Abstract
Introduction
Diagnosis and therapy of cancer remain to be the greatest challenges for all physicians working in clinical oncology and molecular medicine. The statistics speak for themselves with the grim reports of 1,638,910 men and women diagnosed with cancer and nearly 577,190 patients passed away due to cancer in the USA in 2012.
For practicing clinicians, who treat patients suffering from advanced cancers with contemporary systemic therapies, the main challenge is to attain therapeutic efficacy, while minimizing side effects. Unfortunately, all contemporary systemic therapies cause side effects. In treated patients, these side effects may range from nausea to damaged tissues. In cancer survivors, the iatrogenic outcomes of systemic therapies may include genomic mutations and their consequences. Therefore, there is an urgent need for personalized and targeted therapies. Recently, we reviewed the current status of suicide gene therapy for cancer. Herein, we discuss the novel strategy: genetically engineered stem cells’ guided gene therapy.
Review of therapeutic strategies in preclinical and clinical trials
Stem cells have the unique potential for self renewal and differentiation. This potential is the primary reason for introducing them into medicine to regenerate injured or degenerated organs, as well as to rejuvenate aging tissues. Recent advances in genetic engineering and stem cell research have created the foundations for genetic engineering of stem cells as the vectors for delivery of therapeutic transgenes. Specifically in oncology, the stem cells are genetically engineered to deliver the cell suicide inducing genes selectively to the cancer cells only. Expression of the transgenes kills the cancer cells, while leaving healthy cells unaffected. Herein, we present various strategies to bioengineer suicide inducing genes and stem cell vectors. Moreover, we review results of the main preclinical studies and clinical trials. However, the main risk for therapeutic use of stem cells is their cancerous transformation. Therefore, we discuss various strategies to safeguard stem cell guided gene therapy against iatrogenic cancerogenesis.
Perspectives
Defining cancer biomarkers to facilitate early diagnosis, elucidating cancer genomics and proteomics with modern tools of next generation sequencing, and analyzing patients’ gene expression profiles provide essential data to elucidate molecular dynamics of cancer and to consider them for crafting pharmacogenomics-based personalized therapies. Streamlining of these data into genetic engineering of stem cells facilitates their use as the vectors delivering therapeutic genes into specific cancer cells. In this realm, stem cells guided gene therapy becomes a promising new frontier in personalized and targeted therapy of cancer.
Keywords: Cancer, stem cells, suicide gene therapy, stem cell guided delivery of therapeutics, genomic medicine, pharmacogenomics, targeted therapy, personalized therapy
Introduction
Diagnosis and therapy of cancer remain to be the greatest challenge for all of us – physicians working in clinical oncology and molecular medicine. The statistics speak for themselves with the grim reports of 1,638,910 men and women diagnosed with cancer and nearly 577,190 patients passed away due to cancer in the USA in 2012 [1–3].
For practicing clinicians, who treat patients suffering from advanced cancers with contemporary systemic therapies, the challenge is to attain therapeutic efficacy, while minimizing side effects. Unfortunately, all systemic therapies, including chemotherapy, radiation therapy, and radio-immunotherapy, affect to some extent also healthy cells; thus cause side effects [4–26]. In treated patients, these side effects may range from nausea to tissue damage. In cancer survivors, the iatrogenic outcomes may include consequences of genomic mutations in patients themselves or their children. Therefore, there is an urgent need for the patients’ personalized and the cancers’ targeted therapies. Recently, we reviewed the current status and future perspectives of gene therapy for cancer [27–28]. Herein, we discuss genetically engineered stem cells guided gene therapy for cancer as a new frontier in personalized and targeted gene therapy. We provide a short summary in Table 1.
Table 1.
Clinical studies
| Author | Cell line | Design | Materials & Methods | Result | Reference |
|---|---|---|---|---|---|
| Piva et al. | MCF-7-TamR CD44+CD24−/low |
In vitro In vivo |
PCR, Immunofluorecence Western blot FACS, Transient transfection and luciferase assay, Immunohistochemistry | Higher expression of Sox2 in TamR cells and higher expression of SCs | 92 |
| Bergheton et al. | PC9, HCC827, MGH006, NCI-H3122, HCC-78 | In vitro In vivo |
FISH, Western blot, | ROS1 positive patients with NSCLC are sensitive in crizotinib | 96 |
| Liu et al. | A549 LCSLCs | In vitro In vivo |
Western blot, serum-free suspension sphere forming culture method, MTT assay, mtrigel invasion assay, MMP-9 activity assay | Casticin suppress the proliferation of LCSLCs | 98 |
| Lee et al. | PT67.CD PT67.CD.TK HB1.F3 NSCs HFF-1 |
In vitro In vivo |
Migration assay, cell viability assay, NSCs engineered with double prodrug enzymes, PCR | Therapeutic effect of HB1.F3-CD.TK is comparable to HB1.F3-CD, double suicide gene therapy shows efficacy and eradicates NSCs | 58 |
| Altaner et al. | BM-MSCs AT-MSCs CDy-BM-MSCs CDy-AT-MSCs |
In vitro In vivo |
Animal experiments, stereotaxic cell implantation, implantation of 5-FC osmotic pumps and of a miniosmotic pump for ThSCs delivery, MRI | Effective therapy of glioblastoma treated with CDy-BM-MSCs, CDy-AT-MSCs after resection | 56 |
| Bagci-Onder et al. | Gli36, Gli36-EvIII-FmC U87MG U251 Gli79 LN229 A172 |
In vitro In vivo |
Western blot, immunohistochemistry, coculture experiments, lentiviral vectors (Pico2-Fluc.mCherry, LV-S-TRAIL, GFP), viability and caspace assay | Effective, PI3K/mTOR inhibitor, PI-103, increase response of glioma cells in S-TRAIL, reducing tumor volume | 63 |
| Kwon et al. | HB1.F3 NSCs | In vitro In vivo |
Engineering of NSCs in HB1.F3-CD, HB1. F3-CD/5-FC were cocultured with the HNSCC (SNU-1041), labeling of F3-CD cells with ferumoxides | Effective, HB1.F3-CD cells inhibited the growth of an HNSCC cell line in the presence of the 5-FC, with lower toxicity | 84 |
| Malecki et al. | Culture OVCAR Ascites and peritoneal washings | In vitro Ex vivo |
Genetically engineered recombinant DNases | Complete eradication of ovarian cancer cells | 89,69 |
| Kim et al. | SKOV-3 NIH3T3 MCF-7 Hec1a ovarian cells |
In vitro In vivo |
RNA extraction and reverse transcription-PCR, cell growth and migration assays | Effective, GESTCs expressing CD/CE, SKOV-3, with the prodrugs 5-FC or CPT-11 in the presence of HB1.F3.CD or HB1.F3.CE cells inhibits ovarian cancer cell growth | 87 |
| Chai et al. | E.coli DH-5α, plasmid pcDNA3.1, plasmid pIRES, Hep-2 | In vitro In vivo |
Gene transfection, PT-PCR, in vitro experiments on cytocidal effect, bystander effect, detection of TNF-α in the supernatant of in vitro cell culture | Effective, combined gene therapy of CD/5-FC and TNF-α in hep-2 cell line, inhibit tumor cell growth and induced anti-tumor immune response in animal models | 85 |
| Zheng et al. | A2780s HEK293 |
In vitro In vivo |
hpMSCs isolation and culture, hMSCs transfection and protein expression assays, hpMSCs were engineered to deliver endostatin via adenoviral transduction mediated by Lipofectamine 2000, quantitation of cell proliferation and angiogenic microvessel density, alginate encapsulation, analysis of apoptosis in tumor tissues, flow cytometry | Effective antitumor and antimetastatic effect of hpMSCs-Ad-Endo | 86 |
| Li et al. | OVK18#2 RAW 264.7 |
In vitro In vivo |
Synthesis of FA-PEG conjugate, FITC, mass spectrometry, FT-IR, H NMR, Dynamic light scattering and zeta potential, siRNA recovery, ANOVA, flowcytometry, western blot, RT-qPCR | Effective, siRNA/FA-PEG-COL nanoparticles induces inhibition of HIF-1α and tumor proliferation | 90 |
| Shinagawa et al. | hMSCs KM12SM |
In vitro In vivo |
Quantification of MSCs and phosphorylation of PDGFR-β in MSCs, immunohistochemistry, migration and proliferation assay, western blot, RT-PTCR, | Effective, imatinib inhibits tumor-tropism and growth of colon cancer | 99 |
| Lin et al. | HT 29 human colon cancer | In vitro In vivo |
Flowcytometry, western blot, immunohistochemistry, production of shRNA lentiviral vectors | Effective, CD133+ colon cancer cells are responsible for the resistance in antiangiogenetic treatment, through the activation of Hsp27, MAPKAPK2, p38MAPK, PP2A anti-apoptotic signaling pathways | 100 |
| Li et al. | Human pancreatic adenocarcinoma | In vivo | Tissue was minced and digested with collagenase IV, flow cytometry, implantation of PCCs and tumor spheres in NOD/SCID mice, immunoblot, bioluminescent Imaging | Effective, pancreatic tumors express c-Met and its inhibition reduce tumor growth and CSCs | 103 |
| Van den Broeck et al. | Human pancreatic ductal adenocarcinoma | In vivo | Whole-genome expression, analysis by microarray, development of gene signature, n counter analysis, immunohistochemistry | Effective, PDAC contains a subpopulation of CSCs presented chemoresistance | 107 |
| Mohamed et al. | Melanoma tissue microarray | In vitro | Immunohistochemistry | CD 271 expression in melanomas is associated with increased frequency of metastases, c-kit is associated with good prognosis and improved outcome | 110 |
| Schatton et al. | Melanoma cells | In vitro In vivo |
Flow cytometry, RT-PCR, ELISPOT, ELISA | Identification of T cell-modulatory functions of ABCB5+ melanoma SCs subpopulations | 111 |
| Aikawa et al. | AML cells | In vitro In vivo |
Generation of AML mouse models, administration of AP20187, imatinib, or Ki20227, immunofluorescency, immunoprecipitation and immunoblotting | MOZ fusion proteins stimulate PU1-mediated transcription of CSF1R. High expression of CSFR1 can induce AML | 118 |
| Dwyer et al. | BM-MSCs | In vitro In vivo |
Adenoviral infection, detection of NIS expression, PT-PCR, 99mTcO4− imaging of NIS-transfected MSCs, immunohistochemistry, imaging of MSC-NIS engraftment | Effective, in breast cancer MSCs/NIS delivery system decrease tumor growth | 52 |
| Grisendi et al. | HeLa AD-MSCs |
In vitro In vivo |
Isolation of TRAIL cDNA, vector production, and AD-MSC transduction, fluorescence, ELISA, Apoptosis and caspase-8 activation assays, PCR, histochemistry | Effective, MSCs/TRAIL induce apoptosis of cancer cells | 53 |
| Chiocca et al. | Glioma cells | In vivo | Injection of AdV-tk after surgical resection, treated with valacyclovir, MGMT analysis, chemoradiotherapy, QOL, PCR, immunohistochemistry | Effective, AdV-TK/valacyclovir in combination with surgery, radiation and chemotherapy with temozolomide, demonstrated a stable or improved quality of life and increase overall survival | 62 |
TamR: Tamoxifen resistant cells, FACS: fluorescence activating cell sorting, qPCR: quantitative polymerase chain reaction, MX: mitoxantrone, VACV: vaccinia virus, FISH: fluorescent in situ hybridization, MTT: 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, LCSLCs: lung cancer stem-like cells, CDy-BM-MSCs: yeast cytosine deaminase bone marrow mesenchymal stem cells, CDy-AT-MSCs: yeast cytosine deaminase adipose tissue mesenchymal stem cells, HNSCC: Head and neck squamous cell carcinoma, hpMSCs: human placenta derived mesenchymal stem cells, GESTCs: genetically engineered stem cells, CD: cytosine deaminase, CE: carboxyl esterase, 5-FC: 5 fluorocytosine, CTP-11: camptothecin-11, FA-PEG: folic acid–poly ethylene glycol, FITC: Fluorescein isothiocyanate, FT-IR: Fourier transform infrared spectroscopy, H NMR: Proton nuclear magnetic resonance spectroscopy, ANOVA: analysis of variance, PP2A: Protein phosphatase 2A, PCCs: pancreatic cancer cells, ELISPOT: enzyme-linked immunosorbent spot, AML: acute myeloid leukemia, MOZ: leukemia zinc finger gene, CSF1R: macrophage colony stimulating factor receptor 1, NIS: sodium iodide symporter, QOL: quality of life, MGMT: unmethylated O(6)-methylguanine-DNA methyltransferase, AdV-Tk: adenoviral vector containing the herpes simplex virus thymidine kinase gene.
Stem cells have the unique potential for self renewal and differentiation. This potential is the primary reason for introducing them into medicine to regenerate injured or degenerated organs, to correct congenital disorders, or to rejuvenate aging tissues [29–51]. Recent advances in genetic engineering and stem cell research have created the foundations for genetic engineering of stem cells as the vectors for delivery of therapeutic transgenes.
Specifically in oncology, the stem cells are genetically engineered to deliver the cell suicide inducing transgenes selectively to the cancer cells. Expression of the transgenes kills the cancer cells, while leaving healthy cells unaffected [52–63]. Herein, we present various strategies to bioengineer suicide inducing genes and stem cell vectors. Moreover, we review results of the main preclinical studies and clinical trials. However, the main concern for therapeutic use of stem cells is a risk of their cancerous transformation [64–70]. Therefore, we discuss in depth various strategies safeguarding stem cell therapy against iatrogenic cancerogenesis.
Stem cells are defined as undifferentiated cells that have the capacities of self-renewing and differentiation into specialized cell types and tissues [29–51]. These cells can be classified according their potency to differentiate into: unipotent stem cells that can produce only one cell type, multipotent cells able to form all cells of one particular lineage, pluripotent stem cells capable to differentiate into any of the embryonic germ layers and totipotent cells that can give rise to an entire organism. In general, stem cells are classified as embryonic stem cells (ESCs) and adult (ASCs) or non embryonic stem cells [32–36].
Embryonic stem cells
Embryonic stem cells feature totipotency [32–33]. This ability is retained in mammals by the zygote and up to at least 4-cell stage embryos. The embryonic stem cells are derived from the inner cell masses of blastocysts. They have ability to proliferate in an undifferentiated state through multiple passages in culture, as well as to generate any cell of the body. However, the use of ECSs has generated legal, ethical, scientific, and religious opposition, because these cells can only be obtained from human embryos [39].
Adult stem cells
Adult stem cells (ACSs) are self-renewing, multipotent cells obtained from adult tissues. They can be further classified as hemopoietic stem cells (HSCs) and mesenchymal or non-hemopoietic stem cells, stromal, or mesenchymal stem cells (MSCs), based on their origin [35–39]. Bone marrow contains a heterogeneous population of stem cells (BMSCs). Hemopoietic stem cells are derived from bone marrow, peripheral blood, or umbilical cord blood and are capable to differentiate into all blood cells, dendritic cells, lymphocytes and macrophages, so are responsible for the blood renewal each and every day. MSCs are of mesodermal origin and are present in a large number of tissues such as bone marrow, liver, skin, dental pulp, adipose tissue, brain, skeletal muscle. Bone marrow stem cells are able to differentiate into different lineages such as chondrocytes, adipose cells, osteoblasts and muscle cells (12–21).
Human pluripotent induced stem cells
To the repertoire of the natural stem cell therapeutics, human pluripotent induced stem cells have been added [30–39]. They are generated by introducing vectors carrying coding sequences for the transcription factors or the transcription factors themselves, which re-program the adult fully differentiated cells into the undifferentiated state. These induced cells open new therapeutic opportunities, which are practically the same as those of human embryonic stem cells, but without ethical and scientific concerns. In particular, reprogramming the patients’ own cells results in creating human autologous pluripotent induced stem cells, which eliminate the risks of immune response or the need for immuno-suppression.
Trans-differentiated cells
The spectrum of cell therapeutics has been further expanded through directed trans-differentiation of the differentiated cells into the differentiated cells of a different type [71–83]. This is accomplished without the step of reprogramming of the adult stem cells into the undifferentiated cells as outlined above. This novel biotechnology is of paramount importance for bioengineering of the cells with special therapeutic tasks, as they are being recognized by the patients’ immune system as their own cells. These tasks may include guiding the bioengineered stem cells towards selected receptors on cancer cells and delivering suicide genes. This novel technology opens new routes for reprogramming of the patients’ own cells into the therapeutic genes’ carrying vectors.
Sources and therapeutic applications of stem cells
Because of their unique characteristics, self-renewing and multilineage differentiation, stem cells are promising candidates for potential therapeutic uses in regenerative medicine, pharmacogenomics, and bioengineering [29–53]. Ability of MCSs to differentiate into osteoblasts or chondrocytes, has generated considerable interest in using these cells as potential treatment in patients with bone or cartilage disorders through MSCs transplantation. Stem cells ability to differentiate from one type of a tissue lineage into another, carries a great promise for the treatment of a variety of diseases such as cardiovascular disease and heart failure, stroke, Parkinson’s and Huntington disease, diabetes, liver diseases such as cirrhosis to name only a few. Their ability to modulate immune response and their immunosuppressive effect onto B and T lymphocytes proliferation, further expanded possible use of these cells for the treatment of autoimmune diseases, rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis and autoimmune encephalomyelitis. Stem cells can be also obtained from dental tissues and may be isolated from different parts of the teeth, apical papilla, dental follicle, human exfoliated deciduous teeth, dental pulp and periodontal ligament. Stem cell research in dentistry focuses on the regeneration of periodontal ligament, regeneration of coronal dentine, pulp and salivary gland after radiation therapy and repair of craniofacial defects.
Stem cells as the vectors of cancer cell suicide inducing genes
Research on stem cells facilitated opportunities for their genetic engineering to become vectors carrying suicide genes into different types of cancer - suicide gene therapy of cancer [48–63,71–78]. One of the most advanced approaches is based on introduction into tumor cells of genes capable for converting a non-toxic pro-drug into a cytotoxic agent. Among these types of suicide systems, the most studied are: (1) thymidine kinase gene (HSV-TK) of herpes simplex virus in combination with Ganciclovir used as a prodrug and (2) cytosine deaminase (DC) gene of Escherichia coli in combination with 5-flurocytosine (5-FC). These genes utilize different delivery systems, like viral or non-viral vectors, bacteria, parasites and cell-based systems including stem cells. The use of stem cells, as possible vectors, has been investigated due to their easy expansion and advances in genetic engineering. Additional attractive feature is immuno-privileged status of autologous stem cells, as indicated by expression of the major histocompatibility complex 1 (MHC1), but not MHC2, clusters of differentiation 40, 80, and 86. As such, these cells can be used in immuno-competent patients, including those with cancers, while without complications presented from immuno-modulation, with better therapeutic efficacy, and significantly improved safety.
Many treatments result in cells’ death in vitro. The main challenge for practicing clinicians is not to cross the thin line between eradicating cancer cells in vivo, in the patients’ bodies, but not harming these patients’ healthy cells. This is a particularly tough challenge in advanced cancers, which metastasized to multiple and distant locations of the patients’ bodies. These advanced stages are beyond the therapeutic arsenal of local surgery, but require systemic therapies associated with horrendous side effects. In this realm, there is a great promise in genetic engineering of stem cells, so that they are compatible with the patient’s immune system, are guided to the specific tumor, and deliver the therapeutic transgenes into cancer cells, while inducing their death (Figure 1).
Figure 1.
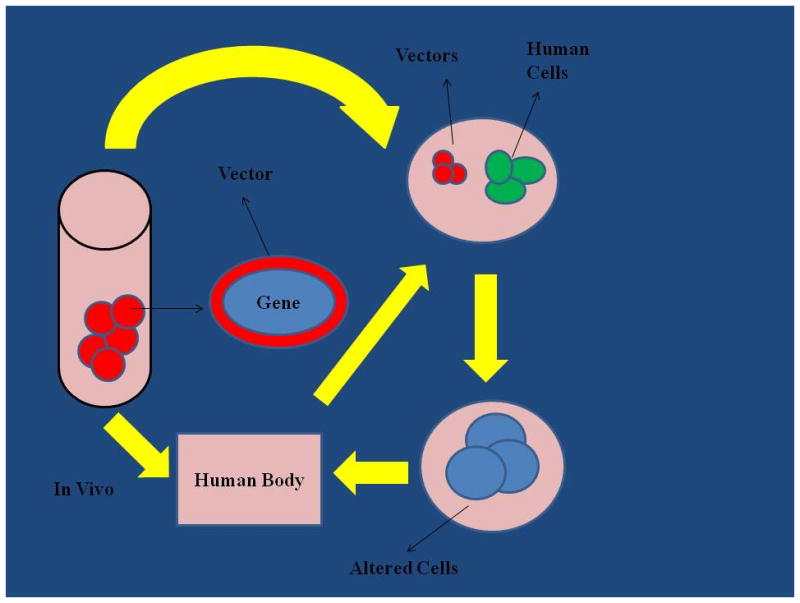
Gene therapy can be administered directly to patients with the aid of transgene vectors. Alternatively, cells from a patient can be acquired, genetically engineered, and returned to this patient. Current research aims at bioengineering of vectors that can deliver therapeutic genes to the targeted cells after injecting into blood circulation or directly into targeted tissues.
However, the recognized risk of stem cell therapy is their cancerogenic potential [64–70]. Therefore, implementing all measures preventing cancerogenic transformation is the stringent sine qua non condition for introducing stem cell therapy into clinical trials.
Review of therapeutic stem cells-guided strategies in preclinical and clinical trials
We have recently reviewed current strategies of cancer suicide gene therapy of cancer [27,28]. Suicide gene therapy has been tried in several types of cancers including those of brain, head and neck, ovary, breast, lung, pancreas, colon, blood, and skin. The use of suicide gene therapy is more efficient and with fewer side effects than chemotherapy or radiotherapy due to selective eradication of the cancer cells. Furthermore, gene therapy aims at blocking specific pathways, growth factors or enzymes that are involved in the carcinogenesis, the tumor growth and cell proliferation. This therapeutic strategy targets the cancers cells directly, while limiting the effects upon the healthy cells and reducing adverse events of systemic chemo- and radiation therapies, which currently remain the cornerstones of cancer treatment. A new frontier in therapy of targeted therapy of cancer is rapidly developing with the stem cells as the vectors delivering therapeutics to the targeted cancer cells. Herein, we review most advanced preclinical studies and clinical trials pursued in this new promising therapeutic frontier.
Glioblastomas
Glioblastomas remain one of the most frequent intracranial tumors with poor prognosis and short survival. Current treatment strategies include surgical resection followed by concomitant chemotherapy and radiation. It is believed that the main contributor to the tumors’ relapses is presence cancer stem cells. On the other hand, stem cells demonstrate natural tropism towards cancerous tissues. This ability was utilized for delivering suicide inducing genes into glioblastomas [57–63]. Use of stem cells as vehicles delivering suicide genes into the brain cancer cells therapy is the most recent and promising therapeutic approach for brain tumors. This is possible due to their unique characteristics of tumor tropism, immunostimulation, tumor infiltration through blood brain barrier. To date, the stem cells were bioengineered with doubled suicide genes: cytosine deaminase (CD) and tyrosine kinase (TK). It was demonstrated that the cells carrying amplified transgenes ensured better treatment results than those bio-engineered with the single suicide gene. The use of double pro-drug enzymes enhanced tumor eradication and offered major safety. Other studies have evaluated mesenchymal cells (MSCs), which were genetically engineered to express cytokines such as IL-2, IL-18, IL-12 and INF-γ. This resulted in increasing the tumors’ immune response. Human MSCs were also engineered in order to express cytosine deaminase: uracil phosphorybosiltranferase (CD:UPRT). In combination with 5-FC, these have shown post operative inhibition of tumor growth in animal models. In other studies, PI103 - systemic PI3K/mTOR inhibitor was combined with stem cells’ delivered tumor necrosis factor related apoptosis inducing ligand (S-TRAIL). This combination inhibited the tumors’ growth in mouse models. As the most encouraging development, the FDA recently approved the first pilot study using CD/5FC and stem cells as the delivery system. The results of this clinical trial will help to evaluate effectiveness and safety of this method.
Overall, bioengineered stem cells used as therapeutic genes’ delivery vectors into glioblastomas, resulted in encouraging results in preclinical studies. Therefore, they appear to be a promising strategy for treatment of brain tumors.
Cancers of Head and Neck
Recurrence and distant metastasis remain critical problem for the treatment of squamous cell carcinomas of head and neck [84–85]. Genetically modified stem cells, may be used as potential targeted treatment. Human stem cells, which were genetically engineered to express cytosine deaminase (HB1.F3-CD) have been used as a delivery system, in combination with 5-FC in vitro and in vivo studies. They resulted in inhibition of the tumors’ growth, when used combined with systemic administration of 5-FC. In laryngeal carcinoma, combined gene therapy of CD/5-FC and TNF-α in hep-2 cell line, has inhibited the tumor cell growth and induced anti-tumor immune response in animal models. As such, stem cells guided gene therapy is a good candidate for streamlining into clinical trials.
Cancers of the Ovaries
Cancers of the ovaries are the most lethal gynecological cancers. Almost 63% of them are diagnosed in advanced stages, which require systemic therapies. These cancers progress into the abdominal cavity without giving specific symptoms. Therefore, by the time of their detection, they are already spread too far for the local surgical resection. Recent studies focus on a possible use of stem cell based therapy [87–91]. Human placenta derived MSCs (hpMSCs) are promising candidates for stem cell therapy due to their ability of homing in tumor sites and modulating the immune response. They have been genetically engineered in order to deliver endostatin - an inhibitor of endothelial cell migration and proliferation; thus of the tumor’s induced angiogenesis. The in vitro and in vivo studies confirmed the homing effect of hpMSCs expressing endostatin in the tumor site, the inhibition of tumor neoangiogenesis, and cell proliferation. Those effects led to starving the neoplasms and reducing their growth. In other studies, genetically engineered stem cells that expressed carboxyl esterase (CE), were engineered to migrate towards ovarian cancers and to deliver therapeutics. These cells induced inhibition of tumor cell proliferation. Over-expression and mutations in the gene coding epidermal growth factor receptor offer a great target for genetically engineered stem cells delivering apoptosis inducing transgenes [89]. Following this targeting approach, genetically engineered stem cells could deliver HSV-TK to EGFR over-expressing cells to eradicate cancer cells.
Breast Cancer
Breast cancer is the most common cancer in females. Endocrine therapy in estrogen receptor α (ERα) positive tumors is largely used with satisfying results, while preventing cancer cells from developing of resistance. Recent studies have shown that Sox2, one of the transcription factors essential for maintaining pluripotency of stem cells, is responsible for resistance to Tamoxifen [92–93]. Sox2 is over-expressed in breast tumor cells through activation of Wnt signaling pathway. In that case, Sox2 is used as an indicator of resistance to treatment. Furthermore, discovery of Wnt pathway inhibitors can lead to new treatment strategies. These encouraging approaches can further be enhanced by recent results obtained by introducing stem cell guided gene therapy [50–52]. Mesenchymal stem cells (MSCs) were used as a vehicle for delivery of the sodium iodine symporter with significant decrease of tumor growth. MSCs were also used as a vehicle for the delivery of TRAIL (MSCs/TRAIL) to induce apoptosis of tumor cells but without toxicity in normal tissues such as in the liver. This therapy represents a promising approach for treatment of the breast cancer.
Lung cancer
Lung cancer is one of the leading causes of death world-wide. Despite successes of platinum-based chemotherapy, there was no major change in rates of long term survival [94–97]. Gene for receptor for tyrosine kinase (ROS1) was recently reported as involved in chromosomal translocations in lung cancer. Patients suffering from non-small cell lung carcinomas (NSCLC) with ROS1 rearrangements appeared to be sensitive to ALK inhibitor Crizotinib. Growing evidence accumulates for cancer stem cells (CSCs) as the source of cancer malignancy, which is associated with poor differentiation, lymph node metastases, and poor prognosis. In NSCLC, subpopulation of the CD133 expressing CSCs was identified. The subpopulation of lung cancer stem cells A549, with high expression of CD133, CD44 and ALDH1, strongly responded to Casticin, that preferentially suppress CSCs proliferation, making it a possible therapeutic for NSCLC.
Different vectors systems have been used to deliver therapeutic genes. Among non-viral vectors, polyethilenimine (PEI) is the most widely used as a gene carrier, due to its low cytotoxicity and high transfection efficacy. Polyspermine and polyethylene glycol (PEG) diacrylate (SPE-alt-PEG) synthesized gene carrier for lung cancer is another synthetic vector with low cytotoxicity, high transfection efficacy, and biocompatibility. SPE-alt-PEG/GFP complexes have been successfully transferred by aerosol into the lungs. Furthermore, the therapeutic effect of SPE-alt-PEG was confirmed by using Pdcd4, decreasing that way the tumor’s size and showing that SPE-alt-PEG is a safe gene carrier for in vivo applications. Many lung cancers express mutations deletion gene for epidermal growth factor receptor variant III. It is the unique receptor, which distinguishes cancer cells from healthy cells. Therefore, it becomes a desired target for genetically engineered stem cells serving as vectors delivering the suicide inducing genes into the lung cancer cells displaying that mutated receptor [127].
Colon cancer
Colon cancer represents one of the most common cancers in Western countries. Studies implicated MSCs in tumor pathogenesis, growth, and metastasis. These cells express platelet-derived growth factor (PDGF) [97–100]. On the other hand, PDGF signaling pathways are determined important for survival and migration of MSCs in colon cancer. Studies in mice have shown that Imatinib - PDGFR inhibitor could decrease tumor growth, angiogenesis and metastatic effect of MSCs. Drugs that target to MSCs could be future treatment for colon cancer. Recently, it was also shown that CSCs, particularly the CD133 subpopulation, are responsible for resistance to anti-angiogenesis treatment, through the activation of Hsp27, MAPKAPK2, p38MAPK and PP2A anti-apoptotic signaling pathways. Targeting these pathways and suppressing the CSCs’ activation, may lead to development of new treatment methods in colorectal cancer. Genetic engineering of the stem cells, so that they deliver endostatin - an inhibitor of endothelial cell migration and proliferation; thus inhibition of tumor’s angiogenesis, may become a venue to break these cancers’ therapeutic resistance.
Pancreatic cancer
Pancreatic cancer is one of the deadliest neoplasms. It is very difficult to diagnose, as it progresses silently without any specific symptoms, thus is mostly diagnosed in very advanced stages. Communicating diagnosis in most cases is equivalent to prognosis of a few months survival. Pancreatic cancer is also very difficult to cure. This features are strong drivers of exploring the stem cells guided therapy [101–107]. The main problem, especially in cases of pancreatic ductal adenocarcinoma (PDAC), is the resistance of pancreatic cells to conventional therapy. The pancreatic cancer stem cells (CSCs) are suggested as contributors to tumor growth and metastasis. They are often associated with epithelial mesenchymal transition (EMT), which leads to cells of similar characteristics with CSCs. Recently, it was shown that pancreatic cancer CD44+/CD24+/ESA+ cell line has stem cells’ properties, characterized by self-renewal and differentiation, while also present high tumorigenicity. Expression of CD133+, CXCR4, SOX2 and c-Met has also been been correlated to tumorigenicity and chemoresistance. Hedgehog, Notch, Wnt signaling pathways are important in cancerogenesis of pancreatic cells and their deregulation such alterations in NF-κB, Akt, TGF-β and miRNA- regulated pathways are very critical for the differentiation, self renewal and tumorigenesis of pancreatic CSCs. Side populations of cells have been identified in PDAC, which express multidrug transporter - ABCB1; thus are capable for expulsion of therapeutics. This leads to developing of chemoresistance. Definition of pancreatic cancers’ biomarkers and exposing mechanisms of resistance opened new routes for new strategies of the targeted therapy which also include genetic engineering of therapeutic stem cells.
Melanoma
Melanoma is one of the most aggressive tumors [108–113]. The major problem for enforcing effective therapy remains drug resistance. Over the last few years, there is a growing interest for the melanoma cancer stem cells (CSCs) and possible alternative ways of prognosis and treatment of this disease. Studies have shown that the melanoma CSCs express surface markers such as ABCB5, CD271, ALDH, SOX10, c-kit, that can play a role in treatment and prognosis. More specifically, CD271 expression is correlated with increased metastatic ability and poor prognosis. Expression of ABCB5 is strongly correlated with disease progression. Stem cells ABCB5+, present lower expression of melanoma antigen recognized by T cells-1 (MART-1); thus are harder to kill by natural immune response or immunotherapy. Similar expression of tumor antigens: MAGE-A, BIRC7/ML-IAP, NY-ESO-1, MHC class 1, inhibition of the production of IL-2, and preferential expression of B7.2, may inhibit antitumor immunity. Future melanoma treatment approaches should consider these biomarkers as possible therapeutic targets for genetically engineered therapeutic stem cells.
Acute Myeloid Leukemia
Studies on acute myeloid leukemias (AMLs) with high incidence of relapses led to detection of the cells’ subpopulations with stem cell-like properties: leukemic stem cells (LSCs) [114–118]. These cells demonstrated resistance to conventional therapies. In several studies, investigators proposed that CD34 and CD38 positive immunophenotypes of LSCs are correlated with lower median survival and with poor outcomes of AML. Different other biomarkers are also associated with the LSCs: CD25, CD71, CD123, HLA- and CLL-1. Among them, CD25+ is correlated to early treatments’ failure. These molecules may be used as prognostic biomarkers, but also as targets for personalized therapies. Specifically, it was shown in animal models that therapeutic targeting of CD123+ in AML cell line led to elimination of LSCs. Signaling pathway for transcription complex NF-κB is another therapeutic target. It is important for proliferation and survival of the LSCs. It can also be involved in mediating drug resistance. Recent studies focus on targeting this pathway with different agents like Bortezomib - an inhibitor used in combination with other agents. Data from the recent study in mice has suggested that eradication of the LSCs could be considered for further tests as a therapeutic approach. The monocytic leukemia zinc finger fusion proteins stimulate PU1-mediated transcription of macrophage colony stimulating factor receptor 1 (CSF1R). High expression of CSFR1 can induce AML, thus a possible therapeutic approach would be apoptosis of CSF1R cells. Identification of the aforementioned biomarkers opens the new perspectives for engineering of the stem cells, which may target these biomarkers and deliver the deadly cargo of suicide inducing genes into the cancer cells.
Safeguarding stem cell therapy against iatrogenic cancerogenesis
“Primum non nocere” is our ultimate creed. Safeguarding stem cell therapy against iatrogenic cancerogenesis should be the primary consideration in designing any therapeutic strategy. Main approaches of such safeguarding, which are currently explored, include negative selection or selective killing of potentially cancerogenic stem cells [69,119–126].
First approach relies upon biomarkers, which are specifically expressed on stem cells. Identification of podocalyxin-like protein-1 on surfaces of embryonic stem cells promoted depletion of these cells to reduce the risks of teratomas [119]. Stage specific embryonic antigen 5 (SSEA5) was identified specifically on human pluripotent stem cells. Monoclonal antibody raised against SSEA-5 was used to deplete the cells expressing this antigen and resulted in reduced numbers of forming teratomas [120]. Claudin 6 is a protein contributing to formation of tight junctions and was identified on stem cells [120]. Anti-claudin-6 antibodies modified with toxins were effective in killing the targeted stem cells. So were the other antibodies followed by toxins [122–123].
In an alternative approach for eliminating stem cells with neoplasmic potential, eradication of the selected cells was accomplished by inhibitor of the oleate synthesis [124]. Stem cells were genetically engineered to express herpes virus thymidine kinase (HSVTK). Thus, they became sensitive to and eliminated by Ganciclovir at the doses far lower than those toxic to the healthy cells [125]. Selectivity of this approach was improved by genetic engineering of truncated herpes simplex virus delta thymidine kinase (λTK) gene under control of EF1α or NANOG promoters [126]. This insertion of λTK gene did not affect pluripotency of the cells, but rendered them sensitive to Ganciclovir. This also created an opportunity to eliminate stem cells expressing genes sustaining pluripotentcy by supplying Ganciclovir, to which the transduced stem cells were more sensitive than were differentiated cells. Finally, in the most direct approach, selective elimination of proliferating and directed-differentiation-resistant stem cells was attained by inducible expression of transgenes for DNases controlled by POLA1 promoter [69]. In this strategy, after providing factors to induce differentiation of the stem cells, the cells, which would resist to directed differentiation and keep proliferating, would express human recombinant DNases executing these stem cells’ death.
Perspectives
Three most current topics of oncology carry great promise for developing effective cancer therapeutics in the near future: defining cancer biomarkers to facilitate early diagnosis, research on cancer heterogeneity and personal genomics with modern tools of next generation sequencing to target specific molecules in cancers, and addressing patients’ gene expression profiles to consider them for crafting personalized and targeted therapies.
Cancer biomarkers for early diagnosis
One of the most effective ways to cure cancer is to capture it early. The solid support for this approach is provided by the statistics reporting 19% five year survival of women diagnosed with ovarian cancer at the advanced stages, but 69% five year survival of women with the cancer diagnosed at the early stages 1 [3]. Cancer captured early can be efficiently treated with the local surgery. On the other hand, invasive and metastatic tumors present poor prognosis and require systemic therapies with horrendous side effects. Hence, a major effort goes toward defining cancer biomarkers and developing screening methods. These include works on circulating tumor cells, free circulating biomarkers, and free circulating DNA. Those works are complemented by refining sensitive methods of molecular imaging. The molecular diagnoses generated by those both approaches should pave the ways for therapies delivered precisely to the targeted cancer cells by bioengineered stem cells.
Targeted therapies addressing cancers’ heterogeneities
All cancers consist of very heterogeneous populations of cancer cells’ clones. Some of these clones respond well to standard therapeutic regimes, but other clones do not respond or develop resistance to those regimes. Cancer stem cells seem to play significant role. Those phenomena lead to clonogenic survival and tumors’ growth propelled by resistant clones. Therefore, analysis of the complete spectrum of all the clones driving cancerous tumors’ growth, while considering that the spectra of these clones may change during or as the result of therapeutic regimes, are really necessary for planning effective therapies [89]. Advances in genomics and proteomics should help to identify these spectra. This would also include the targets for stem cells, which would be delivering cargo of therapeutic genes.
Genomic medicine, pharmacogenomics, and personalized therapy
It is also very important to consider that every patient is different. Susceptibility to diseases and ability to defend against the diseases are the outcomes of the person’s genomic profile. This is the foundation for genomic medicine built on the foundation of the human genome. So is responsiveness to therapeutics. This is the core of pharmacogenomics. Rapid advances towards next generation sequencing of genomes and transcriptomes should help us in defining those differences and crafting therapies adjusted according to the patient’s genomic profile and to the cancer’s molecular profile. Designing and bioengineering of the stem cell guided therapies should play the essential role in these promising endeavors.
Acknowledgments
Sources of funding for the work
This work was supported by the funds from the National Science Foundation [grant numbers: 9420056, 9522771, 9902020, and 0094016]; from the National Institutes of Health [grant numbers: P41 RR000570 and P41 RR002301]; and from the Phoenix Biomolecular Engineering Foundation [grant number: 2006070101] to Marek Malecki MD PhD - Principal Investigator. Administrators of the funding institutions and managers of the facilities had no influence on the project design and the data presented.
Footnotes
Conflict of interest statement
The authors have no conflict of interest.
Authors’ Contributions
MM and PZ wrote the manuscript; KP, IK, SL, LY, RM, KZ edited the manuscript; MM wrote and applied for the grants, edited the manuscript.
Authorship statement
All authors agree with the content of the article and the transfer of copyrights to the “Open Access” publisher.
References
- 1.American Cancer Society. Cancer Facts and Figures 2012. 2012 http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf.
- 2.Jemal A, Siegel R, Hao Y. Cancer statistics. CA Cancer J Clin 2010. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 Jan-Feb;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DK. New issues in systemic therapy for ovarian cancer. J Natl Compr Canc Netw. 2013 May;11(5 Suppl):690–693. doi: 10.6004/jnccn.2013.0203. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo MO, Kavan P, Miller WH, Jr, Panasci L, Assouline S, Johnson N, Cohen V, Patenaude F, Pollak M, Jagoe RT, Batist G. Systemic cancer therapy: achievements and challenges that lie ahead. Front Pharmacol. 2013 May 7;4:57. doi: 10.3389/fphar.2013.00057. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Aharon I, Bar-Joseph H, Tzarfaty G, Kuchinsky L, Rizel S, Stemmer SM, Shalgi R. Doxorubicin-induced ovarian toxicity. Reprod Biol Endocrinol. 2010 doi: 10.1186/1477-7827-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meirow D, Biedermann H, Anderson RA, Wallace WH. Toxicity of chemotherapy and radiation on female reproduction. Clinical Obst and Gynaecol. 2010;53:727–739. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 8.Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. AGING. 2011;3:782–793. doi: 10.18632/aging.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka T, Halicka HD, Traganos F, Seiter K, Darzynkiewicz Z. Induction of ATM Activation, Histone H2AX Phosphorylation and Apoptosis by Etoposide. Cell Cycle. 2007;6:371–376. doi: 10.4161/cc.6.3.3835. [DOI] [PubMed] [Google Scholar]
- 10.Tan X, Wang DB, Wei H, Zhu R, Zhu SS, Jiang H, Yang ZJ. Doxorubicin induces apoptosis in H9c2 cardiomyocytes: role of overexpressed eukaryotic translation initiation factor 5A. Biol Pharm Bull. 2010;10:1666–1672. doi: 10.1248/bpb.33.1666. [DOI] [PubMed] [Google Scholar]
- 11.Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7:535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- 12.Shakir DK, Rasul KI. Chemotherapy induced cardiomyopathy: pathogenesis, monitoring and management. J Clin Med Res. 2009;1:8–12. doi: 10.4021/jocmr2009.02.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 14.Raz A, Fisch B, Okon E, Feldberg D, Nitke S, Raanani H, Abir R. Possible direct cytotoxicity effects of cyclophosphamide on cultured human follicles: an electron microscopy study. J Assist Reprod Genet. 2002;19:500–506. doi: 10.1023/A:1020318704960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pointon AV, Walker TM, Phillips KM, Luo J, Riley J, Zhang SD, Parry JD, Lyon JJ, Marczylo EL, Gant TW. Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase3 activation. PLoS ONE. 2010;5:e12733. doi: 10.1371/journal.pone.0012733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bar-Joseph H, Ben-Aharon I, Rizel S, Stemmer SM, Tzabari M, Shalgi R. Doxorubicin induced apoptosis in germinal vesicle (GV) oocytes. Reprod Toxicol. 2010;30:566–572. doi: 10.1016/j.reprotox.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Adriaens I, Smitz J, Jacquet P. The current knowledge on radiosensitivity of ovarian follicle development stages. Hum Reprod Update. 2009;15:359–377. doi: 10.1093/humupd/dmn063. [DOI] [PubMed] [Google Scholar]
- 18.de La Motte Rouge T, Petrella MC, Michels J, Even C, Balleyguier C, Duclos J, Mazeron R, Morice P, Pautier P, Lhommé C. New drugs and targeted therapeutic agents in ovarian cancer. Bull Cancer. 2009 Dec;96(12):1215–24. doi: 10.1684/bdc.2009.0988. [DOI] [PubMed] [Google Scholar]
- 19.Bouchlariotou S, Tsikouras P, Benjamin R, Neulen J. Fertility sparing in cancer patients. Minim Invasive Ther Allied Technol. 2012 Jul;21(4):282–92. doi: 10.3109/13645706.2011.611520. [DOI] [PubMed] [Google Scholar]
- 20.Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen thawed ovarian tissue. Fertil Steril. 2013 May;99(6):1514–22. doi: 10.1016/j.fertnstert.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Salama M, Winkler K, Murach KF, Seeber B, Ziehr SC, Wildt L. Female fertility loss and preservation: threats and opportunities. Ann Oncol. 2013 Mar;24(3):598–608. doi: 10.1093/annonc/mds514. Epub 2012 Nov 4. [DOI] [PubMed] [Google Scholar]
- 22.Ayensu-Coker L, Bauman D, Lindheim SR, Breech L. Fertility preservation in pediatric, adolescent and young adult female cancer patients. Pediatr Endocrinol Rev. 2012 Nov;10(1):174–87. [PubMed] [Google Scholar]
- 23.Lappi M, Borini A. Fertility preservation in women after the cancer. Curr Pharm Des. 2012;18(3):293–302. doi: 10.2174/138161212799040420. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Lin J, Chu J, Ma Y, Zeng S, et al. Activation of caspase-3 noninvolved in the bystander effect of the herpes simplex virus thymidine kinase gene/ganciclovir (HSV-tk/GCV) system. J Biomed Opt. 2008;13:031209. doi: 10.1117/1.2937830. [DOI] [PubMed] [Google Scholar]
- 25.Beltinger C, Fulda S, Kammertoens T, Meyer E, Uckert W, et al. Herpes simplex virus thymidine kinase/ganciclovir induced apoptosis involves ligand-independent death receptor aggregation and activation of caspases. Proc Natl Acad Sci U S A. 1999;96:8699–8704. doi: 10.1073/pnas.96.15.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012 Sep-Oct;18(5):525–35. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 27.Joo WD, Visintin I, Mor G. Targeted cancer therapy - Are the days of systemic chemotherapy numbered? Maturitas. 2013 Sep 20; doi: 10.1016/j.maturitas.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malecki M. Frontiers in suicide gene therapy of cancer. Journal of Genetic Syndromes and Gene Therapy. 2012;4(1):1–41. doi: 10.4172/2157-7412.1000e114. Open Access. http://www.ncbi.nlm.nih.gov/pubmed/2333007029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarogoulidis P, Darwiche K, Sakkas A, Varmus L, Huang H, Li Q, Freitag L, Zarogoulidis K, Malecki M. Suicide Gene Therapy for Cancer: Current Strategies. Journal of Genetic Syndromes and Gene Therapy. Open Access. 2013;4(1):1–29. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3842193/ [Google Scholar]
- 30.Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013 Jun;140(12):2457–61. doi: 10.1242/dev.092551. [DOI] [PubMed] [Google Scholar]
- 31.Scadden D, Srivastava A. Advancing stem cell biology toward stem cell therapeutics. Cell Stem Cell. 2012;10:149–150. doi: 10.1016/j.stem.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Chen M, Han W, Fu X. How far are induced pluripotent stem cells from the clinic? Ageing Res Rev. 2010 Jul;9(3):257–64. doi: 10.1016/j.arr.2010.03.001. Epub 2010 Ap 4. [DOI] [PubMed] [Google Scholar]
- 33.Fortier LA. Stem cells: classifications, controversies, and clinical applications. Vet Surg. 2005 Sep-Oct;34(5):415–23. doi: 10.1111/j.1532-950X.2005.00063.x. [DOI] [PubMed] [Google Scholar]
- 34.Mitalipov S, Wolf D. Totipotency, pluripotency and nuclear reprogramming. Adv Biochem Eng Biotechnol. 2009;114:185–99. doi: 10.1007/10_2008_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005 May 15;19(10):1129–55. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 36.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004 Jul-Sep;8(3):301–16. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004 Apr;36(4):568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Nor JE. Tooth regeneration in operative dentistry. Oper Dent. 2006 Nov-Dec;31(6):633–42. doi: 10.2341/06-000. [DOI] [PubMed] [Google Scholar]
- 39.Tuch BE. Stem cells--a clinical update. Aust Fam Physician. 2006 Sep;35(9):719–21. [PubMed] [Google Scholar]
- 40.Hyun I. The bioethics of stem cell research and therapy. J Clin Invest. 2010 Jan;120(1):71–5. doi: 10.1172/JCI40435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000 Jun;28(6):707–15. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 42.Wu G, Cui Y, Ma L, Pan X, Wang X, Zhang B. Repairing cartilage defects with bone marrow mesenchymal stem cells induced by CDMP and TGF-beta. Cell Tissue Bank. 2013 doi: 10.1007/s10561-013-9369-x. [DOI] [PubMed] [Google Scholar]
- 43.Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007 Feb;15(2):226–31. doi: 10.1016/j.joca.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013;2013:496218. doi: 10.1155/2013/496218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Intern Med. 2013 Jul;28(4):387–402. doi: 10.3904/kjim.2013.28.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cipriani P, Carubbi F, Liakouli V, Marrelli A, Perricone C, Perricone R, et al. Stem cells in autoimmune diseases: Implications for pathogenesis and future trends in therapy. Autoimmun Rev. 2012 May;12(7):709–49. 16. doi: 10.1016/j.autrev.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Cipriani P, Di Benedetto P, Liakouli V, Del Papa B, Di Padova M, Di Ianni M, et al. Mesenchymal stem cells (MSCs) from scleroderma patients (SSc) preserve their immunomodulatory properties although senescent and normally induce T regulatory cells (Tregs) with a functional phenotype: implications for cellular-based therapy. Clin Exp Immunol. 2013 Aug;173(2):195–206. doi: 10.1111/cei.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cipriani P, Marrelli A, Benedetto PD, Liakouli V, Carubbi F, Ruscitti P, et al. Scleroderma Mesenchymal Stem Cells display a different phenotype from healthy controls; implications for regenerative medicine. Angiogenesis. 2013 Jul;16(3):595–607. doi: 10.1007/s10456-013-9338-9. [DOI] [PubMed] [Google Scholar]
- 49.Loebinger MR, Janes SM. Stem cells as vectors for antitumour therapy. Thorax. 2010 Apr;65(4):362–9. doi: 10.1136/thx.2009.128025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madjd Z, Gheytanchi E, Erfani E, Asadi-Lari M. Application of stem cells in targeted therapy of breast cancer: a systematic review. Asian Pac J Cancer Prev. 2013;14(5):2789–800. doi: 10.7314/apjcp.2013.14.5.2789. [DOI] [PubMed] [Google Scholar]
- 51.Yi BR, Choi KJ, Kim SU, Choi KC. Therapeutic potential of stem cells expressing suicide genes that selectively target human breast cancer cells: evidence that they exert tumoricidal effects via tumor tropism (review) Int J Oncol. 2012 Sep;41(3):798–804. doi: 10.3892/ijo.2012.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dwyer RM, Khan S, Barry FP, O’Brien T, Kerin MJ. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res Ther. 2010;1(3):25. doi: 10.1186/scrt25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grisendi G, Bussolari R, Cafarelli L, Petak I, Rasini V, Veronesi E, et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. 2010 May 1;70(9):3718–29. doi: 10.1158/0008-5472.CAN-09-1865. [DOI] [PubMed] [Google Scholar]
- 54.Mohit E, Rafati S. Biological delivery approaches for gene therapy: Strategies to potentiate efficacy and enhance specificity. Mol Immunol. 2013 Dec 31;56(4):599–611. doi: 10.1016/j.molimm.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Cihova M, Altanerova V, Altaner C. Stem cell based cancer gene therapy. Mol Pharm. 2011 Oct 3;8(5):1480–7. doi: 10.1021/mp200151a. [DOI] [PubMed] [Google Scholar]
- 56.Altaner C, Altanerova V. Stem cell based glioblastoma gene therapy. Neoplasma. 2012;59(6):756–6055. doi: 10.4149/neo_2012_95. [DOI] [PubMed] [Google Scholar]
- 57.Altaner C, Altanerova V, Cihova M, Ondicova K, Rychly B, Baciak L. Complete regression of glioblastoma by mesenchymal stem cells mediated prodrug gene therapy simulating clinical therapeutic scenario. Int J Cancer. 2013 Aug 26; doi: 10.1002/ijc.28455. [DOI] [PubMed] [Google Scholar]
- 58.Lee JY, Lee DH, Kim HA, Choi SA, Lee HJ, Park CK, et al. Double suicide gene therapy using human neural stem cells against glioblastoma: double safety measures. J Neurooncol. 2013:60. doi: 10.1007/s11060-013-1264-6. [DOI] [PubMed] [Google Scholar]
- 59.Wang C, Natsume A, Lee HJ, Motomura K, Nishimira Y, Ohno M, et al. Neural stem cell-based dual suicide gene delivery for metastatic brain tumors. Cancer Gene Ther. 2012 Nov;19(11):796–801. doi: 10.1038/cgt.2012.63. [DOI] [PubMed] [Google Scholar]
- 60.Binello E, Germano IM. Stem cells as therapeutic vehicles for the treatment of high-grade gliomas. Neuro Oncol. 2012 Mar;14(3):256–65. doi: 10.1093/neuonc/nor204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim SU. Neural stem cell-based gene therapy for brain tumors. Stem Cell Rev. 2011 Mar;7(1):130–40. doi: 10.1007/s12015-010-9154-1. [DOI] [PubMed] [Google Scholar]
- 62.Chiocca EA, Aguilar LK, Bell SD, Kaur B, Hardcastle J, Cavaliere R. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011 Sep 20;29(27):3611–9. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bagci-Onder T, Wakimoto H, Anderegg M, Cameron C, Shah K. A dual PI3K/mTOR inhibitor, PI-103, cooperates with stem cell-delivered TRAIL in experimental glioma models. Cancer Res. 2010 Jan 1;71(1):154–63. doi: 10.1158/0008-5472.CAN-10-1601. [DOI] [PubMed] [Google Scholar]
- 64.Sharkis SJ, Jones RJ, Civin C, Jang YY. Pluripotent stem cell-based cancer therapy: promise and challenges. Sci Transl Med. 2012 Mar 28;4(127):127ps9. doi: 10.1126/scitranslmed.3003920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knoepfler PS. Key anticipated regulatory issues for clinical use of human induced pluripotent stem cells. Regen Med. 2012;7(5):713–720. doi: 10.2217/rme.12.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldring CE, Duffy PA, Benvenisty N, Andrews PW, Ben-David U, Eakins R, French N, Hanley NA, Kelly L, Kitteringham NR, Kurth J, Ladenheim D, Laverty H, McBlane J, Narayanan G, Patel S, Reinhardt J, Rossi A, Sharpe M, Park BK. Assessing the safety of stem cell therapeutics. Cell Stem Cell. 2011 Jun 3;8(6):618–28. doi: 10.1016/j.stem.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 67.Lowry WE, Quan WL. Roadblocks en route to the clinical application of induced pluripotent stem cells. J Cell Sci. 2010 Mar 1;123(Pt 5):643–51. doi: 10.1242/jcs.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fong CY, Gauthaman K, Bongso A. Teratomas from pluripotent stem cells: A clinical hurdle. J Cell Biochem. 2010;111:769–781. doi: 10.1002/jcb.22775. [DOI] [PubMed] [Google Scholar]
- 69.Malecki M, LaVanne C, Alhambra D, Dodivenaka C, Nagel S, Malecki R. Safeguarding stem cell-based regenerative therapy against iatrogenic cancerogenesis: Transgenic expression of DNASE1, DNASE1L3, DNASE2, DFFB controlled by POLA promoter in directed-differentiation-resistant and proliferating human autologous pluripotent induced stem cells. J Stem Cell Res Ther. 2013;S9–005:1–11. doi: 10.4172/2157-7633.S9-005. Epub 2013 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011 Apr;11(4):268–77. doi: 10.1038/nrc3034. Epub 2011 Mar 10. [DOI] [PubMed] [Google Scholar]
- 71.Kooreman NG, Wu JC. Tumorigenicity of pluripotent stem cells: biological insights from molecular imaging. J R Soc Interface. 2010 Dec 6;7( Suppl 6):S753–63. doi: 10.1098/rsif.2010.0353.focus. Epub 2010 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shekhani MT, Jayanthy AS, Maddodi N, Setaluri V. Cancer stem cells and tumor transdifferentiation: implications for novel therapeutic strategies. Am J Stem Cells. 2013 Mar 8;2(1):52–61. Print 2013. [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Z, Gong Y, Guo Y, Hai Y, Yang H, Yang S, Liu Y, Ma M, Liu L, Li Z, Gao WQ, He Z. Direct transdifferentiation of spermatogonial stem cells to morphological, phenotypic and functional hepatocyte-like cells via the ERK1/2 and Smad2/3 signaling pathways and the inactivation of cyclin A, cyclin B and cyclin ECell Commun Signal. 2013 Sep 18;11:67. doi: 10.1186/1478-811X-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swales N, Martens GA, Bonné S, Heremans Y, Borup R, Van de Casteele M, Ling Z, Pipeleers D, Ravassard P, Nielsen F, Ferrer J, Heimberg H. Plasticity of adult human pancreatic duct cells by neurogenin3-mediated reprogramming. PLoS One. 2012;7(5):e37055. doi: 10.1371/journal.pone.0037055. Epub 2012 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, Hill JA, DiMaio JM, Baker LA, Bassel-Duby R, Olson EN. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci U S A. 2013 Apr 2;110(14):5588–93. doi: 10.1073/pnas.1301019110. Epub 2013 Mar 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malecki M, Putzer E, Sabo C, Foorohar A, Quach C, Stampe C, Beauchaine M, Tombokan X, Anderson M. Directed cardiomyogenesis of human, autologous, pluripotent, induced, stem cells reprogrammed from peripheral blood, mononuclear cells and retained to infarcted myocardium with bioengineered antibodies: Novel strategy aimed at improving cardiac regenerative therapy. Molecular and Cellular Therapies. 2013;1( 4):1–32. doi: 10.1186/2052-8426-1-4. [DOI] [Google Scholar]
- 77.Buganim Y, Jaenisch R. Transdifferentiation by defined factors as a powerful research tool to address basic biological questions. Cell Cycle. 2012 Dec 15;11(24):4485–6. doi: 10.4161/cc.22665. Epub 2012 Nov 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011 Sep 2;9(3):205–18. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Efe JA, Ding S. Reprogramming, transdifferentiation and the shifting landscape of cellular identity. Cell Cycle. 2011 Jun 15;10(12):1886–7. doi: 10.4161/cc.10.12.15591. Epub 2011 Jun 15. No abstract available. [DOI] [PubMed] [Google Scholar]
- 80.Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A. 2011 May 10;108(19):7838–43. doi: 10.1073/pnas.1103113108. Epub 2011 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Adsani A, Burke ZD, Eberhard D, Lawrence KL, Shen CN, Rustgi AK, Sakaue H, Farrant JM, Tosh D. Dexamethasone treatment induces the reprogramming of pancreatic acinar cells to hepatocytes and ductal cells. PLoS One. 2010 Oct 27;5(10):e13650. doi: 10.1371/journal.pone.0013650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Masip M, Veiga A, Izpisúa Belmonte JC, Simón C. Reprogramming with defined factors: from induced pluripotency to induced transdifferentiation. Mol Hum Reprod. 2010 Nov;16(11):856–68. doi: 10.1093/molehr/gaq059. Epub 2010 Jul 8. Review. [DOI] [PubMed] [Google Scholar]
- 83.Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, Loh KM, Carter AC, Di Giorgio FP, Koszka K, Huangfu D, Akutsu H, Liu DR, Rubin LL, Eggan K. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009 Nov 6;5(5):491–503. doi: 10.1016/j.stem.2009.09.012. Epub 2009 Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chu K, Kim M, Chae SH, Jeong SW, Kang KS. Distribution and in situ proliferation patterns of injected immortalized human neural stem-like focal cerebral ischemia. Neurosci Res. 50:459–465. doi: 10.1016/j.neures.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 85.Kwon SK, Kim SU, Song JJ, Cho CG, Park SW. Selective delivery of a therapeutic gene for treatment of head and neck squamous cell carcinoma using human neural stem cells. Clin Exp Otorhinolaryngol. 2013 Sep;6(3):176–83. doi: 10.3342/ceo.2013.6.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chai LP, Wang ZF, Liang WY, Chen L, Chen D, Wang AX, et al. In vitro and in vivo effect of 5-FC combined gene therapy with TNF-alpha and CD suicide gene on human laryngeal carcinoma cell line Hep-2. PLoS One. 2013;8(4):e61136. doi: 10.1371/journal.pone.0061136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng L, Zhang D, Chen X, Yang L, Wei Y, Zhao X. Antitumor activities of human placenta-derived mesenchymal stem cells expressing endostatin on ovarian cancer. PLoS One. 2012;7(7):e39119. doi: 10.1371/journal.pone.0039119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim KY, Kim SU, Leung PC, Jeung EB, Choi KC. Influence of the prodrugs 5-fluorocytosine and CPT-11 on ovarian cancer cells using genetically engineered stem cells: tumor-tropic potential and inhibition of ovarian cancer cell growth. Cancer Sci. 2010 Apr;101(4):955–62. doi: 10.1111/j.1349-7006.2009.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malecki M, Dahlke J, Haig M, Wohlwend L, Malecki R. Eradication of ovarian cancer cells by transgenic expression of recombinant DNASE1, DNASE1L3, DNASE2, and DFFB controlled by EGFR promoter: Novel strategy for personalized therapy of cancer. J Genetic Syndromes and Gene Therapy. 2013;4(6):152:1–11. doi: 10.4172/2157-7412.1000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li TS, Yawata T, Honke K. Efficient siRNA delivery and tumor accumulation mediated by ionically cross-linked folic acid-poly(ethylene glycol)-chitosan oligosaccharide lactate nanoparticles: For the potential targeted ovarian cancer gene therapy. Eur J Pharm Sci. 2013 Oct 28; doi: 10.1016/j.ejps.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 91.Kim KH, Dmitriev I, O’Malley JP, Wang M, Saddekni S, You Z, et al. A phase I clinical trial of Ad5.SSTR/TK.RGD, a novel infectivity-enhanced bicistronic adenovirus, in patients with recurrent gynecologic cancer. Clin Cancer Res. 2012 Jun 15;18(12):3440–51. doi: 10.1158/1078-0432.CCR-11-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Piva M, Domenici G, Iriondo O, Rabano M, Simoes BM, Comaills V. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol Med. 2013 Oct 31; doi: 10.1002/emmm.201303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Brien CS, Howell SJ, Farnie G, Clarke RB. Resistance to endocrine therapy: are breast cancer stem cells the culprits? J Mammary Gland Biol Neoplasia. 2009 Mar;14(1):45–54. doi: 10.1007/s10911-009-9115-y. [DOI] [PubMed] [Google Scholar]
- 94.Zarogoulidis P, Darwiche K, Hohenforst-Schmidt W, Huang H, Li Q, Freitag L, et al. Inhaled gene therapy in lung cancer: proof-of-concept for nano-oncology and nanobiotechnology in the management of lung cancer. Future Oncol. 2013 Aug;9(8):1171–94. doi: 10.2217/fon.13.67. [DOI] [PubMed] [Google Scholar]
- 95.Kim YK, Cho CS, Cho MH, Jiang HL. Spermine-alt-poly(ethylene glycol) polyspermine as a safe and efficient aerosol gene carrier for lung cancer therapy. J Biomed Mater Res A. 2013 Aug 8; doi: 10.1002/jbm.a.34905. [DOI] [PubMed] [Google Scholar]
- 96.Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012 Mar 10;30(8):863. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qu H, Li R, Liu Z, Zhang J, Luo R. Prognostic value of cancer stem cell marker CD133 expression in non-small cell lung cancer: a systematic review. Int J Clin Exp Pathol. 2013;6(11):2644–50. [PMC free article] [PubMed] [Google Scholar]
- 98.Liu F, Cao X, Liu Z, Guo H, Ren K, Quan M, et al. Casticin suppresses self-renewal and invasion of lung cancer stem-like cells from A549 cells through down-regulation of pAkt. Acta Biochim Biophys Sin (Shanghai) 2013 Nov 17; doi: 10.1093/abbs/gmt123. [DOI] [PubMed] [Google Scholar]
- 99.Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Onoyama M, Ohnishi M, et al. Stroma-directed imatinib therapy impairs the tumor-promoting effect of bone marrow-derived mesenchymal stem cells in an orthotopic transplantation model of colon cancer. Int J Cancer. 2013 Feb 15;132(4):813–23. doi: 10.1002/ijc.27735. [DOI] [PubMed] [Google Scholar]
- 100.Lin SP, Lee YT, Yang SH, Miller SA, Chiou SH, Hung MC, et al. Colon cancer stem cells resist antiangiogenesis therapy-induced apoptosis. Cancer Lett. 2012 Jan 28;328(2):226–34. doi: 10.1016/j.canlet.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 101.Abel EV, Simeone DM. Biology and clinical applications of pancreatic cancer stem cells. Gastroenterology. 2013 Jun;144(6):1241–8. doi: 10.1053/j.gastro.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 102.Li Y, Kong D, Ahmad A, Bao B, Sarkar FH. Pancreatic cancer stem cells: emerging target for designing novel therapy. Cancer Lett. 2013 Sep 10;338(1):94–100. doi: 10.1016/j.canlet.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007 Feb 1;67(3):1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 104.Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011 Dec;141(6):2218–27. e5. doi: 10.1053/j.gastro.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 105.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007 Sep 13;1(3):313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Herreros-Villanueva M, Zhang JS, Koenig A, Abel EV, Smyrk TC, Bamlet WR, et al. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis. 2013;2:e61. doi: 10.1038/oncsis.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van den Broeck A, Vankelecom H, Van Delm W, Gremeaux L, Wouters J, Allemeersch J, et al. Human pancreatic cancer contains a side population expressing cancer stem cell-associated and prognostic genes. PLoS One. 2013;8(9):e73968. doi: 10.1371/journal.pone.0073968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fukunaga-Kalabis M, Martinez G, Nguyen TK, Kim D, Santiago-Walker A, Roesch A, et al. Tenascin-C promotes melanoma progression by maintaining the ABCB5-positive side population. Oncogene. 2010 Nov 18;29(46):6115–24. doi: 10.1038/onc.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee N, Barthel SR, Schatton T. Melanoma stem cells and metastasis: mimicking hematopoietic cell trafficking? Lab Invest. 2013 Oct 14; doi: 10.1038/labinvest.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mohamed A, Gonzalez RS, Lawson D, Wang J, Cohen C. Tumor Stem Cells (CD271, c-kit, SOX10) in Melanomas: Prognostic and Outcome Implications. Appl Immunohistochem Mol Morphol. 2013 Aug 16; doi: 10.1097/PAI.0b013e3182910a3d. [DOI] [PubMed] [Google Scholar]
- 111.Schatton T, Schutte U, Frank NY, Zhan Q, Hoerning A, Robles SC, et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 2010 Jan 15;70(2):697–708. doi: 10.1158/0008-5472.CAN-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murphy GF, Wilson BJ, Girouard SD, Frank NY, Frank MH. Stem cells and targeted approaches to melanoma cure. Mol Aspects Med. 2013 Oct 19; doi: 10.1016/j.mam.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011 Sep;17(9):1086–93. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- 114.Felipe Rico J, Hassane DC, Guzman ML. Acute myelogenous leukemia stem cells: from Bench to Bedside. Cancer Lett. 2013 Sep 10;338(1):4–9. doi: 10.1016/j.canlet.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roboz GJ. Novel approaches to the treatment of acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:43–50. doi: 10.1182/asheducation-2011.1.43. [DOI] [PubMed] [Google Scholar]
- 116.Attar EC, Johnson JL, Amrein PC, Lozanski G, Wadleigh M, DeAngelo DJ, et al. Bortezomib added to daunorubicin and cytarabine during induction therapy and to intermediate-dose cytarabine for consolidation in patients with previously untreated acute myeloid leukemia age 60 to 75 years: CALGB (Alliance) study 10502. J Clin Oncol. 2013 Mar 1;31(7):923–9. doi: 10.1200/JCO.2012.45.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Attar EC, De Angelo DJ, Supko JG, D’Amato F, Zahrieh D, Sirulnik A, et al. Phase I and pharmacokinetic study of bortezomib in combination with idarubicin and cytarabine in patients with acute myelogenous leukemia. Clin Cancer Res. 2008 Mar 1;14(5):1446–54. doi: 10.1158/1078-0432.CCR-07-4626. [DOI] [PubMed] [Google Scholar]
- 118.Aikawa Y, Katsumoto T, Zhang P, Shima H, Shino M, Terui K. PU.1-mediated upregulation of CSF1R is crucial for leukemia stem cell potential induced by MOZ-TIF2. Nat Med. 2010 May;16(5):580–5. doi: 10.1038/nm.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Choo AB, Tan HL, Ang SN, Fong WJ, Chin A, Lo J, Zheng L, Hentze H, Philp RJ, Oh SK, Yap M. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells. 2008 Jun;26(6):1454–63. doi: 10.1634/stemcells.2007-0576. Epub 2008 Mar 20. [DOI] [PubMed] [Google Scholar]
- 120.Tang C, Lee AS, Volkmer JP, Sahoo D, Nag D, Mosley AR, Inlay MA, Ardehali R, Chavez SL, Pera RR, Behr B, Wu JC, Weissman IL, Drukker M. An antibody against SSEA-5 glycan on human pluripotent stem cells enables removal of teratoma-forming cells. Nat Biotechnol. 2011 Aug 14;29(9):829–34. doi: 10.1038/nbt.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ben-David U, Nudel N, Benvenisty N. Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nature Communications. 2013;4:#1992. doi: 10.1038/ncomms2992151. [DOI] [PubMed] [Google Scholar]
- 122.Schriebl K, Satianegara G, Hwang A, Tan HL, Fong WJ, Yang HH, Jungbauer A, Choo A. Selective removal of undifferentiated human embryonic stem cells using magnetic activated cell sorting followed by a cytotoxic antibody. Tissue Eng Part A. 2012 May;18(9–10):899–909. doi: 10.1089/ten.TEA.2011.0311. Epub 2012 Jan 4. [DOI] [PubMed] [Google Scholar]
- 123.Lim DY, Ng YH, Lee J, Mueller M, Choo AB, Wong VV. Cytotoxic antibody fragments for eliminating undifferentiated human embryonic stem cells. J Biotechnol. 2011 May 20;153(3–4):77–85. doi: 10.1016/j.jbiotec.2011.03.017. Epub 2011 Mar 30. [DOI] [PubMed] [Google Scholar]
- 124.Ben-David U, Gan QF, Golan-Lev T, Arora P, Yanuka O, Oren YS, Leikin-Frenkel A, Graf M, Garippa R, Boehringer M, Gromo G, Benvenisty N. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013 Feb 7;12(2):167–79. doi: 10.1016/j.stem.2012.11.015. Epub 2013 Jan 11. [DOI] [PubMed] [Google Scholar]
- 125.Schuldiner M, Itskovitz-Eldor J, Benvenisty N. Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cells. 2003;21(3):257–65. doi: 10.1634/stemcells.21-3-257. [DOI] [PubMed] [Google Scholar]
- 126.Cheng F, Ke Q, Chen F, Cai B, Gao Y, Ye C, Wang D, Zhang L, Lahn BT, Li W, Xiang AP. Protecting against wayward human induced pluripotent stem cells with a suicide gene. Biomaterials. 2012 Apr;33(11):3195–204. doi: 10.1016/j.biomaterials.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 127.Malecki M, Szybalski W. Isolation of single, intact chromosomes from single, selected ovarian cancer cells for in situ hybridization and next generation sequencing. Gene. 2012;493(1):132–9. doi: 10.1016/j.gene.2011.11.044. http://www.ncbi.nlm.nih.gov/pubmed/22155315. [DOI] [PubMed] [Google Scholar]


