Abstract
Objective
The current definition of severe sepsis and septic shock includes a heterogeneous profile of patients. Although the prognostic value of hyperlactatemia is well established, hyperlactatemia is observed in patients with and without shock. The present study aimed to compare the prognosis of septic patients by stratifying them according to two factors: hyperlactatemia and persistent hypotension.
Methods
The present study is a secondary analysis of an observational study conducted in ten hospitals in Brazil (Rede Amil - SP). Septic patients with initial lactate measurements in the first 6 hours of diagnosis were included and divided into 4 groups according to hyperlactatemia (lactate >4mmol/L) and persistent hypotension: (1) severe sepsis (without both criteria); (2) cryptic shock (hyperlactatemia without persistent hypotension); (3) vasoplegic shock (persistent hypotension without hyperlactatemia); and (4) dysoxic shock (both criteria).
Results
In total, 1,948 patients were analyzed, and the sepsis group represented 52% of the patients, followed by 28% with vasoplegic shock, 12% with dysoxic shock and 8% with cryptic shock. Survival at 28 days differed among the groups (p<0.001). Survival was highest among the severe sepsis group (69%, p<0.001 versus others), similar in the cryptic and vasoplegic shock groups (53%, p=0.39), and lowest in the dysoxic shock group (38%, p<0.001 versus others). In the adjusted analysis, the survival at 28 days remained different among the groups (p<0.001) and the dysoxic shock group exhibited the highest hazard ratio (HR=2.99, 95%CI 2.21-4.05).
Conclusion
The definition of sepsis includes four different profiles if we consider the presence of hyperlactatemia. Further studies are needed to better characterize septic patients, to understand the etiology and to design adequate targeted treatments.
Keywords: Infection, Sepsis, Shock, Lactic acid
Abstract
Objetivo
A definição atual de sepse grave e choque séptico inclui um perfil heterogêneo de pacientes. Embora o valor prognóstico de hiperlactatemia seja bem estabelecido, ela está presente em pacientes com ou sem choque. Nosso objetivo foi comparar o prognóstico de pacientes sépticos estratificando-os segundo dois fatores: hiperlactatemia e hipotensão persistente.
Métodos
Este estudo é uma análise secundária de um estudo observacional conduzido em dez hospitais no Brasil (Rede Amil - SP). Pacientes sépticos com valor inicial de lactato das primeiras 6 horas do diagnóstico foram incluídos e divididos em 4 grupos segundo hiperlactatemia (lactato >4mmol/L) e hipotensão persistente: (1) sepse grave (sem ambos os critérios); (2) choque críptico (hiperlactatemia sem hipotensão persistente); (3) choque vasoplégico (hipotensão persistente sem hiperlactatemia); e (4) choque disóxico (ambos os critérios).
Resultados
Foram analisados 1.948 pacientes, e o grupo sepse grave constituiu 52% dos pacientes, seguido por 28% com choque vasoplégico, 12% choque disóxico e 8% com choque críptico. A sobrevida em 28 dias foi diferente entre os grupos (p<0,001), sendo maior para o grupo sepse grave (69%; p<0,001 versus outros), semelhante entre choque críptico e vasoplégico (53%; p=0,39) e menor para choque disóxico (38%; p<0,001 versus outros). Em análise ajustada, a sobrevida em 28 dias permaneceu diferente entre os grupos (p<0,001), sendo a maior razão de risco para o grupo choque disóxico (HR=2,99; IC95% 2,21-4,05).
Conclusão
A definição de pacientes com sepse inclui quatro diferentes perfis, se considerarmos a presença de hiperlactatemia. Novos estudos são necessários para melhor caracterizar pacientes sépticos e gerar conhecimento epidemiológico, além de possível adequação de tratamentos dirigidos.
INTRODUCTION
Sepsis remains a major challenge to public health, even after years of study and progression in the understanding of the condition.(1-3) In recent years, the incidence of sepsis has been increasing, and the associated mortality remains high, with great variability between countries and continents.(3-6) To better stratify sepsis, serum lactate levels have been used worldwide,(7-12) and the current literature demonstrates good results for the use of serum lactate as a prognostic measure, as well as for therapeutic decisions and clinical classification for inclusion in randomized studies and benchmarking.(9-11,13-16)
The current definition of severe sepsis requires the presence of organ dysfunction associated with infection, and lactatemia is included as a variable.(7) Septic shock is defined by the presence of sepsis associated with persistent hypotension after adequate volume replacement and the need for vasoactive drugs.(7) However, septic patients classified as being in severe sepsis or septic shock exhibit great variability with respect to phenotype, clinical outcomes, and prognosis(7,17-21) The two patient profiles of sepsis are classic septic shock and cryptic shock, which is characterized as severe sepsis associated with serum lactate levels above 4mmol/L. Two studies have reported that there is no difference in the mortality of patients with these two sepsis diagnoses.(19,21) Recently, two other studies reclassified patients with classic septic shock as dysoxic shock patients if the patients exhibited hyperlactatemia and as vasoplegic shock patients if the patients exhibited persistent hypotension without hyperlactatemia.(17,20) Patients with vasoplegic shock exhibited better outcomes compared to patients with dysoxic shock.
Few studies have addressed this topic in the current literature, and the topic is of fundamental importance when managing and classifying sepsis. Furthermore, no study has compared the new groups among themselves. Thus, the present study aimed to compare patients with severe sepsis without hypoperfusion and patients with cryptic shock, vasoplegic shock and dysoxic shock. Secondarily, we aimed to assess whether intermediate initial values of lactate have a role in the prognosis of patients with sepsis.
METHODS
The present study constitutes a post-hoc analysis of a retrospective, multicenter, observational cohort study conducted by analyzing a prospectively collected database.(22) Patients admitted to ten hospitals of the Rede Amil from May 2010 to January 2012 in São Paulo were included. Of these hospitals, one specializes in heart diseases and the remaining nine are general hospitals, providing 1,650 beds in total, 191 of which are located in intensive care units (ICU).
The database was built in partnership with the Latin America Sepsis Institute (LASI).(23) Data were collected using the electronic form provided by LASI based on the Surviving Sepsis Campaign (SSC) bundles.(24) In each hospital, a nurse was responsible for including data in the database. These data were prospectively collected using a data collection sheet designed specifically for the present study that was completed by the healthcare team from the time sepsis was diagnosed until the first 24 hours of resuscitation. To collect data, the nurse also reviewed the charts. Throughout the collection, storage, and analysis of data, the patient's privacy was maintained, and all cases were identified only by an identification number. The Research Ethics Committee of the reference hospital, the Hospital Pró-Cardíaco, approved the retrospective analysis and the publication of data on behalf of the entire network (protocol number 104,931), so a signed informed consent was not necessary.
The inclusion criteria consisted of patients diagnosed with severe sepsis or septic shock according to the sepsis consensus conference definitions(7,25) in all areas of the hospital (emergency room, ward and ICU), and only the first sepsis episode was included. The exclusion criteria consisted of patients under 18 years, receiving palliative care or those who refused intensive care. Patients whose initial lactate levels were not measured within the 6 first hours of diagnosis were excluded from the present study. This cutoff value for inclusion was chosen because it is the recommended time window for resuscitation therapeutic approaches.(7)
Definitions
Severe sepsis was defined by the presence of two or more signs of systemic inflammatory response syndrome resulting from a proven or suspected infectious process and at least one organ dysfunction associated with sepsis. Septic shock was considered when the hypotension associated with sepsis was refractory to adequate volume replacement with the subsequent need for vasopressors. The following were considered to be organ dysfunctions: hypotension (systolic blood pressure <90mmHg or mean arterial pressure <65mmHg or decrease >40mmHg in the systolic pressure); bilateral infiltrates on chest thorax radiograph and arterial oxygen pressure/fraction of inspired oxygen ratio (PaO2/FiO2) ≤300 or the need for supplemental oxygen to maintain oxygen saturation >90% (excluding the prior need for oxygen); total serum bilirubin >2mg/dL; urine output ≤0.5mL/kg/h for more than 2 hours or creatinine >2mg/dL; platelet count ≤100x109/L, international normalized ratio >1.5 or activated partial thromboplastin time >60 seconds; and serum lactate ≥2mmol/L.(7)
To define systemic hypoperfusion, blood lactate or central venous lactate was used as a hypoperfusion marker. A cutoff value of 4mmol/L was used instead of the 2-2.5mmol/L used by others because 4mmol/L is the value that currently determines the change in the resuscitation strategy of these patients, according to the SSC.(7) The presence of cryptic shock was considered when patients exhibited severe sepsis criteria and systemic hypoperfusion.(19) Patients with septic shock criteria without systemic hypoperfusion were considered vasoplegic shock patients,(20) and the presence of dysoxic shock was considered when patients exhibited septic shock criteria and systemic hypoperfusion.(20)
To classify the patients in the four groups defined above, we performed a retrospective classification using two variables already specified in our database: persistent hypotension despite adequate volume expansion and the initial serum lactate level.
Demographic data (age and gender), clinical characteristics (temperature, heart and respiratory rate, systemic blood pressure, consciousness level, and chills) and laboratory data (blood glucose levels, blood lactate levels, and leukocyte counts) were collected at diagnosis. The severity scores were also collected at diagnosis (Acute Physiologic and Chronic Health Evaluation (APACHE II) and Sequential Organ Failure Assessment (SOFA),(26) as were the setting of sepsis diagnosis (ward, emergency room, or ICU), the site of infection and the compliance with measures for the resuscitation of septic patients. As the primary outcome, we evaluated the survival at 28 days after the sepsis diagnosis. As a secondary outcome, we evaluated the hospital mortality and the length of stay in the ICU and in the hospital.
Statistical analysis
To analyze the data distribution, a visual analysis of histograms and the Kolmogorov-Smirnov or the Shapiro-Wilk test, when appropriate, were performed. For continuous variables, data were presented as the mean and standard deviation if they exhibited a normal distribution or as the median and interquartile range if they did not exhibit a normal distribution. For categorical variables, data were presented as percentages.
According to the criteria defined above, patients were divided into four groups: (1) severe sepsis; (2) cryptic shock; (3) vasoplegic shock; and (4) dysoxic shock. The continuous variables were compared among the four groups using analysis of variance (ANOVA) for those with a normal distribution and the Kruskal-Wallis test for those without a normal distribution. For post-hoc comparisons, the correction proposed by Bonferroni was used. The categorical variables were compared using the chi-square test or, when appropriate, the Fisher's exact test or.
To analyze the survival at 28 days among the 4 groups, we used the Kaplan-Meier method. The probability of survival between the groups was analyzed by the log-rank test; for multiple post-hoc comparisons, we used the Holm-Sidak method. We evaluated the effect of each group on the survival at 28 days using the Cox regression model, both unadjusted and adjusted. For the adjusted model, we inserted pre-specified variables based on the current literature using the enter method. We created two models: one that considers age, APACHE II, and SOFA in Model A, and one that considers age, APACHE II, SOFA, early use of antibiotics, local of diagnosis, and source hospital in Model B. We calculated the hazard ratio (HR) and its respective confidence interval (95%CI) for each group, and the severe sepsis group was used as a reference. For the Cox regression models, we tested second order interactions between age, APACHE II, and SOFA. To analyze the continuous variable lactate and the hospital mortality outcomes in patients with severe sepsis and septic shock, a nonlinear locally weighted function called Locally Weighted Scatterplot Smoothing (LOESS) was adjusted.
A two-tailed p value ≤0.05 was considered significant. The analyses and graphs were created using the programs Statistical Package for the Social Sciences (SPSS) version 19.0 (SPSS Inc, Chicago, IL), SigmaPlot 12.0 (Systat Software, San Jose, CA), and R v3.0.2 (R Development Core Team 2013).(27)
RESULTS
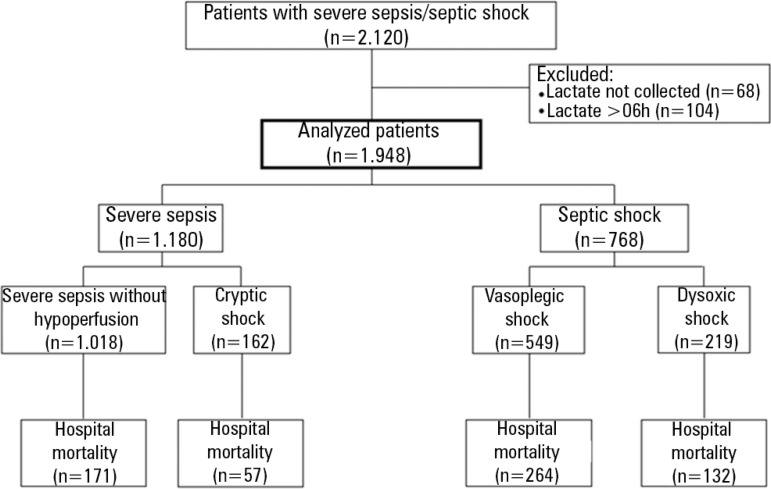
Within May 2010 and January 2012, there were 2,120 patients with severe sepsis or septic shock. Lactate collection was not performed in 68 patients and in 104 patients the first collection occurred more than 6 hours after diagnosis. Thus, 1,948 patients were analyzed and 172 (8%) patients were excluded (Figure 1). The average time between diagnosis and lactate collection was 19 (5-45) minutes.
Figure 1.
Study diagram.
There were 1,180 (61%) initial diagnoses of severe sepsis and 768 (39%) of septic shock. Among patients with severe sepsis, 1,018 (86%) exhibited no hypoperfusion criteria, whereas 162 (14%) were classified as cryptic shock patients. Vasoplegic shock was present in 549 (72%) patients with an initial diagnosis of septic shock, whereas dysoxic shock was diagnosed in 219 (28%) patients (Table 1).
Table 1.
General characteristics of the sample according to the groups
| Severe sepsis | Septic shock | ||||
| Lactate <4mmol/L (N=1.018) | Lactate >4mmol/L (N=162) | Lactate <4mmol/L (N=549) | Lactate >4mmol/L (N=219) | p value | |
| Severe sepsis | Cryptic shock | Vasoplegic shock | Dysoxic shock | ||
| Age (years) | 60±22b | 60±21d | 66±18 | 63±18 | <0.001 |
| Males | 458 (45) | 81 (50) | 267 (49) | 109 (50) | 0.321 |
| APACHE II | 15±6a,b,c | 17±7d,e | 20±8f | 24±8 | <0.001 |
| SOFA | 3 [2-5]b,c | 4[2-6]d,e | 9 [7-10]f | 10 [8-13] | <0.001 |
| Lactate (mmol/L) | 1.78 [1.2-2.5] | 5.25 [4.5-6.6] | 2.0 [1.3-2.7] | 6.10 [4.8-8.8] | <0.001 |
| Place of sepsis diagnosis | <0.001 | ||||
| Emergency room | 500 (49)b | 95 (59)d | 201 (37) | 110 (50) | <0.001 |
| Ward | 390 (38)b | 46 (28) | 167 (30) | 72 (33) | 0.004 |
| ICU | 128 (13)b | 21 (13) | 181 (33) | 37 (17) | <0.001 |
| Site of infection | 0.001 | ||||
| Pneumonia | 350 (34)b | 55 (34) | 149 (27) | 60 (27) | 0.012 |
| Urinary tract | 165 (16)c | 32 (20) | 99 (18) | 54 (25) | 0.027 |
| Abdominal | 147 (14) | 28 (17) | 94 (17) | 34 (16) | 0.501 |
| Catheter-related | 63 (6) | 10 (6) | 48 (9) | 23 (11) | 0.070 |
| Surgical wound | 51 (5) | 14 (9) | 43 (8) | 15 (7) | 0.084 |
| Meningitis | 58 (6) | 7 (4) | 16 (3) | 11 (5) | 0.100 |
| Bone | 25 (3) | 1 (1) | 9 (2) | 3 (1) | 0.317 |
| Endocarditis | - | 1 (1) | 2 (1) | 1 (1) | 0.188 |
| Others | 159 (16)a,c | 14 (9)d | 89 (17)e | 18 (9) | 0.004 |
| Clinical presentation at diagnosis | |||||
| Fever | 359 (35)c | 47 (29) | 152 (28) | 46 (21) | <0.001 |
| Hypothermia | 165 (16)c | 29 (18) | 102 (19) | 60 (27) | 0.002 |
| Tachycardia | 780 (77)a | 143 (88)d,e | 416 (76) | 172 (79) | 0.006 |
| Tachypnea | 562 (55) | 100 (62) | 290 (53) | 131 (60) | 0.121 |
| Leukopenia | 51 (5)c | 8 (5)e | 31 (6)f | 25 (11) | 0.003 |
| Leukocytosis | 490 (48) | 80 (49) | 284 (52) | 101 (46) | 0.444 |
| Hyperglycemia | 156 (15)b | 34 (21) | 138 (25) | 49 (22) | <0.001 |
| Decreased level of consciousness | 316 (31)b | 62 (38) | 224 (41) | 89 (41) | <0.001 |
| Arterial hypotension | 496 (49)b,c | 61 (38)d,e | 549 (100) | 219 (100) | 0.001 |
| Treatment received at diagnosis | |||||
| Collection of blood culture | 870 (86)c | 141 (87) | 445 (81) | 177 (81) | 0.050 |
| Antibiotics | 903 (89) | 141 (87) | 475 (87) | 193 (88) | 0.632 |
| Time to first antibiotic dose (hours) | 0.6 [0.2-1.6] | 0.5 [0.1-1.8] | 0.7 [0.2-2.3] | 0.5 [0.0-2.3] | 0.234 |
| Adequate volume expansion and vasopressor if necessary | 1.010 (99)c | 156 (96) | 512 (93) | 214 (98) | <0.001 |
| Outcomes | |||||
| Hospital mortality | 16.8 (14.4-19.1) | 35.2 (28.4-43.2) | 48.1 (43.7-52.1) | 60.3 (53.9-67.1) | <0.001 |
| Length of stay (days) | |||||
| ICU | 3 [1-7]d,e | 4 [2-9]d | 7 [3-15]f | 4 [1-11] | <0.001 |
| Non-survivors | 8 [3-16]c | 6 [2-17]e | 8 [3-16]f | 2 [1-8] | <0.001 |
| Survivors | 3 [1-5]b,c | 3 [1-7]d,e | 7 [4-15] | 8 [4-13] | <0.001 |
| Hospital | 10 [6-17]b,c | 9 [5-18]d | 14 [7-28]f | 8 [1-18] | <0.001 |
| Non-survivors | 12 [6-23]b,c | 9 [3-22]e | 9 [3-19]f | 2 [1-9] | <0.001 |
| Survivors | 10 [6-17]b,c | 10 [7-17]d>,e | 19 [11-33] | 17 [11-27] | <0.001 |
APACHE - Acute Physiologic Chronic Health Evaluation; SOFA - Sequential Organ Failure Assessment; ICU - intensive care unit. Results expressed as number (%), mean±standard deviation or median (25%-75%).
* Results s expressed in % (confidence interval 95%).
Severe sepsis versus cryptic shock;
severe sepsis versus vasoplegic shock;
severe sepsis versus dysoxic shock;
cryptic shock versus vasoplegic shock;
cryptic shock versus dysoxic shock;
vasoplegic shock versus dysoxic shock.
The patients had an average age of 60 years, with a significant difference between the four groups (p<0.001). The age of the patients in the vasoplegic shock group was higher when compared with those with severe sepsis without hypoperfusion and those with cryptic shock. In total, 47% of the patients were male and the majority of the patients were diagnosed in the emergency room. The vasoplegic shock group exhibited a different pattern and was less often diagnosed in the emergency room when compared with both the severe sepsis without hypoperfusion group and the cryptic shock group. The most frequent site of infection was the lung, followed by the urinary tract and the abdominal tract (Table 1).
At diagnosis, the prevalence of fever, hypothermia, tachycardia, and leukopenia differed among the groups (p<0.001). Fever, hypothermia, and leukopenia were more frequent in the group with severe sepsis without hypoperfusion when compared with the dysoxic shock group. Among patients with severe sepsis, arterial hypotension responsive to volume was present in 49% of patients without hypoperfusion and in 38% of patients with hypoperfusion, respectively. The severity scores of the patients at diagnosis also differed; the APACHE II score ranged from 15±6 in patients with severe sepsis without hypoperfusion to 24±8 in patients with dysoxic shock (p<0.001), with a significantly progressive increase among the four groups (Table 1). With respect to the SOFA score, patients with dysoxic shock exhibited higher scores (10 [8-13]), with no significant difference between patients with severe sepsis without hypoperfusion and patients with cryptic shock (3 [2-5] versus 4 [2-6]; p=0.20, respectively).
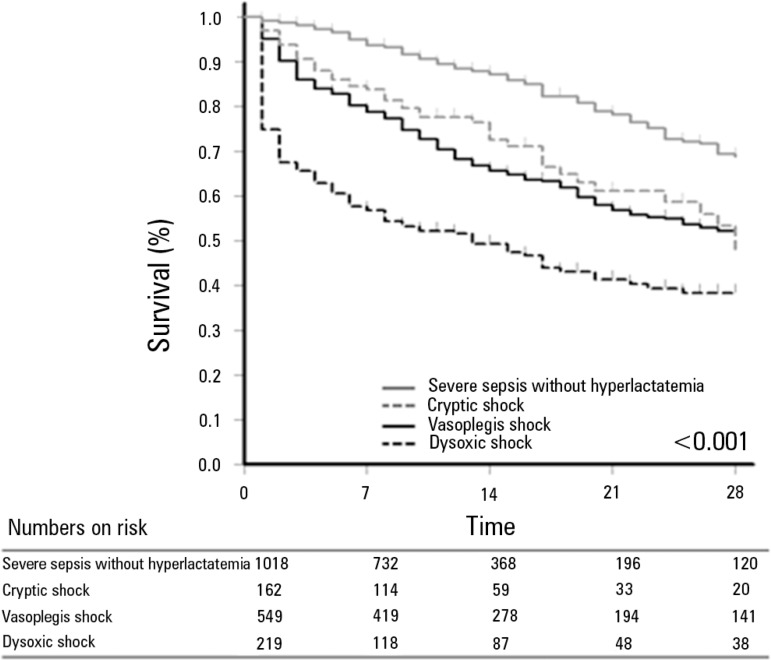
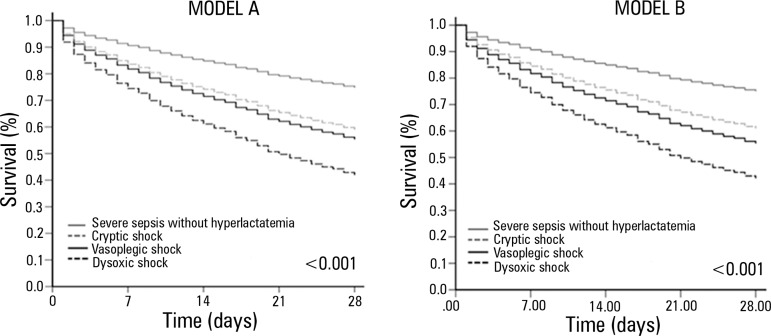
The survival at 28 days differed among the 4 groups (log-rank test: p<0.001, Figure 2). In the analysis of the subgroups, patients from the group with cryptic shock and the group with vasoplegic shock exhibited similar survival (p=0.39), whereas in other groups, the difference was significant (p<0.001). After adjusting for age, APACHE II, and SOFA (Model A, Table 2), the survival difference among the 4 groups persisted (p<0.001), and the dysoxic shock group exhibited increased adjusted risk (Beta=1.095, HR=2.99, 95% CI=2.21-4.05; p<0.001). The results were similar for model B (Table 2). None of the interactions tested were significant, so they were not included in the models. Figure 3 presents the adjusted survival curves of the four groups from models A and B. The hospital mortality was 624 (32%), ranging from 16.8% (95% CI=14.4-19.1) for patients with severe sepsis without hyperlactatemia to 60.3% (95% CI=53.9-67.1) for patients with dysoxic shock.
Figure 2.
Survival curve 28 days after the sepsis diagnosis. A significant difference is observed among the four sepsis phenotypes (log-rank test, p<0.001). In post-hoc comparisons, the survival was different among the four groups, except for the comparison between the cryptic shock group and the vasoplegic shock group (p=0.387).
Table 2.
Survival at 28 days according to the study groups
| Univariate analysis | Model A | Model B | |||||||
| Beta | HR (CI95%) | p value | Beta | HR (CI95%) | p value | Beta | HR (CI95%) | p value | |
| Severe sepsis without hyperlactatemia | Ref. | 1 | <0.001 | Ref. | 1 | <0.001 | Ref. | 1 | <0.001 |
| Cryptic shock | 0.792 | 2.21 (1.59-3.06) | <0.001 | 0.712 | 2.01 (1.47-2.83) | <0.001 | 0.768 | 2.16 (1.54-3.01) | <0.001 |
| Vasoplegic shock | 0.919 | 2.51 (2.03-3.10) | <0.001 | 0.597 | 1.82 (1.41-2.34) | <0.001 | 0.618 | 1.86 (1.43-2.40) | <0.001 |
| Dysoxic shock | 1.522 | 4.58 (3.59-5.84) | <0.001 | 1.095 | 2.99 (2.21-4.05) | <0.001 | 1.171 | 3.23 (2.37-4.39) | <0.001 |
Model A - adjusted for age, APACHE II, and total SOFA. Model B - adjusted for age, APACHE II, SOFA total, place of diagnosis, use of adequate antibiotic treatment within the period (1 hour/3 hours), and the source hospital.
Figure 3.
Adjusted survival curves 28 days after the sepsis diagnosis. Model A is adjusted for age, APACHE II, and SOFA, and model B is adjusted for age, APACHE II, SOFA, early use of antibiotics, place of sepsis diagnosis, and source hospital. In both models, the group with dysoxic shock exhibited a higher hazard ratio when compared with the group with severe sepsis without hyperlactatemia.
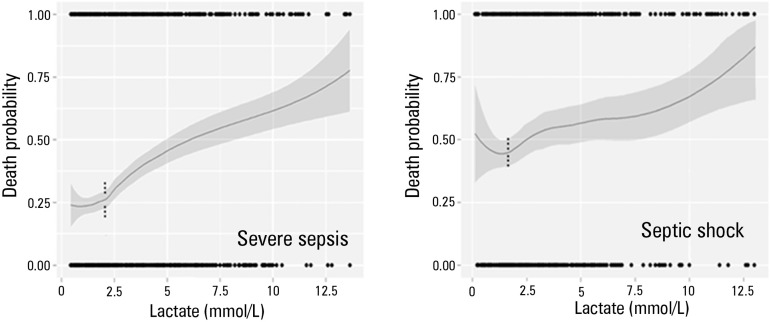
Figure 4 presents the relationship between the continuous values of lactate levels and hospital mortality in patients with a diagnosis of severe sepsis and septic shock separately. Interestingly, the risk of death begins to increase significantly in both groups when the lactate level exceeds 2.0mmol/L. Furthermore, the risk of death is greater among patients with shock.
Figure 4.
Role of intermediate lactate values in patients with severe sepsis and septic shock. An important increase in the risk of death can be observed from the 2 mmol/L value in both groups, although patients with septic shock exhibit a higher risk of death. The risk of death was adjusted by a nonlinear locally weighted function called Locally Weighted Scatterplot Smoothing (LOESS). The gray area corresponds to a confidence interval of 95%.
DISCUSSION
Our results confirm that patients with a clinical diagnosis of severe sepsis or septic shock exhibit variable presentations and outcomes. We demonstrated that the percentage of patients with severe sepsis and hypoperfusion (cryptic shock) at diagnosis is relatively low. Similarly, among patients with septic shock, the number of patients with hypoperfusion (dysoxic shock) is also low. Moreover, the survival at 28 days differed among the groups; survival was lowest in the dysoxic shock group, but similar between patients with cryptic shock and vasoplegic shock, although the prognostic scores differed between the latter two groups. We also demonstrated that among patients with sepsis, the risk of death increases significantly when the initial lactate values are greater than 2mmol/L.
The initial serum lactate level is accepted as a prognostic marker and as a method for evaluating tissue perfusion in several populations of critically ill patients.(10,11,15,28) In both retrospective and prospective studies performed with patients with suspected infection, lactate levels exhibited prognostic value irrespective of the number of organ dysfunctions.(8) Moreover, the initial lactate values are often used for screening, as they are the trigger for the beginning of sepsis resuscitation measures. In the present study, patients with classic severe sepsis and septic shock(3) were divided into new groups according to the serum lactate level. Thus, we add to the current literature the comparison between the four phenotypes of septic patients, given that previous studies analyzed patients with septic shock only with respect to the presence or absence of hypoperfusion(17,18,20) or compared cryptic shock with septic shock without considering the presence or absence of hyperlactatemia in patients with septic shock.(19,21) Among patients with persistent hypotension, we observed that 72% did not exhibit hyperlactatemia, which is higher than has been reported in previous studies (31% and 50%),(17,18,20) although these prior studies involved smaller sample sizes and focused on emergency room patients. Among patients with severe sepsis, only 14% exhibited hyperlactatemia, consistent with the previously described range of 8 to 25%.(8,21)
Occult hypoperfusion has been reported in the literature both in septic patients and in other profiles of critically ill patients.(21,29) The fact that occult hypoperfusion exists and is not diagnosed is unsurprising. The imbalance between the supply and consumption of oxygen is a characteristic of shock conditions, and other markers such as blood pressure, heart rate, are urine output exhibit a low sensitivity of detecting the presence of shock.(21) Thus, even when vital signs are normal, the serum lactate may increase through other mechanisms. This condition is called cryptic shock and is associated with evidence of hypoperfusion, despite the ability of the organism to maintain normal blood pressure with compensatory mechanisms. Moreover, patients with hypotension refractory to volume may also exhibit varying levels of serum lactate, and the relationship between these levels and other clinical parameters is unclear. Hernandez et al.(18) reported that the presence or absence of hyperlactatemia in patients with septic shock did not correlate with age, comorbidities, source of sepsis, or macrohemodynamic parameters, including cardiac output. Conversely, patients with normal levels of lactate exhibited better microcirculation parameters and exhibited a microcirculatory flow close to normal, as evaluated using images of the sublingual microcirculation.(18) Wacharasint et al.(30) also evaluated the importance of intermediate lactate values (cut-off for the Q4 quartile ≥4.4mmol/L) in patients with septic shock. In the original cohort (VASST study), the mean arterial pressure did not differentiate the lactate groups, but the heart rate and central venous pressure was able to differentiate among the groups. In the validation cohort, the heart rate was similar between the groups.(30) In 2013, Sterling et al. analyzed 247 patients with septic shock, dividing them in vasoplegic shock and dysoxic shock, reasserting previous findings that reported no clinical manifestation difference between the groups.(20)
However, in sepsis, tissue hypoxia is not the only factor involved in increasing lactate levels.(31) There are several other possible causes for increased lactate levels, which could potentially explain the absence of clinical manifestations directly related to hyperlactatemia. Mitochondrial dysfunction associated with sepsis can reduce the effective utilization of oxygen by cells despite adequate perfusion, leading to hyperlactatemia associated with normal or even increased venous saturations. This is one of the potential mechanisms of organ dysfunction associated with sepsis. Moreover, lactate clearance is also decreased in septic patients, and increased lactate levels are a result of its decreased metabolism.(32) Additionally, there may be exacerbations of the glycolytic pathway mediated by the use of adrenergic drugs,(11,33) i.e., patients using adrenaline or high doses of noradrenaline may exhibit hyperlactatemia. In the present study, due to the early collection of our samples, this potential cause of increased lactate levels is not likely. Because hyperlactatemia may not reflect reduced tissue supply with hypoperfusion, the prognostic value of increased lactate levels may represent the intrinsic severity of the patient's condition.
The severity scores differed among the four groups for both APACHE II (p<0.001) and SOFA (p<0.001). In the intergroup comparisons, we observed a growing severity continuum from the severe sepsis group without hypoperfusion to the dysoxic shock group, except for SOFA in patients with severe sepsis. The survival at 28 days, both in the univariate and in the adjusted analysis, differed among the four groups. Patients with cryptic shock and vasoplegic shock exhibit similar risks when compared with the group with severe sepsis without hypoperfusion, although patients with vasoplegic shock exhibited higher scores on both scales used in the study. Patients with cryptic shock and vasoplegic shock differed, however, from patients with dysoxic shock for whom the survival was shorter, which suggests greater severity. To our knowledge, this fact is infrequently reported in the literature and may contribute to a better understanding of the disease. Previously, Puskarich et al.(19) reported that the mortality associated with cryptic shock is similar to that associated with the classically defined septic shock (21% versus 19%). Hernandez et al.(17) also observed longer survival times in patients with vasoplegic shock when compared with patients with dysoxic shock (92.3% versus 57.1%).
Guided by the study of Rivers et al.,(34) the current guidelines recommend similar treatment for patients with septic shock and severe sepsis with hypoperfusion using the criterion of initial serum lactate greater than 4mmol/L. Some recent studies demonstrated that even intermediate levels of lactate are associated with an increased risk of unfavorable outcomes in patients with sepsis, suggesting that therapeutic strategies should be established for this group. Indeed, a recent study(13) that used an initial lactate value of 3 mmol/L reported that management guided by lactate clearance and by central venous oxygen saturation is equivalent. In our study, we observed an increased risk of death in patients with sepsis with lactate values greater than 2mmol/L, suggesting that patients with severe sepsis and lactate levels lower than the currently established cutoff must be further studied to develop better therapeutic treatments for this patient population.(30,35)
Our study had limitations that should be emphasized. The present was an observational and retrospective study; thus, there is no information on the etiological diagnosis, inflammatory response, and other important hemodynamic variables of patients with sepsis, such as central venous oxygen saturation and other markers of hypoperfusion, that would better support the findings observed. In addition, we have no data on variables that can interfere with the lactate values, such as the presence of liver disease, the dose of vasoactive drugs, or enzyme deficiencies, which could allow subgroup analyses. The finding of an increased risk of death with lactate values greater than 2mmol/L also suggests that the use of different values to define hyperlactatemia could influence the reported results.
CONCLUSION
In a multicenter study of septic patients, we demonstrated that there are at least four different phenotypes within the two current classic sepsis classifications. The differentiation between the groups is of fundamental epidemiological importance for a possible adaptation of the targeted treatments and can inform better patient selection in future studies.
Footnotes
Conflicts of Interest: None.
REFERENCES
- 1.Silva E, Pedro Mde A, Sogayar AC, Mohovic T, Silva CL, Janiszewski M, Cal RG, de Sousa EF, Abe TP, de Andrade J, de Matos JD, Rezende E, Assunção M, Avezum A, Rocha PC, de Matos GF, Bento AM, Corrêa AD, Vieira PC, Knobel E, Brazilian Sepsis Epidemiological Study Brazilian Sepsis Epidemiological Study (BASES study) Crit Care. 2004;8(4):R251–R260. doi: 10.1186/cc2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva E, Akamine N, Salomao R, Townsend SR, Dellinger RP, Levy M. Surviving sepsis campaign: a project to change sepsis trajectory. Endocr Metab Immune Disord Drug Targets. 2006;6(2):217–222. doi: 10.2174/187153006777442378. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phua J, Koh Y, Du B, Tang YQ, Divatia JV, Tan CC, Gomersall CD, Faruq MO, Shrestha BR, Gia Binh N, Arabi YM, Salahuddin N, Wahyuprajitno B, Tu ML, Wahab AY, Hameed AA, Nishimura M, Procyshyn M, Chan YH, MOSAICS Study Group Management of severe sepsis in patients admitted to Asian intensive care units: prospective cohort study. BMJ. 2011;342: doi: 10.1136/bmj.d3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sogayar AM, Machado FR, Rea-Neto A, Dornas A, Grion CM, Lobo SM, Tura BR, Silva CL, Cal RG, Beer I, Michels V, Safi J, Kayath M, Silva E, Costs Study Group - Latin American Sepsis Institute A multicentre, prospective study to evaluate costs of septic patients in Brazilian intensive care units. Pharmacoeconomics. 2008;26(5):425–434. doi: 10.2165/00019053-200826050-00006. [DOI] [PubMed] [Google Scholar]
- 6.Teles JM, Silva E, Westphal G, Filho RC, Machado FR. Surviving sepsis campaign in Brazil. Shock. 2008;30(Suppl 1):47–52. doi: 10.1097/SHK.0b013e318181a128. Review. [DOI] [PubMed] [Google Scholar]
- 7.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37(5):1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 9.Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg. 1996;171(2):221–226. doi: 10.1016/S0002-9610(97)89552-9. [DOI] [PubMed] [Google Scholar]
- 10.Bakker J, Jansen TC. Don't take vitals, take a lactate. Intensive Care Med. 2007;33(11):1863–1865. doi: 10.1007/s00134-007-0679-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3(1):12. doi: 10.1186/2110-5820-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machado FR, Salomão R, Rigato O, Ferreira EM, Schettino G, Mohovic T, et al. Late recognition and illness severity are determinants of early death in severe septic patients. Clinics (Sao Paulo) 2013;68(5):586–591. doi: 10.6061/clinics/2013(05)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J, LACTATE study group Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 14.Maciel AT, Park M. Unmeasured anions account for most of the metabolic acidosis in patients with hyperlactatemia. Clinics (Sao Paulo) 2007;62(1):55–62. doi: 10.1590/s1807-59322007000100009. [DOI] [PubMed] [Google Scholar]
- 15.Noritomi DT, Soriano FG, Kellum JA, Cappi SB, Biselli PJ, Libório AB, et al. Metabolic acidosis in patients with severe sepsis and septic shock: a longitudinal quantitative study. Crit Care Med. 2009;37(10):2733–2739. doi: 10.1097/ccm.0b013e3181a59165. [DOI] [PubMed] [Google Scholar]
- 16.Park M, Taniguchi LU, Noritomi DT, Libório AB, Maciel AT, Cruz-Neto LM. Clinical utility of standard base excess in the diagnosis and interpretation of metabolic acidosis in critically ill patients. Braz J Med Biol Res. 2008;41(3):241–249. doi: 10.1590/s0100-879x2006005000199. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez G, Castro R, Romero C, de la Hoz C, Angulo D, Aranguiz I, et al. Persistent sepsis-induced hypotension without hyperlactatemia: is it really septic shock? J Crit Care. 2011;26(4):435.e9–435.e14. doi: 10.1016/j.jcrc.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez G, Bruhn A, Castro R, Pedreros C, Rovegno M, Kattan E, et al. Persistent Sepsis-Induced Hypotension without Hyperlactatemia: A Distinct Clinical and Physiological Profile within the Spectrum of Septic Shock. Crit Care Res Pract. 2012;2012: doi: 10.1155/2012/536852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puskarich MA, Trzeciak S, Shapiro NI, Heffner AC, Kline JA, Jones AE, Emergency Medicine Shock Research Network (EMSHOCKNET) Outcomes of patients undergoing early sepsis resuscitation for cryptic shock compared with overt shock. Resuscitation. 2011;82(10):1289–1293. doi: 10.1016/j.resuscitation.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterling SA, Puskarich MA, Shapiro NI, Trzeciak S, Kline JA, Summers RL, Jones AE, Emergency Medicine Shock Research Network (EMSHOCKNET) Characteristics and outcomes of patients with vasoplegic versus tissue dysoxic septic shock. Shock. 2013;40(1):11–14. doi: 10.1097/SHK.0b013e318298836d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33(11):1892–1899. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 22.Noritomi DT, Ranzani OT, Monteiro MB, Ferreira EM, Santos SR, Leibel F, et al. Implementation of a multifaceted sepsis education program in an emerging country setting: clinical outcomes and cost-effectiveness in a long-term follow-up study. Intensive Care Med. 2013 Oct 22; doi: 10.1007/s00134-013-3131-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Latin American Sepsis Institute [Internet] [cited 2012 feb 13]. Available from: http://www.sepsisnet.org/
- 24.Surviving Sepsis Campaign [Internet] [cited 2012.Feb 13]. Available from: http://www.survivingsepsis.org.
- 25.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. Review. [DOI] [PubMed] [Google Scholar]
- 26.Ranzani OT, Battaini LC, Moraes CE, Prada LF, Pinaffi JV, Giannini FP, et al. Outcomes and organ dysfunctions of critically ill patients with systemic lupus erythematosus and other systemic rheumatic diseases. Braz J Med Biol Res. 2011;44(11):1184–1193. doi: 10.1590/s0100-879x2011007500132. [DOI] [PubMed] [Google Scholar]
- 27.R Development Core Team . R: a language and environment for statistical computing. Viena, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 28.Maciel AT, Noritomi DT, Park M. Metabolic acidosis in sepsis. Endocr Metab Immune Disord Drug Targets. 2010;10(3):252–257. doi: 10.2174/187153010791936900. Review. [DOI] [PubMed] [Google Scholar]
- 29.Park M, Maciel AT, Noritomi DT, Brunialti MK, Salomão R, Schettino GP, et al. Is persistent hypotension after transient cardiogenic shock associated with an inflammatory response? Braz J Med Biol Res. 2008;41(8):648–656. doi: 10.1590/s0100-879x2008000800002. [DOI] [PubMed] [Google Scholar]
- 30.Wacharasint P, Nakada TA, Boyd JH, Russell JA, Walley KR. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012;38(1):4–10. doi: 10.1097/SHK.0b013e318254d41a. [DOI] [PubMed] [Google Scholar]
- 31.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. Pt 1J Physiol. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levraut J, Ciebiera JP, Chave S, Rabary O, Jambou P, Carles M, et al. Mild hyperlactatemia in stable septic patients is due to impaired lactate clearance rather than overproduction. Pt 1Am J Respir Crit Care Med. 1998;157(4):1021–1026. doi: 10.1164/ajrccm.157.4.9705037. [DOI] [PubMed] [Google Scholar]
- 33.Mizock BA, Falk JL. Lactic acidosis in critical illness. Crit Care Med. 1992;20(1):80–93. doi: 10.1097/00003246-199201000-00020. [DOI] [PubMed] [Google Scholar]
- 34.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 35.Song YH, Shin TG, Kang MJ, Sim MS, Jo IJ, Song KJ, et al. Predicting factors associated with clinical deterioration of sepsis patients with intermediate levels of serum lactate. Shock. 2012;38(3):249–254. doi: 10.1097/SHK.0b013e3182613e33. [DOI] [PubMed] [Google Scholar]