Abstract
Background
Prostate cancer is the most common noncutaneous malignancy in men in the United States. Patients with metastatic castration-resistant prostate cancer (mCRPC) may be treated with secondary hormonal therapy or with chemotherapy, and potentially with concomitant corticosteroids. Corticosteroids can help manage the side effects of chemotherapy and secondary hormonal therapy and ameliorate prostate cancer–related symptoms, although corticosteroids are also associated with adverse effects. With an increasing number of available treatment options for mCRPC, evaluating the real-world concomitant use of corticosteroids in this patient population is important.
Objective
To evaluate the utilization patterns of corticosteroids for the treatment of patients with mCRPC based on real-world data from 2 large claim databases.
Methods
This retrospective analysis included medical and pharmacy claims from 2 large publicly available healthcare claims databases covering more than 31 million individuals to identify treatment patterns in adult patients with mCRPC. A total of 2593 patients with mCRPC were identified in data set 1 and 626 patients in data set 2 between 2005 and 2011. The appropriate treatment for castration-resistant prostate cancer (CRPC) was defined as chemotherapy, an antiandrogen, an adrenal androgen blocker, or estrogen. The index date was the date of the first CRPC treatment or the first metastasis diagnosis, whichever occurred later. The observation period spanned from the index date to the end of health insurance eligibility. Study end points included population characteristics, the distribution of mCRPC therapies, and corticosteroid utilization patterns.
Results
The study population came from the 2 data sets and included 3219 men who were treated for mCRPC. Bone and lymph nodes were the predominant metastatic sites. Bicalutamide was the most common secondary hormonal therapy, and docetaxel was the most common chemotherapy used for these patients. Overall, 73.4% of the patients in data set 1 received concomitant corticosteroids, as did 71.6% of patients in population 2 during the entire period from the index date to the end of eligibility date. In addition, 62.8% and 60.4% of patients, respectively, received concomitant corticosteroids during the secondary hormonal therapy period, and 93.8% and 95.1% of patients, respectively, received concomitant corticosteroids during the chemotherapy period. Similar patterns of corticosteroid use were observed across geographic areas of the United States.
Conclusion
This study shows consistently similar utilization patterns of corticosteroids in patients with mCRPC in 2 large national databases. Using real-world data to inform concomitant corticosteroid use in the treatment of patients with mCRPC may assist healthcare providers with treatment selection and with sequencing decision. Future research is warranted to investigate evolving treatment options for patients with mCRPC.
KEY POINTS
-
▸
Corticosteroids can help manage the side effects of chemotherapy or secondary hormonal therapy in patients with metastatic castration-resistant prostate cancer (mCRPC) and ameliorate prostate cancer-related symptoms.
-
▸
Real-world data on corticosteroid use and treatment patterns are infrequently reported in patients with mCRPC.
-
▸
Using real-world evidence from medical and pharmacy claims in 2 large claims databases covering >31 million lives, this study identified corticosteroid utilization patterns in patients with mCRPC.
-
▸
Results show that, in actual practice, the use of concomitant corticosteroid therapy in patients with mCRPC is consistent with the drug's labeling information across US regions.
-
▸
In this study, patients with mCRPC were exposed to corticosteroid therapy approximately 33% of the time between 2005 and 2011.
-
▸
Such real-world data may offer providers positive feedback regarding their treatment selection and sequencing decisions for this patient population.
Prostate cancer is the most frequently diagnosed noncutaneous malignancy in males and is a leading cause of cancer-related morbidity and mortality among men in the United States.1,2 The American Cancer Society has estimated that approximately 238,600 new cases of prostate cancer will be diagnosed in 2013, and that approximately 29,700 men will die of the disease.3
Many patients will be cured with a primary therapy of surgery, radiation therapy, and potential adjuvant hormonal therapy, but a subset of patients will experience disease progression with metastases spreading to other organs or to tissues in the body, such as to the bladder or bone. For such patients, androgen-deprivation therapy (ADT) is a widely accepted treatment option. Disease progression despite ADT and castrate levels of testosterone is considered castration-resistant prostate cancer (CRPC).4,5 Up to 85% of patients with CRPC are estimated to have metastatic CRPC (mCRPC) and may be considered for secondary hormonal therapy or for chemotherapy.6,7
Historically, treatment for mCRPC was predominantly palliative; however, recent pharmacologic advances have led to increased treatment options showing survival benefits in addition to pain reduction and improvements in quality of life.7,8 An early trial showed that monotherapy with prednisone may contribute to pain relief and a reduced need for analgesics in a subset of patients.9 In addition, several treatment regimens approved by the US Food and Drug Administration (FDA) for mCRPC involve corticosteroids.
Prednisone in combination with mitoxantrone has been associated with pain reduction and with improvement in quality of life in men with advanced, hormone-refractory prostate cancer, but not with improved survival.10 Treatment with docetaxel in combination with prednisone was the first chemotherapy regimen shown to increase survival among patients with mCRPC.10 Men receiving treatment every 3 weeks with docetaxel plus a daily dose of prednisone had a 24% lower probability of death (hazard ratio, 0.76) compared with men treated with mitoxantrone plus prednisone.10
More recent FDA-approved treatment regimens administered with concomitant corticosteroids include abiraterone acetate in combination with prednisone, an oral androgen biosynthesis inhibitor for the treatment of patients with mCRPC, and cabazitaxel in combination with prednisone, a cytotoxic regimen for patients with metastatic hormone-refractory prostate cancer whose disease progresses and who have previously received docetaxel.11,12
Other recently approved treatment regimens without specific corticosteroid requirements include sipuleucel-T, an autologous cellular immunotherapy for asymptomatic or minimally symptomatic patients with mCRPC, and enzalutamide, an androgen receptor inhibitor for patients with progressed mCRPC who have previously received treatment with docetaxel.13,14 Corticosteroids can help manage the side effects of chemotherapy and secondary hormonal therapy and can ameliorate prostate cancer–related symptoms, although they are also associated with adverse effects.
An increasing number of treatment options for mCRPC included in the National Comprehensive Cancer Network guidelines involve corticosteroids, but real-world data on corticosteroid use and treatment patterns have been reported infrequently in this patient population.15 The goal of the present study was to address this gap by evaluating the real-world corticosteroid treatment patterns for patients with mCRPC in the United States. A retrospective study design was used to describe the treatment patterns of patients with mCRPC.
Methods
Data Source
This retrospective study used data from the Truven Health Analytics MarketScan Research Databases (quarter 1 of 2005 through quarter 1 of 2011) and from the Optum Insight Clinformatics Data Mart database (quarter 1 of 2005 through quarter 2 of 2012). The Truven database (data set 1) includes data from 45 large employers, health plans, and government and public organizations covering more than 18 million lives through a commercial insurer or through Medicare. Enrollees in the database include employees, dependents, and retirees in the United States with primary or Medicare supplemental coverage through privately insured fee-for-service, point of service, or capitated health plans. All census regions are represented, although most enrollees are from the South and North Central (ie, Midwest) regions.
The Optum database (data set 2) contains claims from more than 13 million privately insured individuals covered by 69 self-insured Fortune 500 companies with locations in all census areas of the United States. Data are available for all company beneficiaries, including employees, spouses, dependents, and retirees.
Both databases contain information from integrated, individual-level claims of commercial health insurances, including medical claims for outpatient and inpatient services, pharmacy claims for prescription drugs, and eligibility information on demographics and health plan characteristics. The data were deidentified in compliance with the Health Insurance Portability and Accountability Act of 1996 to preserve patient confidentiality. Institutional Review Board approval and informed consent were not required for this study.
Study Design and Population Selection
Adult patients who were treated for mCRPC after ADT, defined as either having at least 1 dispensing of luteinizing hormone-releasing hormone (LHRH) or an orchiectomy, who had at least 1 diagnosis of a malignant neoplasm of the prostate (International Classification of Diseases, Ninth Revision [ICD-9], 185.xx) and at least 1 additional diagnosis of any history of prostate cancer (ICD-9, 185.xx or V10.46) within 1 year of at least 1 metastatic diagnosis (ICD-9, 196–199) were included in the study population.
Eligible treatments for CRPC included chemotherapy (eg, docetaxel, doxorubicin, estramustine phosphate, etoposide, mitoxantrone, cabazitaxel, paclitaxel, vinblastine, carboplatin, and vinorelbine); antiandrogens (eg, bicalutamide, flutamide, and nilutamide) unless initiated within 90 days of LHRH initiation; adrenal androgen blockers (eg, ketoconazole and aminoglutethimide); and estrogens (eg, diethylstilbestrol, conjugated estrogens, and other estrogens).
To ensure that metastases stemmed from prostate cancer, men with another cancer diagnosis (ICD-9, 140.xx-184.xx, 186.xx-209.xx) within 1 year of the first metastatic diagnosis were excluded from the sample. Continuous eligibility for health insurance was required for at least 1 year before the first metastatic diagnosis and at least 6 months before the first CRPC treatment. To obtain a sufficiently long observation period, patients who had an index date less than 3 months before the end of the availability of the data (ie, cutoff date) were excluded from the analysis.
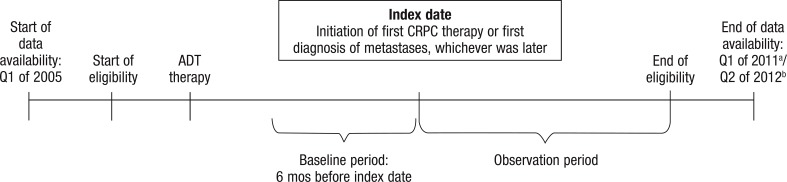
Figure 1 illustrates the study design. The index date was defined as either the initiation of the first CRPC therapy or the date of the first diagnosis of metastases, whichever occurred later. The 6 months preceding the index date constituted the baseline period. The observation period spanned from the index date until the end of the individual patient's health insurance eligibility or until the end of data availability.
Figure 1. Study Design.
aData availability for data set 1 (Truven Health Analytics MarketScan) ended in Q1 of 2011.
bData availability for data set 2 (Optum Insight Clinformatics Data Mart) ended in Q2 of 2012.
ADT indicates androgen-deprivation therapy; CRPC, castration-resistant prostate cancer; Q, quarter.
Study End Points
Population baseline characteristics
The demographic and clinical characteristics of the study population were evaluated during the baseline period. These characteristics included age, payer type, region, year of index date, clinical history (including score on the Quan-Charlson comorbidity index [CCI]16,17), sites of metastases at the first metastatic diagnosis, and length of observation period.
Evaluation of mCRPC therapies
The distribution of mCRPC therapies was evaluated to assess the treatment patterns of patients with mCRPC. The therapies were stratified into secondary hormonal therapies and chemotherapies. Secondary hormonal therapies included antiandrogens, excluding any antiandrogens initiated within 90 days of the initiation of LHRH therapy; adrenal androgen blockers; and estrogen. Relative frequencies for the first treatment of mCRPC and for all treatments during the observation period were calculated.
Evaluation of corticosteroid use
Because corticosteroid utilization may be different when accompanying chemotherapy or secondary hormonal therapy, the use of corticosteroids was evaluated over 3 study periods: the “overall mCRPC period,” the “secondary hormonal therapy period,” and the “chemotherapy period.” The overall mCRPC period was defined as the entire observation period that spans from index date to the end of eligibility or data availability.
The secondary hormonal therapy period was from the first date of treatment with secondary hormonal therapy to the initiation of chemotherapy or the end of eligibility or data availability, whichever occurred first.
The chemotherapy period started with the first date of chemotherapy and ended with the start of a secondary hormonal therapy treatment or the end of eligibility or data availability, whichever occurred earlier. If there were more than 1 secondary hormonal therapy or chemotherapy periods for a single patient, the periods were summed to obtain the total secondary hormonal therapy and chemotherapy periods.
To describe corticosteroid utilization patterns, the number of patients using corticosteroids (overall and stratified by region), the number of prescriptions per patient, the proportion of days covered, and the length of treatment exposure were reported for each of the periods. The proportion of days covered was calculated as the sum of nonoverlapping days of drug supply in a given period divided by the number of days in that given period. The number of patients receiving prednisone and dexamethasone (stratified by oral and injection administration forms) and the daily prednisone dose were also reported. The daily dose of pharmacy dispensings was obtained by multiplying the medication strength with the prescribed quantity and then dividing the result by the days of supply.
Statistical analysis
Univariate descriptive statistics included the mean ($pL standard deviation) and the median values for continuous variables and relative frequencies for categorical variables. All analyses were conducted using SAS Version 9.2 (SAS Institute; Cary, NC) or a newer version.
Results
Population Baseline Characteristics
Table 1 (page 311) shows the demographic and clinical baseline characteristics of the study population. The mean age was 73.3 years in population 1 and 68.4 years in population 2. The populations from the 2 databases differed substantially with respect to payer types; although most (73.2%) patients from data set 1 were enrolled in Medicare, almost all (99.5%) patients from data set 2 were covered by commercial insurance. In contrast, the distribution across regions was similar in both databases, with the Midwest and the South accounting for the majority of patients in both databases (38.1% and 32.7% in data set 1 and 31.6% and 42.3% in data set 2, respectively).
Table 1.
Patient Population Baseline Characteristics in the 2 Data Sets
| Characteristics | Data set 1 (N = 2593) | Data set 2 (N = 626) |
|---|---|---|
| Age, mean ± SD [median] | 73.3 ± 10.4 [75.0] | 68.4 ± 10.6 [66.0] |
| Age group, N (%) | ||
| <55 yrs | 102 (3.9) | 46 (7.3) |
| 55–64 yrs | 557 (21.5) | 238 (38.0) |
| 65–74 yrs | 607 (23.4) | 158 (25.2) |
| ≥75 yrs | 1327 (51.2) | 184 (29.4) |
| Payer type, N (%) | ||
| Medicare enrollees | 1898 (73.2) | 2 (0.3) |
| Commercial claims enrollees | 695 (26.8) | 623 (99.5) |
| HMO | 70 (2.7) | 63 (10.1) |
| PPO | 440 (17.0) | 75 (12.0) |
| POS | 84 (3.2) | 310 (49.5) |
| Indemnity | 0 (0.0) | 135 (21.6) |
| EPO | 0 (0.0) | 38 (6.1) |
| Other/unknown | 101 (3.9) | 2 (0.3) |
| Medicaid enrollees | 0 (0.0) | 1 (0.2) |
| Region, N (%) | ||
| Midwest | 988 (38.1) | 198 (31.6) |
| Northeast | 263 (10.1) | 40 (6.4) |
| South | 848 (32.7) | 265 (42.3) |
| West | 491 (18.9) | 123 (19.6) |
| Unknown | 3 (0.1) | 0 (0.0) |
| Year of index date, N (%) | ||
| 2005 | 369 (14.2) | 5 (0.8) |
| 2006 | 475 (18.3) | 93 (14.9) |
| 2007 | 565 (21.8) | 103 (16.5) |
| 2008 | 547 (21.1) | 110 (17.6) |
| 2009 | 504 (19.4) | 100 (16.0) |
| 2010 | 133 (5.1) | 89 (14.2) |
| 2011 | 0 (0.0) | 104 (16.6) |
| 2012 | 0 (0.0) | 22 (3.5) |
| Clinical history, N (%) | ||
| Charlson comorbidity index, mean ± SD [median] | 0.8 ± 1 [0.0] | 1.0 ± 1.4 [1.0] |
| Diabetes | 516 (19.9) | 150 (24.0) |
| Hypertension | 907 (35.0) | 336 (53.7) |
| Cardiovascular diseases | 892 (34.4) | 199 (31.8) |
| Seizures | 21 (0.8) | 10 (1.6) |
| Metastatic sites at first metastatic diagnosis, N (%) | ||
| Secondary and unspecified malignant neoplasm of lymph nodes | 240 (9.3) | 68 (10.9) |
| Secondary malignant neoplasm of respiratory and digestive systems | 167 (6.4) | 39 (6.2) |
| Secondary malignant neoplasm of other specified sites | 1955 (75.4) | 461 (73.6) |
| Kidney | 1 (0.0) | 0 (0.0) |
| Other urinary organs | 63 (2.4) | 15 (2.4) |
| Skin | 3 (0.1) | 0 (0.0) |
| Brain and spinal cord | 45 (1.7) | 5 (0.8) |
| Other parts of nervous system | 2 (0.1) | 0 (0.0) |
| Bone and bone marrow | 1630 (62.9) | 400 (63.9) |
| Adrenal gland | 3 (0.1) | 1 (0.2) |
| Other specified sites | 208 (8.0) | 40 (6.4) |
| Malignant neoplasm without specification of site | 231 (8.9) | 58 (9.3) |
| Observation period, mean days ± SD [median] | 493.3 ± 409.1 [403.0] | 478.0 ± 434.3 [347.0] |
EPO indicates exclusive provider organization; HMO, health maintenance organization; POS, point of service; PPO, preferred provider organization; SD, standard deviation.
The mean CCI score was 0.8 in population 1 and 1.0 in population 2. In terms of comorbidities, 19.9% and 24.0% of patients had diabetes in populations 1 and 2, respectively; 35.0% and 53.7%, had hypertension; and 34.4% and 31.8%, had cardiovascular diseases. In both populations, the most common metastatic sites were bone and bone marrow (62.9% in population 1, 63.9% in population 2). The mean observation period lasted 493.3 days in population 1 and 478.0 days in population 2.
Evaluation of mCRPC Therapies
Table 2 (page 312) shows the distribution of mCRPC therapies in the study populations. The type of first treatment at or after the index date was similar across the study populations, with 35.7% and 31.6% of patients in population 1 and population 2, respectively, undergoing chemotherapy, and 51.7% and 55.9%, respectively, receiving secondary hormonal therapy. Docetaxel was the most common chemotherapy used, accounting for 25.2% of the first treatment and 41.1% of all treatments in population 1, and for 20.3% of the first treatment and 37.5% of all treatments in population 2.
Table 2.
Distribution of Therapies for Metastatic Castration-Resistant Prostate Cancer
| Data set 1 (N = 2593) | Data set 2 (N = 626) | |||
|---|---|---|---|---|
| Therapy | First treatment, N (%) | All treatments, N (%) | First treatment, N (%) | All treatments, N (%) |
| Chemotherapy | 926 (35.7) | 1345 (51.9) | 198 (31.6) | 309 (49.4) |
| Docetaxel | 653 (25.2) | 1067 (41.1) | 127 (20.3) | 235 (37.5) |
| Carboplatin | 52 (2.0) | 146 (5.6) | 14 (2.2) | 41 (6.5) |
| Estramustine | 31 (1.2) | 107 (4.1) | 9 (1.4) | 24 (3.8) |
| Mitoxantrone | 28 (1.1) | 262 (10.1) | 7 (1.1) | 43 (6.9) |
| Cyclophosphamide | 23 (0.9) | 96 (3.7) | 6 (1.0) | 15 (2.4) |
| Secondary hormonal therapy | 1340 (51.7) | 1496 (57.7) | 350 (55.9) | 391 (62.5) |
| Antiandrogena | 1060 (40.9) | 1193 (46.0) | 288 (46.0) | 319 (51.0) |
| Bicalutamide | 961 (37.1) | 1076 (41.5) | 255 (40.7) | 282 (45.0) |
| Flutamide | 48 (1.9) | 88 (3.4) | 17 (2.7) | 25 (4.0) |
| Nilutamide | 51 (2.0) | 124 (4.8) | 16 (2.6) | 32 (5.1) |
| Adrenal androgen blocker | 261 (10.1) | 507 (19.6) | 60 (9.6) | 119 (19.0) |
| Ketoconazole | 259 (10.0) | 506 (19.5) | 60 (9.6) | 119 (19.0) |
| Aminoglutethimide | 2 (0.1) | 3 (0.1) | 0 (0.0) | 0 (0.0) |
| Estrogens | 19 (0.7) | 53 (2.0) | 2 (0.3) | 8 (1.3) |
| Diethylstilbestrol | 3 (0.1) | 13 (0.5) | 0 (0.0) | 0 (0.0) |
| Conjugated estrogens | 5 (0.2) | 10 (0.4) | 1 (0.2) | 4 (0.6) |
| Other estrogens | 11 (0.4) | 30 (1.2) | 1 (0.2) | 4 (0.6) |
| None of the above | 327 (12.6) | 327 (12.6) | 78 (12.5) | 78 (12.5) |
Antiandrogens initiated within 90 days before or after the initiation of luteinizing hormone-releasing hormone were not considered as a castration-resistant prostate cancer therapy.
Antiandrogens constituted the largest part of secondary hormonal therapies in both populations, with 40.9% of new therapies in data set 1 and 46.0% in data set 2. Bicalutamide was the predominant antiandrogen used, accounting for 37.1% and 40.7% of first treatment and 41.5% and 45.0% of all treatments in population 1 and population 2, respectively. Approximately 13% of the entire study population did not receive any treatment for CRPC after the mCRPC index date.
Evaluation of Corticosteroid Use
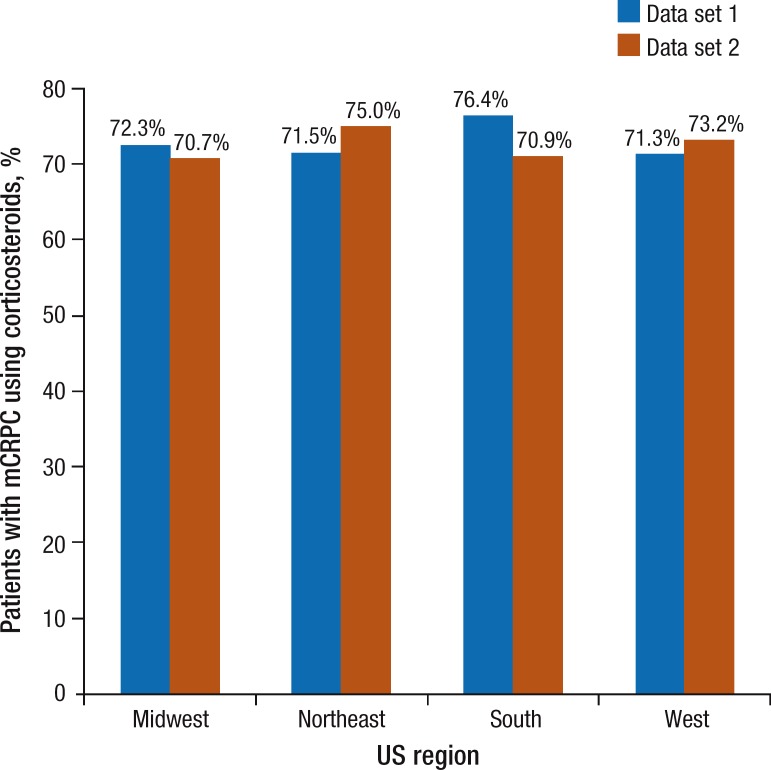
Table 3 (page 313) outlines the patterns of corticosteroid use. In population 1, 73.4% of patients had at least 1 prescription for corticosteroids during the overall period of mCRPC, 62.8% during secondary hormonal therapy, and 93.8% during chemotherapy. In population 2, 71.6% of patients received corticosteroids during the overall mCRPC period, 60.4% during secondary hormonal therapy, and 95.1% during chemotherapy. During the overall mCRPC period, the proportion of days covered was 35% in data set 1 and 38% in data set 2. The regional stratification of corticosteroid use during the overall period of mCRPC in Figure 2 (page 314) shows that the utilization patterns are very similar across all US regions.
Table 3.
Corticosteroid Use in Patients with mCRPC
| Data set 1 | Data set 2 | |||||
|---|---|---|---|---|---|---|
| Overall mCRPC period (N = 2593) | Secondary hormonal therapy period (N = 1496) | Chemotherapy period (N = 1345) | Overall mCRPC period (N = 626) | Secondary hormonal therapy period (N = 391) | Chemotherapy period (N = 309) | |
| Corticosteroid use | ||||||
| Patients, N (%) | 1903 (73.4) | 939 (62.8) | 1262 (93.8) | 448 (71.6) | 236 (60.4) | 294 (95.1) |
| Drugs dispensed, N | 12.5 ± 13.7 [8.0] | 5.0 ± 5.9 [3.0] | 14.3 ± 14.0 [10.0] | 12.0 ± 10.7 [10.0] | 6.0 ± 7.0 [3.0] | 12.7 ± 10.2 [10.0] |
| Proportion of days covered,a mean ± SD [median] | 35% ± 39 [25] | 36% ± 33 [26] | 37% ± 32 [29] | 38% ± 39 [28] | 39% ± 33 [32] | 41% ± 35 [29] |
| Exposure period, days,b mean ± SD [median] | 540 ± 408 [540] | 406 ± 354 [406] | 419 ± 353 [419] | 536 ± 441 [388] | 414 ± 417 [281] | 383 ± 370 [285] |
| Prednisone use | ||||||
| Patients, N (%) | 1129 (43.5) | 493 (33.0) | 780 (58.0) | 285 (45.5) | 148 (37.9) | 185 (59.9) |
| Daily dose, mg,c mean ± SD [median] | 14.7 ± 15.4 [10.4] | 16.9 ± 20.6 [10.1] | 12.8 ± 10.7 [10.3] | 13.8 ± 10.8 [10.3] | 15.0 ± 13.8 [10.1] | 12.3 ± 7.4 [10.2] |
| Dexamethasone use | ||||||
| Patients, N (%) | 1409 (54.3) | 412 (27.5) | 1180 (87.7) | 347 (55.4) | 110 (28.1) | 283 (91.6) |
| Dexamethasone oral, N (%) | 878 (33.9) | 347 (23.2) | 651 (48.4) | 214 (34.2) | 97 (24.8) | 149 (48.2) |
| Dexamethasone injection, N (%) | 1092 (42.1) | 97 (6.5) | 1039 (77.2) | 274 (43.8) | 25 (6.4) | 262 (84.8) |
The proportion of days covered was calculated as the sum of nonoverlapping days of supply in a given period divided by the number of days in that given period.
For the subset of patients with corticosteroid use, the exposure period was defined as the number of days of observation for each period.
The daily dose was obtained by multiplying the medication strength with the prescribed quantity and then dividing the result by days of supply.
mCRPC indicates metastatic castration-resistant prostate cancer.
Figure 2. Corticosteroid Use in Patients with mCRPC, by Geographic Region.
mCRPC indicates metastatic castration-resistant prostate cancer.
The use of prednisone also followed similar patterns in both populations, with 43.5% and 45.5% of patients receiving at least 1 prescription during the overall period of mCRPC, 33.0% and 37.9% during secondary hormonal therapy, and 58.0% and 59.9% during chemotherapy in populations 1 and 2, respectively. In population 1, the median daily prednisone dose was 10.4 mg during the overall period of mCRPC, 10.1 mg during secondary hormonal therapy, and 10.3 mg during chemotherapy. This corresponds to the numbers in population 2, where the median daily dose was 10.3 mg, 10.1 mg, and 10.2 mg in the mCRPC, the secondary hormonal therapy, and the chemotherapy periods, respectively.
Discussion
This observational analysis of data from 2 commercially available administrative claims databases shows that corticosteroid utilization was observed in an overwhelming majority of patients with mCRPC; the analysis of the 2 databases showed that 73.4% of patients with mCRPC in population 1 and 71.6% of patients with mCRPC in population 2 received corticosteroids overall and that 93.8% and 95.1% of patients received corticosteroids after chemotherapy initiation. Similarly, the analysis of the 2 populations showed that patterns of corticosteroid use were consistent across US geographic population subsets. To our knowledge, this study is the first to investigate corticosteroid treatment patterns in a population of patients with mCRPC using real-world data.
The findings of this study are consistent with the indications of FDA-approved therapies for patients with mCRPC: many of these regimens involve corticosteroids, including mitoxantrone plus prednisone, docetaxel plus prednisone, abiraterone plus prednisone, and cabazitaxel plus prednisone. Two agents recently approved by the FDA for mCRPC—enzalutamide and sipuleucel-T—do not include corticosteroids as part of the treatment regimen. However, a recent post-hoc analysis of the enzalutamide randomized controlled trial reported that 48% of patients had received corticosteroids during treatment with enzalutamide.18 To our knowledge, the concurrent use of immunosuppressive agents (including corticosteroids) with sipuleucel-T has not been evaluated. Because sipuleucel-T aims at stimulating the immune system, the concurrent use of immunosuppressive agents may alter its efficacy and safety profile.13
Reviews of clinical trial designs of investigational treatments for mCRPC have also reported the widespread use of corticosteroids in this patient population. MacVicar and Hussain reviewed investigational treatments and found that a majority of phase 3 randomized controlled trials of patients with mCRPC included corticosteroids in the interventional arm, the control arm, or both arms.19 The inclusion of corticosteroids in clinical trials of immunotherapy for patients with mCRPC differs across trials. Clinical trials of some investigational immunologic agents, such as intetumumab, have included corticosteroids in their design,20 whereas trials of other agents, such as ipilimumab, have not.21
Corticosteroids are prescribed concomitantly with chemotherapies and secondary hormonal therapies to manage the adverse effects of these therapies and to ameliorate the symptoms of the underlying malignancy. However, corticosteroids are themselves associated with potential adverse effects that may require monitoring.
Our study shows that, in the real world, corticosteroids have been widely used in this patient population, at a median daily dose of approximately 10 mg for prednisone, which is the FDA-approved dose for several treatment regimens involving prednisone. This supports the fact that real-world corticosteroid utilization is consistent with the labeling information for mCRPC treatment regimens.
We also found that patients with mCRPC were exposed to corticosteroids for approximately 33% of the entire observation period (proportion of days covered, 35%-38%), which may potentially attenuate the adverse events associated with long-term corticosteroid treatment. In the context of emerging treatment options that are indicated with or without concomitant use of corticosteroids, this information may assist healthcare providers and patients in their treatment selection and treatment-sequencing decisions.
Limitations
As with all analyses, some limitations must be taken into consideration. First, analyses based on claims data have inherent limitations, such as potential miscoding or incomplete coding of diagnoses.
Second, the study presented here was limited to treatment patterns of men with mCRPC in the United States. Additional findings may be reported for other global geographic regions.
Furthermore, because of data availability at the time the analyses were conducted, the study time period was limited to the period from quarter 1 of 2005 to quarter 1 of 2011 in the first database and from quarter 1 of 2005 to quarter 2 of 2012 in the second database. Therefore, it is possible that more recent corticosteroid treatment patterns will differ from those described in this article.
Conclusion
The findings in this study show that there is widespread use of corticosteroids in patients with mCRPC across 2 large national databases and provide new insight into the treatment patterns of this class of medications in patients with prostate cancer. The consistency of utilization patterns across US geographic areas shows that these results are not limited to a specific regional pattern. The finding about the real-world prevalence and treatment patterns of concomitant corticosteroid use in the treatment of patients with mCRPC confirms that, overall, corticosteroids are used in accordance with the FDA labeling indications. Future observational research on treatment patterns is warranted as emerging treatments become available for this patient population.
Study Funding
This study was funded by Janssen Scientific Affairs.
Author Disclosure Statement
Ms Lafeuille, Mr Gravel, Ms Grittner, and Mr Lefebvre are employees of Groupe d'Analyse Ltée; Dr Ellis and Dr McKenzie are employees of Janssen Scientific Affairs and stockholders of Johnson & Johnson.
Contributor Information
Marie-Hélène Lafeuille, Ms Lafeuille is Senior Economist, Groupe d'Analyse, Ltée, Montreal, Quebec, Canada.
Jonathan Gravel, Mr Gravel is Economist, Groupe d'Analyse, Ltée, Montreal, Quebec, Canada.
Amanda Grittner, Ms Grittner is Economist, Groupe d'Analyse, Ltée, Montreal, Quebec, Canada.
Patrick Lefebvre, Mr Lefebvre is Vice President, Groupe d'Analyse, Ltée, Montreal, Quebec, Canada.
Lorie Ellis, Dr Ellis is Associate Director, Janssen Scientific Affairs, Titusville, NJ..
R. Scott McKenzie, Dr McKenzie is Senior Director, Janssen Scientific Affairs, Titusville, NJ..
References
- 1.Kohli M, Tindall DJ. New developments in the medical management of prostate cancer. Mayo Clin Proc. 2010; 85: 77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sartor O, Michels RM, Massard C, de Bono JS. Novel therapeutic strategies for metastatic prostate cancer in the post-docetaxel setting. Oncologist. 2011; 16: 1487–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. What are the key statistics about prostate cancer? Revised May 15, 2013. www.cancer.org/cancer/prostatecancer/detailedguide/prostatecancer-key-statistics Accessed March 3, 2013.
- 4.Scher HI, Halabi S, Tannock I, et al; for the Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008; 26: 1148–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saad F, Hotte SJ; for the Canadian Urologic Oncology Group, and the Canadian Urological Association. Guidelines for the management of castrate-resistant prostate cancer. Can Urol Assoc J. 2010; 4: 380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011; 65: 1180–1192 [DOI] [PubMed] [Google Scholar]
- 7.Toren PJ, Gleave ME. Evolving landscape and novel treatments in metastatic castrate-resistant prostate cancer. Asian J Androl. 2013; 15: 342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seruga B, Tannock IF. Chemotherapy-based treatment for castration-resistant prostate cancer. J Clin Oncol. 2011; 29: 3686–3694 [DOI] [PubMed] [Google Scholar]
- 9.Tannock I, Gospodarowicz M, Meakin W, et al. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J Clin Oncol. 1989; 7: 590–597 [DOI] [PubMed] [Google Scholar]
- 10.Tannock IF, de Wit R, Berry WR, et al; for the TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004; 351: 1502–1512 [DOI] [PubMed] [Google Scholar]
- 11.Zytiga (abiraterone acetate) tablets [prescribing information]. Horsham, PA: Janssen Biotech, Inc; December 2012. [Google Scholar]
- 12.Jevtana (cabazitaxel) injection [prescribing information]. Bridgewater, NJ: sano-fi-aventis US LLC; 2013. [Google Scholar]
- 13.Provenge (sipuleucel-T) suspension [prescribing information]. Seattle, WA: Dendreon Corporation; June 2011. [Google Scholar]
- 14.Xtandi (enzalutamide) capsules [prescribing information]. Northbrook, IL: Astellas Pharma US, Inc; August 2012. [Google Scholar]
- 15.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline®): prostate cancer. Version 2.2013. March 11, 2013. www.nccn.org/professionals/physician_gls/pdf/prostate.pdf Accessed May 21, 2013.
- 16.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005; 43: 1130–1139 [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40: 373–383 [DOI] [PubMed] [Google Scholar]
- 18.Scher HI, Fizazi K, Saad F, et al. Impact of on-study corticosteroid use on efficacy and safety in the phase III AFFIRM study of enzalutamide (ENZA), an androgen receptor inhibitor. J Clin Oncol. 2013; 31 (6 suppl 6): Abstract 6. [Google Scholar]
- 19.MacVicar GR, Hussain MH. Emerging therapies in metastatic castration-sensitive and castration-resistant prostate cancer. Curr Opin Oncol. 2013; 25: 252–260 [DOI] [PubMed] [Google Scholar]
- 20.Heidenreich A, Rawal SK, Szkarlat K, et al. A randomized, double-blind, multicenter, phase 2 study of a human monoclonal antibody to human $aLv integrins (intetumumab) in combination with docetaxel and prednisone for the first-line treatment of patients with metastatic castration-resistant prostate cancer. Ann Oncol. 2013; 24: 329–336 [DOI] [PubMed] [Google Scholar]
- 21.Higano CS, Crawford ED. New and emerging agents for the treatment of castration-resistant prostate cancer. Urol Oncol. 2011; 29 (6 suppl): S1–S8 [DOI] [PubMed] [Google Scholar]