Abstract
Objective
To examine the factors associated with acute kidney injury and outcome in patients with lung disease.
Methods
A prospective study was conducted with 100 consecutive patients admitted to a respiratory intensive care unit in Fortaleza (CE), Brazil. The risk factors for acute kidney injury and mortality were investigated in a group of patients with lung diseases.
Results
The mean age of the study population was 57 years, and 50% were male. The incidence of acute kidney injury was higher in patients with PaO2/FiO2<200 mmHg (54% versus 23.7%; p=0.02). Death was observed in 40 cases and the rate of mortality of the acute kidney injury group was higher (62.8% versus 27.6%; p=0.01). The independent factor that was found to be associated with acute kidney injury was PaO2/FiO2<200 mmHg (p=0.01), and the independent risk factors for death were PEEP at admission (OR: 3.6; 95%CI: 1.3-9.6; p=0.009) and need for hemodialysis (OR: 7.9; 95%CI: 2.2-28.3; p=0.001).
Conclusion
There was a higher mortality rate in the acute kidney injury group. Increased mortality was associated with mechanical ventilation, high PEEP, urea and need for dialysis. Further studies must be performed to better establish the relationship between kidney and lung injury and its impact on patient outcome.
Keywords: Acute kidney injury, Respiratory insufficiency, Lung diseases, Prognosis, Mortality, Risk factors
Abstract
Objetivo
Investigar os fatores associados à lesão renal aguda e o prognóstico em pacientes com doença pulmonar.
Métodos
Foi realizado estudo prospectivo com cem pacientes consecutivos admitidos em uma unidade de terapia intensiva respiratória em Fortaleza (CE). Foram investigados fatores de risco para lesão renal aguda e mortalidade em um grupo de pacientes com doenças pulmonares.
Resultados
A média de idade foi de 57 anos, sendo 50% do gênero masculino. A incidência de lesão renal aguda foi maior nos pacientes com PaO2/FiO2<200 mmHg (54% versus 23,7%; p=0,02). O óbito ocorreu em 40 casos. A mortalidade no grupo com lesão renal aguda foi maior (62,8% versus 27,6%; p=0,01). A relação PaO2/FiO2<200 mmHg foi fator independente associado à lesão renal aguda (p=0,01); PEEP na admissão (OR: 3,6; IC95%: 1,3-9,6; p=0,009) e necessidade de hemodiálise (OR: 7,9; IC95%: 2,2-28,3; p=0,001) foram fatores de risco independentes para óbito.
Conclusão
Houve maior mortalidade no grupo com lesão renal aguda. Mortalidade aumentada foi associada com ventilação mecânica, PEEP alta, ureia e necessidade de diálise. Estudos futuros devem ser realizados para melhor estabelecer as inter-relações entre lesão renal e pulmonar e seu impacto no prognóstico.
INTRODUCTION
Acute kidney injury (AKI) is a frequent complication in critically ill patients and its incidence has been increasing.(1) AKI occurs in approximately 36 to 67% of patients admitted to intensive care units (ICUs). AKI is associated with increased mortality,(1,2) and even small increases in serum creatinine are associated with an increased risk of death.(2,3) The causes of AKI in the ICU are frequently "multi-factorial", and in most cases, AKI develops from a combination of hypovolemia, sepsis, nephrotoxins, and hemodynamic perturbations.(1)
Recent studies have demonstrated that AKI impacts the function of other organs, which is associated with increased mortality.(2) Patients with AKI have an increased risk for developing sepsis, respiratory insufficiency, hemorrhage and central nervous system dysfunction.(2) Experimental studies have demonstrated that ischemic AKI leads to an increase in circulating cytokines, chemokines and activated leucocytes, resulting in the infiltration of cells into a number of different organ systems, including the lungs, heart and central nervous system.(4-6) The association between AKI and the need for mechanical ventilation (MV) has been demonstrated in past studies, suggesting a possible link between AKI and lung involvement.(3,7)
AKI can increase pulmonary vascular permeability and downregulate ion channels critical for fluid absorption in the lungs, leading to pulmonary inflammation, hemorrhage, septal edema and apoptosis.(8)
The occurrence of lung injury can also affect the kidneys and ventilator-induced lung injury is the most studied example of the lung-kidney interaction.(8) MV causes hemodynamic abnormalities, which can, in turn, affect renal perfusion by reducing cardiac output and stimulating hormonal and sympathetic pathways.(9)
The aim of this study was to examine the risk factors associated with AKI and outcome in patients with lung disease.
METHODS
Study subjects
A prospective study was conducted on all consecutive patients admitted to the respiratory ICU of Hospital de Messejana, Fortaleza city, northeast of Brazil, in the period between May and December 2009. The study protocol was reviewed and approved by the Committees of Ethics of the Hospital de Messejana, and signed informed consent was obtained from all patients or their legal representative, as appropriate.
The inclusion criteria were all patients with primary lung disease admitted to the ICU in the study period who required mechanical or noninvasive ventilation. Patients with acute lung injury (ALI) from non-pulmonary origin were not included. Patients under MV were considered to be invasively ventilated. The exclusion criteria were patients with preexisting chronic kidney disease (CKD), arterial hypertension, diabetes mellitus and other co-morbidities that could chronically affect renal function.
Parameters
The patients' demographic and clinical characteristics, such as age, gender, cause of pulmonary disease that led to ICU admission, length of hospital stay, need for MV and 24-hour urinary volume, were recorded. The laboratory data collected at admission, including urea, creatinine, hemoglobin, positive-end expiratory pressure (PEEP), arterial carbon dioxide tension (PaCO2) and arterial oxygen tension/fractional inspired oxygen ratio (PaO2/FiO2), were analyzed. The hospital mortality data were also evaluated.
Definitions
The patients were classified according to the RIFLE criteria ("Risk", "Injury", "Failure", "Loss" and "End-stage renal disease") based on the creatinine criteria because most patients did not have their urinary volumes recorded in their medical charts.(10) Baseline creatinine was taken to be the value at the moment of hospital admission or the lowest creatinine level before admission. The Acute Physiology and Chronic Health Evaluation II (APACHE II) was used as the gold-standard severity score.(11) Oliguria was defined as urinary volume <400 mL/day despite appropriate fluid replacement.
Patient groups
The risk factors for AKI and mortality were investigated in a group of critically ill pulmonary patients. The factors associated with AKI and death were investigated through univariate and multivariate analyses. The glomerular filtration rate (GFR) was estimated through the MDRD (Modification of Diet in Renal Disease) formula, as previously described.(12) We also compared the GFR of the surviving patients at hospital discharge, using 60 mL/min/1.73 m2 as the cutoff.
Statistical analyses
Statistical analyses were performed with the programs Statistics Package for the Social Science (SPSS) 17.0 for Windows (SPSS Inc., Chicago, IL, USA) and Epi-Info 6.04b (Centers for Disease Control and Prevention, Atlanta, GA, USA) and consisted of univariate and multivariate analyses. The comparisons between the two groups of patients were conducted using Student t test, Fischer exact test, Mann Whitney test and chi-square test, as appropriate. A logistic regression model was built for quantitative variables, and association measures were calculated (adjusted odds ratio - OR), with a confidence interval of 95% (95%CI). Multiple logistic regression analysis was used to identify the independent variables used as indicators of the predictors of mortality (dependent variable). The variables were chosen from the univariate model and entered into separate models using the stepwise method. Only those of statistical significance (p<0.05) were included in the final regression model. These models were built taking into account the problems of confounding factors and co-linearity. The following variables were entered into the model in the first block: PEEP, APACHE, need for MV and urea. The following variables were entered into the model in the second block: hemodialysis, urinary volume and GFR. For the investigation of independent risk factors for AKI, a multivariate analysis was performed. This analysis included the factors that presented a significance level <20% in the univariate analysis (Mann-Whitney and chi-square tests). Values below 5% (p<0.05) were considered statistically significant.
RESULTS
A total of 100 patients were included in the study. The mean age of the study population was 57.2±18.3 years, and 50% were male. The causes of pulmonary disease that led to hospital admission were pulmonary infection (54%), chronic obstructive pulmonary disease (24%), lung neoplasm (9%), pulmonary tuberculosis (7%) and others (6%). AKI, as defined by the RIFLE criteria, was found in 30 cases (30%) at the moment of ICU admission and in 35 cases during ICU stay (35%). The patients were classified as "Risk" (34%), "Injury" (18%) and "Failure" (48%). There was no association between RIFLE classification and the study parameters.
There was no difference in age between patients with and without AKI (59.0±18.3 versus 56.2±18.4 years; p=0.47). There was also no difference in ICU stay between these two groups of patients (10.6±9.9 versus 8.5±6.6 days; p=0.26).
A total of 86 patients (86%) required mechanical ventilation, and the remaining cases used noninvasive ventilation. There was no significant association between the need for MV and AKI. MV was necessary for 32 patients with AKI (91.4%) and for 54 non-AKI patients (83%; p=0.32). There was a significant association between PaO2/FiO2 at admission and AKI. There were 12 patients with PaO2/FiO2 <200 mmHg, among which the AKI incidence was 54% at admission. In contrast, patients with PaO2/FiO2 >200 mmHg had an AKI incidence of only 23.7%. This difference was statistically significant (p=0.02). The mean PEEP at admission was similar in patients with and without AKI (6.8±2.4 versus 6.3±2.1; p=0.28), and the levels of hemoglobin were lower among AKI patients (8.8±2.6 versus 10.2±2.6 g/dL; p=0.001). The APACHE II score was higher among AKI patients (24±8.1 versus 20±10; p=0.04). The use of vasoactive drugs was not more frequent in patients with AKI, but sepsis was more frequent in patients with AKI (48.5% versus 20%; p=0.005). A comparison of the patients with and without AKI is shown in table 1.
Table 1.
The comparison of critically ill patients with lung disease according to the occurrence of acute kidney injury
| AKI (N=35) | Non-AKI (N=65) | p value | |
| Age (years) | 59.0±18.3 | 56.2±18.4 | 0.47 |
| Gender | |||
| Male | 19 (54) | 31 (48) | 0.67 |
| Female | 16 (46%) | 34 (52) | |
| Pulmonary disease | |||
| Infection | 20 (57.1) | 34 (52.3) | 0.67 |
| CPOD | 6 (17.1) | 18 (27.6) | 0.32 |
| Neoplasm | 4 (11.4) | 5 (7.6) | 0.71 |
| Tuberculosis | 4 (11.4) | 3 (4.6) | 0.23 |
| ICU length of stay (days) | 10.6±9.9 | 8.5±6.6 | 0.26 |
| Use of vasoactive drugs during ICU stay | 18 (51.4) | 40 (61.5) | 0.72 |
| Sepsis | 17 (48.5) | 13 (20) | 0.005 |
| Need for mechanical ventilation | 32 (91.4) | 54 (83) | 0.32 |
| PEEPadm (cmH2O) | 6.8±2.4 | 6.3±2.1 | 0.28 |
| PaCO2 adm (mmHg) | 44±15 | 51±18 | 0.05 |
| PaO2/FiO2 adm (mmHg) | 200±135 | 322±215 | 0.01 |
| PaO2/FiO2≤200 mmHg | 20 (57.1) | 17 (26.1) | 0.02 |
| Hbadm (g/dL) | 8.8±2.6 | 10.2±2.6 | 0.01 |
| Uradm (mg/dL) | 74.4±44.8 | 59.4±37.7 | 0.07 |
| Cradm (mg/dL) | 1.8±1.6 | 1.0±0.5 | 0.0003 |
| GFRadm (mL/min) | 60±39 | 87±45 | 0.003 |
| Urinay volume (mL/day)adm | 293±35 | 1025±369 | 0.0001 |
| APACHE II | 24±8.1 | 20±10 | 0.04 |
| Death | 22 (62.8) | 18 (27.6) | 0.01 |
AKI - acute kidney injury; CPOD - chronic obstructive pulmonary disease; ICU - intensive care unit; PEEP - positive-end expiratory pressure; PaCO2 - arterial carbon dioxide tension; PaO2 - arterial oxygen tension; FiO2 - fractional inspired oxygen ratio; Hb - hemoglobin; Ur - urea; Cr - creatinine; GFR - glomerular filtration rate (MDRD); APACHE II - Acute Physiology and Chronic Health Evaluation II. Student t test and Fisher’s exact test were used for analysis and significance was set at p<0.05. The results are expressed as numbers (%) and median±SD.
Death occurred in 40 cases (40%). Patients with AKI had a mortality of 62.8%, which was higher than the mortality of non-AKI patients (27.6%; p=0.01). The comparison of survivors and nonsurvivors showed that nonsurvivors had a higher frequency of MV requirement, a higher PEEP at admission, an elevated urea level at admission and a higher APACHE II score and required dialysis more often (Table 2). The use of vasoactive drugs, as well as sepsis, was more frequent among nonsurvivors (72.5% versus 1.6%; p=0.0001). The following independent risk factors were associated with death: PEEP at admission (OR: 3.6; 95%CI: 1.3-9.6; p=0.009) and need for hemodialysis (OR: 7.9, 95%CI: 2.2-28.3; p=0.001). The risk factors for AKI and death, as analyzed by logistic regression, are shown in tables 3 and 4.
Table 2.
The comparison of critically ill patients with lung disease according to the occurrence of death
| Survivors (N=60) | Non-survivors (N=40) | p value | |
| Age (years) | 54.9±18.3 | 60.6±18.1 | 0.13 |
| Gender | |||
| Male | 30 (50) | 20 (50) | 1.0 |
| Female | 30 (50) | 20 (50) | |
| ICU length of stay (days) | 9.7±7.0 | 8.7±9.2 | 0.56 |
| Use of vasoactive drugs | 22 (36.6) | 36 (90) | 0.008 |
| Sepsis | 1 (1.6) | 29 (72.5) | 0.0001 |
| Need for mechanical ventilation | 47 (78.3) | 39 (97.5) | 0.001 |
| PEEPadm (cmH2O) | 5.8±1.7 | 7.2±2.5 | 0.009 |
| PaCO2 adm (mmHg) | 46±13 | 51±22 | 0.23 |
| PaO2/FiO2 adm (mmHg) | 310±211 | 232±171 | 0.05 |
| PaO2/FiO2 ≤200 mmHg | 7 (11.6) | 5 (12.5) | 1.0 |
| Hbadm (g/dL) | 10±2.5 | 9.2±2.8 | 0.08 |
| Uradm (mg/dL) | 55.9±35.5 | 78.2±44.9 | 0.01 |
| Cradm (mg/dL) | 1.3±1.2 | 1.4±0.9 | 0.53 |
| GFRadm (mL/min) | 81±48 | 62±35 | 0.03 |
| APACHE II | 20±10 | 24±9.6 | 0.04 |
| Urinary volume (mL/day) | 595±42 | 350±43 | 0.0001 |
| Hemodialysis | 5 (8.3) | 15 (37.5) | 0.001 |
ICU - intensive care unit; PEEP - positive-end expiratory pressure; PaCO2 - arterial carbon dioxide tension; PaO2 - arterial oxygen tension; FiO2 - fractional inspired oxygen ratio; Hb - hemoglobin; Ur - urea; Cr - creatinine; GFR - glomerular filtration rate (MDRD); APACHE II - Acute Physiology and Chronic Health Evaluation II. The Student t test and Fisher’s exact test were used for analysis and significance was set at p<0.05. The results are expressed as numbers (%) and median±SD.
Table 3.
Risk factors for acute kidney injury and death in critically ill patients with lung disease, as determined by univariate analysis
| OR | 95%CI | p value | |
| Risk factors for AKI | |||
| Hemoglobin <8 | 3.12 | 1.18-8.25 | 0.02 |
| APACHE >20 | 5.27 | 2.05-13.5 | 0.01 |
| PaO2/FiO2≤200 mmHg | 3.48 | 1.47-8.24 | 0.005 |
| Risk factors for death | |||
| PEEP>5 cmH2O | 2.08 | 1.31-9.70 | 0.01 |
| Hemodialysis need | 6.41 | 1.74-23.5 | 0.005 |
| Need for mechanical ventilation | 10.7 | 1.35-86.1 | 0.01 |
OR - odds ratio; 95%CI - 95% confidence interval; AKI - acute kidney injury; APACHE - acute physiology and chronic health evaluation; PaO2 - arterial oxygen tension; FiO2 - fractional inspired oxygen ratio; PEEP - positive-end expiratory pressure.
Table 4.
Risk factors for acute kidney injury and death in critically ill patients with lung disease, as determined by multivariate analysis
| OR | 95%CI | p value | |
| Risk factors for AKI | |||
| PaO2/FiO2 ≤200 mmHg | 2.9 | 1.4-7.9 | 0.03 |
| Hemoglobin <8 | 4.7 | 1.3-12.6 | 0.01 |
| APACHE >20 | 5.23 | 1.5-14.8 | 0.02 |
| Risk factors for death | |||
| PEEP | 3.6 | 1.3-9.6 | 0.009 |
| Hemodialysis need | 7.9 | 2.2-28.3 | 0.001 |
OR - odds ratio; 95% CI - 95% confidence interval; PaO2 - arterial oxygen tension; FiO2 - fractional inspired oxygen ratio; APACHE - Acute Physiology and Chronic Health Evaluation; PEEP - positive-end expiratory pressure; AKI - acute kidney injury.
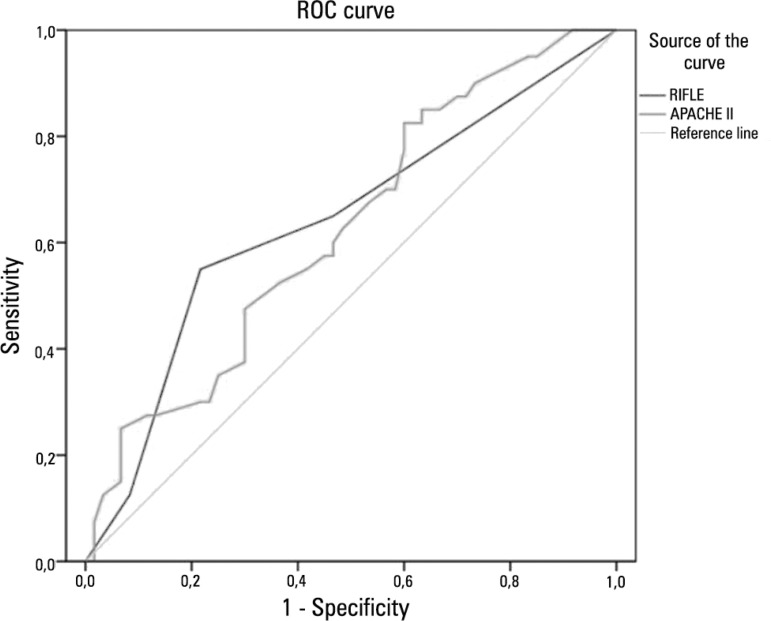
The sensitivity of RIFLE and APACHE II classifications in predicting mortality were shown by the areas under the curve of 0.64 (p=0.01) and 0.62 (p=0.03), respectively, as illustrated in figure 1.
Figure 1.
The sensitivity of the RIFLE and APACHE II classifications in predicting mortality in patients with acute kidney injury and lung disease (AUC=0.64, p=0.01; AUC=0.62, p=0.03, respectively).
DISCUSSION
The kidney and lungs are the two organs that are most frequently involved in the so-called multi-organ failure syndrome. Additionally, there is some evidence that kidney failure can adversely affect pulmonary function and that pulmonary injury can lead to AKI.(9) Volume overload, which may occur during renal impairment, may increase pulmonary capillary hydrostatic pressure. Cytokines play a major role in the initiation and progression of both AKI and ALI and appear to be the prime factors mediating the local and systemic effects of kidney injury.(13) Here, we found important associations between kidney and lung disease among critically ill patients with pulmonary disease.
In the present study, AKI, as defined by the RIFLE criteria, was found in 30% of cases at the moment of ICU admission and in 35% cases during ICU stay. This result is in accordance with previous studies in which the RIFLE criteria was validated.(14) In our cohort, the patients with AKI were of similar age to those without AKI, and their age was similar to the findings reported by other studies conducted in our region.(7,15,16) The ICU length of stay was similar in patients with and without AKI, most likely due to the severity of pulmonary disease in both groups.
Mechanical ventilation was instituted for 86% of the studied cases, and there was no significant association between the need for MV and AKI. There was, however, a significant association between PaO2/FiO2 at admission and AKI, evidencing the impact of AKI in lung function. An independent risk factor associated with AKI was PaO2/FiO2<200 mmHg. Positive pressure ventilation alters venous return, cardiac preload, pulmonary vascular resistance and cardiac afterload. Accordingly, a decrease in several parameters of renal function, including GFR, renal blood flow and free water clearance, has been noted during positive pressure ventilation.(17) Blood gas changes induced by ALI/ARDS can also adversely affect renal hemodynamics and function. Severe hypoxemia and hypercapnia have been demonstrated to reduce renal blood flow due to increased renal vascular resistance related to the activation of vasoactive factors, including angiotensin II and endothelin, and a decrease in nitric oxide, which stimulate noradrenaline release and induce vasoconstriction, respectively.(18) In our cohort, the patients with AKI were more severely ill than those without AKI, as evidenced by a higher APACHE II score. This could have an impact on lung function.
Vieira et al.(19) reported that the median duration of MV, length of ICU stay and ICU mortality rate were longer in AKI patients compared with non-AKI patients. In the present study, there was no significant difference in the length of ICU stay between patients with and without AKI. In the present study, the patients with AKI had a mean duration of hospital stay of 10.6 days, compared to 8.5 days among those without AKI. This difference was not statistically significant. Similar results were also found for the frequency of MV requirement.
AKI-associated mortality remains high, ranging from 50 to 60%, despite the advances in critical care. This may be explained by the use of more aggressive medical and surgical interventions in an aging population.(2) In our cohort, the mortality rate was higher among patients with AKI (62.8%) and was significantly higher than in patients without AKI (27.6%). Previous studies have described AKI, as well as respiratory insufficiency, as independent risk factors for death in critically ill patients.(7,20) It is known that ALI and the consequent hypoxemia, hypercapnia and MV that arise from it worsens renal hemodynamics. This could have increased mortality among the studied cases.(18)
Death occurred in 40% of cases. The comparison of survivors and nonsurvivors showed that nonsurvivors had a higher frequency of MV requirement, higher PEEP at admission and higher urea levels at admission and required dialysis more often. The independent risk factors associated with death were PEEP at admission and need for hemodialysis. High values of PEEP are known to decrease the urinary flow rate, urinary sodium excretion and creatinine clearance.(18) It is currently accepted that much of the increased risk of death during AKI is actually due to extrarenal complications that are usually related to distant organ dysfunction.(21) As such, ALI and AKI synergistically worsen the outcome of critically ill patients.(22) In a study of patients with severe AKI who required dialysis, Chertow et al.(23) found a markedly higher mortality rate in patients who needed MV (81%). Both RIFLE and APACHE II were not good predictors of mortality, as shown in the ROC curve (AUC was under 0.7).
AKI is associated with a high risk for developing CKD.(24,25) Recent studies have shown that patients admitted with AKI are frequently discharged with decreased GFR and later develop CKD. In a study of 187 patients with acute tubular necrosis without previous kidney disease, Liaño et al.(26) showed that after a median follow-up of 7.2 years, 81% of patients had normal renal function. In a study of 206 ICU patients with AKI, 96 (46%) remained alive after 90 days. Of these patients, 89 (94%) had data after 3 years, 32 (35%) had CKD from disease onset and 25 (28%) developed CKD following the AKI episode.(25)
The exact mechanisms by which AKI can become CKD and its risk factors are still poorly understood. In a study of 131 CKD patients who developed dialysis-requiring AKI, Lee et al.(27) showed that 21 (16%) successfully withdrew from acute dialysis. The factors associated with dialysis withdrawal were larger kidney size (OR=1.755; p=0.018), lower predialysis creatinine (OR=0.722; p=0.002), and non-diabetes (OR=0.271; p=0.037). All patients in the non-withdrawal group remained on chronic dialysis after 5 years and 8 of 21 (38%) patients in the withdrawal group developed end-stage renal disease.
This study has some limitations. Due to the small number of patients included in the study, the power of the statistical analysis may have been compromised. As such, it is possible that other risk factors that were not identified here may also be involved in the interactions between the kidney and lung in critically ill patients. It is also possible that other variables that were missed could have influenced the occurrence of AKI and outcome.
CONCLUSIONS
The present study found a higher mortality rate in the acute kidney injury group. However, there was no association between the need for mechanical ventilation and acute kidney injury. The independent factor associated with acute kidney injury was PaO2/FiO2 <200 mmHg. Increased mortality was associated with mechanical ventilation, high PEEP, urea and need for dialysis. Further studies must be performed to better establish the relationship between kidney and lung injury and its impact on patient outcome.
ACKNOWLEDGEMENTS
We are very grateful to the team of physicians, nurses and medical students at the Hospital de Messejana for the assistance provided to the patients.
Footnotes
Conflicts of interest: None.
This study was conducted at the Hospital de Messejana - Fortaleza (CE), Brazil.
REFERENCES
- 1.Dennen P, Douglas IS, Anderson R. Acute kidney injury in the intensive care unit: an update and primer for the intensivist. Crit Care Med. 2010;38(1):261–275. doi: 10.1097/CCM.0b013e3181bfb0b5. [DOI] [PubMed] [Google Scholar]
- 2.Gopaluni S, Lines S, Lewington AJ. Acute kidney injury in the critically ill patient. Curr Anaestesia Crit Care. 2010;21(2):60–64. [Google Scholar]
- 3.Ostermann M, Chang R, Riyadh ICU Program Users Group Correlation between the AKI classification and outcome. Crit Care. 2008;12(6):R144. doi: 10.1186/cc7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int. 2003;63(2):600–606. doi: 10.1046/j.1523-1755.2003.00753.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19(7):1360–1370. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14(6):1549–1558. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 7.Silva Júnior GB, Daher Ede F, Mota RM, Menezes FA. Risk factors for death among critically ill patients with acute renal failure. São Paulo Med J. 2006;124(5):257–263. doi: 10.1590/S1516-31802006000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floege J, Uhlig S. Kidney calling lung and call back: how organs talk to each other. Nephrol Dial Transplant. 2010;25(1):32–34. doi: 10.1093/ndt/gfp464. [DOI] [PubMed] [Google Scholar]
- 9.Ricci Z, Ronco C. Pulmonary/renal interaction. Curr Opin Crit Care. 2010;16(1):13–18. doi: 10.1097/MCC.0b013e328334b13b. Retraction in Curr Opin Crit Care. 2012;18(3):294. [DOI] [PubMed] [Google Scholar]
- 10.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73(5):538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 11.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 12.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 13.Feltes CM, Van Eyk J, Rabb H. Distant-organ changes after acute kidney injury. Nephron Physiol. 2008;109(4):80–84. doi: 10.1159/000142940. [DOI] [PubMed] [Google Scholar]
- 14.Kellum JA. Acute kidney injury. Crit Care Med. 2008;36(4) Suppl:S141–S145. doi: 10.1097/CCM.0b013e318168c4a4. [DOI] [PubMed] [Google Scholar]
- 15.Mataloun SE, Machado FR, Senna AP, Guimarães HP, Amaral JL. Incidence, risk factors and prognostic factors of acute renal failure in patients admitted to an intensive care unit. Braz J Med Biol Res. 2006;39(10):1339–1347. doi: 10.1590/s0100-879x2006001000010. [DOI] [PubMed] [Google Scholar]
- 16.Daher EF, Marques CN, Lima RS, Silva Júnior GB, Barbosa AS, Barbosa ES, et al. Acute kidney injury in an infectious disease intensive care unit - an assessment of prognostic factors. Swiss Med Wkly. 2008;138(9-10):128–133. doi: 10.4414/smw.2008.12062. [DOI] [PubMed] [Google Scholar]
- 17.Kuiper JW, Groeneveld AB, Slutsky AS, Plötz FB. Mechanical ventilation and acute renal failure. Crit Care Med. 2005;33(6):1408–1415. doi: 10.1097/01.ccm.0000165808.30416.ef. [DOI] [PubMed] [Google Scholar]
- 18.Ko GJ, Rabb H, Hassoun HT. Kidney-lung crosstalk in the critically ill patient. Blood Purif. 2009;28(2):75–83. doi: 10.1159/000218087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vieira Jr JM, Castro I, Curvello-Neto A, Demarzo S, Caruso P, Pastore Jr L, et al. Effect of acute kidney injury on weaning from mechanical ventilation in critically ill patients. Crit Care Med. 2007;35(1):184–191. doi: 10.1097/01.CCM.0000249828.81705.65. [DOI] [PubMed] [Google Scholar]
- 20.Alves GC, Silva Júnior GB, Lima RS, Sobral JB, Mota RM, Abreu KL, et al. Risk factors for death among critically ill elderly patients. Rev Bras Ter Intensiva. 2010;22(2):138–143. [PubMed] [Google Scholar]
- 21.Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30(9):2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Doi K, Ishizu T, Fujita T, Noiri E. Lung injury following acute kidney injury: kidney-lung crosstalk. Clin Exp Nephrol. 2011;15(4):464–470. doi: 10.1007/s10157-011-0459-4. [DOI] [PubMed] [Google Scholar]
- 23.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155(14):1505–1511. [PubMed] [Google Scholar]
- 24.Basile C. The long-term prognosis of acute kidney injury: acute renal failure as a cause of chronic kidney disease. J Nephrol. 2008;21(5):657–662. [PubMed] [Google Scholar]
- 25.Triverio PA, Martin PY, Romand J, Pugin J, Perneger T, Saudan P. Long-term prognosis after acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant. 2009;24(7):2186–2189. doi: 10.1093/ndt/gfp072. [DOI] [PubMed] [Google Scholar]
- 26.Liaño F, Felipe C, Tenorio MT, Rivera M, Abraira V, Sáez-de-Urturi JM, et al. Long-term outcome of acute tubular necrosis: a contribution to its natural history. Kidney Int. 2007;71(7):679–686. doi: 10.1038/sj.ki.5002086. [DOI] [PubMed] [Google Scholar]
- 27.Lee PH, Wu VC, Hu FC, Lai CF, Chen YM, Tsai TJ, Wu KD, National Taiwan University Study Group on Acute Renal Failure Outcomes following dialysis for acute kidney injury among different stages of chronic kidney disease. Am J Nephrol. 2011;34(2):95–103. doi: 10.1159/000329082. [DOI] [PubMed] [Google Scholar]