Abstract
We investigated the administration of intravenous (i.v.) busulfan (Bu) combined with melphalan (Mel) in patients with advanced lymphoid malignancies undergoing autologous SCT. Bu 130 mg/m2 was infused daily for 4 days, either as a fixed dose per BSA, or to target an average daily AUC of 5,000 uMol-min, determined by a test dose of i.v. Bu at 32 mg/m2 given 48 hours prior to the high dose regimen, followed by a rest day, followed by two daily doses of Mel at 70mg/m2. Stem cells were infused the following day. 80 patients had i.v. Bu delivered per test dose guidance. The median daily systemic Bu exposure was 4867 uMol-min. 102 patients [Hodgkin's Lymphoma (HL) n=49, Non Hodgkin's Lymphoma (NHL) n=12, Multiple Myeloma (MM) =41] with median age 44 years (range 19 to 65 years) were treated. 2-year overall survival (OS) and progression-free survival (PFS) rates were 85% and 57%, respectively, for patients with HL, 67% and 64%, respectively, for patients with NHL, and 82% and 42%, respectively, for patients with MM. The regimen was very well-tolerated with treatment-related mortality at 100 days, 1 year, and 2 years of 1%, 3%, and 3%, respectively. Intravenous Bu-Mel is well-tolerated. Disease control is encouraging, and should be explored in larger phase II studies.
INTRODUCTION
High dose chemotherapy with autologous hematopoietic stem cell transplantation (SCT) is an accepted treatment option for patients with advanced lymphoid malignancies. Such therapy prolongs both progression-free survival (PFS) and overall survival (OS) in patients with chemotherapy-sensitive recurrent disease compared with standard salvage chemotherapy. For patients with chemotherapy-sensitive Hodgkin's lymphoma (HL), freedom from treatment failure at 3 years was significantly better following BEAM-SCT compared with Dexa-BEAM and no transplant in a randomized study (55% vs. 34%, p=.019) [1]. Similarly, significantly better treatment outcomes following autologous SCT in patients with non-Hodgkin's lymphoma (NHL), as compared with chemotherapy alone, were reported in the PARMA study, with survival of 53% at 5 years [2]. However, long-term disease-control is much less impressive after SCT in patients with chemotherapy-refractory disease, with expected 10-year OS of only 10% to 20% following SCT in patients with primary refractory HL [3, 4] and 5-year OS of 10% in NHL patients [5, 6]. Thus, considerable success has been gained with autologous SCT in patients with chemotherapy-sensitive recurrent HL and NHL. However, these seeming advances, yielding long-term disease control in about half of the patients with recurrent chemotherapy-sensitive disease only highlight the need for further improvement of high-dose regimens, such that an overall significant improvement can be made for all patients categories; Currently, none of the most commonly used high-dose regimens provides any significant benefit in patients with chemotherapy-refractory disease [5] [7-10].
In the efforts to develop more effective and less toxic high-dose chemotherapy regimens, it has further been assumed that alkylating agents, which form the backbone of most pre-transplant regimens, can “break through” (limited) resistance to chemotherapy based on their multiple intracellular mechanistic targets. We also recognized that the systemic exposure of activated cyclophosphamide (Cy) is, at a minimum, very difficult to standardize [11]. As an alternative, several groups have evaluated the combination of busulfan (Bu) and melphalan (Mel). Neither of these alkylators needs to be activated and they both display linear pharmacokinetics in the dose range(s) to be utilized [12-14]. Further, the good central nervous system (CNS) penetration for both Bu and Mel,[15] and their relative non-overlapping clinical toxicity profiles should make this combination an effective, high-dose chemotherapy regimen [16, 17]. The Bu-Mel combination has been most utilized in multiple myeloma (MM). Thus, the Spanish GETH and PETHEMA groups reviewed the outcomes of myeloma patients receiving melphalan 200 mg/m2, melphalan 140 mg/m2 + radiation, or oral busulfan 12 mg/kg + melphalan 140 mg/m2 for autologous SCT conditioning reported to the Spanish transplant registry[18]. In this retrospective analysis, the Bu-Mel combination yielded significantly better overall response rates (97% vs. 89% and 92%, p=.003), although the 5-year OS of 47% was not significantly improved [18]. Similarly, high CR rates were observed following autologous SCT with oral Bu and Mel conditioning in a multicenter trial for patients with myeloma[19]. A recent update, however, documented a significant 5-year PFS advantage for patients treated with a combination of i.v. Bu and Mel compared with patients treated with Mel alone (The European Group for Blood and Marrow Transplantation Conference Proceedings, March 2010). This was attributed primarily to the significantly increased safety for patients who received i.v. versus oral Bu in the combination.
The PETHEMA group noted higher rates of hepatic veno-occlusive disease (VOD) in the patients with myeloma treated with oral versus i.v. Bu as part of their transplant conditioning regimen[20]. This confirmed earlier observations of a decreased risk for VOD and multiorgan failure in patients undergoing allogeneic SCT for myeloid malignancies[21]. Furthermore, once daily i.v. Bu administration was noted to be safe [22], and to have linear PK with highly reproducible intra- and inter-patient systemic exposure,[14]and, finally, it allowed identification of an optimized therapeutic interval represented by the Bu area under the plasma concentration-versus-time curve (AUC) [23]. Based on these considerations, we hypothesized that i.v. Bu given once daily for 4 days with PK-guidance, followed by Mel given over 2 days, would constitute a safe and highly efficacious salvage regimen when delivered with autologous progenitor cell support. Here we report the results of this combination in patients undergoing autologous SCT for advanced lymphoid malignancies. The results confirmed our expectations and encourage further refinement of this safe and highly cytoreductive regimen.
PATIENTS AND METHODS
Patient eligibility
The data were collected prospectively from patients treated from February 2005 to August 2008 on a Phase 2, single arm trial investigating the combination of i.v. Bu and Mel. The study was approved by the institutional review board, and written informed consent was obtained from all patients according to institutional guidelines. Patients were eligible for this study if they were between 18 and 65 years of age with advanced lymphoid malignancies, specifically MM, HL, and NHL beyond first complete remission (CR1). Additional eligibility criteria included acceptable renal and hepatic function with creatinine of ≤1.5 mg% (clearance of ≥60 ml/min) and alanine aminotransferase ≤ 3 times the upper normal limit, a Zubrod performance status of 0 or 1, no evidence of uncontrolled infection, and negative serology for hepatitis B, C and HIV. Patients were required to have adequate cardiac function demonstrated by left ventricular ejection fraction > 40%, and good lung function demonstrated by forced expiratory volume in 1 second, forced vital capacity, and diffusing capacity of lung for CO2 corrected for hemoglobin of more than 50% of predicted. Patients with active CNS disease were excluded.
Restaging studies were obtained within 30 days before SCT, and subsequently at 1 month, 3 months, and 6 months following SCT, and then every 6 months for 3 years, and annually thereafter, as feasible. Staging studies for patients with lymphoma included CT and PET scans, and bone marrow biopsy when applicable; patients with myeloma had serum and urine electrophoresis, immunofixation studies, free light chains, bone marrow biopsy and bone survey.
Conditioning regimen
The transplant conditioning regimen consisted of i.v. Bu administered either as a fixed dose of 130 mg/m2 infused over 3 hours once daily for 4 days or to target an average daily AUC of 5,000 μM-min + 12%. In the latter scenario, the therapeutic dose was determined by a test dose of i.v. Bu administered at 32 mg/m2 and infused over 45 minutes approximately 48 hours before the first therapeutic Bu dose. The Bu administrations were followed by a rest day to allow for glutathione repletion, and Mel was administered at a fixed dose of 70 mg/m2 infused over 30 minutes once daily for 2 days. The autologous progenitor cells were infused on the following day.
Peripheral blood progenitor cell mobilization and collection
The methods of peripheral blood progenitor cell (PBPC) mobilization, collection, storage, and infusion have been described. [24] Patients received non-purged autologous PBPC collected after mobilization with filgrastim alone (n=41) or filgrastim plus chemotherapy (n=61) depending on the protocols for PBPC collection that were active at the time of study entry. The target progenitor cell dose was 4×106 CD34+ cells/kg with a minimal acceptable dose of 2×106 CD34+ cells/kg. Patients who failed to reach that target could undergo bone marrow harvest at the discretion of the treating physician. Bone marrow was obtained by multiple aspirations from the right and left iliac crest under general anesthesia, with a target total nucleated cell dose of 3×108 cells/kg. All products were cryopreserved using standard techniques.
Supportive care
Phenytoin 600 mg orally was used during and one day after completion of i.v. Bu therapy, starting the evening before the first dose [25]. Institutional transplant guidelines for antimicrobial, antifungal, and antiviral prophylaxis were followed. All patients received 5 microgram/kg filgrastim subcutaneously daily from day +1 until their absolute neutrophil count was greater than 0.5 × 109/L for 3 consecutive days. Packed red blood cells were administered to maintain hemoglobin levels ≥8 g/dl. Platelet transfusions were administered to keep platelet counts ≥10 × 109/L. All blood products were filtered and irradiated.
Busulfan Pharmacokinetic/therapeutic drug monitoring
A total of 10 blood samples were collected for Bu concentration determination during and up to about 14 hours following infusion of both the test dose and first therapeutic dose of Bu. The target daily AUC was 5000 +/- 12% μMol-min. If necessary, a second Bu dose adjustment was made following the first therapeutic dose analysis in efforts to keep the total course AUC at 20,000 umol-min. Blood for PK analyses was collected from a peripheral line to avoid sample contamination caused by the proximity between the different central venous catheter ports. Samples were collected in heparinized tubes and transported to the laboratory on wet ice. Plasma was separated and analyzed with high-pressure liquid chromatography after derivatization with diethyldithiocarbamate (DDTC) [25], or during the latter phase of the study, by a newer LC-MS technique, which significantly shortens processing- and sample-run times, but with retained sensitivity and accuracy [26] (Timothy Madden, personal communication). All concentration-time plasma busulfan data were analyzed using an open one-compartment PK model, using the ADAPT II software program, version 4.0 (Biomedical Simulation Resource, University of Southern California, Los Angeles, CA)[14, 27].
Definitions and clinical outcome variables
Standardized disease response criteria were used for disease staging and response to transplant in HL and NHL patients[28]. A CR was defined as the disappearance of all measurable lesions for >30 days. A residual PET negative mass in a previously PET positive patient was counted as CR. Partial remission (PR) was defined as a >50% decrease in measurable disease without the appearance of new lesions, stable disease (SD) was defined as a <50% decrease in measurable disease without the appearance of new lesions, and progressive disease (PD) was defined as a >25% increase in measurable disease or the appearance of a new lesion. For patients with MM, CR was defined as the disappearance of the monoclonal protein on immunofixation analysis. A PR was defined as a reduction of >50% in serum paraprotein levels and/or a reduction of >90% in urinary paraprotein levels for at least 6 weeks. For patients with non-secretory MM, a reduction of >50% in bone marrow plasma cells was considered indicative of a PR, while the observation of <5% bone marrow plasma cells was considered indicative of a CR. Disease progression was defined by an increase of >25% in serum or urinary paraprotein levels on at least 2 occasions, an increase in the number or size of osteolytic lesions, or the development of hypercalcemia[29]. For all disease types, patients who achieved at least a PR with salvage chemotherapy administered before transplantation were considered to have chemotherapy-sensitive disease and patients who had less than a PR were classified as chemotherapy-resistant.
Hematologic recovery was defined on the date which the patient had an absolute neutrophil count ≥0.5 109/L for 3 consecutive days. Platelet recovery was defined as occurring on the first of 7 consecutive days with a platelet count ≥20 109/L without transfusion support. Failure to engraft by day +30 was considered primary engraftment failure. Overall survival was defined as the time from the date of transplant until date of death from any cause, and patients still alive at last follow-up were considered censored. Progression-free survival (PFS) was defined as the date of transplantation until date of progression or death from any cause. Treatment-related mortality was defined as death from any cause other than disease progression or relapse. Toxicity was scored using the modified National Cancer Institute Common Toxicity Criteria version 3.0 (NCI, Bethesda, MD).
Adverse events and hematologic parameters were monitored daily and clinical chemistry parameters at least twice weekly during the initial hospitalization period. Subsequently, patients were followed up at least quarterly during the first year with physical examinations, blood counts, and CT and/or PET scans, bone marrow aspiration and biopsy as clinically indicated.
Statistical methods
The primary outcomes for this single-arm trial were safety and overall survival. Patients were categorized by disease type, with safety monitoring performed separately within each disease subtype based on accruing survival time data. Bayesian early stopping rules based on the observed rates of these 2 outcomes, as compared to historical data, were implemented separately for each disease subtype[30]. The Kaplan-Meier estimator [31] was used to assess OS and PFS probabilities in months. Descriptive statistics were used to summarize patient demographics. Three patients with multiple myeloma received a second transplant in stable disease that was not part of the originally planned treatment program; these 3 patients were censored at the time of their second SCT.
RESULTS
Patient and treatment characteristics
Patient demographics and baseline disease characteristics are listed in Tables 1 and 2. One hundred-two patients (49 HL, 12 NHL, 41 MM) with median age of 44 years (range 19–65) were evaluated on this study. The median number of prior treatment regimens was 3 (range 1-6), with two MM patients having had a prior auto-SCT. The majority of patients had advanced disease at time of transplant; only 13% (n=13) were in clinical remission, while 87% (n=89) had active disease. Among those transplanted with active disease, 21% were documented as chemotherapy-refractory. Eighty patients (78%) had PK-directed Bu dosing.
Table 1.
Patient characteristics at diagnosis
| Characteristic | No. Patients |
|---|---|
| Total patients | 102 |
| Sex, female/male | 38/64 |
| Disease histology at diagnosis | |
| Hodgkin's lymphoma | |
| Nodular sclerosis | 43 |
| Mixed cellularity | 4 |
| Lymphocyte deplete | 1 |
| Nodular lymphocyte predominant | 1 |
| Non-Hodgkin's lymphoma | |
| Diffuse large cell | 6 |
| Follicular, large cell | 1 |
| Follicular, mixed small and large cell | 3 |
| Anaplastic | 1 |
| Mantle cell | 1 |
| Multiple Myeloma | |
| IgG | 23 |
| IgA | 9 |
| IgM plasma cell leukemia | 1 |
| Non-secretory | 2 |
| Light chain only | 6 |
| Disease stage at diagnosis | |
| Hodgkins or Non-Hodgkin's lymphoma | |
| I | 2 |
| II | 21 |
| III | 18 |
| IV | 17 |
| Unknown | 3 |
| Multiple Myeloma | |
| Durie-Salmon I | 9 |
| Durie-Salmon II | 15 |
| Durie-Salmon III | 17 |
| LDH | |
| Elevated | 9 |
| Unknown | 66 |
| B2 microglobulin | |
| Elevated | 31 |
| Unknown | 57 |
| B symptoms in NHL, HL | |
| Present | 32 |
| Unkown | 7 |
| Bulky disease in NHL, HL | |
| Yes | 23 |
| Unknown | 24 |
Table 2.
Patient, graft characteristics at transplant, and hematopoietic recovery
| Characteristic | No. Patients |
|---|---|
| Total patients | 102 |
| Median age at transplant (range) | 44 (19-65) years |
| Hodgkin's and non-Hodgkin's | 61 |
| Median months to SCT (range) | 18 (8-140) |
| Median lines prior therapy (range) | 3 (1-6) |
| Disease status at transplant | |
| Complete remission 2 | 13 |
| Sensitive relapse | 36 |
| Refractory relapse | 12 |
| Multiple Myeloma | 41 |
| Median months to SCT (range) | 8 (3-116) |
| Median lines prior therapy (range)* | 2 (1-6) |
| Disease status at transplant | |
| Persistent disease, chemo-sensitive | 34 |
| Persistent disease, chemo-refractory | 7 |
| Stem Cell Source | |
| Bone marrow | 4 |
| Peripheral blood | 98 |
| Graft Composition, median (range) | |
| Total nucleated cells (×108/kg) | 6.5 (1.6-33.9) |
| CD34+ (×106/kg) | 5.2 (0.7-12.5) |
| Days to ANC >0.5 ×109/L, median (range) | 10 (8-44) |
| Days to Platelet >20 ×109/L, median (range) | 9 (7-141) |
2 prior autologous SCT
Graft content and engraftment
Stem cell graft characteristics and hematopoietic recovery data are summarized in Table 2. The source of stem cells was peripheral blood for the majority of patients. The median TNC count and CD34+ cell doses were 6.8 × 108/kg (range 1.56-33.9) and 5.26 × 106/kg (range 2.49-12.49), respectively. The median time to neutrophil and platelet recovery were 10 (range 8–13) and 9 days (range 7–31), respectively.
Four patients received bone marrow infusions after peripheral blood progenitor cell mobilization was unsuccessful. The TNC recovery from the marrow product was marginal in 3 of the 4 patients with median TNC count and CD34+ cell doses 1.9 × 108/kg (range 1.69-4.67) and 0.9 × 106/kg (range 0.72-1.21), respectively. All 4 patients had delayed engraftment, with an average 47 days to neutrophil recovery; only 1 patient recovered platelets at 141 days. Three patients were rescued with allogeneic SCT; one patient died with rapidly progressive disease while awaiting an allogeneic stem cell transplant.
Overall survival
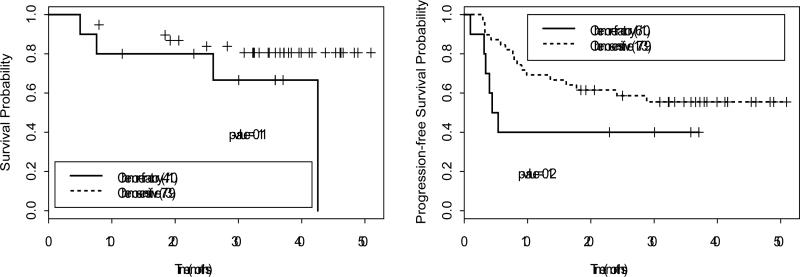
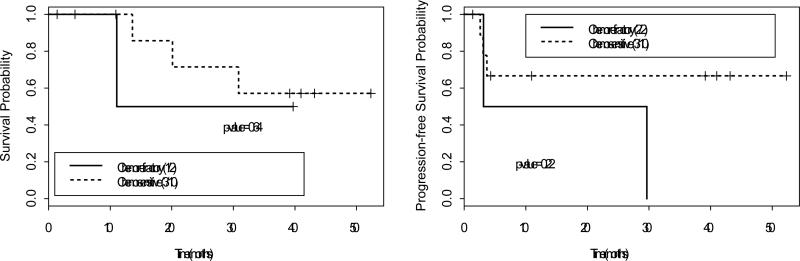
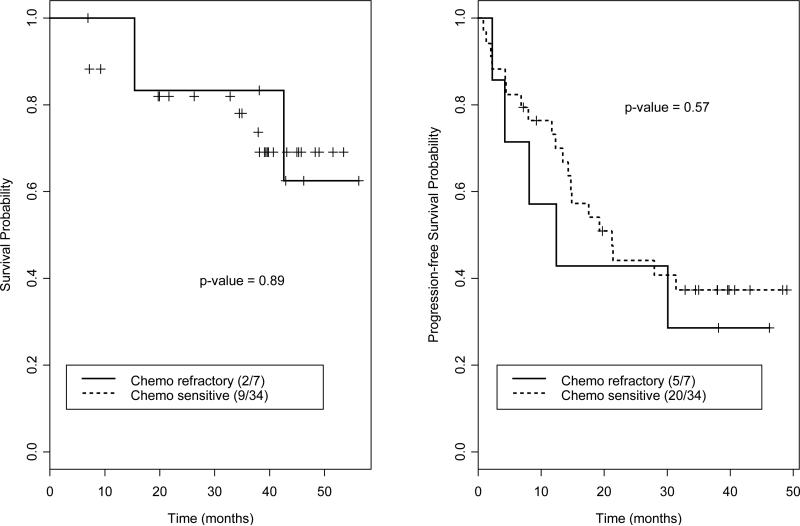
Among 49 patients with HL, 38 were alive at a median follow-up of 34 months (range 4-51) with 1- and 2-year OS rates of 90% and 85%, respectively (Figure 1). Among 12 patients with NHL, 8 were alive at a median follow-up of 35 months (range 4-53) with 1- and 2-year OS rates of 87% and 67%, respectively (Figure 2). Among 41 MM patients, 30 were alive at a median follow-up of 38 months (range 3-57), with 1- and 2-year OS rates of 91% and 82%, respectively (Figure 3). Sensitivity to chemotherapy did not significantly impact OS in any of the disease types (Figures 1-3).
Figure 1.
Overall survival and progression-free survival for patients with Hodgkin's lymphoma. Sensitivity to chemotherapy does not significantly impact outcome.
Figure 2.
Overall survival and progression-free survival for patients with Non-Hodgkin's lymphoma. Sensitivity to chemotherapy does not significantly impact outcome.
Figure 3.
Overall survival and progression-free survival for patients with multiple myeloma. Sensitivity to chemotherapy does not significantly impact outcome.
Response, relapse, and progression-free survival
Among 49 patients with HL, 34 achieved a complete response and 11 achieved a partial response for an overall response rate of 92%. Twenty-three patients relapsed, with 1- and 2-year PFS rates of 63% and 57%, respectively (Figure 1). Among 12 patients with NHL, 6 achieved a complete response and 3 achieved a partial response for an overall response rate of 75%. Five patients relapsed, with 1- and 2-year PFS rates stable at 64% (Figure 2). Among 41 MM patients, 7 achieved a CR and 16 achieved a PR for an overall response rate of 58%. Twenty-five patients relapsed, with 1- and 2-year PFS rates of 67% and 42%, respectively (Figure 3). Sensitivity to chemotherapy did not significantly impact PFS in any of the disease types (Figures 1-3).
Treatment toxicity
Regimen-related toxicities are detailed in Table 3. No grade IV toxicity was noted. The most commonly observed toxicities were grade I or II nausea and vomiting (77%), mucositis (67%), and diarrhea (55%). Grade I or II reversible elevation of liver function tests occurred in approximately 23% of patients; grade III liver enzyme toxicity was noted in 4% of patients. Only 1 case of mild-to-moderate VOD was ascertained using Jones' criteria[32]. This was a 42 year-old patient who had received 2 lines of chemotherapy (ABVD, ESHAP) for refractory Hodgkin's disease prior to transplant; VOD developed approximately one month after SCT and resolved with supportive measures. Significant CNS toxicity was not observed, but one patient who was unable to take his prophylactic phenytoin because of nausea and vomiting suffered a seizure. There were 3 cases of grade I pulmonary toxicity manifested as mild shortness of breath, and 6 cases of possible grade II pulmonary toxicity manifested as pulmonary infiltrates and shortness of breath without isolation of definite infectious causes; this symptomatology resolved with inhaled or oral steroid therapy; 5 of these cases were HL patients who had received bleomycin therapy, and additionally one of the HL patients had received therapeutic mediastinal radiation therapy, and one was a MM patient who had received a prior autologous SCT. The cumulative incidences of TRM at 100 days, 1 year, and 2 years were 1%, 3%, and 3%, respectively. There were a total 3 non-relapse deaths, all related to infection. One death resulted following complications from an allogeneic SCT which was required after a patient with NHL developed therapy-related myelodysplastic syndrome within 100 days following the autologous transplant.
Table 3.
Regimen-related toxicity in 102 patients
| Grade, n (%) |
||||
|---|---|---|---|---|
| Toxicity | Grade I | Grade II | Grade III | Grade IV |
| Liver | ||||
| Transaminase elevation | 6 (6) | 0 (0) | 1 (1) | 0 (0) |
| Bilirubin elevation | 6 (6) | 5 (5) | 2 (2) | 0 (0) |
| VOD* | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Alkaline phosphatase elevation | 6 (6) | 0 (0) | 1 (1) | 0 (0) |
| Gastrointestinal tract | ||||
| Diarrhea | 37 (36) | 19 (19) | 5 (5) | 0 (0) |
| Nausea and vomiting | 49 (48) | 30 (29) | 7 (7) | 0 (0) |
| Mucositis | 18 (18) | 50 (49) | 7 (7) | 0 (00 |
| Urinary tract/kidney | ||||
| Creatinine elevation | 2 (2) | 0 (0) | 0 (0) | 0 (0) |
| Hemorrhagic cystitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Skin | ||||
| Rash | 7 (7) | 2 (2) | 1 (1) | 0 (0) |
| Neurologic | ||||
| Seizures | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Pulmonary/pleural | ||||
| Shortness of breath | 1 (1) | 2 (2) | 0 (0) | 0 (0) |
| Pulmonary infiltrate | 2 (2) | 5 (5) | 0 (0) | 0 (0) |
| Cardiovascular | ||||
| Tachycardia | 1 (1) | 0 (0) | 3 (3) | 0 (0) |
VOD; veno-occlusive disease of the liver.
PK studies
Busulfan PK parameters were calculated from the data obtained from blood samples of 80 consenting patients. The drug is known to be cleared in less than 24 hours without drug accumulation observed over the 4-day dosing interval. Over 85% of patients had inter-dose variation of calculated clearance (ClT) estimates of less than 20% between the test dose and first therapeutic dose; with overall mean difference of calculated test-to-therapeutic ClT of 5.0% (± 12.7%) for all patients. The mean (CV%) population ClT, volume of distribution (Vd), and plasma half-life (t1/2) for once-daily dosing were 97.9 ml/min/m2 (15.7%), 22.9 L/m2 (14.0%), and 2.7 hours (15.0%) from the first therapeutic dose. The mean and median daily AUCs from the first therapeutic dose were 4869 and 4867 μMol-min, respectively.
DISCUSSION
The robust anti-tumor activity of Bu and Mel has been demonstrated in the autologous transplant setting in children and adults with advanced myeloid and lymphoid malignancies[17, 18, 33][34]. All these earlier studies used oral Bu, either in daily or divided doses; severe mucositis and VOD were the most significant toxicities reported. We hypothesized that i.v., PK-guided Bu administration that allows for more precise dose delivery with a tighter range in systemic drug exposure would result in decreased regimen-related toxicity, improved efficacy, and ultimately improved overall and progression-free survival.
The disease control noted with this regimen appears at least equivalent with reported results from similar alkylator-based combinations. The 2-year PFS rate of 40% noted in patients with chemotherapy-refractory HL compares favorably with previously reported results with both chemotherapy-only and TBI-based transplant regimens in similar high-risk populations[3, 4], albeit our findings are limited by the small sample size. Similarly, the 2 patients with chemotherapy-refractory NHL had a better PFS rate compared to historic results [5, 6], but a larger patient cohort is needed to evaluate disease control in the NHL population. We are continuing to explore this combination in a subsequent trial where we have added gemcitabine to the Bu-Mel combination, in an attempt to utilize the previous experience with synergistic enhancement of cytotoxic effects when combining nucleoside analogs and alkylating agents [22, 35-38]. Results from several other alkylating agent combinations in various hematologic malignancies suggest that the addition of a nucleoside analogue may be synergistic [9, 35, 37, 39, 40]. Thiotepa was combined with BuMel (BuMelTT) in patients with NHL treated with auto-SCT in a study reported by Zaucha et al [9]. Patients treated with BuMelTT had a significantly higher CR rate compared to patients treated with BEAM in a retrospective analysis (56% vs 31%, p=0.03). Of importance, oral busulfan was used in this regimen, with 4 cases of liver failure noted and a higher TRM rate compared to the BEAM group, and ultimately no difference in OS [9].
Similarly, the outcomes noted with the Bu-Mel combination in myeloma compare favorably with the standard Mel 200 mg/m2 transplant preparative regimen used in myeloma. In a study of a single transplant with Mel 200 conditioning in myeloma patients younger than 65 years, the CR rate was 44% and median survival 54.1 months.[41] We reported data from our center with Mel 200 mg/m2 for a single autologous transplant that showed an overall response rate of 69% and median PFS of 20 months[42]. Intensification of this transplant regimen has been attempted, with no additional benefit[42, 43]. However, in a recent report, 55 patients received i.v. Bu at 3.2 mg/kg daily ×3 and Mel 140mg/2 daily ×1 followed by autologous SCT; The high overall response rate of 87% (CR 20%) and 1-year PFS of 87% were impressive, although follow-up is short at a median of 15 months [44]. These data encouraged us to initiate a randomized study of Bu-Mel compared with Mel alone at 200mg/m2 to prospectively compare these SCT conditioning regimens in patients with myeloma.
In addition to good disease control, the 2-year TRM rate of 3% in this study compares favorably to rates reported for other myeloma and lymphoma studies. Only one case of reversible VOD was noted in a heavily pre-treated patient. Additionally, despite the significant CNS penetration of both Bu and Mel, a seizure was noted in one patient who was unable to tolerate the prophylactic oral phenytoin, underscoring the need for seizure prophylaxis with this regimen. There were no unexpected toxicities and prompt engraftment in all patients receiving peripheral blood stem cell grafts. Delayed count recovery and 1 case of graft failure was noted only in the 4 patients receiving bone marrow grafts after peripheral blood stem cell yield was inadequate, suggesting a fundamental weakness of the available progenitor cell products. There were 6 cases of (probable) pulmonary toxicity during the first 100 days following transplant in patients who had previously been heavily exposed to Bleomycin and/or chest XRT. All of them responded promptly to steroid therapy. No excess pulmonary toxicity was noted in earlier studies utilizing either oral or i.v. Bu with Mel. However, when oral Bu was combined with etoposide and cyclophosphamide, the 5-year incidence of pulmonary mortality was noted to be 3.6% occurring at a median of 5 months following transplant [45].
It may be likely that the use of i.v. Bu, which provides complete dose assurance and avoids the first-pass hepatic effect, which contributes significantly to the regimen's impressive safety profile. The assumed benefits from PK-directed Bu dosing were extrapolated from our previous observations of an optimal therapeutic interval for Bu when used in the i.v. BuCy2 regimen in patients receiving an allogeneic SCT for chronic myeloid leukemia [23]. In that report the risk of regimen-related toxicity, acute GVHD, and death were analyzed as functions of the per dose i.v. Bu AUC. Probabilities of developing gastrointestinal toxicity, hepatotoxicity, mucositis, and acute GVHD were significantly associated with elevated AUC. Most important, the risk of death was significantly associated with AUC, with risk significantly lower for patients receiving a per-dose AUC between 950 and 1520 μMol-min [23]. A similar trend was recently reported by Russell and coworkers, who observed increased toxicity when the average daily AUC exceeded 6,000 μMol-min in the 4-day Bu-Flu regimen[36]. Extrapolating from this data, the optimal daily Bu dose AUC would be within the window of 3800 to 6080 micromol-min. Thus, we targeted a (median) daily dose AUC of 5000 μMol-min for the present study. In a prior study, we demonstrated that a daily Bu dose of 130 mg/m2 would be expected to yield a median daily AUC of approximately 4,900 μMol-min [35]. Therefore, patients who refused PK-directed Bu dosing received the fixed daily Bu dose 130 mg/m2. The contribution of PK-directed dosing to better overall outcome in this study remains somewhat speculative, since the majority of patients received PK-directed dosing, making it difficult to discern any differences in those receiving PK-guided versus fixed dose Bu with respect to toxicity or disease control.
In conclusion, the results observed with the combination of i.v. Bu-Mel are encouraging in both myeloma and lymphoma patients. Larger phase II studies are warranted to further explore this combination. Additionally, i.v., PK-directed Bu administration provides a safe, controlled platform for introducing additional agents to the combination to further augment disease control.
Reference
- 1.Schmitz N, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. 2002;359(9323):2065–71. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 2.Philip T, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333(23):1540–5. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 3.Popat U, et al. Prognostic factors for disease progression after high-dose chemotherapy and autologous hematopoietic stem cell transplantation for recurrent or refractory Hodgkin's lymphoma. Bone Marrow Transplant. 2004;33(10):1015–23. doi: 10.1038/sj.bmt.1704483. [DOI] [PubMed] [Google Scholar]
- 4.Sureda A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005;16(4):625–33. doi: 10.1093/annonc/mdi119. [DOI] [PubMed] [Google Scholar]
- 5.Caballero MD, et al. High-dose therapy in diffuse large cell lymphoma: results and prognostic factors in 452 patients from the GEL-TAMO Spanish Cooperative Group. Ann Oncol. 2003;14(1):140–51. doi: 10.1093/annonc/mdg008. [DOI] [PubMed] [Google Scholar]
- 6.Mills W, et al. BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 1995;13(3):588–95. doi: 10.1200/JCO.1995.13.3.588. [DOI] [PubMed] [Google Scholar]
- 7.Jo JC, et al. BEAC or BEAM high-dose chemotherapy followed by autologous stem cell transplantation in non-Hodgkin's lymphoma patients: comparative analysis of efficacy and toxicity. Ann Hematol. 2008;87(1):43–8. doi: 10.1007/s00277-007-0360-0. [DOI] [PubMed] [Google Scholar]
- 8.Ulrickson M, et al. Busulfan and cyclophosphamide (Bu/Cy) as a preparative regimen for autologous stem cell transplantation in patients with non-Hodgkin lymphoma: a single-institution experience. Biol Blood Marrow Transplant. 2009;15(11):1447–54. doi: 10.1016/j.bbmt.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Zaucha R, et al. High-dose chemotherapy with BEAM or Busulphan/Melphalan and Thiotepa followed by hematopoietic cell transplantation in malignant lymphoma. Leuk Lymphoma. 2008;49(10):1899–906. doi: 10.1080/10428190802340184. [DOI] [PubMed] [Google Scholar]
- 10.Salar A, et al. Autologous stem cell transplantation for clinically aggressive non-Hodgkin's lymphoma: the role of preparative regimens. Bone Marrow Transplant. 2001;27(4):405–12. doi: 10.1038/sj.bmt.1702795. [DOI] [PubMed] [Google Scholar]
- 11.McDonald GB, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101(5):2043–8. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 12.Hassan M, et al. Busulfan bioavailability. Blood. 1994;84(7):2144–50. [PubMed] [Google Scholar]
- 13.Jones RB. Clinical pharmacology of melphalan and its implications for clinical resistance to anticancer agents. Cancer Treat Res. 2002;112:305–22. doi: 10.1007/978-1-4615-1173-1_15. [DOI] [PubMed] [Google Scholar]
- 14.Madden T, et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biol Blood Marrow Transplant. 2007;13(1):56–64. doi: 10.1016/j.bbmt.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Balis FM, Poplack DG. Central nervous system pharmacology of antileukemic drugs. Am J Pediatr Hematol Oncol. 1989;11(1):74–86. doi: 10.1097/00043426-198921000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Vey N, et al. A pilot study of busulfan and melphalan as preparatory regimen prior to allogeneic bone marrow transplantation in refractory or relapsed hematological malignancies. Bone Marrow Transplant. 1996;18(3):495–9. [PubMed] [Google Scholar]
- 17.Cony-Makhoul P, et al. Busulphan and melphalan prior to autologous transplantation for myeloid malignancies. Bone Marrow Transplant. 1995;16(1):69–70. [PubMed] [Google Scholar]
- 18.Lahuerta JJ, et al. Conditioning regimens in autologous stem cell transplantation for multiple myeloma: a comparative study of efficacy and toxicity from the Spanish Registry for Transplantation in Multiple Myeloma. Br J Haematol. 2000;109(1):138–47. doi: 10.1046/j.1365-2141.2000.01979.x. [DOI] [PubMed] [Google Scholar]
- 19.Tribalto M, et al. Autologous peripheral blood stem cell transplantation as first line treatment of multiple myeloma: an Italian Multicenter Study. Haematologica. 2000;85(1):52–8. [PubMed] [Google Scholar]
- 20.Carreras E, et al. Veno-occlusive disease of the liver after high-dose cytoreductive therapy with busulfan and melphalan for autologous blood stem cell transplantation in multiple myeloma patients. Biol Blood Marrow Transplant. 2007;13(12):1448–54. doi: 10.1016/j.bbmt.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Kashyap A, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8(9):493–500. doi: 10.1053/bbmt.2002.v8.pm12374454. [DOI] [PubMed] [Google Scholar]
- 22.Russell JA, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8(9):468–76. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 23.Andersson BS, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8(9):477–85. doi: 10.1053/bbmt.2002.v8.pm12374452. [DOI] [PubMed] [Google Scholar]
- 24.Hosing C, et al. High-dose rituximab does not negatively affect peripheral blood stem cell mobilization kinetics in patients with intermediate-grade non-Hodgkin's lymphoma. Leuk Lymphoma. 2006;47(7):1290–4. doi: 10.1080/10428190500468584. [DOI] [PubMed] [Google Scholar]
- 25.Andersson BS, et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant. 2000;6(5A):548–54. doi: 10.1016/s1083-8791(00)70064-4. [DOI] [PubMed] [Google Scholar]
- 26.Kazerooni R, Madden T, McAdams P, de Lima M, Jones RB, Kebriaei P, Champlin R, Andersson B. BMT Tandem Meetings. Tampa, Florida.: 2009. Development and comparison of limited sampling strategies for pharmacokinetically-guided high-dose, intravenous busulfan. [Google Scholar]
- 27.Schumitzky D.D.A.a.A. ADAPT II User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Los Angeles, CA.: 1997. [Google Scholar]
- 28.Cheson BD, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 29.Blade J, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102(5):1115–23. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 30.Thall PF, Wathen JK. Covariate-adjusted adaptive randomization in a sarcoma trial with multi-stage treatments. Stat Med. 2005;24(13):1947–64. doi: 10.1002/sim.2077. [DOI] [PubMed] [Google Scholar]
- 31.Meier K.a. Non-parametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53 [Google Scholar]
- 32.Jones RJ, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44(6):778–83. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Meloni G, et al. Ten-year follow-up of a single center prospective trial of unmanipulated peripheral blood stem cell autograft and interferon-alpha in early phase chronic myeloyd leukemia. Haematologica. 2001;86(6):596–601. [PubMed] [Google Scholar]
- 34.Grigg AP, et al. Phase II study of autologous stem cell transplant using busulfan-melphalan chemotherapy-only conditioning followed by interferon for relapsed poor prognosis follicular non-Hodgkin lymphoma. Leuk Lymphoma. 51(4):641–9. doi: 10.3109/10428191003611428. [DOI] [PubMed] [Google Scholar]
- 35.de Lima M, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104(3):857–64. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 36.Russell JA, et al. Transplantation from matched siblings using once-daily intravenous busulfan/fludarabine with thymoglobulin: a myeloablative regimen with low nonrelapse mortality in all but older patients with high-risk disease. Biol Blood Marrow Transplant. 2008;14(8):888–95. doi: 10.1016/j.bbmt.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002;41(2):93–103. doi: 10.2165/00003088-200241020-00002. [DOI] [PubMed] [Google Scholar]
- 38.Andersson BS, et al. Once daily i.v. busulfan and fludarabine (i.v. Bu-Flu) compares favorably with i.v. busulfan and cyclophosphamide (i.v. BuCy2) as pretransplant conditioning therapy in AML/MDS. Biol Blood Marrow Transplant. 2008;14(6):672–84. doi: 10.1016/j.bbmt.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(5):523–36. doi: 10.1016/j.bbmt.2008.12.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valdez BC, et al. 5-Aza-2′-deoxycytidine sensitizes busulfan-resistant myeloid leukemia cells by regulating expression of genes involved in cell cycle checkpoint and apoptosis. Leuk Res. 34(3):364–72. doi: 10.1016/j.leukres.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Child JA, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 42.Anagnostopoulos A, et al. Comparison of high-dose melphalan with a more intensive regimen of thiotepa, busulfan, and cyclophosphamide for patients with multiple myeloma. Cancer. 2004;100(12):2607–12. doi: 10.1002/cncr.20294. [DOI] [PubMed] [Google Scholar]
- 43.Comenzo RL, et al. Results of a phase I/II trial adding carmustine (300 mg/m2) to melphalan (200 mg/m2) in multiple myeloma patients undergoing autologous stem cell transplantation. Leukemia. 2006;20(2):345–9. doi: 10.1038/sj.leu.2404003. [DOI] [PubMed] [Google Scholar]
- 44.Blanes M, et al. Single daily dose of intravenous busulfan and melphalan as a conditioning regimen for patients with multiple myeloma undergoing autologous stem cell transplantation: a phase II trial. Leuk Lymphoma. 2009;50(2):216–22. doi: 10.1080/10428190802630170. [DOI] [PubMed] [Google Scholar]
- 45.Kalaycio M, et al. High-dose busulfan and the risk of pulmonary mortality after autologous stem cell transplant. Clin Transplant. 2006;20(6):783–7. doi: 10.1111/j.1399-0012.2006.00581.x. [DOI] [PubMed] [Google Scholar]