Abstract
Background
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability with cognitive and behavioral impairments, and is associated with a lifetime of care-taking challenges. There is a paucity of data on the economic burden of FXS.
Objective
To analyze the direct costs associated with healthcare and medication utilization for patients with FXS by using commercial and Medicare/Medicaid administrative claims data.
Methods
All-cause direct healthcare and prescription drug utilization were analyzed from the Thomson Reuters Healthcare MarketScan Commercial, the Medicare Supplemental, and the MarketScan Medicaid databases between 2004 and 2009. Inclusion criteria were ≥1 diagnosis of FXS (International Classification of Diseases, Ninth Revision, 759.83) and ≥12 months of continuous enrollment in the current health plan. Emergency department, hospitalization, outpatient visit, nonspecified procedures, and prescription drug data were analyzed for a 12-month follow-up period. Because the number of Medicare patients was <50, commercial and Medicare databases were combined into a single cohort. Descriptive statistics were used to summarize the results.
Results
A total of 1505 patients were included in the study; of these, 784 patients had commercial/Medicare insurance and 721 patients had Medicaid. The mean age was 18 years. In all age-groups, the median all-cause healthcare cost per patient was significantly lower in the commercial/Medicare cohort (range, $2222-$2955) than in the Medicaid cohort (range, $4548-$9702). The annual median costs per patient for those who had any medical procedures were $1614 and $3064 for commercial or Medicare and for Medicaid, respectively. The annual median costs per patient for those with at least 1 hospitalization was $7740 in the commercial/Medicare cohort (9.4% of patients) and $4468 in the Medicaid cohort (12.5% of patients).
Conclusion
This first descriptive US claims analysis supports the overall results from surveys on the economic burden related to FXS. The cost drivers in this population included medical procedures, hospitalizations in a subset of patients, and medications to a lesser extent. This information may be relevant to payers for benefit design and allocation of resources. A more targeted assessment of resource utilization is needed to estimate the value of interventions that reduce costs and improve the outcomes of patients with FXS.
Fragile X syndrome (FXS) is the most common form of inherited intellectual disability, with cognitive and behavioral impairments of varying degrees that are associated with distinct physical features.1 This neuro-developmental disorder is caused by the silencing of a single X-linked gene, the fragile X mental retardation 1 gene, and hence manifests primarily in males. The disorder has a prevalence of 1 in 3717 to 1 in 8918 for Caucasian males, and 1 in 8000 to 1 in 9000 females in the general population.2 In a 2003 literature review, Song and colleagues reported that FXS affected an estimated 1 in 4000 males and 1 in 8000 females.3 Higher prevalence rates have been observed among certain ethnic groups, such as African American males (1 in 2500) and Tunisian Jews.2,4
KEY POINTS
-
▸
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability, including impaired visual and working memory, sustained attention, and executive function; it also involves behavioral impairments and an increased rate of comorbidities.
-
▸
Many patients with FXS have difficulty with basic functional skills and need specialized therapy, such as speech and language therapy, occupational therapy, and physical therapy.
-
▸
This is the first analysis of the direct costs associated with healthcare and medication utilization in patients with FXS, using claims data from commercial/Medicare and Medicaid databases.
-
▸
The median all-cause healthcare cost per patient with FXS was significantly lower in the commercial/Medicare cohort ($2222-$2955) than in the Medicaid cohort ($4548-$9702).
-
▸
Tranquilizers, antipsychotics, antidepressants, and amphetamine-type stimulants were often prescribed for management of behavioral symptoms.
-
▸
The cost drivers for this patient population include medical procedures, hospitalization, and to a lesser extent, medications.
-
▸
These data suggest that FXS is a costly condition with considerable economic and psychosocial burden on patients and on the healthcare system as a whole.
No recent studies are available that provide direct estimates for the prevalence of the full mutation allele together with clinical symptoms in the general population. However, estimates of the prevalence of FXS based on screening studies in newborn boys are in agreement with extrapolations from populations with learning difficulties. Song and colleagues conducted a systematic review to compare population screening strategies in FXS and to estimate the costs associated with these strategies; prevalence data were reported, but they are now considered outdated.3
Cognitive and developmental deficits are often evident as early as age 3 years and are usually the manifestations that lead to a diagnosis of FXS. Males usually display mild-to-moderate intellectual disability, with an average intelligence quotient (IQ) of 40 to 50 in adults; females with FXS have an average IQ of approximately 80; only 25% of females with FXS are considered to have intellectual disability. Cognitive difficulties include impairments in visual memory, working memory, sustained attention, and executive function.
Typical behavioral symptoms of FXS include aggression, irritability, social avoidance, and autistic features. Approximately 30% of individuals with FXS also meet the criteria for autistic disorder.5 Moreover, a number of secondary conditions are more frequent in individuals with FXS than in the general population, including anxiety, hyperactivity, self-injurious behavior, and seizures.6
Although individuals with FXS face a lifetime of challenges, there is a paucity of data on the economic burden associated with FXS for caregivers and for society. One of the earliest reports, from a survey conducted in 1992 in the state of Colorado, estimated the average lifetime cost up to age 72 years for the care and support of an individual with FXS to be nearly $2 million in 1992 dollars.7 This translates to an annual out-of-pocket (OOP) medical cost to families—including hospitalization, therapy, medications, and physician visit costs—of $17,016 annually per child in 2002 dollars.7
Surveys offer a relatively rapid and cost-effective way to collect useful data in a standardized manner across a large population of interest. Based on a 2007–2008 national survey conducted in the United States, individuals with FXS are prescribed atypical antipsychotics, antidepressants, stimulants, and other miscellaneous centrally acting agents to manage maladaptive behaviors.8 Speech and hearing therapy, as well as occupational therapy are interventions that may also be used to improve communication and daily functioning skills in this patient population.9 However, surveys cannot replace large population studies because of methodologic limitations. Data in surveys are self-reported, and survey samples may not be representative of the general population of individuals with FXS.
Analyzing data from a variety of sources deepens our understanding of the unmet needs in a target population. There are no US Food and Drug Administration–approved medications indicated for the treatment of patients with FXS or the specific behavioral symptoms associated with this syndrome; therefore, new therapies will be challenged to demonstrate clinical and economic value to key healthcare stakeholders. The use of claims data to describe direct costs can therefore help to facilitate decisions on benefit design and on allocation of resources for services and treatments by health plan administrators and policymakers.
To update the information on the economic burden of FXS in the United States, we analyzed administrative claims data from a commercially insured population, with the goal of descriptively characterizing direct costs for healthcare resource utilization and medication utilization in patients diagnosed with FXS.
Methods
This study analyzed data from the Truven Health Analytics (formerly Thomson Reuters Healthcare at the time the data were licensed) MarketScan Commercial Claims and Encounters Database, the Medicare Supplemental and Coordination of Benefits Database, and the MarketScan Medicaid Database from January 1, 2004, through December 31, 2009. These 3 databases represented the inpatient and outpatient prescription drug claims of more than 33 million persons in the United States and provided detailed enrollment, cost, utilization, and dates of utilization for healthcare services. The inclusion of commercial, Medicare, and Medicaid databases were used to obtain an understanding of the utilization of healthcare resources and of prescription medication according to provider benefit plans.
The MarketScan Commercial Claims and Encounters Database provided data on healthcare services for individuals aged <65 years with coverage under a variety of fee-for-service (FFS), fully capitated, and partially capitated health plans. The Medicare Supplemental Database provided data on healthcare services for individuals with Medicare supplemental insurance paid for by employers. Both the Medicare-covered portion of payment and the employer-paid portion were included in this database, which contained predominantly FFS plan data. The Medicaid database contained the healthcare services for Medicaid enrollees from 10 states covered under a variety of FFS and managed care plans.
Because of the number of patients with FXS in the Medicare Supplemental Database was <50, the commercial and Medicare supplemental databases were combined, and hereafter referred to as commercial/Medicare. This approach was possible because deidentified patients were coded the same across the 2 databases, thereby avoiding double entries.
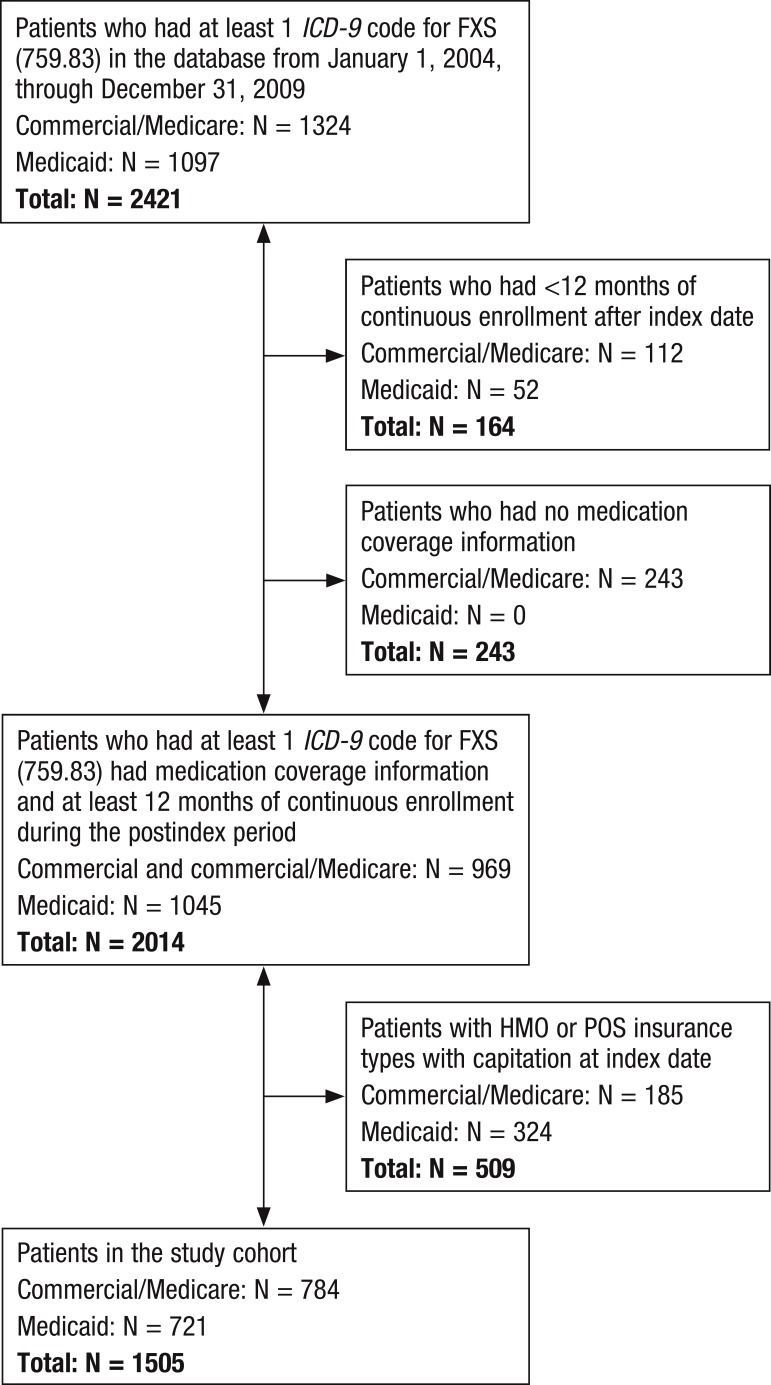
The study population included patients from inpatient and outpatient tables in commercial/Medicare and Medicaid databases who had at least 1 diagnosis of FXS (International Classification of Diseases, Ninth Revision [ICD-9] code 759.83) at any diagnosis position within the study period from January 1, 2004, through December 31, 2009. The first date within the study period, after which a patient had 12 months of continuous enrollment in the current health plan, was considered the index date for that patient. The follow-up period for each patient was 12 months after the index date. Patients who had coverage of HMOs or point of service (POS) insurance were excluded. The patient selection scheme for this analysis is described in Figure 1.
Figure 1. Patient Selection Scheme.
FXS indicates fragile X syndrome; ICD-9, International Classification of Diseases, Ninth Revision; POS, point of service.
Because the objective of this study was to characterize direct costs for healthcare resource utilization and medication utilization, patients for whom information on medication prescriptions was not available were excluded from the study. The commercial/Medicare database contained an indicator variable characterizing availability of information on medication use; commercial/Medicare patients were only included if information on medication use was indicated as available. The Medicaid database, however, does not contain this variable, so we assumed that all patients in the Medicaid database had information on medication use.
All-cause healthcare resource utilization was evaluated during the 1-year postindex date and included costs resulting from emergency department visits, hospitalizations, outpatient visits, and medical procedures. The rate and proportion of patients with at least 1 hospitalization or outpatient visit during the 12 months of follow-up were presented for analysis.
Nonspecified procedures were assessed to determine what types of procedures patients with FXS were most often receiving. Procedures were identified with Current Procedural Terminology codes. The top 20 procedures incurred after the index date during the 1-year follow-up period were presented for analysis.
Statistical Analysis
This study was descriptive in nature, and data are presented for commercial/Medicare and Medicaid databases separately. For continuous or count variables, the mean, median, standard deviation, minimum, maximum, and 95% confidence interval (CI) limits are reported. For categorical or binary variables, the frequency distribution was estimated together with 95% CIs for proportions. To test for an association between categorical variables, the chi-squared or Fisher's exact test (in case of expected cell counts <5) were performed. The Kruskal-Wallis test was used to evaluate whether costs have the same distribution for each group defined by a categorical variable.
Results
Demographics
A total of 1505 patients with FXS were included in the study population. Of these patients, 784 received care covered by commercial/Medicare insurance and 721 had Medicaid insurance coverage.
Table 1 lists patient demographic characteristics at the index date. The mean age in the commercial/Medicare and the Medicaid cohorts was 18 years. In both benefit plans, approximately 15% to 20% of patients were adolescents. The percentages of adults were similar in the commercial/Medicare and Medicaid cohorts (35.7% and 41.5%, respectively). In the commercial/Medicare cohort, 71.2% of patients were male compared with 80.7% in the Medicaid cohort.
Table 1.
Demographic Characteristics of Patients with Fragile X Syndrome
| Variable | Commercial/Medicare | Medicaid |
|---|---|---|
| Patients, N (%) | 784 (100) | 721 (100) |
| Mean age, yrs (± SD) | 17.5 (± 15.7) | 18.5 (± 15.7) |
| Age-group stratification, yrs | ||
| Children, 0–11, N (%) | 354 (45.2) | 302 (41.9) |
| Adolescents, 12–17, N (%) | 150 (19.1) | 120 (16.6) |
| Adults, ≥18, N (%) | 280 (35.7) | 299 (41.5) |
| Sex | ||
| Male, N (%) | 558 (71.2) | 582 (80.7) |
| Female, N (%) | 226 (28.8) | 139 (19.3) |
SD indicates standard deviation.
Comorbidities
The 6 most prevalent comorbid conditions in our study sample cohorts are listed in Table 2. In general, developmental disorders, otitis media, and symptoms concerning nutrition were among the most prevalent comorbid conditions in patients with FXS. Of the 6 conditions listed in Table 2, most were more common in the Medicaid cohort. Specific delays in development were reported twice as prevalent in the Medicaid population (27.9%) than in the commercial/Medicare population (12.5%), and pervasive developmental disorders were reported more frequently in Medicaid patients (13.5%) than in commercial/Medicare patients (11.0%).
Table 2.
Prevalence of the Most Common Comorbidities in Patients with Fragile X Syndrome
| Comorbiditiesa | Commercial/Medicare, N (%) | Medicaid, N (%) |
|---|---|---|
| Suppurative and unspecified otitis media | 151 (19.3) | 119 (16.5) |
| Specific delays in development | 98 (12.5) | 201 (27.9) |
| Hyperkinetic syndrome of childhood | 96 (12.2) | 151 (20.9) |
| Symptoms concerning nutrition, metabolism, and development | 88 (11.2) | 130 (18.0) |
| Nonsuppurative otitis media and eustachian tube disorders | 86 (11.0) | 72 (10.0) |
| Pervasive developmental disorders | 86 (11.0) | 97 (13.5) |
As identified by ICD-9 codes.
ICD-9 indicates International Classification of Diseases, Ninth Revision.
Healthcare Utilization and Costs
Table 3 shows all-cause healthcare utilization and costs per patient with FXS. All-cause healthcare utilization and the mean costs per patient for each age-group in the commercial/Medicare and the Medicaid cohorts were substantially higher than the corresponding median costs. Outliers were identified in the data, so median costs may be more representative of the findings; therefore, we report median costs for the results.
Table 3.
Healthcare Utilization and Costs Per Patient
| Parameter | Commercial/Medicare (N = 784) | Medicaid (N = 721) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Mean, $ | Median, $ | 95% CI | P value | N (%) | Mean, $ | Median, $ | 95% CI | P value | |
| Age, yrs | ||||||||||
| Children, 0–11 | 354 (45.2) | 7851.6 | 2955.0 | 5415.8–10,287.4 | <.049a | 302 (41.9) | 15,016.9 | 4547.8 | 9418.2–20,615.6 | <.001a |
| Adolescents, 12–17 | 150 (19.1) | 5667.9 | 2222.1 | 3015.2–8320.7 | 120 (16.6) | 10,490.6 | 4580.6 | 7974.4–13,006.8 | ||
| Adults, ≥18 | 280 (35.7) | 8752.2 | 2384.3 | 5649.0–11,855.4 | 299 (41.5) | 22,538.8 | 9701.7 | 19,158.0–25,919.5 | ||
| Sex | ||||||||||
| Male | 558 (71.2) | 7153.17 | 2619.2 | 5419.1–8887.2 | .584a | 582 (80.7) | 16,662.2 | 5973.3 | 14,142.0–19,182.5 | .135a |
| Female | 226 (28.8) | 9242.54 | 2356.1 | 5492.9–12,992.2 | 139 (19.3) | 20,400.3 | 5153.6 | 10,521.6–30,279.1 | ||
| Care received | ||||||||||
| No treatment intervention | 24 (3.1) | 95.5 | 0.0 | 0–255.2 | NA | 13 (1.8) | 0.0 | 0.0 | − | NA |
| Drug therapy only | 18 (2.3) | 664.9 | 246.2 | 175.8–1154.0 | 7 (1.0) | 1418.1 | 1577.9 | 115.8–2720.5 | ||
| Procedure only | 186 (23.7) | 4466.2 | 1614.2 | 3415.7–5516.7 | 85 (11.8) | 7068.1 | 3063.9 | 5118.0–9018.2 | ||
| Drug therapy and procedure | 556 (70.9) | 9416.0 | 3453.2 | 7148.0–11,684.1 | 616 (85.4) | 19,354.5 | 6641.7 | 16,143.0–22,566.0 | ||
| Inpatient and outpatient costs | ||||||||||
| Patients with hospitalizationa | 74 (9.4) | 21,676.7 | 7740.3 | 10,902.9–32,450.5 | NA | 90 (12.5) | 25,847.3 | 4467.7 | 11,363.0–40,331.6 | NA |
| Patients with outpatient visita | 747 (95.3) | 4642.8 | 1751.4 | 3717.3–5568.3 | 702 (97.4) | 12,608.3 | 3355.2 | 10,957.9–14,258.8 | ||
Kruskal-Wallis test.
CI indicates confidence interval; NA, not available.
The median costs in the commercial/Medicare cohort were consistently lower than for the Medicaid cohort. In the overall study population, costs per patient were 1.5 to 2 times lower in the commercial/Medicare cohort than in the Medicaid cohort; among adults, costs were almost 4-fold lower ($2384) in the commercial/Medicare cohort than in the Medicaid cohort ($9702).
The median healthcare cost for males with FXS receiving commercial/Medicare benefits was $2619 compared with $5973 for males with Medicaid benefit coverage for the same 12-month period. Only a small proportion of patients received no treatment—3.1% in the commercial/Medicare group and 1.8% in the Medicaid group—during the 12 months of follow-up. “No treatment” was defined as not receiving any medication or a procedure during the study period; no differentiation was made for procedures that were used for diagnostic purposes or for those potentially used for treatment. The majority of patients in both cohorts received a combination of medications and procedures—70.9% in commercial/Medicare and 85.4% in Medicaid.
The majority of patients with FXS received care in an outpatient environment. In the commercial/Medicare care cohort, 95.3% of patients had at least 1 outpatient visit during the 12-month follow-up period compared with 97.4% in the Medicaid cohort. In the commercial/Medicare cohort, 9.4% of patients had at least 1 hospitalization annually compared with 12.5% of patients in the Medicaid cohort. The mean all-cause healthcare utilization costs for a patient with at least 1 hospitalization was $21,677 (median, $7740) in the commercial/Medicare cohort and $25,847 (median, $4468) in the Medicaid cohort.
During the 12-month follow-up period, we reported the percentage of patients who only received a procedure as part of their care, without concomitant drug therapy. Almost 24% of commercial/Medicare patients received a procedure alone—with a median cost of $1614 per patient—compared with approximately 12% of Medicaid patients, with a median cost of $3064 per patient. The 20 most frequently utilized procedures are shown in Table 4.
Table 4.
The 20 Most Frequently Utilized Procedures in Each Cohort, Identified with CPT Code
| CPT Code/Commercial/Medicare Cohort (N = 784) | Patients, N (%) | CPT Code/Commercial/Medicare Cohort (N = 721) | Patients, N (%) |
|---|---|---|---|
| 104-Office visits, established patient | 681 (86.9) | 104-Office visits, established patient | 545 (75.6) |
| 115-Preventive care visits | 270 (34.4) | 190-Case management services | 342 (47.4) |
| 120-Outpatient consults | 269 (34.3) | 499-Unmapped codes | 251 (34.8) |
| 130-Injections: immunizations | 235 (30.0) | 331-Blood count, automated | 220 (30.5) |
| 101-Office visits, new patient | 223 (28.4) | 303-Laboratory tests, organ/disease panel | 208 (28.8) |
| 319-Other chemistry tests | 199 (25.4) | 111-Emergency department visits | 201 (27.9) |
| 031-Venipuncture (draw blood) | 194 (24.7) | 101-Office visits, new patient | 200 (27.7) |
| 369-Other microbiology tests | 178 (22.7) | 186-Physical medicine: other procedures | 196 (27.2) |
| 331-Blood count, automated | 177 (22.6) | 149-Speech/hearing therapy | 193 (26.8) |
| 306-Routine urinalysis | 158 (20.2) | 120-Outpatient consults | 190 (26.4) |
| 303-Laboratory tests, organ/disease panel | 157 (20.0) | 031-Venipuncture (draw blood) | 187 (25.9) |
| 111-Emergency department visits | 145 (18.5) | 319-Other chemistry tests | 171 (23.7) |
| 186-Physical medicine: other procedures | 116 (14.8) | 139-Therapeutic psychiatric services | 164 (22.7) |
| 372-Surgical pathology | 111 (14.2) | 306-Routine urinalysis | 137 (19.0) |
| 131-Injections: therapeutic/IV | 109 (13.9) | 130-Injections: immunizations | 132 (18.3) |
| 349-Immunology tests | 107 (13.6) | 198-Medical supplies and devices | 125 (17.3) |
| 470-Anesthesia services | 103 (13.1) | 115-Preventive care visits | 125 (17.3) |
| 198-Medical supplies and devices | 101 (12.9) | 131-Injections: therapeutic/IV | 122 (16.9) |
| 149-Speech/hearing therapy | 95 (12.1) | 369-Other microbiology tests | 111 (15.4) |
| 133-Other preventive medical services | 91 (11.6) | 150-Other ENT services (nonsurgical) | 109 (15.1) |
CPT indicates Current Procedural Terminology; ENT, ear, nose, throat; IV, intravenous.
Drug Utilization
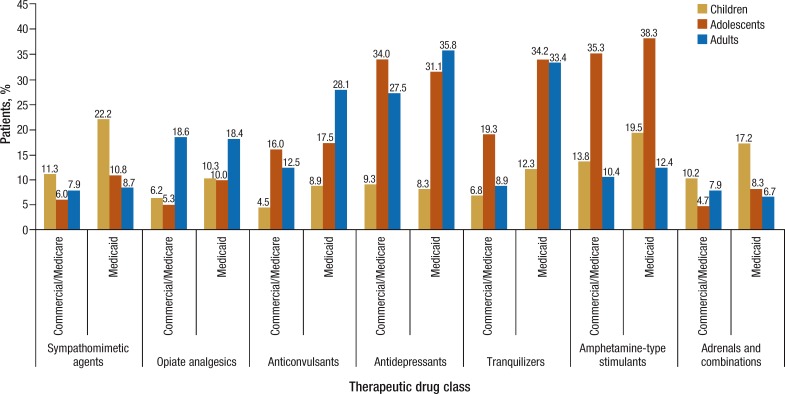
In both cohorts, the most frequently prescribed therapeutic drug classes were similar. Tranquilizers, antidepressants, amphetamine-type stimulants, and anticonvulsants were often prescribed, but, in general, were more frequent in the Medicaid population, as shown in Figure 2.
Figure 2. Most Frequently Prescribed Therapeutic Drug Classes for Patients with Fragile X Syndrome.
A detailed analysis of psychotropic medications prescribed to manage behavioral symptoms is shown in Table 5. In general, the utilization of specific medications is similar between the commercial/Medicare and Medicaid cohorts; however, the overall percentage of patients receiving risperidone in the commercial/Medicare population was 4.9% versus 14.0% in the Medicaid cohort. Within each cohort, adolescents had the highest percentage of utilization of risperidone. Similarly, clonidine was used in only 3.3% of the commercial/Medicare patients versus in 10.4% of Medicaid patients, with the highest utilization being found in adolescents. There were also substantially higher percentages of adolescents receiving sertraline, aripiprazole, and methylphenidate in both cohorts compared with the child and adult age-groups.
Table 5.
Percentage of Patients with Fragile X Syndrome Prescribed Psychotropic Medications
| Drug | Commercial/Medicare | Medicaid | ||||||
|---|---|---|---|---|---|---|---|---|
| Children, % (n = 354) | Adolescents, % (n = 150) | Adults, % (n = 280) | Total, % (N = 784) | Children, % (n = 302) | Adolescents, % (n = 120) | Adults, % (n = 299) | Total, % (N = 721) | |
| Antidepressants | ||||||||
| Sertraline hydrochloride | 3.7 | 10.0 | 6.8 | 6.0 | 2.0 | 10.0 | 9.0 | 6.2 |
| Fluoxetine hydrochloride | 2.0 | 6.7 | 3.6 | 3.4 | 2.3 | 5.9 | 5.0 | 4.0 |
| Escitalopram oxalate | 1.1 | 4.0 | 5.4 | 3.2 | 0.7 | 3.3 | 3.7 | 2.4 |
| Citalopram hydrobromide | 1.4 | 5.3 | 2.1 | 2.4 | 1.7 | 0.0 | 4.7 | 2.6 |
| Paroxetine hydrochloride | 0.6 | 4.0 | 2.9 | 2.0 | 2.0 | 2.5 | 6.0 | 3.7 |
| Bupropion hydrochloride | 0.6 | 0.0 | 4.6 | 1.9 | 0.0 | 3.3 | 2.3 | 1.5 |
| Venlafaxine hydrochloride | 0.0 | 1.3 | 4.3 | 1.8 | 0.0 | 1.7 | 2.7 | 1.4 |
| Trazodone hydrochloride | 0.0 | 2.0 | 2.5 | 1.3 | 0.3 | 4.2 | 3.3 | 2.2 |
| Antipsychotic agents | ||||||||
| Risperidone | 4.0 | 8.0 | 4.3 | 4.9 | 10.3 | 20.8 | 15.1 | 14.0 |
| Aripiprazole | 4.0 | 8.7 | 2.9 | 4.5 | 1.7 | 8.3 | 5.4 | 4.3 |
| Quetiapine fumarate | 0.9 | 4.0 | 2.9 | 2.2 | 0.7 | 6.7 | 7.7 | 4.6 |
| Stimulants | ||||||||
| Methylphenidate hydrochloride | 7.9 | 19.3 | 5.4 | 9.2 | 9.9 | 15.0 | 6.7 | 9.4 |
| Dexmethyl-phenidate hydrochloride | 3.7 | 2.0 | 1.1 | 2.4 | 3.3 | 1.7 | 0.3 | 1.8 |
| Lisdexamfetamine dimesylate | 1.1 | 2.7 | 0.0 | 1.0 | 0.0 | 1.7 | 0.0 | 0.3 |
| Miscellaneous | ||||||||
| Clonidine | 2.6 | 8.0 | 1.8 | 3.3 | 9.6 | 19.2 | 7.7 | 10.4 |
| Guanfacine | 2.6 | 6.7 | 1.4 | 2.9 | 3.0 | 1.7 | 1.0 | 1.9 |
| Minocycline | 0.0 | 2.7 | 2.9 | 1.5 | 0.7 | 0.0 | 1.0 | 0.7 |
| Lithium carbonate | 0.0 | 0.0 | 0.7 | 0.3 | 0.0 | 0.8 | 1.7 | 0.8 |
Discussion
To our knowledge, this is the first study in the United States to use administrative claims data to report direct healthcare costs and medication utilization in patients who are diagnosed with FXS. This is also the first study to report findings for healthcare costs for patients with FXS based on longitudinal data. Although FXS is an orphan disease, and the budget impact is presumably modest compared with many other diseases, the findings are relevant to payers who are tasked with allocating resources and with the redesigning of private health benefit plans and Medicaid programs to implement cost-saving initiatives.
A notable observation was the higher overall costs in Medicaid patients versus commercial/Medicare patients. Medicaid-insured patients received more prescribed drugs and incurred higher outpatient costs than patients in the commercial/Medicare cohort. These differences between benefit plans appear to be contrary to what has been reported from the national survey of parents, which found that the type of insurance (private or public) was not associated with reported financial burden on families.10 However, the survey did not look at actual healthcare costs, and the majority of the population was in the high-income bracket.
In a recent study by Bailey and colleagues, caregivers reported 9.2 hours daily of family caregiving for males with FXS and an additional 5.5 hours daily of paid help.11 Most families reported that FXS had at least some financial impact on the family, and caregivers had to take an average of 19.4 hours from work monthly to care for their child's needs.11 With these new emerging data on the indirect costs associated with caring for a child with FXS, it is likely that the overall economic burden is underestimated, independent of the socioeconomic status of the patient. Teasing out factors that drive economic disparities and differences between the Medicaid and commercial/Medicare cohorts will require further investigation.
The outliers that we observed in the data set reflect the fact that high expenditures tend to concentrate among a minority of families that have a member with FXS, a finding that was also reported by Ouyang and colleagues.10 Although the mean costs per patient in our analysis were similar to those reported in the 1992 survey,7,12 the median costs per patient in both cohorts were ≤50% of the corresponding mean costs in the study cohorts. The main cost drivers we identified were medical procedures and hospitalizations, which were distributed differently—procedures (nonspecified) drove costs in the majority of patients, whereas hospitalizations (all-cause) clustered in a subset of patients with FXS. In addition, overall we found that medications were a much weaker cost driver than procedures or hospitalizations.
One or more comorbidities were present in 10% to 30% of the patients, with higher percentages in the Medicaid cohort. We note that developmental disorders, otitis media, and nutrition-related symptoms were more prevalent in the Medicaid cohort than in the commercial/Medicare cohort.
Whether insurer selection leads to a bias toward healthier populations in the commercial/Medicare cohort cannot be determined from our data; there is limited scope for such selection, because eligibility for Medicaid is determined by economic circumstances and not primarily by insurers. Less favorable socioeconomic circumstances in patients who qualify for Medicaid benefits may adversely impact general health status; all explanations, however, remain speculative based on the current evidence.
Delays in development, otitis media, pervasive developmental disorders, and autism-like psychiatric presentation, particularly in males with FXS, are important causes of healthcare utilization.13 Autistic disorder is a strong predictor of outcomes for individuals with FXS, and the combination of pervasive developmental disorders and autistic disorders leads to more limited independence in adults, greater need for daily assistance, and less possibility to coreside with family.14,15
In the US national parent survey, problems with inattention-hyperactivity and affect were reported in a large majority of men (84.7% and 71.7%, respectively), and in >50% of women (65.5% and 58.8%, respectively).16 More than 33% of men had also been diagnosed with or treated for aggression (43.0%), self-injury (47.3%), and autism spectrum disorder (37.3%).16
In our FXS study sample, 11% to 13% of patients also had a diagnosis of pervasive developmental disorders, which suggests that there is substantial potential economic burden in this subpopulation. Furthermore, our sample showed a trend toward higher median costs in the men in both cohorts, but we did not conduct a comparison of costs between men and women. We did find that individuals with FXS were frequently prescribed psychotropic drugs, such as antidepressants, stimulants, tranquilizers, and antipsychotics, that are often used to treat disorders such as hyperactivity, anxiety, and inattention, similar to findings reported elsewhere.17
Many patients with FXS have difficulty achieving basic functional skills and need specialized therapy, such as speech and language therapy, occupational therapy, and physical therapy to address developmental challenges. Such expenditures may account for approximately 30% of the total employment impact and financial burden on families of children with FXS.10 A recent survey of families in the United States who had at least 1 child who was a carrier of FXS or who had the full mutation reported that 72% of males and 47% of females were receiving at least 1 type of therapy, most frequently speech and language therapy and occupational therapy, in males and females.9 In our study, 12.1% and 26.7% of commercial/Medicare and Medicaid patients, respectively, were receiving speech and language therapy during the 12-month follow-up period (data not shown). Although the numbers are not directly comparable between studies, the similarities highlight the relevance of speech and language therapy and occupational therapy in the medical care of individuals with FXS.
The strengths of this analysis are derived from the use of claims databases, which reduce responder bias, compared with surveys, and ensure a diagnosis of FXS for all included patients. We were able to confirm comorbidities and prescription drug utilization, which deepens our understanding of the potential unmet medical needs in individuals with FXS. Finally, the database captures patients who received inpatient services at a hospital and patients who received outpatient medical care, thus reflecting real-world healthcare resource utilization.
Limitations
Several limitations can be noted when interpreting our study results. Patients were included based on the specific ICD-9 code for FXS; therefore, the size of the population of interest may have been underestimated. For Medicaid patients, the long-term care database that potentially covers older patients with FXS was not included. However, we do not believe that excluding patients who are aged >65 years substantially biases the results, because the diagnostic test for FXS was only commercially available in 1999, and newborn screening is not mandated in the United States. There is no published epidemiologic study reporting the prevalence of patients with FXS who are aged >65 years that would further inform this assumption.
Also, patients with HMO or POS insurance coverage were excluded from the study, because FFS equivalents for HMO and POS insurance types are not reported in the database and could have biased the results.
Furthermore, it was assumed that all patients in the Medicaid database have information on prescriptions, which may result in an underestimation of results related to prescription drug use in these patients. In this initial assessment of medication utilization, we did not analyze the treatment patterns of medications used, because the objective was to take a global view of all of the medications used rather than preselecting treatment patterns.
We may have underestimated the overall economic burden, because only medical services and prescription drug costs reimbursed by the health plan were captured in the claims database; medical services that patients received, but that were not covered by the benefit plan in this database, may have been paid for privately, thereby incurring OOP expenses for families.
We did not assess indirect costs (eg, loss in work productivity) for caregivers or other paid assistance required by the family. Costs described in this study resulted from all causes and were not specifically related to FXS.
The data were not analyzed in relation to total patient expenditures or to costs in patients without FXS, which would be relevant for a payer to consider.
Given the sometimes large variations in costs, it will be useful for future analyses to include a more detailed view of the patients who may be considered outliers, both with very low and very high costs of care.
Finally, because these data are from a population with FXS in the United States alone, these results may not be generalizable to healthcare systems outside of the United States.
Conclusion
These results from administrative claims support and complement results from surveys on the economic burden related to the care of individuals who are diagnosed with FXS. In our study, we identified the procedures and hospitalizations clustered in a subset of patients as the main drivers of direct healthcare costs. Prescription drug utilization appeared to be less of a contributor to overall cost. It is the opinion of the authors that a more targeted assessment of resource utilization is needed to identify FXS-specific cost drivers to characterize the incremental direct and indirect costs associated with this intellectual disability. Such data will be critical to capturing the true value of future treatment interventions that reduce healthcare and social care resource utilization and improve patient outcomes.
Author Disclosure Statement
Ms Sacco is an Employee and Stockholder of Novartis Pharmaceuticals Corp; Dr Capkun-Niggli is an Employee of Novartis Pharma AG; Ms Zhang is an Employee of Beijing Novartis Pharma Co, Ltd; and Dr Jose is an Employee of Novartis Healthcare Private Limited. No additional financial compensation was provided to the authors for this study.
Contributor Information
Patricia Sacco, Director of Global Health Economics and Outcomes Research, Novartis Pharmaceuticals Corporation, East Hanover, NJ.
Gorana Capkun-Niggli, Director of Global Health Economics and Outcomes Research, Novartis Pharma AG, Basel, Switzerland.
Xin Zhang, Statistical Analyst, Beijing Novartis Pharma Co, Ltd, Shanghai, China.
Rosemary Jose, Evidence Analyst, Global Health Economics and Outcomes Research, Novartis Healthcare Private Limited, Hyderabad, India.
References
- 1.Hersh JH, Saul RA; Committee on Genetics. Health supervision for children with fragile X syndrome. Pediatrics. 2011; 127: 994–1006 [DOI] [PubMed] [Google Scholar]
- 2.Crawford DC, Acuña JM, Sherman SL. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 2001; 3: 359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song FJ, Barton P, Sleightholme V, et al. Screening for fragile X syndrome: a literature review and modelling study. Health Technol Assess. 2003; 7: 1–106 [DOI] [PubMed] [Google Scholar]
- 4.Falik-Zaccai TC, Shachak E, Yalon M, et al. Predisposition to the fragile X syndrome in Jews of Tunisian descent is due to the absence of AGG interruptions on a rare Mediterranean haplotype. Am J Hum Genet. 1997; 60: 103–112 [PMC free article] [PubMed] [Google Scholar]
- 5.Hagerman RJ, Berry-Kravis E, Kaufmann WE, et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009; 123: 378–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey DB, Jr, Skinner D, Sparkman KL. Discovering fragile X syndrome: family experiences and perceptions. Pediatrics. 2003; 111: 407–416 [DOI] [PubMed] [Google Scholar]
- 7.Lauria DP, Webb MJ, McKenzie P. The economic impact of the fragile X syndrome on the state of Colorado. International Fragile X Conference Proceedings. 1992: 393–405
- 8.Bailey DB, Jr, Raspa M, Bishop E, et al. Medication utilization for targeted symptoms in children and adults with fragile X syndrome: US survey. J Dev Behav Pediatr. 2012; 33: 62–69 [DOI] [PubMed] [Google Scholar]
- 9.Martin GE, Ausderau KK, Raspa M, et al. Therapy service use among individuals with fragile X syndrome: findings from a US parent survey. J Intellect Disabil Res. 2012 Sep 14.. [Epub ahead of print]. [DOI] [PubMed]
- 10.Ouyang L, Grosse S, Raspa M, Bailey D. Employment impact and financial burden for families of children with fragile X syndrome: findings from the National Fragile X Survey. J Intellect Disabil Res. 2010; 54: 918–928 [DOI] [PubMed] [Google Scholar]
- 11.Bailey DB, Jr, Raspa M, Bishop E, et al. Health and economic consequences of fragile X syndrome for caregivers. J Dev Behav Pediatr. 2012; 33: 705–712 [DOI] [PubMed] [Google Scholar]
- 12.Beckett L, Yu Q, Long AN. The impact of Fragile X: prevalence, numbers affected, and economic impact. A white paper prepared for the National Fragile X Foundation. September 2005
- 13.Tranfaglia MR. Fragile X syndrome: a psychiatric perspective. Results Probl Cell Differ. 2012; 54: 281–295 [DOI] [PubMed] [Google Scholar]
- 14.Bailey DB, Raspa M, Olmsted MG. Using a parent survey to advance knowledge about the nature and consequences of fragile X syndrome. Am J Intellect Dev Disabil. 2010; 115: 447–460 [DOI] [PubMed] [Google Scholar]
- 15.Bailey DB, Jr, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet A. 2008; 146A: 2060–2069 [DOI] [PubMed] [Google Scholar]
- 16.Hartley SL, Seltzer MM, Raspa M, et al. Exploring the adult life of men and women with fragile X syndrome: results from a national survey. Am J Intellect Dev Disabil. 2011; 116: 16–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKinney C, Renk K. Atypical antipsychotic medications in the management of disruptive behaviors in children: safety guidelines and recommendations. Clin Psychol Rev. 2011; 31: 465–471 [DOI] [PubMed] [Google Scholar]