Abstract
Purpose.
To characterize visual field (VF) loss at the baseline visit and to evaluate VF quality control (QC) procedures in the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT).
Methods.
The Visual Field Reading Center (VFRC) evaluated 660 baseline VFs (1320 hemifields) from 165 enrolled patients. Three readers independently classified each superior and inferior hemifield and identified any abnormalities. A subset (20%) of the hemifields was reread to evaluate within- and between-reader agreements. The QC system addressed test parameters, patient data, and shipment errors.
Results.
The majority (60%) of the baseline hemifields consisted of localized nerve fiber bundle-type VF loss. Approximately one-third (31.5%) of all the classifications consisted of partial arcuate defects combined with an enlarged blind spot, making this the most common type of hemifield classification. Inferior hemifield loss was greater than superior loss for both study and nonstudy eyes. Reader agreements were >90% for both inferior and superior hemifields for two out of three readers. Test–retest reliability agreement for individual readers was 95% for both hemifields. There were few QC errors with only 5.48 error points per 100-point VF.
Conclusions.
The most common type of IIHTT baseline hemifield abnormality was a localized nerve fiber bundle-like defect. Localized inferior hemifield loss was more common than superior hemifield loss. Quality control and within- and between-reader agreement were excellent for the IIHTT (ClinicalTrials.gov number, NCT01003639).
Keywords: visual fields, intracranial idiopathic hypertension, optic neuropathy, quality control
The VFRC at the University of California-Davis was to provide consistent expert analysis and interpretation of visual field data and to ensure high-quality data for subjects participating in a clinical trial. The latter role was accomplished through the use of technician certification and QC.
Introduction
Idiopathic intracranial hypertension (IIH) is a disorder of elevated intracranial pressure (ICP) of unknown cause.1–4 A variety of pharmacologic and surgical procedures have been suggested to preserve vision, but all these investigations were uncontrolled, nonrandomized, and/or retrospective.5 Well-designed prospective randomized placebo-controlled clinical trials for IIH have not been performed. The Idiopathic Intracranial Hypertension Treatment Trial (IIHTT) is a multicenter, double-blind, randomized, placebo-controlled study seeking to evaluate the efficacy of a weight-reduction and low-sodium diet plus acetazolamide versus the same diet plus placebo in reducing or reversing visual field loss.6
The roles of the Visual Field Reading Center (VFRC) at the University of California-Davis in this trial were to provide consistent expert analysis and interpretation of visual field data and to ensure high-quality data for subjects participating in a clinical trial. The latter role was accomplished through the use of technician certification and quality control (QC).
In this paper, we describe the baseline visual field abnormality classifications and QC procedures for 165 subjects from 38 sites who underwent eligibility visual field testing for entry to the IIHTT.
Methods
The IIHTT is a multicenter, double-blind, placebo-controlled study with 165 participants who met the modified Dandy criteria for IIH. They were enrolled at 38 Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC) network sites, having met the IIHTT inclusion criteria as previously published.6–9 The IIHTT followed the tenets of the Declaration of Helsinki; informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study; the research was approved by the institutional human experimentation committee or institutional review board (IRB); and a Data Safety and Monitoring Committee was in place to monitor the ethical conduct of the study and the accumulation of data for evidence of adverse and beneficial treatment effects.
Major inclusion criteria included diagnosis of IIH at ages 18 to 60 years, presence of bilateral papilledema, reproducible visual field loss with an average perimetric mean deviation (PMD) of −2 dB to −7 dB, elevated ICP, and no other causes of other elevated ICP. Those meeting entry criteria were randomized to one of two treatment groups, either diet + acetazolamide or the diet + placebo, and were followed for 6 months. The change in PMD from baseline visit to the 6-month visit was the primary outcome variable.
Visual Field Testing Inclusion Criteria
Participants underwent at least two screening visual field examinations at least 30 minutes apart using the Swedish Interactive Threshold Algorithm (SITA) Standard 24-2 test pattern on the Humphrey Field Analyzer (HFA; Carl Zeiss Meditec, Dublin, CA, USA) II perimeter (Model 750). If one of the visual fields was unreliable or there was a large difference in sensitivity and pattern of visual field loss between the two examinations, a third visual field was performed and the two reliable visual fields that were most similar in sensitivity were retained as the baseline perimetric examinations. Since lowering cerebral spinal fluid (CSF) pressure may transiently improve visual field function, performance of at least one set of screening visual fields was required after the lumbar puncture. All visual fields were required (1) to have adequate gaze tracking (fixation monitoring) and meet reliability standards of fixation loss errors < 33% and false-positive errors < 15%, (2) to have reproducible visual loss on both sets of fields, and (3) to have an average PMD between −2 and −7 dB. Since the frequency of false-negative errors increases with increasing visual field damage, we believe that the false-negative response rate can be a misleading indicator of reliability.10,11 Although false-negative rate was not used as a criterion of reliability, it was employed as an indicator of potential difficulties in performing the visual field examination on a particular clinic visit. The eye with the most visual loss based on the worse PMD was designated as the study eye, but both eyes were tested and followed. The two sets of visual fields from the study and nonstudy eyes were averaged for the mean baseline PMD.
Visual field examinations from the same participant that differed in PMD by >4 dB were regarded as too variable, requiring an additional visual field test. If the visual field was unreliable or if pattern of the visual field loss was not reproducible, the visual fields were sometimes repeated at the discretion of the VFRC.
Abnormality Classifications
Two of the authors in this study (JLK and CAJ) characterized the types and severity of visual field defects in the Optic Neuritis Treatment Trial (ONTT) and in the Ocular Hypertension Treatment Trial (OHTS).12,13 As with the ONTT and OHTS, the authors developed a classification system for the IIHTT for the purpose of identifying visual field abnormalities associated with IIH patients observed at baseline. A total of 11 categories representing five general types of IIH visual loss (localized, enlarged blind spot [EBS], diffuse, other, and neurologic-like) were developed to include patterns of visual field loss characteristic of ocular and neurologic-like diseases, as well as patterns that were associated with testing artifact (see Table 1, Supplementary Table S1). The two experienced readers trained a third visual field reader (KEC).
Table 1.
Classification of Visual Field Abnormalities in the IIHTT
| Localized nerve fiber bundle | Listed from least severe to most severe |
| Nasal step (NS) | Limited field loss adjacent to the nasal horizontal meridian with at least 1 abnormal point at or outside 15° on the meridian. One or more points must persist on both the total and pattern deviation plots. |
| Pericentral (Pc) | A relatively small visual field abnormality (2 or more adjacent locations that are outside normal limits) that is outside the papillomacular bundle region and beyond the 9° of fixation, where 1 or more points are within this region and appear on both the total and pattern deviation probability plots. |
| Partial arcuate (Parc) | Visual field loss in the nerve fiber bundle region that extends incompletely from the blind spot to the nasal meridian. The defect is generally contiguous with either the blind spot or the nasal meridian and must include at least 1 abnormal location in the temporal visual field. One or more points must persist on both the total and pattern deviation plots. |
| Arcuate (Arc) | Significant visual field loss in the nerve fiber bundle region, extending across contiguous abnormal points from the blind spot to at least 1 point outside 15° adjacent to the nasal meridian. The majority of the points must persist on both the total and pattern deviation plots. |
| Enlarged blind spot (EBS) | A visual field abnormality in the nerve fiber bundle region that involves at least 1 point at the 0.5% or 1% level or 2 or more points at the P < 5% level, and is contiguous with the blind spot. In addition, the grayscale abnormality will be weighted heavily for this determination. |
| Diffuse | Listed from least severe to most severe |
| Widespread (Wsp) | Diffuse visual field loss that includes all 4 quadrants. The GHT may show a general reduction of sensitivity or the MD must show P < 5%. The PSD must not show a P < 5% value. The majority of abnormal points on the total deviation plot are not abnormal on the pattern deviation plot. |
| Neurologic-like | Chiasmal, retrochiasmal, optic nerve chiasm (listed from least severe to most severe) |
| Vertical step (VS) | Limited visual field loss that respects the vertical meridian and that includes at least 2 abnormal points at or outside 15° along the vertical meridian. One or more points must persist on both the total and pattern deviation plots. |
| Quadrant (Q) | Significant visual field loss throughout an entire quadrant that respects the vertical midline. Essentially all points must have a P < 5% value on the total deviation plot, and 1 or more points must persist on both the total and pattern deviation plots. |
| Other | Listed from least severe to most severe |
| Paracentral (Pc) | A relatively small visual field abnormality that is within 9° of fixation, where 1 or more points are within this region and appear on both the total and pattern deviation probability plots, and is generally not contiguous with the blind spot or the nasal meridian. In particular, it does not involve points outside 15° that are adjacent to the nasal meridian. |
| Superior depression (SD) | Two or more abnormal points in the very superior region. One or more points must persist on both the total and pattern deviation plots. |
| Inferior depression (ID) | Two or more abnormal points in the very inferior region. One or more points must persist on both the total and pattern deviation plots. |
| Partial peripheral rim (PPR) | Generally continuous field loss outside 15°, but not in all quadrants; must have some curvature. One or more points must persist on both the total and pattern deviation plots. |
| Normal | All locations are within normal limits on the total deviation plot. |
The three readers independently evaluated 660 baseline visual fields (1320 superior and inferior hemifields) from the 165 participants and classified visual field abnormalities into the 11 different monocular categories. An abnormal visual field was defined as having a Glaucoma Hemifield Test (GHT) outside of normal limits and/or a pattern standard deviation (PSD) P < 5%. The GHT compares 24-2 perimetry results in 10 regions, with five inferior regions representing mirror images of five corresponding superior regions. Differences between corresponding superior and inferior zones are compared with the differences present in the population of normal controls. The GHT is described as “outside normal limits” when differences between a matched pair of corresponding zones exceed the difference found in 99% of the normal population, or when both members of a pair of zones are more abnormal than those of 99.5% of the individuals within the normative population.14 In addition to the first definition, a secondary definition for the abnormality of each hemifield was created to describe the type and extent of the visual field defect (see Supplementary Table S1). Thus, for a hemifield to be classified as normal, it must not meet any of the criteria for hemifield abnormality as described in Supplementary Table S1.
The superior and inferior hemifields that met abnormality criteria were evaluated separately. Readers assigned classifications to both the superior and inferior hemifields using primarily the total and pattern deviation probability plots, with emphasis on the plot showing the greater number of abnormal points. However, the numeric deviation plots and the grayscale were also evaluated to confirm the appropriateness of the classification. In general, one or more abnormal test locations had to persist on both the total and pattern deviation plots. A final classification was determined by agreement among at least two of the three readers. If three readers did not agree, then the visual fields were adjudicated by group consensus.
After a final classification was made for each visual field, an interreader agreement (agreement among readers for a given hemifield) and test–retest reader agreement (agreement between the first and second reading) were performed. A test–retest reader agreement was determined by reassessing a 20% sample (132 visual fields) from the 660 baseline visual fields. This sample reflected a random distribution of the 11 abnormality classifications. In addition to the agreement among at least two of the three readers, Fleiss' κ statistical measure for multiple raters and multiple categories was computed to summarize agreement in the visual ratings of the inferior and superior hemifields.
Mean deviation (MD) and PSD ranges were determined, and repeated measures models, accounting for correlation of visual fields coming from the same individuals, were used to assess associations with MD index (severity of loss) and with the inferior and superior hemifield classifications.
Technician Certification
Each clinical site was required to have at least two visual field technicians certified for IIHTT perimetry. The certification process involved a telephone session with a VFRC staff member, after which the candidate submitted two sets of visual fields performed on both eyes of two nonstudy participants. Certification was awarded if these data were successfully submitted per the IIHTT VFRC Operations Manual. To maintain certification, a repeat certification session was required for any technician who did not perform an IIHTT visual field for a period of 1 year.
Quality Control Measures
Between January 2010 and November 2012, 118 certified visual field technicians from 38 clinical sites performed 660 baseline visual fields on 165 participants in the IIHTT. To ensure the validity and integrity of all visual field data collected, the VFRC maintained rigorous QC measures. The IIHTT visual field QC system addressed three areas of clinic performance: test parameter errors, patient data errors, and shipment errors (see Supplementary Table S2). Each visual field was graded on a 100-point scale and processed using the 4th Dimension database software (4D, San Jose, CA, USA). Mean test errors (test parameter errors) are the average error points (out of a possible 100 points) generated for visual field test errors (e.g., wrong test, fluctuation off, or foveal threshold off) based on the total number of visual fields received and processed. Mean patient errors (patient data errors) are the average error points generated for visual field patient data errors (e.g., wrong data entry, visual acuity missing, or wrong birth date), and mean shipment errors (e.g., shipped late or incorrectly) are based on the total number of visual fields received and processed. A score was assigned to each QC error, with zero representing a perfect score. Factors that rendered the data unusable for the study (e.g., using the wrong test procedure) had higher point values, whereas those that produced minor errors (e.g., incorrect data entry) had lower point values. The VFRC routinely conducted QC assessments of visual field data by providing continuous feedback regarding technician performance and expertise in the collection and inspection of the data.
Results
Baseline Characteristics
A total of 165 subjects (161 women, 4 men) were enrolled. The average age of enrollees was 29.2 years, with an average of 14.0 years of education. Subject ethnicity included 65% white, 25% black, 2% Native American, and 8% another race (or did not report a race). Only 5% of the subjects identified family members with IIH. Other baseline characteristics for the 165 participants are listed in a previous publication.6
Of the 165 subjects enrolled, 53 (32%) subjects required at least three baseline fields for eligibility determination. The additional fields (retests) were performed due to the previous screening fields having a nonreproducible visual field defect or an unreliable field, or as part of our protocol to perform at least one visual field examination after lumbar puncture. The baseline visual field consisted of the average of two visual field results or (in the case of three baseline visual fields) the average of the two visual fields that were mostly closely matched in terms of severity and pattern of loss.
Visual Field Classification Reader Agreement
Using the complete sample of visual fields, agreement between two of the three readers of ≥91% was obtained for both the superior and inferior hemifields as shown in Table 2. Reader agreement using Fleiss' κ statistic was in the moderate range for both the superior (κ = 0.63, P < 0.001) and the inferior (κ = 0.65, P < 0.001) hemifields. A 20% sample of the 660 baseline fields (132 visual fields) was reassessed for test–retest agreement. Agreement between two of the three readers of 95% was obtained for both the superior and inferior hemifields (Table 2). Fleiss' κ statistics were reduced slightly but were still in the moderate range (superior: κ = 0.53, P < 0.001; inferior: κ = 0.58, P < 0.001). A total of two or more readers were in agreement with the initial (original) reading 81% of the time as exhibited in Table 2. An overall agreement of 81% was considered good agreement, as these hemifields were cases near the boundaries of various classification criteria. Agreement using Fleiss' κ was substantial when the original read was compared to the second retest read for both hemifields (superior: κ = 0.70, P < 0.001; inferior: κ = 0.71, P < 0.001).
Table 2.
Inter- and Intrareader Hemifield Agreement
|
% of Times 2/3 or 3/3 Readers Agreed With Each Other for Initial Read,
n
= 660 |
% of Times 2/3 or 3/3 Readers Agreed With Each Other for Second Read, 20% Retest,
n
= 132 |
% of Times Second Read, Retest, Agreed With the Initial Read,
n
= 132 |
|
| Superior agreement, 2 or more in agreement, no. of times (%) | 628 (95) | 125 (95) | 104 (79) |
| Fleiss' κ | κ = 0.63, P < 0.001 | κ = 0.53, P < 0.001 | κ = 0.70, P < 0.001 |
| Inferior agreement, 2 or more in agreement, no. of times (%) | 597 (91) | 126 (95) | 109 (83) |
| Fleiss' κ | κ = 0.65, P < 0.001 | κ = 0.58, P < 0.001 | κ = 0.71, P < 0.001 |
| Total agreement, 2 or more in agreement, no. of times (%) | 1225 (93) | 251 (95) | 213 (81) |
Visual Field Abnormality Characteristics
Of the 660 baseline fields, 482 (73%) met the primary abnormality criteria listed in Supplementary Table S1 (2a, 2b, or both) with a GHT outside of normal limits or PSD < 5%. A total of 178 (27%) fields had normal GHT and PSD indices but contained at least one hemifield that met the secondary abnormality criteria listed in Supplementary Table S1 (2c–f).
For the 1320 hemifields from both the study and nonstudy eyes at baseline, Table 3 and Supplementary Table S3 show that the majority (60%) of the hemifield abnormalities consisted of localized nerve fiber bundle-type loss. A total of 15.2% were due to an EBS; 5.8% were due to diffuse loss (widespread loss); 1.2% consisted of neurologic-like loss (vertical step and quadrant); 6.1% were due to other abnormalities (paracentral, superior and inferior depression, and partial peripheral rim); and 11.8% of the hemifields were classified as normal.
Table 3.
IIHTT Hemifield Abnormality Classification Frequency for the Study and Nonstudy Eyes
|
Classification |
Study Eye Total |
% |
Nonstudy Eye Total |
% |
Both Eyes Total |
% |
| Localized nerve fiber bundle (NFB) defects, with and without an enlarged blind spot (EBS) | 472 | 71.5 | 318 | 48.2 | 790 | 59.9 |
| EBS, no localized NFB defects | 78 | 11.8 | 123 | 18.6 | 201 | 15.2 |
| Diffuse | 36 | 5.5 | 40 | 6.1 | 76 | 5.8 |
| Neurologic-like | 9 | 1.3 | 7 | 1.1 | 16 | 1.2 |
| Other | 36 | 5.5 | 45 | 6.8 | 81 | 6.1 |
| Normal | 29 | 4.4 | 127 | 19.2 | 156 | 11.8 |
| Total | 660 | 100.0 | 660 | 100.0 | 1320 | 100.0 |
The hemifield abnormality classifications were also evaluated separately for the study and the nonstudy eye. For the 660 hemifields from the study eye at baseline, 71.5% of the hemifield abnormalities consisted of localized nerve fiber bundle-type loss; 11.8% were due to an EBS; 5.5% were due to diffuse loss; 1.3% were due to neurologic-like loss; 5.5% were due to other abnormalities; and 4.4% of the hemifields were classified as normal (Table 3). For the 660 hemifields from the nonstudy eye at baseline, 48.2% of the hemifield abnormalities consisted of localized optic nerve visual field loss; 18.6% were due to an EBS; 6.1% were due to diffuse loss; 1.1% were due to neurologic-like loss; 6.8% were due to other abnormalities; and 19.2% of the hemifields were classified as normal (Table 3).
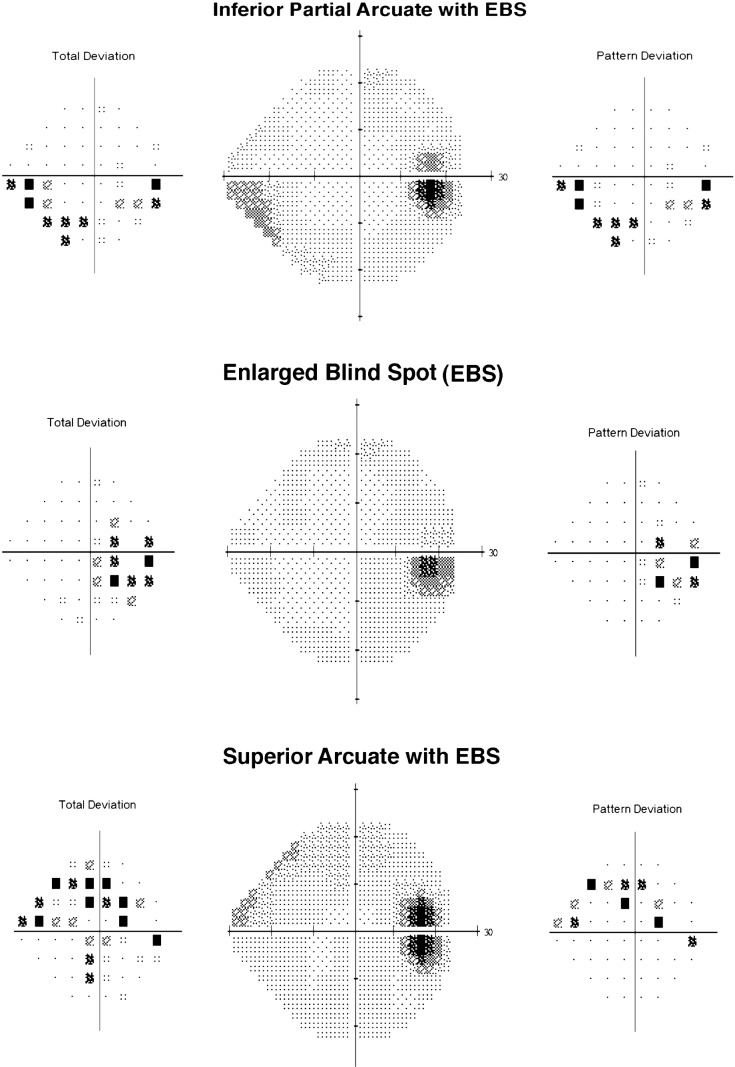
Table 4 shows localized nerve fiber bundle visual field loss (with and without an EBS) as the most common type of abnormality with inferior localized nerve fiber bundle defects greater than superior for both study and nonstudy eye, respectively (P < 0.001). Examples of the most common type of hemifield abnormalities are shown in the Figure.
Table 4.
Visual Field Defects of the Study and Nonstudy Eyes of IIHTT Subjects at Study Entry by Superior and Inferior Hemifield
|
Classification |
Study Eye |
Fellow Eye |
||||||
|
Superior Hemifield |
Inferior Hemifield |
Superior Hemifield |
Inferior Hemifield |
|||||
|
n |
% |
n |
% |
n |
% |
n |
% |
|
| Localized nerve fiber bundle (NFB) defects, with and without an enlarged blind spot (EBS) | 226 | 68.5 | 246 | 74.6 | 151 | 45.8 | 167 | 50.6 |
| EBS, no localized NFB defects | 37 | 11.2 | 41 | 12.4 | 56 | 17 | 67 | 20.3 |
| Diffuse | 19 | 5.8 | 17 | 5.2 | 20 | 6.1 | 20 | 6.1 |
| Neurologic-like | 6 | 1.8 | 3 | <1.0 | 3 | <1.0 | 4 | 1.2 |
| Other | 19 | 5.8 | 17 | 5.2 | 24 | 7.3 | 21 | 6.3 |
| Normal | 23 | 6.9 | 6 | 1.8 | 76 | 23.0 | 51 | 15.5 |
| Total | 330 | 100.0 | 330 | 100.0 | 330 | 100.0 | 330 | 100.0 |
Figure.
Examples of the most common types of IIHTT baseline visual field abnormalities.
Summaries for the MD index are as follows: mean, −2.90 dB; 25th percentile, −3.71 dB; median, −2.74 dB; 75th percentile, −2.04 dB; range, −7.6 to 1.68 dB; 95% confidence interval for the mean (from repeated measures model), −3.06 to 2.75 dB; 5th percentile of the data, −5.36 dB; and 95th percentile of the data, −0.77 dB. Age and race/ethnicity were not significantly associated with the visual field MD index. Severity of visual field classifications was increased in the left eye relative to the right eye (P < 0.001) and in visual fields with defects relative to normal fields (P < 0.001) in both the superior and inferior hemispheres. Thus, the left eyes, which were the majority (62%) of the study eyes, had a greater amount of visual field loss compared to the right eyes. When predicting classifications in either hemifield, there was no difference by eye (P > 0.1), race/ethnicity (P > 0.4), or age (P > 0.2).
Visual Field Quality Control
A total of 214 (32%) of the 660 baseline visual fields were performed without any test, patient data, or shipment errors. The 450 (68%) remaining baseline fields had one or more errors or a combination of errors producing an average of only 5.48 error points per 100-point visual field, reflecting minor QC errors. The findings in Supplementary Table S4 indicate that there was outstanding adherence to the protocol and testing procedures, providing the highest quality of visual field data.
Discussion
Baseline perimetry of subjects in the IIHTT demonstrates that patients with IIH and mild visual loss have optic nerve–related visual field deficits in localized nerve fiber bundle-like patterns (Table 3, Fig.). The inferior hemifields exhibit more abnormalities than the superior hemifields (Table 4), with the most common pattern being a partial inferior arcuate defect with an EBS (Fig.). Isolated central and cecocentral scotomas as a visual field defect were rare at baseline examination in our subjects.
A number of previous investigators have developed systems for classifying the severity and pattern of glaucomatous visual field loss.15–29 Three of the authors (JLK, CAJ, KEC) have reported classifications of visual field defects in the ONTT12 and the OHTS.13 In the ONTT, over 90% of the abnormal baseline visual fields exhibited central and cecocentral scotomas, in addition to over 20% consisting of localized nerve fiber bundle-type loss. At 1 year, over 50% of the abnormal visual field results reverted back to normal. However, of the remaining abnormalities, >25% of the visual field defects still consisted of localized nerve fiber optic nerve loss.12,30,31 In the OHTS, entrance criteria required all baseline visual fields to be normal. Over the 8-year study duration, the same three authors reviewed approximately 40,000 visual fields and determined that only 2500 (6.25%) were abnormal. Also, as one might expect, over 50% of the visual field abnormalities consisted of localized nerve fiber bundle-type loss. This is to be expected in ocular hypertensive patients who eventually convert to glaucoma. Visual acuity and central vision were unaffected in the OHTS patients, in contrast to the ONTT patients. Past studies examining glaucomatous visual loss have reported that approximately 60% of the visual fields contained inferior retinal nerve fiber layer (RNFL) deficits and 40% contained superior RNFL deficits,32 although this finding has been disputed.33,34 When the visual field loss in the IIHTT was compared to that in the OHTS, the loss in patients with IIH was similar to the loss in patients with glaucoma in that similar nerve fiber bundle-like defects were present. However, the frequently present EBS and occasional loss in the central 10° due to neurosensory detachments or choroidal folds are not glaucoma-like.
The IIHTT enrolled patients with IIH and mild visual field loss. Thus the visual field information from this trial cannot be generalized to what might be seen in patients with more severe optic nerve damage from IIH.
However, another study by Wall and George1 prospectively evaluated 50 consecutive IIH patients with both manual Goldmann kinetic and threshold static automated perimetry at each visit and enrolled subjects with all degrees of damage. The IIH patients had all levels of optic nerve damage from none to severe. The authors found visual loss in at least one eye in 96% of patients with Goldmann perimetry and in 92% with automated perimetry. Similar to observations in the IIHTT cohort, they commonly found visual field constriction and loss of inferonasal portions of the visual field with arcuate defects, nasal step defects, and combinations of both. Altitudinal defects were uncommon, and temporal sector defects were rare. Fourteen percent of the patients had central or paracentral defects with automated perimetry. It appears that cases with more severe damage have morphology similar to that seen in the IIHTT.
There is considerable evidence that the optic nerve head is the main site of damage for papilledema based on the patterns of visual loss that are similar to those seen in glaucoma.1 The primary insult in papilledema is a slowing of axonal transport due to elevated CSF pressure that is transmitted down the optic nerve sheath.35 This elevated CSF pressure disturbs the normal gradient between IOP and retrolaminar pressure and results in increased tissue pressure within the optic nerve, interfering with axoplasmic flow and producing flow stasis. Hayreh and Tso36–41 showed that reduction of both slow and fast axoplasmic transport resulted in intra-axonal edema. Ischemia is also a possible contributing factor, as Hayreh showed delays in prelaminar arterial filling on fluorescein angiography.38 We believe that the main mechanism of visual loss in papilledema is likely axoplasmic flow stasis leading to high pressure within the optic nerve head with resultant intraneuronal optic nerve ischemia. Other mechanisms causing visual loss are neurosensory retinal detachments from fluid tracking from the optic disc to the fovea, macular exudates or hemorrhage, hyperopic shifts related to optic nerve sheath-related globe flattening with elevation of peripapillary retina from papilledema (EBS), and choroidal folds.40,41
We found that the eye with the most damage was the left eye in 62% of cases. We are not certain of the reason for this, but preferred sleeping position has been found to correlate with the presence of greater visual field loss (determined by a worse MD) in glaucoma.42 It has been suggested that the left-sided sleeping preference promotes gastric emptying and less gastric discomfort that might be important in obesity. We found no differences in visual field classification group when analyzing by eye, race/ethnicity, or age.
We were able to obtain high-quality perimetric data from the IIHTT. Technicians who are trained and certified in a standard fashion generally produce reliable, high-quality data by reducing errors and have less artifactual data related to testing conditions, patient cooperation, and performance problems.43–45 Sites received frequent feedback from the VFRC; similar feedback in the ONTT was time linked to improvement in QC measures.12 Supplementary Tables S2 and S4 demonstrate minimal testing errors and unreliability rates in the IIHTT for visual field testing. Similar to the situation with ONTT and OHTS, we believe that the use of visual field QC and high-quality study investigators are essential for any clinical trial using perimetry as an outcome measure.
In summary, the majority (60%) of the baseline visual fields contained localized nerve fiber bundle defects. A partial arcuate abnormality combined with an EBS was the most common type of hemifield classification for both the study eye and nonstudy eye. There was a higher occurrence of visual field defects in the inferior hemifield and greater visual field loss in the left eye. Similarly to what has been seen in prior studies, nerve fiber bundle-like defects, similar to glaucoma, are characteristic for optic nerve damage from increased intracranial pressure.
Acknowledgments
The authors acknowledge Laura Leming (Department of Ophthalmology and Vision Science, University of California-Davis, California) for her assistance in processing the visual field data and Danielle Harvey, PhD, for her assistance with the statistical analyses. There was no compensation received for their contributions.
Disclosure: J.L. Keltner, None; C.A. Johnson, None; K.E. Cello, None; M. Wall, None
APPENDIX
Nordic Idiopathic Intracranial Hypertension Study Group
Authors/Writing Committee: The following investigators of the NORDIC Idiopathic Intracranial Hypertension Study Group take authorship responsibility for the study results: Michael Wall, MD; Michael P. McDermott, PhD; Karl D. Kieburtz, MD, MPH; James J. Corbett, MD; Steven E. Feldon, MD; Deborah I. Friedman, MD, MPH; David M. Katz, MD; John L. Keltner, MD; Eleanor B. Schron, PhD; Mark J. Kupersmith, MD
Affiliations of Authors/Writing Committee: University of Iowa, Iowa City, Iowa, United States (Wall); University of Rochester School of Medicine & Dentistry, Rochester, New York, United States (McDermott, Kieburtz); University of Mississippi Medical Center, Jackson, Mississippi, United States (Corbett); University of Rochester Eye Institute, Rochester, New York, United States (Feldon); University of Texas Southwestern Medical Center, Dallas, Texas, United States (Friedman); Bethesda Neurology LLC, Bethesda, Maryland, United States (Katz); University of California-Davis Medical Center, Sacramento, California, United States (Keltner); National Eye Institute, Bethesda, Maryland, United States (Schron); Roosevelt Hospital, New York, New York, United States (Kupersmith).
Footnotes
See the appendix for the IIHTT investigators of the NORDIC Study Group.
References
- 1. Wall M, George D. Idiopathic intracranial hypertension. A prospective study of 50 patients. Brain. 1991; 114: 155–180 [PubMed] [Google Scholar]
- 2. Corbett JJ, Savino PJ, Thompson HS, et al. Visual loss in pseudotumor cerebri: follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982; 39: 461–474 [DOI] [PubMed] [Google Scholar]
- 3. Sorenson PS, Krogsaa B, Gjerris F. Clinical course and prognosis of pseudotumor cerebri. A prospective study of 24 patients. Acta Neurol Scand. 1988; 77: 164–172 [DOI] [PubMed] [Google Scholar]
- 4. Wall M, George D. Visual loss in pseudotumor cerebri: incidence and defects related to visual field strategy. Arch Neurol. 1987; 44: 170–175 [DOI] [PubMed] [Google Scholar]
- 5. Lee AG, Wall M. Papilledema: are we any nearer to a consensus on pathogenesis and treatment? Curr Neurol Neurosci Rep. 2012; 12: 334–339 [DOI] [PubMed] [Google Scholar]
- 6. Wall M, Kupersmith MJ, Kieburtz KD, et al. The Idiopathic Intracranial Hypertension Treatment Trial: clinical profile at baseline. JAMA Neurol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friedman DI, McDermott MP, Kieburtz K, et al. The Idiopathic Intracranial Hypertension Treatment Trial (IIHTT): design considerations and methods. J Neuroophthalmol. In press [DOI] [PubMed] [Google Scholar]
- 8. Smith JL. Whence pseudotumor cerebri? J Clin Neuroophthalmol. 1985; 5: 55–56 [PubMed] [Google Scholar]
- 9. Friedman DI, Rausch EA. Headache diagnoses in patients with treated idiopathic intracranial hypertension. Neurology. 2002; 58: 1551–1553 [DOI] [PubMed] [Google Scholar]
- 10. Bengtsson B, Heijl A. False-negative responses in glaucoma perimetry: indicators of patient performance or test reliability? Am J Ophthalmol. 2000; 130: 689 [DOI] [PubMed] [Google Scholar]
- 11. Bengtsson B, Heijl A. False-negative responses in glaucoma perimetry: indicators of patient performance or test reliability? Invest Ophthalmol Vis Sci. 2000; 41: 2201–2204 [PubMed] [Google Scholar]
- 12. Keltner JL, Johnson CA, Cello KE, Dontchev M, Gal RL, Beck RW; for the Optic Neuritis Study Group. Visual field profile of optic neuritis. A final follow-up report from the Optic Neuritis Treatment Trial from baseline through 15 years. Arch Ophthalmol. 2010; 128: 330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keltner JL, Johnson CA, Cello KE. et al. for the OHTS Study Group. Classification of visual field abnormalities in the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2003; 121: 643–650 [DOI] [PubMed] [Google Scholar]
- 14. Bosworth CF, Sample PA, Johnson CA, Weinreb RN. Current practice with standard automated perimetry. Semin Ophthalmol. 2000; 15: 172–181 [DOI] [PubMed] [Google Scholar]
- 15. Aulhorn E, Harms H. Early visual field defects in glaucoma. In: Twentieth International Congress of Ophthalmology. Munich: S. Karger; 1967: 151–186 [Google Scholar]
- 16. Drance SM. Doyne Memorial Lecture, 1975. Correlation of optic nerve and visual field defects in simple glaucoma. Trans Ophthalmol Soc U K. 1975; 95: 288–296 [PubMed] [Google Scholar]
- 17. Aulhorn E, Karmeyer H. Frequency distribution in early glaucomatous visual field defects. In: Second International Visual Field Symposium. Tubingen: The Hague; 1976: 75–83 [Google Scholar]
- 18. Johnson CA, Keltner JL. Computer analysis of visual field loss and optimization of automated perimetric test strategies. Ophthalmology. 1981; 88: 1058–1065 [DOI] [PubMed] [Google Scholar]
- 19. Hart WM, Becker B. The onset and evolution of glaucomatous visual field defects. Ophthalmology. 1982; 89: 268–279 [DOI] [PubMed] [Google Scholar]
- 20. Flanagan JG, Wild JM, Barnes DA, Gilmartin BA, Good PA, Crews SJ. The qualitative comparative analysis of the visual field using computer assisted, semi-automated and manual instrumentation: III. Clinical analysis. Doc Ophthalmol. 1984; 58: 341–350 [DOI] [PubMed] [Google Scholar]
- 21. Henson DB, Hobley A. Frequency distribution of early glaucomatous visual field defects. Am J Optom Physiol Opt. 1986; 63: 455–461 [DOI] [PubMed] [Google Scholar]
- 22. Asman P, Heijl A, Olsson J, Rootzen H. Spatial analyses of glaucomatous visual fields; a comparison with traditional visual field indices. Acta Ophthalmol (Copenh). 1992; 70: 679–686 [DOI] [PubMed] [Google Scholar]
- 23. Mutlukan E, Keating D. Visual field interpretation with a personal computer based neural network. Eye. 1994; 8: 321–323 [DOI] [PubMed] [Google Scholar]
- 24. Spenceley SE, Henson DB, Bull DR. Visual field analysis using artificial neural networks. Ophthalmic Physiol Opt. 1994; 14: 239–248 [DOI] [PubMed] [Google Scholar]
- 25. Brusini P. Clinical use of a new method for visual field damage classification in glaucoma. Eur J Ophthalmol. 1996; 6: 402–407 [DOI] [PubMed] [Google Scholar]
- 26. Henson DB, Spenceley SE, Bull DR. Spatial classification of glaucomatous visual field loss. Br J Ophthalmol. 1996; 80: 526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hilton S, Katz J, Zeger S. Classifying visual field data. Stat Med. 1996; 15: 1349–1364 [DOI] [PubMed] [Google Scholar]
- 28. Anton A, Maquet JA, Mayo A, Tapia J, Pastor JC. Value of logistic discriminant analysis for interpreting initial visual field defects. Ophthalmology. 1997; 104: 525–531 [DOI] [PubMed] [Google Scholar]
- 29. Kocak I, Zulauf M, Hendrickson P, Stumpfig D. Evaluation of the Brusini glaucoma staging system for follow-up in glaucoma. Eur J Ophthalmol. 1997; 7: 345–350 [DOI] [PubMed] [Google Scholar]
- 30. Keltner JL, Johnson CA, Spurr JO, Beck RW. Baseline visual field profile of optic neuritis: the experience of the optic neuritis treatment trial. Arch Ophthalmol. 1993; 111: 231–234 [DOI] [PubMed] [Google Scholar]
- 31. Keltner JL, Johnson CA, Spurr JO, Beck RW. Visual field profile of optic neuritis: one-year follow-up in the Optic Neuritis Treatment Trial. Arch Ophthalmol. 1994; 112: 946–953 [DOI] [PubMed] [Google Scholar]
- 32. Gardiner SK, Johnson CA, Cioffi GA. Evaluation of the structure-function relationship in glaucoma. Invest Ophthalmol Vis Sci. 2005; 46: 3712–3717 [DOI] [PubMed] [Google Scholar]
- 33. Heijl A, Lundqvist L. The frequency distribution of earliest glaucomatous visual field defects documented by automatic perimetry. Acta Ophthalmol (Copenh). 1984; 62: 658–664 [DOI] [PubMed] [Google Scholar]
- 34. Henson DB, Hobley AJ. Frequency distribution of early glaucomatous visual field defects. Am J Optom Physiol Opt. 1986; 63: 455–461 [DOI] [PubMed] [Google Scholar]
- 35. Grehn F, Knorr-Held S, Kommerell G. Glaucomatouslike visual field defects in chronic papilledema. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1981; 217: 99–109 [DOI] [PubMed] [Google Scholar]
- 36. Hayreh SS. Pathogenesis of optic disc oedema in raised intracranial pressure. Trans Ophthalmol Soc U K. 1976; 96: 404–407 [PubMed] [Google Scholar]
- 37. Hayreh SS. Optic disc edema in raised intracranial pressure. V. Pathogenesis. Arch Ophthalmol. 1977; 95: 1553–1565 [DOI] [PubMed] [Google Scholar]
- 38. Hayreh SS, March W, Anderson DR. Pathogenesis of block of rapid orthograde axonal transport by elevated intraocular pressure. Exp Eye Res. 1979; 28: 515–523 [DOI] [PubMed] [Google Scholar]
- 39. Tso MO, Hayreh SS. Optic disc edema in raised intracranial pressure. IV. Axoplasmic transport in experimental papilledema. Arch Ophthalmol. 1977; 95: 1458–1462 [DOI] [PubMed] [Google Scholar]
- 40. Corbett JJ, Jacobson DM, Mauer RC, Thompson HS. Enlargement of the blind spot caused by papilledema. Am J Ophthalmol. 1988; 105: 261–265 [DOI] [PubMed] [Google Scholar]
- 41. Frisén L, Holm M. Visual field defects associated with choroidal folds. In: Glaser JS. ed Symposium of the University of Miami. Vol 9. St. Louis: Mosby; 1977: 248–257 [Google Scholar]
- 42. Kim KM, Jeoung JW, Park KH, Kim DM, Ritch R. Relationship between preferred sleeping position and asymmetric visual field loss in open-angle glaucoma patients. Am J Ophthalmol. 2014; 157: 739–745 [DOI] [PubMed] [Google Scholar]
- 43. Johnson CA, Keltner JL, Cello KE. et al. and the Ocular Hypertension Treatment Study Group. Baseline visual field characteristics in the Ocular Hypertension Treatment Study (OHTS). Ophthalmology. 2002; 109: 432–437 [DOI] [PubMed] [Google Scholar]
- 44. Keltner JL, Johnson CA, Beck RW, Cleary PA, Spurr JO. Optic Neuritis Study Group. Quality control functions of the Visual Field Reading Center (VFRC) for the Optic Neuritis Treatment Trial (ONTT). Control Clin Trials. 1993; 14: 143–159 [DOI] [PubMed] [Google Scholar]
- 45. Keltner JL, Johnson CA, Cello KE. et al. and the Ocular Hypertension Treatment Study Group. Visual field quality control in the Ocular Hypertension Treatment Study (OHTS). J Glaucoma. 2007; 16: 665–669 [DOI] [PubMed] [Google Scholar]