Abstract
Objective
To assess the effect of the application of a managed protocol for the maintenance care of deceased potential multiple organ donors at two hospitals.
Methods
A before (Phase 1)/after (Phase 2) study conducted at two general hospitals, which included consecutively potential donors admitted to two intensive care units. In Phase 1 (16 months), the data were collected retrospectively, and the maintenance care measures of the potential donors were instituted by the intensivists. In Phase 2 (12 months), the data collection was prospective, and a managed protocol was used for maintenance care. The two phases were compared in terms of their demographic variables, physiological variables at diagnosis of brain death and the end of the process, time to performance of brain death confirmatory test and end of the process, adherence to bundles of maintenance care essential measures, losses due to cardiac arrest, family refusal, contraindications, and the conversion rate of potential into actual donors. Student's t- and chi-square tests were used, and p-value < 0.05 was considered to be significant.
Results
A total of 42 potential donors were identified (18 in Phase 1 and 24 in Phase 2). The time interval between the first clinical assessment and the recovery decreased in Phase 2 (Phase 1: 35.0±15.5 hours versus Phase 2: 24.6±6.2 hours; p = 0.023). Adherence increased to 10 out of the 19 essential items of maintenance care, and losses due to cardiac arrest also decreased in Phase 2 (Phase 1: 27.8 versus 0% in Phase 2; p = 0.006), while the convertion rate increased (Phase 1: 44.4 versus 75% in Phase 2; p = 0.044). The losses due to family refusal and medical contraindication did not vary.
Conclusion
The adoption of a managed protocol focused on the application of essential measures for the care of potential deceased donors might reduce the loss of potential donors due to cardiac arrest.
Keywords: Tissue donors, Brain death, Clinical protocols, Heart arrest
Abstract
Objetivo
Avaliar o efeito da aplicação de um protocolo gerenciado de manutenção de potenciais doadores falecidos de múltiplos órgãos em duas unidades hospitalares.
Métodos
Estudo antes (Fase 1)/depois (Fase 2) realizado em dois hospitais gerais que incluiu, consecutivamente, os potenciais doadores ingressados em duas unidades de terapia intensiva. Na Fase 1 (16 meses), os dados foram coletados retrospectivamente e as medidas de manutenção do potencial doador foram instituídas a critério do intensivista. Na Fase 2 (12 meses), a coleta de dados foi prospectiva e a manutenção foi guiada por um protocolo gerenciado. As duas fases foram comparadas entre si de acordo com variáveis demográficas, variáveis fisiológicas no diagnóstico da morte encefálica e ao final do processo, tempo necessário para realização do exame confirmatório de morte encefálica e final do processo, aderência aos conjuntos de medidas essenciais de manutenção (pacotes), perdas por parada cardíaca, perdas por negativa familiar, perdas por contraindicação e taxa de conversão de potenciais doadores em doadores reais. Foram aplicados os testes de t-Student e do qui-quadrado, e o valor de p<0,05 foi considerado significativo.
Resultados
Identificaram-se 42 potenciais doadores (18 na Fase 1 e 24 na Fase 2). Houve diminuição do tempo entre a primeira exploração clínica e o explante (Fase 1: 35,0±15,5 horas versus Fase 2: 24,6±6,2 horas; p=0,023). Houve aumento na aderência em 10 dos 19 itens essenciais de manutenção, e redução nas perdas por parada cardíaca (Fase 1: 27,8 versus 0% na Fase 2; p=0,006) com aumento de doadores reais (Fase 1: 44,4 versus 75% na Fase 2; p=0,044). Não houve mudança nas perdas por negativa familiar ou por contraindicação médica.
Conclusão
A adoção de um protocolo gerenciado promove a aplicação de medidas essenciais no cuidado do potencial doador falecido e pode reduzir as perdas de potenciais doadores por parada cardíaca.
INTRODUCTION
The difference between the large organ demand and the low number of transplantations performed represents a serious public health problem worldwide.(1,2) In Brazil, the loss of transplantable organs from deceased potential donors as a function of medical contraindications, family refusal, or cardiac arrest is notably high.(3)
The strategies to improve the rate of transplants include the enhancement of living-donor programs, the adoption of donation after circulatory death programs, and the increase of donations from brain-dead individuals. This last option includes to improve brain death reporting, decreasing family refusal, adjusting the contraindication criteria, and reducing losses due to cardiac arrest.(1,2,4-7)
In Brazil, the donation rate per million population (pmp) increased 43.6% in the last five years, reaching 10.6 donations pmp. The Brazilian state that exhibits the best performance in this regard is Santa Catarina, where the main reason for the increase in the donation rate is the professionalization and training of the coordinators of Transplant Commissions. Such training is in particular focused on the skills to communicate bad news to the relatives of potential organ donors, as well as on the revision of the criteria used to define the medical contraindications to donation.(3,8,9)
The application of these measures began in 2008 and resulted in the reduction of losses by contraindication (2007: 10% versus 2011: 5.2%; p<0.02) and family refusal (2007: 44.9% versus 2011: 24.7%; p<0.001) with a net increase in the actual donations from 14.6 pmp in 2007 to 25.7 pmp in 2011. Nevertheless, the rate of losses due to cardiac arrest (27.8%) remained high.(8,10)
The recent publication of Brazilian guidelines for multiple organ maintenance care in deceased adult potential donors represents an important step forwards to achieve uniform management criteria for intensive care teams and might reduce the loss of organs due to cardiac arrest.(11-13) However, the wide-scale application of the best scientific findings available to clinical practice depends upon the dissemination and incorporation of such information, which might require many years to occur. Although the introduction of institutional protocols is crucial to reduce that time, the mere availability of such protocols is no guarantee that they will be actually applied.(4) In addition to the protocols, healthcare professionals charged with their application play a crucial role by warning the attending staff during management and promoting real-time "course correction".(14) Thus, a managed protocol for potential organ donor maintenance care might reduce losses due to cardiac arrest and increase the number of actual donations.(2,5-7,15)
The aim of the present pilot study was to investigate the impact of a managed multiple organ maintenance care protocol in potential deceased donors at two hospitals.
METHODS
Study design and definitions
An intervention (quasi-experimental) before-and-after study was conducted from January 2010 to January 2012 at the intensive care unit (ICU) of two general high-complexity hospitals in southern Brazil, one of which is public and includes 195 beds and 14 ICU beds, and the other is private with 164 beds and 15 ICU beds. All of the brain-dead patients admitted to both ICUs were consecutively included in the study. A diagnosis of brain death was established according to resolution 1,480/97 of the Federal Medical Council.(16) The study was approved by the Research Ethics Committee of Hospital Municipal São José (No. 12,029). Informed consent was waived, as the study investigated the application of an institutional protocol.
Maintenance care protocols
The study was divided into two phases: in Phase 1, which lasted from January 2010 to April 2011, the measures for the maintenance care of potential donors were freely established by each intensivist. In that phase, data collection was retrospective using the patients' clinical records and the documentation available at the In-Hospital Coordination of Organ and Tissue Donation for Transplantation (Coordenação Intra-Hospitalar de Doação de Órgãos e Tecidos para Transplantes - CIHDOTT).
In Phase II, which lasted from May 2011 to April 2012, the managed protocol for deceased potential donor maintenance care based on the Brazilian guidelines published in 2011 was implemented.(11-13) The protocol consisted of the adoption of the guidelines,(13) which describes the main clinical measures that must be taken in chronological order. Table 1 describes the physiological data and clinical goals that must be accomplished. The continuous assessment of the fulfillment of the various items in the guide was performed at the bedside by CIHDOTT nurses and/or ICU medical interns. The changes introduced in Phase 2 were exclusively organizational and did not demand additional human or structural resources.
Table 1.
Goals and physiological variables analyzed during maintenance care
| Time goals | Ventilatory goals |
| a. Δtª - first clinical assessment: graphical complementary test <6 hours | a. PEEP = 8-10 cmH2O (t1) |
| b. Δtb - first clinical assessment: recovery <24 hours | b. Tidal volume = 5-8 mL/kg (t1) |
| c. Fulfillment of all time goals | c. Plateau pressure <30 cm H2O (t1) |
| d. PaO2/FiO2 >300 (t1) | |
| Temperature goals | e. Fulfillment of all ventilatory goals |
| a. Measurement of central temperature | e'. Fulfillment of goals without PaO2/FiO2 |
| b. Temperature > 35 ºC (t0) | |
| c. Temperature > 35 ºC (t1) | Endocrine-metabolic goals |
| d. Fulfillment of all temperature goals | a. Methylprednisolone 15 mg/kg every 24 hours |
| b. Levothyroxine 300 μ g every 24 hours per enteral route | |
| Hemodynamic goals | c. Glucose <180 mg% (t1) |
| a. PAM >65 mm Hg (t1) | d. Insulin IV when glycemia >180 mg/dL |
| b. Urine output >0.5 mL/kg/h and < 4 mL/kg/h (t1) | e. Na+ from 130-150 mEq/L (t1) |
| c. Fulfillment of all hemodynamic goals | f. Fulfillment of all hormone replacement goals |
| Physiological variables | Outcome indicators |
| a. pH, base excess, PaO2/FiO2 (t0 e t1) | a. Losses due to cardiac arrest |
| b. Heart rate, MAP (t0 and t1) | b. Actual donation |
| c. Fluid infusion, vasopressor agents, urine output, and fluid balance (t0 e t1) | c. Transplanted organs/donor |
| d. Glycemia (t0 and t1) | d. Losses due to family refusal |
| e. Lactate, hematocrit, sodium, and creatinine (t1) | e. Losses due to medical contraindication |
t0 - time of the first clinical assessment; t1 - immediately before recovery; MAP - mean arterial pressure; PEEP - positive end-expiratory pressure; PaO2/FiO2 - partial pressure of oxygen/fraction of inspired oxygen; IV - per intravenous route.
Data collection and analysis
The data collected from the healthcare form were transferred to an Excel spreadsheet for later analysis.
The groups of potential donors in Phases 1 and 2 were compared with regard to their age; gender; cause of brain death; physiological variables at the first clinical assessment of brain death (t0) and upon discontinuation of care - by family refusal, medical contraindication, or recovery (t1); time interval between the first clinical assessment and the graphical confirmatory test of brain death (Δta); time interval between the first clinical examination and recovery (Δtb); and the fulfillment of the maintenance goals described in table 1. The doses of vasopressor agents were made uniform and converted to norepinephrine (NE) units (U): a 5 µg/kg/min dose of dopamine corresponds to 0.2 U of NE, and 0.04 µg/min of vasopressin to 0.3 U of NE.(17,18)
The software Statistical Package for the Social Sciences (SPSS) version 15.0 was used to perform statistical analysis. Continuous variables were expressed as means ± standard deviation and were compared using Student's t-test. The categorical variables were expressed as absolute and relative values and were compared using the chi-square test. A p-value < 0.05 was considered to be statistically significant.
RESULTS
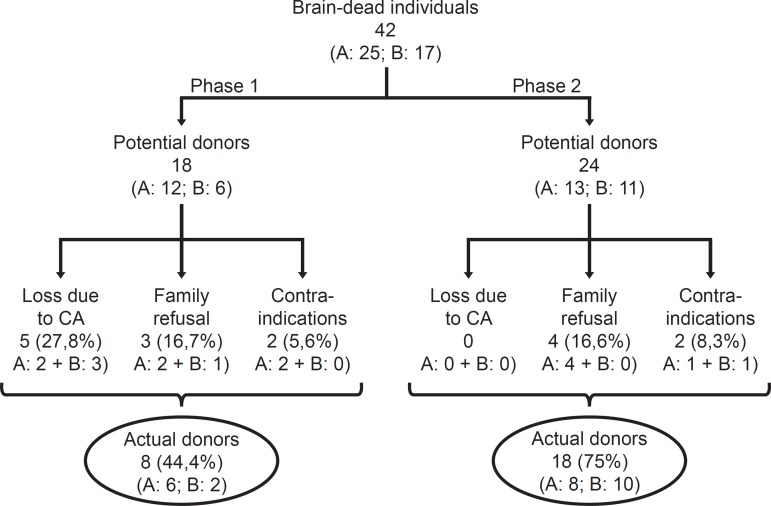
A total of 42 brain-dead patients were included in the study: 18 in Phase 1 and 24 in Phase 2 (Figure 1). All of the patients remained at the ICU during assessment and maintenance. Most patients were male, and the distribution was similar in both phases.
Figure 1.
Distribution of the included potential donors according to the study phase. A - public hospital; B - private hospital; CA - cardiac arrest.
Table 2 describes the demographic and clinical variables of the sample at the time of the first clinical assessment (t0) and immediately before recovery (t1).
Table 2.
Clinical and demographic characteristics of the potential donors included in the two study phases
| Characteristics | Phase 1 | Phase 2 | p value | |
| (N = 18) | (N=24) | |||
| Male gender | 11 (61.1) | 16 (64) | 0.71 | |
| Age (years) | 39.4±15 | 49.8±19.6 | 0.07 | |
| Cause of brain death | ||||
| Brain trauma | 9 (50) | 8 (32) | 0.27 | |
| Stroke | 6 (33.3) | 14 (60) | 0.21 | |
| Other | 3 (16.6) | 2 (8) | 0.41 | |
| Temperature (ºC) | ||||
| t0 | 36.4±1.1 | 36.3±1.6 | 0.83 | |
| t1 | 36.4±0.9 | 36.8±0.7 | 0.17 | |
| HR (bpm) | ||||
| t0 | 107±25 | 106±27 | 0.83 | |
| t1 | 104±28 | 106±23 | 0.82 | |
| MAP (mmHg) | ||||
| t0 | 88±22 | 95±22 | 0.25 | |
| t1 | 73±30 | 93±11 | <0.02 | |
| Vasopressor (NA units#) | ||||
| t0 | 0.14±0.16 | 0.18±0.23 | 0.63 | |
| t1 | 0.12±0.23 | 0.07±0.10* | 0.36 | |
| Fluid infusion (mL) | ||||
| t0 (24 hours before) | 4.972±5.096 | 4.759±4.224 | 0.88 | |
| t1 | 2.897±3.604 | 5.247±4.073 | 0.07 | |
| Urine output (mL/kg/h) | ||||
| t0 (24 hours before) | 2.21±2.17 | 2.35±2.14 | 0.83 | |
| t1 | 0.95±1.03 | 2.5±1.73 | <0.05 | |
| pH | ||||
| t0 | 7.26±0.16 | 7.26±0.15 | 0.93 | |
| t1 | 7.24±0.12 | 7.36±0.12** | <0.02 | |
| Base deficit | ||||
| t0 | -7.0±6.4 | -4.9±4.4 | 0.21 | |
| t1 | -7.8±6.6 | -3.5±3.9 | <0.03 | |
| PaO2/FiO2 | ||||
| t0 | 209±77 | 304±182 | 0.08 | |
| t1 | 194±116 | 388±334 | 0.10 | |
| Glycemia (mg/dL) | ||||
| t0 | 153±49 | 157±35 | 0.65 | |
| t1 | 151±39 | 215±71*** | <0.008 | |
| Lactate (mmol/L) (t1) | 1.4±0.5 | 1.9±0.9 | 0.24 | |
| Hematocrit (%) (ti) | 30.5±7.6 | 30.1±5.8 | 0.84 | |
| Sodium (mEq/L) (ti) | 147±8.7 | 149±7.2 | 0.46 | |
| Creatinine (t1) | 1.8±2.3 | 0.8±0.6 | <0.05 | |
t0 - first clinical assessment; t1 - before recovery; HR - heart rate; MAP - mean arterial pressure; PaO2/FiO2 - partial pressure of oxygen/fraction of inspired oxygen.
Uniformed doses of vasopressor agents in noradrenaline units. Differences between t0 and t1 in Phase 2:
p=0.053;
p=0.015;
p=0.001. Results expressed as absolute number and percentage, mean ± standard deviation.
The losses due to cardiac arrest decreased with a concomitant increase in actual donations. The losses due to family refusal and medical contraindication did not exhibit variation (Table 3).
Table 3.
Main indicators of performance comparing phase 1 and 2 in the state of Santa Catarina and Brazil
| Indicators | Phase 1 | Phase 2 | p value | Santa Catarina |
| (N = 18) | (N = 24) | 2011 | ||
| Actual donations | 8 (44.4) | 18 (75) | <0.05 | (41.5) |
| Losses due to cardiac arrest | 5 (27.8) | 0 | <0.007 | (27.5) |
| Losses due to family refusal | 3 (16.7) | 4 (16.6) | 1 | (24.7) |
| Losses due to contraindication | 2 (11.1) | 2 (8.3) | 0.76 | (6.3) |
Results expressed as absolute number and percentage
The time interval between the first clinical assessment and recovery decreased (Phase 1: 35.0±15.5 hours versus Phase 2: 24.6±6.2 hours; p<0.03), and the number of donors explanted in less than 24 hours was greater in Phase 2 (p<0.05) (Table 4). Adherence to the performance of the graphical complementary test within six hours from the first clinical assessment increased (p<0.05) (Table 4).
Table 4.
Adherence to goals during maintenance care
| Goals | Phase 1 | Phase 2 | p value | |
| (N=18) | (N = 24) | |||
| Time goals | ||||
| Δtª 1st clin assessmt - confirm test. | 3 (16.7) | 11 (45.8) | <0.05 | |
| <6 h | ||||
| Δtb 1st clin assessmt - expiant <24 h | 1/8 (12.5)# | 10/18 (55.5)# | <0.05 | |
| Fulfillment of all goals | 1/8 (12.5) # | 7/18(38.9) # | 0.178 | |
| Temperature goals | ||||
| Measurement of central temperature | 1 (5.6) | 13 (52) | <0.001 | |
| Temperature >35ºC at 1st assessment | 16 (88.9) | 24 (100) | 0.09 | |
| Temperature >35ºC on ICU exit | 16 (88.9) | 21 (87.5) | 0.89 | |
| Fulfillment of all goals | 0 | 11 (45.8) | <0.001 | |
| Ventilatory goals | ||||
| PEEP=8-10 cmH2O | 2 (11.1) | 10 (41.7) | <0.03 | |
| Tidal volume = 5-8 mL/kg | 0 | 9 (37.5) | <0.004 | |
| Plateau pressure <30 cm H2O | 0 | 11 (45.8) | <0.001 | |
| PaO2/FiO2 >300 on recovery | 2 (11.1) | 9 (37.5) | 0.06 | |
| Fulfillment of all goals | 0 | 4 (16.6) | 0.07 | |
| Fulfillment of goals without PaO2/FiO2 | 0 | 7 (29.1) | <0.02 | |
| Hemodynamic goals | ||||
| MAP >65 mmHg | 11 (61.1) | 21 (87.5) | <0.05 | |
| Urine output >0.5 mL/kg/h - <4 ml/kg/h | 6 (33.3) | 15 (62.5) | 0.06 | |
| Fulfillment of all goals | 3 (16.7) | 7 (29.1) | 0.34 | |
| Hormone replacement goals | ||||
| Methylprednisolone 15 mg/kg every 24 h | 6 (33.3) | 24 (100) | <0.001 | |
| Levothyroxine 300 µ g enteral every 24 h | 0 | 21 (87.5) | <0.001 | |
| Urine output >0.5 mL/kg/h - <4 mL/kg/h | 6 (33.3) | 15 (62.5) | 0.06 | |
| Glucose <180 mg% on explant | 8 (44.4) | 8 (33.3) | 0.46 | |
| Insulin IV when glycemia >180 mg/dL | 0 | 11 (45.8) | <0.001 | |
| Sodium 130-150 mEq/L on explant | 12 (66.7) | 16 (66.6) | 1 | |
| Fulfillment of all goals | 0 | 4 (16.6) | 0.07 | |
ICU - intensive care unit; PEEP - positive end-expiratory pressure; PaO2 - partial pressure of oxygen; FiO2 - fraction of inspired oxygen; MAP - mean arterial pressure. # Percentage of the actual donor number. Results expressed as absolute number and percentage.
The central temperature exhibited a substantial increase in Phase 2, as did the global fulfilling of the temperature goals (Table 4).
Adherence to a mean arterial pressure (MAP) > 65 mmHg increased (Table 4), urine output increased, and the creatinine concentration decreased (Table 2). Despite the greater use of vasopressin in Phase 2 (Phase 1 = 22.2% versus Phase 2 = 54. 2%; p<0.04), the uniform vasopressor doses were similar in both phases. With regard to the acid-base balance, the arterial pH increased, and the base deficit decreased, whereas the pH rose from t0 to t1 in Phase 2 (Table 2).
As observed in the case of vasopressin, adherence to the administration of methylprednisolone and levothyroxine was greater in Phase 2 (Table 4). Conversely, glycemia increased in Phase 2 (Table 4).
Care with the adoption of protective ventilation was greater in Phase 2, where also the number of potential donors exhibiting PaO2/FiO2>300 was higher (Table 4).
DISCUSSION
The present study shows that a managed treatment protocol for deceased potential donors reduces the incidence of cardiac arrest before organ recovery.
Although the present study was a pilot study conducted with a small sample, the causal association between the systematization and management of the maintenance measures and the results are undeniable. Recently, Salim et al. showed that the implementation of an aggressive strategy to handle deceased potential donors correlated with a 57% increase in brain death reporting (p<0.001), an 87% reduction in losses due to cardiac arrest (p < 0.001), and an 82% increase in the number of actual donors (p<0.001).(5) Similarly, the measures implemented in our study resulted in a 100% reduction in losses due to cardiac arrest during the investigated period. As the losses due to medical contraindications and family refusal did not vary, the relative increase of 53.7% in the actual donors might be attributed to the reduction of losses due to cardiac arrest.
The time interval between the first clinical assessment and recovery decreased (Δtb), and the adherence to the time-related goals increased, which show that the protocol made it shorter the maintenance and donation process. Diagnostic delay and correction of the physiological disorders associated with brain death most likely reduced the oxygen delivery to tissues (DO2) and amplified the inflammatory response, thus affecting the use of organs in transplants and post-transplant survival.(17,18)
In 2011, we investigated septic patients who were treated using a managed protocol that was similar to the one in the present study, and we found that a reduction in the time to diagnosis of severe sepsis reduced the mortality of that population.(19) Although in severe sepsis, the main factor that influences mortality is early antibiotic treatment, many other therapeutic measures seem to influence a non-measurable variable that might be defining for the outcomes: greater attention paid to patients.(20) Thus, the analogy between goal-managed care in sepsis and deceased potential donors seems quite appropriate. On these grounds, we might infer that the reduction in losses due to cardiac arrest resulted from the accomplishment of the basic protocol goals, such as maintenance of the temperature,(21) hemodynamic conditions,(5,18) electrolytic balance,(22,23) hormone replacement,(24,25) and an appropriate ventilatory regimen.(26,27)
The control of the potential donors' temperature was quite appropriate in both study phases, and in Phase 2, the monitoring of the central temperature by means of esophageal thermometer increased and there was complete adherence to the temperature goals. Due to the wide thermal lability of the skin temperature and its strong influence by the environmental temperature, the central temperature affords more precise data, especially when hypothermia occurs. The maintenance of normal temperature is crucial to ensure several biological phenomena, especially hemodynamic control.(11)
Hemodynamic instability is the major challenge posed to the management of potential donors as hypotension is a common occurrence and reduces the perfusion of organs. The maintenance of a MAP>65 mmHg is one of the essential goals. Adherence to that goal was reached in 87.5% of the individuals in Phase 2, compared to 61.1% in Phase 1, and the MAP levels were also higher. With regard to that goal, attention must be paid to the appropriate volume expansion to reduce the risk of damage that is associated with vasoconstriction secondary to the use of vasopressor agents. In this regard, there was a tendency to increase the volume infused in Phase 2 compared to Phase 1 (p=0.068). This idea is reinforced by the increased urine output, reduced creatinine concentration, higher pH, and lower base deficit. The increase in the pH from t0 to t1 in Phase 2 might also denote improved perfusion.
Most brain-dead individuals requiring vasopressor agents are vasopressin-depleted.(11,28) Thus, the greater use of vasopressin in Phase 2 might account for the increased pressure levels, despite the similarity between the uniform doses of vasopressor agents that were used in both phases.
The high rate of levothyroxine use in Phase 2 (87.5%) denotes a higher degree of care with regard to the potential donors; however, it is not possible to attribute pharmacological advantages to this drug, due to the lack of information on the action of its enteral administration and the overall lack of understanding of the actual role of thyroid hormones in the maintenance of potential donors. Conversely, methylprednisolone, which was administered in high doses to 95% of the potential donors in Phase 2, might have contributed to compensating for an eventual adrenal insufficiency and the consequent achievement of better hemodynamic control. Nevertheless, the use of that corticosteroid might have been the cause of the frequent occurrence of hyperglycemia in Phase 2 (Table 2). The detrimental effects of hyperglycemia on kidney function following transplantation and on the water-electrolytic balance emphasize the need for the strict control of the potential donors' glycemia, especially following the administration of corticosteroids.(12,29)
Although the benefits of protective ventilation for the use of lungs for transplantation are evident (27% increase; p=0.004),(24) the rate of use of that strategy in the present study was low (0% in Phase 1; 16.6% in Phase 2).-
The availability of clinical evidence, even in prestigious journals, does not ensure their transformation into actual care practices.(14) The adoption of the protocol described in the present study induced changes to actual practice, as certain relevant actions and goals were pre-defined, provided opportunities to perform therapeutic adjustments, and decreased variation and subjectivity in the handling of deceased potential donors. The protocol is an adaptation of the medical early warning systems - MEWS, which are traditionally divided into (1) screening, (2) diagnosis, (3) treatment, (4) analysis of indicators, and (5) process revision.(1,4,30) The published good practice guidelines by Spain's National Transplant Organization (Organización Nacional de Transplantes - ONT) suggest several basic management principles, including a MEWS-based protocol. One year after the publication of such guidelines, the number of organ donors increased by approximately 15% in Spain.(4,31) Even though a portion of the measures that were standardized by a given protocol might lack robust evidence, the management of activities and real-time "route corrections" place the healthcare staff at the bedside with a consequent improvement in assistance.
The present study had several limitations. In addition to the small sample, the retrospective data collection in Phase 1 might have impaired the quality of the information. Due to the disputable quality of the Phase 1 records, comorbidities were not assessed, which also impaired the analysis of the data.
Taking the study limitations into consideration and placing the results into the proper perspective, the findings of the present study are of paramount importance for the state of Santa Catarina. Despite the reduction in losses due to family refusal and medical contraindications, the rate of losses due to cardiac arrest during the clinical management of potential donors remains high.(3,8) An analysis of the ten main multiple organ donation hospitals, which provide 75% (n=120/159) of the brain-dead donors in the state, showed that the rate of losses due to cardiac arrest was also high in 2011 (68/266; 25.5% of the brain deaths reported by those hospitals).(8) Based on these findings and the results of the present study, one might infer that the large-scale application of similar systematics might reduce the loss of potential donors due to cardiac arrest, both in the state of Santa Catarina and in Brazil as a whole.
CONCLUSION
The results of the present study demonstrate the crucial role of coordinated actions in improving the quality of the maintenance of potential organ donors. The use of this protocol was correlated with the performance of essential measures in the maintenance of the potential donors, leading to a reduction in donation losses due to cardiac arrest.
Footnotes
Conflict of interest: None.
This study was conducted at Centro Hospitalar Unimed - CHU - Joinville (SC), Brazil.
REFERENCES
- 1.The Madrid resolution on organ donation and transplantation: national responsibility in meeting the needs of patients, guided by the WHO principles. Transplantation. 2011;91(Suppl 11):S29–S31. doi: 10.1097/01.tp.0000399131.74618.a5. [DOI] [PubMed] [Google Scholar]
- 2.DuBose J, Salim A. Aggressive organ donor management protocol. J Intensive Care Med. 2008;23(6):367–375. doi: 10.1177/0885066608324208. [DOI] [PubMed] [Google Scholar]
- 3.Associação Brasileira de Transplantes de Órgãos (ABTO) Estatística de transplantes. [citado 2012 Fev 12];Registro Brasileiro de Transplantes (RBT) 2011 17(4) Available from: http://www.abto.org.br/abto02/portugues/populacao/rbt/lista.aspx. [Google Scholar]
- 4.España. Ministério de Sanidad, Política Social e Igualdad. Organización Nacional de Trasplantes . Guía de buenas prácticas en el proceso de la donación de órganos. España: 2011. Disponível em: http://www.ont.es/publicaciones/Documents/GUIA_BUENAS_PRACTICAS_DONACION_ORGANOS.pdf. [Google Scholar]
- 5.Salim A, Velmahos GC, Brown C, Belzberg H, Demetriades D. Aggressive organ donor management significantly increases the number of organs available for transplantation. J Trauma. 2005;58(5):991–994. doi: 10.1097/01.ta.0000168708.78049.32. [DOI] [PubMed] [Google Scholar]
- 6.Powner D. Aggressive donor care--to what end? J Intensive Care Med. 2008;23(6):409–411. doi: 10.1177/0885066608324198. [DOI] [PubMed] [Google Scholar]
- 7.Helms AK, Torbey MT, Hacein-Bey L, Chyba C, Varelas PN. Standardized protocols increase organ and tissue donation rates in the neurocritical care unit. Neurology. 2004;63(10):1955–1957. doi: 10.1212/01.wnl.0000144197.06562.24. [DOI] [PubMed] [Google Scholar]
- 8.SC Transplantes - Central de Captação, Notificação e Distribuição de Órgãos e Tecidos de Santa Catarina (CNCDO/SC) [citado 2012 Fev 12];Estatísticas 2011. Disponível em: http://www.sctransplantes.saude.sc.gov.br/images/2011/2011%20final.pdf.
- 9.Andrade J, Galán Torres JB. Analisis del proceso de donación de órganos en el estado de Santa Catarina. Tesina presentada al Máster Alianza en Donación y Trasplante de Órganos Tejidos y Células. 2008. [Google Scholar]
- 10.España. Organización Nacional de Trasplantes (ONT) [citado 2012 Fev 12];Datos Globales de Donación e Trasplante. Memorias de actividad de donación y trasplante. Disponível em: http://www.ont.es/infesp/Paginas/DatosdeDonacionyTrasplante.aspx.
- 11.Westphal GA, Caldeira M, Filho, Vieira KD, Zaclikevis VR, Bartz MC, Wanzuita R, et al. Diretrizes para manutenção de múltiplos órgãos no potencial doador adulto falecido: parte I. Aspectos gerais e suporte hemodinâmico. Rev Bras Ter Intensiva. 2011;23(3):255–268. [PubMed] [Google Scholar]
- 12.Westphal GA, Caldeira M, Filho, Vieira KD, Zaclikevis VR, Bartz MC, Wanzuita R, et al. Diretrizes para manutenção de múltiplos órgãos no potencial doador adulto falecido: parte II. Ventilação mecânica, controle endócrino metabólico e aspectos hematológicos e infecciosos. Rev Bras Ter Intensiva. 2011;23(3):269–282. [PubMed] [Google Scholar]
- 13.Westphal GA, Caldeira M, Filho, Vieira KD, Zaclikevis VR, Bartz MC, Wanzuita R, et al. Diretrizes para manutenção de múltiplos órgãos no potencial doador adulto falecido: parte III. Recomendações órgãos específicas. Rev Bras Ter Intensiva. 2011;23(4):410–425. [PubMed] [Google Scholar]
- 14.Titler MG. The evidence for evidence-based practice implementation. In: Hughes RG, editor. Patient safety and quality: an evidence-based handbook for nurses. 6th ed. Vol. 1. Rockville: Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 15.Straznicka M, Follette DM, Eisner MD, Roberts PF, Menza RL, Babcock WD. Aggressive management of lung donors classified as unacceptable: excellent recipient survival one year after transplantation. J Thorac Cardiovasc Surg. 2002;124(2):250–258. doi: 10.1067/mtc.2002.123813. [DOI] [PubMed] [Google Scholar]
- 16.Brasil. Conselho Federal de Medicina . Resolução CFM Nº 1480/97 de 8 de agosto de 1997. Critérios diagnósticos de morte encefálica. Brasília: CFM; 1997. [Google Scholar]
- 17.Murugan R, Venkataraman R, Wahed AS, Elder M, Hergenroeder G, Carter M, Madden NJ, Powner D, Kellum JA, HIDonOR Study Investigators Increased plasma interleukin-6 in donors is associated with lower recipient hospital-free survival after cadaveric organ transplantation. Crit Care Med. 2008;36(6):1810–1816. doi: 10.1097/CCM.0b013e318174d89f. [DOI] [PubMed] [Google Scholar]
- 18.Murugan R, Venkataraman R, Wahed AS, Elder M, Carter M, Madden NJ, Kellum JA, HIDonOR Study Investigators Preload responsiveness is associated with increased interleukin-6 and lower organ yield from brain-dead donors. Crit Care Med. 2009;37(8):2387–2393. doi: 10.1097/CCM.0b013e3181a960d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westphal GA, Koenig A, Caldeira M, Filho, Feijó J, de Oliveira LT, Nunes F, et al. Reduced mortality after the implementation of a protocol for the early detection of severe sepsis. J Crit Care. 2011;26(1):76–81. doi: 10.1016/j.jcrc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36(2):222–231. doi: 10.1007/s00134-009-1738-3. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keegan MT, Wood KE, Coursin DB. An update on ICU management of the potential organ donor. In: Vincent JL, editor. Year book of intensive care and emergency medicine. Berlin: Springer-Verlag; 2010. pp. 547–559. [Google Scholar]
- 22.Powner DJ, Kellum JA, Darby JM. Abnormalities in fluids, electrolytes, and metabolism of organ donors. Prog Transplant. 2000;10(2):88–94. doi: 10.1177/152692480001000204. quiz 95-6. [DOI] [PubMed] [Google Scholar]
- 23.Ramos HC, Lopez R. Critical care management of the brain-dead organ donor. Curr Opin Organ Transplant. 2002;7(1):70–75. [Google Scholar]
- 24.Amado JA, López-Espadas F, Vázquez-Barquero A, Salas E, Riancho JA, López-Cordovilla JJ, et al. Blood levels of cytokines in brain-dead patients: relationship with circulating hormones and acute-phase reactants. Metabolism. 1995;44(6):812–816. doi: 10.1016/0026-0495(95)90198-1. [DOI] [PubMed] [Google Scholar]
- 25.Shemie SD, Ross H, Pagliarello J, Baker AJ, Greig PD, Brand T, Cockfield S, Keshavjee S, Nickerson P, Rao V, Guest C, Young K, Doig C, Pediatric Recommendations Group Organ donor management in Canada: recommendations of the forum on Medical Management to Optimize Donor Organ Potential. CMAJ. 2006;174(6):S13–S32. doi: 10.1503/cmaj.045131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascia L, Pasero D, Slutsky AS, Arguis MJ, Berardino M, Grasso S, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA. 2010;304(23):2620–2627. doi: 10.1001/jama.2010.1796. [DOI] [PubMed] [Google Scholar]
- 27.Lebovitz DJ, Reis K, Yun J, Herman L, McCurry KR. An aggressive lung recruitment protocol increases the percentage of lung donors with no increased adverse effect in lung recipients: 3173. Transplantation. 2010;90(2 ) Suppl:356–356. [Google Scholar]
- 28.Chen JM, Cullinane S, Spanier TB, Artrip JH, John R, Edwards NM, et al. Vasopressin deficiency and pressor hypersensitivity in hemodynamically unstable organ donors. Circulation. 1999;100(19 ) Suppl:II244–II246. doi: 10.1161/01.cir.100.suppl_2.ii-244. [DOI] [PubMed] [Google Scholar]
- 29.Blasi-Ibanez A, Hirose R, Feiner J, Freise C, Stock PG, Roberts JP, et al. Predictors associated with terminal renal function in deceased organ donors in the intensive care unit. Anesthesiology. 2009;110(2):333–341. doi: 10.1097/ALN.0b013e318194ca8a. [DOI] [PubMed] [Google Scholar]
- 30.Morgan RJ, Willams F, Wright MM. An early warning scoring system for detecting developing critical illness. Clin Intensive Care. 1997;8:100. [Google Scholar]
- 31.García Rada A. Number of organ donors rises by 15% in Spain after doctors are given good practice guide. BMJ. 2011;342:d2181. doi: 10.1136/bmj.d2181. [DOI] [PubMed] [Google Scholar]