Abstract
Hyperglycemia is a commonly encountered issue in critically ill patients in the intensive care setting. The presence of hyperglycemia is associated with increased morbidity and mortality, regardless of the reason for admission (e.g., acute myocardial infarction, status post-cardiovascular surgery, stroke, sepsis). However, the pathophysiology and, in particular, the treatment of hyperglycemia in the critically ill patient remain controversial. In clinical practice, several aspects must be taken into account in the management of these patients, including blood glucose targets, history of diabetes mellitus, the route of nutrition (enteral or parenteral), and available monitoring equipment, which substantially increases the workload of providers involved in the patients' care. This review describes the epidemiology, pathophysiology, management, and monitoring of hyperglycemia in the critically ill adult patient.
Keywords: Hyperglycemia, Glucose, Critical care, Diabetes mellitus, Myocardial infarction
INTRODUCTION
Despite advances in the management of patients with diabetes mellitus (DM), this population still has a poorer prognosis after ischemic events compared with nondiabetic patients. Hyperglycemia is a common issue in critically ill patients, even in the absence of pre-existing DM, and is associated with increased morbidity and mortality.(1) Stress hyperglycemia can be defined as a blood glucose level >140 mg/dL without a previous history of diabetes or glycated hemoglobin (HbA1c) >6.5%.(2) Although the Diabetes Insulin Glucose in Acute Myocardial Infarction (DIGAMI) trial and studies conducted in Leuven demonstrated the benefit of intensive glucose control in critically ill patients, later studies failed to replicate these findings.(3-5)
The objective of the present review is to analyze various aspects related to glucose control in the critically ill patient and to define the optimal management of hyperglycemia in these patients.
EPIDEMIOLOGY
It is difficult to define the incidence of acute hyperglycemia, which may vary from 40-90%, depending on the threshold used to define abnormal levels of glucose.(1,6,7) Hyperglycemia in the critical care setting is associated with a poor prognosis in patients with no history of DM. This association is well documented for both admissions and the mean glucose level during the hospital stay.(6,7) In a prospective cohort study that evaluated patients with community-acquired pneumonia, increased blood glucose levels on admission were associated with increased mortality in patients with no history of diabetes.(8)
The worldwide prevalence of diabetes is 2.8%. This rate increases to approximately 15-30% among critically ill patients.(1,2) In patients with pre-existing DM, the presence of hyperglycemia has not been consistently associated with a worse prognosis.(3,4) Patients with diabetes exhibited increased mortality in a cohort of patients with community-acquired pneumonia, but this outcome was not influenced by the levels of glucose on admission.(8)
PATHOGENESIS
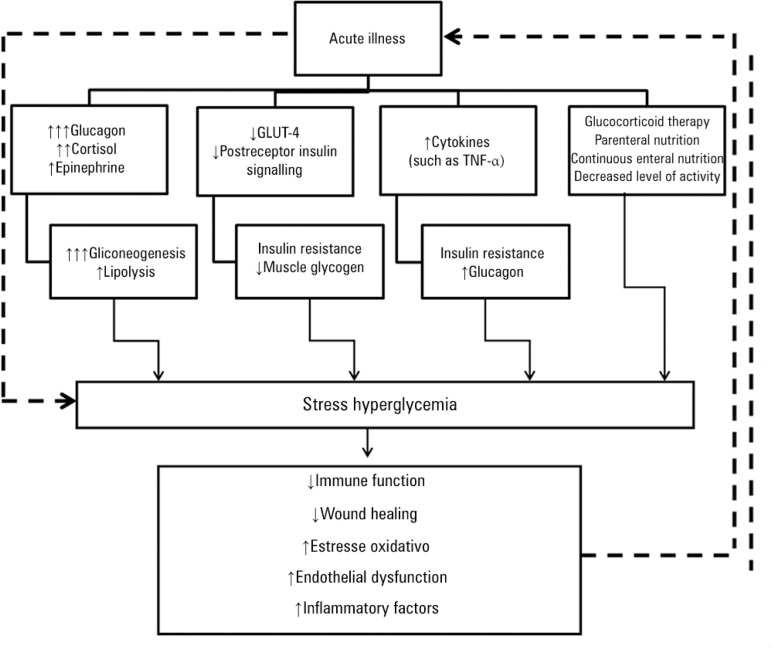
Hyperglycemia may be an independent determinant of the prognosis of critically ill patients or only a marker of disease severity. The mechanisms underlying the development of hyperglycemia in critical illness include a release of counter-regulatory stress hormones (corticosteroids and catecholamines) and proinflammatory mediators and the administration of exogenous corticosteroids, vasopressors, and parenteral solutions containing dextrose. Gluconeogenesis, with glucagon as the prime mediator (but also cortisol and epinephrine), seems to be the most important contributor to stress hyperglycemia.(5,6) Critical illness also deranges the immune system and the inflammatory response. This response becomes nonspecific, causing oxidative stress, mitochondrial dysfunction, cell death, and tissue injury and ultimately leading to organ failure (Figure 1).(5-7)
Figure 1.
Mechanism of stress hyperglycemia.
BENEFITS OF INTENSIVE GLUCOSE CONTROL IN THE INTENSIVE CARE UNIT SETTING
A series of clinical trials has been conducted to ascertain the benefits of achieving strict blood glucose control targets in critically ill patients (Table 1).(8-10)
Table 1.
Findings of the main clinical trials designed to assess the efficacy of intensive glucose management in the intensive care unit setting
| Trial | Leuven 1 (N=1,548) | Leuven 2 (N=1,200) | NICE-SUGAR (N=6,104) |
|---|---|---|---|
| ICU type | Surgical | Medical | Medical/Surgical |
| CG target (mg/dL) | 180-215 | 180-215 | 140-180 |
| IG target (mg/dL) | <110 | <110 | <108 |
| Diabetes, n (%) | 204 (13) | 203 (17) | 1211(20) |
| Monitoring | Gas analyzer | Gas analyzer, HemoCue | Gas analyzer, laboratory tests, glucose meter |
| Glucose measurement | Arterial | Arterial | Arterial/Capillary* |
| Parenteral nutrition (%) | 87 | 87 | 29.5 |
| Energy intake (24 hours, Kcal) | 1,100 | 1,100 | 800 |
| Insulin protocol | Central line | Central line | Any route |
| Mortality (%) | 4.6 in IG versus 8 in CG (p<0.04) | 37.3 in IG versus 40 in CG (p=0.33) | 27.5 in IG versus 24.9 in CG (p=0.02) |
| Hypoglycemia (%) | 5 in IG versus 0.8 in CG | 18.1 in IG versus 3.1 in CG | 6.8 in IG versus 0.5 in CG |
ICU - intensive care unit; IG - intervention group; CG - control group.
In the NICE-SUGAR trial, blood glucose was measured in arterial samples whenever possible, and capillary blood glucose measurement was actively discouraged.
In a Belgian clinical trial conducted in the city of Leuven, the achievement of a strictly normoglycemic target range (blood glucose 80-110mg/dL) by intravenous insulin administration led to a 32% reduction in mortality compared with more flexible glucose control (target range 180-215mg/dL) in a surgical intensive care unit (ICU) setting.(8) The same team of investigators conducted a similar trial in patients admitted to a medical ICU and found a reduction in mortality only among patients who stayed in the ICU for more than three days.(9) However, in this study, there was no difference in overall mortality. Additionally, in a subgroup of patients staying in the ICU for less than three days, mortality was higher in the intensive-treatment group than in the conventional-treatment group (hazard ratio 1.09; p=0.05).(9)
The Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial, the largest randomized trial conducted to date with this objective, compared two insulin-based glucose control strategies (target blood glucose <180mg/dL in the control group versus a target range of 81-108mg/dL in the intervention group) in a sample of 6104 ICU patients.(10) In this trial, intensive glucose control was associated with increased cardiovascular mortality, with an absolute difference of 5.8%.(10) A series of meta-analysis conducted after the NICE-SUGAR trial found no benefit for intensive glucose control and confirmed that this strategy is associated with an increased risk of hypoglycemia.(11-14) One factor that may explain this difference in findings is the amount of energy provided by parenteral nutrition in the Belgian study, which provided significantly greater caloric intake compared with the NICE-SUGAR trial. A metaregression analysis demonstrated that there is a significant relationship between the treatment effect (28-day mortality) and the proportion of calories provided parenterally, suggesting that strict glucose control may be beneficial when parenteral nutrition is particularly energy rich.(11)
SPECIFIC SITUATIONS
Postoperative period
In a study of 263 patients undergoing vascular surgery, intensive glucose control was associated with a reduction in the composite endpoint of all-cause death, myocardial infarction, and acute heart failure.(15) Moderately strict blood glucose control (target range 110-150mg/dl) throughout the hospital stay, added to the usual standard of care in patients undergoing heart surgery, was associated with a 6% reduction in infection rates and a 12% reduction in atrial fibrillation, with no between-group differences in mortality.(16) However, other studies have failed to show any benefit, even in this subgroup of patients.(14)
Neurocritical care
In a study of 933 patients with an admission diagnosis of stroke, strict glucose control was not beneficial in reducing mortality or improving neurological outcomes. However, this study was ended prematurely due to difficulties in enrollment, thus limiting its statistical power.(17) These findings were replicated in a later study, which compared aggressive blood glucose control (target range <130mg/dL) with conventional control (target <200mg/dL) in 46 patients with ischemic stroke.(18) However, in another study of acute ischemic stroke patients who developed hyperglycemia and did not have a previous history of diabetes, intensive glucose control was associated with improved 30-day neurological performance, as measured by the National Institutes of HealthStroke Scale (NIHSS) score, compared with performance following conventional blood glucose control.(19) In a meta-analysis that included studies on only neurocritical patients, strict glycemic control had no impact in mortality, although a less strict glycemic target (140-180mg/dL) was associated with fewer unfavorable neurological outcomes.(20)
Myocardial infarction
It is not known whether intensive blood glucose control is associated with better outcomes in patients with acute myocardial infarction (AMI). In the DIGAMI trial, patients were randomized to receive either an insulin/glucose infusion during the first 24 hours after admission, followed by subcutaneous administration of intermediate- and short-acting insulin four times daily for at least 3 months, or standard DM treatment at the discretion of their care providers. High cardiac risk was defined as meeting two or more of the following criteria: age ≥70 years, a history of previous AMI, a history of congestive heart failure, and ongoing digitalis treatment. The patients were classified into four predefined strata according to their history of insulin use and their cardiac risk: 1, no insulin and low risk; 2, insulin and low risk; 3, no insulin and high-risk; and 4, insulin and high risk. All other aspects of AMI management were similar between the two groups. Although patients in the intervention group (who received the insulin/glucose infusion) had a slight reduction in in-hospital mortality (9.1% versus 11.1%; nonsignificant) and 3-month mortality (12.4% versus 15.6%; nonsignificant), only 1-year mortality was significantly lower in the intervention group (18.6% versus 26.1%; relative mortality reduction, 28%; 95% CI, 8-45%), questioning whether the benefit was due to acute management in the ICU or to later intensive control. An analysis of mortality in the pre-stratified risk groups showed that the greatest reduction occurred among patients with no prior insulin treatment. In this group, the relative reduction in mortality was 51% (19-70%; p=0.004) at the 1-year follow-up.(21)
A meta-analysis of 11 randomized clinical trials including over 23,000 AMI patients showed no benefit for the use of intensive glucose control protocols.(22)
SEPSIS
The relationship between glycemic control and the severity of sepsis was analyzed in a cohort of 191 patients treated with intensive glucose control (target of 80-140mg/dL). The researchers concluded that among patients with severe sepsis or septic shock, the risk of hypoglycemia and hyperglycemia was higher.(23) In a multicenter randomized trial (the Efficacy of Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP study), conventional therapy was compared with intensive insulin therapy, and fluids for resuscitation (10% pentastarch versus modified Ringer's lactate) were also compared.(24) There was no benefit for strict glucose control in patients with severe sepsis, and the trial was stopped early for safety reasons, given the high rate of hypoglycemia.(24) In the NICE-SUGAR trial, an analysis of subgroups did not show any improvement in mortality in patients with severe sepsis.(10) The Surviving Sepsis Campaign Guidelines recommend starting insulin therapy after two consecutive blood glucose measurements above 180mg/dl and an upper target level ≤180mg/dL.(25)
IMPACT OF BLOOD GLUCOSE VARIABILITY ON THE CRITICALLY ILL PATIENT
The administration of vasopressors, corticosteroids, and enteral and parenteral nutrition, as well as the discontinuation of this therapy due to a variety of procedures, leads to significant variability in blood glucose levels.(26)
Retrospective studies have shown a relationship between increased blood glucose variability and increase mortality.(26,27) A retrospective analysis of the Leuven dataset showed that patients with the greatest blood glucose fluctuations had the worst outcomes, regardless of allocation within the study.(27)
The optimal method to measure the amplitude of blood glucose levels is not defined. In a systematic review, 13 different indicators were reported, without a clear definition of the best method for the assessment of glycemic variability.(28) However, a prospective cohort study evaluated the standard deviation and mean amplitude of the glycemic index, the absolute glucose change per hour, and the glycemic lability index. The standard deviation was the only measure that was consistently associated with hospital mortality.(29)
Further clinical trials are required to determine whether the use of indices of glucose control variability in critically ill patients can reduce morbidity and mortality.
IMPACT OF PRIOR GLUCOSE CONTROL ON ACUTE HYPERGLYCEMIA
A need for an assessment of baseline blood glucose prior to ICU admission by HbA1c measurement has been suggested, particularly in patients with no history of DM.(30) In hospitalized patients with random admission, hyperglycemia with HbA1c >6% had a specificity of 100% for the diagnosis of diabetes and a sensibility of 57%.(31)
Acute hyperglycemia does not appear to be a marker of mortality in critically ill patients with pre-existing DM.(4)
In a retrospective study, pre-existing hyperglycemia affected the relationship between acute blood glucose levels and mortality, suggesting a significant interaction between chronic and acute glycemic control.(4)
The importance of glucose variability in critically ill patients with DM is also unclear. There is no association between high glucose variability and mortality in diabetic critically ill patients.(32,33) Patients with DM also seem to tolerate lower glucose levels than do non-DM patients. In retrospective cohort, Sechterberger et al. reported that the cutoffs for detrimentally low glucose levels were 63mg/dL in DM and 88mg/dl in the absence of DM.(32)
HbA1c levels were shown to be predictive of mortality in a study of diabetic patients with sepsis.(34) This finding was not replicated in a later study conducted in the ICU of Hospital de Clínicas de Porto Alegre, Porto Alegre (RS), Brazil.(35)
GLUCOSE MONITORING IN THE CRITICALLY ILL PATIENT
In critically ill patients, who are usually in a hypercatabolic state, capillary recruitment is increased. As these patients also have poor peripheral perfusion, the proportion of glucose reaching the periphery is even lower, increasing the efficiency of capillary glucose uptake. Consequently, capillary blood glucose measurements become less representative of the arterial and central compartments' glucose levels.(36)
Real-time continuous glucose monitoring is a new technology that may provide important additional information on trends and fluctuations in glucose control. This method may predict progression to hypoglycemia and hyperglycemia, thus aiding in the clear determination of insulin dose adjustments and reduction in blood glucose variability, a factor that has been independently associated with mortality in critically ill patients.(26,27)
This method, known as the Continuous Glucose Monitoring System (CGMS), is based on a sensor placed in the subcutaneous tissue. The sensor measures the glucose level of the interstitial fluid every 10 seconds and sends the results to a monitor, which then calculates the average glucose level every 5 minutes.(26) The CGMS has been assessed in critically ill patients and has been found to correlate adequately (r=0.89) with arterial blood glucose measurements.(27) A comparative study of conventional monitoring compared with CGMS in critically ill patients found a 9.9% reduction in the absolute risk of hypoglycemia with the continuous monitoring method.(37)
In a small pilot study, the use of continuous glucose measurement by microdialysis in a central vein had a high correlation with arterial plasma values.(38) This potentially useful technique still needs further studies to prove its utility in clinical practice.
FINAL CONSIDERATION
Glucose control in the intensive care unit adds yet another facet to the routine care of a highly complex patient profile. Although observational studies and certain interventional trials have suggested that intensive glucose control can reduce mortality in this setting, recent studies have failed to confirm these findings.
The contradictory findings of the existing literature may be explained by differences in critically ill patients' profiles and by the management of hyperglycemia using different intensive care unit protocols. Patients with diabetes mellitus, for instance, appear to derive less benefit from strict glucose control. Conversely, patients receiving parenteral nutrition may benefit from more intensive control. The impact of the higher incidence of hypoglycemia secondary to intensive glucose control has yet to be defined in critically ill patients; hypoglycemia may simply be a marker of disease severity or may be directly associated with adverse events in these patients.
Therefore, current evidence shows that an optimal glucose target does not exist for all critically ill patients and that a target should be determined on a case-by-case basis. Furthermore, the methods used to achieve proposed glucose targets are often ineffective, as demonstrated by the NICE-SUGAR trial, in which less than 50% of patients in the intervention group reached the predefined target. The use and standardization of new glucose monitoring methods may help patients to reach the desired glucose levels, perhaps with greater safety. The authors suggest a target blood glucose range of 140-180mg/dl, as used in the control group of the NICE-SUGAR trial. Furthermore, the best available method for blood glucose monitoring should be used in the care of critically ill patients with hyperglycemia.
ACKNOWLEDGMENTS
Funding grant: Fundo de Incentivo a Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre (FIPE-HCPA).
AR Fabrin and MF Santos received undergraduate research grants from Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and the Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq), respectively.
Footnotes
Conflicts of interest: None.
REFERÊNCIAS
- 1.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, et al. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36(8):2249–2255. doi: 10.1097/CCM.0b013e318181039a. [DOI] [PubMed] [Google Scholar]
- 2.Smith FG, Sheehy AM, Vincent JL, Coursin DB. Critical illness-induced dysglycaemia: diabetes and beyond. Crit Care. 2010;14(6):327. doi: 10.1186/cc9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P. Clinical Guidelines Committee of the American College of Physicians. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;154(4):260–267. doi: 10.7326/0003-4819-154-4-201102150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, et al. The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit Care Med. 2011;39(1):105–111. doi: 10.1097/CCM.0b013e3181feb5ea. [DOI] [PubMed] [Google Scholar]
- 5.Mizock BA. Alterations in fuel metabolism in critical illness: hyperglycaemia. Best Pract Res Clin Endocrinol Metab. 2001;15(4):533–551. doi: 10.1053/beem.2001.0168. [DOI] [PubMed] [Google Scholar]
- 6.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355(18):1903–1911. doi: 10.1056/NEJMcp060094. [DOI] [PubMed] [Google Scholar]
- 8.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 9.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 10.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 11.Marik PE, Preiser JC. Toward understanding tight glycemic control in the ICU: a systematic review and metaanalysis. Chest. 2010;137(3):544–551. doi: 10.1378/chest.09-1737. [DOI] [PubMed] [Google Scholar]
- 12.Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821–827. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ling Y, Li X, Gao X. Intensive versus conventional glucose control in critically ill patients: a meta-analysis of randomized controlled trials. Eur J Intern Med. 2012;23(6):564–574. doi: 10.1016/j.ejim.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Kansagara D, Fu R, Freeman M, Wolf F, Helfand M. Intensive insulin therapy in hospitalized patients: a systematic review. Ann Intern Med. 2011;154(4):268–282. doi: 10.7326/0003-4819-154-4-201102150-00008. Review. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniam B, Panzica PJ, Novack V, Mahmood F, Matyal R, Mitchell JD, et al. Continuous perioperative insulin infusion decreases major cardiovascular events in patients undergoing vascular surgery: a prospective, randomized trial. Anesthesiology. 2009;110(5):970–977. doi: 10.1097/ALN.0b013e3181a1005b. [DOI] [PubMed] [Google Scholar]
- 16.Leibowitz G, Raizman E, Brezis M, Glaser B, Raz I, Shapira O. Effects of moderate intensity glycemic control after cardiac surgery. Ann Thorac Surg. 2010;90(6):1825–1832. doi: 10.1016/j.athoracsur.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 17.Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, Bamford JM, James OF, Alberti KG, GIST Trialists Collaboration Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;6(5):397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- 18.Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, et al. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008;39(2):384–389. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]
- 19.Staszewski J, Brodacki B, Kotowicz J, Stepien A. Intravenous insulin therapy in the maintenance of strict glycemic control in nondiabetic acute stroke patients with mild hyperglycemia. J Stroke Cerebrovasc Dis. 2011;20(2):150–154. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Kramer AH, Roberts DJ, Zygun DA. Optimal glycemic control in neurocritical care patients: a systematic review and meta-analysis. Crit Care. 2012;16(5):R203. doi: 10.1186/cc11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malmberg K, Norhammar A, Wedel H, Rydén L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99(20):2626–2632. doi: 10.1161/01.cir.99.20.2626. [DOI] [PubMed] [Google Scholar]
- 22.Zhao YT, Weng CL, Chen ML, Li KB, Ge YG, Lin XM, et al. Comparison of glucose-insulin-potassium and insulin-glucose as adjunctive therapy in acute myocardial infarction: a contemporary meta-analysis of randomised controlled trials. Heart. 2010;96(20):1622–1626. doi: 10.1136/hrt.2010.194563. [DOI] [PubMed] [Google Scholar]
- 23.Waeschle RM, Moerer O, Hilgers R, Herrmann P, Neumann P, Quintel M. The impact of the severity of sepsis on the risk of hypoglycaemia and glycaemic variability. Crit Care. 2008;12(5):R129. doi: 10.1186/cc7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K, German Competence Network Sepsis (SepNet) Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 25.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 26.Ali NA, O'Brien JM, Jr, Dungan K, Phillips G, Marsh CB, Lemeshow S, et al. Glucose variability and mortality in patients with sepsis. Crit Care Med. 2008;36(8):2316–2321. doi: 10.1097/CCM.0b013e3181810378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyfroidt G, Keenan DM, Wang X, Wouters PJ, Veldhuis JD, Van den Berghe G. Dynamic characteristics of blood glucose time series during the course of critical illness: effects of intensive insulin therapy and relative association with mortality. Crit Care Med. 2010;38(4):1021–1029. doi: 10.1097/CCM.0b013e3181cf710e. [DOI] [PubMed] [Google Scholar]
- 28.Eslami S, Taherzadeh Z, Schultz MJ, Abu-Hanna A. Glucose variability measures and their effect on mortality: a systematic review. Intensive Care Med. 2011;37(4):583–593. doi: 10.1007/s00134-010-2129-5. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meynaar IA, Eslami S, Abu-Hanna A, van der Voort P, de Lange DW, de Keizer N. Blood glucose amplitude variability as predictor for mortality in surgical and medical intensive care unit patients: a multicenter cohort study. J Crit Care. 2012;27(2):119–124. doi: 10.1016/j.jcrc.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Kavanagh BP, McCowen KC. Clinical practice. Glycemic control in the ICU. N Engl J Med. 2010;363(26):2540–2546. doi: 10.1056/NEJMcp1001115. Erratum in. N Engl J Med. 2011;364(12):1182. [DOI] [PubMed] [Google Scholar]
- 31.Greci LS, Kailasam M, Malkani S, Katz DL, Hulinsky I, Ahmadi R, et al. Utility of HbA(1c) levels for diabetes case finding in hospitalized patients with hyperglycemia. Diabetes Care. 2003;26(4):1064–1068. doi: 10.2337/diacare.26.4.1064. [DOI] [PubMed] [Google Scholar]
- 32.Sechterberger MK, Bosman RJ, Oudemans-van Straaten HM, Siegelaar SE, Hermanides J, Hoekstra JB, et al. The effect of diabetes mellitus on the association between measures of glycaemic control and ICU mortality: a retrospective cohort study. Crit Care. 2013;17(2):R52. doi: 10.1186/cc12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krinsley JS. Glycemic variability and mortality in critically ill patients: the impact of diabetes. J Diabetes Sci Technol. 2009;3(6):1292–1301. doi: 10.1177/193229680900300609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gornik I, Gornik O, Gasparovic V. HbA1c is outcome predictor in diabetic patients with sepsis. Diabetes Res Clin Pract. 2007;77(1):120–125. doi: 10.1016/j.diabres.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Viana MV, Moraes RB, Fabbrin AR, Santos MF, Vieira SR, Canani LH, et al. Estimativa da frequência de diabetes melito através de seu diagnóstico pelos níveis de hemoglobina glicada e sua relação com escore de gravidade e mortalidade na unidade de terapia intensiva. Rev Bras Ter Intensiva. 2011;(Supl 1):S21. [Google Scholar]
- 36.Fahy BG, Coursin DB. Critical glucose control: the devil is in the details. Mayo Clin Proc. 2008;83(4):394–397. doi: 10.4065/83.4.394. [DOI] [PubMed] [Google Scholar]
- 37.Holzinger U, Warszawska J, Kitzberger R, Wewalka M, Miehsler W, Herkner H, et al. Real-time continuous glucose monitoring in critically ill patients: a prospective randomized trial. Diabetes Care. 2010;33(3):467–472. doi: 10.2337/dc09-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blixt C, Rooyackers O, Isaksson B, Wernerman J. Continuous on-line glucose measurement by microdialysis in a central vein. A pilot study. Critical Care. 2013;17(3):R87. doi: 10.1186/cc12713. [DOI] [PMC free article] [PubMed] [Google Scholar]