Abstract
Image-guided thermal interventions have been proposed for potential palliative and curative treatments of pancreatic tumors. Catheter-based ultrasound devices offer the potential for temporal and 3D spatial control of the energy deposition profile. The objective of this study was to apply theoretical and experimental techniques to investigate the feasibility of endogastric, intraluminal and transgastric catheter-based ultrasound for MR guided thermal therapy of pancreatic tumors. The transgastric approach involves insertion of a catheter-based ultrasound applicator (array of 1.5 mm OD x 10 mm transducers, 360° or sectored 180°, ~7 MHz frequency, 13–14G cooling catheter) directly into the pancreas, either endoscopically or via image-guided percutaneous placement. An intraluminal applicator, of a more flexible but similar construct, was considered for endoscopic insertion directly into the pancreatic or biliary duct. An endoluminal approach was devised based on an ultrasound transducer assembly (tubular, planar, curvilinear) enclosed in a cooling balloon which is endoscopically positioned within the stomach or duodenum, adjacent to pancreatic targets from within the GI tract. A 3D acoustic bio-thermal model was implemented to calculate acoustic energy distributions and used a FEM solver to determine the transient temperature and thermal dose profiles in tissue during heating. These models were used to determine transducer parameters and delivery strategies and to study the feasibility of ablating 1–3 cm diameter tumors located 2–10 mm deep in the pancreas, while thermally sparing the stomach wall. Heterogeneous acoustic and thermal properties were incorporated, including approximations for tumor desmoplasia and dynamic changes during heating. A series of anatomic models based on imaging scans of representative patients were used to investigate the three approaches. Proof of concept (POC) endogastric and transgastric applicators were fabricated and experimentally evaluated in tissue mimicking phantoms, ex vivo tissue and in vivo canine model under multi-slice MR thermometry. RF micro-coils were evaluated to enable active catheter-tracking and prescription of thermometry slice positions. Interstitial and intraluminal ultrasound applicators could be used to ablate (t43>240 min) tumors measuring 2.3–3.4 cm in diameter when powered with 20–30 W/cm2 at 7 MHz for 5–10 min. Endoluminal applicators with planar and curvilinear transducers operating at 3–4 MHz could be used to treat tumors up to 20–25 mm deep from the stomach wall within 5 min. POC devices were fabricated and successfully integrated into the MRI environment with catheter tracking, real-time thermometry and closed-loop feedback control.
Keywords: hyperthermia, ablation, pancreatic cancer, MR temperature imaging, ultrasound, modeling
1. INTRODUCTION
Pancreatic cancer is the fourth leading cause and accounts for 10% of cancer mortality in the US (American Cancer Society - 2013). Surgery is the preferred treatment modality, but the vast majority of patients (~80–90%) are not surgical candidates when they present with disease. Additional standard treatment options include radiation therapy and chemotherapy. However in many cases the response and survival rates remain dismal. Thermal ablation can provide palliative relief by denervation/neurolysis1 and debulking, and has potential to improve response in small localized disease. To date, applied ablation technologies include RF ablation,2 cryotherapy,3 electroporation4 and external ultrasound-guided high-intensity focused ultrasound (HIFU).5 HIFU offers the potential for non-invasive thermal therapy of pancreatic targets; however, the limited acoustic window (due to air interfaces within the GI tract) and motion of the pancreas pose a significant challenge. Hyperthermia therapy (HT) in the 40–45°C range has been shown to enhance efficacy of standard chemotherapy regimens, and also to have a role in hyperthermia targeted thermal sensitive liposomes or HT targeted drug delivery in desmoplastic pancreatic tumors.6
Compared to other minimally-invasive energy modalities, catheter-based ultrasound offers the potential for 3D spatial control and selectivity of the energy deposition profile to target either thermal ablation or hyperthermia. Numerous transducer configurations and heating patterns are possible, and it is compatible with MR temperature guidance.7,8 The goals of this study are to perform a preliminary investigation of MR guided (MRg) thermal therapy of pancreatic targets using high intensity ultrasound with transgastric, endoluminal and intraluminal approaches (Fig. 1). Specific objectives include: development of 3D bioacoustic and thermal models, parametric analysis, and patient specific evaluations to investigate designs and performance; Proof of concept (POC) designs are fabricated and evaluated on the bench, and in phantom, ex vivo tissues and in vivo animal model under MR temperature imaging; techniques for MRTI and localization are explored.
Figure 1.

The general schema and concepts for catheter-based ultrasound devices for MR guided ablation or hyperthermia of pancreas tumors. (a) Interstitial transgastric applicator as inserted via an endoscope into a pancreatic tumor, similar to endoscopic biopsy. Similarly, an intraluminal applicator can be inserted directly into the pancreatic duct. (b) Larger endoluminal device positioned with the stomach or duodenum with acoustic energy directed toward tumor target.
2. THEORETICAL INVESTIGATIONS
2.1 3D Acoustic and Biothermal Models
A 3D acoustic and bioheat transfer model was implemented to calculate the sonication patterns and resultant transient temperature profiles produced by the transgastric, intraluminal and endogastric ultrasound applicators under consideration. An implicit FEM solver (COMSOL Multiphysics) was used to solve the Pennes bioheat transfer equation9 (Equation 1):
| (1) |
T is tissue temperature, k is thermal conductivity, ωb is blood perfusion, Cb is specific heat of blood, Tb is blood temperature, ρ is density and Ct is specific heat of tissue. Q is the acoustic power deposition within tissue, calculated using an analytical expression for the cylindrical applicators10,11 and the rectangular radiator method12 ρ for the planar and curvilinear transducers. A direct implicit stationary solver (PARDISO) available in COMSOL was used to compute temperature solutions. Dirichlet boundary conditions were set to constant body/basal temperature (37 °C) at the extremities of the simulation domain and to constant cooling water temperature (10–22°C) at the applicator-GI tract or balloon boundary. For the transgastric (interstitial) & intraluminal approach, the applicator constructs were modeled as 1.5 mm (OD) × 10 mm tubular transducers with 360°, 180°, or dual 180° sectors operating at 7 – 8 MHz and 20–30 W/ cm2 for a 5–10 min power application. The intraluminal device also had a 4 mm OD inflatable balloon. The endoluminal devices were modeled in three different configurations: as tubular (10 mm OD × 10 L), planar (10 mm × 12.5 mm) and lightly focused (radius of curvature ROC = 20 mm, 10 mm × 12.5 mm) transducer arrays operating between 3–6 MHz. The tissue parameters were as follows: pancreas attenuation = 11×f 0.78 Np/m and perfusion = 10 kg/m3/s, pancreatic desmoplastic tumor attenuation = 1–1.5 ×αpanc Np/m and perfusion =1–3 kg/m3/s and stomach wall attenuation = 5 f Np/m and perfusion = 5 kg/m3/s. f is the frequency of acoustic energy in MHz. The stomach wall was modeled with a 3 mm thickness.
2.2 Parametric Analysis of Configurations
Parametric simulations were used to investigate the general performance of the three applicator configurations, as well as to study the range of optimum design parameters to consider for device development. As depicted in Fig 2, the interstitial devices can localize ablation to the pancreatic tumor target area by dynamic changes and tailoring of power control in length and time, to produce coagulation zones 2.3 cm –3.1 cm diameter for the 360° sonications, and 1.2–1.7 cm radial depth for the 180° directional heating. By adjusting the power to the array elements independently, the length of the ablation zone could be controlled between 2.4–4.2 cm, with other lengths possible but dependent upon number of array elements.
Figure 2.
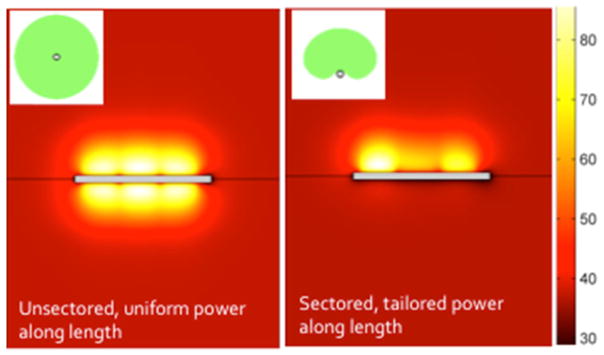
Simulated ablation zones from a 3x360° and 3x180° directional interstitial ultrasound applicator inserted within desmoplastic pancreatic tumor volumes surrounded by normal pancreas tissue, after 5 min. Applied power to elements can be independently controlled to tailor the length and radial penetration. The directional applicators can be applied to protect regions of tissue (such as a duct).
As depicted in Fig. 3 and Table 1, the endoluminal devices, which can be larger in diameter, can penetrate effectively through the stomach wall and into pancreatic target regions and normal pancreas tissue, but with performance differences between configurations. Tubular, planar and curvilinear lightly focused transducer sections were considered, each measuring 10 mm width or OD by 12 mm length. The applicators were modeled with water cooled balloon at 10–22°C to protect the stomach wall, push the maximum temperature into the pancreas, and to couple the ultrasound energy. The findings include that the tubular radiators required operation at a low frequency of 3 MHz to effectively penetrate the stomach wall into the pancreas. At appropriate power levels, zones of thermal coagulation n (t43>240 min) within target tissue could extend 15–20 mm beyond the stomach wall within 5–10 min without damage to the stomach wall itself. The 3 MHz planar transducer configuration could penetrate ~20 mm from the stomach wall within 3 min. 3–4 MHz was the optimal frequency for the planar transducer in order to effectively penetrate into the pancreas and avoid overheating the stomach wall. Within 3 min the curvilinear approach could penetrate slightly deeper to ~25 mm depth from the stomach wall while limiting stomach wall heating to <43°C, but it produced narrower heated zones. All configurations could be developed as multi-element arrays to be dynamically controlled as well as repositioned to produce variable length coagulation patterns.
Figure 3.

Parametric simulations of temperature distributions and thermal dose contours (t43>240 min) for (a) cylindrical, (b) planar and (c) curvilinear transducer configurations under consideration, operated at 3 MHz with 10–22°C cooling.
Table 1.
Summary of parametric simulations of the endoluminal devices for pancreas targeting from within the stomach or duodenum. The depth of the “tumor” from the stoma ach wall, the treatment time and the frequency were varied. Ablation zones determined by t43 thresholds greater than 240 min are listed.
| Transducer | f | Time | Tumor depth | Tstomach wall | Ablation zone dimensions |
|---|---|---|---|---|---|
| Tubular | 3 MHz | 5 min | 2 mm | 41.3 °C | 13.1 mm x 21.3 mm |
| Tubular | 3 MHz | 10 min | 2 mm | 45.6 °C | 18.0 mm x 24.5 mm |
| Planar | 3 MHz | 3 min | 5 mm | 44.4 °C | 12.5 mm x 14.2 mm x 12.5 mm |
| Planar | 3 MHz | 3 min | 2 mm | 44.1 °C | 17.8 mm x 14.9 mm x 13.1 mm |
| Planar | 4 MHz | 3 min | 2 mm | 51.4 °C | 17.6 mm x 14.6 mm x 12.5 mm |
| Lightly focused | 3 MHz | 3 min | 5 mm | 42.4 °C | 20.3 mm x 12.4 mm x 8.9 mm |
| Lightly focused | 3 MHz | 3 min | 2 mm | 44.0 °C | 19.3 mm x 12.9 mm x 9.4 mm |
| Lightly focused | 4 MHz | 3 min | 2 mm | 46.8 °C | 16.9 mm x 11.9 mm x 8.9 mm |
2.3 Patient Specific Simulations
Patient-specific 3D anatomic models were created from axial CT scans of three representative cases, with tumors in the head, tail and body of the pancreas. Anatomical structures such as stomach wall, duodenum, pancreas and tumor target were segmented and a FEM mesh was generated using Mimics and 3-Matic (Materialise, Inc.). The acoustic and thermal distributions were calculated using COMSOL MultiPhysics. Applicator positions and configurations were varied. The theoretical models described above were adapted to calculate the energy deposition and transient temperature profiles on meshes derived from patient-specific anatomies. Fig. 4(a–c) depicts the CT scan, 3D FEM model and temperature distributions of a tumor localized to the tail of the pancreas, with an interstitial ultrasound applicator inserted through the stomach wall into the tumor. Control of the power level to the separate elements of a 360° applicator generated thermal coagulative temperatures extending beyond the tumor boundary in under 5 min. Similarly, a duodenal approach for a tumor in the head of pancreas is shown for a multi-sector dual transducer applicator for a 4 min ablation. Fig. 5 depicts a 10 mm x 12 mm curvilinear applicator with 10 °C cooling flow positioned within the duodenum and adjacent to the pancreatic tumor within the head of the pancreas. The target volume was ablated (t43>240 min) in 5 min while preserving the stomach wall. An intraluminal approach was also investigated with patient specific models. Fig 6 depicts a tumor located around the pancreatic duct. An intraluminal applicator, which could be placed within the duct under endoscopy, was modeled as 1.5 mm OD x three 5-10-5 mm long 360° transducer segments mounted on a 2 mm OD catheter, with an inflatable 4 mm OD cooling balloon on the distal end. For this case, 7 MHz power at 20 W/cm2 was applied, and PID control was implemented based upon Tmax=85 °C with Tmax and boundary control. These devices were able to ablate circumferential or adjacent tumors greater than 30 mm diameter within 5–10 min. Additionally, given the recent interest in hyperthermia for enhancing drug delivery or chemotherapy, lower power applications for long duration was applied to generate hyperthermia. Results indicate that hyperthermic temperature distributions generated intraductally can extend 40°C contours at steady-state to tumor boundaries greater than 25 mm diameter within desmoplastic tumors.
Figure 4.
Patient-specific models depicting interstitial ultrasound ablation of pancreatic tumors in the tail and head of the pancreas. A transgastric approach is applied for the (top row) tail, and a trans-duodenal approach is used for the head (bottom row). Multi-element multi-sector devices were applied to coagulate 2 cm diameter tumor volumes in under 5 min. 3D anatomy and final temperature distributions are depicted.
Figure 5.

Patient-specific model of endgastric ultrasound ablation of a pancreatic tumor in the head of the pancreas. (a) and (b) show the anatomy and tumor location in a CT scan and 3D model, respectively. (c) depicts simulations of temperature distributions and thermal dose contours (t43>240 min) transducers operated at 3 MHz with 10°C cooling.
Figure 6.

Patient specific simulations of intraductal ultrasound depicting (a) anatomy and location of tumor and applicator within the pancreatic duct, (b) ablative temperature isosurface under 5 min of ablation and (c) steady-state hyperthermia temperatures (>40°C) extending beyond tumor boundary.
3. INITIAL DEVICES AND EVALUATIONS
3.1 Proof of Concept Devices
A transgastric compatible construct was fabricated using MR compatible 1.5 mm x 10 mm long tubular radiators, sectored for 180° heating pattern, to form a dual element array in a catheter-cooled configuration.13 A 3 mm diameter x 40 cm long PEEK catheter was blended to a 6 cm section of a 13g Celcon implant catheter to form the catheter-cooled sheath, with the applicator inserted from within an endoscope (Fig 7a). A POC endoluminal device was fabricated using a 12 mm OD nylon transducer assembly with ports for water cooling and a balloon, integrated to a proximal 4 mm diameter nylon extrusion with water cooling channels and RF leads. A single 10 mm x 12 mm PZT4 transducer segment with a center frequency of 5.6 MHz was sealed in place with air-backing. A vinyl balloon is attached to encapsulate cooling flow, to reduce heating of the stomach wall and to couple the ultrasound energy. Active MR tracking coils can be added to the proximal catheter material to more efficiently localize the device and to setup control slices for MRTI and guidance e.
Figure 7.

Initial proof-of-concept devices: (a) interstitial device for transgastric insertion and (b) endoluminal device for placement within stomach or duodenum adjacent to pancreas.
3.2 MRTI and Heating Performance within Ex Vivo Tissue/Phantom and In Vivo Canine
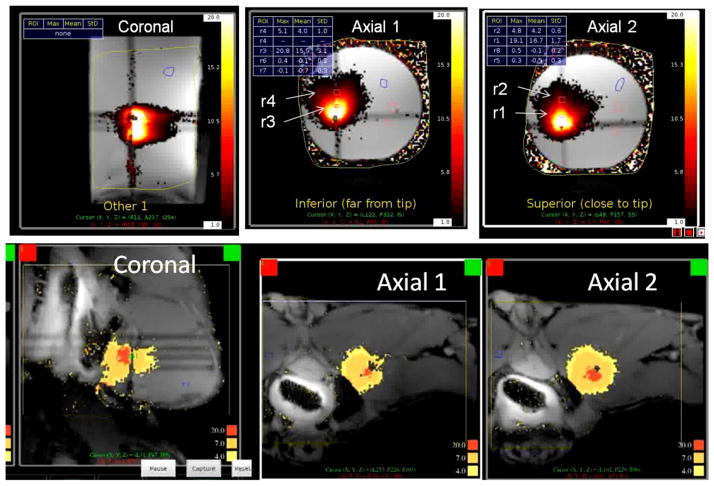
The devices were evaluated in a tissue equivalent phantom,14 ex vivo tissues and in vivo muscle using MRTI for monitoring and guidance. All devices are MR compatible. The experiments were conducted in a 3T GE MRI scanner. MR images were acquired using surface imaging coils (with an endorectal coil for the in vivo case). The MR images were recorded with sequences optimized for MR temperature monitoring (echo time = 7 ms,, field-of-view = 15 cm and image size = 128×128 pixels). MR thermometry was performed in real time using RTHawk (HeartVista Inc, Palo Alto, CA), a commercially available software platform which enabled real time access to the MR scanner’s settings and parameters, volumetric multi-plane imaging, dynamic image plane positioning and fast temperature reconstruction from the MR images. The transgastric interstitial applicator with dual transducer elements was evaluated in phantom, with MRTI slices aligned with each element to provide temperature feedback for control of Tmax and temperature at the outer boundary. All devices were MR compatible. Fig. 8 depicts the directional temperature profiles measured in a coronal slice along the length of the applicator, as well as two axial slices through the applicator that were used for real-time feedback control, both in phantom and in vivo. Similarly, the endogastric POC device was inserted into a tissue equivalent phantom and monitored in an axial and a sagital slice through the transducer (Fig. 9). This was done within phantom and within ex vivo muscle tissue surrounded by stomach tissue. The temperature profiles and heating distributions were well over ablative requirements and correlate qualitatively with theoretical predictions.
Figure 8.
Multi-slice MRTI for monitoring and control of transgastric interstitial heating shown (top) within tissue equivalent phantom and (bottom) within in vivo canine muscle, demonstrating penetrating and effective directional heating patterns with control along the length and angle of the applicator.
Figure 9.
Multi-slice MRTI for monitoring and control of an endoluminal applicator with a planar transducer shown (top) within tissue equivalent phantom and (bottom) within ex vivo porcine muscle (a pancreas tumor mimic) surrounded by porcine stomach, demonstrating MR compatibility as well as penetrating and effective directional heating patterns through the stoma ach and into the surrounding tissue.
Both pancake type and an angled-oblique coil were integrated on the delivery catheters and evaluated using MR tracking sequences. The SNR and ability to localize the position of the catheter using these MR tracking coils under MR tracking pulses controlled by RTHawk is demonstrated in Fig. 10. These results are preliminary, but indicate that the integration of tracking coils can be utilized to quickly determine applicator position and setup the multi-slice MRTI and control.
Figure 10.

Two types of coils, (left) angled oblique and pancake coils, were evaluated for active MR tracking of applicator position within ex vivo tissue. The applicator image (center) with the (right) high-signal spatial localization from the tracking coil could potentially be used to demarcate the precise position and orientation of the applicator within complex anatomy when setting up MRTI.
4. SUMMARY
Preliminary results from theoretical simulations and experiments indicate that MR guided catheter-based ultrasound has potential for conformal thermal therapy of pancreatic tumors. We investigated and defined operational constraints and capabilities of transgastric, intraluminal and endoluminal approaches. The transgastric and intraluminal approaches are realizable and can generate 30 mm diameter ablation n zones, which can be tailored to the shape of the tumor, within a 5–10 min time frame. The intraluminal applicator configuration appears feasible to deliver effective, localized hyperthermia to tumors circumferential or adjacent to an accessible duct, with significant potential for enhancing chemotherapy or targeted drug delivery in desmoplastic tumors. The endoluminal approach, with planar or curvilinear arrays, appears capable of treating targets extending ~25 mm deep from GI tract. All devices can be fabricated and operated under MRTI for real-time control and guidance. Further development and evaluation of MR guided ultrasound devices for endoluminal and intraluminal delivery is warranted.
Acknowledgments
This study was supported by grants from the National Institutes of Health R01CA122276, R21CA137472 and P01CA159992.
References
- 1.Bahn BM, Erdek MA. Celiac plexus block and neurolysis for pancreatic cancer. Curr Pain Headache Rep. 2013;17(2):310. doi: 10.1007/s11916-012-0310-y. [DOI] [PubMed] [Google Scholar]
- 2.Cantore M, Girelli R, Mambrini A, Frigerio I, Boz G, Salvia R, Giardino A, Orlandi M, Auriemma A, Bassi C. Combined modality treatment for patients with locally advanced pancreatic adenocarcinoma. Br J Surg. 2012;99(8):1083–1088. doi: 10.1002/bjs.8789. [DOI] [PubMed] [Google Scholar]
- 3.Arcidiacono PG, Carrara S, Reni M, Petrone MC, Cappio S, Balzano G, Boemo C, Cereda S, Nicoletti R, Enderle MD, Neugebauer A, von Renteln D, Eickhoff A, Testoni PA. Feasibility and safety of eus-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. 2012;76(6):1142–1151. doi: 10.1016/j.gie.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Martin RC, 2nd, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: Potential improved overall survival. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2736-1. [DOI] [PubMed] [Google Scholar]
- 5.Khokhlova TD, Hwang JH. HIFU for palliative treatment of pancreatic cancer. J Gastrointest Oncol. 2011;2(3):175–184. doi: 10.3978/j.issn.2078-6891.2011.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tschoep-Lechner KE, Milani V, Berger F, Dieterle N, Abdel-Rahman S, Salat C, Issels RD. Gemcitabine and cisplatin combined with regional hyperthermia as second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. Int J Hyperthermia. 2013;29(1):8–16. doi: 10.3109/02656736.2012.740764. [DOI] [PubMed] [Google Scholar]
- 7.Diederich CJ, Nau WH, Kinsey A, Ross T, Wootton J, Juang T, Butts-Pauly K, Rieke V, Chen J, Bouley DM, Sommer G. Catheter-based ultrasound devices and MR thermal monitoring for conformal prostate thermal therapy. Conf Proc IEEE Eng Med Biol Soc 2008. 2008:3664–3668. doi: 10.1109/IEMBS.2008.4650002. [DOI] [PubMed] [Google Scholar]
- 8.Pauly KB, Diederich CJ, Rieke V, Bouley D, Chen J, Nau WH, Ross AB, Kinsey AM, Sommer G. Magnetic resonance-guided high-intensity ultrasound ablation of the prostate. Top Magn Reson Imaging. 2006;17(3):195–207. doi: 10.1097/RMR.0b013e31803774dd. [DOI] [PubMed] [Google Scholar]
- 9.Pennes HH. Analysis of tissue and aterial blood temperatures in the resting human forearm. Journal of Applied Physiology. 1948;1:93–122. doi: 10.1152/jappl.1948.1.2.93. [DOI] [PubMed] [Google Scholar]
- 10.Diederich CJ, Hynynen K. Induction of hyperthermia using an intracavitary multielement ultrasonic applicator. IEEE Trans Biomed Eng. 1989;36(4):432–438. doi: 10.1109/10.18749. [DOI] [PubMed] [Google Scholar]
- 11.Tyreus PD, Diederich CJ. Theoretical model of internally cooled interstitial ultrasound applicators for thermal therapy. Phys Med Biol. 2002;47(7):1073–1089. doi: 10.1088/0031-9155/47/7/306. [DOI] [PubMed] [Google Scholar]
- 12.Ocheltree KB, Frizzel LA. Sound field calculation for rectangular sources. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 1989;36(2):242–248. doi: 10.1109/58.19157. [DOI] [PubMed] [Google Scholar]
- 13.Nau WH, Diederich CJ, Burdette EC. Evaluation of multielement catheter-cooled interstitial ultrasound applicators for high-temperature thermal therapy. Medical Physics. 2001;28(7):1525–1534. doi: 10.1118/1.1381550. [DOI] [PubMed] [Google Scholar]
- 14.King RL, Liu Y, Maruvada S, Herman BA, Wear KA, Harris GR. Development and characterization of a tissue-mimicking material for high-intensity focused ultrasound. IEEE Trans Ultrason Ferroelectr Freq Control. 2011;58(7):1397–1405. doi: 10.1109/TUFFC.2011.1959. [DOI] [PubMed] [Google Scholar]