Abstract
Carotid artery stenting (CAS) is less invasive and has a lower incidence of systemic complications such as myocardial infarction compared with carotid endarterectomy. However, CAS is known to have a high incidence of ischemic complications due to distal thromboembolism. Progress has been made in the development of various distal protection devices and protection methods aimed at preventing thromboembolism. Similar to these methods, perioperative antiplatelet therapy is also able to play a very important role in the prevention of ischemic events. Dual antiplatelet therapy has become standard for perioperative management of CAS.
Key Words : Carotid artery, Stenting, Ischemic stroke
Introduction
Cerebrovascular disease is a leading cause of morbidity and mortality in most developed countries. Extracranial carotid artery stenosis, which is a part of systemic arteriosclerotic disease, is an important cause of ischemic stroke. Even in Japan, the number of patients with this disease is increasing with westernization of the lifestyle. For patients with mild-to-moderate asymptomatic carotid stenosis, which poses a lower risk of ischemic stroke, medical treatments such as antiplatelet therapy are recommended [1,2,3]. However, for patients with severe carotid stenosis, which poses a higher risk of ischemic stroke, antiplatelet therapy alone does not provide a sufficient preventive effect against ischemic stroke, and surgical revascularization is necessary [4,5].
Carotid endarterectomy has been the standard surgical procedure for extracranial carotid artery stenosis. This procedure entails direct exposure of the carotid artery and surgically removal of the atherosclerotic plaque. There is a high level of evidence suggesting its superiority to medical treatment alone in preventing stroke in patients with symptomatic or asymptomatic extracranial carotid artery stenosis (table 1). However, an important disadvantage is that such a highly invasive procedure sometimes causes perioperative adverse events, especially in high-surgical-risk populations.
Table 1.
Results of randomized clinical trials of endarterectomy for carotid artery stenosis
| Trial | Status | Follow-up | Primary end point | CEA | Medical therapy | p value |
|---|---|---|---|---|---|---|
| NASCET [4] | Symptomatic ≥70% | 2 years | Ipsilateral stroke | 9.0% | 26.0% | <0.001 |
| ECST [5] | Symptomatic ≥80% | 3 years | Stroke or death | 14.9% | 26.5% | <0.001 |
| ACAS [1] | Asymptomatic ≥60% | 5 years | Ipsilateral stroke, | 5.1% | 11.0% | 0.004 |
| periprocedual death | ||||||
| ACST [2] | Asymptomatic ≥60% | 5 years | Any stroke | 6.4% | 11.8% | 0.0001 |
In the 1990s, carotid artery stenting (CAS) emerged as a less invasive revascularization procedure, providing an alternative to endarterectomy. Several large-scale randomized clinical studies comparing the therapeutic results of CAS and endarterectomy have been reported [6,7,8,9,10]. However, whereas some studies have proven the noninferiority of CAS to endarterectomy, others have been unable to do so. Thus, we cannot say which therapeutic technique is superior yet (table 2). It is currently considered appropriate for high-surgical-risk populations, such as elderly patients or those with severe comorbidities or restenosis after carotid endarterectomy.
Table 2.
Results of randomized clinical trials compared to endarterectomy and carotid artery stenting
| Trial | Status | End point | CAS | CEA | p value |
|---|---|---|---|---|---|
| SAPPHIRE [6] | CEA high risk symptomatic ≥50% asymptomatic ≥80% | 30 days: MI; 1 year: ipsilateral stroke or death | 12.2% | 20.1% | 0.05 |
| SPACE [7] | Symptomatic ≥50% | 30 days: ipsilateral stroke or death | 6.8% | 6.3% | NS |
| EVA-3S [8] | Symptomatic ≥70% | 30 days: death; 4 years: ipsilateral stroke | 9.6% | 3.9% | NS |
| CREST [9] | Symptomatic ≥50% asymptomatic ≥80% | 4 years: ipsilateral stroke or surgical death | 7.2% | 6.8% | NS |
| ICSS [10] | Symptomatic ≥50% | 120 days: stroke, death, or procedural MI | 8.5% | 5.2% | 0.006 |
Among those clinical studies, the CREST [9] trial has shown some interesting results that reflect the respective characteristics of each procedure, i.e. CAS and carotid endarterectomy. This randomized trial compared the therapeutic results of CAS and endarterectomy in patients who had symptomatic lesions with ≥50% stenosis or asymptomatic lesions with ≥60% stenosis. The incidence of periprocedural stroke, myocardial infarction (MI), death, or ipsilateral stroke within 4 years of the procedure did not differ significantly between the two groups (7.2% with CAS and 6.8% with endarterectomy, p = 0.51), thus proving the noninferiority of CAS to carotid endarterectomy. However, the most noteworthy point of the CREST trial is that manifesting events differed greatly between the two procedures. That is, the incidence of periprocedural ischemic stroke was higher with CAS (4.1 vs. 2.3%, p = 0.01), whereas the incidence of perioperative MI was higher with endarterectomy (1.1 vs. 2.3%, p = 0.03).
CAS often leads to ischemic events by thromboembolism that are normally rarely seen in carotid endarterectomy. During CAS, percutaneous interventions cause intimal injury of the arterial vessel, which releases procoagulant tissue factor and exposes collagen in the subendothelium, thereby triggering the activation of platelets and subsequent formation of a thrombus. Symptomatic ischemic stroke occurred with an incidence of 2-9%, and asymptomatic ischemic lesions detected on diffusion-weighted imaging by distal embolism were observed with an incidence of 25-41% after protected CAS [11,12,13].
Perioperative Ischemic Complications of Carotid Stenting
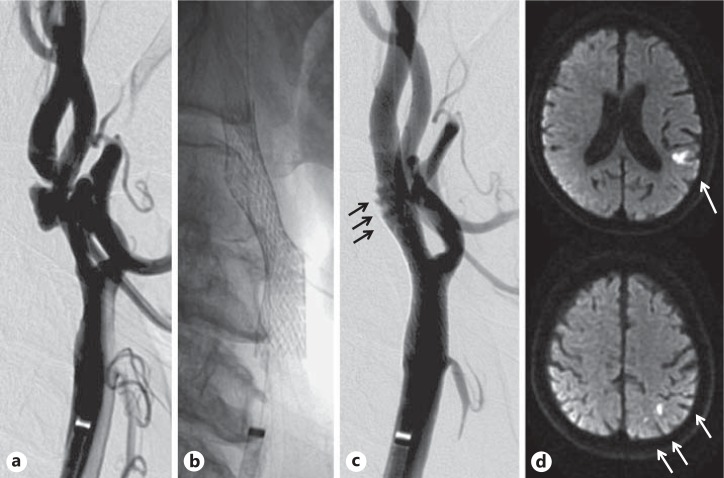
Two mechanisms are considered for the occurrence of ischemic stroke during and after CAS: (1) distal embolism due to ruptured plaque and (2) formation of a mural thrombus due to platelet activation caused by intimal injury or a foreign body placed in the blood vessel. However, it is difficult to recognize the cause using imaging diagnosis only (fig. 1). Antiplatelet therapy plays a key role in the prevention of mechanism 2.
Fig. 1.
Representative images of distal embolism by CAS. a Prestenting angiogram of left internal carotid artery stenosis. b Stenting was performed with distal protection with a balloon. c Poststenting angiogram showing in-stent thrombus formation (black arrows). d Diffusion-weighted magnetic resonance imaging. The white arrows show multiple ischemic lesions detected in the left hemisphere after the procedure.
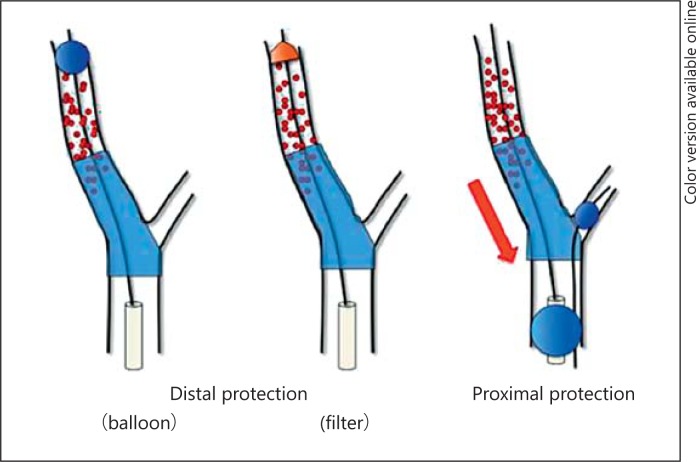
Various protection devices to prevent distal embolism have been developed to prevent ischemic stroke by mechanism 1. According to the global registry of Wholey et al. [14], the incidence of perioperative stroke and death was high at 5.2% in a patient group for which protection devices were not used, but it was significantly lower at 2.2% in a patient group for which protection devices were used. These results contributed to recognition of the importance of protection devices for the prevention of periprocedural ischemic stroke of CAS [14]. Protection methods can be divided into distal protection with balloon or filter devices and proximal protection using double balloon catheter (fig. 2). The distal protection method is simple, but there is a possibility that debris will leak out through gaps in the vascular wall. With proximal protection, the recovery rate of debris is higher, but this method has some disadvantages such as that it is a complicated procedure and that it requires placement of a larger sheath than usual.
Fig. 2.
Various protection devices to prevent distal embolism by CAS.
Moreover, there have been recent advances in plaque-imaging techniques using magnetic resonance imaging, etc., that provide preoperative information regarding vulnerable plaque that might readily cause distal embolism. Distal embolism after CAS was reportedly more often observed in patients with symptomatic echolucent plaque [15], a necrotic core in the plaques on virtual histology intravascular ultrasound [16], or high-intensity signals in the plaques detected by black-blood MRI [17] or time-of-flight MRA [18]. CAS or endarterectomy should be selected on the basis of the patient's background, i.e. age, coexisting diseases, or character of the plaque. If vulnerable plaque is detected by preoperative plaque imaging, endarterectomy is the best option because it has a lower risk of ischemic stroke than CAS [19,20].
For the prevention of ischemic stroke by mechanism 2, antiplatelet therapy is essential. In coronary intervention, which has a longer history than CAS, standard perioperative antithrombotic therapy was carried out using an anticoagulant regimen of heparin or warfarin and aspirin monotherapy. However, subacute stent thrombosis occurred as a serious complication in approximately 5-20% of patients [21,22]. Comparative studies of dual antiplatelet therapies (DAPT) and anticoagulant therapies were performed in the late 1990s. Schömig et al. [22] undertook a prospective study of postoperative antithrombotic therapy in patients who had undergone coronary artery stenting by randomizing them into an oral aspirin plus heparin/warfarin anticoagulant therapy group and an oral aspirin plus ticlopidine DAPT group. The incidence of in-stent thrombosis after 30 days was 5.4% in the anticoagulant therapy group and 0.8% in the DAPT group, and they reported the superiority of DAPT. Based on that history, the superiority of DAPT became common knowledge with regard to periprocedural management in coronary artery intervention.
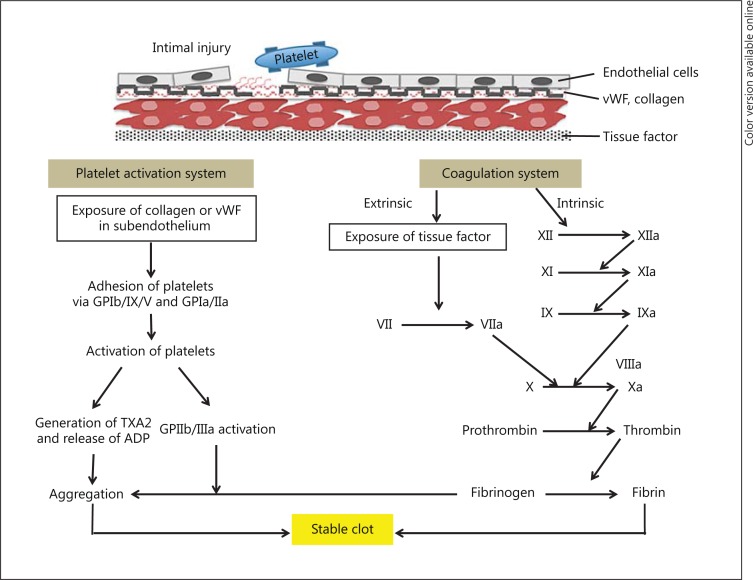
The process of thrombus formation is known to involve a mutual interaction between the coagulation system and the platelet activation system. If there is damage to the vascular endothelium, glycoprotein (GP) Ia/IIa and Ib/V/IX receptors on the platelets react with von Willebrand factor and collagen in the subendothelial tissue and initiate a platelet adhesion reaction. Platelet adhesion stimulates platelet activation, which leads to a release of adenosine diphosphate (ADP) and other intragranular substances, generation of thromboxane A2 from arachidonic acid, etc., and culminates in activation of the GPIIb/IIIa receptor, which binds to fibrinogen. Simultaneously, in the coagulation system, there is activation of various coagulation factors from tissue factors, etc., that are released at the site of endothelial damage, and fibrinogen is generated. Finally, the generated fibrinogen binds to the activated GPIIb/IIIa on the surface of platelets, these platelets adhere to each other, and a fibrin clot is formed [23] (fig. 3).
Fig. 3.
Important steps in thrombus formation. vWF = von Willebrand factor; TXA2 = thromboxane A2.
Red thrombi are formed from the activation of the coagulation system at sites of flow stagnation, such as inside catheters, and have red blood cells and fibrin as their main constituents, whereas white thrombi are formed from the activation of platelets at sites of endothelial damage by balloon angioplasty or stenting and have platelets as their main constituent [24]. Red thrombi can be prevented by anticoagulants such as heparin, but white thrombi cannot.
Status of Antiplatelet Therapy for CAS
As antithrombotic therapy for CAS, coadministration of aspirin and a thienopyridine antiplatelet drug has been reported to be more effective than antiplatelet monotherapy plus combination therapy with anticoagulants, such as aspirin alone and a 24-hour continuous drip infusion of heparin. Coadministration of two antiplatelet agents (DAPT) is now considered to be the norm during the perioperative period for CAS. In a randomized control trial for perioperative antithrombotic treatment for CAS, McKevitt et al. [25] compared the perioperative complications of aspirin plus heparin as an anticoagulant group and aspirin plus clopidogrel as a DAPT group. Compared to the anticoagulant group, the DAPT group showed lower incidences of both ischemic complications (0 vs. 25%, respectively) and hemorrhagic complications (9 vs. 17%, respectively), and the authors reported that the incidence of any kind of perioperative complications was reduced. Dalainas et al. [26] also reported the efficacy of DAPT in patients undergoing CAS (table 3). From the results of these studies, it would become known that DAPT was associated with lower ischemic complications and lower hemorrhagic complications than anticoagulant therapy. On the other hand, cases of fatal strokes in CAS patients who underwent CAS who were not administered DAPT (aspirin alone or no antiplatelet agent) were reported [27]. With regard to the currently recommended perioperative antithrombotic treatment at the time of CAS, five US academic societies released a Consensus Document on Carotid Stenting [28]. That document stated that ‘the patient should be orally administered two antiplatelet agents (aspirin, clopidogrel) for at least 4 days prior to the operation when possible, and at least 24 h prior to the operation’, and ‘postoperatively, clopidogrel should be continued for at least 30 days, and aspirin should be continued for the rest of the patient's life’. This has become the standard perioperative antiplatelet therapy.
Table 3.
Randomized trial compared with aspirin plus anticoagulant therapy and dual antiplatelet therapy
| First author | Outcome | Aspirin + heparin | Aspirin + clopidogrel | p value |
|---|---|---|---|---|
| McKevitt [25] | 30-day incidence of adverse neurological events | 25% | 0% | 0.02 |
| Bleeding complication | 17% | 9% | 0.035 | |
| Dalainas [26] | 30-day incidence of adverse neurological events | 16% | 2% | <0.05 |
| Bleeding complication | 4% | 2% | NS | |
| Subacute stent thrombosis | 2% | 0% | NS | |
Which Antiplatelet Agents Are Better for CAS?
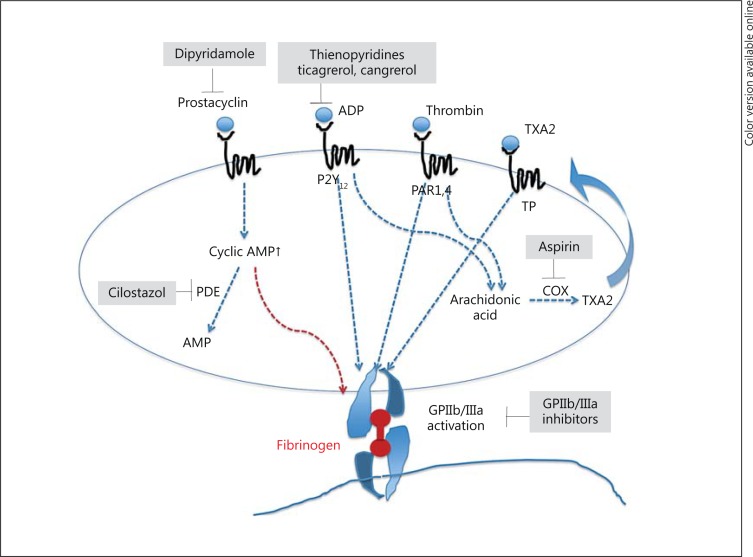
The combination of aspirin and clopidogrel has become common for DAPT in CAS based on evidence generated in coronary artery intervention. However, several antiplatelet agents are available (fig. 4). The dose, regimen, or combination of these antiplatelet agents for CAS is not established.
Fig. 4.
Target of antiplatelet agents. TXA2 = Thromboxane A2; TP = thromboxane receptor; PAR = protease-activated receptor; COX = cyclooxygenase; AMP = adenosine monophosphate; PDE = phosphodiesterase.
Aspirin
Aspirin irreversibly acetylates the enzyme cyclooxygenase-1 at serine 529 preventing the conversion of arachidonic acid to thromboxane A2 in a dose-dependent manner [29]. Aspirin is the most widely used medicine for the prevention of cardiovascular diseases and reduces the risk of MI, stroke, and cardiovascular death by about 20% in a broad range of patients at high risk for future cardiovascular events [30]. Based on randomized trials, aspirin is superior to CEA for patients with mild-to-moderate-grade carotid stenosis [2,4,31]. For patients undergoing endarterectomy, low-dose aspirin is beneficial and reduces the perioperative risk of stroke, MI, and death [32].
It is known that not all patients derive the same benefit from aspirin and up to 40% may be resistant to its effects due to many potential causes [33,34]. Although there are various laboratory assays to assess aspirin resistance (table 4), no currently developed assay is specific for aspirin resistance and no accepted definition of aspirin resistance has been established [35,36,37].
Table 4.
Platelet function test
| Test | Details | Blood sample |
|---|---|---|
| Light transmission aggregometry | Platelet aggregation by several antagonists (collagen, ADP, shear stress, epinephrine, arachidonic acid) | Platelet-rich plasma |
| Point of care analysis | PFA-100, verify now, platelet works, etc. | Whole blood |
| Flow cytometry | VASP phosphorylation, P-selectin | Whole blood |
| ELISA | Urine 11-dehydroTXB2, serum TXB2, platelet-derived microparticles | Serum |
TXB2 = Thromboxane; ELISA = enzyme-linked immunosorbent assay; VASP = vasodilator-stimulated phosphoprotein.
Thienopyridines
Thienopyridines are prodrugs that selectively irreversibly inhibit the adenosine 5′-diphosphate P2Y12 receptor, first-generation thienopyridine, second-generation clopidogrel, and third-generation prasugrel.
For the prevention of stroke in secondary prevention trials, clopidogrel has been shown to be more effective in reducing the risk for MI, ischemic stroke, and vascular death in patients at risk of ischemic events [38].
In patients with acute coronary syndrome treated with coronary interventions, long-term DAPT with clopidogrel and aspirin is more effective than aspirin alone in preventing major cardiovascular events which include stent thrombosis [39,40]. The onset of the effect of clopidogrel is relatively slow because clopidogrel is a prodrug that requires metabolism for conversion to its active metabolite. So, achievement of effective platelet inhibition requires some hours after a loading dose [41]. Clopidogrel has platelet inhibition variability as does aspirin; approximately 15-48% of patients have a poor platelet inhibition response to clopidogrel [42,43,44]. The antiplatelet effects achieved with a loading dose of clopidogrel are not always rapid or platelet reactivity measured by laboratory assays may persist in some patients despite the adjunctive use of clopidogrel. Certain common genetic variants of the hepatic cytochrome P-450 system that are involved in the conversion of clopidogrel to its active metabolite have been suggested as one of the causes [45]. Specifically, in patients who are carriers of a loss-of-function CYP2C19 allele (including the *2 and *3 alleles), the conversion of clopidogrel to its active metabolite may be reduced, resulting in decreased inhibition of platelet function and increased poor cardiovascular outcomes [46,47]. Conversely, carriers of the ultrarapid enzyme activity allele *17 have an increased platelet response to clopidogrel and an increased risk of bleeding [48]. On the contrary, it has also been reported that these variants of CYP2C19 do not modify the efficacy and safety of clopidogrel clinically, so larger studies will be needed to definitively assess a genetic effect on the patients [49].
For carotid artery stenosis, a randomized control trial in CAS which compared heparin and clopidogrel as treatment in addition to aspirin revealed a superiority of clopidogrel as mentioned above [25,26]. In addition, the Clopidogrel and Aspirin for the Reduction of Emboli in Symptomatic carotid Stenosis (CARESS) trial [50] showed that DAPT with clopidogrel and aspirin reduced the incidence of microembolisms determined by transcranial Doppler ultrasonography by 73% compared with aspirin monotherapy.
Other Adenosine 5′-Diphosphate P2Y12 Receptor Antagonists
These reversible selective P2Y12 receptor antagonists were not evaluated in clinical trials for perioperative antithrombotic therapy of CAS.
Ticagrelor
Ticagrelor is a reversible adenosine 5′-diphosphate P2Y12 receptor antagonist. Unlike the thienopyridines, ticagrelor is not a prodrug and does not require metabolic activation to inhibit the P2Y12 receptor. Conversely, due to its shorter half-life, it requires a twice daily administration. The Phase III Study of Platelet Inhibition and Patients Outcomes (PLATO) trial reported that ticagrelor is more effective than clopidogrel in vascular death, MI, or stroke in the patients with acute coronary syndrome [51]. Contrary to this, it was also reported that ticagrelor was associated with a higher rate of major bleeding compered to clopidogrel [52].
Cangrelor
Cangrelor is an adenosine triphosphate analogue, which directly determines reversible inhibition of the P2Y12 receptor without needing hepatic biotransformation [53]. Cangrelor is administered intravenously, with a very rapid onset of its effect and a short half-life. Because it quickly returns to pretreatment levels, cangrelor may be advantageous for patients who need urgent surgery. Cangrelor was associated with a significant reduction in early ischemic events when compared with clopidogrel in patients with non-ST-elevation acute coronary syndrome undergoing PCI [53].
Dipyridamole
Dipyridamole is not recommended for primary prevention of cardiovascular stroke. In the ESPRIT, extended-release dipyridamole plus aspirin was superior to aspirin alone for secondary prevention of MI, stroke, or vascular death [54]. In other trials, compared with clopidogrel, extended-release dipyridamole plus aspirin could not show superiority to clopidogrel [55,56].
Cilostazol
Cilostazol, a reversible selective antagonist of phosphodiesterase III, reduces recurrent stroke with fewer bleeding complications by inhibiting platelet aggregation and augmenting vasodilation [57,58,59,60]. It has a unique suppressive effect on the proliferation of vascular smooth muscle, as shown by its reduction of intimal hyperplasia and restenosis in patients after coronary intervention [61]. Especially in drug-eluting stents, triple antiplatelet therapy with cilostazol, aspirin, and clopidogrel decreased angiographic restenosis, resulting in a reduced risk of target lesion revascularization compared with DAPT in diabetic patients [62] or in drug-eluting stent implantation for long coronary lesions [63]. A preventive effect of cilostazol on restenosis is similarly recognized after CAS [64,65], so these results suggest that cilostazol may be a treatment option as perioperative antiplatelet therapy for CAS. Moreover, although cilostazol is expected to have side effects such as headache and tachycardia [59], cilostazol may exert preventive effects with regard to prolonged bradycardia due to factors such as the carotid sinus reflex following CAS. Poor platelet inhibition response to cilostazol has been not reported.
GPIIb/IIIa Antagonist
With regard to GPIIb/IIIa antagonists, when administered starting 24 h prior to CAS, the incidence of ischemic complications reportedly decreased compared to the group with no administration, but cases of fatal intracranial hemorrhage were encountered [66,67]. There is a need for a controlled, prospective trial to clarify this safety aspect.
The optimal drug combination for antithrombotic therapy remains unclear, but care seems warranted regarding the possibility of poor platelet inhibition response to drugs, and verification of antiplatelet effects by performing platelet function tests prior to stenting procedures is needed. Platelet function tests should also be performed with the objective of preventing hemorrhagic complications of CAS, since the procedure could – as in the case of carotid endarterectomy – lead to intracranial bleeding due to postoperative hyperperfusion [68,69] (fig. 5), and use of a large-diameter system might cause puncture site hematoma [70]. An individual tailored antiplatelet therapy based on platelet function tests may eliminate resistance to antiplatelet therapy and reduce perioperative complications [71].
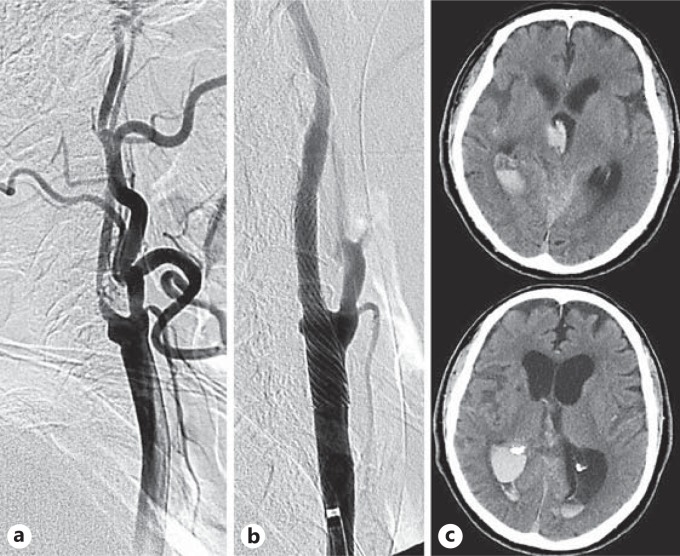
Fig. 5.
The representative case of intracranial hemorrhage due to hyperfusion syndrome after carotid stenting. a Prestenting angiogram of right internal carotid artery stenosis. b Stenting was performed with proximal protection. c The consciousness level of the patients gradually deteriorated 2 h after the procedure. Computed tomography revealed subarachnoid hemorrhage in the right sylvian fissure and intraventricular hemorrhage.
Loading at the Time of Emergency Carotid Stenting
The most troubling problems are encountered when emergency CAS is required. When emergency recanalization is performed for acute occlusion, various situations may present, such as the discovery of stenosis of the cervical internal carotid artery or dissection of the intracranial internal carotid artery, or onset of severe stenosis causing progressive stroke. These situations are encountered surprisingly frequently. In these situations, loading administration of antiplatelet agents is necessary to prevent subacute stent thrombosis.
Aspirin shows the most rapid effect, seen within about 1 h. Clopidogrel loading at 300 mg is also approved in the coronary artery field, but efficacy with 600 mg has recently been reported [72]. We perform loading with 200 mg of aspirin and 600 mg of clopidogrel. In extreme emergencies, we sometimes add 200 mg of cilostazol.
Conclusion
CAS is a widely accepted alternative for patients at high risk for endarterectomy. However, evidence for many antiplatelet agents that are used for preventing periprocedural ischemic events has not been established. Clopidogrel 75 mg and low-dose aspirin are commonly used in CAS but have not been standardized, and the dose and regimen of other agents such as the newly developed P2Y12 receptor antagonists, dipyridamole, cilostazol, or the GPIIbIIIa antagonist used for CAS have not been not established, although there are some reports of their effectiveness. A prospective comparative study involving a greater number of patients undergoing CAS may be needed in the future to properly assess these antiplatelet agents.
References
- 1.Endarterectomy for asymptomatic carotid artery stenosis – Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 2.Halliday A, Mansfield A, Marro J, MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 3.Norris JW, Zhu CZ, Bornstein NM, Chambers BR. Vascular risks of asymptomatic carotid stenosis. Stroke. 1991;22:1485–1490. doi: 10.1161/01.str.22.12.1485. [DOI] [PubMed] [Google Scholar]
- 4.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis – North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Eng J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 5.Randomised trial of endarterectomy for recently symptomatic carotid stenosis final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- 6.Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 7.SPACE Collaborative Group. Ringleb PA, Allenberg J, et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–1247. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 8.Mas JL, Chatellier G, Beyssen B, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Eng J Med. 2006;355:1660–1671. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 9.Brott TG, Hobson RW, 2nd, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Eng J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Carotid Stenting Study Investigators. Ederle J, Dobson J, Featherstone RL, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–997. doi: 10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kastrup A, Nägele T, Gröschel K, et al. Incidence of new brain lesions after carotid stenting with and without cerebral protection. Stroke. 2006;37:2312–2316. doi: 10.1161/01.STR.0000236492.86303.85. [DOI] [PubMed] [Google Scholar]
- 12.Hammer FD, Lacroix V, Duprez T, et al. Cerebral microembolization after protected carotid artery stenting in surgical high-risk patients: results of a 2-year prospective study. J Vasc Surg. 2005;42:847–853. doi: 10.1016/j.jvs.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 13.Flach HZ, Ouhlous M, Hendriks JM, et al. Cerebral ischemia after carotid intervention. J Endovasc Ther. 2004;11:251–257. doi: 10.1583/03-1128.1. [DOI] [PubMed] [Google Scholar]
- 14.Wholey MH, Wholey M, Mathias K, et al. Global experience in cervical carotid artery stent placement. Catheter Cardiovasc Interv. 2000;50:160–167. doi: 10.1002/(sici)1522-726x(200006)50:2<160::aid-ccd2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 15.Giannakopoulos TG, Moulakakis K, Sfyroeras GS, et al. Association between plaque echogenicity and embolic material captured in filter during protected carotid angioplasty and stenting. Eur J Vasc Endovasc Surg. 2012;43:627–631. doi: 10.1016/j.ejvs.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Timaran CH, Rosero EB, Martinez AE, et al. Atherosclerotic plaque composition assessed by virtual histology intravascular ultrasound and cerebral embolization after carotid stenting. J Vasc Surg. 2010;52:1188–1194. doi: 10.1016/j.jvs.2010.05.101. [DOI] [PubMed] [Google Scholar]
- 17.Yamada K, Kawasaki M, Yoshimura S, et al. Prediction of silent lesions after carotid artery stenting using integrated backscatter ultrasound and magnetic resonance imaging. Atherosclerosis. 2010;208:161–166. doi: 10.1016/j.atherosclerosis.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura S, Yamada K, Kawasaki M, et al. High-intensity signal on time-of-flight magnetic resonance angiography indicates carotid plaques at high risk for cerebral embolism during stenting. Stroke. 2011;42:3132–3137. doi: 10.1161/STROKEAHA.111.615708. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura S, Yamada K, Kawasaki M, et al. Selection of carotid artery stenting or endarterectomy based on magnetic resonance plaque imaging reduced periprocedural adverse events. J Stroke Cerebrovasc Dis 2012, E-pub ahead of print. [DOI] [PubMed]
- 20.Yamada K, Yoshimura S, Kawasaki M, et al. Embolic complications after carotid artery stenting or carotid endarterectomy are associated with tissue characteristics of carotid plaques evaluated by magnetic resonance imaging. Atherosclerosis. 2011;215:399–404. doi: 10.1016/j.atherosclerosis.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Serruys PW, Strauss BH, Beatt KJ, et al. Angiographic follow-up after placement of a self-expanding coronary-artery stent. N Engl J Med. 1991;324:13–17. doi: 10.1056/NEJM199101033240103. [DOI] [PubMed] [Google Scholar]
- 22.Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after placement of coronary-artery stents. N Eng J Med. 1996;334:1084–1089. doi: 10.1056/NEJM199604253341702. [DOI] [PubMed] [Google Scholar]
- 23.Qureshi AI, Luft AR, Sharma M, et al. Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures. 1. Pathophysiological and pharmacological features. Neurosurgery. 2000;46:1344–1359. doi: 10.1097/00006123-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Parsson H, Cwikiel W, Johansson K, et al. Deposition of platelets and neutrophilis in porcine iliac arteries after angioplasty and Wallstent placement compared with angioplasty alone. Cardiovasc Intervent Radiol. 1994;17:190–196. doi: 10.1007/BF00571533. [DOI] [PubMed] [Google Scholar]
- 25.McKevitt FM, Randall MS, Cleaveland TJ, et al. The benefits of combined anti-platelet treatment in carotid artery stenting. Eur J Vasc Endovasc Surg. 2005;29:522–527. doi: 10.1016/j.ejvs.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Dalainas I, Nano G, Bianchi P, et al. Dual antiplatelet regime versus acetyl-acetic acid for carotid artery stenting. Cardiovasc Intervent Radiol. 2006;29:519–521. doi: 10.1007/s00270-005-5288-y. [DOI] [PubMed] [Google Scholar]
- 27.Chaturvedi S, Sohrab S, Tselis A. Carotid stent thrombosis: report of 2 fatal cases. Stroke. 2001;32:2700–2702. [PubMed] [Google Scholar]
- 28.Bates ER, Babb JD, Casey DE, Jr, American College of Cardiology Foundation Task Force, American Society of Interventional and Therapeutic Neuroradiology, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society for Interventional Radiology ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting. Vasc Med. 2007;12:35–83. [Google Scholar]
- 29.Loll PJ, Picot D, Garavito RM. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat Struct Biol. 1995;2:637–643. doi: 10.1038/nsb0895-637. [DOI] [PubMed] [Google Scholar]
- 30.Antithrombotic Trialists' Collaboration Collaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high-risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endarterectomy for asymptomatic carotid artery stenosis – Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 32.Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial – ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet. 1999;353:2179–2184. doi: 10.1016/s0140-6736(99)05388-x. [DOI] [PubMed] [Google Scholar]
- 33.Grotemeyer KH, Scharafinski HW, Husstedt IW. Two-year follow-up of aspirin responder and aspirin non-responder: a pilot study including 180 post-stroke patients. Thromb Res. 1993;71:397–403. doi: 10.1016/0049-3848(93)90164-j. [DOI] [PubMed] [Google Scholar]
- 34.Gum PA, Kottke-Marchant K, Welsh PA, et al. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41:961–965. doi: 10.1016/s0735-1097(02)03014-0. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald R, Pirmohamed M. Aspirin resistance: effect of clinical, biochemical and genetic factors. Pharmacol Ther. 2011;130:213–225. doi: 10.1016/j.pharmthera.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Hankey GJ, Eikelboom JW. Aspirin resistance. Lancet. 2006;367:606–617. doi: 10.1016/S0140-6736(06)68040-9. [DOI] [PubMed] [Google Scholar]
- 37.Tan HA, Anand SS, Hankey GJ. Aspirin resistance. Thromb Res. 2007;120:337–346. doi: 10.1016/j.thromres.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 38.CAPRIE Steering Committee A randomized, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 39.Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 40.Steinhubl SR, Berger PB, Mann JT, 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 41.Wallentin L, Varenhorst C, James S, et al. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J. 2008;29:21–30. doi: 10.1093/eurheartj/ehm545. [DOI] [PubMed] [Google Scholar]
- 42.Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 43.Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–3175. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 44.Gurbel PA, Bliden KP, Hiatt BL, et al. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 45.Holmes DR, Jr, Dehmer GJ, Kaul S, et al. ACCF/AHA clopidogrel clinical alert: approaches to the FDA ‘boxed warning’: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Caridol. 2010;56:321–341. doi: 10.1016/j.jacc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Shuldiner AR, O'Connel JR, Bliden KP, et al. Association of cytochrome p450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–858. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 48.Sibbing D, Koch W, Gebhard D, et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121:512–518. doi: 10.1161/CIRCULATIONAHA.109.885194. [DOI] [PubMed] [Google Scholar]
- 49.Pare G, Mehta SR, Yusuf S, et al. Effects of CYP2C19 genotype on outcome of clopidogrel treatment. N Eng J Med. 2010;363:1704–1714. doi: 10.1056/NEJMoa1008410. [DOI] [PubMed] [Google Scholar]
- 50.Markus HS, Droste DW, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. 2005;111:2233–2240. doi: 10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 51.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 52.Bellemain-Appaix A, Brieger D, Beygui F, et al. New P2Y12 inhibitors versus clopidogrel in percutaneous coronary intervention: a meta-analysis. J Am Coll Cardiol. 2010;56:1542–1551. doi: 10.1016/j.jacc.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 53.White HD, Chew DP, Dauerman HL, Mahaffey KW, Gibson CM, Stone GW, Gruberg L, Harrington RA, Bhatt DL. Reduced immediate ischemic events with cangrelor in PCI: a pooled analysis of the CHAMPION trials using the universal definition of myocardial infarction. Am Heart J. 2012;163:182–190. doi: 10.1016/j.ahj.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 54.ESPRIT Study Group. Halkes PH, van Gijn J, Kappelle LJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665–1673. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- 55.Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bath PM, Cotton D, Martin RH, PRoFESS Study Group Effect of combined aspirin and extended-release dipyridamole versus clopidogrel on functional outcome and recurrence in acute, mild ischemic stroke: PRoFESS subgroup analysis. Stroke. 2010;41:732–738. doi: 10.1161/STROKEAHA.109.564906. [DOI] [PubMed] [Google Scholar]
- 57.Huang Y, Cheng Y, Wu J, Cilostazol versus Aspirin for Secondary Ischaemic Stroke Prevention cooperation investigators Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double-blind, pilot study. Lancet Neurol. 2008;7:494–499. doi: 10.1016/S1474-4422(08)70094-2. [DOI] [PubMed] [Google Scholar]
- 58.Shinohara Y, Katayama Y, Uchiyama S, CSPS 2 group Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010;9:959–968. doi: 10.1016/S1474-4422(10)70198-8. [DOI] [PubMed] [Google Scholar]
- 59.Lee YS, Bae HJ, Kang DW, et al. Cilostazol in Acute Ischemic Stroke Treatment (CAIST Trial): a randomized double-blind non-inferiority trial. Cerebrovasc Dis. 2011;32:65–71. doi: 10.1159/000327036. [DOI] [PubMed] [Google Scholar]
- 60.Weintraub WS. The vascular effects of cilostazol. Can J Cardiol. 2006;22:56B–60B. doi: 10.1016/s0828-282x(06)70987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Douglas JS, Jr, Holmes DR, Jr, Kereiakes DJ, Cilostazol for Restenosis Trial (CREST) Investigators Coronary stent restenosis in patients treated with cilostazol. Circulation. 2005;112:2826–2832. doi: 10.1161/CIRCULATIONAHA.104.530097. [DOI] [PubMed] [Google Scholar]
- 62.Lee SW, Park SW, Kim YH, et al. Drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus the DECLARE-DIABETES Trial (a randomized comparison of triple antiplatelet therapy with dual antiplatelet therapy after drug-eluting stent implantation in diabetic patients) J Am Coll Cardiol. 2008;51:1181–1187. doi: 10.1016/j.jacc.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 63.Lee SW, Park SW, Kim YH, DECLARE-LONG II Study Investigators A randomized, double-blind, multicenter comparison study of triple antiplatelet therapy with dual antiplatelet therapy to reduce restenosis after drug-eluting stent implantation in long coronary lesions: results from the DECLARE-LONG II (Drug-Eluting Stenting Followed by Cilostazol Treatment Reduces Late Restenosis in Patients with Long Coronary Lesions) trial. J Am Coll Cardiol. 2011;57:1264–1270. doi: 10.1016/j.jacc.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 64.Takigawa T, Matsumaru Y, Hayakawa M. Cilostazol reduces restenosis after carotid artery stenting. J Vasc Surg. 2010;51:51–56. doi: 10.1016/j.jvs.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 65.Takayama K, Taoka T, Nakagawa H, et al. Effect of cilostazol in preventing restenosis after carotid artery stenting using the carotid wall stent: a multicenter retrospective study. AJNR Am J Neuroradiol. 2012;33:2167–2170. doi: 10.3174/ajnr.A3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qureshi AI, Suri MF, Ali Z, et al. Carotid angioplasty and stent placement: a prospective analysis of perioperative complications and impact of intravenously administered abciximab. Neurosurgery. 2002;50:466–473. doi: 10.1097/00006123-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Wholey MH, Wholey MH, Eles G, et al. Evaluation of glycoprotein IIb/IIIa inhibitors in carotid angioplasty and stenting. J Endovasc Ther. 2003;10:33–41. doi: 10.1177/152660280301000108. [DOI] [PubMed] [Google Scholar]
- 68.Abou-Chebl A, Yadav JS, Reginelli JP, et al. Intracranial hemorrhage and hyperperfusion syndrome following carotid artery stenting: risk factors, prevention, and treatment. J Am Coll Cardiol. 2004;43:1596–1601. doi: 10.1016/j.jacc.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 69.Morrish W, Grahovac S, Douen A, et al. Intracranial hemorrhage after stenting and angioplasty of extracranial carotid stenosis. Am J Neuroradiol. 2000;21:1911–1191. [PMC free article] [PubMed] [Google Scholar]
- 70.Taha MM, Sakaida H, Asakura F, et al. Access site complications with carotid angioplasty and stenting. Surg Neurol. 2007;68:431–437. doi: 10.1016/j.surneu.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 71.Neubauer H, Kaiser AF, Endres HG, et al. Tailored antiplatelet therapy can overcome clopidogrel and aspirin resistance: the Bochum Clopidogrel and Aspirin Plan (BOCLA-Plan) to improve antiplatelet therapy. [DOI] [PMC free article] [PubMed]
- 72.Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233–1243. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]