Abstract
Intravenous administration of tissue plasminogen activator within 4.5 h of symptom onset is presently the ‘golden rule’ for treating acute ischemic stroke. However, many patients miss the time window and others reject this treatment due to a long list of contraindications. Mechanical embolectomy has recently progressed as a potential alternative for treating patients beyond the time window for IV thrombolysis. In this paper, recent progress in mechanical embolectomy, angioplasty, and stenting in acute stroke is reviewed. Despite worries concerning the long-term clinical outcomes and increased risk of intracranial hemorrhage, favorable clinical outcomes may be achieved after mechanical embolectomy in carefully selected patients even 4.5 h after stroke onset. Potential steps should be prepared and attempted in these patients whose opportunity for recovery will elapse in a flash.
Key Words: Mechanical embolectomy, Angioplasty, Stenting
Introduction
Based on the results of the National Institute of Neurological Disorders and Stroke Study (NINDS) in 1995 [1] and the Prolyse in Acute Cerebral Thromboembolism (PROACT II) study in 1999 [2], the intravenous (IV) and intra-arterial (IA) tissue plasminogen activators (tPA) for the treatment of acute stroke were approved by the US Food and Drug Administration (FDA). Since then, acute ischemic stroke within 3 and 6 h has been treated by IV and IA tPA, respectively. Recently, the European Cooperative Acute Stroke Study (ECASS III) expanded the time window of IV tPA to 4.5 h after stroke onset [3]. This is a giant progress in stroke treatment. However, only 3–8.5% of stroke patients could receive the tPA treatment [4]. Several studies show that 1 of 3 patients will benefit from IV tPA within 3 h, 1 of 7 will benefit from IV tPA within 4.5 h, and 1 of 5 will benefit from IA fibrinolysis within 6 h [5,6,7].
There are several possible reasons for the low efficiency of tPA. Not all of the patients know the exact time of stroke onset. As many as 15–25% of stroke patients are cases of wake-up strokes who are not generally offered the thrombolytic therapy according to the recommendations of the FDA [8]. Not all patients can get to the hospital within 4.5 h. Only 20–25% of all acute stroke patients meet time window for IV thrombolysis [9] and the rate is even lower in underdeveloped countries [10]. Not all patients are suitable for receiving the IV tPA treatment even if they meet the treatment window. There are many contraindications for tPA, mainly involving a history of and/or propensity for intracerebral hemorrhage [11]. Not all of the lesions can be removed by tPA. Large proximal clots such as terminal internal carotid artery occlusion are less susceptible to IV tPA [12], especially when the thrombi are longer than 8 mm [13]. Only 10% of internal carotid artery and 25% of proximal middle cerebral artery occlusions are recanalizable [14]. Not all the recanalizations are complete. Angiographically confirmed residual thrombus requiring IA therapy was found among 70% of patients who were treated with IV tPA [15]. Not all patients can benefit from successful recanalization. Downstream perfusion can be hampered by distal thromboemboli and inflammatory changes in the microcirculation which is a no-reflow phenomenon [16].
Several emerging therapies aim to overcome the limitations of tPA. First, one approach uses novel thrombolytic or defibrinogenating agents such as tenecteplase [17], desmoteplase [18], plasmin [19], and ancrod [20] to extend the time window of treatment or decrease the complications of rtPA. Second, combinatory approaches which involve using rtPA plus other agents or methods such as Argatroban [21], low-molecular-weight heparin [22], acetylsalicylic acid [23], GP IIb/IIIa inhibitors [24], and sonothrombolysis [25] are used to enhance the efficacy of fibrinolytics, prevent reclusion, and improve microcirculatory flow. There are some noninvasive methods to augment cerebral blood flow (CBF) such as noninvasive ventilator support [26], sphenopalatine ganglion stimulation [27], and partial aortic occlusion. Finally, endovascular treatments have been introduced to treat ischemic stroke to achieve local lytic application and greater rates of arterial recanalization. For the details of advantages and disadvantages, please see the recent review of Barreto and Akexandrov [28]. In this paper, we highlight the recent progress of endovascular treatments for ischemic stroke beyond the therapy time window and treatment ranges of IV tPA.
Possible Mechanisms for Recanalization beyond 4.5 h
The major advantage of endovascular therapy is the high rate of recanalization, which is associated with good clinical outcomes [29]. The underlying mechanism of the good outcomes after recanalization is the existence of ischemic penumbra which is characterized by hypoperfusion with cellular dysfunction but not neuron death, undetermined fate, and salvage requiring successful treatment [30,31]. Preclinical studies have indicated that restoration of blood flow even after a long time (>24 h) could prevent a substantial amount of penumbra from developing infarction independent of the time to reperfusion [32]. In clinical trials, 90–100% of patients with acute ischemic stroke have penumbra in the first 3 h after stroke onset [33]; 75–80% of stroke patients have penumbral tissues 6 h after stroke onset [34]. Even 18 h after stroke onset, up to one-third of patients exhibit large volumes of penumbra [35]. Markus et al. [36] observed that hypoxic tissue could survive independent of time for up to 48 h after stroke onset using a hypoxic marker and positron emission tomography (PET). The results of both basic research and clinical trials indicate that patients beyond the therapy time window could benefit from endovascular treatments. However, penumbra varies from patient to patient because it depends upon a variety of factors such as the location of vessel lesions [15], the location of ischemic lesions [37], and collateral circulation [38]. Evaluation of the penumbra and selection of the patients is necessary for endovascular treatment.
Selecting Patients for Endovascular Treatment
Thanks to great progress in neuroimaging, it is now possible to detect the penumbra and select suitable patients for endovascular treatments. Computed tomography (CT), CT perfusion, CT angiography (CTA), magnetic resonance imaging (MRI), perfusion and diffusion MRI (PWI/DWI), single-photon emission CT (SPECT), PET, and digital subtraction angiography are now used to select patients, especially those beyond the time window of tPA.
CT is a fast, widely used, and easily available method to evaluate acute ischemic stroke. It has been used to select patients for endovascular treatment in the main studies conducted to date [39,40,41]. The Alberta Stroke Program Early CT Scale (ASPECTS) score is a semiquantitative measure and has been confirmed to be an excellent prognostic tool [42]. In the Penumbra Pivotal Stroke Trial, the results showed that patients could benefit from endovascular treatments (3-fold compared to the control group) when the ASPECTS score was >7 [43]. However, the limitations of CT are that it cannot show imaging of penumbra and is less sensitive to early strokes and lesions of posterior circulation.
There are two types of CT perfusion, i.e. whole brain and dynamic CT perfusion, which could provide information of cerebral blood volume and CBF, and mean transit time and time to peak, respectively [31]. Natarajan et al. [44] used CT perfusion to guide endovascular treatments for patients more than 8 h after stroke onset. They found that based on cerebral blood volumes >30% within the hemisphere compared with the contralateral hemisphere, the recanalization rate was 66.7%, while 20% of patients possessed a modified Rankin Scale (mRS) score ≤2 and a 3.5-point decrease in the National Institutes of Health Stroke Scale (NIHSS) [44]. Using mechanical embolectomy in patients with lesions in less than one-third of the middle cerebral artery territory beyond the time window (18.6 ± 16 h after stroke onset), 85.7% of patients had recanalization and 42.9% of patients achieved an mRS score ≤2 [45]. An ongoing clinical trial [DWI/PWI and CTP Assessment in the Triage of Wake-up and Late Presenting Strokes Undergoing Neurointervention (DAWN) trial] is evaluating the safety and efficacy of perfusion-based endovascular therapy in patients presenting more than 8 h after symptom onset. Preliminary data of the DAWN trial showed that 73% of the patients achieved successful recanalization and 83% of the patients had an mRS score ≤3 while the rates of symptomatic intracerebral hemorrhage and mortality were 10.4 and 22% when compared to the control group, respectively [46]. There are some limitations of CT perfusion such as interobserver variability, inconsistencies between different software programs, and a lack of standardization in diagnostic criteria.
The penumbra could be estimated by MRI based on the hypothesis of mismatch between the acute PWI and DWI lesions [47]. In MR RESUE research, mechanical embolectomy was based on the presentation of penumbra detected by PWI/DWI. The results showed that acute stroke patients even beyond 4.5 h after stroke onset still benefited from the endovascular treatment when the mismatch was present [48]. When using DWI analysis to predict the clinical outcome of the patients, it was found that patients could only benefit from IV tPA thrombolysis when the DWI lesion volume was less than 25 ml and the excellent outcome (mRS ≤2) from treatment was substantially increased in patients with volumes less than 18 ml [49]. In anterior circulation stroke patients receiving IA thrombolysis, a DWI lesion volume greater than 70 ml predicted poor outcomes [50]. These findings were confirmed by a subsequent study using endovascular treatment. Patients with initial DWI >70 ml have poor outcomes despite a 50% recanalization rate [51]. The limitations of MRI are that it needs more time to obtain the images of mismatch and there are motion artifacts and questions regarding the validity of the mismatch [52].
SPECT defines a 40–70% reduction of the tracer signal as penumbra. However, it is semiquantitative, with radiation exposure and lack of standardized protocols; therefore, the clinical application of SPECT is very limited [53]. Another possible tool is Xenon CT, which can detect CBF and define the penumbra as CBF 7–30 ml/100 g/min. Xenon CT is technically complex and not a validated way to find penumbra [54]. Some studies have applied PET to detect the penumbra; however, PET is an expensive and time-consuming technique with radiation exposure and arterial access [55].
There are some other imaging tools to suggest the existence of the penumbra and direct the endovascular treatment. Hyperintense vessels on fluid-attenuated inversion recovery imaging are frequently observed in acute ischemic stroke patients [56,57], which shows the presence of large PWI/DWI mismatch in patients with proximal middle cerebral artery occlusion [58]. The existence of hyperintense vessels in distal territory of the middle cerebral artery is predictive of prolonged tissue viability on acute evaluation, and it can be used to identify patients who will benefit from treatment beyond the current thrombolysis time window [58].
Clinical profiles may also predict the outcomes after endovascular treatment. The results of a post hoc analysis of the PROACT II trial suggested that the endovascular therapy should be restricted up to the age of 80–85 years with consideration given to concomitant cardiac and other comorbidities [59]. In one study, the authors evaluated the cost and effectiveness of mechanical thrombectomy compared with standard medical therapy in patients who are ineligible to receive tPA. For patients older than 82 years of age, the treatment was only borderline cost-effective [60].
The NIHSS score is often applied to assess the outcome of stroke patients. One study found that NIHSS scores below 10 are correlated with an arterial occlusion amenable to IA treatment while NIHSS scores above 20 are linked to heightened chances of intracerebral hemorrhage [40]. The major limitations of the NIHSS are that it underestimates stroke severity, particularly for posterior circulation, and it has limited use for assessing functional status, disability, and the final outcome [61]. Hyperglycemia may be another predictor of poor outcomes [62]. Hyperglycemia in the first 48 h after endovascular treatments was associated with disadvantageous outcomes [63].
Some studies have developed scores to predict the clinical outcomes after endovascular treatment. The Houston IA recanalization therapy score (Houston IAT) was developed by the University of Texas Houston Stroke Center. It divided the Houston IAT score into 3 categories: 1 point for age >75 years; 1 point for NIHSS score >18, and 1 point for glucose >150 mg/dl. The percentage of poor outcomes using the Houston IAT score was: score of 0, 44%; score of 1, 67%; score of 2, 97%, and score of 3, 100%. Recanalization rates were similar across the scores [64]. In the MERCI and Multi MERCI trials, the totaled health risk in vascular event (THRIVE) score was applied to assess the patients. The THRIVE score, which reflects the contributions of chronic disease, age, and stroke severity, included a 10-level predictive score. Patients with a low THRIVE score of 0–2 had good outcomes in 64.7% of cases, patients with a moderate THRIVE score of 3–5 had good outcomes in 43.5% of cases, and patients with a high THRIVE score of 6–9 had good outcomes in 10.6% of cases [65]. Both of the scores were recently validated by another group [66].
Selecting Devices for Recanalization
Stroke is a heterologous-cause disease. Different causes induce the clot of cerebral vessels, such as embolism, thrombosis, and atherosclerosis [67]. To date, there are many kinds of endovascular treatment devices and each device has its own advantages and disadvantages; therefore, selecting the proper device for each patient is necessary (table 1). We divided the devices into three categories: snare-based devices, aspiration devices, and stent-based devices.
Table 1.
Devices used in different studies for recanalization in patients with acute stoke
| Study | Merci [41] | Multi Merci [100] | Penumbra [101] | POST [102] | SARIS [94] | Wingspan [94] | Solitaire [96] | Trevo [98] |
|---|---|---|---|---|---|---|---|---|
| Device | Merci | Merci | Penumbra | Penumbra | Wingspan/Enterprise | Wingspan/Enterprise | Solitaire | Trevo |
| Sample, n | 141 | 164 | 20 | 125 | 20 | 12 | 20 | 13 |
| Age, years | 67 | 68 | 60 | 64 | 63 | 49 | 66 | 74 |
| NIHSS | 20.1 | 19 | 21 | 18 | 14 | 17 | 19 | 19 |
| Hyperglycemia | none | none | none | none | none | none | none | none |
| Imaging | CT | CT | CT | CT | CT | CT | CT | CT |
| Time to treatment, h | 4.3 | 3–8 | ≥3 | 4.5 | 5.25 | 66.1 | 5.9 | 5.0 |
| Recanalization, % | 48 | 57.3 | 100 | 81.6 | 100 | 92.7 | 90 | 77 |
| mRS ≤2, % | 46 | 36 | 45 | 25 | 60 | 50 | 45 | 30.8 |
| Mortality, % | 23.9 | 34 | 45 | 32.8 | 25 | 16.7 | 20 | 30.8 |
| Symptomatic ICH, % | 7.8 | 9.8 | 10 | 11.2 | 5 | 16.7 | 10 | 0 |
ICH = Intracerebral hemorrhage.
Snare-Based Devices
In the 1990s, the snare was initially used to retrieve foreign bodies from cerebral vessels, such as fracture of electrolytically detachable coils during treatment of intracerebral aneurysms [68]. Chopko et al. [69] used a 4-mm goose-neck snare through a microcatheter to snare and withdraw a clot in a 38-year-old man with near-total occlusion of the right middle cerebral artery at the M1-M2 junction. Subsequently four devices were developed to remove cerebral clots: the Microsnare (Microvena, Minneapolis, Minn., USA), the Neuronet (Guidant, Temecula, Calif., USA), the In-Time Retriever (Target, Fremont, Calif., USA), and the EnSnare (Medical Device Technologies, Gainesville, Fla., USA) [70]. The Microsnare is a 90° angled loop that comes in 2-, 4-, and 7-mm diameters and can be placed through any standard 0.018-in microcatheter [71]; the Neuronet is a self-expanding nitinol basket portion tapering to a shapeable platinum-tipped wire [72]; the In-Time Retriever is a Dormia-type design that is delivered without a separate microcatheter and is opened by applying traction to the central wire that leads to foreshortening and opening of the device [73]; the EnSnare is similar to the In-Time device. In the meantime, there have been three other devices, i.e. Catch, Attactor-18, and Alligator. The Catch device consists of a self-expanding basket-like device fixed to a pusher wire [74]. The Attracter-18 device is a radiopaque distal platinum coil with a wire tip [75]. The Alligator retrieval device contains 4 small jaws mounted on a solid core shaft within a microcatheter, which once advanced from the microcatheter forms a 4-pronged grabbing device [76]. Next, the Merci clot retriever (Concentric Medical, Mountain View, Calif., USA) was developed and first approved by the FDA in 2004. The Merci device is a flexible nitinol wire with a multi-loop helix when it is advanced out of the microcatheter. There are currently V, L, and X6 series of the Merci device [41,77]. Another retriever device approved in Europe is the Phenox clot retriever (Phenox, Bochum, Germany) which is a wire surrounded by a dense palisade of perpendicularly oriented stiff polyamide microfilaments [78].
Besides these devices, some researchers have tried to combine the microwire with other techniques. The LaTIS laser device uses laser technology which contains a laser at its tip to heat the clot to make it break down [79]. The Endovascular Photo Acoustic Recanalization (EPAR) laser system creates microcavitation bubbles by converting photonic energy into acoustic energy at the fiber optic tip to break the clot down [80]. The EndoWave Infusion Catheter System (EKOS) ultrasound device with a small ultrasound transducer at the tip of the device could increase the permeability of drugs into the clot to accelerate clot dissolution [81].
Aspiration Devices
The snare-based techniques generally dissolve the clots, and clot fragmentation may cause potential proximal occlusion. To overcome this potential risk, aspiration devices were developed. Initially the aspiration devices were applied to remove foreign species in the cerebral vessels, such as dialysis-related access grafts. There are four kinds of devices: the AngioJet System, the Oasis System, the Hydrolyzer, and the Amplatz device. The AngioJet System, based on the Venturi-Bernoulli effect, uses high-pressure saline jets which are introduced through orifices in the distal tip of the catheter to create a localized low-pressure zone, resulting in a vacuum effect with entrainment and dissociation of bulky thrombi [82]. The other three early systems are similar to the AngioJet System and were only experimental for acute embolic stroke [83,84]. Subsequently, the NeuroJet came out; it also used the Venturi effect to disrupt and aspirate the clot via a high-pressure saline jet at the distal tip that was then immediately aspirated. The system was specifically designed to remove cerebral vessel thromboemboli [83]. Recently, a new-generation neuroembolectomy device, the Penumbra System, was approved by the FDA for the treatment of acute stroke via debulking and aspiration of acute clots [85]. The Penumbra consists of 3 main components: a reperfusion catheter, a separator, and a thrombus removal ring required to remove the residual thrombus. The reperfusion catheter is used to separate the thrombus and aspirate it from the occluded vessel.
Stent-Based Devices
Angioplasty and stenting has become a widely used mechanical treatment for atherosclerosis in craniocervical vessels in China [86,87,88,89]. It was first used to treat asymptomatic patients or subacute and chronic stroke patients [90]. For acute ischemic strokes, balloon-mounted stents were used in an early small cohort study until the self-expanding stent came out [91,92]. The NeuroForm self-expanding stent, which was designed for cerebral aneurysm, was used to treat occlusion of the middle cerebral artery after failure of thrombolysis in an acute stroke patient [93]. Then the Wingspan self-expanding stent, which is an extra-flexible self-expanding stent, was approved by the FDA as the primary intervention for 20 cases of acute stroke [94]. Another self-expanding stent is the Enterprise stent, which is a new highly flexible nitinol stent with a fixed closed-cell design [95].
The Solitaire device, a laser-cut, self-expanding, split-design, closed-cell nitinol stent device, was developed to treat acute stroke [96] (fig. 1). The Aperio device, a hybrid-design stent-based clot retriever with alternating segments of small closed cells and wider hybrid cells for high flexibility, could improve vessel apposition and provide better thrombus fixation during retrieval [97]. The Trevo System is a stent-like device which can integrate the clot into the stent structure and retract the device to remove the clot from the vessel [98].
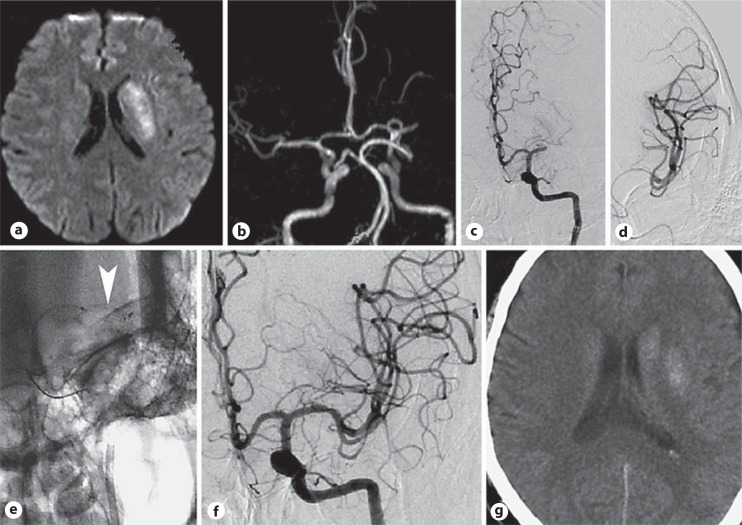
Fig. 1.
A 58-year-old male with aphasia and right hemiparesis reached our hospital 6 h after symptom onset (NIHSS = 15). a A diffusion-weighted image showed a slightly high signal in the left insular area. b Magnetic resonance angiogram. c Digital subtraction angiography demonstrated M1 segment occlusion in the left middle cerebral artery. d Angiogram via a microguide indicated complete patency of the distal middle cerebral artery. e A solitaire stent was deployed (arrowhead). f A final angiogram of the left middle cerebral artery after retrieval of the stent showed complete recanalization of the middle cerebral artery. g CT showed a small infarction in the left internal capsule 18 h after recanalization. The patient was discharged 7 days later with an NIHSS score of 4.
Evidence for Mechanical Embolectomy beyond the Time Window
There are several clinical trials in which the enrolled patients were beyond the therapy time window or indications for tPA. Based on these results, the FDA approved the Merci device and the Penumbra System to treat acute ischemic stroke.
In a phase I clinical trial of the Merci device, 28 patients with a mean age of 68 years were studied [99]. The median baseline NIHSS score was 22. The median time from onset to completion of treatment was 6.25 h, and 6 patients presented within 3 h of symptom onset but had contraindications for IV tPA. One patient was treated 11 h after stroke onset because of a large diffusion/perfusion mismatch on MRI. Successful recanalization (thrombolysis in myocardial infarction, TIMI, grade 2 or 3) was achieved in 18 (64%) patients. One month later, in the 13 patients who presented with moderate to severe stroke (NIHSS 10–20), 6 out of 10 revascularized patients had achieved significant recovery (mRS ≤2) while none of the 15 patients with mild stroke (NIHSS >20) had achieved significant recovery. Though there were 12 patients suffering intracerebal hemorrhage, no symptomatic intracranial hemorrhages occurred. This study showed the safety and efficacy of cerebral embolectomy with the Merci retriever even when performed in an extended 8-hour time window.
Then the same group expanded the number of enrolled patients to further assess the Merci retriever [41]. The mean age of 151 enrolled patients (141 in whom the device was deployed) was 67.7 years and the mean NIHSS score was 20.1. The CT scan was applied before the embolectomy. Recanalization was achieved in 48% (68/141) of patients. Good neurological outcomes (mRS ≤2) were more frequent at 90 days in patients with successful recanalization compared with patients with unsuccessful recanalization (46 vs. 10%; p < 0.0001) and mortality was less (32 vs. 54%; p < 0.05). Symptomatic intracranial hemorrhage occurred in 7.8% of patients.
To gain a better grasp of the thrombus, the new-generation Merci device L5 was developed, and the Multi MERCI trial was designed to test the L5 device versus first-generation devices [100]. The L5 retriever was used in 131 patients among 164 receiving thrombectomy at 3–8 h of stroke onset. The mean age was 68 years and the baseline NIHSS score was 19. Successful recanalization was observed in 75 of 131 (57.3%) treatable vessels. Thirty-six percent of patients had favorable clinical outcomes (mRS 0–2) and mortality was 34%. Symptomatic intracerebral hemorrhage occurred in 16 patients (9.8%). Higher rates of recanalization and good clinical outcomes, as well as lower mortality rates, were associated with the new-generation Merci device.
The safety and performance of the Penumbra System in reducing large-vessel occlusive clots in acute stroke patients were assessed [85]. Twenty-three subjects, with a mean age of 60 years and a baseline NIHSS score of 21, were enrolled, and 21 target vessels were treated in 20 subjects using the Penumbra System. Ten patients presented more than 3 h after symptom onset while, of the remaining 50% who presented within 3 h, 6 were refractory to rtPA therapy and 4 were not eligible for thrombolytic therapy. All of the patients were successfully revascularized (TIMI ≥2). At the 30-day follow-up, 45% of subjects had good clinical outcomes (4-point or greater NIHSS improvement or mRS ≤2). Subjects with a higher baseline (NIHSS ≥20) tended to have a lower probability of a good outcome compared with patients with NIHSS scores of less than 20 (27 vs. 63%). The all-cause mortality rate was 45% (9 of 20) and 8 patients had intracranial hemorrhage (2 of these patients were symptomatic as defined by CT evidence of a bleeding event and a 4-point deterioration in the NIHSS score).
Then a prospective, multicenter, single-arm study (the penumbra pivotal stroke trial) was designed to further assess the safety and efficacy of the Penumbra System [101]. A total of 125 patients were enrolled and treated using the Penumbra System. The mean age was 63.5 years and the baseline NIHSS score was 17.6; 33 patients had a higher score (≥20). Subjects within 3 h of symptom onset were ineligible or refractory to tPA therapy. CT scan was used to evaluate patients before the thrombectomy. The rate of successful recanalization was 81.6% (TIMI ≥2). Among the treated patients, 41.6% achieved good clinical outcomes at 30 days (defined by a 4-point or greater NIHSS improvement or mRS ≤2). The higher baseline NIHSS score (≥20) tended to induce a lower probability of a good outcome (mRS ≤2, 6.3 vs. 32.2%) and cause higher mortality (48.5 vs. 17.6%) compared to patients with lower NIHSS scores (less than 20). A total of 35 patients had intracranial hemorrhage on 24-hour CT; 14 (11.2%) of them were symptomatic and the mortality was 32.8% at 90 days.
Later, the POST trial was conducted to assess the initial postmarket experience of the Penumbra System and how it compared with the results of the Penumbra Pivotal trial [102]. A total of 157 subjects were treated in 7 international centers. The mean age was 65 years and the baseline NIHSS score was 16. The median time from stroke onset to arterial puncture was 4.5 h. Successful recanalization was observed in 87% of the treated vessels with TIMI 2 (54%) or 3 (33%). Patients who were successfully revascularized (TIMI ≥2) by the Penumbra System had significantly better outcomes (mRS ≤2, 45 vs. 13%) and lower mortality (16 vs. 50%) than those who were not (TIMI ≤1). Nine (5.7%) procedural serious adverse events occurred.
Levy et al. [103] first used self-expanding stents, including NeuroForm and Wingspan, for recanalization of acute cerebrovascular occlusions within 8 h of stroke onset in 18 patients. The mean age of patients from the participating institutions was 75.1 years and the median admission NIHSS score was 18. All of the patients underwent a CT scan before the procedure. Recanalization was achieved in 79% of cases. Good outcomes (mRS ≤3) were observed in 33% of all patients and 43% of the revascularized patients. The baseline NIHSS score did not contribute to the clinical results. Intracerebral hemorrhage occurred in a total of 7 patients; 2 of these cases resulted in progression to death, and 3 were asymptomatic and resolved on subsequent CT scans. The good clinical outcome (3.5 vs. 10.5 h) and successful recanalization (8.5 vs. 6 h) were independent of the time from stroke onset. It was noticeable that all of the 3 patients treated using Wingspan achieved complete recanalization (TIMI ≥2). Therefore, stent-assisted recanalization in acute ischemic stroke (SARIS) used Wingspan and Enterprise (1 patient) as the primary intervention in 20 cases of acute ischemic stroke [94]. The mean age was 63 years and the mean baseline NIHSS score was 14. The mean time from stroke onset to intervention was 5.25 h (1 at 8.5 h; 1 at 9.75 h). Recanalization was achieved in all of the patients (TIMI = 3, 60%; TIMI = 2, 40%). Only 1 symptomatic and 2 asymptomatic intracranial hemorrhages occurred. At the 1-month follow-up, 60% of the patients had good clinical outcomes (mRS ≤3) and 45% of the subjects achieved complete recovery (mRS ≤1). Five patients had died at the 1-month follow-up. A further study confirmed these findings [104]. Nineteen patients with acute ischemic stroke with a mean of 6.4 h from symptom onset were treated using 6 Enterprise and 13 Wingspan stents. The median NIHSS score on admission was 19 and the mean age was 63 years. Recanalization was achieved in 95% of occlusions (TIMI ≥2). A good clinical outcome was achieved in 42% of cases, and 12 patients achieved an mRS score ≤3. Symptomatic intracerebral hemorrhage occurred in 3 patients, 2 of whom died.
Conclusion
Intracranial recanalization using mechanical embolectomy is a safe and feasible approach in strictly selected patients presenting with acute ischemic stroke beyond the therapy time window for IV thrombolysis.
References
- 1.Tissue plasminogen activator for acute ischemic stroke – the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study – a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 3.Bluhmki E, Chamorro A, Davalos A, et al. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8:1095–1102. doi: 10.1016/S1474-4422(09)70264-9. [DOI] [PubMed] [Google Scholar]
- 4.Reeves MJ, Arora S, Broderick JP, et al. Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke. 2005;36:1232–1240. doi: 10.1161/01.STR.0000165902.18021.5b. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL. Hemorrhage after thrombolytic therapy for stroke: the clinically relevant number needed to harm. Stroke. 2007;38:2279–2283. doi: 10.1161/STROKEAHA.107.487009. [DOI] [PubMed] [Google Scholar]
- 6.Saver JL. Number needed to treat estimates incorporating effects over the entire range of clinical outcomes: novel derivation method and application to thrombolytic therapy for acute stroke. Arch Neurol. 2004;61:1066–1070. doi: 10.1001/archneur.61.7.1066. [DOI] [PubMed] [Google Scholar]
- 7.Saver JL, Gornbein J, Grotta J, et al. Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3-to 4.5-hour window: joint outcome table analysis of the ECASS 3 trial. Stroke. 2009;40:2433–2437. doi: 10.1161/STROKEAHA.108.543561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fink JN, Kumar S, Horkan C, et al. The stroke patient who woke up: clinical and radiological features, including diffusion and perfusion MRI. Stroke. 2002;33:988–993. doi: 10.1161/01.str.0000014585.17714.67. [DOI] [PubMed] [Google Scholar]
- 9.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–1955. doi: 10.1161/STROKEAHA.110.612358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Liao X, Zhao X, et al. Using recombinant tissue plasminogen activator to treat acute ischemic stroke in China: analysis of the results from the Chinese National Stroke Registry (CNSR) Stroke. 2011;42:1658–1664. doi: 10.1161/STROKEAHA.110.604249. [DOI] [PubMed] [Google Scholar]
- 11.Abou-Chebl A. Intra-arterial therapy for acute ischemic stroke. Neurotherapeutics. 2011;8:400–413. doi: 10.1007/s13311-011-0059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38:948–954. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 13.Riedel CH, Zimmermann P, Jensen-Kondering U, Stingele R, Deuschl G, Jansen O. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke. 2011;42:1775–1777. doi: 10.1161/STROKEAHA.110.609693. [DOI] [PubMed] [Google Scholar]
- 14.Wolpert SM, Bruckmann H, Greenlee R, Wechsler L, Pessin MS, del Zoppo GJ. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator – the rt-PA Acute Stroke Study Group. AJNR Am J Neuroradiol. 1993;14:3–13. [PMC free article] [PubMed] [Google Scholar]
- 15.Lum C, Stys PK, Hogan MJ, Nguyen TB, Srinivasan A, Goyal M. Acute anterior circulation stroke: recanalization using clot angioplasty. Can J Neurol Sci. 2006;33:217–222. doi: 10.1017/s0317167100005011. [DOI] [PubMed] [Google Scholar]
- 16.del Zoppo GJ. Virchow's triad: the vascular basis of cerebral injury. Rev Neurol Dis. 2008;5(suppl 1):S12–S21. [PMC free article] [PubMed] [Google Scholar]
- 17.Haley EC, Jr, Lyden PD, Johnston KC, Hemmen TM. A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke. 2005;36:607–612. doi: 10.1161/01.STR.0000154872.73240.e9. [DOI] [PubMed] [Google Scholar]
- 18.Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8:141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marder VJ, Jahan R, Gruber T, Goyal A, Arora V. Thrombolysis with plasmin: implications for stroke treatment. Stroke. 2010;41(suppl):S45–S49. doi: 10.1161/STROKEAHA.110.595157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hennerici MG, Kay R, Bogousslavsky J, Lenzi GL, Verstraete M, Orgogozo JM. Intravenous ancrod for acute ischaemic stroke in the European Stroke Treatment with Ancrod Trial: a randomised controlled trial. Lancet. 2006;368:1871–1878. doi: 10.1016/S0140-6736(06)69776-6. [DOI] [PubMed] [Google Scholar]
- 21.Sugg RM, Pary JK, Uchino K, et al. Argatroban tPA stroke study: study design and results in the first treated cohort. Arch Neurol. 2006;63:1057–1062. doi: 10.1001/archneur.63.8.1057. [DOI] [PubMed] [Google Scholar]
- 22.Mikulik R, Dufek M, Goldemund D, Reif M. A pilot study on systemic thrombolysis followed by low molecular weight heparin in ischemic stroke. Eur J Neurol. 2006;13:1106–1111. doi: 10.1111/j.1468-1331.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 23.Zinkstok SM, Vermeulen M, Stam J, de Haan RJ, Roos YB. Antiplatelet therapy in combination with rt-PA thrombolysis in ischemic stroke (ARTIS): rationale and design of a randomized controlled trial. Cerebrovasc Dis. 2010;29:79–81. doi: 10.1159/000256651. [DOI] [PubMed] [Google Scholar]
- 24.Topol EJ. Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomised trial. Lancet. 2001;357:1905–1914. doi: 10.1016/s0140-6736(00)05059-5. [DOI] [PubMed] [Google Scholar]
- 25.Alexandrov AV, Molina CA, Grotta JC, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 26.Tsivgoulis G, Zhang Y, Alexandrov AW, et al. Safety and tolerability of early noninvasive ventilatory correction using bilevel positive airway pressure in acute ischemic stroke. Stroke. 2011;42:1030–1034. doi: 10.1161/STROKEAHA.110.600221. [DOI] [PubMed] [Google Scholar]
- 27.Toda N, Tanaka T, Ayajiki K, Okamura T. Cerebral vasodilatation induced by stimulation of the pterygopalatine ganglion and greater petrosal nerve in anesthetized monkeys. Neuroscience. 2000;96:393–398. doi: 10.1016/s0306-4522(99)00557-6. [DOI] [PubMed] [Google Scholar]
- 28.Barreto AD, Alexandrov AV. Adjunctive and alternative approaches to current reperfusion therapy. Stroke. 2012;43:591–598. doi: 10.1161/STROKEAHA.111.617902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahan R. Solitaire flow-restoration device for treatment of acute ischemic stroke: safety and recanalization efficacy study in a swine vessel occlusion model. AJNR Am J Neuroradiol. 2010;31:1938–1943. doi: 10.3174/ajnr.A2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron JC. Perfusion thresholds in human cerebral ischemia: historical perspective and therapeutic implications. Cerebrovasc Dis. 2001;11(suppl 1):2–8. doi: 10.1159/000049119. [DOI] [PubMed] [Google Scholar]
- 31.Kumar G, Goyal MK, Sahota PK, Jain R. Penumbra, the basis of neuroimaging in acute stroke treatment: current evidence. J Neurol Sci. 2010;288:13–24. doi: 10.1016/j.jns.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 32.Bardutzky J, Shen Q, Henninger N, Schwab S, Duong TQ, Fisher M. Characterizing tissue fate after transient cerebral ischemia of varying duration using quantitative diffusion and perfusion imaging. Stroke. 2007;38:1336–1344. doi: 10.1161/01.STR.0000259636.26950.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darby DG, Barber PA, Gerraty RP, et al. Pathophysiological topography of acute ischemia by combined diffusion-weighted and perfusion MRI. Stroke. 1999;30:2043–2052. doi: 10.1161/01.str.30.10.2043. [DOI] [PubMed] [Google Scholar]
- 34.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 35.Baron JC. Mapping the ischaemic penumbra with PET: implications for acute stroke treatment. Cerebrovasc Dis. 1999;9:193–201. doi: 10.1159/000015955. [DOI] [PubMed] [Google Scholar]
- 36.Markus R, Reutens DC, Kazui S, et al. Hypoxic tissue in ischaemic stroke: persistence and clinical consequences of spontaneous survival. Brain. 2004;127:1427–1436. doi: 10.1093/brain/awh162. [DOI] [PubMed] [Google Scholar]
- 37.Koga M, Reutens DC, Wright P, et al. The existence and evolution of diffusion-perfusion mismatched tissue in white and gray matter after acute stroke. Stroke. 2005;36:2132–2137. doi: 10.1161/01.STR.0000181066.23213.8f. [DOI] [PubMed] [Google Scholar]
- 38.Choi JH, Bateman BT, Mangla S, et al. Endovascular recanalization therapy in acute ischemic stroke. Stroke. 2006;37:419–424. doi: 10.1161/01.STR.0000198808.90579.65. [DOI] [PubMed] [Google Scholar]
- 39.IMS II Trial Investigators The Interventional Management of Stroke (IMS) II Study. Stroke. 2007;38:2127–2135. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- 40.Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke. 2009;40:3777–3783. doi: 10.1161/STROKEAHA.109.561431. [DOI] [PubMed] [Google Scholar]
- 41.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 42.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group – Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 43.Goyal M, Menon BK, Coutts SB, Hill MD, Demchuk AM. Effect of baseline CT scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke. 2011;42:93–97. doi: 10.1161/STROKEAHA.110.594481. [DOI] [PubMed] [Google Scholar]
- 44.Natarajan SK, Snyder KV, Siddiqui AH, Ionita CC, Hopkins LN, Levy EI. Safety and effectiveness of endovascular therapy after 8 hours of acute ischemic stroke onset and wake-up strokes. Stroke. 2009;40:3269–3274. doi: 10.1161/STROKEAHA.109.555102. [DOI] [PubMed] [Google Scholar]
- 45.Abou-Chebl A. Endovascular treatment of acute ischemic stroke may be safely performed with no time window limit in appropriately selected patients. Stroke. 2010;41:1996–2000. doi: 10.1161/STROKEAHA.110.578997. [DOI] [PubMed] [Google Scholar]
- 46.Amenta PS, Ali MS, Dumont AS, et al. Computed tomography perfusion-based selection of patients for endovascular recanalization. Neurosurg Focus. 2011;30:E6. doi: 10.3171/2011.4.FOCUS10296. [DOI] [PubMed] [Google Scholar]
- 47.Schlaug G, Benfield A, Baird AE, et al. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology. 1999;53:1528–1537. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- 48.Kidwell CS, Alger JR, Saver JL. Evolving paradigms in neuroimaging of the ischemic penumbra. Stroke. 2004;35 (suppl 1):2662–2665. doi: 10.1161/01.STR.0000143222.13069.70. [DOI] [PubMed] [Google Scholar]
- 49.Parsons MW, Christensen S, McElduff P, et al. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab. 2010;30:1214–1225. doi: 10.1038/jcbfm.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanak D, Nosal V, Horak D, et al. Impact of diffusion-weighted MRI-measured initial cerebral infarction volume on clinical outcome in acute stroke patients with middle cerebral artery occlusion treated by thrombolysis. Neuroradiology. 2006;48:632–639. doi: 10.1007/s00234-006-0105-0. [DOI] [PubMed] [Google Scholar]
- 51.Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–2054. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sobesky J, Zaro Weber O, Lehnhardt FG, et al. Which time-to-peak threshold best identifies penumbral flow? A comparison of perfusion-weighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke. Stroke. 2004;35:2843–2847. doi: 10.1161/01.STR.0000147043.29399.f6. [DOI] [PubMed] [Google Scholar]
- 53.Heiss WD. Ischemic penumbra: evidence from functional imaging in man. J Cereb Blood Flow Metab. 2000;20:1276–1293. doi: 10.1097/00004647-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Kaufmann AM, Firlik AD, Fukui MB, Wechsler LR, Jungries CA, Yonas H. Ischemic core and penumbra in human stroke. Stroke. 1999;30:93–99. doi: 10.1161/01.str.30.1.93. [DOI] [PubMed] [Google Scholar]
- 55.Heiss WD, Kracht LW, Thiel A, Grond M, Pawlik G. Penumbral probability thresholds of cortical flumazenil binding and blood flow predicting tissue outcome in patients with cerebral ischaemia. Brain. 2001;124:20–29. doi: 10.1093/brain/124.1.20. [DOI] [PubMed] [Google Scholar]
- 56.Liu W, Xu G, Yue X, et al. Hyperintense vessels on FLAIR: a useful non-invasive method for assessing intracerebral collaterals. Eur J Radiol. 2011;80:786–791. doi: 10.1016/j.ejrad.2010.09.043. [DOI] [PubMed] [Google Scholar]
- 57.Liu W, Yin Q, Yao L, et al. Decreased hyperintense vessels on FLAIR images after endovascular recanalization of symptomatic internal carotid artery occlusion. Eur J Radiol 2011, E-pub ahead of print.
- 58.Lee KY, Latour LL, Luby M, Hsia AW, Merino JG, Warach S. Distal hyperintense vessels on FLAIR: an MRI marker for collateral circulation in acute stroke? Neurology. 2009;72:1134–1139. doi: 10.1212/01.wnl.0000345360.80382.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wechsler LR, Roberts R, Furlan AJ, et al. Factors influencing outcome and treatment effect in PROACT II. Stroke. 2003;34:1224–1229. doi: 10.1161/01.STR.0000068782.15297.28. [DOI] [PubMed] [Google Scholar]
- 60.Patil CG, Long EF, Lansberg MG. Cost-effectiveness analysis of mechanical thrombectomy in acute ischemic stroke. J Neurosurg. 2009;110:508–513. doi: 10.3171/2008.8.JNS08133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raychev R, Ovbiagele B. Endovascular therapy of acute ischemic stroke. Exp Opin Pharmacother. 2011;12:913–930. doi: 10.1517/14656566.2011.543897. [DOI] [PubMed] [Google Scholar]
- 62.Balucani C, Grotta JC. Selecting stroke patients for intra-arterial therapy. Neurology. 2012;78:755–761. doi: 10.1212/WNL.0b013e318248e558. [DOI] [PubMed] [Google Scholar]
- 63.Natarajan SK, Dandona P, Karmon Y, et al. Prediction of adverse outcomes by blood glucose level after endovascular therapy for acute ischemic stroke. J Neurosurg. 2011;114:1785–1799. doi: 10.3171/2011.1.JNS10884. [DOI] [PubMed] [Google Scholar]
- 64.Hallevi H, Barreto AD, Liebeskind DS, et al. Identifying patients at high risk for poor outcome after intra-arterial therapy for acute ischemic stroke. Stroke. 2009;40:1780–1785. doi: 10.1161/STROKEAHA.108.535146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flint AC, Cullen SP, Faigeles BS, Rao VA. Predicting long-term outcome after endovascular stroke treatment: the totaled health risks in vascular events score. AJNR Am J Neuroradiol. 2010;31:1192–1196. doi: 10.3174/ajnr.A2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishkanian AA, McCullough-Hicks ME, Appelboom G, et al. Improving patient selection for endovascular treatment of acute cerebral ischemia: a review of the literature and an external validation of the Houston IAT and THRIVE predictive scoring systems. Neurosurg Focus. 2011;30:E7. doi: 10.3171/2011.3.FOCUS1144. [DOI] [PubMed] [Google Scholar]
- 67.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics – 2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 68.Standard SC, Chavis TD, Wakhloo AK, Ahuja A, Guterman LR, Hopkins LN. Retrieval of a Guglielmi detachable coil after unraveling and fracture: case report and experimental results. Neurosurgery. 1994;35:994–998. doi: 10.1227/00006123-199411000-00038. discussion 999. [DOI] [PubMed] [Google Scholar]
- 69.Chopko BW, Kerber C, Wong W, Georgy B. Transcatheter snare removal of acute middle cerebral artery thromboembolism: technical case report. Neurosurgery. 2000;46:1529–1531. doi: 10.1097/00006123-200006000-00046. [DOI] [PubMed] [Google Scholar]
- 70.Nesbit GM, Luh G, Tien R, Barnwell SL. New and future endovascular treatment strategies for acute ischemic stroke. J VascI Interv Radiol. 2004;15:S103–S110. doi: 10.1097/01.rvi.0000112578.95689.66. [DOI] [PubMed] [Google Scholar]
- 71.Fourie P, Duncan IC. Microsnare-assisted mechanical removal of intraprocedural distal middle cerebral arterial thromboembolism. AJNR Am J Neuroradiol. 2003;24:630–632. [PMC free article] [PubMed] [Google Scholar]
- 72.Mayer TE, Hamann GF, Brueckmann HJ. Treatment of basilar artery embolism with a mechanical extraction device: necessity of flow reversal. Stroke. 2002;33:2232–2235. doi: 10.1161/01.str.0000024524.71680.c6. [DOI] [PubMed] [Google Scholar]
- 73.Zaidat OO, Tolbert M, Smith TP, Alexander MJ. Primary endovascular therapy with clot retrieval and balloon angioplasty for acute basilar artery occlusion. Pediatr Neurosurg. 2005;41:323–327. doi: 10.1159/000088735. [DOI] [PubMed] [Google Scholar]
- 74.Mourand I, Brunel H, Costalat V, et al. Mechanical thrombectomy in acute ischemic stroke: catch device. AJNR Am J Neuroradiol. 2011;32:1381–1385. doi: 10.3174/ajnr.A2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schumacher HC, Meyers PM, Yavagal DR, et al. Endovascular mechanical thrombectomy of an occluded superior division branch of the left MCA for acute cardioembolic stroke. Cardiovasc Intervent Radiol. 2003;26:305–308. doi: 10.1007/s00270-003-2719-5. [DOI] [PubMed] [Google Scholar]
- 76.Kerber CW, Wanke I, Bernard J, Jr, Woo HH, Liu MW, Nelson PK. Rapid intracranial clot removal with a new device: the alligator retriever. AJNR Am J Neuroradiol. 2007;28:860–863. [PMC free article] [PubMed] [Google Scholar]
- 77.Ansari S, Rahman M, McConnell DJ, Waters MF, Hoh BL, Mocco J. Recanalization therapy for acute ischemic stroke. 2. Mechanical intra-arterial technologies. Neurosurg Rev. 2011;34:11–20. doi: 10.1007/s10143-010-0294-1. [DOI] [PubMed] [Google Scholar]
- 78.Henkes H, Reinartz J, Lowens S, et al. A device for fast mechanical clot retrieval from intracranial arteries (Phenox clot retriever) Neurocrit Care. 2006;5:134–140. doi: 10.1385/NCC:5:2:134. [DOI] [PubMed] [Google Scholar]
- 79.Schild AF, Baltodano NM, Elias R, Livingstone J, Raines JK. Latis™ catheter: new technology for thrombectomy of vascular access grafts. J Vasc Access. 2003;4:118–122. [PubMed] [Google Scholar]
- 80.Berlis A, Lutsep H, Barnwell S, et al. Mechanical thrombolysis in acute ischemic stroke with endovascular photoacoustic recanalization. Stroke. 2004;35:1112–1116. doi: 10.1161/01.STR.0000124126.17508.d3. [DOI] [PubMed] [Google Scholar]
- 81.Owens CA. Ultrasound-enhanced thrombolysis: EKOS EndoWave Infusion Catheter System. Semin Intervent Radiol. 2008;25:37–41. doi: 10.1055/s-2008-1052304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee MS, Singh V, Wilentz JR, Makkar RR. AngioJet thrombectomy. J Invasive Cardiol. 2004;16:587–591. [PubMed] [Google Scholar]
- 83.Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke. 2005;36:2311–2320. doi: 10.1161/01.STR.0000182100.65262.46. [DOI] [PubMed] [Google Scholar]
- 84.Muller-Hulsbeck S, Grimm J, Leidt J, Jahnke T, Heller M. Comparison of in vitro effectiveness of mechanical thrombectomy devices. J Vasc Interv Radiol. 2001;12:1185–1191. doi: 10.1016/s1051-0443(07)61678-9. [DOI] [PubMed] [Google Scholar]
- 85.Bose A, Henkes H, Alfke K, et al. The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol. 2008;29:1409–1413. doi: 10.3174/ajnr.A1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu X, Xu G. Endovascular treatments of atherosclerotic carotid diseases in China. Int J Stroke. 2010;5:417–420. doi: 10.1111/j.1747-4949.2010.00471.x. [DOI] [PubMed] [Google Scholar]
- 87.Yue X, Xi G, Zhou Z, Xu G, Liu X. Combined intraarterial and intravenous thrombolysis for severe cerebral venous sinus thrombosis. J Thromb Thrombolysis. 2010;29:361–367. doi: 10.1007/s11239-009-0386-3. [DOI] [PubMed] [Google Scholar]
- 88.Yue X, Yin Q, Xi G, et al. Comparison of BMSs with SES for symptomatic intracranial disease of the middle cerebral artery stenosis. Cardiovasc Intervent Radiol. 2011;34:54–60. doi: 10.1007/s00270-010-9885-z. [DOI] [PubMed] [Google Scholar]
- 89.Zhu SG, Zhang RL, Liu WH, et al. Predictive factors for in-stent restenosis after balloon-mounted stent placement for symptomatic intracranial atherosclerosis. Eur J Vasc Endovasc Surg. 2010;40:499–506. doi: 10.1016/j.ejvs.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 90.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levy EI, Ecker RD, Horowitz MB, et al. Stent-assisted intracranial recanalization for acute stroke: early results. Neurosurgery. 2006;58:458–463. doi: 10.1227/01.NEU.0000199159.32210.E4. discussion 458–463. [DOI] [PubMed] [Google Scholar]
- 92.Levy EI, Ecker RD, Hanel RA, et al. Acute M2 bifurcation stenting for cerebral infarction: lessons learned from the heart – technical case report. Neurosurgery. 2006;58:E588. doi: 10.1227/01.NEU.0000197522.11613.0C. discussion E588. [DOI] [PubMed] [Google Scholar]
- 93.Fitzsimmons BF, Becske T, Nelson PK. Rapid stent-supported revascularization in acute ischemic stroke. AJNR Am J Neuroradiol. 2006;27:1132–1134. [PMC free article] [PubMed] [Google Scholar]
- 94.Levy EI, Siddiqui AH, Crumlish A, et al. First Food and Drug Administration-approved prospective trial of primary intracranial stenting for acute stroke: SARIS (stent-assisted recanalization in acute ischemic stroke) Stroke. 2009;40:3552–3556. doi: 10.1161/STROKEAHA.109.561274. [DOI] [PubMed] [Google Scholar]
- 95.Weber W, Bendszus M, Kis B, Boulanger T, Solymosi L, Kuhne D. A new self-expanding nitinol stent (Enterprise) for the treatment of wide-necked intracranial aneurysms: initial clinical and angiographic results in 31 aneurysms. Neuroradiology. 2007;49:555–561. doi: 10.1007/s00234-007-0232-2. [DOI] [PubMed] [Google Scholar]
- 96.Machi P, Costalat V, Lobotesis K, et al. Solitaire FR thrombectomy system: immediate results in 56 consecutive acute ischemic stroke patients. J Neurointerv Surg. 2012;4:62–66. doi: 10.1136/jnis.2010.004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caloro M, Lionetto L, Cuomo I, et al. An improved simple LC-MS/MS method for the measurement of serum aripiprazole and its major metabolite. J Pharm Biomed Anal. 2012;62:135–139. doi: 10.1016/j.jpba.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 98.Mendonca N, Flores A, Pagola J, et al. Trevo System: single-center experience with a novel mechanical thrombectomy device. J Neuroimaging 2011, E-pub ahead of print.
- 99.Gobin YP, Starkman S, Duckwiler GR, et al. MERCI 1: a phase 1 study of mechanical embolus removal in cerebral ischemia. Stroke. 2004;35:2848–2854. doi: 10.1161/01.STR.0000147718.12954.60. [DOI] [PubMed] [Google Scholar]
- 100.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 101.Penumbra Pivotal Stroke Trial Investigators The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 102.Tarr R, Hsu D, Kulcsar Z, et al. The POST trial: initial post-market experience of the Penumbra system: revascularization of large vessel occlusion in acute ischemic stroke in the United States and Europe. J Neurointerv Surg. 2010;2:341–344. doi: 10.1136/jnis.2010.002600. [DOI] [PubMed] [Google Scholar]
- 103.Levy EI, Mehta R, Gupta R, et al. Self-expanding stents for recanalization of acute cerebrovascular occlusions. AJNR Am J Neuroradiol. 2007;28:816–822. [PMC free article] [PubMed] [Google Scholar]
- 104.Linfante I, Samaniego EA, Geisbusch P, Dabus G. Self-expandable stents in the treatment of acute ischemic stroke refractory to current thrombectomy devices. Stroke. 2011;42:2636–2638. doi: 10.1161/STROKEAHA.111.618389. [DOI] [PubMed] [Google Scholar]