Abstract
Obesity is a metabolic state in which excess fat is accumulated in peripheral tissues, including the white adipose tissue, muscle, and liver. Sustained obesity has profound consequences on one’s life, which can span from superficial psychological symptoms to serious co-morbidities that may dramatically diminish both the quality and length of life. Obesity and related metabolic disorders account for the largest financial burden on the health care system. Together, these issues make it imperative that obesity be cured or prevented. Despite the increasing wealth of knowledge on the etiology of obesity (see below), there is no successful medical strategy that is available for the vast majority of patients. We suggest that brain temperature control may be a crucial component in obesity development and that shortcutting the brain metabolic centers by hypothalamic temperature alterations in a non-invasive remote manner will provide a revolutionary approach to the treatment of obesity.
Keywords: hypothalamus, obesity, temperature regulation, circuit activity
Introduction
Compelling evidence has been gathered during the last 100 years to suggest that the underlying cause for fat accumulation in peripheral tissues arises, at least in part, from the central nervous system [1-9]. From the dawn of modern neurobiology at the end of the 19th century, both intellectual as well as experimental attempts have been made to identify and isolate the brain’s role in energy metabolism regulation. For example, based on the emerging experimental observations on brain stem regulation of respiration at the time, Sherrington suggested that a similar blood link between the periphery and brain should exist for the regulation of food intake [10]. Conclusions on signals from the periphery to the brain regarding feeding regulation were also drawn by others implicating gut-born humoral signals for appropriate brain responses to changing metabolic needs [11,12]. It is still remarkable to realize that these minds, and undoubtedly many more in or before the late 19th and early 20th centuries without the luxury of modern technology and widespread availability of knowledge, had the intellectual capability to predict basic principles that, 100 years later, proved to be correct. These past 100 years, however, have not elapsed without other advancements in the understanding of the brain-obesity relationship.
The involvement of various hypothalamic regions in the regulation of energy homeostasis stems from degeneration studies in the 1940s and 1950s: destruction of the hypothalamic ventromedial (VMH), paraventricular (PVH), and dorsomedial (DMH) nuclei induced hyperphagia [1,13-16]. In contrast, discrete lesions placed in the lateral hypothalamus (LH) [15] reduced food intake. While these approaches may be considered crude by today’s standards of research, they were pivotal in pinpointing the hypothalamus as a major regulator of energy homeostasis. In fact, in retrospect to our current knowledge of specific neurotransmitter- and neuromodulator-containing hypothalamic neuronal populations (see below), these lesion studies were strikingly precise in distinguishing between subregions of the hypothalamus that house circuits that either promote or suppress feeding. The degeneration studies in conjunction with physiological observations on strains of animals that were obese [17,18] confirmed the proposition that humoral signals arising from the periphery may exist to inform brain sites about overall energy needs.
A fundamental breakthrough came in 1994 and 1995 when the gene encoding the adipose signal leptin was discovered and its product tested on leptin deficient obese ob/ob mice [19-22]. Subsequently, receptors for leptin were cloned and localized to structures of the hypothalamus [23], some of which had been implicated in metabolism regulation by lesion studies. However, leptin binding and its receptors accumulated predominantly in a ventromedial hypothalamic structure, the arcuate nucleus [24], an area that was not specifically suggested by the lesion studies. The appreciation of this small hypothalamic nucleus gained momentum regarding energy homeostasis when, due partially to serendipity, the melanocortin system was discovered as a player in obesity. In a quest to better understand the role of various melanocortin receptors in the determination of skin color [25,26], the striking revelation was made that when melanocortin receptor 4 (MC4R) was eliminated, animals became morbidly obese [27]. Since the melanocortin system had long been known to exist in neurons of the arcuate nucleus [28], it was reasonable to test whether leptin exerts its effect on metabolism by the mediation of the arcuate nucleus melanocortin system. And this was found to be the case [29]. Further analysis of components of the central melanocortin system [30] delivered an attractive model, which today is considered to be the primun movens of metabolism regulation.
The realization of the critical role that the arcuate nucleus melanocortin system plays in mediating leptin’s effect on metabolism and the subsequent recognition of a distinct local counterpart of the pro-opiomelanocortin (POMC) cells, the arcuate nucleus agouti-related protein (AgRP)- neuropeptide Y (NPY)-producing cells [31], provided an exceptionally attractive and simple model of the “heart” of the central feeding center. In this model, activation of the POMC neurons by leptin [32,33] triggers release of α-melanocyte stimulating hormone (α-MSH) from POMC axon terminals, which in turn, appropriately activates MC4Rs, leading to suppressed food intake and increased energy expenditure. Simultaneously, leptin suppresses the activity of arcuate nucleus NPY/AgRP neurons [32,33], which otherwise, through the release of AgRP, antagonizes the effect of α-MSH on MC4R [30]. The NPY/AgRP system not only antagonizes anorexigenic melanocortin cells at their target sites where MC4Rs are located, but it also very robustly and directly inhibits POMC perikarya using both NPY as well as the small inhibitory amino acid neurotransmitter, GABA [33], an event that occurs through basket-like synaptic innervations of POMC cells by NPY/AgRP terminals [34]. This unidirectional [34] interaction between the NPY/AgRP and POMC perikarya is of critical significance because it provides a tonic inhibition of the melanocortin cells whenever the NPY/AgRP neurons are active. Because there is no constitutive direct feedback mechanism from the POMC cells to disengage NPY/AgRP neurons, this simple anatomical constellation (Figure 1) may provide the easiest explanation why the baseline blueprint of feeding circuits is more likely to promote feeding then satiety. While this bias toward positive energy balance is a necessity from an evolutionary perspective, it is also a likely contributor to the etiology of metabolic disorders, such as obesity.
Both neuronal systems are directly targeted by leptin and can also be affected by other peripheral metabolic signals, such as ghrelin, glucose, insulin, and peptide YY [35-38]. Their localization in ventromedial aspects of the arcuate nucleus in close vicinity to the neuroheamal (regions without blood-brain barrier) median eminence warrants that these neurons may be reached by circulating metabolic signals in the earliest and most effective manner. In fact, it is not unusual to find either NPY/AgRP or POMC perikarya or dendrites in direct contact with capillaries (Figure 1), furthering the likelihood that these neurons are in excellent position to respond to changes in characteristics of the blood, such as its temperature fluctuation.
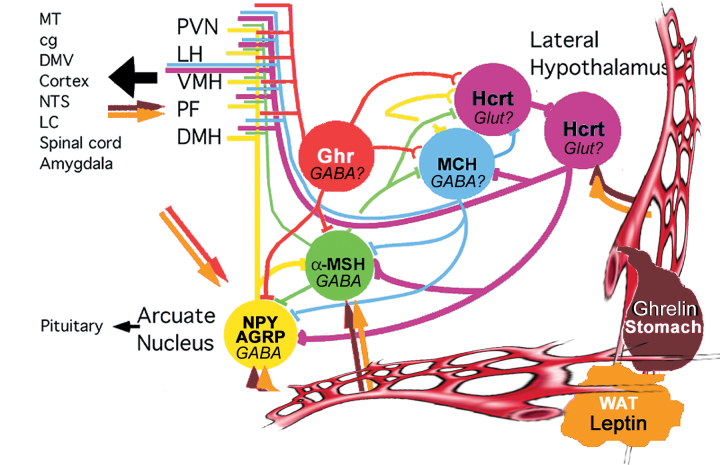
Figure 1.
Schematic drawing showing known interactions and projection targets of various hypothalamic peptidergic systems, including lateral hypothalamic neurons producing hypocretin/orexin (Hcrt) and melanin concentrating hormone (MCH) and components of the melanocortin system, the NPY/AGRP- and MSH-producing neurons. These neuronal populations are targeted from the circulation by white adipose tissue-derived leptin and stomach-derived ghrelin. They are synaptically interconnected and project, to some degree, in an overlapping manner to intra- and extra-hypothalamic sites. It is a recurring mistake to consider peptide circuits as independent entities. Almost without exception, peptidergic neurons contain various other neuromodulators, including the classical neurotransmitters GABA or glutamate (Glut). With varying artistic characteristics, most of the schemes proposed to date are similar to Figure 1 in that they depict connectivity as a still picture implying hard wiring. While such mapping of connectivity will continue to provide important building blocks for evolving concepts, they lack both synaptic resolution as well as indication of soft wiring or plasticity.
The simplicity of the putative hypothalamic feeding center is remarkable and suggests that manipulation of either component of the melanocortin system should shift energy balance. More importantly, if this system is the primum movens of energy homeostasis, then it should be possible to correct metabolic disorders such as obesity by altering either the AgRP/NPY or POMC circuit. Indeed, genetic manipulations of either component can lead to altered metabolic phenotype [39]. To date, no successful medical strategies have emerged that target this system for the treatment of either positive (obesity) or negative (cachexia) energy balance, in part because of the redundant interaction of the melanocortin system with other hypothalamic and extrahypothalamic neuronal systems. Developments of the past decade also suggest that the flexibility of the hypothalamic center of metabolism is not only due to redundant interconnectivity but also the consequence of its recently uncovered soft wiring [9,40].
An Apparent Paradox: The Increasing Body of Knowledge on Perceived Feeding Centers and the Lack of Successful Therapies for Obesity
The discovery of hypophyseotropic hormones in the 1960s rapidly helped reveal the regulation of various peripheral tissues, including the ovaries and testes, by the brain and how the feedback from these tissues influenced the brain. For example, the characterization and synthesis of luteinizing hormone releasing hormone (LHRH) and its influence on the pituitary had immediate academic as well as clinical implications for issues associated with ovarian cycles and infertility [41]. This rapid translation of newfound principles for clinical medicine occurred at a time when genetics and molecular biology, in general, were not common practice and, by and large, were not utilized. Thus, the anticipation since the discovery of leptin in 1994/1995 that medical approaches for metabolic disorders would soon follow seemed to be well founded. These medical breakthroughs have not yet occurred, however, and it is unlikely that the status quo will change in the immediate future. Nevertheless, the paradox remains, considering the amount of intellectual talent and monetary resources being directed at this issue.
The argument can be made that, from an evolutionary perspective, the regulation of energy balance is at least as important as reproduction. Thus, if it turns out to be overly redundant, a method should be found to successfully shortcut the central nervous system component of metabolism regulation similar to what was done regarding reproduction. It is worthwhile to note that although the principal hypothalamic neurons in the regulation of ovarian and testicular function, the LHRH cells, have been found and extensively studied [42], the blueprint of gonadal feedback on the hypothalamus remains largely ill-defined. This fascinating and biologically critical subject, however, is becoming more academic as successful approaches bypassing the brain to regulate reproduction have emerged [43]. Whether or not it is reasonable to anticipate similar breakthroughs in metabolism regulation is yet to be seen. Controlling hypothalamic temperature may offer that very possibility.
The Temperature Hypothesis
We suggest that hypothalamic temperature is a determinant of the output of the arcuate nucleus metabolic center, which, in turn, underlies feeding behavior and energy expenditure. It is intriguing to note that a similar hypothesis was proposed more than 50 years ago by Brobeck [44], albeit his impetus was more intuition than experimentally based. It is important to clarify that our temperature hypothesis (and that of Brobeck) is not to be mistaken (although it most likely relates) with the regulation and role of body temperature in metabolism regulation.
Core Body Temperature and Metabolism
The primary mode of energy expenditure in mammals is by dissipation of energy in the form of heat. Thus, body temperature and its regulation is a major determinant of a metabolic phenotype. Humans rely primarily on behavioral thermoregulation to protect themselves against the cold. They wear clothing, use various heat generating devices, and seek out a warm environment. When these behaviors are unavailable or inadequate to defend body temperature homeostasis during cold exposure, lower peripheral tissue temperatures stimulate peripheral thermoreceptors, and the resultant afferent flow elicits autonomic homoeostatic responses (thermogenesis and vasoconstriction) to restore body temperature [45-49]. Both core and skin temperatures modulate temperature regulation responses [50-52], which are finely tuned by thermoregulation centers located in the preoptic/anterior hypothalamus. The hypothalamus contributes to all core temperature signals, both hypothalamic and extrahypothalamic, such as spinal cord or blood temperature. The hypothalamus also regulates metabolism (see above), so hypothalamic temperature and metabolic responses may be tightly coupled. Intriguingly, this possibility has never been explored by prospective studies, and, consequently, our current understanding of the effect of hypothalamic temperature on thermoregulatory and metabolic responses is limited.
Hypothalamic Temperature and Metabolism
Our interest in hypothalamic temperature emerged when the mitochondrial uncoupler, UCP2, was discovered and localized to the central nervous system [53]. Uncoupling proteins encoded by nuclear DNA are located in the inner membrane of the mitochondria, and their primary function is thought to be to leak hydrogen protons from the intermembrane space to the matrix of the mitochondria. Through this process, they deprive the driving force of ATP synthase from catalyzing ATP synthesis, dissipate energy in the form of heat, and by enhancing the speed of oxidation, they may diminish the production of superoxides and block entry of calcium to the mitochondrial matrix [35,36]. The most well-characterized uncoupling protein, UCP1, is expressed solely in the brown adipose tissue and is responsible for thermogenesis in small rodents. In the last few years, however, several other members of the uncoupling protein family have been discovered and found to promote partial uncoupling of oxidation from phosphorylation in vitro. These proteins include UCP2, UCP3, UCP4, and brain mitochondrial carrier protein 1 (BMCP1). The five UCPs differ greatly in tissue distribution and regulation and may have distinct physiological roles. While UCP1 and UCP3 are expressed only in peripheral tissues (UCP1 only in brown adipose tissue, and UCP3 solely in skeletal muscle and the heart in humans), and UCP4 and BMCP1 are predominantly expressed in the CNS, UCP2 is expressed in muscle, adipose tissue, spleen, and the CNS [41,47,48]. In the healthy brain, UCP2 is expressed predominantly in neuronal populations of subcortical regions that are involved in the central regulation of autonomic, endocrine, and metabolic processes. These regions include the arcuate nucleus, a key brain region in determining energy balance.
We hypothesized that if UCP2 in neuronal circuits is a functional uncoupler in a manner similar to what was found regarding UCP1 in the brown fat, the proton leak of mitochondria in UCP2-containing brain regions should be increased. Indeed, we found that the mitochondrial respiratory control ratio (RCR) in rat mitochondrial extracts from regions with abundant UCP2 expression (hypothalamus) was significantly higher than that measured in regions that lack UCP2 expression (the striatum-lateral thalamus region). The presence of decreased mitochondrial energy coupling efficiency (increased proton leak) in UCP2 containing brain regions supported our hypothesis that a thermogenic mechanism is intrinsic to distinct neuronal pathways. To test this further, brain tissue temperature in the face of steady core body temperature was determined at several dorso-ventral and medio-lateral locations in rats, and it was found that UCP2-containing brain regions have a significantly higher local temperature when compared to other sites or to the core body temperature. These observations raised the possibility that a mitochondrial uncoupling mechanism participates in the central regulation of whole body metabolism and that in this process, temperature is a determinant factor [53]. To address this, we analyzed feeding responses of UCP2 overexpressing and UCP2 knockout animals. In these studies, we found that overexpression of UCP2 in the hypothalamus, where it is co-expressed with the most potent orexigenic neuropeptide, NPY [53], results in elevated levels of hypothalamic NPY levels and food intake. In contrast, UCP2 knockout animals exhibited blunted rebound feeding after a short fast [54,55]. In further support of the idea that a hypothalamic thermogenic mechanism may contribute to the regulation of feeding circuits is our observation during the development of diet-induced obesity of the induction of mRNA levels of a heat-inducible protein, heat-responsive protein 12, in the hypothalamus [56]. This is a time when peroxisome-driven beta oxidation of fats appears to be increased in the hypothalamus [57], and mitochondrial fusion emerges [58-60].
Proposed Approach for the Testing of the Conceptual and Practical Basis of the Temperature Hypothesis
If hypothalamic temperature is a major determinant of the outflow of the arcuate nucleus metabolic circuitry, then approaches that selectively up- or down-regulate local temperature would provide non-invasive and non-pharmacological tools to regulate energy expenditure. If such a methodology succeeds, therapeutic approaches to obesity and related disorders would be dramatically changed and enhanced. Our proposal aims to clarify the conceptual and practical correlates of such approaches.
Conceptual Questions Regarding Hypothalamic Temperature and Metabolic Output
The hypothesis predicts that if hypothalamic temperature is critical for the output of the central metabolic circuitry, then metabolic malfunctions (such as obesity and type 2 diabetes) may be accompanied by altered hypothalamic temperature. Thus, it will be important to determine hypothalamic temperature and its correlation to core body temperature in lean and obese subjects. Brain temperature monitoring has been very difficult to accomplish. Although small temperature probes are available for deep brain temperature measurements [53], it nevertheless remains an invasive approach that involves destruction of blood vessels and induction of acute inflammatory responses, all of which will have a direct impact on local temperature. Of course, assessing fluctuating hypothalamic temperature in relation with metabolic parameters will provide only temporal relationship between these events. For this, it will be necessary to assess the effect of hypothalamic temperature rise and fall on feeding behavior, energy expenditure, electrophysiology, and synapse organization of the melanocortin system.
Practical Aspects of Hypothalamic Temperature and Metabolic Output
While animal models will allow for the testing of conceptual and some practical issues, they will not answer the critical question, i.e., how hypothalamic temperature manipulations could aid the human metabolic condition. In humans, environmental cold exposure produces peripheral vasoconstriction resulting in decreases in peripheral blood flow, thus reducing convective transfer between the body’s core and shell (i.e., skin, peripheral fat and skeletal muscle). Responses to cold exposure are moderated by factors that influence heat production and heat loss, including the magnitude of the metabolic response, and individual characteristics such as body composition, age, and sex [49,61-63]. Individuals with a large surface area to mass ratio (lean) experience greater declines in body temperature during environmental cold exposure than those with smaller surface area to mass ratio (obese), and insulation is often correlated with subcutaneous fat thickness. Fatter individuals shiver less and experience smaller declines in body temperature during cold exposure than lean individuals [64]. In normal weight individuals, whole-body cold exposure results in vasoconstriction in the periphery when oral temperature reaches ~35°C and becomes maximal when skin temperature is ~31°C [65]. The metabolic response to mild exposure in both moderate and extremely obese adults [66] is reduced relative to lean subjects [67]. Similar data in our laboratory support these findings in children in whom metabolic rate was much lower than expected during cold exposure [68]. Moreover, tympanic temperature vasoconstriction threshold is delayed in obese subjects (35.5°C versus 36.0°C, for lean and obese, respectively) during general anesthesia [69].
The attenuated thermogenic response to cold exposure in obese subjects [66-68] is to conserve energy so this may also predispose some individuals to obesity. A number of studies have demonstrated the phenomenon of low or normal body temperature combined with low metabolic rate in individuals prone to obesity, leading to the description of an “obesity prone syndrome” [70]. This syndrome includes the hypothesis that individuals who are not yet obese but are prone to obesity might start with a lower body temperature. As their body weight increases, their temperature might rise and become normal, thus diminishing any relationship between body temperature and obesity. Part of this normalization of body temperature might be related to the insulating properties of body fat or related to defects at the hypothalamic level. Studies in Pima Indians demonstrated that individuals with low metabolic rate and low body temperature were especially prone to become obese [70-71]. Moreover, low sympathetic nervous system activity is associated with body weight gain, and low activity of the adrenal medulla is associated with the development of central adiposity [72]. The data in humans are consistent with those in ob/ob mice, in whom characteristics of this syndrome include poor temperature responses to cold and low metabolic rate, both present before the animals become obese [73]. Although studies in obese humans have measured metabolic responses to acute cold exposure [70,71], these studies have not included metabolic rate, sympathetic nerve stimulation (SNS) responses, and peripheral vasoconstriction measurements in conjunction with skin (Tsk) and core (Tc) temperature measurements. More importantly, there has been no attempt to isolate the effect of hypothalamic cooling on metabolic or sympathetic responses in a population at risk for obesity.
Although the monitoring of hypothalamic temperature of humans is difficult, it is possible to determine the temporal and causal relationship between skin temperature, esophageal temperature, and the metabolic phenotype. Testing heating and cooling devices that can be used on human subjects for the purpose of evoking metabolic alterations.
Concluding Remarks
We suggest that brain temperature control is a crucial component in obesity development and that shortcutting the brain metabolic centers by hypothalamic temperature alterations in a non-invasive remote manner could provide a revolutionary approach to the treatment of obesity and related metabolic disorders.
Abbreviations
- VMH
ventromedial hypothalamus
- PVH
paraventricular hypothalamus
- DMH
dorsomedial hypothalamus
- LH
lateral hypothalamus
- MC4R
melanocortin receptor 4
- POMC
pro-opiomelanocortin
- AgRP
arcuate nucleus agouti-related protein
- NPY
neuropeptide Y
- α-MSH
α-melanocyte stimulating hormone
- LHRH
luteinizing hormone releasing hormone
- BMCP1
brain mitochondrial carrier protein 1
- RCR
respiratory control ratio
- Tsk
skin temperature
- Tc
core temperature
- Hcrt
hypocretin/orexin
- Glut
glutamate
References
- Brobeck JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol Rev. 1946;26(4):541–559. doi: 10.1152/physrev.1946.26.4.541. [DOI] [PubMed] [Google Scholar]
- Strominger JL, Brobeck JR. A mechanism of regulation of food intake. Yale J Biol Med. 1953;25(5):383–390. [PMC free article] [PubMed] [Google Scholar]
- Leibel RL, Chung WK, Chua SC Jr.. The molecular genetics of rodent single gene obesities. J Biol Chem. 1997;272(51):31937–31940. doi: 10.1074/jbc.272.51.31937. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 2007;30:367–398. doi: 10.1146/annurev.neuro.30.051606.094324. [DOI] [PubMed] [Google Scholar]
- Myers MG Jr., Olson DP. Central nervous system control of metabolism. Nature. 2012;491(7424):357–363. doi: 10.1038/nature11705. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Woods SC. Monitoring of stored and available fuel by the CNS: implications for obesity. Nat Rev Neurosci. 2003;4(11):901–909. doi: 10.1038/nrn1245. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S, Tschop M. Brain circuits regulating energy homeostasis. Neuroscientist. 2004;10(3):235–246. doi: 10.1177/1073858403262151. [DOI] [PubMed] [Google Scholar]
- Sherrington C. In: Text-book of physiology. Sharpey-Schäfer EA, editor. Edinburgh: YJ Pentland; 1898. Cutaneous Sensation; pp. 920–1001. [Google Scholar]
- Cannon WB, Washburn AL. An explanation of hunger. 1911. Obes Res. 1993;1(6):494–500. doi: 10.1002/j.1550-8528.1993.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Carlson AJ. The control of hunger in health and disease. Chicago: The University of Chicago Press; 1916. [Google Scholar]
- Hetherington AW, Ranson SW. The relation of various hypothalamic lesions to adiposity in the rat. J Comp Neurol. 1942;76(3):475–499. [Google Scholar]
- Hetherington AW. Non-production of hypothalamic obesity in the rat by lesions rostral or dorsal to the ventro-medial hypothalamic nuclei. J Comp Neurol. 1944;80(1):33–45. [Google Scholar]
- Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24(2):123–140. [PMC free article] [PubMed] [Google Scholar]
- Hetherington AW. The relation of various hypothalamic lesions to adiposity and other phenomena in the rat. Am J Physiol. 1941;133:326. [Google Scholar]
- Coleman DL. Diabetes-obesity syndromes in mice. Diabetes. 1982;31(Suppl 1 Pt 2):1–6. doi: 10.2337/diab.31.1.s1. [DOI] [PubMed] [Google Scholar]
- Zucker LM, Zucker TF. Fatty, a new mutation in the rat. Journal of Heredity. 1961;52(6):275–278. [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T. et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387(2-3):113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98(5):1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257(5074):1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E. et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72(6):827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88(1):131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Pelletier G, Dube D. Electron microscopic immunohistochemical localization of alpha-msh in the rat brain. Am J Anat. 1977;150(1):201–205. doi: 10.1002/aja.1001500115. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G. et al. Melanocortin receptors in leptin effects. Nature. 1997;390(6658):349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1(4):271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C. et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23(4):775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Naftolin F, Kalra SP, Leranth C. Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology. 1992;131(5):2461–2467. doi: 10.1210/endo.131.5.1425443. [DOI] [PubMed] [Google Scholar]
- Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L. et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377(6549):530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology. 1997;138(11):5063–5066. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Smith MA, Mirshamsi S, Routh VH, Ashford ML. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nat Neurosci. 2000;3(8):757–758. doi: 10.1038/77660. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL. et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;4318(8):589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X. et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304(5667):110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Schally AV, Kastin AJ, Arimura A, Coy D, Coy E, Debeljuk L. et al. Basic and clinical studies with luteinizing hormone-releasing hormone (LH-RH) and its analogues. J Reprod Fertil Suppl. 1973;20(0):119–136. [PubMed] [Google Scholar]
- Pfaff DW, Schwanzel-Fukuda M, Parhar IS, Lauber AH, McCarthy LM, Kow LM. GnRH neurons and other cellular and molecular mechanisms for simple mammalian reproductive behaviors. Recent Prog Horm Res. 1994;49:1–25. doi: 10.1016/b978-0-12-571149-4.50005-2. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Kolesnick RN. Realizing the promise of apoptosis-based therapies: separating the living from the clinically undead. Cell Death Differ. 2003;10(5):493–495. doi: 10.1038/sj.cdd.4401217. [DOI] [PubMed] [Google Scholar]
- Brobeck JR. Neural control of hunger, appetite, and satiety. Yale J Biol Med. 1957;29(6):565–574. [PMC free article] [PubMed] [Google Scholar]
- Nielsen B, Astrup A, Samuelsen P, Wengholt H, Christensen NJ. Effect of physical training on thermogenic responses to cold and ephedrine in obesity. Int J Obes Relat Metab Disord. 1993;17(7):383–390. [PubMed] [Google Scholar]
- Emmett JD. A review of heart rate and blood pressure responses in the cold in healthy subjects and coronary artery disease patients. J Cardiopulm Rehabil. 1995;15(1):19–24. doi: 10.1097/00008483-199501000-00003. [DOI] [PubMed] [Google Scholar]
- Granberg PO. Human physiology under cold exposure. Arctic Med Res. 1991;50(Suppl 6):23–27. [PubMed] [Google Scholar]
- Matsumoto T, Miyawaki T, Ue H, Kanda T, Zenji C, Moritani T. Autonomic responsiveness to acute cold exposure in obese and non-obese young women. Int J Obes Relat Metab Disord. 1999;23(8):793–800. doi: 10.1038/sj.ijo.0800928. [DOI] [PubMed] [Google Scholar]
- Stocks JM, Taylor NA, Tipton MJ, Greenleaf JE. Human physiological responses to cold exposure. Aviat Space Environ Med. 2004;75(5):444–457. [PubMed] [Google Scholar]
- Mekjavic IB, Eiken O. Inhibition of shivering in man by thermal stimulation of the facial area. Acta Physiol Scand. 1985;125(4):633–637. doi: 10.1111/j.1748-1716.1985.tb07765.x. [DOI] [PubMed] [Google Scholar]
- Frank SM, Higgins MS, Fleisher LA, Sitzmann JV, Raff H, Breslow MJ. Adrenergic, respiratory, and cardiovascular effects of core cooling in humans. Am J Physiol. 1997;272(2 Pt 2):R557–R562. doi: 10.1152/ajpregu.1997.272.2.R557. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Takamata A, Ito T, Sessler DI, Kitamura Y, Shimosato G. et al. Upright posture reduces thermogenesis and augments core hypothermia. Anesth Analg. 2002;94(6):1646-51, table of contents. doi: 10.1097/00000539-200206000-00053. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci. 1999;19(3):1072–1087. doi: 10.1523/JNEUROSCI.19-03-01072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM. et al. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 2007;5(1):21–33. doi: 10.1016/j.cmet.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM. et al. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454(7206):846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO. et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano S, Liu ZW, Jeong JK, Dietrich MO, Ruan HB, Kim E. et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med. 2011;17(9):1121–1127. doi: 10.1038/nm.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Liu ZW, Horvath TL. Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell. 2013;155(1):188–199. doi: 10.1016/j.cell.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger M, Dietrich MO, Sebastian D, Imbernon M, Castano C, Garcia A. et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155(1):172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U. Mitofusins: mighty regulators of metabolism. Cell. 2013;155(1):17–18. doi: 10.1016/j.cell.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Halliday D, Hesp R, Stalley SF, Warwick P, Altman DG, Garrow JS. Resting metabolic rate, weight, surface area and body composition in obese women. Int J Obes. 1979;3(1):1–6. [PubMed] [Google Scholar]
- Bogardus C, Lillioja S, Ravussin E, Abbott W, Zawadzki JK, Young A. Familial dependence of the resting metabolic rate. N Engl J Med. 1986;315(2):96–100. doi: 10.1056/NEJM198607103150205. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78(6):1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toner MM, Sawka MN, Pandolf KB. Thermal responses during arm and leg and combined arm-leg exercise in water. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(5):1355–1360. doi: 10.1152/jappl.1984.56.5.1355. [DOI] [PubMed] [Google Scholar]
- Veicsteinas A, Ferretti G, Rennie DW. Superficial shell insulation in resting and exercising men in cold water. J Appl Physiol Respir Environ Exerc Physiol. 1982;52(6):1557–1564. doi: 10.1152/jappl.1982.52.6.1557. [DOI] [PubMed] [Google Scholar]
- Jequier E, Gygax PH, Pittet P, Vannotti A. Increased thermal body insulation: relationship to the development of obesity. J Appl Physiol. 1974;36(6):674–678. doi: 10.1152/jappl.1974.36.6.674. [DOI] [PubMed] [Google Scholar]
- Vallerand AL, Zamecnik J, Jacobs I. Plasma glucose turnover during cold stress in humans. J Appl Physiol (1985) 1995;78(4):1296–1302. doi: 10.1152/jappl.1995.78.4.1296. [DOI] [PubMed] [Google Scholar]
- Yeckel CW, Taksali SE, Dziura J, Weiss R, Burgert TS, Sherwin RS. et al. The normal glucose tolerance continuum in obese youth: evidence for impaierment in beta-cell function independent of insulin resistance. J Clin Endocrinol Metab. 2005;90(2):747–754. doi: 10.1210/jc.2004-1258. [DOI] [PubMed] [Google Scholar]
- Kasai T, Hirose M, Matsukawa T, Takamata A, Yaegashi K, Tanaka Y. Preoperative blood pressure and catecholamines related to hypothermia during general anesthesia. Acta Anaesthesiol Scand. 2003;47(2):208–212. doi: 10.1034/j.1399-6576.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG. et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318(8):467–472. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- Rising R, Keys A, Ravussin E, Bogardus C. Concomitant interindividual variation in body temperature and metabolic rate. Am J Physiol. 1992;263(4 Pt 1):E730–E734. doi: 10.1152/ajpendo.1992.263.4.E730. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Young JB, Bogardus C, Ravussin E. A low sympathoadrenal activity is associated with body weight gain and development of central adiposity in Pima Indian men. Obes Res. 1997;5(4):341–347. doi: 10.1002/j.1550-8528.1997.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Bray GA, York DA, Fisler JS. Experimental obesity: a homeostatic failure due to defective nutrient stimulation of the sympathetic nervous system. Vitam Horm. 1989;45:1–125. doi: 10.1016/s0083-6729(08)60393-3. [DOI] [PubMed] [Google Scholar]