Abstract
Unstable carotid plaques are associated with an increased incidence of embolic complications after carotid artery stenting (CAS). Magnetic resonance (MR) imaging assessment of the unstable components of carotid plaque such as intraplaque hemorrhage and the lipid-rich necrotic core has a high sensitivity and specificity and is capable of volumetric analysis. This is a review of the current understanding of the association between MR carotid plaque imaging and silent ischemic lesions or ischemic complications after CAS.
Key Words: Magnetic resonance plaque imaging, Carotid artery stenting, Carotid plaque
Carotid Artery Stenting and Preoperative Carotid Plaque Imaging
Carotid artery stenting (CAS) has recently emerged as a potential alternative to carotid endarterectomy (CEA) because it is less invasive and requires a shorter hospital stay [1]. A randomized controlled trial, SAPPHIRE, showed that CAS and CEA had similar efficacy in patients at high risk for CEA [2]. Moreover, another recent randomized trial, CREST, showed that the risk of the composite primary outcome of stroke, myocardial infarction, or death did not differ significantly between CAS and CEA in patients at any risk for CEA [3]. Although several advantages of CAS have been reported, one of its disadvantages is a high incidence of distal emboli, which can be detected by diffusion-weighted MRI (DWI-MRI) regardless of the patient's symptoms. It has recently been reported that plaque instability, e.g. intraplaque hemorrhage (IPH) and a lipid-rich necrotic core (LRNC), is associated with an increased number of emboli after CAS; therefore, preoperative carotid plaque imaging is essential [4,5,6,7].
Magnetic Resonance Carotid Plaque Imaging
Preoperative imaging for carotid plaque morphology can be performed by B-mode ultrasound (US), computed tomography (CT), conventional angiography (MRA), intravascular ultrasound (IVUS), and MRI. Each of these modalities has strengths and limitations. B-mode US is usually the first in line in preoperative imaging. It is readily available, inexpensive, and provides information about the size of the plaque and the turbulence of flow. It can also give a good approximation of plaque composition with regard to whether the core contains water (blood) or lipids or is calcified or fibrous. The limitations of US are poor wall and lumen definition, lack of quantitation capabilities, and obstruction of the view due to calcifications. It is also very heavily operator dependent. CT has good wall and lumen definition and is fast and reproducible. Its drawbacks are radiation exposure and poor soft tissue contrast and definition. Conventional angiography (MRA) is ubiquitous and provides excellent information about stenosis and the lumen surface; however, it can give no information about plaque composition. IVUS can give information about lumen, wall, and plaque composition but it has the same limitations as B-mode US in that calcifications, which are common in the carotid plaque, produce restrictive shadows. In addition, IVUS is invasive and carries the risk of embolization. MRI also has distinct drawbacks. It is expensive, slow, and not applicable if patients are claustrophobic. It is coil (hardware) dependent and not readily available in some areas. However, the strengths of this modality in preoperative screening are the focus of this review.
Noninvasive magnetic resonance (MR) has advantages over other modalities in that it can visualize the vessel lumen and wall [8,9] and has good soft tissue contrast which allows for evaluation of the compositional and morphologic features of carotid atherosclerotic plaques [10,11]. By identifying the unique combination of signal intensities displayed by each component in different contrast weightings, MRI is able to detect plaque components. In early initial experiments involving ex vivo imaging of tissue components of CEA specimens, including the LRNC, IPH, and calcification, components could be differentiated with sensitivities and specificities ranging from 84 to 100% [12]. Translation of these findings to in vivo imaging produced a sensitivity of 85% and a specificity of 92% [10] (table 1). Saam et al. [11] demonstrated the in vivo capability of MRI to quantify major components of carotid plaque by comparing results with histological findings. The MRI assessment of plaque composition was statistically equivalent to those of histology for the LRNC and fibrous tissue. Intra- and interobserver reproducibility was good to excellent for all tissue components, with intraclass correlation coefficients ranging from 0.73 to 0.95 [11]. Multicontrast MRI assessment of carotid plaque uses fast spin-echo-based T1-weighted (T1W), T2-weighted (T2W) images and time-of-flight (TOF) images [8,11] (table 2).
Table 1.
Performance of multicontrast MRI in the identification of carotid plaque components
Table 2.
MRI criteria used to identify plaque tissue components
| TOF | T1W | T2W | |
|---|---|---|---|
| LRNC with | |||
| no or little IPH | Iso | Iso to Hyper | Hypo to Iso |
| fresh IPH | Hyper | Hyper | Hyper |
| recent IPH | Hyper | Hyper | Hyper |
The classification is based on the following signal intensities relative to sternocleidomastoid muscle: Hyper = Hyperintense; Iso = isointense; Hypo = hypointense.
Detection of High-Risk Features for CAS Using MR Carotid Plaque Imaging
A large LRNC and the presence of IPH are considered to be the important factors determining the amount of debris produced during carotid stenting [7,13,14]. Therefore, preoperative assessment and exclusion of such plaques is necessary to reduce the rate of complications after CAS.
The LRNC is generally located in the bulk of the plaque and produces an isointense image on T1W images. However, depending on the amount and age of hemorrhage present in the core, the LRNC may have varied signal intensities in T2W images [11] (table 2). Yoshida et al. [15] reported that the signal intensity ratio (SIR) (intensity of plaques/intensity of sternocleidomastoid muscle) of the LRNC with IPH was significantly higher than that of fibrous tissue and calcification (1.569 ± 0.251; 1.099 ± 0.312, and 0.717 ± 0.251). The group also showed that unstable plaques (% LRNC area more than 50% on cross section) could be differentiated from stable plaques with a sensitivity of 79% and a specificity of 84% when the SIR cutoff value was 1.25 using a cross-sectional analysis of T1W images [15].
Fresh hemorrhage appears as a hyperintense signal on T1W and TOF images but has an iso-to hypointense signal on T2W images (fig. 1). Recent hemorrhage is identified by a hyperintense signal on all three contrast weightings. Area measurements of hemorrhage generally combine fresh and recent hemorrhage [11] (table 2). Moreover, recent works by Yim et al. [16] and Yoshimura et al. [7] have indicated that maximum intensity projection (MIP) images from TOF MRA can identify IPH as a high-intensity signal (HIS) in the plaque [7,16]. MIP images from TOF MRA are widely used for screening carotid artery stenosis [17] and allow for rapid determination of the degree of stenosis and other anatomical findings by use of rotational views. Therefore, this recent finding raises the possibility of an additional application of MIP images from TOF MRA used for the investigation of carotid artery stenosis (fig. 2).
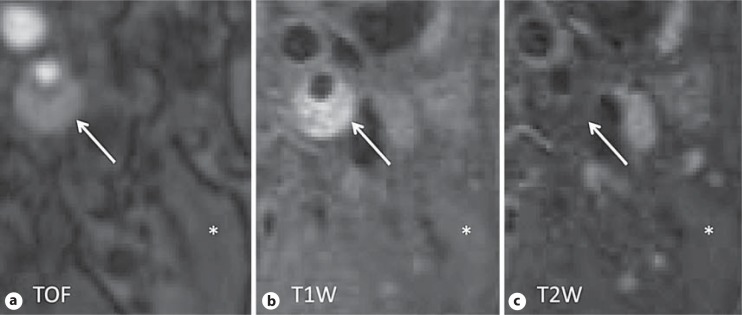
Fig. 1.
Representative images of a large carotid IPH. IPH produces a high signal intensity on both TOF (a) and T1W (b) images, and iso- or hypointense signals on T2W (c) images (arrows). The asterisks denote sternocleidomastoid muscle.
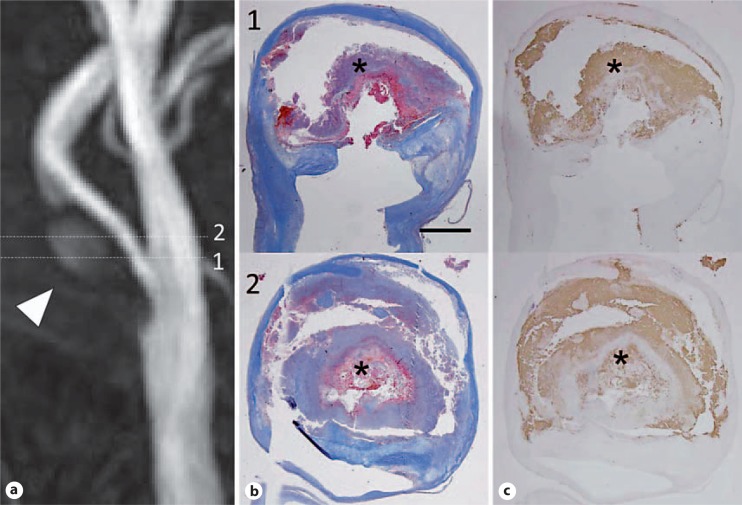
Fig. 2.
a Example of an HIS-positive plaque on MIP images of TOF MRA. Images were obtained from a 61-year-old man who had asymptomatic severe carotid artery stenosis. HIS in the plaque was observed on MIP images on TOF MRA (arrowhead). Numbers mean the slice level of MRA. Numbers in MRA and histology are matched as the same slice level. b The matching histology Masson's trichrome staining showed large regions of IPH (asterisk). c The antibody to glycophorin A also produced a strong signal in areas of hemorrhage (asterisk). Scale bar = 2 mm. Reprinted with permission from Yoshimura et al. [7].
Association between Preoperative MR Carotid Plaque Image Findings and Ischemic Complications after CAS
An electronic literature search of MR carotid plaque imaging and CAS produced the following results, the main characteristics of which are summarized in table 3.
Table 3.
Preoperative MR plaque imaging and CAS
| Reference | Year | Procedures n | MR plaque imaging sequence | Definition of plaque characteristics | Plaque characteristics (category and number) | New DWI lesion | Slow-flow phenomenon | Any new stroke | |
|---|---|---|---|---|---|---|---|---|---|
| Sakamoto et al. [13] | 2010 | 31 | T1W, T2W, TOF | Presence of main components (IPH, LRNC); | Vulnerable plaques: 14 | 6 | 8 | 1 | |
| Presence of main components (Fib, CA) | Stable plaques: 17 | 10 | 2 | 1 | |||||
| Yamada et al. [20] | 2011 | 56 | T1W | SIR ≥1.25 | High SIR group: 28 | 17 | NG | 2 | |
| SIR <1.25 | Low SIR group: 28 | 6 | NG | 0 | |||||
| Yoshimura et al. [7] | 2011 | 112 | TOF | HIS positive in the plaque | HIS-positive group: 38 | 25 | NG | 7 | |
| HIS negative in the plaque | HIS-negative group: 74 | 26 | NG | 1 | |||||
NG = Not given; Fib = fibrous tissue; CA = calcification.
Sakamoto et al. [13] studied the relation between preoperative carotid plaque features by MR imaging and arterial flow impairment (slow-flow phenomenon) during CAS in 31 patients. Flow impairment such as antegrade flow reduction or flow arrest in the internal carotid artery is sometimes observed as a spurious angiographic finding. This phenomenon is considered to be ‘pre-complication status’ and is associated with an increasing risk of periprocedural embolic stroke [18]. The group chose this slow-flow phenomenon as a marker of high risk for CAS. Plaques containing either IPH or LRNC were assigned to the vulnerable plaque group and plaques composed of fibrous tissue and dense calcification were assigned to the stable plaque group according to previously established criteria [8]. The slow-flow phenomenon was observed in ten CAS procedures (five flow arrests and five flow reductions). The slow-flow phenomenon occurred significantly more frequently in patients in the vulnerable plaque group than in the stable plaque group (p < 0.01). The authors concluded that vulnerable carotid plaques have a significantly higher risk of slow-flow phenomenon than stable plaques and the occurrence of the slow-flow phenomenon can be predicted by MR plaque imaging before CAS [13].
Yamada et al. [6] performed quantitative analysis of plaque characteristics in carotid arteries using the SIR (intensity of plaques/intensity of sternocleidomastoid muscle) of T1W images before CAS in 56 patients. Plaque with SIR ≥1.25 was defined as high SIR plaque and plaque with SIR <1.25 was defined as low SIR plaque according to previously reported criteria [6,15]. DWI-MRI of the brain was performed before and after CAS. The incidence of newly appearing ipsilateral silent ischemic lesions was significantly greater in high SIR plaques (17/28; 68%) than in low SIR plaques (6/28; 21%: p = 0.003). The number of new DWI-MRI lesions was higher in patients who developed a minor or major stroke within 30 days after CAS than in patients who did not [19]. It was concluded that it may be possible to avoid neurological complications and improve the clinical outcome by assessing tissue characteristics of carotid plaques using MRI and performing CEA for carotid stenosis with high SIR [20] (fig. 3).
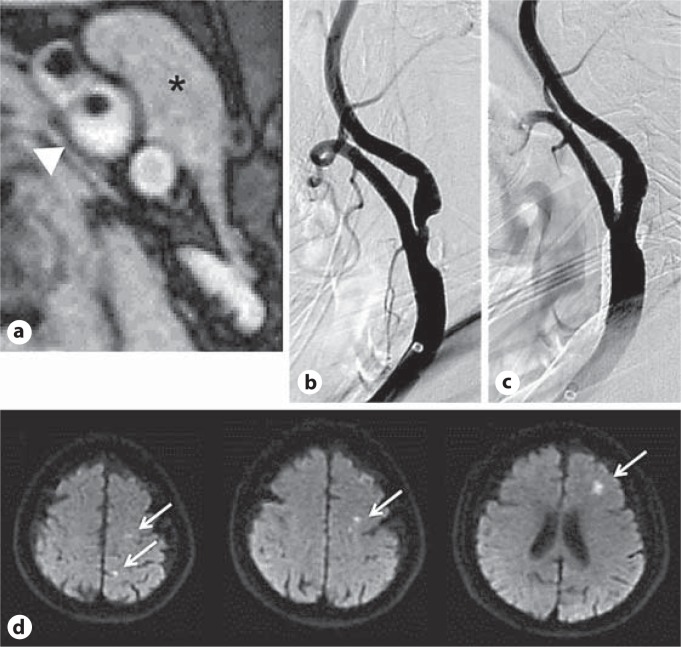
Fig. 3.
Representative images of CAS for left carotid artery stenosis in a 74-year-old woman with a left-sided stroke. a The arrowhead shows an axial image of the most stenotic lesion of the plaque on the T1W image. The SIR was 1.37. The asterisk indicates sternocleidomastoid muscle. b Angiogram of the left carotid artery stenosis before stenting. c Angiogram of the stenosis after stenting shows successful dilatation of the carotid arterial lumen. d DWI-MRI. The arrows indicate multiple ischemic lesions which are detected in the left cerebral hemisphere after the stenting procedure. Reprinted with permission from Yamada et al. [20].
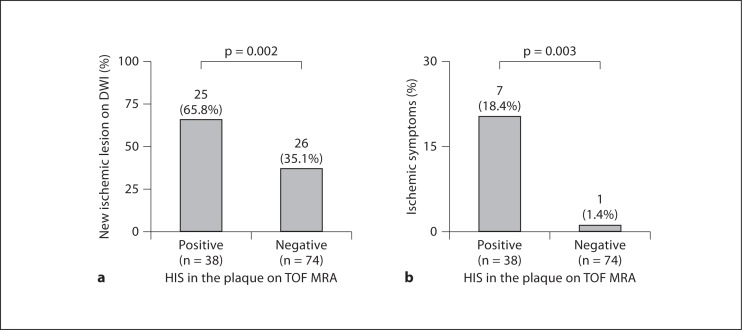
The authors also reported the association between the presence of HIS in the plaque on MIP images from TOF MRA and cerebral embolism during CAS. They validated the relation of high signals in the plaque on MIP images from TOF MRA and the presence of IPH with histology. Then, as a clinical study, the HIS in the plaque on MIP images from TOF MRA were assessed with regard to the ability to discriminate plaque at high risk for cerebral embolism during CAS in 112 patients. The authors found that postoperative ischemic lesions on DWI-MRI were more frequent in the HIS-positive plaques (25/38; 65.8%) than in the HIS-negative groups (26/74; 35.1%; p = 0.002). Periprocedural ischemic symptoms were more frequently observed in HIS-positive plaques (7/38; 18.4%) than in HIS-negative plaques (1/74; 1.4%; p = 0.003). In multivariate logistic regression analysis, HIS on MIP image from TOF MRA was an independent predictor of periprocedural ischemic symptoms. Therefore, it was concluded that HIS in the plaque on MIP image from TOF MRA could discriminate plaques at high risk for cerebral embolism during CAS [7] (fig. 2, 4).
Fig. 4.
Relationship between the presence of HIS in the plaque on TOF MRA and new ischemic lesions on DWI (a) and periprocedural ischemic symptoms (b). a New ischemic lesions on DWI were more frequently observed in the HIS-positive group than in the HIS-negative group. b Periprocedural ischemic symptoms were more frequently observed in the HIS-positive group than in the HIS-negative group. Reprinted with permission from Yoshimura et al. [7].
The above three studies emphasized the possibility of MR carotid plaque imaging using T1W and TOF images for predicting ischemic complications during CAS. However, these studies were performed in a retrospective manner; prospective studies will be required in the future. The studies also did not consider the volume of components or plaques. The volumes of hemorrhage, necrotic core, or plaque wall may be important indicators of distal embolism as these might correlate with the amount of debris produced during CAS and will be part of future work [13,14].
Conclusions
This review was undertaken to highlight recent studies of MR plaque imaging and the association between preoperative MR carotid plaque imaging and CAS. MRI is well suited for preoperative carotid plaque imaging because it is noninvasive, enables visualization of the carotid vessel lumen and wall, and can quantify plaque components. Few clinical studies have focused on the possibility of preoperative MR plaque imaging for the prediction of new ischemic lesions or ischemic complications during CAS. Precise plaque evaluation and a prospective clinical study will be required to establish the importance of preoperative MR plaque imaging for the prediction or the reduction of complications during CAS.
Acknowledgment
The authors thank Marina S. Ferguson for her manuscript editing advice.
References
- 1.Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg. 1996;3:42–62. doi: 10.1583/1074-6218(1996)003<0042:SITCAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 3.Brott TG, Howard RW, 2nd, Howard G, CREST Investigators Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohki T, Marin ML, Lyon RT, Berdejo GL, Soundararajan K, Ohki M, Yuan JG, Faries PL, Wain RA, Sanchez LA, Suggs WD, Veith FJ. Ex vivo human carotid artery bifurcation stenting: correlation of lesion characteristics with embolic potential. J Vasc Surg. 1998;27:463–471. doi: 10.1016/s0741-5214(98)70321-0. [DOI] [PubMed] [Google Scholar]
- 5.Biasi GM, Froio A, Diethrich EB, Deleo G, Galimberti S, Mingazzini P, Nicolaides AN, Griffin M, Raithel D, Reid DB, Valsecchi MG. Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation. 2004;110:756–762. doi: 10.1161/01.CIR.0000138103.91187.E3. [DOI] [PubMed] [Google Scholar]
- 6.Yamada K, Kawasaki M, Yoshimura S, Enomoto Y, Asano T, Minatoguchi S, Iwama T. Prediction of silent ischemic lesions after carotid artery stenting using integrated backscatter ultrasound and magnetic resonance imaging. Atherosclerosis. 2010;208:161–166. doi: 10.1016/j.atherosclerosis.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura S, Yamada K, Kawasaki M, Asano T, Kanematsu M, Takamatsu M, Hara A, Iwama T. High intensity signal on time-of-flight MR angiography indicates carotid plaques at high risk for cerebral embolism during stenting. Stroke. 2011;42:3132–3137. doi: 10.1161/STROKEAHA.111.615708. [DOI] [PubMed] [Google Scholar]
- 8.Yuan C, Mitsumori LM, Beach KW, Maravilla KR. Carotid atherosclerotic plaque: noninvasive MR characterization and identification of vulnerable lesions. Radiology. 2001;221:285–299. doi: 10.1148/radiol.2212001612. [DOI] [PubMed] [Google Scholar]
- 9.Saam T, Hatsukami TS, Takaya N, Chu B, Underhill HR, Kerwin WS, Cai J, Ferguson MS, Yuan C. The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology. 2008;244:64–77. doi: 10.1148/radiol.2441051769. [DOI] [PubMed] [Google Scholar]
- 10.Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, Small R, Davies JW, Kerwin WS, Hatsukami TS. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051–2056. doi: 10.1161/hc4201.097839. [DOI] [PubMed] [Google Scholar]
- 11.Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, Hatsukami TS, Yuan C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25:234–239. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 12.Shinnar M, Fallon JT, Wehrli S, Levin M, Dalmacy D, Fayad ZA, Badimon JJ, Harrington M, Harrington E, Fuster V. The diagnosis accuracy of ex vivo MRI for human atherosclerotic plaque characterization. Arterioscler Thromb Vasc Biol. 1999;19:2756–2761. doi: 10.1161/01.atv.19.11.2756. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto M, Taoka T, Nakagawa H, Takayama K, Wada T, Myouchin K, Akashi T, Miyasaka T, Fukusumi A, Iwasaki S, Kichikawa K. Magnetic resonance plaque imaging to predict the occurrence of the slow-flow phenomenon in carotid artery stenting procedures. Neuroradiology. 2010;52:275–283. doi: 10.1007/s00234-009-0623-7. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto S, Nakahara I, Higashi T, Iwamuro Y, Watanabe Y, Takezawa M, Murata D, Yokota T, Kita J, Yamada T. Fibro-fatty volume of culprit lesions in virtual histology intravascular ultrasound is associated with the amount of debris during carotid artery stenting. Cerebrovac Dis. 2010;29:468–475. doi: 10.1159/000297962. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida K, Narumi O, Chin M, Inoue K, Tabuchi T, Oda K, Nagayama M, Egawa N, Hojo M, Goto Y, Watanabe Y, Yamagata S. Characterization of carotid atherosclerosis and detection of soft plaque with use of black-blood MR imaging. AJNR Am J Neuroradiol. 2008;29:868–874. doi: 10.3174/ajnr.A1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yim YJ, Choe YH, Ko Y, Kim ST, Kim KH, Jeon P, Byun HS, Kim DI. High signal intensity halo around the carotid artery on maximum intensity projection images of time-of-flight MR angiography: a new sign for intraplaque hemorrhage. J Magn Reson Imaging. 2008;27:1341–1346. doi: 10.1002/jmri.21284. [DOI] [PubMed] [Google Scholar]
- 17.De Marco JK, Nesbit GM, Wesbey GE, Richardso D. Prospective evaluation of extracranial carotid stenosis: MR angiography with maximum-intensity projections and multiplanar reformation compared with conventional angiography. AJR Am J Roentgenol. 1994;163:1205–1212. doi: 10.2214/ajr.163.5.7976902. [DOI] [PubMed] [Google Scholar]
- 18.Casserly IP, Abou-Chebl A, Fathi RB, Lee DS, Saw J, Exaire JE, Kapadia SR, Bajzer CT, Yadav JS. Slow-flow phenomenon during carotid artery intervention with embolic protection devices: predictors and clinical outcome. J Am Coll Cardiol. 2005;46:1466–1472. doi: 10.1016/j.jacc.2005.05.082. [DOI] [PubMed] [Google Scholar]
- 19.Kastrup A, Nagele T, Groschel K, Schmidt F, Vogler E, Schulz J, Ememann U. Incidence of new brain lesions after carotid stenting with and without cerebral protection. Stroke. 2006;37:2312–2316. doi: 10.1161/01.STR.0000236492.86303.85. [DOI] [PubMed] [Google Scholar]
- 20.Yamada K, Yoshimura S, Kawasaki M, Enomoto Y, Asano T, Hara A, Minatoguchi S, Iwama T. Embolic complications after carotid artery stenting or carotid endarterectomy are associated with tissue characteristics of carotid plaques evaluated by magnetic resonance imaging. Atherosclerosis. 2011;215:399–404. doi: 10.1016/j.atherosclerosis.2011.01.002. [DOI] [PubMed] [Google Scholar]