Abstract
Objectives
Thromboembolic complications are well recognized during the endovascular management of intracranial aneurysms. In this study, we present a case series of 40 patients with intraprocedural thrombotic complications who were treated with intra-arterial eptifibatide (IAE), and a review of the literature.
Methods
Twenty-five patients with ruptured intracranial aneurysms (RIA), 10 with unruptured intracranial aneurysms (UIA) and 5 with aneurysmal subarachnoid hemorrhage-induced vasospasm (VSP) received IAE for intraprocedural thrombi during endovascular treatment. Rates of recanalization, strokes, and hemorrhagic complications were assessed.
Results
Recanalization was achieved in 96% (24/25) of the RIA patients [72% (18/25) complete; 24% (6/25) partial], in 100% (10/10) of the UIA patients [90% (9/10) complete; 10% (1/10) partial], and in 100% (5/5) of the VSP patients [80% (4/5) complete; 20% (1/5) partial]. Strokes following intraprocedural thrombosis were coil-related (20%, 5/25) or stent-related (12%, 3/25) in RIA patients, stent-related (10%, 1/10) in UIA patients, and heparin-induced thrombocytopenia type II-related (60%, 3/5) or vasospasm-related (20%, 1/5) in VSP patients. There were no intracerebral hemorrhagic complications in UIA. Intracerebral hemorrhage was observed in 20% of the RIA patients (5/25), all of whom had received intra-arterial thrombolytics and/or high-dose heparin infusion in addition to IAE; in 12%, this was external ventricular drain-related (3/25), 4% had parenchymal hematoma type 1 (1/25), and 4% parenchymal hematoma type 2 (1/25). One of the 5 VSP patients, who had received argatroban in addition to IAE, had parenchymal hematoma type 1. No clinically significant systemic hemorrhage was observed in this study.
Conclusion
Treatment of thromboembolic complications with IAE during endovascular management of aneurysms was effective in achieving recanalization and overall well tolerated in this series.
Key Words : Eptifibatide, Intracranial aneurysm, Intra-arterial thrombus
Introduction
Endovascular methods are used to treat the majority of intracranial aneurysms. Intraprocedural thromboembolic complications are a well-recognized challenge stemming from that approach. They occur with a frequency of 3-11% [1,2] and can contribute to periprocedural morbidity and mortality [3]. Rapid dissolution of the platelet-rich thrombus is important in limiting the risk of ischemic sequelae.
Despite the standard use of intravenous heparin infusion during most cases of embolization, thrombus formation occurs [2], generally requiring additional boluses of heparin and/or the use of other agents [4]; in prior studies, fibrinolytics [5,6] achieved suboptimal recanalization and were associated with symptomatic intracerebral hemorrhage [7]. Intraprocedural thrombi are platelet rich and have limited amounts of fibrin. Hence, mechanistically, inhibiting platelet aggregation offers an attractive approach. Glycoprotein IIb/IIIa is an important surface molecule that mediates platelet aggregation. Eptifibatide, a glycoprotein IIb/IIIa inhibitor, is one of several available inhibitors of platelet aggregation which has been used intravenously [8] or intra-arterially in a case report [9] to treat thrombotic complications during the endovascular management of aneurysms. Here we present a case series of 40 patients who were treated with intra-arterial eptifibatide (IAE) in the endovascular management of intracranial aneurysms, and a review of the literature.
Methods
A single-center, prospectively collected series of 40 consecutive patients who had undergone endovascular treatment of intracranial aneurysms between 2003 and 2008 and received IAE for the management of intraluminal thrombosis were retrospectively reviewed. This was an off-label use of an FDA-approved medication. Of the 40 patients, 25 patients had ruptured intracranial aneurysms (RIA), 10 patients presented for elective management of unruptured intracranial aneurysms (UIA), and 5 patients received intra-arterial infusion of nicardipine and/or milrinone for aneurysmal subarachnoid hemorrhage (SAH)-induced vasospasm (VSP). Medical charts were reviewed to gather data on demographics (age and gender), clinical factors (Hunt-Hess grade; prehospital antiplatelet and/or anticoagulant use; need for external ventricular drain placement; development of heparin-induced thrombocytopenia type II, HIT II), angiographic factors (aneurysm size; ruptured/unruptured status; location of the aneurysm; Hunt-Hess/Fisher grade; coil compaction; degree of recanalization following treatment of intraprocedural thrombus) and laboratory information (activated coagulation time, complete blood count, coagulation studies and antiplatelet antibodies). Data were handled in accordance with the Health Insurance Portability and Accountability Act.
Transfemoral angiography was performed under general anesthesia in a biplane suite. All catheterizations were performed under continuous infusion of heparinized saline (4,000 U/l). Heparin was routinely used prior to coiling of UIA and after formation of the coil basket in RIA to achieve an activated coagulation time of >200 s. One VSP patient received heparin, 2 received argatroban and 2 did not receive any anticoagulation prior to the thrombotic event. IAE (range: 2.25-12 mg; <90 μg/kg) was used for intraprocedural thrombi, administered according to the patient's body weight, with the starting dose calculated at 25% of the dose limit (90 μg/kg). On the posttreatment angiogram, if there still was persistence of the intraluminal thrombus, additional doses were administered as calculated above. Intravenous infusion (1.5-3.75 mg/h; 0.5 μg/kg/min) was continued postprocedurally in 20 cases for 2-48 h at the discretion of the neurointerventionalist. Two cases each of RIA and VSP also involved the use of intra-arterial urokinase for the treatment of intraprocedural thrombi. Six of the 40 procedures employed stent assistance (Neuroform stent), all of whom received a clopidogrel loading dose followed by daily clopidogrel for 3 months and aspirin indefinitely. Four procedures employed balloon remodeling. Postprocedurally, the patients were managed in the neurointensive care unit. Seventeen patients had an external ventricular drain for the management of hydrocephalus.
Intraprocedural thromboembolic complication was defined as any filling defect or complete occlusion of a previously visualized vessel by a thrombus. Recanalization, stroke and hemorrhage rates were calculated after adjudication of the imaging data (by P.R. and R.G.N.). All patients underwent immediate postangiographic CT scanning, and again within 24-48 h after the procedure, to detect ischemic and/or hemorrhagic complications. New focal deficits or the presence of ischemic or hemorrhagic complications in the territory of the parent artery present within 48 h after the procedure were recorded as a complication of either intraprocedural thrombosis or due to the use of IAE. Other intraprocedural complications such as rupture of an intracranial aneurysm, hemorrhage adjacent to or along the external ventricular drain catheter tract, groin complications and systemic hemorrhage within 48 h after the procedure were also recorded.
Results
Forty cases of intraprocedural thromboembolic complications during the endovascular treatment of RIA (n = 25), UIA (n = 10) and VSP (n = 5) were managed with IAE. The patients' characteristics were as summarized in table 1. Complete/partial thrombotic occlusions were seen in 44/56% of RIA, 50/50% of UIA and 80/20% of VSP cases. Overall rates of recanalization with IAE were 96% in RIA, and 100% each in the UIA and VSP groups; representative examples of each are shown in figure 1. Complete recanalization was noted in 72% of RIA, 90% of UIA and 80% of VSP cases. Partial recanalization was achieved in 24% (RIA), 10% (UIA) and 20% (VSP) of the cases (table 2).
Table 1.
Patient characteristics
| RIA | UIA | VSP | |
|---|---|---|---|
| Patients, n | 25 | 10 | 5 |
| Average age ± SD, years | 52.3 ± 12.0 | 50 ± 12.6 | 48 ± 10.6 |
| Women, n/total n | 19/25 (76%) | 9/10 (90%) | 4/5 (80%) |
| Aneurysm size ≥10 mm, n/total n | 12/25 (48%) | 1/10 (10%) | none |
| Hunt-Hess grade ≥4, n/total n | 9/25 (36%) | none | 1/5 (20%) |
| Fisher grade ≥3, n/total n | 17/25 (68%) | none | 3/5 (60%) |
| Coil compaction, n/total n | n/a | 3/10 (30%) | n/a |
| Posterior circulation, n/total n | 5/25 (20%) | 4/10 (40%) | 2/5 (40%) |
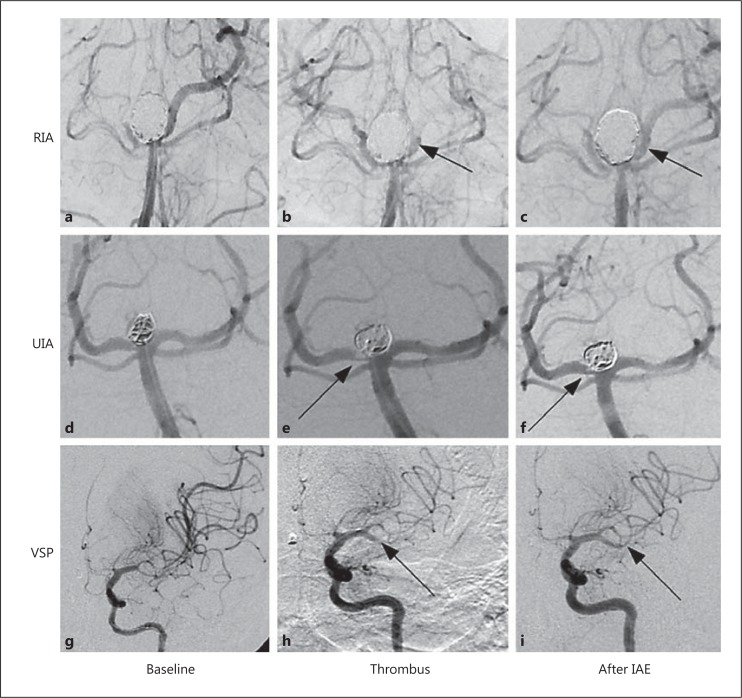
Fig. 1.
Illustrative examples showing the successful rescue treatment of thromboembolic complications with IAE. a-c Left vertebral artery cerebral angiogram. Ruptured basilar tip aneurysm (11 × 8 mm) with a broad neck. a Good opacification of the bilateral SCA and PCA with washout of the right PCA from the right posterior communicating artery. b Good positioning of the coil mass with near-complete occlusion of the basilar tip aneurysm. Note the diminished opacification of the left PCA just distal to the P1 segment (arrow) consistent with a partly occlusive thrombus. c After 2.25 mg of IAE, complete recanalization of the left PCA (arrow) is seen without evidence of distal embolization. d-f Right vertebral artery angiogram. Unruptured basilar tip aneurysm (5 × 6 mm). d Good flow is detected within the aneurysm and in the basilar artery and bilateral SCA and PCA. e Good coil packing within the aneurysm is noted. There is a prominent filling defect at the right lateral aspect of the coil mass adjacent to the right PCA origin (arrow). f Near-complete resolution of the filling defect of the right PCA (arrow) after 5.25 mg of IAE. g-i Left internal carotid artery angiogram. Severe vasospasm of the left MCA and anterior cerebral artery. g Severe vasospasm in the left MCA involving the superior and inferior divisions. h A filling defect is noted in the mid-distal inferior division of the left MCA (arrow). i Complete recanalization of the inferior division of the left MCA (arrow) after 5 mg of IAE. SCA = Superior cerebellar artery; PCA = posterior cerebral artery; MCA = middle cerebral artery.
Table 2.
Rates of recanalization with IAE (n/total n)
| RIA | UIA | VSP | |
|---|---|---|---|
| Pretreatment complete occlusion | 11/25 (44%) | 5/10 (50%) | 4/5 (80%) |
| Pretreatment partial occlusion | 14/25 (56%) | 5/10 (50%) | 1/5 (20%) |
| All recanalizations | 24/25 (96%) | 10/10 (100%) | 5/5 (100%) |
| Full recanalization | 18/25 (72%) | 9/10 (90%) | 4/5 (80%) |
| Partial recanalization | 6/25 (24%) | 1/10 (10%) | 1/5 (20%) |
| No recanalization | 1/25 (4%) | none | none |
All-cause ischemic stroke rates were 36% in RIA, 10% in UIA and 80% in VSP cases (table 3). Large ischemic strokes occurred in 36% of RIA and 80% of VSP cases, whereas punctate strokes occurred in 10% of UIA cases. Despite complete recanalization, strokes occurred in 32% of RIA and 60% of VSP cases. Cases related to coil protrusion or stent assistance accounted for 20 and 12% of ischemic strokes, respectively, in the RIA group. Strokes related to stent assistance accounted for 10% of the UIA cases. In the VSP group, 3/5 patients developed HIT II-related strokes, whereas 1/5 developed a stroke due to vasospasm.
Table 3.
Rates of stroke in the endovascular management of intracranial aneurysms (n/total n)
| RIA | UIA | VSP | |
|---|---|---|---|
| No stroke | 16/25 (64%) | 9/10 (90%) | 1/5 (20%) |
| Stroke | 9/25 (36%) | 1/10 (10%) | 4/5 (80%) |
| Punctate | none | 1/10 (10%) | none |
| Large | 9/25 (36%) | none | 4/5 (80%) |
| Despite complete recanalization | 8/25 (32%) | none | 3/5 (60%) |
| Coil-related | 5/25 (20%) | none | n/a |
| Stent-related | 3/25 (12%) | 1/10 (10%) | n/a |
| HIT II-related | n/a | n/a | 3/5 (60%) |
| Vasospasm-related | n/a | n/a | 1/5 (20%) |
The overall intracerebral hemorrhage rates were 20, 0 and 20% in the RIA, UIA and VSP groups, respectively (table 4). Of those, parenchymal hematoma type 1 was present in 4% of RIA and 20% of VSP cases. Parenchymal hematoma type 2 was present in 4% of the RIA cases. Twelve percent of hemorrhagic complications in the RIA group were related to external ventricular drain placement. Notably, any type of intracerebral hemorrhage was seen in those cases where thrombolytics and/or high-dose anticoagulants were used in addition to IAE. Groin hematoma complicated a case of UIA, and there was hemoptysis in one case of VSP, but these were not clinically significant.
Table 4.
Rates of hemorrhagic complications with IAE (n/total n)
| RIA | UIA | VSP | |
|---|---|---|---|
| Intracerebral hemorrhage | 5/25 (20%) | none | 1/5 (20%) |
| Parenchymal hematoma type 1 | 1/25 (4%) | none | 1/5 (20%) |
| Parenchymal hematoma type 2 | 1/25 (4%) | none | none |
| External ventricular drain-related | 3/25 (12%) | n/a | none |
| Systemic hemorrhage | none | 1/10 (10%) | 1/5 (20%) |
Discussion
Eptifibatide is a highly specific, reversible small-molecular-weight glycoprotein IIb/IIIa inhibitor with a rapid onset of action, lower binding affinity and a plasma half-life of 1.5 h; normal platelet function is restored within 2-4 h after discontinuation of the drug [10]. In comparison, abciximab is a chimeric monoclonal antibody that not only binds to the glycoprotein IIb/IIIa receptor with a much higher affinity, but possibly to other cell surface receptors with unclear significance [11]. Despite its shorter plasma half-life than that of eptifibatide, it takes 48 h or longer for the restoration of platelet function after discontinuation of abciximab. These properties have been implicated in the higher bleeding rates seen with abciximab and possibly in delaying necessary surgeries in the face of its prolonged antiplatelet effect. Compared with eptifibatide, higher rates of immunogenicity and thrombocytopenia have also been reported with abciximab use [11]. With tirofiban, another nonpeptide glycoprotein IIb/IIIa inhibitor, the bleeding time returns to normal 4 h after its cessation, compared with about 15 min with eptifibatide [12]. These differences, along with a lower cost profile, make eptifibatide a more attractive rescue agent in endovascular thromboembolic complications.
Intraprocedural thromboembolic complications are attributed to a combination of the presence of foreign materials (stents, coils, catheters and guide wires), coil protrusion into the parent vessel and vessel injury [1,2]. Additionally, patients undergoing acute neurointerventional treatment can be hypercoagulable. Theoretically, dissolution of platelet-rich thrombi can be better achieved with the use of glycoprotein IIb/IIIa inhibitors. Hypothetically, lower doses of rescue treatment may be given intra-arterially, thus limiting the risk of bleeding complications. In our retrospective case series, IAE use was safe and well tolerated in the management of thromboembolic complications during endovascular treatment of intracerebral aneurysms.
Recanalization with IAE
The number of published cases using eptifibatide in the management of thromboembolic complications during aneurysm treatment has been limited to date. Intravenous eptifibatide was used in treating periprocedural multifocal distal occlusions during the management of RIA [3]; prophylactic intravenous eptifibatide use in 77/84 endovascular procedures for aneurysm coiling was safe [8], and IAE was successful for rescue treatment in a case series with 4 patients [9].
To the best of our knowledge, our study is the largest report of IAE use in the rescue of thromboembolic complications occurring during the endovascular management of aneurysms. The overall recanalization rate was 97.5% with complete (Thrombolysis in Myocardial Infarction score 3) recanalization achieved in 77.5% of the cases (table 2). The fact that in 20 of these cases intravenous eptifibatide infusion was continued does not detract from the efficacy of the intra-arterial route, because the intravenous infusion was post hoc and appears to have been discretionary rather than driven by either partial recanalization (Thrombolysis in Myocardial Infarction score 1 or 2), coil protrusion, or due to stent and/or balloon assistance.
Pooling our data with those published by Katsaridis et al. [9] gives rise to an average rate of complete recanalization of 88.8% for IAE use (table 5). In comparison, the collective experience with similar use of intra-arterial abciximab (IAA) or tirofiban shows an average rate of complete recanalization of 73.9 and 67.7%, respectively (table 5) [9,13,14,15,16,17,18,19,20,21,22,23,24]. More studies are warranted in further exploring the efficacy of IAE.
Table 5.
Published recanalization and stroke rates with intra-arterial glycoprotein IIb/IIIa inhibitors in the endovascular management of intracranial aneurysms
| Number of aneurysms | Drug | Recanalization, C/P1 | Stroke2 | |
|---|---|---|---|---|
| Duncan and Fourie, 20023 [13] | 1 RIA | abciximab | 100% C | 100.00% |
| Mounayer et al., 2003 [14] | 13 (4 RIA, 9 UIA) | abciximab | 92.3% C/7.7% P | 7.70% |
| Song et al., 2004 [15] | 7 (4 RIA, 3 UIA) | abciximab | 57.1% C/28.6% P | 42.90% |
| Fiorella et al., 2004 [16] | 3 RIA | abciximab | 100% C | 66.60% |
| Park et al., 2008 [17] | 32 (16 RIA, 16 UIA) | abciximab | 53% C/47% P | 12.90% |
| Ries et al., 2009 [18] | 3 (NR) | abciximab | 66.6% (C/P n/a) | 66.60% |
| Jones et al., 2008 [19] | 15 (12 RIA, 3 UIA) | abciximab | 40% C/46.7% P | 6.60% |
| Linfante et al., 2010 [20] | 19 (8 RIA, 11 UIA) | abciximab | 100% (C/P n/a) | 0.00% |
| Aggour et al., 2010 [21] | 23 (11 RIA, 9 UIA, 3 recanalized) | IA → IV abciximab | 56.5% C/17.4% P | 47.80% |
| Average | 116 (56 RIA, 51 UIA, 6 other) | abciximab | 73.9% C/16.4% P | 39.00% |
| Kang et al., 2008 [22] | 25 (11 RIA, 14 UIA) | tirofiban | 16% C/80% P | 20.00%4 |
| Kulcsár et al., 2010 [23] | 1 RIA | tirofiban | 100% C | 0.00% |
| Cho et al., 2012 [24] | 39 RIA | tirofiban | 87.2% C/7.7% P | 20.50% |
| Average | 65 (51 RIA, 14 UIA) | tirofiban | 67.7% C/29.2% P | 13.50% |
| Katsaridis et al., 2008 [9] | 4 (3 RIA, 1 VSP) | eptifibatide | 100% C | 0.00% |
| Present study, 2013 | 40 (25 RIA, 10 UIA, 5 VSP) | eptifibatide | 77.5% C/20% P | 35.00% |
| Average | 44 (28 RIA, 10 UIA, 6 VSP) | eptifibatide | 88.8% C/10% P | 17.50% |
C = Complete recanalization; P = partial recanalization; NR = not reported; IA = intra-arterial; IV = intravenous.
Assumed complete recanalization if breakdown not given.
Clinically or radiographically.
Only cases with treated aneurysms are tabulated.
Resolved after 2 weeks.
Ischemic Strokes
The incidence of intraprocedural thromboembolic complications during endovascular management of aneurysms has been reported to be between 2.4 and 69% [1,25], a subset of which is followed by permanent neurologic deficit. Given the modest efficacy of fibrinolytics in the treatment of platelet-rich thrombi and their association with increased intracerebral hemorrhage rates, in our opinion these should be thought of as second-line agents [5,6,17].
Intravenous abciximab has been successful in preventing or mitigating the extent of strokes due to intraprocedural thromboembolic complications in about 60-70% [18,26] and 50-86% for IAA [19,21] (table 5). Published data on the use of eptifibatide are limited but suggest that it is comparable in this regard: a single stroke was noted among 77 patients who received eptifibatide prophylaxis [8], and in 4 cases of IAE use, none suffered a stroke as a consequence of intraprocedural thromboembolism [9].
In our study, the success rate of IAE in preventing strokes in the target vessel territory following intraprocedural clot dissolution was 64% for RIA, 90% for UIA and 20% for VSP cases (table 3). Not surprisingly, ruptured and re-treated aneurysms have higher rates of procedure-related complications than unruptured aneurysms [3], and this might partly explain our findings. Inherent differences in the types of initial pathology (higher representation of VSP cases in our study), defining strokes radiographically versus clinically, and the time frame used to identify periprocedural strokes might also account for the differences between our findings and those of other such studies. After pooling our results with those of Katsaridis et al. [9], albeit still with a small sample size overall, the stroke-free rate with IAE is favorable at 82.5% (table 5). More studies are warranted to explore this finding.
In our series, large strokes were identified in 36% (n = 9) of RIA cases, which were attributed to proximal vessel occlusion at the coil-parent vessel interface (n = 5) or related to the use of the Neuroform stent (n = 3). In a retrospective study of 64 patients [27], the rate of thromboembolic events was 9.3% with the use of stents. In the previously published, large prospective multicenter trial CLARITY, aneurysms measuring >10 mm (28.0 vs. 10.7%) and those with a neck of >4 mm (20.8 vs. 11.0%) were associated with a higher rate of thromboembolic complications in RIA [28]. In univariate analysis, aneurysm size of >10 mm and neck of >4 mm were significantly associated with higher cumulative morbidity and mortality rates; in multivariate analysis, aneurysm size of >10 mm had a significant effect on thromboembolic events and on cumulative morbidity and mortality rates [28]. In our RIA cohort with large strokes, 7 had aneurysms measuring >10 mm and 1 had a neck size of 4.2 mm. Given that stent assistance is a commonly used tool in the treatment of complex aneurysms that are either deemed large or have a wide neck, it is not surprising that most of the strokes in our series were in this group.
In our UIA group, a punctate stroke occurred in a single case that required the use of a Neuroform stent. Importantly, this aneurysm measured >10 mm in its largest dimension. Thromboembolic complications in a prospectively studied UIA group (ATENA trial) [29] were 9.9% for aneurysms measuring 7-15 mm and 10.5% for those treated by stenting. Our findings are in keeping with this study. Taken together, the strokes in our RIA and UIA groups occurred in high-risk subsets where an endovascular treatment approach may not have been feasible but for stent assistance. Conceivably, stroke rates might be lowered in this group with the judicious and cautious use of prophylactic treatment as previously published [8], but additional studies are necessary.
In the VSP group, 4 of the 5 cases developed large strokes: 1 was attributed to the development of symptomatic vasospasm, a factor known to complicate aneurysmal SAH in as many as 23% of cases [30]. The other 3 strokes occurred despite complete recanalization of the target vessel with IAE. In those 3 cases, the patients were found to have heparin-platelet factor 4 antibodies accompanied by a decrease of 14-65% in the platelet count, thus supporting a diagnosis of HIT II. Hoh et al. [31] reported a 15% incidence of HIT II in aneurysmal SAH patients. In their study, new ischemic strokes on CT were significantly more frequent in patients with HIT II than in those without this complication (66 vs. 40%), which corroborates our findings. The HIT II group also had a less favorable clinical outcome and more deaths than those without HIT II [31].
In 2 of the patients with HIT II in our VSP group, intra-arterial urokinase was successfully used in addition to IAE to disrupt intraprocedural clots. The combined use of an antiplatelet and a fibrinolytic agent suggests that in HIT II the thrombi may be more complex in their composition than initially believed. Thrombi in HIT II were previously characterized as almost entirely rich in platelets with limited fibrin content [32]. However, serum from patients with HIT II can interact with endothelial cells and promote the release of tissue factor and trigger the coagulation cascade; this effect was blocked in the presence of a glycoprotein IIb/IIIa antagonist, suggesting that activated platelets play a key role in triggering the coagulation cascade in HIT II [33]. Arguably, early glycoprotein IIb/IIIa antagonist use may be effective in the management of HIT II-induced thrombi, but insufficient if a hypercoagulable state follows. This might explain the need for fibrinolytic therapy in the 2 VSP cases with HIT II in our group.
Hemorrhagic Complications
One of the most feared complications of rescue therapy for intraprocedural thromboembolism is the risk of precipitating or worsening hemorrhage. In our series, no cases of intracerebral or systemic hemorrhage were detected when only IAE and therapeutic heparinization was used. In a retrospective case series of 31 patients with either RIA or UIA complicated by intraprocedural thromboembolism, the postprocedural hemorrhage rate was 9.7% after IAA use [17]. All 3 hemorrhagic complications occurred in patients with RIA in the setting of thrombocytopenia; it was unclear whether the thrombocytopenia was due to abciximab or heparin use. In comparison, there were no instances of thrombocytopenia attributable to eptifibatide use in our study. In a multicenter retrospective series of 51 patients, the overall rate of hemorrhagic complications following abciximab use was 18%; 1 patient with RIA who received IAA suffered worsening of the preexisting SAH and intraventricular hemorrhage, and developed a new intracerebral hemorrhage along the external ventricular drain tract [34]. Another study reported a single case of fatal hemorrhage (2.4%) complicating IAA use during the treatment of an RIA [26]. A recent retrospective RIA series reported 3 cases each of hyperacute rebleeding following the use of IAA or intra-arterial tirofiban for intraprocedural thromboembolism [35]. Thus far, no intracerebral hemorrhagic complications have been reported with isolated IAE use in the acute endovascular management of aneurysms.
In cases where hemorrhages were noted in our study (table 4), many risk factors were associated with this complication: combined use of antiplatelet agents, fibrinolytics and/or anticoagulants, external ventricular drain placement, and the development of thrombocytopenia due to HIT II. In the RIA group, there was 1 case each of parenchymal hematoma type 1 and parenchymal hematoma type 2 where, in addition to the standard heparin infusion, intra-arterial urokinase was used; in the case complicated by parenchymal hematoma type 2, intravenous eptifibatide was continued for 48 h after the procedure as well. Both cases had a partial thromboplastin time (PTT) of >150 s after the endovascular procedure. In the 1 case of parenchymal hematoma type 1 in the VSP group, argatroban was used in addition to IAE because of HIT II.
In a collagenase-induced animal model of intracerebral hemorrhage [36], unlike aspirin and a novel glycoprotein IIb/IIIa antagonist, both recombinant tissue plasminogen activator and heparin increased the hemorrhagic volume in a dose-dependent fashion. Pathophysiologically, the latter might have an effect on fibrin and matrix metalloproteinase-9 (MMP-9), thus tipping the balance in favor of hemorrhage. MMP-9 belongs to a class of matrix metalloproteinases that has been implicated in vascular remodeling of aneurysms. In one study, MMP-9 was disproportionately overexpressed in human RIA compared with UIA [37]. This not only offers a plausible explanation for what sets the RIA and UIA groups apart, but it might also give one pause before using fibrinolytic agents in the former setting. Based on our experience here, extreme caution should be exercised in combining antiplatelet, antithrombotic and fibrinolytic agents to minimize the risk of hemorrhagic complications.
The other risk factor for developing intracerebral hemorrhage that emerged in our series was the presence of an external ventricular drain. Of the 17 cases with an external ventricular drain, 3 (17.6%) were associated with drain-related hemorrhages in the RIA group: 2 had a supratherapeutic PTT and 2 underwent drain manipulation. In a prospectively collected series of 24 patients [38], 3 cases (12.5%) had symptomatic external ventricular drain-related hemorrhages due to drain manipulation, which were possibly exacerbated by the concurrent use of anticoagulants and antiplatelet drugs. In a meta-analysis of 1,790 patients with external ventricular drain, a hemorrhage rate of 10.6% was observed on routine postplacement CT; symptomatic hemorrhages accounted for 0.91% [39]. A much higher rate of external ventricular drain-related symptomatic hemorrhage was reported [40] for patients whose aneurysms were treated with stent assistance than for those without (8 vs. 0.9%). The authors ascribed this difference to the use of antiplatelet agents for thromboprophylaxis. In a retrospective review of a prospectively collected aneurysm database [41] there was no difference in external ventricular drain-related hemorrhage rates between patients who underwent heparinization during coiling and those who did not. The 1 patient who had a symptomatic external ventricular drain-related hemorrhage in the heparinized group had a supratherapeutic PTT of >150 s, which was felt to be the proximal reason for the hemorrhage, and not heparin use per se. Taken together, our findings are in line with these reports and support the notion that drain manipulation and having supratherapeutic levels of anticoagulation might have contributed to the external ventricular drain-related hemorrhages.
There were 2 cases of systemic hemorrhages in our study: one with hemoptysis in the UIA group who had been treated with both IAE and had a supratherapeutic PTT, another in a VSP case where the patient developed a groin access site hematoma after being treated with IAE and intra-arterial urokinase in addition to argatroban for HIT II. Neither of these systemic hemorrhages was life-threatening.
Limitations
We acknowledge that there are a few limitations to our study. It is retrospective in nature and does not have a control group. The sample size is small. Treatment decisions were variable with regard to the use of rescue agents and were driven by the experience of the neurointerventionalist, and this may confound our data set. Modifying some of these variables and conducting a prospective analysis might be helpful in furthering our results.
Conclusion
In this retrospective study we described the successful use of IAE as a rescue agent in the treatment of thromboembolic complications during the endovascular management of RIA and UIA, and in the management of patients with ruptured aneurysms being treated for vasospasm. Additional studies are needed to confirm these findings with a larger sample size and in a prospective manner.
Disclosure Statement
R.G.N. is a member of the scientific advisory boards for Concentric Medical Inc., ev3 Neurovascular Inc., CoAxia Inc., Rapid Medical Inc. and Neurointervention Inc., but he donates all fees from industry consulting services to a Stroke Research and Educational Fund. A.J.Y. receives research grant support from Penumbra Inc.
References
- 1.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475–482. doi: 10.3171/jns.1997.86.3.0475. [DOI] [PubMed] [Google Scholar]
- 2.Cognard C, Weill A, Castaings L, Rey A, Moret J. Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology. 1998;206:499–510. doi: 10.1148/radiology.206.2.9457205. [DOI] [PubMed] [Google Scholar]
- 3.Park HK, Horowitz M, Jungreis C, Genevro J, Koebbe C, Levy E, Kassam A. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2005;26:506–514. [PMC free article] [PubMed] [Google Scholar]
- 4.Workman MJ, Cloft HJ, Tong FC, Dion JE, Jensen ME, Marx WF, Kallmes DF. Thrombus formation at the neck of cerebral aneurysms during treatment with Guglielmi detachable coils. AJNR Am J Neuroradiol. 2002;23:1568–1576. [PMC free article] [PubMed] [Google Scholar]
- 5.Cronqvist M, Pierot L, Boulin A, Cognard C, Castaings L, Moret J. Local intraarterial fibrinolysis of thromboemboli occurring during endovascular treatment of intracerebral aneurysm: a comparison of anatomic results and clinical outcome. AJNR Am J Neuroradiol. 1998;19:157–165. [PMC free article] [PubMed] [Google Scholar]
- 6.Hähnel S, Schellinger PD, Gutschalk A, Geletneky K, Hartmann M, Knauth M, Sartor K. Local intra-arterial fibrinolysis of thromboemboli occurring during neuroendovascular procedures with recombinant tissue plasminogen activator. Stroke. 2003;34:1723–1728. doi: 10.1161/01.STR.0000078372.76670.83. [DOI] [PubMed] [Google Scholar]
- 7.Arnold M, Fischer U, Schroth G, Nedeltchev K, Isenegger J, Remonda L, Windecker S, Brekenfeld C, Mattle HP. Intra-arterial thrombolysis of acute iatrogenic intracranial arterial occlusion attributable to neuroendovascular procedures or coronary angiography. Stroke. 2008;39:1491–1495. doi: 10.1161/STROKEAHA.107.506279. [DOI] [PubMed] [Google Scholar]
- 8.Yi HJ, Gupta R, Jovin TG, Tayal A, Genevro J, Gologorsky Y, Horowitz M. Initial experience with the use of intravenous eptifibatide bolus during endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2006;27:1856–1860. [PMC free article] [PubMed] [Google Scholar]
- 9.Katsaridis V, Papagiannaki C, Skoulios N, Achoulias I, Peios D. Local intra-arterial eptifibatide for intraoperative vessel thrombosis during aneurysm coiling. AJNR Am J Neuroradiol. 2008;29:1414–1417. doi: 10.3174/ajnr.A1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarborough RM. Development of eptifibatide. Am Heart J. 1999;138:1093–1104. doi: 10.1016/s0002-8703(99)70075-x. [DOI] [PubMed] [Google Scholar]
- 11.Coller BS. Anti-GPIIb/IIIa drugs: current strategies and future directions. Thromb Haemost. 2001;86:427–443. [PubMed] [Google Scholar]
- 12.Fiehler J, Ries T. Prevention and treatment of thromboembolism during endovascular aneurysm therapy. Klin Neuroradiol. 2009;19:73–81. doi: 10.1007/s00062-009-8029-9. [DOI] [PubMed] [Google Scholar]
- 13.Duncan IC, Fourie PA. Catheter-directed intra-arterial abciximab administration for acute thrombotic occlusions during neurointerventional procedures. Interv Neuroradiol. 2002;8:159–168. doi: 10.1177/159101990200800208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mounayer C, Piotin M, Baldi S, Spelle L, Moret J. Intraarterial administration of abciximab for thromboembolic events occurring during aneurysm coil placement. AJNR Am J Neuroradiol. 2003;24:2039–2043. [PMC free article] [PubMed] [Google Scholar]
- 15.Song JK, Niimi Y, Fernandez PM, Brisman JL, Buciuc R, Kupersmith MJ, Berenstein A. Thrombus formation during intracranial aneurysm coil placement: treatment with intra-arterial abciximab. AJNR Am J Neuroradiol. 2004;25:1147–1153. [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorella D, Albuquerque FC, Han P, McDougall CG. Strategies for the management of intraprocedural thromboembolic complications with abciximab (ReoPro) Neurosurgery. 2004;54:1089–1097. doi: 10.1227/01.neu.0000119351.86658.1d. discussion 1097-1098. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Kim JE, Sheen SH, Jung CK, Kwon BJ, Kwon OK, Oh CW, Han MH, Han DH. Intraarterial abciximab for treatment of thromboembolism during coil embolization of intracranial aneurysms: outcome and fatal hemorrhagic complications. J Neurosurg. 2008;108:450–457. doi: 10.3171/JNS/2008/108/3/0450. [DOI] [PubMed] [Google Scholar]
- 18.Ries T, Siemonsen S, Grzyska U, Zeumer H, Fiehler J. Abciximab is a safe rescue therapy in thromboembolic events complicating cerebral aneurysm coil embolization: single center experience in 42 cases and review of the literature. Stroke. 2009;40:1750–1757. doi: 10.1161/STROKEAHA.108.539197. [DOI] [PubMed] [Google Scholar]
- 19.Jones RG, Davagnanam I, Colley S, West RJ, Yates DA. Abciximab for treatment of thromboembolic complications during endovascular coiling of intracranial aneurysms. AJNR Am J Neuroradiol. 2008;29:1925–1929. doi: 10.3174/ajnr.A1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linfante I, Etezadi V, Andreone V, DeLeo M, Alehashemi S, Shaw K, Wakhloo A. Intra-arterial abciximab for the treatment of thrombus formation during coil embolization of intracranial aneurysms. J Neurointerv Surg. 2010;2:135–138. doi: 10.1136/jnis.2009.001933. [DOI] [PubMed] [Google Scholar]
- 21.Aggour M, Pierot L, Kadziolka K, Gomis P, Graftieaux JP. Abciximab treatment modalities for thromboembolic events related to aneurysm coiling. Neurosurgery. 2010;67:503–508. doi: 10.1227/NEU.0b013e3181f8d1db. [DOI] [PubMed] [Google Scholar]
- 22.Kang HS, Kwon BJ, Roh HG, Yoon SW, Chang HW, Kim JE, Han MH. Intra-arterial tirofiban infusion for thromboembolism during endovascular treatment of intracranial aneurysms. Neurosurgery. 2008;63:230–237. doi: 10.1227/01.NEU.0000320440.85178.CC. discussion 237-238. [DOI] [PubMed] [Google Scholar]
- 23.Kulcsár Z, Wetzel SG, Augsburger L, Gruber A, Wanke I, Rüfenacht DA. Effect of flow diversion treatment on very small ruptured aneurysms. Neurosurgery. 2010;67:789–793. doi: 10.1227/01.NEU.0000372920.39101.55. [DOI] [PubMed] [Google Scholar]
- 24.Cho YD, Lee JY, Seo JH, Kang HS, Kim JE, Jung KH, Han MH. Intra-arterial tirofiban infusion for thromboembolic complication during coil embolization of ruptured intracranial aneurysms. Eur J Radiol. 2012;81:2833–2838. doi: 10.1016/j.ejrad.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Soeda A, Sakai N, Murao K, Sakai H, Ihara K, Yamada N, Imakita S, Nagata I. Thromboembolic events associated with Guglielmi detachable coil embolization with use of diffusion-weighted MR imaging. Part II. Detection of the microemboli proximal to cerebral aneurysm. AJNR Am J Neuroradiol. 2003;24:2035–2038. [PMC free article] [PubMed] [Google Scholar]
- 26.Gralla J, Rennie AT, Corkill RA, Lalloo ST, Molyneux A, Byrne JV, Kuker W. Abciximab for thrombolysis during intracranial aneurysm coiling. Neuroradiology. 2008;50:1041–1047. doi: 10.1007/s00234-008-0457-8. [DOI] [PubMed] [Google Scholar]
- 27.Fiorella D, Albuquerque FC, Deshmukh VR, McDougall CG. Usefulness of the Neuroform stent for the treatment of cerebral aneurysms: results at initial (3-6-mo) follow-up. Neurosurgery. 2005;56:1191–1201. doi: 10.1227/01.neu.0000159645.86823.af. discussion 1201-1202. [DOI] [PubMed] [Google Scholar]
- 28.Pierot L, Cognard C, Anxionnat R, Ricolfi F, CLARITY Investigators Ruptured intracranial aneurysms: factors affecting the rate and outcome of endovascular treatment complications in a series of 782 patients (CLARITY study) Radiology. 2010;256:916–923. doi: 10.1148/radiol.10092209. [DOI] [PubMed] [Google Scholar]
- 29.Pierot L, Spelle L, Vitry F, ATENA Investigators Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke. 2008;39:2497–2504. doi: 10.1161/STROKEAHA.107.512756. [DOI] [PubMed] [Google Scholar]
- 30.Keyrouz SG, Diringer MN. Clinical review: prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit Care. 2007;11:220. doi: 10.1186/cc5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoh BL, Aghi M, Pryor JC, Ogilvy CS. Heparin-induced thrombocytopenia Type II in subarachnoid hemorrhage patients: incidence and complications. Neurosurgery. 2005;57:243–248. doi: 10.1227/01.neu.0000166539.02280.e5. discussion 243-248. [DOI] [PubMed] [Google Scholar]
- 32.Hermanns B, Janssens U, Handt S, Füzesi L. Pathomorphological aspects of heparin-induced thrombocytopenia II (HIT-II syndrome) Virchows Arch. 1998;432:541–546. doi: 10.1007/s004280050203. [DOI] [PubMed] [Google Scholar]
- 33.Herbert JM, Savi P, Jeske WP, Walenga JM. Effect of SR121566A, a potent GP IIb-IIIa antagonist, on the HIT serum/heparin-induced platelet mediated activation of human endothelial cells. Thromb Haemost. 1998;80:326–331. [PubMed] [Google Scholar]
- 34.Walsh RD, Barrett KM, Aguilar MI, Lanzino G, Hanel RA, Miller DA, Chong BW, Freeman WD. Intracranial hemorrhage following neuroendovascular procedures with abciximab is associated with high mortality: a multicenter series. Neurocrit Care. 2011;15:85–95. doi: 10.1007/s12028-010-9338-1. [DOI] [PubMed] [Google Scholar]
- 35.Cho YD, Lee JY, Seo JH, Kang HS, Kim JE, Kwon OK, Chung YS, Han MH. Early recurrent hemorrhage after coil embolization in ruptured intracranial aneurysms. Neuroradiology. 2012;54:719–726. doi: 10.1007/s00234-011-0950-3. [DOI] [PubMed] [Google Scholar]
- 36.Mihara K, Aoki T, Moriguchi A, Maeda M, Furuichi Y, Matsuoka N, Mutoh S. Prohemorrhagic and bleeding time activities of recombinant tissue plasminogen activator, heparin, aspirin, and a glycoprotein IIb/IIIa antagonist. J Neurotrauma. 2005;22:1362–1373. doi: 10.1089/neu.2005.22.1362. [DOI] [PubMed] [Google Scholar]
- 37.Jin D, Sheng J, Yang X, Gao B. Matrix metalloproteinases and tissue inhibitors of metalloproteinases expression in human cerebral ruptured and unruptured aneurysm. Surg Neurol. 2007;68(suppl 2):S11–S16. doi: 10.1016/j.surneu.2007.02.060. discussion S16. [DOI] [PubMed] [Google Scholar]
- 38.Ross IB, Dhillon GS. Ventriculostomy-related cerebral hemorrhages after endovascular aneurysm treatment. AJNR Am J Neuroradiol. 2003;24:1528–1531. [PMC free article] [PubMed] [Google Scholar]
- 39.Binz DD, Toussaint LG, 3rd, Friedman JA. Hemorrhagic complications of ventriculostomy placement: a meta-analysis. Neurocrit Care. 2009;10:253–256. doi: 10.1007/s12028-009-9193-0. [DOI] [PubMed] [Google Scholar]
- 40.Kung DK, Policeni BA, Capuano AW, Rossen JD, Jabbour PM, Torner JC, Howard MA, Hasan D. Risk of ventriculostomy-related hemorrhage in patients with acutely ruptured aneurysms treated using stent-assisted coiling. J Neurosurg. 2011;114:1021–1027. doi: 10.3171/2010.9.JNS10445. [DOI] [PubMed] [Google Scholar]
- 41.Hoh BL, Nogueira RG, Ledezma CJ, Pryor JC, Ogilvy CS. Safety of heparinization for cerebral aneurysm coiling soon after external ventriculostomy drain placement. Neurosurgery. 2005;57:845–849. doi: 10.1227/01.neu.0000180814.95032.07. discussion 845-849. [DOI] [PubMed] [Google Scholar]