Abstract
Background
No curative therapy is available for Parkinson's disease; therefore, one of the main goals of treatment is to control motor symptoms, often via the use of levodopa (also known as L-dopa). However, prolonged levodopa treatment in Parkinson's disease has been associated with the development of motor fluctuations and the occurrence of levodopa-induced dyskinesias (LIDs).
Objective
To gain a clear, empirical understanding of the current real-world approach to treatment and patient outcomes associated with Parkinson's disease and LIDs.
Methods
This study used a mixed methodology, combining a cross-sectional survey of neurologists practicing in the United States, a retrospective chart review of patients with Parkinson's disease and LIDs, and cross-sectional surveys of health-related quality of life (QOL) and physical functioning in patients with Parkinson's disease. The surveys included the 39-item Parkinson's Disease Questionnaire, the Unified Parkinson's Disease Rating Scale, the Parkinson Disease Dyskinesia 26-item Scale, and the modified Abnormal Involuntary Movement Scale (mAIMS). Survey and chart data were collected between May 2010 and July 2011. Descriptive analyses were used to evaluate the distribution of study variables, treatment patterns, patient QOL, and patient physical functioning.
Results
Data from 7 neurologists and from 172 patients with Parkinson's disease and LIDs were collected. Results from the physician survey indicate that prescribing patterns depend largely on the severity of LIDs, assessed via mAIMS. Most patients (88%) received pharmacologic therapy as first-line treatment for LIDs, with monotherapy favored in patients with mild LIDs and combination therapy in patients with moderate-to-severe LIDs. The mean time from the diagnosis of LID to the administration of first-line treatment for the condition was 10.7 months (standard deviation, 14.0 months). The study population reflects a mean time from levodopa initiation to the onset of LIDs of slightly more than 5 years, regardless of the levodopa dosage. Results from the chart review and the physician survey suggest a strong alignment in severity classification among the assessment scales used.
Conclusion
These findings indicate that the diagnosis and the treatment of Parkinson's disease and LIDs are not optimal, because of the length of time from diagnosis to treatment, and because of the variability in treatment selection and response. Additional real-world studies are recommended to better understand treatment patterns, compliance with guidelines, and their potential impact on patient outcomes.
Parkinson's disease is a degenerative disorder that is characterized by muscle rigidity, tremors, and motor impairment that often results in progressive disability and severe complications that seriously affect a patient's health-related quality of life (QOL) and physical functioning. The worldwide prevalence rates for Parkinson's disease range from 0.5% to 1% among individuals aged 65 to 69 years, and from 1% to 3% among those aged ≥80 years.1 Parkinson's disease often develops after age 60,2 and is the second-most common neurodegenerative disorder among the elderly population.1
KEY POINTS
-
▸
Parkinson's disease is the second-most common neurodegenerative disorder in the elderly population, and the prevalence is greatest in those aged ≥80 years.
-
▸
Prolonged use of levodopa, the cornerstone treatment for Parkinson's disease, is associated with painful and disabling dyskinesias, which limit the ability to optimize treatment and reduce the patient's functional ability.
-
▸
ASTROID is the first study to quantify real-world data of treatment patterns and patient outcomes associated with levodopa-induced dyskinesias (LIDs).
-
▸
The prevalence of LIDs is underestimated; once established, LIDs are difficult to manage, and efforts should be focused on preventive measures rather than on reducing their severity.
-
▸
Overall, 56% of dyskinesias occur when levodopa levels are highest, suggesting that dosages may often be too high.
-
▸
Based on this study, the mean time from a LID diagnosis to treatment initiation exceeds 10 months, indicating a less-than-optimal approach to diagnosis and treatment of this condition.
Although no curative therapy is available for Parkinson's disease, one of the main goals of treatment is to control the motor symptoms of the disease.3,4 Levodopa (also known as L-dopa), which is widely considered the cornerstone of treatment for Parkinson's disease, is effective in reducing many symptoms associated with Parkinson's disease during the early stages of the disease, thereby offering patients an acceptable QOL and a reasonable functional ability with regard to the activities of daily living (ADLs).3 However, levodopa has been associated with several side effects, and prolonged levodopa treatment in Parkinson's disease has been associated over time with increased motor fluctuations and the development of levodopa-induced dyskinesias (LIDs).5 The effectiveness of levodopa treatment decreases with the progression of the disease, because of the persistent loss of nigrostriatal neurons (ie, dopamine-containing neurons located in the substantia nigra in the brain). Typically, the first sign of this loss is the gradual return of Parkinson's disease symptoms before the next dose of the medication is due, which is called “wearing off.” Wearing off generally necessitates increases in levodopa dosage and frequency.6
LIDs often present as chorea or choreoathetosis. “Chorea” refers to abnormal, involuntary, nonrepetitive movements that are characterized by brief, irregular contractions that appear to flow from one muscle to the next. The severity of these movements can vary from occasional abnormal movements that are absent at rest and provoked only during active movement (eg, walking, talking) to violent, large-amplitude flinging and flailing arm movements (ie, ballismus). Often, twisting or writhing athetoid movements (ie, choreoathetosis) are added onto these movements.7 LIDs usually first appear on the side of the patient that is most affected by Parkinson's disease, and generally present in the legs before the arms.7 Although dyskinesias may predominantly affect the legs and arms, they may spread to other body parts, such as the torso, head, and neck, or to the speech and respiratory muscles.7,8
The second-most common form of LIDs is dystonia, presenting as sustained muscle contractions. Dystonia can occur either alone or in combination with the chorea. When combined with chorea, the dystonia can manifest as twisting of the leg when walking or when the arm is being pulled behind the back. “On” and “off” phases are used to describe the presence of levodopa's benefit. Off-time dystonias, which occur when levodopa plasma levels are low, are usually quite painful and account for greater disability than chorea.7
Based on the relationship between LIDs and levodopa dosing, LIDs are classified as peak-dose, diphasic, “off-state,” “on-state,” or “yo-yo” dyskinesias (Table 1).7,9 Because some dyskinesias represent a response to the concentration of levodopa, such effects may be eliminated or decreased by the reduction of the levodopa dose. However, this dose reduction can be problematic when the reduced dose results in the recurrence of Parkinsonian symptoms. Because dyskinesia may recur with exposure to other dopamine agonists, the prevalence of LIDs may not be correctly diagnosed and, therefore, the rates of LIDs may be underestimated. The prevalence data for LIDs are limited.8
Table 1.
Types of Dyskinesias
| Type of dyskinesia | Characteristics |
|---|---|
| Peak-dose dyskinesias |
|
| Diphasic dyskinesias |
|
| “Off-state” dystonias |
|
| “On-state” dystonias |
|
| “Yo-yo” dyskinesias |
|
The incidence of LIDs appears to vary by the age at Parkinson's disease onset, the duration and progression of the disease, the levodopa dosage, and the duration of levodopa treatment.10 Earlier studies have reported prevalence rates of LIDs between 30% and 80% in patients with Parkinson's disease.11 Although the biologic mechanisms for the development of LIDs have not been established, it is clear that LIDs have a severe, negative impact on a patient's QOL and physical functioning.11
Consensus is lacking among experts regarding the optimal scale or instrument to be used to measure dyskinesias accurately and reliably. Given the intermittent nature of dyskinesias, these events may not be present during a clinical evaluation by the physician. Some patients may have difficulty in remembering or accurately reporting dyskinesia symptoms, especially when the symptoms are mild and intermittent. The assessment of dyskinesia, therefore, remains largely subjective and often inaccurate. There is an underlying need for more objective, easy-to-use, validated scales that can be applied by patients and physicians to accurately evaluate and report dyskinesias; such scales will improve clinical evaluation and aid physicians in prescribing the proper treatment. The Movement Disorder Society was the first organization to conduct a comprehensive, systematic review of the psychometric properties of the scales used to measure dyskinesia in Parkinson's disease, and this organization published its recommendations.12 Scales that have been recommended for clinician use in a population of patients with Parkinson's disease include the Abnormal Involuntary Movement Scale (AIMS)5,13 and the Unified Parkinson's Disease Rating Scale (UPDRS).5,12 Patient-rated scales include the 39-item Parkinson's Disease Questionnaire (PDQ-39)14,15 and the Parkinson Disease Dyskinesia 26-item Scale (PDYS-26)5 (Table 2).5,12–15
Table 2.
Outcomes and Patient-Rated Instruments
| Instrument name | Purpose | Scale | Score rangea |
|---|---|---|---|
| Clinician-rated instruments | |||
| mAIMS (modified Abnormal Involuntary Movement Scale)5,13 | Assess the severity of abnormal movements in 6 different areas of the body | 5-point scale, with ratings from 0–4 (absent, minimal, mild, moderate, severe), with higher scores indicating more severe abnormal movements |
0–24: Mild = 0–12 Moderate = 13–18 Severe = 19+ |
| UPDRS (Unified Parkinson's Disease Rating Scale)5,12 | Assess the severity of Parkinson's disease symptoms using a 5-point scale with ratings from 0 (normal) to 4 (severe), with higher scores indicating greater disability from Parkinson's disease | The UPDRS is made up of the following sections5,12:
These are evaluated by interview and clinical observation Some sections require multiple grades assigned to each extremity |
This study included only select questions from Part IV (Complications of therapy, questions 32 and 33) |
| Patient-rated instruments | |||
| PDYS-26 (Parkinson Disease Dyskinesia 26-item Scale)5 | Quantify the impact of dyskinesia on ADLs during the past week | 5-point scale, where 0 = not at all and 4 = activity impossible |
0–104: Mild = 0–26 Moderate = 27–52 Severe = 53+ |
| PDQ-39 (39-item Parkinson's Disease Questionnaire)14,15 | Measure health status, covering 8 aspects of quality of life | 5-point scale, where 0 = never and 4 = always |
0–156: Mild = 0–39 Moderate = 40–78 Severe = 79+ |
Mild, moderate, and severe ranges were determined by multiplying the number of questions by the score assigned to that severity (eg, in the PDYS-26, a moderate severity score = 2; therefore 2 * 26 = 52) to determine the maximum score for the moderate range.
ADLs indicates activities of daily living.
Once established, LIDs are difficult to manage, and therefore efforts should be made to prevent them. Preventive and therapeutic measures for LIDs include a variety of pharmacologic strategies and/or neuro-surgery; however, current medical therapies focus only on reducing the severity of dyskinesia.16 Ultimately, dyskinesias limit the ability to optimize the Parkinson's disease treatment regimen and have a negative impact on the patient's health-related QOL and functional ability with ADLs.16 An important unmet need for patients with Parkinson's disease includes the prevention of LIDs, as well as the early identification of LIDs and effective management that does not further complicate underlying Parkinson's disease management.
The purpose of the ASTROID (Assessment of Treatment Patterns and Patient Outcomes in Levodopa-Induced Dyskinesia) study was to provide an overview of current real-world treatment practices and patient-reported outcomes (PROs) for QOL and physical functioning in patients with Parkinson's disease and LIDs, using 3 objectives—quantify the medication use and treatment patterns in LIDs management; characterize the current levels of health status, QOL, and physical functioning; and identify patient characteristics by LIDs severity.
Methods
Study Design
This mixed-methodology study design included a cross-sectional survey of neurologists practicing in the United States, a retrospective chart review of patients with Parkinson's disease and LIDs from their respective neurologists, and a cross-sectional survey of these same patients' health-related QOL and physical functioning. Survey and chart data were collected between May 2010 and July 2011.
Physician and Patient Selection
The physicians recruited for this study represent a convenience sample based on their ability to serve as principal investigators and on their willingness to complete the necessary questionnaires, recruit patients, obtain patients' consent, complete the Institutional Review Board process, and supervise the conduct of the study in compliance with the protocol's requirements.
A third-party vendor sent e-mail invitations to neurologists with whom the vendor had established a previous relationship and faxed invitations to physicians at Parkinson's Disease and Movement Disorders Centers across the United States. The neurologists who expressed initial interest received a follow-up telephone call to discuss the project in more detail and to verify their willingness and ability to fulfill all participation requirements as outlined above.
The physician practices that were selected for the study provided broad US geographic coverage and varied in size, ranging from single-physician to multiple-physician practices. The physicians selected patients for the study in accordance with the screening criteria and the patient's willingness to participate in the study. All physicians completed the Institutional Review Board approval process, and the patients provided written informed consent per the study protocol and the Institutional Review Board requirements.
Study Protocol
The participating physicians completed a 13-item customized questionnaire that was developed by external experts and a study team with expertise in Parkinson's disease and LIDs. The 13-item questionnaire included questions that asked physicians to (1) quantify medication use and treatment pathways; (2) characterize current levels of health status, QOL, and physical functioning, such as ADLs; (3) estimate the prevalence of LIDs among their patients with Parkinson's disease; and (4) assess treatment timelines for patients with LIDs across various lines of therapy (ie, first, second, and third). The overall objective of this questionnaire was to provide a brief overview of physician-reported treatment practices and outcomes for patients with Parkinson's disease and LIDs.
The physician or study nurse at each center extracted patient data from charts; the data were de-identified and entered into an electronic data-collection tool. Questionnaires completed by the patients were sent to a third-party vendor for entry into the study database. All patient data were de-identified, and the patients' responses were verified to be within the range of possible values.
The study eligibility criteria included the following requirements: patients had to be aged between 50 and 90 years, be treated with levodopa and have expressed LIDs, have the ability to comply with procedures for cognitive and other testing, provide full written informed consent before the performance of any protocol-specified procedure, and have a caregiver or family informant if they were unable to care for themselves. The study was conducted after the review and approval by the Goodwyn Institutional Review Board (Cincinnati, OH) of all study documents, patient consent forms, and investigators' ability.
Variables of Interest
To capture clinical outcomes of interest from the physicians' perspective and the PROs of interest, several scales were used. A 33-item, multipart chart abstraction form included information on patients' age, sex, comorbidities, Parkinson's disease treatment history, LID severity and treatments, and drug interactions. A component of the physician-applied questionnaire asked the physicians to evaluate these patients with scales that included questions from Part IV of the UPDRS (Complications of Therapy, questions 32 and 33) and the modified Abnormal Involuntary Movement Scale (mAIMS).5,13 In addition, these same patients were asked to complete a survey that included PROs of interest using the PDYS-26 and the PDQ-39. Table 2 outlines the PROs and physician-reported scales used in this study.
To assess treatment patterns, the key data elements collected included medication dosage and frequency, treatment patterns (ie, first- and second-line treatment selection), the length of time between changes in therapy, and the time from a diagnosis of LIDs to first-line therapy initiation. Monotherapies were categorized as a dopamine agonist, a catechol-O-methyltransferase (COMT) inhibitor, a monoamine oxidase inhibitor, amantadine, or an atypical antipsychotic. Fixed combinations included carbidopa plus levodopa enteral infusion; carbidopa plus levodopa immediate-release; carbidopa plus levodopa controlled-release; and carbidopa with levodopa and entacapone.
Analytic Plan
Descriptive analyses were used to evaluate the distribution of all variables of interest. When appropriate, survey questions were stratified by the severity of LIDs (ie, mild, moderate, or severe) based on the mAIMS. The assessment of treatment patterns to characterize changes in medications over time and correlations between medication administration and disease progression were recorded.
Results
Physician-Reported Sample Characteristics
The physician survey included 7 neurologists who provided real-world information on treatment patterns and clinical outcomes of interest in patients with Parkinson's disease and LIDs. Each of the 7 physicians reported treating between 97 and 375 patients (mean, N = 189) with Parkinson's disease, for a total of 1322 patients.
Overall, the physicians estimated that 62% of the patients were being treated with a form of levodopa. Of the patients being treated with levodopa, the physicians estimated that 27.6% demonstrated symptoms of LIDs; however, of the total of 1322 patients with Parkinson's disease, these 7 physicians indicated that 856 (64.8%) patients had symptoms of LIDs based on the mAIMS, indicating an underestimation of the proportion of the population experiencing LIDs or the inclusion of choreas, but not dystonias, in these estimates. Based on the mAIMS, the physicians estimated symptom severity of LIDs as mild in 39% of patients, moderate in 38%, and severe in 23%. LIDs can be classified based on disease course and clinical phenomenology after a regular or an over-threshold dose of levodopa.11 Common categories are diphasic, off-state, and on-state (Table 1). The physician-reported occurrences of dyskinesia among patients included on-state dyskinesia in 56% of patients, diphasic dyskinesia in 26%, and off-state dyskinesia in 18%.
Physician-Reported Treatment and Prescribing Patterns
Among the 7 surveyed physicians, the preferred therapeutic strategy in patients with Parkinson's disease and LIDs was symptomatic treatment (N = 4; 57%), followed by restorative or neuroprotective treatment (N = 2; 29%), and other (“ideally, both”; N = 1; 14%). All 7 physicians reported that they considered increased disability associated with functioning as the most important disease aspect in assessing the progression of Parkinson's disease.
Among this group, 6 physicians believed that the duration of treatment with levodopa was the most important risk factor implicated in the onset of LIDs in patients with Parkinson's disease (1 physician indicated that “severity and duration” of Parkinson's disease symptoms was the most influential risk factor).
The physicians were divided on their preferred strategy to minimize the duration of the “off” times in patients with motor fluctuations, as well as their preferred strategy to maximize the duration of the “on” times in these patients. Specifically, the most frequently selected answers to minimize off times and maximize on times were the use of a controlled-release form (N = 2) and the addition of a COMT inhibitor (N = 2).
Monotherapy was favored as the first-line treatment for mild LIDs, and combination therapy was more frequently used with disease progression. Table 3 outlines physician-reported prescribing patterns by LID severity. Of note, not all of the physicians answered each of the survey questions completely, rendering some responses not evaluable.
Table 3.
Physician-Reported Prescribing Patterns, by LID Severity
| Disease severity | Physicians, N (N = 7) | Drugs, doses, and frequency |
|---|---|---|
| Mild LIDs, 32% (278 patients) | ||
| Monotherapy (includes fixed-dose combination medications) | 4 | Dopamine agonists: |
| Combination therapy | 1 |
|
| No medication | 1 | Stated did not understand the question |
| No answer | 1 | NA |
| Moderate LIDs, 37% (316 patients) | ||
| Monotherapy (includes fixed-dose combination medications) | 2 | Dopamine agonists:
|
| Combination therapy | 4 | Cited medicationsb:
|
| No medication | 1 | Stated did not understand the question |
| Severe LIDs, 31% (262 patients) | ||
| Monotherapy (includes fixed-dose combination medications) | 1 | Dopamine agonists: Pramipexole (Mirapex)a every day |
| Combination therapy | 5 | Cited medicationsb:
|
| No medication | 1 | Stated did not understand the question |
No dose was provided.
Doses and frequencies varied by respondent; in other instances, no dose or frequency was selected. Therefore, dose and frequency are not listed.
LIDs indicates levodopa-induced dyskinesias; NA, not applicable.
For mild LIDs, 5 of the 7 physicians indicated they would prescribe a medication for the management of mild LIDs, with 4 of these 5 indicating that they would choose monotherapy with a dopamine agonist, specifically, pramipexole (Mirapex) or ropinirole (Requip). For moderate LIDs, 4 of the 7 physicians indicated that they would prescribe combination therapy from among several medications, including carbidopa plus levodopa and entacapone (Stalevo 100); entacapone (Comtan); pramipexole; rasagiline mesylate (Azilect); ropinirole; generic amantadine; or branded amantadine (Symmetrel). In severe LIDs, 5 of the 7 physicians indicated that they would prescribe combination therapy from among several medications, including entacapone; carbidopa plus levodopa and entacapone; pramipexole; rasagiline mesylate; ropinirole; generic amantadine; or carbidopa plus levodopa (Sinemet).
Medical Chart Data Sample Characteristics
Medical chart data were collected from 172 patients (79 male, 93 female; age range, 50–90 years, with approximately 80% falling into the range of 61–80 years) between May 2010 and July 2011. Table 4 presents baseline characteristics from the chart data. The mean lowest initial total daily dose of levodopa (216 mg) was administered in the population with moderate LIDs; the mean time to LID onset was 4.8 years in this subgroup. Overall, the sample reflects a mean time from levodopa initiation to LID onset of slightly more than 5 years and a mean daily dose of levodopa of 241 mg.
Table 4.
Patient Characteristics, by LID Severity
| LID severity | ||||
|---|---|---|---|---|
| Characteristics | Mild, 39% (N = 67) | Moderate, 31% (N = 53) | Severe, 30% (N = 52) | Total, 100% (N = 172) |
| Sex, N (%) | ||||
| Male | 33 (49) | 23 (43) | 23 (44) | 79 (46) |
| Female | 34 (51) | 30 (57) | 29 (56) | 93 (54) |
| Age, N (%) | ||||
| ≤50 yrs | 2 (3) | 0 (0) | 0 (0) | 2 (1) |
| 51–60 yrs | 8 (12) | 10 (19) | 5 (10) | 23 (13) |
| 61–70 yrs | 43 (64) | 30 (57) | 24 (46) | 97 (56) |
| 71–80 yrs | 14 (21) | 9 (17) | 19 (37) | 42 (24) |
| 81–90 yrs | 0 (0) | 4 (8) | 4 (8) | 8 (5) |
| Mean time from Parkinson's disease diagnosisa to levodopa initiation, yrs | 0.9 | 1.7 | 1.7 | 1.4 |
| Mean total initial daily dose of levodopa, mgb | 250 | 216 | 255 | 241 |
| Mean time from levodopa initiationc to LID onset, yrsb | 4.8 | 4.8 | 6.4 | 5.3 |
| PDQ-39 sum score, mean (SD) | 43 (20) | 75 (14) | 92 (16) | 82 (20) |
| mAIMS, mean (SD) | 10 (10) | 13 (6) | 15 (6) | 14 (6) |
Mean date range of Parkinson's disease diagnosis: 1998–2001.
Two patients were removed from the analysis of the mild LIDs and the overall analysis because total daily doses of levodopa were recorded in error as 1 mg daily.
Mean date range of levodopa initiation: 1999–2002.
LID indicates levodopa-induced dyskinesia; mAIMS, modified Abnormal Involuntary Movement Scale; PDQ-39, 39-item Parkinson's Disease Questionnaire; SD, standard deviation.
Physician- and Patient-Reported Outcomes of Interest
In comparing total scores on the PDYS-26 with the mAIMS and PDQ-39, a strong alignment was seen in severity classification among these scales (eg, a patient who scored as “mild” on one scale was likely to score as “mild” on the other scales). The moderate and severe categories followed a similar pattern. Across all PDYS-26 severity categories, the physicians reported that dyskinesia was present between 26% and 50% of the waking day, according to the UPDRS. Of note, the physicians reported that the dyskinesias were moderately disabling in the patients who scored as “mild” on the PDYS-26, but only mildly disabling in the moderate and severe groups, according to the UPDRS.
Medical Chart Timeline of LID Diagnosis to Treatment Initiation, Progression
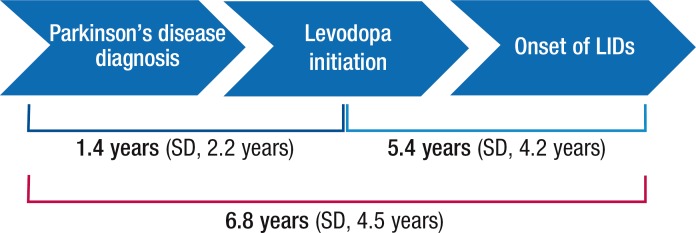
Figure 1 presents the timeline of progression to LIDs. The mean time from the diagnosis of Parkinson's disease to levodopa initiation was 1.4 years (standard deviation [SD], 2.2 years), and the mean time from levodopa initiation to the onset of LIDs was 5.4 years (SD, 4.2 years). The total mean time from the diagnosis of Parkinson's disease to the onset of LIDs was 6.8 years (SD, 4.5 years). The mean time from the diagnosis of LIDs to initiation of first-line treatment for LIDs was 10.7 months (SD, 14.0 months).
Figure 1. Timeline to Diagnosis of LIDs in Chart Review of 172 Patients with Parkinson's Disease.
LIDs indicates levodopa-induced dyskinesias; SD, standard deviation.
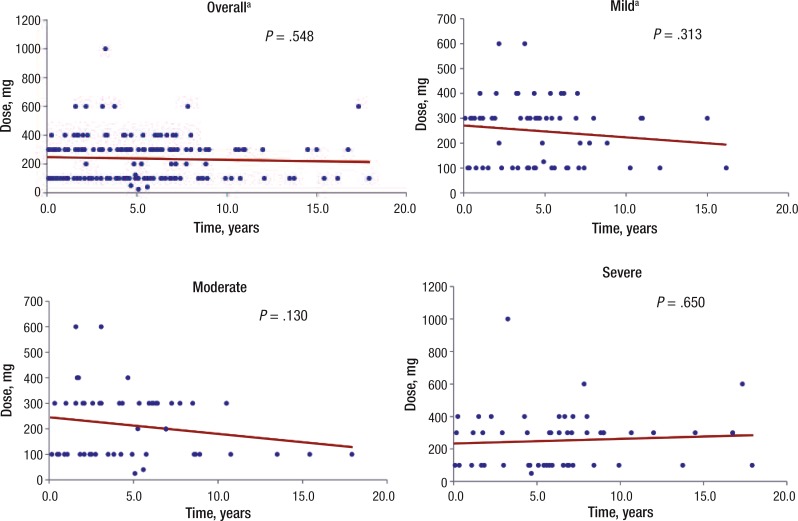
Linear regression models were used to determine whether there was an association between the average levodopa dosage and the time to the onset of LIDs. Four regression models were used—1 overall model that included all severity levels of LIDs and 3 models stratified by LID severity (ie, mild, moderate, and severe). Figure 2 displays results for the overall model, indicating that the relationship between the dosage and the time to the onset of LIDs is not significant (P = .548). The results for each model that was stratified by LIDs severity also indicate that the relationship between the dosage and the time to LID onset is not statistically significant (Figure 2).
Figure 2. Scatterplots of the Relationship between Levodopa Dose and LID Onset.
aTwo patients were removed from the “mild” and the “overall” analyses because total daily doses of levodopa were recorded in error as 1 mg daily.
LID indicates levodopa-induced dyskinesia.
Medical Chart Results for LID Treatment
Nearly all (84.3%) patients with LIDs were treated pharmacologically across first, second, and third lines of treatment, and 10.5% of patients with LID were treated with surgical intervention only. The first-line LID treatment selection was most influenced by the type of control and effects, particularly the ability to control worsening motor symptoms and ensure more stable levodopa plasma levels. Of 172 patients, 151 (approximately 88%) received pharmacologic therapy as first-line treatment for LIDs, with 17% receiving monotherapy; nearly 50% of the monotherapy consisted of dopamine agonists, and approximately 33% consisted of fixed-dose combination drugs. (Based on the study protocol, fixed-dose combinations were considered “monotherapy.”) In addition, 121 patients (approximately 70%) received combination therapy, with 49 of the 121 (approximately 40%) receiving ropinirole and rasagiline mesylate and a branded, or generic, carbidopa plus levodopa combination (25/100-mg dose). Of the approximately 11% of patients who received surgical intervention, 17 of 18 were categorized as having severe LIDs.
For the 27 patients (16%) who required a second-line treatment, a lack of efficacy was the most frequently cited (74%) reason for the change in treatment. Of these 27 patients, 21 (78%) received pharmacologic treatment, and 33% of these patients had another medication added. Nineteen percent received surgical intervention (4 of the 5 were classified as severe LIDs). Of the 27 patients, 9 (33%) progressed from a lesser severity category of LIDs by the time the second-line therapy started.
Of the patients requiring second-line treatment, 6 (22%) also required a third-line treatment. A lack of efficacy was the reason cited for the change in treatment in all cases, 100% of whom received pharmacologic therapy. Figure 3 presents the timeline of disease progression for Parkinson's disease diagnosis through third-line treatment for LIDs.
Figure 3. Timeline of Disease Progression: Parkinson's Disease Diagnosis Through Third-Line Treatment for LIDs.
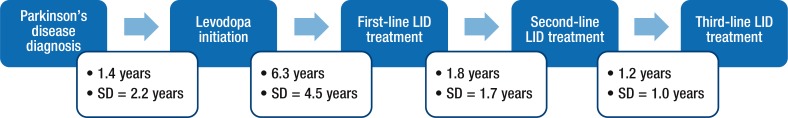
LIDs indicates levodopa-induced dyskinesias; SD, standard deviation.
Discussion
The purpose of the ASTROID study was to provide an overview of current real-world treatment practices and PROs for health-related QOL and physical functioning in patients with Parkinson's disease and LIDs. One of the main objectives was to quantify the medication use and treatment patterns in the management of LIDs. Results from the physician survey indicated that monotherapy was the preferred treatment for mild LIDs and that combination therapy was preferred for moderate and severe cases. However, there was no preferred strategy among physicians to minimize the duration of the off times or to maximize the duration of the on times in patients with motor fluctuations.
That the physicians reported the proportion of dyskinesias in the on-state phase to be 56% suggests that levodopa dosages are too high and require dose reduction to minimize dyskinesias. This may indicate a need for physician education regarding appropriate levodopa dosing. Alternatively, it may also indicate the need for patient and caregiver education about not self-medicating at a higher-than-prescribed dose to avoid complications of therapy.
In this study, patients with Parkinson's disease expressing symptoms of LIDs were prescribed a mean daily levodopa dose of only 241 mg. This dose is considerably lower than the dose used in the population in the DATATOP study, in which the average daily levodopa dose of 387 mg was found to produce symptoms of LIDs,17 raising a question about what levodopa dose patients are actually consuming.
The physician survey reports that the duration of levodopa treatment was selected as the most influential risk factor for the development of LIDs. The chart review findings were consistent with this result, demonstrating the onset of LIDs at approximately 5 years, regardless of dose and across all LID severity categories. Based on these findings, the implementation of screening for LIDs at regular intervals after the initiation of levodopa treatment would seem to be a logical approach to proactively identify and treat patients with LIDs. Of note, however, the mean time from the diagnosis of LIDs to first-line treatment for these events was 10.7 months (SD, 14.0 months), suggesting that there is a need to raise awareness of the importance of regular and early LID screening.
Treatment guidelines from the American Academy of Neurology recommend using entacapone and rasagiline to reduce off time (Level A evidence), and Level B evidence supports the use of pergolide, pramipexole, ropinirole, and tolcapone to reduce off time.18 However, none of the medications on the Level A evidence list of recommended drugs was named by the participating physicians as those used to treat patients with mild LIDs, although some of the medications listed by these physicians were supported by Level B evidence.18 This result may be driven by the cost of the medications, patients' insurance coverage, insurance company pharmacy management strategies, or patient or prescriber preferences. Further study of the reasons for such deviations from the guidelines is warranted.
A key objective of this study was to characterize the current levels of health status, QOL, and physical functioning, such as ADLs, in patients with Parkinson's disease and LIDs. Results from the chart review and physician survey suggest that there was a strong alignment in severity classification among the PRO scales used (ie, a patient who scored as “mild” on one scale was likely to score as “mild” on the other scales); however, there were some variations. For example, the physicians reported that the dyskinesias were moderately disabling in the patients who scored as “mild” on the PDYS-26, but only mildly disabling in the patients who scored as “moderate” or “severe” on the PDYS-26. Considering the wide variability of the disease state and the subjective nature of the assessment tools used, it is not surprising that the scales were not exactly aligned.
Another objective of the study was to identify patient characteristics and treatment selection by the severity of LIDs. The surveyed physicians estimated that the prevalence of LIDs in their practices was 28%; the true rate based on the mAIMS was 65%, indicating either an underestimation of the magnitude of the population with LIDs or estimates that did not take dystonias into account but rather were based on on-state choreas only. The physicians also underestimated the severity of LIDs in their patient populations, estimating that 23% of them had severe LIDs versus an actual rate of 31% based on the chart data. For first-line treatment for LIDs, 88% of patients received pharmacologic therapy, and most (70%) of them received combination therapy. Of the patients who received surgical treatment, 94% were categorized as having severe LIDs. A consideration for physicians will be to have patients complete a validated questionnaire or scale that measures their QOL and functional ability as part of the routine visit. Assessment at regular visits can provide the physician with a longitudinal record of patient response to treatment.
To our knowledge, this is the first study to analyze current real-world treatment practices and outcomes for patients with Parkinson's disease and LIDs. In addition, we used a mixed-methodology approach to assess how patients' QOL, health status, and physical functioning were affected by the severity of LIDs. The concordant results from the patient-reported QOL and ADL scales and the clinician-assessed scales support the use of these instruments in a population of patients with Parkinson's disease and LIDs.
Although the sample of 7 physicians is small, these physicians reported practice patterns based on their entire population of patients with Parkinson's disease (N = 1322) in addition to chart review data for 172 patients, making the results more robust. These 7 physicians use different approaches to minimize LIDs, and not all of the medications they prescribe are on the list of recommended medications from the American Academy of Neurology treatment guidelines. Perhaps these findings represent a need to improve compliance with recommended guidelines to optimize patient outcomes.
Limitations
Because of the retrospective, observational nature of this study, a true causal link cannot be made between any of the variables of interest and the outcomes, and study designs such as these are predisposed to selection bias.
Self-reported surveys are subject to recall bias and may not accurately reflect characteristics of the general population. In addition, not all patients were required to have a minimum number of years of data available in their record for inclusion in the study. As such, the results might have been influenced by the length of time a patient was associated with a provider.
Furthermore, not all physicians had the same number of patients, and some of the results may possibly be over- or underrepresented by a group of patients from a particular practice.
Finally, we surveyed a sample of US neurologists; therefore, we recognize that the results may not be generalizable to healthcare systems outside of the United States.
Conclusion
Our findings indicate that the diagnosis and the treatment of Parkinson's disease and LIDs are not optimal because of the length of time from diagnosis to treatment and the variability in treatment selection and response. Increased awareness and education for physicians to screen for LIDs and initiate treatment sooner are needed. Additional real-world studies are recommended to better understand the treatment patterns, patient adherence, compliance with guidelines, and impact on patient outcomes.
Acknowledgments
The authors would like to acknowledge Amit M. Shelat, DO, MPA, FACP, Attending Neurologist, Diplomate, American Board of Psychiatry & Neurology, for his assistance in editing this manuscript.
Biography

Barb Lennert
Study Funding
This study was funded by Novartis Pharmaceuticals Corporation.
Author Disclosure Statement
Ms Lennert and Ms Farrelly are employees of Xcenda, a consulting company that received funding from Novartis for this study; Dr Bibeau was an employee of Xcenda at the time of the study analysis and during preparation of the manuscript; Ms Sacco is a shareholder of Novartis; and Dr Schoor is an employee of Medimix International, a research company that received funding from Novartis for this study.
Contributor Information
Barb Lennert, Senior Director, Process Improvement, Xcenda, LLC, Palm Harbor, FL.
Wendy Bibeau, Former Health Economics & Outcomes Research Analyst, Xcenda, LLC, Palm Harbor, FL.
Eileen Farrelly, Associate Director of Data Analytics and Trends, Xcenda, LLC, Palm Harbor, FL.
Patricia Sacco, Director, Global Health Economics & Outcomes Research, Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Tessa Schoor, Chief Medical Officer, Medimix International, Miami, FL.
References
- 1.Nussbaum RL, Ellis CE. Alzheimer's disease and Parkinson's disease. N Engl J Med. 2003; 348: 1356–1364 [DOI] [PubMed] [Google Scholar]
- 2.Van Den Eeden SK, Tanner CM, Bernstein AL, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003; 157: 1015–1022 [DOI] [PubMed] [Google Scholar]
- 3.Parkinson's disease: hope through research. National Institute of Neurological Disorders website. www.ninds.nih.gov/disorders/parkinsons_disease/detail_parkinsons_disease.htm Accessed February 7, 2012.
- 4.Puente V, De Fabregues O, Oliveras C, et al. Eighteen-month study of continuous intraduodenal levodopa infusion in patients with advanced Parkinson's disease: impact on control of fluctuations and quality of life. Parkinsonism Relat Disord. 2010; 16: 218–221 [DOI] [PubMed] [Google Scholar]
- 5.Colosimo C, Martínez-Martín P, Fabbrini G, et al. Task force report on scales to assess dyskinesia in Parkinson's disease: critique and recommendations. Mov Disord. 2010; 25: 1131–1142 [DOI] [PubMed] [Google Scholar]
- 6.Aviles-Olmos I, Martinez-Fernandez R, Foltynie T. L-dopa-induced dyskinesias in Parkinson's disease. Euro Neurolog J. 2010; 2: 91–100 [Google Scholar]
- 7.Thanvi B, Lo N, Robinson T. Levodopa induced dyskinesia in Parkinson's disease: clinical features, pathogenesis, prevention and treatment. Postgrad Med J. 2007; 83: 384–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson's disease: a community-based study. Brain. 2000; 123: 2297–2305 [DOI] [PubMed] [Google Scholar]
- 9.Fahn S. The spectrum of levodopa-induced dyskinesias. Ann Neurol. 2000; 47: S2–S9, S9–S11. [PubMed] [Google Scholar]
- 10.Berg D, Godau J, Trenkwalder C, et al. AFQ056 treatment of levodopa-induced dyskinesias: results of 2 randomized controlled trials. Mov Disord. 2011; 26: 1243–1250 [DOI] [PubMed] [Google Scholar]
- 11.Fabbrini G, Brotchie JM, Grandas F, et al. Levodopa-induced dyskinesias. Mov Disord. 2007; 22: 1379–1389 [DOI] [PubMed] [Google Scholar]
- 12.Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003; 18: 738–750 [DOI] [PubMed] [Google Scholar]
- 13.Abnormal Involuntary Movement Scale (117-AIMS). In: Guy W, ed. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare; 1976: 534–537 DHEW publication (ADM) 76–338. [Google Scholar]
- 14.Martínez-Martín P, Jeukens-Visser M, Lyons KE, et al. Health-related quality-of-life scales in Parkinson's disease: critique and recommendations. Mov Disord. 2011; 26: 2371–2380 [DOI] [PubMed] [Google Scholar]
- 15.Hagell P, Nygren C. The 39-item Parkinson's disease questionnaire (PDQ-39) revisited: implications for evidence-based medicine. J Neurol Neurosurg Psychiatr. 2007; 78: 1191–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottwald MD, Aminoff MJ. Therapies for dopaminergic-induced dyskinesias in Parkinson disease. Ann Neurol. 2011; 69: 919–927 [DOI] [PubMed] [Google Scholar]
- 17.Parkinson Study Group. Impact of deprenyl and tocopherol treatment on Parkinson's disease in DATATOP patients requiring levodopa. Ann Neurol. 1996; 39: 37–45 [DOI] [PubMed] [Google Scholar]
- 18.Pahwa R, Factor SA, Lyons KE, et al. Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006; 66: 983–995 [DOI] [PubMed] [Google Scholar]