Abstract
The focus of the review is on the behavioral and physiological manifestations of stress versus depression. The purpose of the review is to evaluate the conceptual approach of using stress models as surrogates for depression. Social stress and depression have many characteristics in common and promote each other. Both have adverse effects on social relationships and the quality of life, and increase risk of other diseases. However, they are not the same constructs. In human and nonhuman primates, the behavior and neurobiology of stressed individuals differ from that of depressed individuals. Some similarities in stress physiology in socially stressed and depressed individuals have been used to support the use of stressed animals as models of depression, and much has been learned from stress models of depression. However, the studies reviewed here also suggest that the depressed state also has different characteristics than the stressed state, and studying the differences may be important to furthering our understanding of each of these constructs as well as their mutual relationship.
Keywords: Social stress, Depression, Nonhuman primate, Animal models, Heart rate, Cortisol, Behavior, Coronary artery atherosclerosis, Body composition
Introduction
The focus of the review is on the behavioral and physiological manifestations of stress versus depression. The purpose of the review is to promote a re-evaluation of the conceptual approach of using stress models as surrogates for depression. Social stress and depression have many characteristics in common and promote each other. Both have adverse effects on social relationships and the quality of life, and increase risk of other diseases. However, they are not the same constructs in human beings. Here, the relationship between social stress and depression is reviewed, a nonhuman primate model of social stress and depression is presented, and the similarities and differences between socially stressed monkeys and depressed monkeys are compared. The implications of similarities and differences between socially stressed versus depressed monkeys for animal modeling of social stress and depression, and for furthering our understanding of each of these constructs, as well as their mutual relationship, are considered.
The relationship between stress and depression
Stress exposure model of depression
It is well-established in the literature that stressful life events are associated with risk for depression. This relationship holds for acute stresses and for chronic stress. Stressors may precipitate a depressive bout rather quickly or be temporally remote, as early life events predispose to later life depression. The relationship between stress and depression holds for all ages from childhood through late life, and for males and females. The relationship may be strongest for the first or first few bouts of depression but is also associated with recurrences (see Liu and Alloy, 2010).
Stress generation model of depression
More recently, the bidirectional nature of stress and depression has been acknowledged in the stress generation model of depression. This model posits that depression generates stress, which serves to fuel the cycle of stress and depression (Liu and Alloy, 2010).
The loose association of stress with depression
Even though these relationships between stress and depression are well-documented, not all stressful events precipitate depression, and the reasons for this are not well understood. Improving our understanding of why depression does not occur following all stressful events may help uncover new avenues for prevention and therapeutic intervention. Likely factors influencing whether stress will result in depression are the nature of the stressor and the resiliency of the stressed individual. Depression is heritable, fueling the search for genetic factors that influence resiliency. Recent work suggests gene–environment interactions may be important; that is, a certain genetic makeup may increase resiliency to certain stressors, at least at certain times of life (Aguilera et al., 2009; Elzinga et al., 2011). However, conflicting reports characterize this literature. Epigenetic modification of gene expression by early stress exposure is a likely contributor to the probability of stress precipitating depression; however, much research is needed to establish these links (Plazas-Mayorca and Vrana, 2011).
Animal models: stress or depression?
One of the factors blocking progress in understanding the relationship between stress and depression is that the effects of stress can be temporally remote, and early experiences may modify later stress responses. Long-term studies that are large enough to assess stressful events and depression prospectively for the decades that are needed to understand these relationships are prohibitively expensive. Thus, clinical investigators are mostly left with retrospective studies in which stressful events are difficult to accurately assess.
Animal models are a viable alternative but typically capture only certain aspects of a complex, multifaceted disease such as depression. Animal models are evaluated based on their construct (etiological), face (similarity in phenotype to the disease/disorder being modeled), and predictive (responses to interventions predict human responses) validity (McKinney, 1984; Willner, 1984). Animal models used in depression research are almost entirely rodent models that provide shorter lifespans to study under controlled conditions. Genetic models have been useful in identifying single genes that may contribute to depression, but lack construct validity (Nestler and Hyman, 2010).
Behavioral models capture more of the complexity of depression. Commonly used rodent behavioral models include the forced-swim and tail-suspension models, which characterize behavioral and physiologic responses to uncontrollable stress. These models are thought to reflect behavioral despair which is quantified as the time to immobility and have provided a fast screening tool for novel pharmacotherapies. To avoid adaptation to repeated presentation of the same stressor, the chronic mild stress model has been used. This involves a more complicated paradigm of presentation of a variety of types of stressors and is thus more labor intensive, time and space demanding, and more difficult to replicate than the forced swim and tail suspension tasks. However, this paradigm does produce anhedonia, a hallmark feature of depression, which, like human depression, can be ameliorated by chronic, but not acute, antidepressant treatment (Willner, 2005).
Social defeat is another stress paradigm commonly used as a behavioral model of depression in rodents and tree shrews (Tupaia belangeri), a solitary species phylogenetically closely related to primates. Briefly, the paradigm involves introducing an experimental animal into the territory of an aggressive, and usually larger, animal for short time periods repeatedly over several days (Fuchs, 2005; Yan et al., 2010). The anhedonia and social withdrawal in these defeat models are also responsive to chronic, but not acute, antidepressant treatment (Krishnan et al., 2007). Moreover, not all animals exposed to social defeat stress will exhibit the depression-like syndrome, which provides the opportunity to study susceptibility and resiliency to stress (Charney, 2004; Krishnan et al., 2007). Thus, these chronic stress models hold the potential to provide invaluable insights into novel neurobiological mechanisms because they model specific characteristics of behavior (Krishnan and Nestler, 2010).
Recently Perera and colleagues reported the results of a small study of adult female bonnet macaque (Macaca radiata) behavioral responses to social isolation stress (Perera et al., 2011). Briefly, 6 bonnet macaques housed in a social group pen were socially isolated 2 days/week for 15 weeks, and 3 of the monkeys were simultaneously treated with the selective serotonin reuptake inhibitor fluoxetine. The three untreated monkeys appeared to gradually increase anhedonic behaviors (defined as a behavioral composite of collapsed postures, inactivity, and blank stares) and decrease social status scores calculated as total subordinate behaviors subtracted from total dominant behaviors. Fluoxetine appeared to ameliorate the behavioral effects of the social isolation stress in the three treated monkeys. Further study will reveal utility of this potential model.
Each of the stress paradigms results in one or a few specific characteristics of human depression. However, none of them capture the complexity and heterogeneity that is characteristic of human depression which limits their face validity. The predictive validity of these models is being reconsidered as evidence accumulates suggesting that antidepressant pharmacotherapies developed using rodents stress models have limited efficacy in human depression (Rush et al., 2006; Kirsch et al., 2008; Spielmans et al., 2011). Notably, depressive-like behavior in all of these models is induced by experimental stress. Thus, these models cannot clearly distinguish between stress responses and a depression-like state as manifested by human beings. Hence, these behavioral models have limited construct validity (Duman, 2010). The lack of differentiation between behavioral and physiological characteristics of stress versus depression preclude examination of differences between a stressed state and a depressed state, and, perhaps more importantly, the examination of the relationship between stress and depression in animal models. For these reasons, we have been studying a nonhuman primate model of social stress-associated depression in which some behavioral and neurobiological responses to social stress are different from those that accompany depressive behavior. This model is presented below.
A nonhuman primate model of social stress-associated depression
Social status in cynomolgus social groups
We have studied the effects of social stress on the health of adult female cynomolgus monkeys (Macaca fascicularis) for over 20 years. The monkeys in these experiments were wild-caught as adults, in recent years from a purpose-bred free-ranging island colony in Indonesia. The monkeys are housed in small social groups of 3 to 5 females in rooms that are approximately 8–10 m3 in area and enriched with perches, barrels, and manipulanda such as mirrors and toys. The monkeys are fed a diet containing moderate amounts of fat and cholesterol to mimic key dietary constituents consumed in Western societies. When placed in these groups, the monkeys quickly organize themselves into linear social status hierarchies which may be stable for years (Shively and Kaplan, 1991). Social status is evaluated by recording the outcomes of agonistic interactions. The animal to which all others in the group direct submissive behaviors is considered dominant. The monkey that all but the most dominant submits to is considered second-ranking, and so on. Compared to dominant females, subordinates receive more aggression, are groomed less, are more vigilant, and spend more time alone out of arm’s reach of another monkey (Shively et al., 1997; Shively, 1998) (Table A.1). Furthermore, subordinates respond to a standardized stressor with increased heart rates that recover more slowly than dominants, and subordinates are hypercortisolemic and insensitive to glucocorticoid-negative feedback in dexamethasone (Dex) suppression tests (Kaplan et al., 1986, 2010; Shively, 1998; Shively et al., 1997) (Table A.1). This behavioral and physiological profile led to the conclusion that socially subordinate female cynomolgus monkeys in these small laboratory social groups are stressed relative to their dominant counterparts.
Social subordination and social defeat
Acute social defeat is a social stressor used in some rodent and tree shrew stress models of depression. While social subordination includes instances of social defeat, it also includes other features that are likely important to the nature of the stressor: 1) cynomolgus monkeys normally live in social groups characterized by stable linear social status hierarchies all of their lives; 2) these hierarchies generally do not involve much overt aggression once they are established, and they are usually established in a matter of hours or perhaps a few days; 3) while subordinates appear stressed relative to dominants, it is a level of physiological stress with which they survive for a life span, which is not reported to be any shorter than that of dominant monkeys (Table A.1); and 4) the data on social subordination stress reported here are based on studies that include 1–3.5 years of behavioral characterization of the monkeys. Thus, social subordination is a chronic mild social stressor with social validity with respect to the natural history of the species.
The definition and rate of depression in adult female cynomolgus monkeys
In the late 1980s we began observing and recording the percent time spent in a behavior termed depressive, in which the monkeys sat in a slumped or collapsed body posture, with open eyes, accompanied by a lack of responsivity to environmental events (Fig. A.1). This behavior was reminiscent of that described in infant macaques removed from their mothers (Suomi et al., 1975). We have observed depressive behavior in two separate groups of female monkeys (78 animals in all). Interobserver agreement in the identification of depressive behavior was greater than 92% in both experiments. In the first experiment (hereafter referred to as Experiment I), following a 3-month single-cage quarantine, 42 females lived for 26 months in social groups of about 4 females each, and behavior was recorded weekly throughout this time. Sixteen of 42 monkeys (38%) displayed depressive behavior (Shively et al., 1997). The rate of depression was similar in the second experiment (hereafter referred to as Experiment II and described below) in which 42% of the monkeys exhibited depressive behavior (Shively et al., 2005).
Depressive behavior and social status
Over the course of Experiment I, depressive behavior was more common in subordinate than dominant females: 61% of subordinates displayed depressive behavior whereas only 10% of dominants were ever observed in this behavior (Shively et al., 1997). It was concluded that the stress associated with low social status may increase the likelihood of depressive behavior; however, social subordination and depression are not homologous, as 39% of subordinates did not display depressive behavior, and a few dominants did suggesting individual differences in stress sensitivity and resilience (Bethea et al., 2008). It is notable that rates of depression in the human population are inversely related to socioeconomic status (Adler and Rehkopf, 2008; Lorant et al., 2003).
Experiment II included 36 monkeys and was divided into three phases. During Phase 0 the animals were housed in single cages for 12 months, in Phase 1 the monkeys were housed in their first social groups for 12 months, and in Phase 2 the social groups were systematically reorganized so that half of the previous subordinates became dominant and half the previous dominants became subordinate. The monkeys lived in these social groups for 22 months. Percent time spent in depressive behavior was recorded throughout the three phases, and social status was determined monthly throughout Phases I and II. There was no difference between dominants and subordinates in the time spent in depressive behavior in Phase I, whereas in Phase II subordinates displayed significantly more depressive behavior than dominants (Shively et al., 2005). The change in the relationship between social status and depression over time was due in part to attrition and in part to changes in depressive behavior. All but one depressed dominant in Phase I either died or were not depressed in Phase II (Shively et al., 2005).
Variability in sensitivity and responses to stress
The fact that many but not all socially subordinate females, and only a few dominant females exhibit depressive behavior indicates unexplained variability that may be due to variation in the social environment, or to individual differences in sensitivity or resilience to social stress, or in individual responses to social stress. At the social group level it is reasonable to posit that it may be more stressful to live in some social groups than others. However, little data is available that addresses this hypothesis due to the challenge of studying an adequate number of social groups. Variability can also occur at the individual level. Depressive behavior may be only one of a range of potential responses to social subordination stress. Studying the attributes of subordinates that do not become depressed may provide valuable insights about alternative stress responses. Much evidence has accumulated suggesting that this variability may also be due to individual differences in resilience or susceptibility to social stress.
In a series of studies Cameron and Bethea studied individual differences in stress responsivity by assessing changes in ovulation and reproductive hormone secretion when animals were exposed to a combined metabolic and psychosocial stress which included mild psychosocial stress+moderate dieting+moderate exercise. The mild psychosocial stress involved moving single-caged monkeys to a new housing room, where unfamiliar animals surrounded them. The moderate diet was a 20% decrease in calorie intake, and the moderate exercise was provided by running monkeys on a motorized treadmill at 80% of each individual’s maximum speed for 1 h/day, 5 days/week. Stress responsive monkeys were defined as those with the most impairment to ovarian function during the stress. Under nonstress conditions, these stress sensitive monkeys had lower ovarian steroid secretion during normal (nonstressed) menstrual cycles, lower serotonergic function in response to fenfluramine, fewer serotonin neurons, lower expression of pivotal serotonin-related genes, lower expression of 5HT2A and 2C genes in the hypothalamus, higher gene expression of GAD67 and CRH in the hypothalamus, reduced gonadotropin-releasing hormone transport to the anterior pituitary and higher basal heart rates (Centeno et al., 2007; Bethea et al., 2008, 2011).
Predictors of depression
Congruent with these data, in Experiment I discussed above, heart rates were recorded just prior to social housing while the monkeys were still in single cages. Single caging may be considered a stressor as it increases heart rate in adult female cynomolgus monkeys (Watson et al., 1998). Females that later exhibited depression in social groups had higher overnight heart rates in single cages than those that never exhibited behavioral depression suggesting that stress sensitivity may increase the likelihood of a depressive response to social stress (Shively et al., 2002). During Phase 0 of Experiment II discussed above, the monkeys lived in single cages for a year. During this phase, monkeys that subsequently became depressed in Phase 1 social groups had decreased cortisol secretion in a corticotropin-releasing hormone (CRH) challenge test, decreased circulating insulin-like growth factor-1 (IGF-1) concentrations, lower activity levels, and higher total plasma cholesterol (TPC) concentrations and the ratio of TPC:high density lipoprotein cholesterol (HDL-C) concentrations. These characteristics were not associated with depressive behavior during Phase 0 (Shively et al., 2005). Thus, monkeys which subsequently become depressed respond to single caging differently than those that do not become depressed. It may be that these are stress-susceptible monkeys similar to those studied by Cameron and Bethea.
Direct comparisons between social subordination stress and depression (Table A.1)
In the monkey model of social stress-associated depression, depressive behavior is more common in the socially stressed subordinate females, but many subordinates do not exhibit depressive behavior. Likewise, depressive behavior can be observed in a small proportion of socially dominant females that are presumably less socially stressed compared to their subordinate counterparts. This distribution provides the ability to compare the characteristics of socially stressed subordinate females with females that display the depressive behavior. Table A.1 depicts the behavioral and physiological characteristics of socially stressed subordinate monkeys versus monkeys that exhibit depressive behavior. Since the majority of monkeys that exhibit depressive behavior are also socially subordinate, characteristics of monkeys displaying depressive behavior mostly reflect the additive characteristics of subordinate animals that go on to respond to social stress with depressive behavior. The citations refer to mean differences between groups of intact adult female cynomolgus monkeys, unless otherwise specified. Characteristics that appear to differ between stressed monkeys and depressed monkeys are highlighted in gray. Citations in the table are not repeated in text.
Body composition
It appears from the literature that there is little evidence of a relationship between social status and body weight in female cynomolgus macaques, although body weight is higher in dominant than subordinate male and female rhesus monkeys (Macaca mulatta) and male baboons (Papio anubis) (Michopoulos et al., 2009; Sapolsky and Mott, 1987; Zehr et al., 2005). In contrast, nondepressed cynomolgus females were reported to be 17% heavier than depressed females. Body mass index (BMI) or related measures of adiposity appear unrelated to social status (Zehr et al., 2005), whereas BMI was 20% higher in nondepressed than depressed females cynomolgus monkeys.
Hypothalamic–pituitary–adrenal (HPA) function
The HPA axis appears to be perturbed in socially subordinate monkeys compared to dominants, as well as depressed compared to nondepressed monkeys. Subordinate monkeys and depressed monkeys are similar in that they are insensitive to glucocorticoid-negative feedback in dexamethasone suppression tests. Unlike depressed monkeys, subordinates also appear to be hypercortisolemic as reflected in basal cortisol levels and in response to adrenocorticotropin (ACTH) challenge. Depressed monkeys are reported to have higher cortisol responses to CRH challenge, whereas no reports of results of CRH challenge tests could be found in relation to social status. Hypercortisolemia has been reported in association with social subordination in a number of primate species (Abbott et al., 2003).
Ovarian function
Cynomolgus monkeys have menstrual cycles like women. The peak progesterone concentration in the luteal phase is an index of the quality of ovarian function. High values indicate that ovulation occurred, whereas low values indicate impaired ovulation or an anovulatory cycle. By this measure both subordinate and depressed female cynomolgus monkeys have impaired ovarian function. The findings are consistent with those of Cameron and Bethea in stress sensitive cynomolgus macaques (Bethea et al., 2008).
Heart rate as an indicator of autonomic function
In an open field test, subordinates respond to a standardized stressor with higher initial heart rates that recover more slowly than dominants, whereas no significant differences in heart rate are observed in their home-cage social groups. Depressed monkeys maintain higher heart rates around the clock in their social groups compared to nondepressed monkeys. Thus, while both subordinates and depressed monkeys have perturbations in autonomic function, these seem more severe in depressed monkeys.
Neurobiological characteristics
There are some data suggesting that female subordinates have higher prolactin responses to fenfluramine, an indicator of differences in central serotonergic tone during the follicular phase of the menstrual cycle, but the sample size for this study was small (n=4 dominant, 4 subordinate). Much of the serotonin (5-HT) in the brain is produced in the raphe nucleus, and tryptophan hydroxylase (TPH) is the rate limiting factor for 5-HT production. TPH in the raphe nucleus of ovariectomized subordinate cynomolgus monkeys is lower than in their dominant counterparts, supporting differences in central serotonergic function. Additionally, subordinates have lower prolactin responses to haloperidol, and lower concentrations of the dopamine metabolite homovanillic acid (HVA) in their cerebrospinal fluid (CSF), indicating differences in dopaminergic tone. This observation was followed by multiple observations of lower striatal dopamine D2 receptor binding potential (BP), measured with positron emission tomography (PET) in subordinate male and female cynomolgus monkeys, and human beings of low socioeconomic status relative to their high socioeconomic status counterparts (Martinez et al., 2010; Morgan et al., 2002).
The picture is different for depression. There are no reports of neuroendocrine challenge test measures of 5-HT in depressed monkeys. However, 5-HT1A receptor BP measured with PET is lower in multiple areas of the corticolimbic system in depressed monkeys. In this study, social status was not associated with 5-HT1A receptor BP. Also un-like subordinates, depressed monkeys in this study did not have lower striatal D2 receptor BP (Shively unpublished data). Finally, depressed monkeys have smaller hippocampi like depressed human beings (McKinnon et al., 2009). There were no observed differences in hippocampal volume in dominant versus subordinate monkeys in these studies, but there were also not many cases to compare (Willard et al., unpublished data). There are no other reports of social status differences in clinical studies or nonhuman primates, although small, nonsignificant decreases (5–7%) in hippocampal volume have been observed in the tree shrew model of social stress (Czéh et al., 2001, 2006).
Behavioral characteristics
Following the neurobiological differences, subordinate female cynomolgus monkeys also appear behaviorally different from depressed female cynomolgus monkeys. There are no differences between depressed and nondepressed females in the frequency of aggression received, submissions sent, or submissions received, or in the percent time being groomed, all of which occur at different rates in dominant versus subordinate monkeys. Another interesting difference in subordinates versus depressed monkeys is in time spent alone. Subordinates spend more time alone than dominants, and this has been interpreted as evidence of a lack of social support. Although depression in human beings is sometimes interpreted as social withdrawal, depressed monkeys actually spent twice as much time as their nondepressed counterparts sitting in physical contact with conspecifics. In fact, much of their behavioral depression (slumped body posture unresponsive to environmental events) occurs while in body contact. Body contact may serve two purposes for depressed monkeys: First, body contact may provide warmth, and second, since depressed monkeys are unresponsive to environmental events, they may use the startle and orienting behavior of the monkey with which they are in body contact to warn them of threatening environmental events. Another behavior that is different between subordinates and depressed monkeys is activity level. Activity levels of depressed monkeys are 50% lower than nondepressed monkeys, whereas there are no reported differences in activity levels of dominants versus subordinates. Vigilant scanning of the social environment is characteristic of subordinate female cynomolgus monkeys in these small social groups. The behavior consists of head swiveling to visually scan the home pen while in a crouched posture, accompanied by lip smacking and grimacing. These are fear and appeasement behaviors in macaques; indeed these monkeys look fearful and anxious when engaged in this behavior. Vigilance was more common in depressed animals in one study but not another. This behavior deserves further investigation as a possible behavioral indicator of anxiety.
Cardiovascular disease risk
Both social stress and depression are associated with increased cardiovascular disease risk in human beings. Coronary heart disease is caused by coronary artery atherosclerosis and its sequelae. Cynomolgus monkeys have been a useful model to study factors that affect the development of coronary artery atherosclerosis. There is one report of lower HDL-C concentrations in wild subordinate male Anubis baboons (Papio anubis) (Sapolsky and Mott, 1987), and one from a study of male and female cynomolgus monkeys, both from the late 1980s. There have been no other reports of lipid differences between dominants and subordinates, although cardiovascular risk factors have been studied extensively since then in cynomolgus monkeys. Typically, we do not observe reliable differences in plasma cholesterol concentrations in dominants and subordinates. In contrast, the differences in plasma lipid concentrations in depressed versus nondepressed monkeys are notable. Depressed monkeys have lower HDL-C and higher TPC concentrations than nondepressed monkeys. In addition, there are differences in circulating fatty acid concentrations between depressed and nondepressed monkeys.
Among female cynomolgus macaques, subordinates have about twice as extensive coronary artery atherosclerosis as dominants, and this is a reliable difference documented in multiple studies. Thus, subordinate females have poor ovarian function and exaggerated heart rate responses to acute severe stress, both of which have been associated with increased coronary artery atherosclerosis extent. Depressed females have about four times the coronary artery atherosclerosis than nondepressed females. This is consistent with their dyslipidemia, high heart rates, low activity levels, and poor ovarian function, all of which are associated with increased coronary artery atherosclerosis extent (Shively et al., 2008, 2009).
Mortality
Socially dominant rhesus macaques live longer than their subordinate counterparts (Blomquist et al., 2011). Likewise, low social status is associated with increased mortality in the human population (Adler, 2009, Adler et al., 1994). Mortality was associated with depressive behavior in Experiment II described above. Over the course of this 4-year experiment, 56% of the monkeys in the highest quartile of depression died (5/9), whereas there was only one (11%) or two (22%) deaths in each of the other quartiles. Depression is also associated with non suicide mortality in the human population (Bradvik and Berglund, 2001; Kouzis et al., 1995; Rovner et al., 1991; von Ammon Cavanaugh et al., 2001).
Summary and conclusions
This review focuses on the behavioral and physiological manifestations of stress versus depression. The purpose of the review is to evaluate the conceptual approach of using stress models as surrogates for depression. Social stress and depression have many characteristics in common. They promote each other. Both have adverse effects on social relationships and the quality of life. Both promote physiological responses that increase risk of other diseases. However, they are not the same constructs. In human and nonhuman primates, the behavior and neurobiology of stressed individuals differ from that of stressed individuals that go on to exhibit depressive behavior. Socially stressed subordinate monkeys and depressed monkeys have some physiological characteristics in common such as perturbations in HPA, autonomic, and ovarian function, as well as exacerbated coronary artery atherosclerosis and increased mortality. However, the perturbations in some systems seem more extreme in depressed individuals. These observations, coupled with the fact that depressive behavior is more common in socially stressed subordinate monkeys, suggest that depressed monkeys are largely made up of socially subordinate females that are preferentially susceptible to a depressive response to social stress. If this is the case, then comparative studies of subordinates who do and do not exhibit depressive behavior may give us insight into mechanisms that support resiliency to stress, and identify new targets for therapeutic intervention. An aspect of human depression that is relatively unexplored in this monkey model is anhedonia. Lower body weight coupled with lack of interest in environmental events observed in monkeys that exhibit depressive behavior suggests anhedonia, however, further study of this characteristic is needed. Some similarities in stress physiology in socially stressed and depressed individuals have been used to support the contention that stressed animals are valid models of depression. Indeed, much has been learned from stress models of depression. However, the studies reviewed here also suggest that separating stress and depression in animal models may be important to furthering our understanding of each of these constructs as well as their mutual relationship.
Acknowledgments
This work was supported by NIH Grants RO1 HL39789, RO1 HL87103; RO1 MH56881, R21 MH086731, RO1 HL087103, and the John D. and Catherine T. MacArthur Foundation.
Abbreviations
- ACTH
adrenocorticotropic hormone
- BP
binding potential
- BMI
body mass index
- CRH
corticotropin-releasing hormone
- CSF
cerebrospinal fluid
- Dex
dexamethasone
- D2
dopamine receptor D2
- HPA
hypothalamic–pituitary–adrenal
- HDL-C
high density lipoprotein cholesterol
- HVA
homovanillic acid
- IGF-1
insulin-like growth factor-1
- OVX
ovariectomized
- PET
positron emission tomography
- P4
Progesterone
- 5-HT
serotonin
- 5-HT1A
serotonin 1A receptor
- TPC
total plasma cholesterol
- TPH
tryptophan hydroxylase
Appendix A
Fig. A.1.
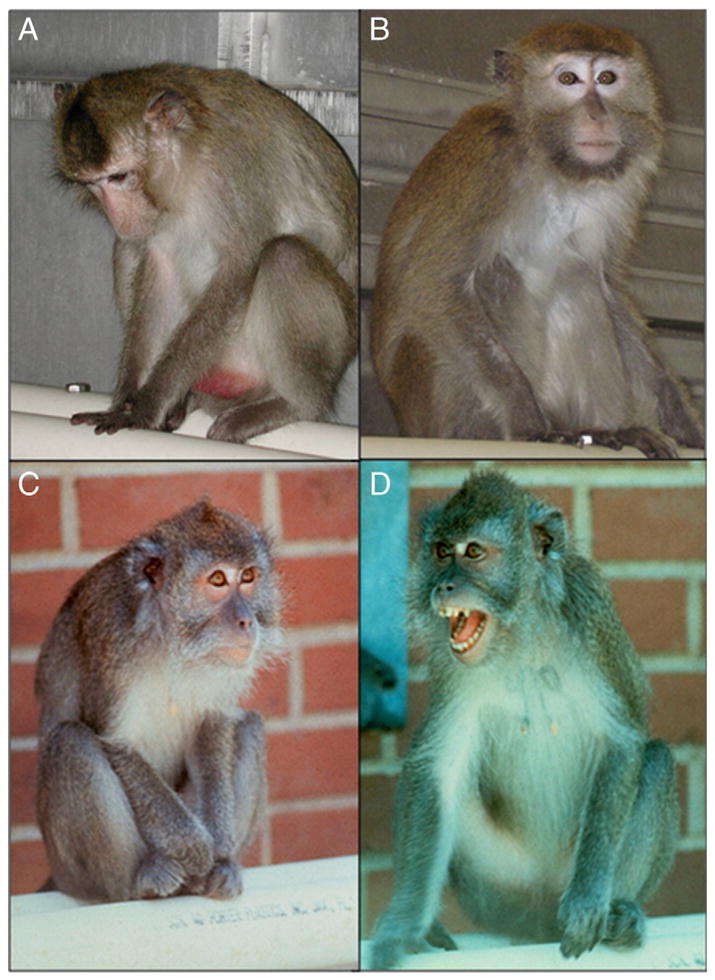
Social subordination versus depression behavior in adult female cynomolgus monkeys: A) Depressed behavior includes a slumped body posture, head dropped below shoulder level, eyes open to distinguish the behavior from sleep, and a lack of orienting and responding to environmental stimuli to which other monkeys attend, in this case the photographer. B) Normal Alert behavior includes visual attention to environmental stimuli such as the photographer. C) Submissive behavior is characterized by the head drawn back, ears back, body small, and vigilance. D) This socially dominant female is displaying the aggressive behavior termed an “open-mouth threat”, her head is forward, ears are forward, and she is directly staring at the individual she is threatening.
Table A.1.
Characteristics of socially subordinate versus depressed monkeys.
| Variables | Social subordination stress | Depression |
|---|---|---|
| Body weight (BW) | Dom = Sub, Kaplan et al. (2002, 2010); BW predicts status in males but not females (Morgan et al. (2000); Riddick et al. (2009) | Nondepressed > Depressed, Shively et al. (2005) |
| Body mass index | Dom = Sub, Kaplan et al. (2010) | Nondepressed > Depressed, Shively et al. (2005) |
| Basal cortisol | Dom < Sub, Shively et al. (1997); Shively (1998) | Nondepressed = Depressed, Shively et al. (1997, 2005) |
| % change dex suppression test | Dom > Sub, Shively et al. (1997); Dom somewhat greater than Sub (p = 0.10), Shively (1998). Post-Dex cortisol Dom < Subs, Shively (1998); Kaplan et al. (2010) | Nondepressed > Depressed, Shively et al. (1997) (among subordinates only, depressed were more affected [had higher post-Dex cortisol] than nondepressed); Shively et al. (2002) |
| Cortisol R to ACTH | Dom < Sub, Kaplan et al. (1986); Shively (1998); Dom somewhat lower than Sub (p = 0.09), Shively et al. (1997) | Nondepressed = Depressed in the Shively et al. (1997) study (data not reported) |
| Cortisol R to CRH | Nondepressed > Depressed, Shively et al. (2005) | |
| Ovarian function (χ̄ peak luteal P4) | Dom > Sub, Shively et al. (1994, 2002); Kaplan et al. (2010) | Nondepressed > Depressed, Shively et al. (2002, 2005) |
| Heart Rate | Social status x time interaction in an open field test: Dom reduce heart rate faster than Sub, Shively (1998) | 24 h heart rate: Nondepressed < Depressed, Shively et al. (2002, 2005) |
| Prolactin R to fenfluramine | Dom = Sub when randomly done with respect to menstrual cycle, Shively (1998); Dom < Sub early follicular phase, Shively et al. (1995) | |
| Prolactin R to haloperidol; CSF HVA | Dom > Sub, Shively (1998); Kaplan et al. (2002) | |
| Tryptophan hydroxylase raphe nucleus | Dom > Sub OVX, Shively et al. (2003) | |
| 5-HT1A receptor BP limbic system | Dom = Sub: social status not correlated with 5-HT1A BP, Shively et al. (2006) | Nondepressed > Depressed, Shively et al. (2006) |
| D2 receptor BP striatum | Dom > Sub, Grant et al. (1998) | Nondepressed = Depressed, Shively et al. (unpublished data) |
| Variables | Social Status | Depression |
|---|---|---|
| Bippocampal volume | Dom = Sub, Willard et al. (unpublished data) but only a few cases available to compare | Nondepressed > Depressed, Willard et al. (2009); in OVX Willard et al. (2011) |
| Aggression sent | Dom > Sub, Shively (1998) | Nondepressed > Depressed, Shively et al. (2005) |
| Aggression received | Dom < Sub, Shively (1998) | Nondepressed = Depressed, Shively et al. (2005) |
| Submissions sent | Dom < Sub, Shively et al. (1997, 1998) | Nondepressed = Depressed, Shively et al. (2005) |
| Submission received | Dom > Sub, Shively et al. (1997, 1998) | Nondepressed = Depressed, Shively et al. (2005) |
| Vigilant scanning | Dom < Sub, Shively et al. (1997, 1998) | Nondepressed < Depressed, Shively et al. (1997); Nondepressed = Depressed, Shively et al. (2005) |
| % time being groomed | Dom > Sub, Shively et al. (1997, 1998) | Nondepressed = Depressed, Shively et al. (2005) |
| % time in body contact | Dom < Sub, Shively (1998); Dom=Sub, Shively et al. (1997) | Nondepressed < Depressed, Shively et al. (2005) |
| % time alone | Dom < Sub, Shively et al. (1997) | Nondepressed > Depressed, Shively et al. (2005) |
| Activity level | Dom = Sub, Shively (1998) | Nondepressed > Depressed, Shively et al. (2005); activity inversely correlated with depression, Shively et al. (2008) |
| % time depressed posture | Sub > Dom, particularly Sub with a history of social subordination, Shively et al. (1997); social status inversely correlated with depression, Shively et al. (2006) | X |
| Lipids (mg/dl) | HDL-C Dom > Sub, Hamm et al. (1983) | Nondepressed differ from Depressed in TPC, HDL-C and fatty acids, Shively et al. (2005); Chilton et al. (2011) |
| Coronary artery atherosclerosis | Dom < Sub, 2-fold difference, Kaplan et al. (2002, 2009) | Nondepressed < Depressed, 4-fold difference, Shively et al. (2008) |
| Mortality | Dom < Sub, Blomquist et al. (2011) | Nondepressed < Depressed: depression was inversely correlated with mortality, Shively et al. (2005) |
Note: Data are mean differences between groups of intact adult female cynomolgus monkeys unless otherwise noted. Empty cells indicate no reports available. Gray boxes highlight characteristics that appear to differ between stressed monkeys and depressed monkeys. Dom: dominant animals; Sub: subordinate animals; R: response; Dex: dexamethasone; ACTH: adrenocorticotropin hormone; CRH: corticotrophin-releasing hormone; P4: progesterone; ovx: ovariectomized; CSF: cerebrospinal fluid; HVA: homovanillic acid; 5-HT1A: Serotonin 1A receptor; BP: binding potential; D2: dopamine receptor D2; TPC: total plasma cholesterol; HDL-C: high density lipoprotein cholesterol. Sources: Chilton et al., 2011; Grant et al., 1998; Hamm et al., 1983; Kaplan et al., 2002; Kaplan et al., 2009; Morgan et al., 2000; Riddick et al., 2009; Shively and Clarkson, 1994; Shively et al., 1995; Shively et al., 2003; Shively et al., 2006; Willard et al., 2009; Willard et al., 2011.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43 (1):67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Adler NE. Health disparities through a psychological lens. Am Psychol. 2009;64:663–673. doi: 10.1037/0003-066X.64.8.663. [DOI] [PubMed] [Google Scholar]
- Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, van Os J, Ibáñez MI, Ruipérez MA, Ortet G, Fañanás L. Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychol Med. 2009;39 (9):1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Centeno ML, Cameron JL. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol Neurobiol. 2008;38:199–230. doi: 10.1007/s12035-008-8042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Lima FB, Centeno ML, Weissheimer KV, Senashova O, Reddy AP, Cameron JL. Effects of citalopram on serotonin and CRF systems in the midbrain of primates with differences in stress sensitivity. J Chem Neuroanat. 2011;41:200–218. doi: 10.1016/j.jchemneu.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomquist GE, Sade DS, Berard JD. Rank-related fitness differences and their demographic pathways in semi-free ranging rhesus macaques (Macaca mulatta) Int J Primatol. 2011;32:193–208. [Google Scholar]
- Bradvik L, Berglund M. Late mortality in severe depression. Acta Psychiatr Scand. 2001;103:111–116. doi: 10.1034/j.1600-0447.2001.00212.x. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Reddy AP, Cameron JL, Bethea CL. Corticotropin-releasing hormone and pro-opiomelanocortin gene expression in female monkeys with differences in sensitivity to stress. Neuroendocrinology. 2007;86:277–288. doi: 10.1159/000109877. [DOI] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Chilton FH, Lee TC, Willard SL, Ivester P, Sergeant S, Reigster TC, Shively CA. Depression and altered serum lipids in cynomolgus monkeys consuming a Western diet. Physiol Behav. 2011;104 (2):222–227. doi: 10.1016/j.physbeh.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98 (22):12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh B, Simon M, Schmelting B, Hiemke C, Fuchs E. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31 (8):1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- Duman CH. Models of depression. Vitam Horm. 2010;82:1–21. doi: 10.1016/S0083-6729(10)82001-1. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Molendijk ML, Oude Voshaar RC, Bus BA, Prickaerts J, Spinhoven P, Penninx BJ. The impact of childhood abuse and recent stress on serum brain-derived neurotrophic factor and the moderating role of BDNF Val66Met. Psychopharmacology (Berl) 2011;214 (1):319–328. doi: 10.1007/s00213-010-1961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Social stress in tree shrews as an animal model of depression: an example of a behavioral model of a CNS disorder. CNS Spectr. 2005;10 (3):182–190. doi: 10.1017/s1092852900010038. [DOI] [PubMed] [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29 (1):80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hamm TE, Jr, Kaplan JR, Clarkson TB, Bullock BC. Effects of gender and social behavior on the development of coronary artery atherosclerosis in cynomolgus macaques. Atherosclerosis. 1983;48 (3):221–233. doi: 10.1016/0021-9150(83)90040-0. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Koritnik DR, Rose JC, Manuck SB. Adrenal responsiveness and social status in intact and ovariectomized Macaca fasciciularis. Am J Primatol. 1986;11:181–193. doi: 10.1002/ajp.1350110209. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Fontenot MB, Mann JJ. Central nervous system monoamine correlates of social dominance in cynomolgus monkeys (Macaca fascicularis) Neuropsychopharmacology. 2002;26 (4):431–443. doi: 10.1016/S0893-133X(01)00344-X. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Manuck SB. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. Am J Primatol. 2009;71 (9):732–741. doi: 10.1002/ajp.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Appt SE, Lees CJ, Franke AA, Berga SL, Wilson ME, Manuck SB, Clarkson TB. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Hum Reprod. 2010;25 (12):3083–3094. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;25 (2):e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzis A, Eaton WW, Leaf PJ. Psychopathology and mortality in the general population. Soc Psychiatry Psychiatr Epidemiol. 1995;30:165–170. doi: 10.1007/BF00790655. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167 (11):1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Liu RT, Alloy LB. Stress generation in depression: a systematic review of the empirical literature and recommendations for future study. Clin Psychol Rev. 2010;30 (5):582–593. doi: 10.1016/j.cpr.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorant V, Deliège D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157 (2):98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, Broft A, Van Heertum R, Kleber HD. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry. 2010;67 (3):275–278. doi: 10.1016/j.biopsych.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney WT. Animal models of depression: an overview. Psychiatr Dev. 1984;2 (2):77–96. [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34 (1):41–54. [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biol Reprod. 2009;81 (6):1154–1163. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA. Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am J Primatol. 2000;52 (3):115–131. doi: 10.1002/1098-2345(200011)52:3<115::AID-AJP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: do-pamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5 (2):169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13 (10):1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, Lange C, Higley JD, Rosoklija G, Hen R, Sackeim HA, Coplan JD. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One. 2011;6 (4):e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plazas-Mayorca MD, Vrana KE. Proteomic investigation of epigenetics in neuropsychiatric disorders: a missing link between genetics and behavior? J Proteome Res. 2011;10 (1):58–65. doi: 10.1021/pr100463y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, Pierre PJ, Bennett A, Garg PK, Garg S, Nader MA. Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience. 2009;158 (4):1257–1265. doi: 10.1016/j.neuroscience.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovner BW, German PS, Brant LJ, Clark R, Burton L, Folstein MF. Depression and mortality in nursing homes. JAMA. 1991;265:993–996. doi: 10.1001/jama.265.8.993. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163 (11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Mott GE. Social subordinance in wild baboons is associated with suppressed high density lipoprotein-cholesterol concentrations: the possible role of chronic social stress. Endocrinology. 1987;121 (5):1605–1610. doi: 10.1210/endo-121-5-1605. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biol Psychiatry. 1998;44 (9):882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. Social status and coronary artery atherosclerosis in female monkeys. Arterioscler Thromb. 1994;14 (5):721–726. doi: 10.1161/01.atv.14.5.721. [DOI] [PubMed] [Google Scholar]
- Shively CA, Kaplan JR. Stability of social status rankings of female cynomolgus monkeys, of varying reproductive condition, in different social groups. Am J Primatol. 1991;23:239–245. doi: 10.1002/ajp.1350230404. [DOI] [PubMed] [Google Scholar]
- Shively CA, Fontenot MB, Kaplan JR. Social status, behavior and central serotonergic responsivity in female cynomolgus monkeys. Am J Primatol. 1995;37:333–339. doi: 10.1002/ajp.1350370408. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom Med. 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Mirkes SJ, Lu NZ, Henderson JA, Bethea CL. Soy and social stress affect serotonin neurotransmission in primates. Pharmacogenomics J. 2003;3 (2):114–121. doi: 10.1038/sj.tpj.6500166. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol Psychol. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, Henderson JA, Smith MA, Buchheimer N. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch Gen Psychiatry. 2006;63 (4):396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Adams MR, Golden DL, Willard SL, Clarkson TB. Depressive behavior and coronary artery atherogenesis in adult female cynomolgus monkeys. Psychosom Med. 2008;70 (6):637–645. doi: 10.1097/PSY.0b013e31817eaf0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neurosci Biobehav Rev. 2009;33 (2):133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmans GI, Berman MI, Usitalo AN. Psychotherapy versus second-generation antidepressants in the treatment of depression: a meta-analysis. J Nerv Ment Dis. 2011;199 (3):142–149. doi: 10.1097/NMD.0b013e31820caefb. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Eisele CD, Grady SA, Harlow HF. Depressive behavior in adult monkeys following separation from family environment. J Abnorm Psychol. 1975;84:576–578. doi: 10.1037/h0077066. [DOI] [PubMed] [Google Scholar]
- von Ammon Cavanaugh S, Furlanetto LM, Creech SD, Powell LH. Medical illness, past depression, and present depression: a predictive triad for in-hospital mortality. Am J Psychiatry. 2001;158:43–48. doi: 10.1176/appi.ajp.158.1.43. [DOI] [PubMed] [Google Scholar]
- Watson SL, Shively CA, Kaplan JR, Line SW. Effects of chronic social separation on cardiovascular disease risk factors in female cynomolgus monkeys. Atherosclerosis. 1998;137:259–266. doi: 10.1016/s0021-9150(97)00277-3. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willard SL, Friedman DP, Henkel CK, Shively CA. Anterior hippocampal volume is reduced in behaviorally depressed female cynomolgus macaques. Psychoneuroendocrinology. 2009;34 (10):1469–1475. doi: 10.1016/j.psyneuen.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard SL, Daunais JB, Cline JM, Shively CA. Hippocampal volume in post-menopausal cynomolgus macaques with behavioral depression. Menopause. 2011;18:582–586. doi: 10.1097/gme.0b013e3181fcb47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. The validity of animal models of depression. Psychopharmacology (Berl) 1984;83 (1):1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Yan HC, Cao X, Das M, Zhu XH, Gao TM. Behavioral animal models of depression. Neurosci Bull. 2010;26 (4):327–337. doi: 10.1007/s12264-010-0323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Van Meter PE, Wallen K. Factors regulating the timing of puberty onset in female rhesus monkeys (Macaca mulatta): role of prenatal androgens, social rank, and adolescent body weight. Biol Reprod. 2005;72 (5):1087–1094. doi: 10.1095/biolreprod.104.027755. [DOI] [PubMed] [Google Scholar]


