3:1879–1883. doi: 10.1111/j.1538-7836.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- 24.Shariat-Madar Z, et al. Bradykinin B2 receptor knockout mice are protected from thrombosis by increased nitric oxide and prostacyclin. Blood. 2006;108:192–199. doi: 10.1182/blood-2006-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marney AM, Ma J, Luther JM, Ikizler TA, Brown NJ. Endogenous bradykinin contributes to increased plasminogen activator inhibitor 1 antigen following hemodialysis. J Am Soc Nephrol. 2009;20:2246–2252. doi: 10.1681/ASN.2009050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barratt-Due A, et al. The role of bradykinin and the effect of the bradykinin receptor antagonist icatibant in porcine sepsis. Shock. 2011;36:517–523. doi: 10.1097/SHK.0b013e3182336a34. [DOI] [PubMed] [Google Scholar]
- 27.Ray MJ, Hales M, Marsh N. Epsilon-aminocaproic acid promotes the release of alpha2-antiplasmin during and after cardiopulmonary bypass. Blood Coagul Fibrinolysis. 2001;12:129–135. doi: 10.1097/00001721-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Greilich PE, Brouse CF, Whitten CW, Chi L, Dimaio JM, Jessen ME. Antifibrinolytic therapy during cardiopulmonary bypass reduces proinflammatory cytokine levels: a randomized, double-blind, placebo-controlled study of epsilon-aminocaproic acid and aprotinin. J Thorac Cardiovasc Surg. 2003;126:1498–1503. doi: 10.1016/s0022-5223(03)00946-2. [DOI] [PubMed] [Google Scholar]
- 29.Greilich PE, et al. Effects of epsilon-aminocaproic acid and aprotinin on leukocyte-platelet adhesion in patients undergoing cardiac surgery. Anesthesiology. 2004;100:225–233. doi: 10.1097/00000542-200402000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Eisses MJ, Seidel K, Aldea GS, Chandler WL. Reducing hemostatic activation during cardiopulmonary bypass: a combined approach. Anesth Analg. 2004;98:1208–1216. doi: 10.1213/01.ane.0000108489.88613.2c. table of contents. [DOI] [PubMed] [Google Scholar]
- 31.Hardy JF, et al. Prophylactic tranexamic acid and epsilon-aminocaproic acid for primary myocardial revascularization. Ann Thorac Surg. 1998;65:371–376. doi: 10.1016/s0003-4975(97)01016-3. [DOI] [PubMed] [Google Scholar]
- 32.Kluger R, Olive DJ, Stewart AB, Blyth CM. Epsilon-aminocaproic acid in coronary artery bypass graft surgery: preincision or postheparin? Anesthesiology. 2003;99:1263–1269. doi: 10.1097/00000542-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Troianos CA, et al. The effect of prophylactic epsilon-aminocaproic acid on bleeding, transfusions, platelet function, and fibrinolysis during coronary artery bypass grafting. Anesthesiology. 1999;91:430–435. doi: 10.1097/00000542-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Kikura M, Levy JH, Tanaka KA, Ramsay JG. A double-blind, placebo-controlled trial of epsilon-aminocaproic acid for reducing blood loss in coronary artery bypass grafting surgery. J Am Coll Surg. 2006;202:216–222. doi: 10.1016/j.jamcollsurg.2005.10.001. quiz A44. [DOI] [PubMed] [Google Scholar]
- 35.Brown JR, Birkmeyer NJ, O’Connor GT. Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation. 2007;115:2801–2813. doi: 10.1161/CIRCULATIONAHA.106.671222. [DOI] [PubMed] [Google Scholar]
- 36.Bennett-Guerrero E, et al. Cost-benefit and efficacy of aprotinin compared with epsilon-aminocaproic acid in patients having repeated cardiac operations: a randomized, blinded clinical trial. Anesthesiology. 1997;87:1373–1380. doi: 10.1097/00000542-199712000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Greilich PE, et al. The effect of epsilon-aminocaproic acid and aprotinin on fibrinolysis and blood loss in patients undergoing primary, isolated coronary artery bypass surgery: a randomized, double-blind, placebo-controlled, noninferiority trial. Anesth Analg. 2009;109:15–24. doi: 10.1213/ane.0b013e3181a40b5d. [DOI] [PubMed] [Google Scholar]
- 38.Fergusson DA, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319–2331. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 39.Butterworth J, James RL, Lin Y, Prielipp RC, Hudspeth AS. Pharmacokinetics of epsilon-aminocaproic acid in patients undergoing aortocoronary bypass surgery. Anesthesiology. 1999;90:1624–1635. doi: 10.1097/00000542-199906000-00019. [DOI] [PubMed] [Google Scholar]
- 40.Greilich PE, Okada K, Latham P, Kumar RR, Jessen ME. Aprotinin but not epsilon-aminocaproic acid decreases interleukin-10 after cardiac surgery with extracorporeal circulation: randomized, double-blind, placebo-controlled study in patients receiving aprotinin and ε-aminocaproic acid. Circulation. 2001;104:I265–I269. doi: 10.1161/hc37t1.094781. [DOI] [PubMed] [Google Scholar]
- 41.Kawahito K, Adachi H, Ino T. Influence of surgical procedures on interleukin-6 and monocyte chemotactic and activating factor responses: CABG vs. valvular surgery. J Interferon Cytokine Res. 2000;20:1–6. doi: 10.1089/107999000312676. [DOI] [PubMed] [Google Scholar]
- 42.Whitten CW, Hill GE, Ivy R, Greilich PE, Lipton JM. Does the duration of cardiopulmonary bypass or aortic cross-clamp, in the absence of blood and/or blood product administration, influence the IL-6 response to cardiac surgery? Anesth Analg. 1998;86:28–33. doi: 10.1097/00000539-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Mehta RL, et al. Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macedo E, Malhotra R, Claure-Del Granado R, Fedullo P, Mehta RL. Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2011;26:509–515. doi: 10.1093/ndt/gfq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaughan DE, Rouleau JL, Ridker PM, Arnold JM, Menapace FJ, Pfeffer MA. Effects of ramipril on plasma fibrinolytic balance in patients with acute anterior myocardial infarction. HEART Study Investigators. Circulation. 1997;96:442–447. doi: 10.1161/01.cir.96.2.442. [DOI] [PubMed] [Google Scholar]
- 46.Pretorius M, Rosenbaum DA, Lefebvre J, Vaughan DE, Brown NJ. Smoking impairs bradykinin-stimulated t-PA release. Hypertension. 2002;39:767–771. doi: 10.1161/hy0302.105767. [DOI] [PubMed] [Google Scholar]