Abstract
Objective
The aim of this study was to systematically review the importance of neuron-specific enolase and S100 beta for diagnosing and monitoring septic encephalopathy.
Methods
A PubMed database search was performed to identify studies that evaluated S100 beta and neuron-specific enolase serum levels in patients with sepsis and that were published between January 2000 and April 2012. Only human studies that employed an additional method of neurological assessment were selected.
Results
Nine studies were identified, seven of which associated high concentrations of S100 beta and neuron-specific enolase with the development of septic encephalopathy. Four studies also associated these concentrations with increased mortality. However, two studies did not find such an association when they evaluated S100 beta levels, and one of these studies did not observe a correlation between neuron-specific enolase and septic encephalopathy.
Conclusion
S100 beta and neuron-specific enolase are promising biomarkers for diagnosing and monitoring patients with septic encephalopathy, but more research is necessary.
Keywords: Sepsis/complications, Brain diseases/etiology, Biological markers, S100 proteins, Phosphopyruvate hydratase, Intensive care
Abstract
Objetivo
O objetivo deste estudo foi revisar sistematicamente a importância da enolase específica neuronal e S100B para diagnóstico e monitorização da encefalopatia séptica.
Métodos
Foi realizada uma busca no banco de dados PubMed selecionando estudos que avaliaram níveis séricos de S 100 B e enolase específica neuronal em pacientes com sepse, publicados entre Janeiro de 2000 e Abril de 2012. Apenas estudos em humanos e que utilizaram um método adicional de avaliação neurológica foram selecionados.
Resultados
Foram identificados nove estudos, dos quais sete associaram concentrações elevadas de S100 beta e enolase específica neuronal ao desenvolvimento de encefalopatia séptica; quatro também as associaram ao aumento de mortalidade. Entretanto, dois trabalhos não encontraram essa associação quando avaliaram S100 beta e um deles não observou correlação entre a enolase específica neuronal e encefalopatia séptica.
Conclusão
A S100 beta e enolase específica neuronal são biomarcadores promissores para diagnóstico e monitorização de pacientes com encefalopatia séptica, mas é necessária uma maior investigação.
INTRODUCTION
Septic encephalopathy (SE) is a common but not well-understood complication of sepsis that affects between 9% and 71% of septic patients,(1-4) depending on the diagnostic criteria used. SE may be defined as a cerebral disorder resulting from metabolic and cellular signaling changes that are mediated by inflammatory components.(2) SE is typically an early event during the natural evolution of the disease and often appears prior to the failure of other organs.(2,5) Moreover, SE is associated with a worse prognosis.(6)
SE not only is associated with high hospital mortality (16%-63%),(2) but also can lead to long-term cognitive and functional limitations in those patients who survive.(7) Because of the possible consequences associated with this organ dysfunction, early diagnosis of brain injury can help identify those patients with more severe disease who require increased surveillance and immediate intervention. However, the clinical signs can vary based on the patient's degree of sedation; moreover, the clinical signs may be nonspecific, as several diseases are commonly associated with a reduced level of consciousness or agitation, disorientation, poor concentration, delirium, and coma.(1,4,5,8) Together, these factors render SE a diagnosis of exclusion.(1,2,9) Thus, clinical criteria, which are based on electrophysiological and biochemical tests, should be used in diagnosing SE.(3) In this context, SE biomarkers(2) would be useful for monitoring brain dysfunction and predicting mortality,(10) although the exact function of these markers in managing septic patients remains unclear.(11) Among the various biomarkers currently in use, neuron-specific enolase (NSE) and S100 beta are the most promising.
NSE is a γγ isomer of the cytoplasmic glycolytic enzyme that is found in neurons and neuroendocrine cells.(12) NSE is released into the blood and cerebrospinal fluid during brain damage.(13) S100 beta is a calcium-binding protein that belongs to the S100 family, which is composed of low-molecular-weight, multigene proteins.(14) S100 beta is produced by astrocytes in the central nervous system (CNS) but has both a neuroectodermal and mesodermal origin(15 ) and can therefore be expressed by other cells such as chondrocytes, adipocytes, and melanocytes. The exact mechanism by which S100 beta is excreted is still unknown but appears to be related to the oxidative stress(16) produced when neural tissue is attacked.
The aim of this systematic review is to highlight the importance of the biomarkers S100 beta and NSE in diagnosing and monitoring SE.
METHODS
A systematic PubMed search was performed for scientific articles related to SE biomarkers, employing the following search terms:
"((((((S-100beta) OR S-100 beta) OR S100beta) OR s100b) OR neuron-specific enolase) OR NSE) AND sepsis".
In addition to this search strategy, the keyword "septic encephalopathy" was used, and abstracts of all the resultant publications were evaluated to identify possibly relevant studies.
For the initial analysis, studies published in English between January 1, 2000 and April 31, 2012 were selected.
To select the studies, the following inclusion criteria were used: prospective cohort studies, clinical trials that used biomarkers as evaluation parameters, and cross-sectional studies. Furthermore, studies were selected that used at least one method of neurological assessment, such as the Glasgow coma scale (GCS), the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU), computed tomography (CT), magnetic resonance imaging (MRI), electroencephalography (EEG), and intracranial pressure (ICP) or direct measurements of cerebral spinal fluid (CSF) markers. Studies were excluded from this review if the methodology did not fit the inclusion criteria, did not assess SE, did not measure NSE or S100 beta levels, included pregnant women in the patient cohort, or employed nonhuman experimental models.
The references in these selected articles were also used to identify additional studies.
The search for articles was performed independently and blindly by all of the present authors, who strictly adhered to the defined inclusion and exclusion criteria. The results obtained by each author were subsequently compared. In the case of disagreement over the selected articles, the publications were reassessed together by the authors, who deliberated on the relevance of the studies and whether to include them in the present review.
RESULTS
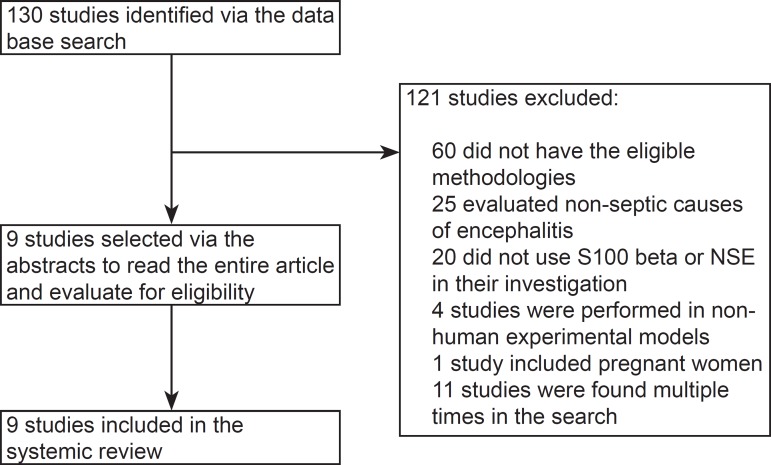
Using the predefined search strategies, 130 studies were identified. Of these studies, 121 were excluded, as shown in figure 1. Some of the excluded studies fulfilled more than one exclusion criterion but were grouped into only one category. No relevant studies were found in the reference sections of the selected articles.
Figure 1.
Flowchart of the studies selected for systematic review. NSE - neuron-specific enolase.
Among the studies included in this review (Table 1), the majority had a non-probabilistic sampling design and included patients who were treated at their respective health services for sepsis,(17-20) severe sepsis,(10,17,19-21) and septic shock.(10,17,20,22,23) The diagnoses for these studies were based on the criteria established by the American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine (SCCM). Only one study was conducted using initially healthy individuals.(23) Three studies analyzed children(18,19,22) who were 15 years of age or younger,(22 ) whereas the other investigations only included adults older than 18 years in their cohorts. The most frequently encountered exclusion criteria in these studies were primary CNS diseases (i.e., meningitis, encephalitis, stroke, epilepsy, and tumors),(10,17-20,22,23) metabolic neurological causes,(10,17,20,22) disorders secondary to non-septic recent surgery for cardiac revascularization,(10,22 , 23) and neurosurgery.(10,19,23) Five studies used control groups to compare their results,(10,18,19,22,24) whereas three studies compared their results between subgroups of septic patients formed based on other evaluation criteria, such as GCS(21,23) and CAM-ICU.(17) One group of researchers performed transcranial Doppler ultrasonography on their patients and used indirect measurements of ICP and cerebral perfusion pressure to compare their results.(20)
Table 1.
Characteristics of the studies investigating biomarkers of septic encephalopathy
| Author | Year | Study design | Population studied | Sample | Biomarker used | Evaluation criteria | Clinical significance |
| Rodríguez-Nuñez et al.(18) | 2001 | Cross-sectional cohort | Children 1 to 15 years of age with sepsis | 182 | NSE | None | The time or intensity of hypoxia was not sufficient to cause neuronal damage |
| Nguyen et al.(10) | 2006 | Prospective cohort | Patients 18 to 89 years of age with severe sepsis and septic shock | 220 | S100 beta NSE | GCS, MRI and CT | S100 beta levels are predictors of mortality in the ICU and more accurately reflect the development of encephalopathy and brain damage |
| Piazza et al.(21) | 2007 | Prospective cohort | Patients between 49 and 84 years of age with severe sepsis | 21 | S100 beta | GCS, EEG, CT | The increased S100 beta levels were not related to the severity of neurological dysfunction |
| Hsu et al.(22) | 2008 | Prospective cohort | Children between 3 months and 21 years of age with septic shock | 56 | S100 beta NSE | EEG | Increased S100 beta and NSE levels strongly suggest neurological injury |
| Pfister et al.(17) | 2008 | Prospective cohort | Patients between 18 and 90 years of age with sepsis, severe sepsis, or septic shock | 16 | S100 beta | CAM-ICU | S100 beta levels are correlated with sepsis associated with delirium, but its diagnostic role needs further study |
| Pfister et al.(20) | 2008 | Prospective cohort | Adults with an average age of 67 years, who had sepsis, severe sepsis, or septic shock | 15 | S100 beta | PPC and ICP | The increased concentration of S100 beta is related to low cerebral perfusion pressures |
| Hamed et al.(19) | 2009 | Prospective cohort | Septic children between 1 and 180 months of age | 75 | S100 beta | GCS, EEG, MRI and CT | S100 beta levels suggest a direct involvement of this biomarker in septic encephalopathy |
| Spapen et al.(23) | 2010 | Clinical trial | Patients between 56 and 82 years of age with septic shock | 54 | S100 beta | GCS | S100 beta is a potential biomarker for diagnosing and monitoring septic encephalopathy |
| van den Boogaard et al.(24) | 2010 | Clinical trial | Previously healthy males between 1 and 25 years of age who volunteered for the administration of LPS, | 25 | S100 beta NSE | EEG | There were no signs that acute systemic inflammation increases the levels of specific proteins in the brain or alters cognitive function |
NSE - neuron-specific enolase; GCS - Glasgow Coma Scale; EEG - electroencephalogram; ICU - intensive care unit; MRI - magnetic resonance imaging; CT - computed tomography; CAM-ICU - confusion assessment method for the intensive care unit; CPP - cerebral perfusion pressure; ICP - intracranial pressure.
The biomarker analyses were performed using different types of tests. Some studies used enzyme immunoassays (ELISAs),(18,19,22 ) one used the LIAISON Sangect 100 commercial kit,(21) two used radioimmunoassays,(10,23) one used a luminometric assay,(23) and two studies(17,20) did not specify the type of assay that was used. Furthermore, different maximum values for normal blood concentrations of S100 beta were observed in the adults (0.105 mg/L, 0.12 mg/L, <0.15 mg/L, and 0.5 mg/L).(10,17,20,21,23,24) Serum levels of NSE that were≤12.5 mg/L were considered normal in three studies.(10,23,24)
NSE:
Among the selected studies, four(10,18,22,24) evaluated NSE. In the pediatric populations, one study demonstrated elevated NSE serum concentrations in patients with septic shock compared with the control group (96.6 mg/±8.9 versus 4.0 mg/±1.3, p<0.001).(22) EEGs performed on six children with shock indicated a 100% incidence of neurological alterations suggesting encephalopathy. The study by Rodríguez-Núñez et al.(18) analyzed the CSF of children with sepsis, who presented higher biomarker concentrations than the control group did (1.58ng/m±0.81 versus 1.52ng/m±1.01), but this difference was not significant.
One study of adults with severe sepsis and septic shock revealed elevated levels of NSE in 70% of patients diagnosed with encephalopathy, which was based on persistent neurological changes for at least 72 hours after weaning from sedation.(10) In the study by van den Boogaard, who analyzed a previously healthy population, blood samples collected during the induction of systemic inflammation by administering Escherichia coli lipopolysaccharide (LPS) presented decreasing concentrations of NSE in the short term (11.1 mg/±0.47 to 7.7 mg/±0.39; p<0.0001).(24)
One study correlated increased patient mortality with higher NSE concentrations,( 22) whereas another study revealed no correlation between these variables, despite having demonstrated that patients who had NSE levels >30 μg/L died.(10)
S100 beta
The association between S100 beta and SE was studies,(10,17,19-24) investigated in eight five of which correlated elevated biomarker levels with the development of SE.(10,17,19,22,23) One study identified high serum concentrations of S100 beta in patients with low cerebral perfusion pressure.(20) The four studies that used GCS to clinically diagnose SE revealed elevated levels of S100 beta in the patients with the lowest scores.( 10,17,19,23) Of the studies that used EEG as an evaluation criterion, two associated electroencephalographic abnormalities with increased serum concentrations of S100 beta.(19,22) Additionally, four studies correlated elevated biomarker levels with increased mortality,(10,17,20,22) and two studies confirmed the use of S100 beta as a method of monitoring brain damage during sepsis.(10,23)
However, two studies found no correlation between the increased S100 beta serum concentrations and the development of SE.(21,24) Both studies used EEG as an evaluation criterion and revealed no correlation between the test standards and the biomarker levels. Moreover, the study that used GCS showed no correlation between the GCS scores and the serum concentrations of S100 beta, suggesting that the severity of brain damage cannot be defined based on the levels of this protein.(21)
DISCUSSION
The present review identified a positive association between elevated levels of NSE and S100 beta and the development of encephalopathy secondary to sepsis. The findings suggest that these biomarkers may facilitate diagnosis of this complication, which is common but often undiagnosed.(6)
The pathophysiology of SE appears to be multifactorial. The disease results from the interaction and overlapping of various mechanisms related to the systemic inflammatory response,(5) including oxidative stress, proinflammatory and anti-inflammatory mediators, the complement cascade, endothelial dysfunction, blood-brain barrier disruption, and microvascular failure.(1,25) This entire process leads to dysfunction, apoptosis, and cell death. Therefore, the development of this disease is more closely related to the inflammatory response than to the infectious agent alone.
Various clinical tools have been used to diagnose SE. CAM-ICU is a validated scale for identifying delirium and is capable of accessing different aspects of a person's mental state, including attention, thought organization, and consciousness.(26) GCS is another scale that is used to diagnose SE and, although initially designed to assess the level of consciousness in trauma patients, is now used in patients with a variety of disorders(4,27) However, during identification of an altered level of consciousness, the clinical findings are frequently nonspecific and cannot specify the cause of the neurological syndrome.
The use of sedation, a practice that is still common in intensive care units, may also contribute to an inaccurate assessment of a patient's state of consciousness. Imaging methods may serve as alternatives; however, CT does not identify definitive changes in SE cases, whereas MRI is often useful for diagnosing brain abnormalities and may eventually facilitate the determination of a prognosis.(28) Furthermore, these imaging methods are expensive, and the transport of patients to undergo the exam has been a major limitation for use.(28) Young et al. investigated EEG and concluded that it is a sensitive method for evaluating brain function in SE,(29) but again, sedation is a limiting factor in employing this method.(4,21) Thus, in the absence of well-defined criteria for diagnosing SE,(1)additional and more accurate methods must be examined.
In this context, the search for biochemical markers appears to be a natural process. Once the sensitivities and specificities of these biomarkers have been established, their application is generally easy because they can be measured when samples are collected from patients (regardless of the clinical status) and because there is no requirement for patient transport or for a specialized professional to perform the procedure. Different studies have used S100 beta and NSE as biomarkers that can correlate patient outcomes with severe traumatic brain injury.(30) Stein et al. found elevated concentrations of S100 beta in patients who were admitted with brain damage following trauma and who had a worse prognosis.(30) Other researchers have identified a correlation between the biomarkers and nontraumatic ischemic brain injury, such as cardiac arrest (CA)(31-33) and surgical cases of cardiac revascularization.(34,35) González-García et al. found significantly elevated serum concentrations of NSE and S100 beta in patients with arterial ischemic stroke (AIS) compared with the control group (NSE: 11.2 µmol/L versus 9.5 µmol/L, with p=0.0135; S100 beta: 127 nmol/L versus 84.6 nmol/L, with p=0.0000).(36)
Few studies have attempted to directly associate S100 beta and NSE with SE. Six studies included in the present review correlated these biomarkers with the development of SE(10,17,19,20,22,23) and thus analyzed distinct populations. The studies by Hsu et al.(22) and Hamed et al.(19) focused on the pediatric population, whereas the remaining studies(10,17,20,23) included adults and seniors. This variation in cohorts associated with similar clinical findings suggests that S100 beta and NSE may be used as biomarkers for SE in the general population. Most of these studies included only patients with severe sepsis and septic shock, i.e., in advanced stages of infection, during which organ dysfunction already exists. Because SE is usually an early event in the natural evolution of sepsis,(3,6) patients in this phase of the disease most likely have already developed encephalopathy, and the use of biomarkers would be one way to confirm this clinical suspicion.
In their study, Hamed et al.(19) also included septic patients who did not display any clinical or EEG evidence of neurological damage. Notably, this group had S100 beta serum concentrations that were higher than in the non-septic patients, suggesting the early occurrence of brain damage during sepsis and highlighting the limited sensitivity of other tests in diagnosing this pathology. Another important finding in the study by Hamed et al. was that the biomarker concentrations in the CSF are higher than those in the blood, which corroborates the theory of increased intrathecal production of S100 beta during sepsis. Conversely, several authors have argued that S100 beta may not be a specific cerebral biomarker,(22) as extracranial foci of elevated levels of this protein (such as in the heart, skeletal muscle, and kidney) have been described.(17,20) Nevertheless, Nguyen et al.(10) used postoperative patients who had undergone surgical revascularization as a control group in their study and did not find elevated concentrations of NSE or S100 beta in this group.
The primary objective of the studies conducted by Spapen(23) and Pfister(20) was not to evaluate the use of S100 beta as a biomarker of SE; however, in the first investigation, high concentrations of this protein were found in patients with GCS scores <13, which is consistent with the results reported in other studies that evaluated S100 beta as a diagnostic criterion for SE. Furthermore, this clinical trial is a good example of using this protein to monitor patients. In addition, Pfister(20) correlated low cerebral perfusion pressures with elevated serum S100 beta levels in patients with sepsis, severe sepsis, and septic shock. Although brain perfusion is not a proven diagnostic method for SE, low perfusion pressure is one of the pathophysiological mechanisms of SE that has been previously cited, and low perfusion pressure may be an indirect signal of CNS injury. Therefore, this finding strengthens the association between S100 beta and SE.
Three other studies included in the present review did not associate SE with elevated concentrations of these biomarkers.(18,21,24) One such study was conducted by Rodríguez-Núñez et al.,(18) who analyzed the CSF concentrations of NSE in children with sepsis. This cross-sectional study model may be considered a limiting factor because the duration of the disease might not have been sufficient for ischemia and neuronal damage to develop.
Based on the close relationship between the inflammatory response and the pathophysiology of SE, van den Boogaard et al.(24) studied the behavior of NSE and S100 beta in previously healthy subjects with a transient systemic inflammatory response induced by the administration of E. coli lipopolysaccharide (LPS). Serum concentrations of cortisol, inflammatory cytokines, NSE, and S100 beta, as well as electroencephalographic abnormalities, were the parameters used to evaluate the results. However, no evidence was found indicating that acute inflammation causes increased serum concentrations of specific cerebral proteins. However, such findings cannot be considered definitive. The timing of the experiment may be a limiting factor in the study because, after 8 hours of monitoring, the inflammatory cytokines, which had increased significantly, returned to their basal levels; therefore, the inflammatory response, which is a key element in the development of encephalopathy, was not perpetuated. However, it is possible that the quantity of LPS administered was one factor responsible for these findings. Viral load is a major determinant of the inflammatory response during infection; thus, the amount of LPS administered might not have been sufficient to produce an inflammatory response that could cause brain damage and consequently increase the serum concentrations of these biomarkers.
Of the studies included in this systematic review, four used GCS as a parameter for correlating biomarker levels in the evaluation of encephalopathy.(10,19,21,23) Piazza et al.(21)reported that S100 beta could not indicate brain injury in septic patients upon admission because the protein concentrations did not correlate with the GCS findings. However, the study did not demonstrate that all patients with a GCS score<8 had elevated serum concentrations of S100 beta, with the exception of one patient who had a Glasgow score of 8. Adequate weaning from sedation might also have influenced the results, but because the study did not report the type of sedation used or the interval between the discontinuation of sedation and application of GCS, there is no way of determining how much the sedation interfered with the patient assessment.
Regarding the use of NSE and S100 beta in monitoring these patients, the studies yielded conflicting results, especially for S100 beta. Seven studies performed serial assessments of the levels of these biomarkers,(10,17,20-24) but only four of them correlated the NSE and S100 beta concentrations with time.(21-24 ) Hsu et al.(22) presented an adjusted biomarker curve in which the highest concentrations were found between the fifth and seventh day following admission. Piazza et al.(21) also found elevated levels of S100 beta by the end of the seventh day of hospitalization, and Spapen et al.(23) noted peak levels of S100 beta between the second and third days of monitoring. However, a common thread in these studies is that, after the initiation of monitoring, patients develop higher-than-normal levels of these markers. This finding suggests that these substances should be assessed upon admission; thereafter, it is still unclear how often the marker levels should be determined for monitoring these patients. Irrespective of the method, it is proposed that monitoring should be performed because of the association between elevated levels of these biomarkers and increased mortality. Thus, it would be possible to establish a prognosis and stratify patients according to their disease severity.
A common feature observed in the studies in this systematic review is the small number of evaluated patients. The largest study included 220 patients, but only 27 patients were diagnosed with SE, thus decreasing the external validation of the results. Furthermore, various studies employed different laboratory assays to measure the S100 beta and NSE concentrations, thereby eliminating the possibility of quantitatively analyzing the results from these investigations. Another relevant fact is that most of the studies evaluated S100 beta, whereas only four studies included the analysis of NSE.(10,18,21,22)
This systematic review has certain limitations that should be noted. The present authors only utilized the PubMed database to search for relevant articles and only included articles that were written in English, which might have limited the findings of this study.
CONCLUSION
NSE and especially S100 beta are potential serum biomarkers for diagnosing and monitoring SE. However, additional studies with a larger number of patients are necessary to establish more definitive outcomes.
Footnotes
Conflicts of interest: None.
REFERENCES
- 1.Ringer TM, Axer H, Romeike BF, Zinke J, Brumkhorst F, Witte OW, et al. Neurological sequelae of sepsis: I) Septic encephalopathy. Open Crit CareMed J. 2011;4:2–7. [Google Scholar]
- 2.Pytel P, Alexander JJ. Pathogenesis of septic encephalopathy. Curr Opin Neurol. 2009;22(3):283–287. doi: 10.1097/WCO.0b013e32832b3101. [DOI] [PubMed] [Google Scholar]
- 3.Ebersoldt M, Sharshar T, Annane D. Sepsis associated delirium. Intensive Care Med. 2007;33(6):941–950. doi: 10.1007/s00134-007-0622-2. [DOI] [PubMed] [Google Scholar]
- 4.Szatmári S, Végh T, Csomós A, Hallay J, Takács I, Molnár C, et al. Impaired cerebrovascular reactivity in sepsis-associated encephalopathy studied by acetazolamide test. Crit Care. 2010;14(2):R50. doi: 10.1186/cc8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papadopoulos MC, Davies DC, Moss RF, Tighe D, Bennett ED. Pathophysiology of septic encephalopathy: a review. Crit Care Med. 2000;28(8):3019–3024. doi: 10.1097/00003246-200008000-00057. [DOI] [PubMed] [Google Scholar]
- 6.Zampieri FG, Park M, Machado FS, Azevedo LC. Sepsis-associated encephalopathy: not just delirium. Clinics (Sao Paulo) 2011;66(10):1825–1831. doi: 10.1590/S1807-59322011001000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term Cognitive Impairment and Functional Disability Among Survivors of Severe Sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamar CD, Hurley RA, Taber HK. Sepsis-associated encephalopathy: review of the neuropsychiatric manifestations and cognitive outcome. J Neuropsychiatry Clin Neurosci. 2011;23(3):236–241. doi: 10.1176/jnp.23.3.jnp237. [DOI] [PubMed] [Google Scholar]
- 9.Davies NW, Sharief MK, Howard RS. Infection-associated encephalopathies: their investigation, diagnosis, and treatment. J Neurol. 2006;253(7):833–845. doi: 10.1007/s00415-006-0092-4. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen DN, Spapen H, Su F, Schiettecatte J, Shi L, Hachimi-Idrissi S, et al. Elevated serum levels of S-100beta protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med. 2006;34(7):1967–1974. doi: 10.1097/01.CCM.0000217218.51381.49. [DOI] [PubMed] [Google Scholar]
- 11.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14(1):R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shinozaki K, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Abe R, et al. S-100B and neuron-specific enolase as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation: a systematic review. Crit Care. 2009;13(4):R121. doi: 10.1186/cc7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Lei L, Shi Y, Wang J, Jiang H, Shen L, et al. Serum concentrations of NSE and S100B in spinocerebellar ataxia type 3/Machado-Joseph disease. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36(6):504–510. doi: 10.3969/j.issn.1672-7347.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Peric B, Zagar I, Novakovic S, Zgajnar J, Hocevar M. Role of serum S100B and PET-CT in follow-up of patients with cutaneous melanoma. BMC Cancer. 2011;11:328. doi: 10.1186/1471-2407-11-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruijff S, Bastiaannet E, Kobold AC, van Ginkel RJ, Suurmeijer AJ, Hoekstra HJ. S-100B concentrations predict disease-free survival in stage III melanoma patients. Ann Surg Oncol. 2009;16(12):3455–3462. doi: 10.1245/s10434-009-0629-8. Erratum in Ann Surg Oncol. 2011;18 Suppl 3:S331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamed SA, Hamed EA, Zakary MM. Oxidative stress and S-100B protein in children with bacterial meningitis. BMC Neurol. 2009;9:51. doi: 10.1186/1471-2377-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfister D, Siegemund M, Dell-Kuster S, Smielewski P, Rüegg S, Strebel SP, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12(3):R63. doi: 10.1186/cc6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodríguez-Núñez A, Cid E, Rodríguez-García J, Camiña F, Rodríguez-Segade S, Castro-Gago M. Concentrations of nucleotides, nucleosides, purine bases, oxypurines, uric acid, and neuron-specific enolase in the cerebrospinal fluid of children with sepsis. J Child Neurol. 2001;16(9):704–706. doi: 10.1177/088307380101600918. [DOI] [PubMed] [Google Scholar]
- 19.Hamed SA, Hamed EA, Abdella MM. Septic encephalopathy: relationship to serum and cerebrospinal fluid levels of adhesion molecules, lipid peroxides and S-100B protein. Neuropediatrics. 2009;40(2):66–72. doi: 10.1055/s-0029-1231054. [DOI] [PubMed] [Google Scholar]
- 20.Pfister D, Schmidt P, Smielewski P, Siegemund M, Strebel SP, Rüegg S, et al. Intracranial pressure in patients with sepsis. Acta Neurochir Suppl. 2008;102:71–5. doi: 10.1007/978-3-211-85578-2_14. [DOI] [PubMed] [Google Scholar]
- 21.Piazza O, Russo E, Cotena S, Esposito G, Tufano R. Elevated S100B levels do not correlate with the severity of encephalopathy during sepsis. Br J Anaesth. 2007;99(4):518–521. doi: 10.1093/bja/aem201. [DOI] [PubMed] [Google Scholar]
- 22.Hsu AA, Fenton K, Weinstein S, Carpenter J, Dalton H, Bell MJ. Neurological injury markers in children with septic shock. Pediatr Crit Care Med. 2008;9(3):245–251. doi: 10.1097/PCC.0b013e3181727b22. [DOI] [PubMed] [Google Scholar]
- 23.Spapen H, Nguyen DN, Troubleyn J, Huyghens L, Schiettecatte J. Drotrecogin alfa (activated) may attenuate severe sepsis-associated encephalopathy in clinical septic shock. Crit Care. 2010;14(2):R54. doi: 10.1186/cc8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Boogaard M, Ramakers BP, van Alfen N, van der Werf SP, Fick WF, Hoedemaekers CW, et al. Endotoxemia-induced inflammation and the effect on the human brain. Crit Care. 2010;14(3):R81. doi: 10.1186/cc9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharshar T, Hopkinson NS, Orlikowski D, Annane D. Science review: The brain in sepsis--culprit and victim. Crit Care. 2005;9(1):37–44. doi: 10.1186/cc2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Siami S, Annane D, Sharshar T. The encephalopathy in sepsis. Crit Care Clin. 2008;24(1):67–82. doi: 10.1016/j.ccc.2007.10.001. viii. [DOI] [PubMed] [Google Scholar]
- 28.Piazza O, Cotena S, De Robertis E, Caranci F, Tufano R. Sepsis associated encephalopathy studied by MRI and cerebral spinal fluid S100B measurement. Neurochem Res. 2009;34(7):1289–1292. doi: 10.1007/s11064-008-9907-2. [DOI] [PubMed] [Google Scholar]
- 29.Young GB, Bolton CF, Archibald YM, Austin TW, Wells GA. The electroencephalogram in sepsis-associated encephalopathy. J Clin Neurophysiol. 1992;9(1):145–152. doi: 10.1097/00004691-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Stein DM, Lindell AL, Murdock KR, Kufera JA, Menaker J, Bochicchio GV, et al. Use of serum biomarkers to predict cerebral hypoxia after severe traumatic brain injury. J Neurotrauma. 2012;29(6):1140–1149. doi: 10.1089/neu.2011.2149. [DOI] [PubMed] [Google Scholar]
- 31.Dauberschmidt R, Zinsmeyer J, Mrochen H, Meyer M. Changes of neuron-specific enolase concentration in plasma after cardiac arrest and resuscitation. Mol Chem Neuropathol. 1991;14(3):237–245. doi: 10.1007/BF03159939. [DOI] [PubMed] [Google Scholar]
- 32.Martens P. Serum neuron-specific enolase as a prognostic marker for irreversible brain damage in comatose cardiac arrest survivors. Acad Emerg Med. 1996;3(2):126–131. doi: 10.1111/j.1553-2712.1996.tb03399.x. [DOI] [PubMed] [Google Scholar]
- 33.Meynaar IA, Oudemans-van Straaten HM, van der Wetering J, Verlooy P, Slaats EH, Bosman RJ, et al. Serum neuron-specific enolase predicts outcome in post-anoxic coma: a prospective cohort study. Intensive Care Med. 2003;29(2):189–195. doi: 10.1007/s00134-002-1573-2. [DOI] [PubMed] [Google Scholar]
- 34.Basile AM, Fusi C, Conti AA, Paniccia R, Trefoloni G, Pracucci G, et al. S-100 protein and neuron-specific enolase as markers of subclinical cerebral damage after cardiac surgery: preliminary observation of a 6-month follow-up study. Eur Neurol. 2001;45(3):151–159. doi: 10.1159/000052114. [DOI] [PubMed] [Google Scholar]
- 35.Astudillo R, Van der Linden J, Radegran K, Hanson LO, Aberg B. Elevated serum levels of S-100 after deep hypothermic arrest correlate with duration of circulatory arrest. Eur J Cardiothorac Surg. 1996;10(12):1107–1112. doi: 10.1016/s1010-7940(96)80358-7. discussion 1113. [DOI] [PubMed] [Google Scholar]
- 36.González-García S, González-Quevedo A, Fernández-Concepción O, Peña-Sánchez M, Menéndez-Saínz C, Hernández-Díaz Z, et al. Short-term prognostic value of serum neuron specific enolase and S100B in acute stroke patients. Clin Biochem. 2012;45(16-17):1302–1307. doi: 10.1016/j.clinbiochem.2012.07.094. [DOI] [PubMed] [Google Scholar]