Abstract
Metabolic syndrome (MetS), characterized by central obesity, dyslipidemias, hypertension, and hyperglycemia, impacts 34 percent of the U.S. adult population. MetS has been demonstrated to be affected by dietary components. Data from epidemiological studies and clinical interventions suggest that one or more dairy components might directly affect MetS parameters. For example, calcium has been postulated to reduce body weight by modulating vitamin D concentrations in plasma and therefore attenuating intracellular calcium effects in activating genes involved in fatty acid synthesis and reducing those involved in lipolysis. Peptides present in milk have been associated with the inhibition of angiotensin converting enzyme and, therefore, with blood pressure reductions. Branched chain amino acids may increase post-prandial insulin secretion and regulate plasma glucose levels, and leucine, an abundant amino acid in milk, may be responsible for decreased plasma glucose through modulation of mTOR. Through different proposed mechanisms, dairy nutrients may target all components of MetS.
Keywords: metabolic syndrome, dairy, calcium, leucine, body weight
introduction
Metabolic syndrome (MetS) is characterized by accumulation of visceral fat, dyslipidemias associated with high plasma triglycerides (TG) and low HDL, hypertension, and high concentrations of fasting plasma glucose, as well as low-grade inflammation [1]. According to the National Cholesterol Education Adult Panel III, an individual has MetS if he/she has three of the following characteristics: waist circumference (WC) > 88 cm for females and > 102 cm for males, TG > 150 mg/dL, HDL < 40 mg/dL for men and < 50 mg/dL for women, fasting blood glucose > 100 mg/dL, and blood pressure > 130/85 mm Hg [2].
MetS is viewed as a precursor condition, which increases the risk for more serious diseases. However, MetS also affects health prior to the development of chronic disease. During a current health risk assessment of 5,012 U.S. employees, subjects with MetS were 31 percent more likely to have >3 sick days/year compared to those without MetS (p < 0.0001) [3]. MetS increases the risk of developing non-alcoholic steatohepatitis [4], cardiovascular disease (CVD) [5], and type 2 diabetes mellitus (T2DM) [6], chronic diseases that are already highly prevalent. According to a recent meta-analysis of 16 cohort studies, the relative risk for developing diabetes in the presence of MetS ranged from 3.53 to 5.17 (95% CI 3.99-6.69), depending on how MetS was defined [7]. Thus, early identification and treatment of MetS could potentially lessen both CVD and T2DM disease incidence and improve health outcomes. To this end, MetS was defined to serve as a clinical tool for the identification of high risk populations [8], as focusing attention on the clinical causes of CVD allows opportunity for prevention while the condition is still in a precursor state [9]. It has therefore become a necessity to control MetS by lifestyle changes before it progresses to T2D or CVD.
Dietary interventions that promote lifestyle changes, weight loss, and improvements in dyslipidemias and blood pressure can be used as an appropriate alternative to reduce MetS. These dietary changes include carbohydrate-restricted diets (CRD) [10], the Mediterranean diet [11], low fat diets [12], eggs as part of a low carbohydrate diet [13], and others [14]. For example, CRD have been shown to efficiently target all parameters of MetS [15]. CRD decrease plasma TG, increase HDL-C, lower blood pressure, reduce plasma glucose, and are very effective in reducing visceral obesity [16]. The Mediterranean diet promotes incorporation of healthy fatty acids and phytonutrient rich foods that are beneficial both under weight loss and weight stable conditions [17]. Eggs as part of a carbohydrate-restricted diet have shown to increase HDL cholesterol and reduce inflammation [13] and insulin resistance in individuals with MetS [18]. Other nutrients that have been identified to reduce MetS are dietary fiber, probiotics, low fat diets, and dairy [14]. In this review, the effects of dairy and dairy components on the parameters of MetS as a dietary approach are discussed.
Milk Components
The composition of milk is approximately 89 percent water, 3.5 percent protein, 4.6 percent carbohydrate, and 3.3 percent lipid [19]. Milk protein consists of casein (80 percent) and whey (20 percent), both of which contain anti-hypertensive peptides [20]. The amino acid profile in milk contains all indispensable amino acids, with a high biological value. The main fatty acids (FA) are palmitic (28 percent), oleic (27 percent), stearic (13 percent), and myristic (11 percent). Approximately 10 percent of the FA are short and medium chain, characterized by being directly absorbed into the portal vein and becoming oxidized, rather than incorporated into triglycerides for storage [21]. Thus, these FA are associated with weight loss [22].
The 2010 Dietary Guidelines for Americans suggest a daily intake of three servings of low-fat milk or milk products per day for most Americans [23]; however, according to National Health and Nutrition Examination Survey (NHANES) 1999-2000, only ~30 percent of adults (aged 19 to 50) and 10 percent of older adults (>50 y) meet these recommendations [24]. More recent data indicate that even with supplementation, calcium density, quantified by mg calcium per energy intake, is still insufficient and the average American only consumes ~ 70 percent of calcium recommendations [25]. Thus, increasing intake of calcium dense food is necessary.
Dairy products provide ~ 50 percent of vitamin D and nearly half the dietary intake of calcium [26]. Based on the benefits of dairy product consumption found in observational and prospective longitudinal studies and randomized control trials (RCT), an estimate of disease burden reduction was calculated to be in the range of $26 billion in the United States if adults consumed three to four servings of dairy per day [27].
Dairy and Metabolic Syndrome
Several cohort studies have examined the association between dairy consumption and MetS. The CARDIA study [28] reported that compared to < 10 servings dairy/week, consuming ≥ 35 servings/week decreased the odds for developing MetS by 72 percent. Dietary patterns in a U.S. cohort of ~16,000 adults (aged 45-64) in the Atherosclerosis Risk in Communities Study, showed an inverse association between MetS and dairy consumption, where those consuming the highest quintile of dairy had a 13 percent decreased risk of developing MetS as compared to the lowest quintile [28]. Similarly, the DESIRE study [29] reported that consumption of >2 servings of dairy products/day decreased the odds for developing MetS by 11 percent compared to no dairy intake, while the Caerphilly study [30] found inverse associations between milk consumption and total dairy and MetS risk. Tremblay et al. [31], in a systematic review of observational studies, reported that three to four servings of dairy decreased the risk of developing MetS by 29 percent compared to consuming < 2 servings/day. Data from the British Women’s Heart and Health study found that abstaining from milk reduced the odds for developing MetS by 45 percent [32], while Snijger et al. [33] found that dairy intake was associated with lower diastolic blood pressure but not with any other parameter of MetS. Beyoun et al. [34] found no association between milk and MetS, but a positive association between cheese intake and MetS and a protective effect of yogurt. A very recent study that evaluated the effects of dairy consumption on MetS in more than 7,000 middle-aged Koreans concluded that daily intake of dairy products protected against MetS mainly by an association with decreased central obesity [35]. In summary, the majority of observational data suggest dairy consumption may protect against MetS; however, these inconsistencies highlight the importance of RCT investigating the effect of dairy on MetS.
Randomized Clinical Trials and Dairy
Increased dairy consumption (≥3 servings compared to ≤ 1 diary serving/day) has been reported to reduce WC [36,37], hypertension [34,38], and inflammatory compounds [39] in weight stable overweight or obese subjects. Some studies reported decreased serum insulin [34,38] and improved insulin resistance [38] with dairy intake. However, dairy’s role in MetS is unclear as these effects are not confirmed in other studies. For example, Van Loan et al. found no difference in MetS parameters with ≥4 dairy servings compared to ≤1serving/day [40], and van Meijl et al. found decreases in systolic blood pressure and tumor necrosis factor α (TNF-α), but no differences in other MetS components or inflammatory markers in those consuming three servings of dairy compared to isocaloric carbohydrate control foods [39]. The discrepancies among these clinical trials can be due to several factors. Dugan et al. [37] and Stancliffe et al. [39] recruited individuals with MetS, while in the study by van Mejil et al. [38], subjects were overweight or obese and it is not clear whether they met MetS criteria per se. It is possible that the metabolic status of the subjects may influence outcomes as those with MetS differ clinically from age and BMI matched controls [1]. In addition, the magnitude between dairy and calcium intake [37-39] differed. Since a threshold effect of calcium intake has been suggested [41] and dietary calcium has been shown to reduce weight and BMI [36], this factor can also explain the discrepancies among these studies.
Some evidence supports individual nutrients in dairy to have a beneficial effect on MetS such as calcium and the attenuation of adiposity, or peptide fractions, which have been found to lower blood pressure. Below, the effects and potential mechanism of dairy intake on individual MetS components are reviewed.
Effects of Calcium and Dairy Consumption on Adiposity
The high intake of calcium, a mineral prevalent in dairy products, may promote weight reduction through modulation of serum 1, 25-hydroxyvitamin D concentrations. Serum calcium concentration is tightly regulated, and the metabolic correction of low serum calcium is suggested to account for the mechanisms by which dietary calcium intake modulates adiposity [42,43]. If serum calcium concentration falls, parathyroid hormone (PTH) is secreted to normalize calcium levels. PTH increases renal calcium reabsorption and activates bone resorption and the kidney hydroxylase enzyme, 25-hydroxyvitamin D-1α hydroxylase, which converts inactive vitamin D into its active form, 1, 25-hydroxyvitamin D (1, 25-OH2-vitD) [44]. 1, 25-OH2-vitD increases intestinal calcium absorption within the enterocyte. Thus, low serum calcium triggers an increase in the synthesis of 1, 25-OH2-vitD.
In adipose tissue, 1, 25-OH2-vitD causes an influx of calcium ions into adipocytes, thus increasing intracellular calcium [Cai]. Functioning as a second messenger, [Cai] activates gene transcription of fatty acid synthase (FAS), which increases fatty acid (FA) synthesis within adipose tissue. [Cai] also inhibits lipolysis through activation of phosphodiesterases, which blunts the release of FA for oxidation through hormone sensitive lipase (HSL) inhibition. According to the theory proposed by Zemel [45], lowered adipocyte [Cai], resulting from increased dietary calcium, mitigates adiposity by decreasing de novo lipogenesis and directly stimulating lipolysis as explained in the above mechanism.
Zemel’s proposed mechanism was demonstrated in a transgenic mouse model expressing the agouti protein in adipose tissue, a model used for diet-induced obesity and shown to have similar adipocyte activity as human adipocytes [46]. Mice were fed diets containing differing calcium levels: low calcium, supplemental calcium, medium dairy, or high dairy for 6 weeks. Mice fat pads were harvested to quantify fat mass and to determine FAS protein expression and activity level in adipocytes. Mice fed low calcium diets had higher body weight gain and fat mass compared to mice fed supplemental calcium or dairy. Both supplemental calcium and high dairy decreased FAS activity by 35 percent and 65 percent, respectively [46]. The reductions in FAS activity resulted from decreases in FAS mRNA, which was 27 percent and 51 percent lower in mice fed high calcium and dairy diets, respectively. High calcium and dairy diet feeding also resulted in 3.4- to 5.2-fold increases in adipocyte lipolysis, as measured by glycerol release following incubation with forskolin, compared to adipocytes from mice fed low calcium. Data obtained from this study, such as the mRNA, activity level of FAS, and measures of lipolysis support the theory that dietary calcium modulates adiposity by reducing adipocyte [Cai].
The above mechanism has support from additional animal studies [4], and treatment of human adipocytes with 1, 25-OH2-vitD was also shown to increase FAS, inhibit lipolysis, and directly suppress uncoupling protein 2 (UCP2). Data support an increase in adipocyte [Cai] [47]. However, in contrast, Bortolotti et al. [48], in a 5-week randomized double-blind placebo controlled study, provided calcium (800 mg/d) or placebo to overweight or obese subjects (n = 10) who were low dairy consumers with usual calcium intake < 800 mg/day. No differences were found in body weight, lipid oxidation, or energy expenditure between the calcium and placebo phases. Additionally, they found no changes in adipose tissue mRNA for HSL. Furthermore, the above mechanism is incongruent with epidemiological data that demonstrate lower levels of vitamin D in overweight and obese populations [49] and in the MetS population [50,51].
Results from RCT investigating a calcium or dairy attenuation of adiposity are mixed. A recent systematic review of 15 cross-sectional studies [22] found inverse associations between increased dairy intake and lower body mass index (BMI) or waist circumference (WC) in seven studies, but authors also found no associations (n = 3) or positive associations (n = 2). However, according to the regression equation based on a mixed-model analysis of 18 prospective studies, increasing dietary calcium intake from 400 to 800 mg/d was associated with a 1.1 kg/m2 decrease in BMI. A recent randomized study by Dugan et al. [37] reported significant decreases in body weight and BMI in women when they were taking the dairy prescribed diet compared to a control diet, which further supports the role of dietary calcium/dairy intake in decreasing adiposity.
Dairy Intake and Blood Pressure
Different mechanisms have been suggested to account for the blood pressure-lowering effect of dairy products. One possible mechanism involves the increase in serum 1, 25-OH2-vitD, which results from low serum calcium. Increased serum concentration of 1, 25-OH2-vitD causes an increase of calcium ion influx into cells. When this occurs in smooth muscle cells, it causes constriction and results in increased blood pressure [52]. Therefore, increasing calcium intake, which is high in dairy products, can decrease serum 1, 25-OH2-vitD concentrations and prevent the calcium-mediated vasoconstriction in smooth muscle cells. This mechanism is supported by studies that show an independent association with 1, 25-dihydroxtvitamin D and increased blood pressure [53]. However, the primary mechanism believed responsible for the blood pressure-lowering effects of dairy involves inhibition of the angiotensin-converting enzyme (ACE). Blood pressure is controlled in part through the Renin-Angiotensin II–Aldosterone pathway. When blood pressure decreases, the kidneys secrete rennin. Rennin converts angiotensinogen into the inactive enzyme, angiotensin I. Angiotensin I is converted into the active form angiotensin II by ACE. Angiotensin II has several effects that collectively increase blood pressure. Angiotensin II directly causes vasoconstriction, increases the release of anti-diuretic hormone via release of vasopressin, thus causing the re-absorption of water at the level of the kidneys, and triggers release of aldosterone from the adrenal cortex, causing the kidney to re-absorb sodium [54]. Thus, inhibiting ACE decreases the production of angiotensin II and therefore decreases blood pressure-raising effects. ACE inhibitors have been developed as pharmaceutical drugs that can decrease vascular resistance and lower blood pressure during hypertension [55]. ACE inhibitors also occur naturally within the protein primary sequence in several foods, including fish, gelatin, corn, and milk protein [56].
Dairy contains two forms of protein, casein and whey. Both include peptide fractions that have ACE-inhibiting properties, called casokinins and lactokinins, respectively. However, these proteins require enzymatic hydrolysis to release the functional peptides. This can be accomplished during product manufacturing, through fermentation from lactic acid producing bacteria, or during the digestive process [57]. Thus, human digestion of dairy foods can release ACE-inhibiting peptide fractions. Since proline-proline dipeptides resist enzymatic degradation [56], they may be especially protected from hydrolysis during digestion, which suggests peptide fractions containing proline may be more effective at lowering blood pressure. Peptide sequences believed to be responsible for ACE inhibition include the lactotripeptides amino acid sequences isoleucine-proline-proline (IPP) and valine-proline-proline (VPP).
Although whey and casein contain ACE-inhibiting peptides and have both been shown to exert blood pressure-lowering effects, results are inconsistent. For example, Yamasue et al. [58] recruited subjects (n = 30, 23-69 y) with blood pressure above 130/85 mm Hg and provided them with 200 mL sour milk, which contained 2.66 mg VPP and 1.38 mg IPP twice a day for 2 weeks. Twenty-four-hour blood pressure was measured with an ambulatory measuring system. Results indicated that night systolic blood pressure was lower at week 4 (120 ± 13.3, p = 0.04) and week 8 (119 ± 14.3, p = 0.03) compared to baseline (124 ± 16.0). In contrast, van der Zander et al. [59] provided casein hydrosylate powder containing ~29 mg IPP and 22 mg VPP or placebo to 275 hypertensive adults for 8 weeks but found no difference in systolic or diastolic blood pressure between peptides and placebo. A possible explanation may be that casein coagulates in the stomach, rendering it less available for enzymatic hydrolysis and slows delivery of amino acids to the intestine for absorption.
In contrast, whey proteins are water soluble and are delivered more rapidly to the intestinal track, thus they could theoretically provide increased opportunity for biological activity. However, Pal et al. [60] provided subjects (70 men and women, 16-65 y) a supplement containing 27 grams of whey, casein, or glucose to be consumed as part of the usual diet for 12 weeks. Supplementation with whey or casein significantly decreased systolic blood pressure by 4.0 and 4.2 percent respectively, compared to baseline. Diastolic blood pressure was decreased by 3 percent in both treatments compared to baseline, with no differences between the types of protein.
The blood pressure-lowering effect of dairy, independent of calcium intake, is supported by cross-sectional [61,62] and prospective data [44,62]. A subset population from the French MONICA study (n = 912, men 45-64 y) completed dietary records and underwent blood pressure testing. Subjects were stratified into quintiles according to dairy and calcium intakes. Using linear regression models, both dairy and calcium consumption were independently associated with decreases in systolic blood pressure for all subjects, regardless of hypertension treatment status. The relationship, which was controlled for calcium intake and other common blood pressure confounders, was stronger for those not taking hypertension medications, with significant reductions (p trend < 0.01) for all tested combinations [62]. A recent meta-analysis was performed to determine the effect of low-fat versus high-fat dairy foods on elevated blood pressure in adults [63]. The overall relative risk (RR) was 0.87 (95% CI: 0.81-0.94). After separation by fat content, only low-fat dairy was significant, with an RR of 0.84 (95% CI: 0.74 -0.95).
Overall, data support a blood pressure-lowering effect of dairy, specifically from low-fat dairy and from the whey fraction. In fact, two meta-analyses reported that lactokinins significantly decreased blood pressure. Xu et al. [64] examined the effect of milk IPP and VPP in studies of subjects with either pre-hypertension or hypertension on blood pressure in randomized controlled trials. Nine clinical trials were included in the analyses. Results showed significant decreases in systolic and diastolic blood pressure. These effects were greater by 2.4 mm Hg for systolic blood pressure for those with hypertension compared to those with pre-hypertension. Additionally, Pripp et al. [65] examined the effect of peptides derived from food proteins on blood pressure in 17 randomized controlled trials. Results were of similar magnitude of the analysis conducted by Xu et al. [64], with peptides decreasing systolic by 5.1 percent and diastolic blood pressure by 2.4 percent.
Effect of Dairy Intake on Serum Glucose Levels
Studies have demonstrated improved glucose control in pre-diabetics and diabetics when consuming diets containing higher amounts of protein. Although not completely understood, mechanisms facilitating improved glucose control have been suggested and include a protein-induced increase in serum insulin, increasing hepatic regulatory control of glucose production, and modulation of insulin signaling by leucine through the mammalian target of rapamycin (mTOR) pathway.
The high amino acid content, specifically the branch chain amino acids, may modulate glucose levels by increasing postprandial insulin secretion. Whey protein has been shown to increase glucose-dependent insulinotropic polypeptide (GIP), a hormone secreted by the endocrine cells of the small intestine, which triggers insulin release by pancreatic β cells [66,67]. This increased insulin secretion can benefit diabetics, potentially decreasing serum glucose concentrations. For example, Frid et al. [68] provided high glycemic foods at breakfast (white bread) and at lunch (mashed potatoes) with and without 28g whey protein to 14 volunteers with type 2 diabetes (T2D). Whey consumption increased serum insulin at breakfast (31 percent) and lunch (57 percent) compared to meals without whey and resulted in decreased postprandial serum glucose by 21 percent compared to the test meal without whey. A decrease in plasma glucose has also been observed after 6 weeks in men with MetS in a randomized trial comparing high dairy intake versus a control food [37]. Increased insulin secretion is especially important in T2D as diabetic individuals typically experience a decreased insulin response to carbohydrates. A beneficial effect of combining the insulin-stimulating effects of whey with carbohydrate intake was recently demonstrated by Manders et al. [69], who provided carbohydrate alone or with a protein hydrolysate/amino acid mixture to 10 volunteers with T2D and measured the plasma insulin response from carbohydrate with and without the protein/amino acid mixture. Consuming protein/amino acids with carbohydrate resulted in higher plasma insulin and reduced plasma glucose compared to those consuming carbohydrate alone. Further, isotope tracers used to measure glucose disposal [(6,6-2H2) glucose] found that diabetics consuming protein with carbohydrate experienced an increase (p < 0.01) in glucose disposal compared to those consuming carbohydrate alone [69].
An additional mechanism explaining how increased protein consumption may positively affect glucose concentrations has been postulated by Layman et al. [70], who suggest that when carbohydrates are replaced with protein, the branched chained amino acids (BCAA), specifically leucine, will “shift” the regulatory control of glucose homeostasis away from the pancreas and insulin secretion toward hepatic control through gluconeogenesis. This “shifting of regulatory control” will provide overall glycemic stabilization via increased utilization of the glucose-alanine cycle and hepatic de novo gluconeogenesis, stabilizing fasting and post-prandial glucose concentrations. It was also postulated that after carbohydrate intake, the body transitions from higher to lower insulin levels while there is a corresponding increase in glucagon to trigger hepatic de novo gluconeogenesis, thus keeping glucose concentrations constant. Since high carbohydrate intake results in correspondingly high insulin secretion, there is a “rebound” hypoglycemia prior to normalization of glucose levels via hepatic de novo gluconeogenesis. However, high protein intake can mitigate these large fluctuations in serum glucose concentrations, while stabilizing glucose at a lower serum concentration [70]. This premise was tested by feeding a 400 kcal breakfast with either high protein (33g protein and 39 g carbohydrate) or high carbohydrate (10g protein and 57g carbohydrate) to 24 women for breakfast [71]. Two-hour post-prandial glucose, which corresponds to the transition time from exogenous to endogenous glucose production, was lower in both groups; however, the carbohydrate group dropped 30 percent below the glucose values compared to the high protein group, indicating decreased baseline glucose stabilization. Additionally, insulin increased more than two-fold and was > 40 percent higher in the high carbohydrate group compared to the high protein group. In addition, plasma-branched chained amino acids alanine and glutamine were increased in the high protein group but were decreased in the high carbohydrate group, suggesting less amino acid availability for participating in hepatic de novo gluconeogenesis and glucose recycling via the glucose-alanine cycle.
A third potential theory of how protein regulates glucose concentrations involves modulation of insulin signaling by leucine through the mTOR pathway. During insulin signaling, insulin binds to the insulin receptor, which triggers the auto-phosphorylation of tyrosine residues on insulin substrate 1 (IRS-1). The resultant signaling pathway leads to the translocation of the glucose transporter-4 (GLUT 4) to the cell membrane for glucose uptake. However, the activation of mTOR leads to the activation of S6 protein kinase 1 (S6K1), which increases the phosphorylation of serine on IRS-1, creating a negative feedback loop by inhibiting IRS-1 and thus halting insulin’s signaling. It is well known that leucine activates mTOR, which is important in increasing muscle synthesis. However, mTOR activation can also increase serine phosphorylation, which inhibits IRS-1. Since this has been shown to increase insulin resistance, it is also believed to facilitate the pathogenesis of obesity and T2D [72]. Yet the effect of leucine is unclear as others have found that leucine improves insulin sensitivity. A recent study supports a protective effect of leucine [73]. In this study, C57BL/6J mice were randomized to consume chow containing 21 percent (control) or 60 percent energy (high fat) from fat with or without leucine (~60mg/d vs. 120 mg/d) added to the water for 8 weeks. Mice consuming the high fat (HF) diet gained more weight regardless of leucine intake, and mice consuming high fat and leucine (HFL) did not differ in food intake or body weight compared to those consuming HF. HF mice developed obesity, fatty liver, and insulin resistance, while HFL mice had improved glucose tolerance and insulin signaling. Steatotic livers from HF mice weighed double compared to control mice, while liver weigh from HFL mice did not differ from controls [73]. All these findings indicate increases in insulin sensitive mediated by leucine.
While it is not completely understood, increased dairy intake has been shown to lower serum glucose through several potential mechanisms, which may include protein-induced increases in serum insulin and increasing hepatic regulatory control of glucose production. However, the effect of leucine on insulin sensitivity requires further study.
Calcium, Dairy and Plasma Lipids, Lipoproteins and Apolipoproteins
Although the current Dietary Guidelines for Americans recommend reducing saturated fat to reduce cardiovascular disease risk [23], a recent meta-analysis did not find an association between saturated fat and cardiovascular disease [74]. In fact, another meta-analysis examining dairy consumption and incidence of vascular disease found a protective effect of dairy. In this study, a higher dairy consumption was associated with 21, 13, and 8 percent decreases in risk for stroke, all-cause mortality, and ischemic heart disease, respectively [75] compared to lower dairy consumption. Although reasons for this are not entirely clear, the high calcium content and the lipid fractions present in dairy products may positively alter the serum lipid profile.
Early studies testing the lipid-lowering effect of calcium found that supplemental calcium (900 mg/day) decreased serum total cholesterol by 15.4 mg/dL and triglycerides by 32.2 mg/dL [76]. The primary mechanism believed to be responsible for this effect is the intestinal binding of calcium to saturated fatty acids, which forms insoluble soaps that are excreted in the feces. A secondary mechanism is thought to occur from calcium binding to bile acids, which also increases their fecal excretion. The loss of bile acids interrupts enterohepatic circulation, and this triggers the removal of circulating cholesterol through upregulation of hepatic LDL receptors and leads to increased bile acid synthesis via hepatic cholesterol.
Denke et al. [77] randomized healthy men (n = 13, aged 38-49 years) with moderately elevated total cholesterol to receive diets containing high (2200 mg/d) or low (240 mg/d) supplemental calcium citrate for 1 week in a crossover study. Higher calcium intake resulted in greater fecal fat excretion of palmitic, stearic, and oleic acids (p < 0.05). The reduction in fat absorption resulted in lower fasting serum total and LDL cholesterol and apolipoprotein B compared to the low calcium diets. In a similar study, the effect of dairy calcium on serum lipids was investigated in 16 healthy men [78]. Subjects consumed milk or yogurt (1 liter/day) containing 1200 mg calcium or a placebo with 120 mg calcium for 1 week in a cross-over design. Fecal FA and bile acid excretion increased during dairy intake and resulted in lower fat absorption compared to the placebo. In addition to these studies, this mechanism has been established in several animal models and in human trials (reviewed in [31]). Christensen et al. [79] performed a systematic review and meta-analysis, including 15 RCT of healthy adults, to determine the effect of calcium consumed through supplementation or from dairy products on fecal fat excretion. Results from the analysis demonstrated that supplemental calcium resulted in an estimated ~2 g/day increased fat excretion from increased calcium intake above 800 mg/d. Intakes of dairy calcium resulted in higher amounts of fat excretion, with study results indicating that an increase of ~1240 mg/d of dairy calcium corresponds to 5.2 g/d increase in fecal fat.
These cholesterol-lowering effects of calcium may attenuate the potential cholesterol-raising effects of palmitic, myristic, and lauric acids found in dairy foods. Additionally, dairy is high in stearic acid, a saturated fatty acid that is not associated with raising cholesterol when exchanged for dietary carbohydrates or other saturated FA [80]. Specific fatty acids provided by dairy may also modulate lipoprotein metabolism. Lauric and myristic acids were shown to raise ApoA1 concentrations when replacing carbohydrates in the diet with no significant effect on ApoB concentrations [81], suggesting these FA may contribute to a more favorable lipoprotein profile. Other lipid components in milk including phospholipids and sphingolipids have been reported to decrease cholesterol absorption in animals [82,83]. The different proposed mechanisms from dairy nutrients associated with reductions in MetS are depicted in Figure 1.
Figure 1.
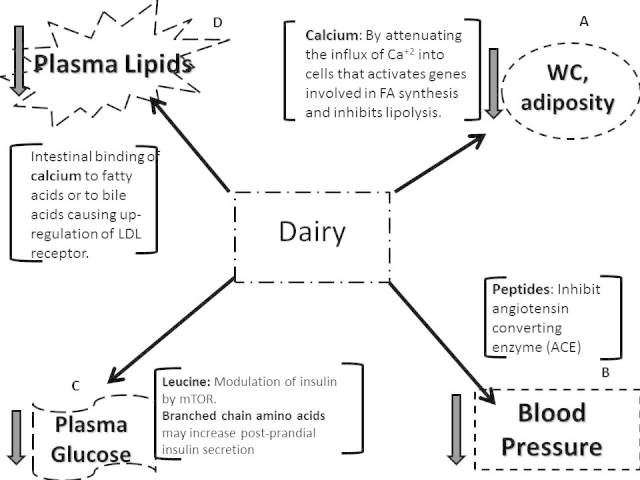
Proposed mechanisms by which dairy intake may reduce parameters of metabolic syndrome including waist circumference (Panel A), blood pressure (Panel B), plasma glucose (Panel C), and dyslipidemias (Panel D).
Conclusions
Based on these reviewed mechanisms and data, dietary inclusion of low-fat dairy may present an alternative strategy to attenuate MetS markers. Epidemiological data for the most part suggest that there is an inverse correlation between dairy consumption and MetS [33-35]. Clinical studies have also shown associations between increased dairy intake and lowering of one or several parameters of metabolic syndrome including weight and waist circumference [45], blood pressure [38], dyslipidemias [77], and hyperglycemia [37]. Additional benefits have been shown in maintaining vascular function and decreasing hyperglycemia [84], inflammation [39], and reductions in type 2 diabetes [85]. Therefore, we conclude that increasing dairy intake for the most part reduces the biomarkers that define MetS in addition to improving low grade and T2D, associated with increased risk for heart disease. Additionally, including ~3 servings of dairy into the diet per day requires only slight dietary modifications, making it an easy lifestyle change to maintain.
Abbreviations
- ACE
angiotensin converting enzyme
- CRD
carbohydrate restricted diets
- FA
fatty acids
- MetS
metabolic syndrome
- RCT
randomized clinical trials
- T2D
type 2 diabetes
- TG
triglycerides
- WC
waist circumference
- NHANES
National Health and Nutrition Examination Survey
- PTH
parathyroid hormone
- 1, 25-OH2-vitD
1, 25-hydroxyvitamin D
- HSL
hormone sensitive lipase
- [Cai]
intracellular calcium
- FAS
fatty acid synthase
- UCP2
uncoupling protein 2
- BMI
body mass index
- TG
triglycerides
- IPP
isoleucine-proline-proline
- VPP
valine-proline-proline
- mTOR
mammalian target of rapamycin
- GIP
glucose-dependent insulinotropic polypeptide
- BCAA
branched chained amino acids
- IRS-1
insulin substrate 1
- GLUT 4
glucose transporter-4
- S6K1
S6 protein kinase 1
- HF
high fat
- HFL
high fat and leucine
- CVD
cardiovascular disease
Author contributions
CED wrote the manuscript. MLF revised, edited, wrote the conclusions, and did the figure.
References
- Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92(2):399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- Burton WN, Chen CY, Schultz AB, Edington DW. The prevalence of metabolic syndrome in an employed population and the impact on health and productivity. J Occup Environ Med. 2008;50(10):1139–1148. doi: 10.1097/JOM.0b013e318188b8eb. [DOI] [PubMed] [Google Scholar]
- Chen SH, He F, Zhou HL, Wu HR, Xia C, Li YM. Relationship between nonalcoholic fatty liver disease and metabolic syndrome. J Dig Dis. 2011;12(2):125–130. doi: 10.1111/j.1751-2980.2011.00487.x. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M. et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes. 2010;2(3):180–193. doi: 10.1111/j.1753-0407.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: Current state of the evidence. Diabetes Care. 2008;31(19):1898–1904. doi: 10.2337/dc08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM. Does a diagnosis of metabolic syndrome have value in clinical practice? Am J Clin Nutr. 2006;83(6):1248–1251. doi: 10.1093/ajcn/83.6.1248. [DOI] [PubMed] [Google Scholar]
- Grundy SM. Metabolic syndrome: A multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92(2):399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- Al-Sarraj T, Saadi H, Calle MC, Volek JS, Fernandez ML. Carbohydrate restriction as a first-line dietary intervention therapy effectively reduces the biomarkers of metabolic syndrome in Emirati adults. J Nutrition. 2009;139(9):1667–1676. doi: 10.3945/jn.109.109603. [DOI] [PubMed] [Google Scholar]
- Jones JL, Fernandez ML, McIntosh M, Najm W, Calle MC, Kalynych C. et al. A Mediterranean-style low-glycemic-load diet improves variables of metabolic syndrome in women, and addition of a phytochemical-rich medical food enhances benefits on lipoprotein metabolism. J Clin Lipidol. 2011;5(3):188–196. doi: 10.1016/j.jacl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Klemsdal TO, Holme I, Nerland H, Pedersen TR, Tonstad S. Effects of a low glycemic load diet versus a low-fat diet in subjects with and without the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2010;20(3):195–201. doi: 10.1016/j.numecd.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Blesso CN, Andersen CJ, Barona J, Volk B, Volek JS, Fernandez ML. Effects of carbohydrate restriction and dietary cholesterol provided by eggs on clinical risk factors of metabolic syndrome. J Clin Lipidol. 2013;7(5):463–471. doi: 10.1016/j.jacl.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Andersen CJ, Fernandez ML. Dietary approaches to improving atheroprotective HDL functions. Food and Function. 2013;14(3):241–254. doi: 10.1039/c3fo60207a. [DOI] [PubMed] [Google Scholar]
- Volek JS, Phinney SD, Forsythe CE, Quann EE, Wood RJ, Puglisi MJ. et al. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids. 2009;44(4):297–309. doi: 10.1007/s11745-008-3274-2. [DOI] [PubMed] [Google Scholar]
- Volek JS, Fernandez ML, Feinman RD, Phinney SD. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog Lipid Res. 2008;47(5):307–318. doi: 10.1016/j.plipres.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Kesse-Guyot E, Ahluwalia N, Lassale C, Hercberg S, Fezeu L, Lairon D. Adherence to Mediterranean diet reduces the risk of metabolic syndrome: A 6-year prospective study. Nutr Metab Cardiovasc Dis. 2013;23(7):677–683. doi: 10.1016/j.numecd.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Blesso CN, Andersen CJ, Barona J, Volek J, Fernandez ML. Whole egg consumption improves lipoprotein profiles and insulin sensitivity in individuals with metabolic syndrome. Metabolism. 2013;62(3):400–410. doi: 10.1016/j.metabol.2012.08.014. [DOI] [PubMed] [Google Scholar]
- German JB, Dillard CJ. Composition, structure and absorption of milk lipids: A source of energy, fat-soluble nutrients and bioactive molecules. Crit Rev Food Sci Nutr. 2006;46(1):57–92. doi: 10.1080/10408690590957098. [DOI] [PubMed] [Google Scholar]
- Miller G, Jarvis J, McBean L, editors. Handbook of Dairy Foods and Nutrition. Boca Raton, FL: CRC Press LLC; 2000. [Google Scholar]
- Molkentin J. Occurrence and biochemical characteristics of natural bioactive substances in bovine milk lipids. Br J Nutr. 2000;84(Suppl 1):S47–S53. doi: 10.1017/s0007114500002245. [DOI] [PubMed] [Google Scholar]
- Dougkas A, Reynolds CK, Givens ID, Elwood PC, Minihane AM. Associations between dairy consumption and body weight: A review of the evidence and underlying mechanisms. Nutr Res Rev. 2011;24(1):72–95. doi: 10.1017/S095442241000034X. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th edition. Washington, DC: U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulgoni VL 3rd, Huth PJ, DiRienzo DB, Miller GD. Determination of the optimal number of dairy servings to ensure a low prevalence of inadequate calcium intake in Americans. J Am Coll Nutr. 2004;23(6):651–659. doi: 10.1080/07315724.2004.10719407. [DOI] [PubMed] [Google Scholar]
- Mangano KM, Walsh SJ, Insogna KL, Kenny AM, Kerstetter JE. Calcium intake in the United States from dietary and supplemental sources across adult age groups: New estimates from the National Health and Nutrition Examination Survey 2003-2006. J Am Diet Assoc. 2011;111(5):687–695. doi: 10.1016/j.jada.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietary intakes for adults 20 years of age and older. Centers for Disease Control and Prevention [Internet] Available from: http://www.cdc.gov/nchs/fastats/diet.htm .
- McCarron DA, Heaney RP. Estimated healthcare savings associated with adequate dairy food intake. Am J Hypertens. 2004;17(1):88–97. doi: 10.1016/j.amjhyper.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Pereira MA, Jacobs DR Jr., Van Horn L, Slattery ML, Kartashov AI, Ludwig DS. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: The CARDIA Study. JAMA. 2002;287(16):2081–2089. doi: 10.1001/jama.287.16.2081. [DOI] [PubMed] [Google Scholar]
- Fumeron F, Lamri A, Emery N, Bellili N, Jaziri R, Porchay-Balderelli I. et al. DESIR Study Group: Dairy products and the metabolic syndrome in a prospective study, DESIR. J Am Coll Nutr. 2011;30(5 Suppl 1):454S–463S. doi: 10.1080/07315724.2011.10719990. [DOI] [PubMed] [Google Scholar]
- Elwood PC, Pickering JE, Fehily AM. Milk and dairy consumption, diabetes and the metabolic syndrome: The Caerphilly prospective study. J Epidemiol Community Health. 2007;61(8):695–698. doi: 10.1136/jech.2006.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay A, Gilbert JA. Milk products, insulin resistance syndrome and type 2 diabetes. J Am Coll Nutr. 2009;28(Suppl 1):91S–102S. doi: 10.1080/07315724.2009.10719809. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Ebrahim S, Timpson N, Davey Smith G. Avoiding milk is associated with a reduced risk of insulin resistance and the metabolic syndrome: Findings from the British Women’s Heart and Health Study. Diabet Med. 2005;22(7):808–811. doi: 10.1111/j.1464-5491.2005.01537.x. [DOI] [PubMed] [Google Scholar]
- Snijder MB, van der Heijden AA, van Dam RM, Stehouwer CD, Hiddink GJ, Nijpels G. et al. Is higher dairy consumption associated with lower body weight and fewer metabolic disturbances? The Hoorn Study. Am J Clin Nutr. 2007;85(4):989–995. doi: 10.1093/ajcn/85.4.989. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Gary TL, Caballero BH, Lawrence RS, Cheskin LJ, Wang Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am J Clin Nutr. 2008;87(6):1914–1925. doi: 10.1093/ajcn/87.6.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Yoon YS, Lee Y, Kim CI, Oh SW. Dairy product intake is inversely associated with metabolic syndrome in korean adults: anseong and ansan cohort of the korean genome and epidemiology study. J Korean Med Sci. 2013;28(10):1482–1488. doi: 10.3346/jkms.2013.28.10.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel MB, Richards J, Milstead A, Campbell P. Effects of calcium and dairy on body composition and weight loss in African-American adults. Obes Res. 2005;13(7):1218–1225. doi: 10.1038/oby.2005.144. [DOI] [PubMed] [Google Scholar]
- Dugan CE, Barona J, Fernandez ML. Increased dairy consumption differentially improves metabolic syndrome markers in male and female adult. Met Synd Rel Dis. 2013 doi: 10.1089/met.2013.0109. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- van Meijl LE, Mensink RP. Low-fat dairy consumption reduces systolic blood pressure, but does not improve other metabolic risk parameters in overweight and obese subjects. Nutr Metab Cardiovasc Dis. 2011;21(5):355–361. doi: 10.1016/j.numecd.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Stancliffe RA, Thorpe T, Zemel MB. Dairy attentuates oxidative and inflammatory stress in metabolic syndrome. Am J Clin Nutr. 2011;94(2):422–430. doi: 10.3945/ajcn.111.013342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loan MC, Keim NL, Adams SH, Souza E, Woodhouse LR, Thomas A. et al. Dairy foods in a moderate energy restricted diet do not enhance central fat, weight, and intra-abdominal adipose tissue losses nor reduce adipocyte size or inflammatory markers in overweight and obese adults: A controlled feeding study. J Obes. doi: 10.1155/2011/989657. Epub 2011 Sep 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH. et al. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes. 2006;30:1529–1534. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- Vaskonen T. Dietary minerals and modification of cardiovascular risk factors. J Nutr Biochem. 2003;14(9):492–506. doi: 10.1016/s0955-2863(03)00074-3. [DOI] [PubMed] [Google Scholar]
- van Meijl LE, Vrolix R, Mensink RP. Dairy product consumption and the metabolic syndrome. Nutr Res Rev. 2008;21(2):148–157. doi: 10.1017/S0954422408116997. [DOI] [PubMed] [Google Scholar]
- Shils M, Shike M, Ross C, Carballero B, Cousins R, editors. Modern Nutrition in Health and Disease. 10th edition. Baltimore: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Zemel MB. Mechanisms of dairy modulation of adiposity. J Nutr. 2003;133(1):252S–256S. doi: 10.1093/jn/133.1.252S. [DOI] [PubMed] [Google Scholar]
- Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J. 2000;14(9):1132–1138. [PubMed] [Google Scholar]
- Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1alpha,25-Dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. FASEB J. 2001;15(14):2751–2753. doi: 10.1096/fj.01-0584fje. [DOI] [PubMed] [Google Scholar]
- Bortolotti M, Rudelle S, Schneiter P, Vidal H, Loizon E, Tappy L. et al. Dairy calcium supplementation in overweight or obese persons: Its effect on markers of fat metabolism. Am J Clin Nutr. 2008;88(4):877–885. doi: 10.1093/ajcn/88.4.877. [DOI] [PubMed] [Google Scholar]
- Parikh SJ, Edelman M, Uwaifo GI, Freedman RJ, Semega-Janneh M, Reynolds J. et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89(3):1196–1199. doi: 10.1210/jc.2003-031398. [DOI] [PubMed] [Google Scholar]
- Ganji V, Zhang X, Shaikh N, Tangpricha V. Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001-2006. Am J Clin Nutr. 2011;94(1):225–233. doi: 10.3945/ajcn.111.013516. [DOI] [PubMed] [Google Scholar]
- Oosterwerff MM, Eekhoff EM, Heymans MW, Lips P, van Schoor NM. Serum 25-hydroxyvitamin D levels and the metabolic syndrome in older persons: A population-based study. Clin Endocrinol (Oxf) 2011;75(5):608–613. doi: 10.1111/j.1365-2265.2011.04110.x. [DOI] [PubMed] [Google Scholar]
- Zemel MB. Calcium modulation of hypertension and obesity: mechanisms and implications. J Am Coll Nutr. 2001;20(5):428S–435S. doi: 10.1080/07315724.2001.10719180. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Grieger JA, Hilpert KF, West SG. Milk products, dietary patterns and blood pressure management. J Am Coll Nutr. 2009;28(Suppl 1):103S–119S. doi: 10.1080/07315724.2009.10719804. [DOI] [PubMed] [Google Scholar]
- Berne R, Levy M, Koeppen B, Stanton B. Physiology. St. Louis, MI: Mosby; 2004. [Google Scholar]
- Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97(14):1411–1420. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RJ, Meisel H. Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme. Br J Nutr. 2000;84(Suppl 1):S33–S37. doi: 10.1017/s0007114500002221. [DOI] [PubMed] [Google Scholar]
- Yamamoto N. Antihypertensive peptides derived from food proteins. Biopolymers. 1997;43(5):129–134. doi: 10.1002/(SICI)1097-0282(1997)43:2<129::AID-BIP5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Yamasue K, Morikawa N, Mizushima S, Tochikubo O. The blood pressure lowering effect of lactotripeptides and salt intake in 24-h ambulatory blood pressure measurements. Clin Exp Hypertens. 2010;32(4):214–220. doi: 10.3109/10641963.2010.491885. [DOI] [PubMed] [Google Scholar]
- van der Zander K, Bots ML, Bak AA, Koning MM, de Leeuw PW. Enzymatically hydrolyzed lactotripeptides do not lower blood pressure in mildly hypertensive subjects. Am J Clin Nutr. 2008;88(6):1697–1702. doi: 10.3945/ajcn.2008.26003. [DOI] [PubMed] [Google Scholar]
- Pal S, Ellis V. The chronic effects of whey proteins on blood pressure, vascular function, and inflammatory markers in overweight individuals. Obesity. 2010;18(7):1354–1359. doi: 10.1038/oby.2009.397. [DOI] [PubMed] [Google Scholar]
- McCarron DA, Reusser ME. Finding consensus in the dietary calcium-blood pressure debate. J Am Coll Nutr. 1999;18(5 Suppl):398S–405S. doi: 10.1080/07315724.1999.10718904. [DOI] [PubMed] [Google Scholar]
- Ruidavets JB, Bongard V, Simon C, Dallongeville J, Ducimetiere P, Arveiler D. et al. Independent contribution of dairy products and calcium intake to blood pressure variations at a population level. J Hypertens. 2006;24(4):671–681. doi: 10.1097/01.hjh.0000217849.10831.16. [DOI] [PubMed] [Google Scholar]
- Ralston RA, Lee JH, Truby H, Palermo CE, Walker KZ. A systematic review and meta-analysis of elevated blood pressure and consumption of dairy foods. J Hum Hypertens. 2012;26(1):3–13. doi: 10.1038/jhh.2011.3. [DOI] [PubMed] [Google Scholar]
- Xu JY, Qin LQ, Wang PY, Li W, Chang C. Effect of milk tripeptides on blood pressure: A meta-analysis of randomized controlled trials. Nutrition. 2008;24(10):933–940. doi: 10.1016/j.nut.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Pripp AH. Effect of peptides derived from food proteins on blood pressure: a meta-analysis of randomized controlled trials. Food Nutr Res. 2008;52 doi: 10.3402/fnr.v52i0.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon MC, Nuttall FQ. Amino acid ingestion and glucose metabolism—a review. IUBMB Life. 2010;62(9):660–668. doi: 10.1002/iub.375. [DOI] [PubMed] [Google Scholar]
- Graf S, Egert S, Heer M. Effects of whey protein supplements on metabolism: evidence from human intervention studies. Curr Opin Clin Nutr Metab Care. 2011;14(6):569–580. doi: 10.1097/MCO.0b013e32834b89da. [DOI] [PubMed] [Google Scholar]
- Frid AH, Nilsson M, Holst JJ, Bjorck IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr. 2005;82(1):69–75. doi: 10.1093/ajcn.82.1.69. [DOI] [PubMed] [Google Scholar]
- Manders RJ, Wagenmakers AJ, Koopman R, Zorenc AH, Menheere PP, Schaper NC. et al. Co-ingestion of a protein hydrolysate and amino acid mixture with carbohydrate improves plasma glucose disposal in patients with type 2 diabetes. Am J Clin Nutr. 2005;82(1):76–83. doi: 10.1093/ajcn.82.1.76. [DOI] [PubMed] [Google Scholar]
- Layman DK, Shiue H, Sather C, Erickson DJ, Baum J. Increased dietary protein modifies glucose and insulin homeostasis in adult women during weight loss. J Nutr. 2003;133(2):405–410. doi: 10.1093/jn/133.2.405. [DOI] [PubMed] [Google Scholar]
- Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C. et al. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133(2):411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- Melnik BC. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J Diabetes. 2012;3(3):38–53. doi: 10.4239/wjd.v3.i3.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K. et al. Dietary leucine—an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6(6):e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91(3):502–509. doi: 10.3945/ajcn.2008.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood PC, Pickering JE, Givens DI, Gallacher JE. The consumption of milk and dairy foods and the incidence of vascular disease and diabetes: an overview of the evidence. Lipids. 2010;45(10):925–939. doi: 10.1007/s11745-010-3412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacowitz H, Fleischman AI, Bierenbaum ML. Effects of oral calcium upon serum lipids in man. Br Med J. 1965;1(5446):1352–1354. doi: 10.1136/bmj.1.5446.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denke MA, Fox MM, Schulte MC. Short-term dietary calcium fortification increases fecal saturated fat content and reduces serum lipids in men. J Nutr. 1993;123(6):1047–1053. doi: 10.1093/jn/123.6.1047. [DOI] [PubMed] [Google Scholar]
- Govers MJ, Termont DS, Lapre JA, Kleibeuker KH, Vonk RJ, Van der Meer R. Calcium in milk products precipitates intestinal fatty acids and secondary bile acids and thus inhibits colonic cytotoxicity in humans. Cancer Res. 1996;56(14):3270–3275. [PubMed] [Google Scholar]
- Christensen R, Lorenzen JK, Svith CR, Bartels EM, Melanson EL, Saris WH. et al. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev. 2009;10(4):475–486. doi: 10.1111/j.1467-789X.2009.00599.x. [DOI] [PubMed] [Google Scholar]
- Hunter JE, Zhang J, Kris-Etherton PM. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: a systematic review. Am J Clin Nutr. 2010;91(1):46–63. doi: 10.3945/ajcn.2009.27661. [DOI] [PubMed] [Google Scholar]
- Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- Noh SK, Koo SI. Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. J Nutr. 2004;134(10):2611–2616. doi: 10.1093/jn/134.10.2611. [DOI] [PubMed] [Google Scholar]
- Kamili A, Wat E, Chung RW, Tandy S, Weir JM, Meikle PJ. et al. Hepatic accumulation of intestinal cholesterol is decreased and fecal cholesterol excretion is increased in mice fed a high-fat diet supplemented with milk phospholipids. Nutr Metab (Lond) 2010;7:90. doi: 10.1186/1743-7075-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard KD, Mah E, Guo Y, Pei R, Volek JS, Bruno RS. Low-fat milk ingestion prevents postprandial hyperglycemia-mediated impairments in vascular endothelial function in obese individuals with metabolic syndrome. J Nutr. 2013;143(10):1602–1610. doi: 10.3945/jn.113.179465. [DOI] [PubMed] [Google Scholar]
- Kalergis M, Leung Yinko SS, Nedelcu R. Dairy products and prevention of type 2 diabetes: Implications for research and practice. Front Endocrinol (Lausanne) 2013;23(4):90. doi: 10.3389/fendo.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]


