Abstract
Nanotechnology is an exciting and powerful discipline of science; the altered properties of which have offered many new and profitable products and applications. Agriculture, food and medicine sector industries have been investing more in nanotechnology research. Plants or their extracts provide a biological synthesis route of several metallic nanoparticles which is more eco-friendly and allows a controlled synthesis with well-defined size and shape. The rapid drug delivery in the presence of a carrier is a recent development to treat patients with nanoparticles of certain metals. The engineered nanoparticles are more useful in increasing the crop production, although this issue is still in infancy. This is simply due to the unprecedented and unforeseen health hazard and environmental concern. The well-known metal ions such as zinc, iron and copper are essential constituents of several enzymes found in the human system even though the indiscriminate use of similar other metal nanoparticle in food and medicine without clinical trial is not advisable. This review is intended to describe the novel phytosynthesis of metal and metal oxide nanoparticles with regard to their shape, size, structure and diverse application in almost all fields of medicine, agriculture and technology. We have also emphasized the concept and controversial mechanism of green synthesis of nanoparticles.
Keywords: Metal, Metal oxide, Carbon nanomaterials, Green synthesis, Agriculture, Medicine
Review
Introduction
Globally, incredible changes in agricultural production patterns have taken place. It has become possible only through the application of modern labour saving technologies for intensive on-farm mechanization, irrigation, postharvest handling and use of improved crop varieties. Despite the tremendous progress made in agricultural productivity, still there exists food insecurity and poverty in many developing countries. Nanotechnology is an area which includes almost all branches of science including biology, chemistry, physics, engineering and medicine, although the main thrust of research focuses on the food and agriculture. The products based on nanotechnologies were estimated to be more than 800 and expected to raise more in the market within the next few years [1,2]. By next year, it is expected that more than 15% of all products on the global market will have some kind of nanotechnology incorporated into their manufacturing process [3].
The major global problem is to increase food production with limited resources and minimum and efficient use of fertilizer and pesticides without polluting the environment. A variety of nanomaterials have been tested against germination of seeds, growth of shoot/root and crop production besides testing their adverse effect on the flora and fauna. The Food and Agriculture Organization (FAO) of the United Nations and World Health Organization (WHO) at their expert meeting on the ‘application of nanotechnologies in the food and agriculture sectors’ in Rome in 2010 have identified the potential of nanotechnology in food and agriculture sectors and are investing heavily in its application to food production at a global level [4]. It was aimed at developing innovative ways to increase food production, water treatment, preservation and packaging besides toxicology and human health risk associated with the use of nanotechnology. Since the engineered nanoparticles of 1- to 100 nm may have different physical and chemical properties than the naturally occurring ones, their impact on human health must be assessed as a function of their size and shape. The committee recognized the potential risk and benefits of nanotechnology but wanted the sponsored researchers to address these issues in their ongoing projects. The global market in nanotechnology is expected to reach US$1 trillion by 2015 [5].
Plants are able to hyperaccumulate metals, up to concentrations several hundreds of times those found in non-hyperaccumulating plants [6-8]. It is thought that this provides a measure of protection for the plant from insects and other herbivores. The use of nanoparticles in the growth of plants and control of plant diseases is a recent practice [9-13]. Nanomaterial can be used in the diagnosis of some plant diseases by labelled nanoparticles. It can be helpful in the increased production of useful small edible plants such as spinach, radish, rye or grain like maize, rice and wheat [14]. Nanotechnology has potential for the controlled release of drug, nutrients and pesticides/agrochemicals for efficient use of trace elements without disturbing the non-target insects [15]. It also provides way to convert organic wastes to useful products [15,16]. Porous hollow silica nanoparticles are used for the controlled delivery of the water-soluble pesticide validamycin [17]. Biodegradable organic waste and plant or fruit peeling have also been used for the synthesis of nanoparticles as all of these contain phenols, flavonoids and reducing agents [18-20]. Nanoparticles reveal completely new or improved properties based on specific characteristics such as size, distribution and morphology, if compared with larger particles of the bulk material they are made of [21]. Since the absorption of minerals by the plant is non-selective, some of the metal ions in conjunction with anions may cause toxicity if they exceed the tolerance limit of the plant. When the nanoparticles are absorbed, they are subsequently translocated and accumulated in different parts of the plants forming complex with carrier proteins. It is, however, not yet clear as to how some plant species select certain nanoparticles and reject others. If they are larger than the pore of root, they get accumulated at the surface, and when they are smaller, they get absorbed and transported to other parts of the plants.
It is the present requirement to produce more food crops from the extant resources. Genetically modified crops are a way to substantially produce better food grain, but it has some implications [22]. The production of food crop from engineered nanoparticle is another alternative. A wide range of metal oxide nanoparticles (ZnO, TiO2, Al2O3, FeO, Fe2O3, etc.), fullerenes, carbon nanotubes, quantum dots, etc. have an increasing range of applications (Figure 1) for different purposes [23] and make their way easily in the environment [24,25]. Their potential adverse effects on the environment and human health are being subjected to intense debate [26]. Although nanoparticles, whether natural or synthetic, are being used in every sphere of life, their exploitation in agriculture is limited. Studies have been directed towards seed germination, root elongation, foliar growth and seed and crop development [27]. The use of nanoparticles without knowing the toxic effect on the plant may sometimes cause mutation, which may be very damaging to both plants and ecosystem. Nanoparticles when sprayed or inoculated will penetrate and transported to various parts of the plant. Some nanoparticles are stored in extracellular space and some within the cell. Some plants reject the nanoparticles and some accept or store them (Figure 2). Inadvertent use of rare and precious metal nanoparticles generally does not show any positive effect on the plant except for their storage and blocking the passage of vessels [28-30]. The process of nanoparticle accumulation in plants may be used to clean up nanoparticle contamination and extraction of metal from such plants. The extraction of metal from such plants is called phytomining or phytoextraction [6,31,32]. An et al. [33] have reported an increase in ascorbate and chlorophyll contents in leaves of asparagus treated with silver nanoparticles. Likewise, soybean treated with nano-iron showed increased weight of beans [34]. The result is not always positive as in some cases no effect or negative effect was noted when the plants were treated with gold, silver or copper nanoparticles [35,36].
Figure 1.
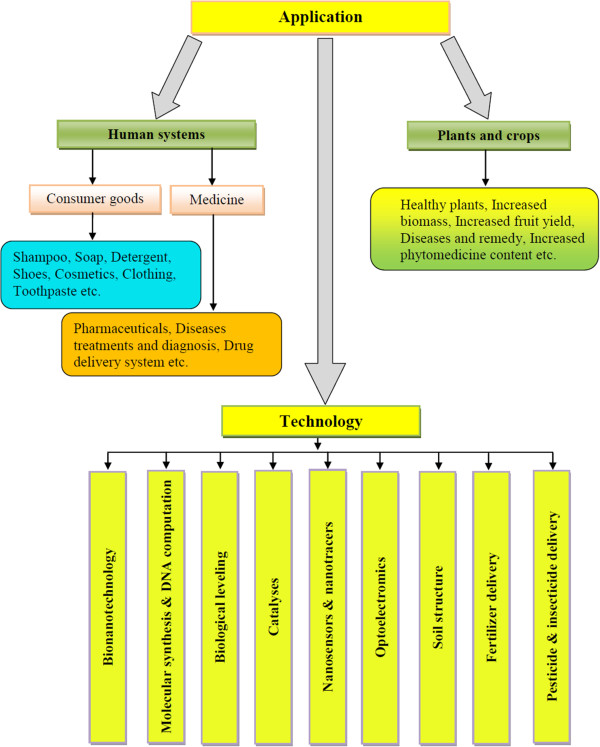
Application of engineered nanoparticles in living systems.
Figure 2.
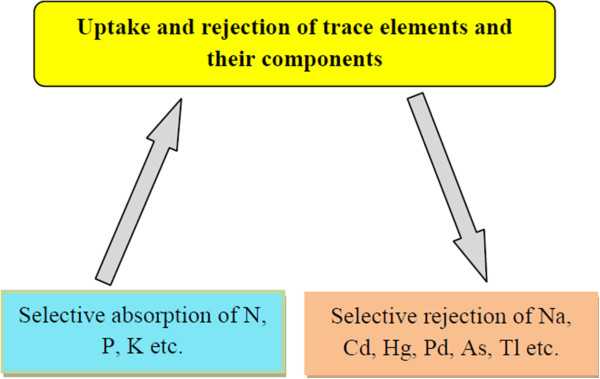
Selective absorption and rejection of nanoparticles.
Nanoparticles of commercial importance are being synthesized directly from metal or metal salts, in the presence of some organic material or plant extract. The creepers and many other plants exude an organic material, probably a polysaccharide with some resin, which help plants to climb vertically or through adventitious roots to produce nanoparticles of the trace elements present, so that they may be absorbed. One such example comes from English ivy (Hedera helix) which produces from its adventitious root hairs' nanocomposite adhesive that contains spherical nanoparticles of 60- to 85 nm diameter. The production of the nanoparticles depends on the proliferation of the adventitious roots. Usually, indole-3-butyric acid (IBA) and α-naphthalene acetic acid (NAA) have been recommended for promoting adventitious roots in shoot cutting propagation in many shrub [37-39] or tree [40-42]. In order to increase the proliferation of the root to produce larger quantity of the composite nanomaterial from English ivy, an auxin namely IBA was used as a root growth enhancer. Maximum root production was achieved by soaking the shoot segments of the climber in 0.1 mg mL-1 IBA [43]. It is worth mentioning that the adventitious root hairs which do not come in touch with the solid surface dry up and abort. The overall production of the composite nanomaterial is only 0.75% which is sufficient to support the plant. It is uncertain whether such material can be used for the production of metal nanoparticles as these are nanomaterial themselves. However, it may be used in hardening and cementing the teeth because it dries up quickly. Further studies from the plant resin and gums may enhance our knowledge in this area.
This review is intended to discuss the phytosynthesis of metal and metal oxide nanoparticles including carbon nanomaterials and their application in agriculture, medicine and technology.
Engineered nanoparticles
The synthesis of nanoparticles (Figure 3) and their application in allied field has become the favourite pursuit of all scientists including biologist, chemists and engineers. It is known that almost all plants (herbs, shrubs or trees) containing aroma, latex, flavonoids, phenols, alcohols and proteins can produce metal nanoparticles from the metal salts (Figure 4). Although nanoparticles can be chemically synthesized by conventional methods, biosynthesis prevents the atmosphere from pollution. The shape and size of nanoparticles may be controlled and a desired type of nanoparticle may be produced by controlling the temperature and concentration of the medium. Engineered nanoparticles may be classified into the metal (or non-metal) and metal oxide nanoparticles.
Figure 3.
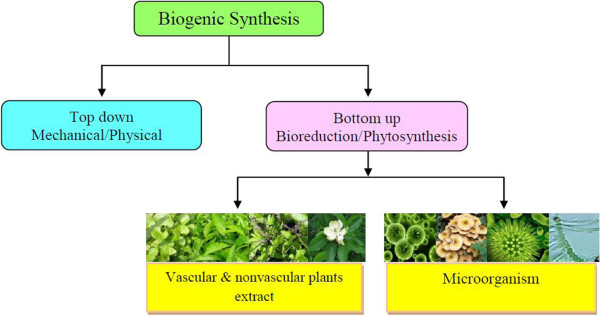
Flow diagram for biogenic synthesis of nanoparticles.
Figure 4.

Herbs, shrubs and trees for nanoparticle fabrication.
Metal nanoparticles
Synthesis of engineered nanoparticles is usually done by the interaction of microorganisms, algae or plant extracts. It is quite obvious that nanomaterials may be useful or harmful in living system depending on their shape, size and above all the nature of specific metal ion. The effect of engineered metal nanoparticles of varying size and concentration on different parts of a variety of plants is given in Table 1.
Table 1.
Effects of engineered metal nanoparticles on plants
| Nanoparticle | Size (nm) | Plant | Concentration | Effect | References |
|---|---|---|---|---|---|
| Aluminium |
|
Corn, cucumber, lettuce, radish, rapeseed |
2,000 mg L-1 |
No effect on germination |
[44] |
| 1 to 100 |
Red kidney beans, ryegrass |
10, 100, 1,000 and 10,000 mg L-1 |
No toxicity |
[45] |
|
| |
Radish, rapeseed |
2,000 mg L-1 |
Improved root growth |
[44] |
|
| |
Ryegrass |
2,000 mg L-1 |
Decreased root length |
[44] |
|
| |
Ryegrass |
2,000 mg L-1 |
Reduced germination |
[44] |
|
| |
Corn, lettuce |
2,000 mg L-1 |
Reduced root length |
[44] |
|
| Copper |
|
Lettuce |
0.013% (w/w) |
No effect on germination, improved shoot/root ratio |
[13] |
| |
Mung bean |
<200 mg L-1 |
Reduced seedling growth |
[30] |
|
| |
Mung bean |
800 mg L-1 |
Reduced shoot growth |
[30] |
|
| |
Wheat |
<200 mg L-1 |
Reduced root and seedling growth |
[30] |
|
| 50 |
Zucchini |
1,000 mg L-1 |
Reduced biomass |
[46] |
|
| 50 |
Zucchini |
1,000 mg L-1 |
Reduced root growth |
[46] |
|
| Dodecanethiol-functionalized gold |
|
Lettuce |
0.013% (w/w) |
No effect on germination, improved shoot/root ratio |
[13] |
| Gold |
10 |
Cucumber, lettuce |
62, 100 and 116 mg L-1 |
Positive effect on germination index |
[47] |
| Iron |
|
Flax, meadow fescue, red clover, white clover |
100, 250 and 500 mg L-1 |
No effect on germination |
[48] |
| |
Barley, ryegrass |
100 and 250 mg L-1 |
No effect on germination |
[48] |
|
| |
Barley, flax, ryegrass |
2,000 and 5,000 mg L-1 |
Completely inhibited germination |
[48] |
|
| |
Barley |
300 mg L-1 |
Reduced germination |
[48] |
|
| |
Flax, barley, ryegrass |
>1,500 mg L-1 |
No germination |
[48] |
|
| Mixture of gold/copper |
|
Lettuce |
0.013% (w/w) |
No effect on germination, improved shoot/root ratio |
[13] |
| Palladium entrapped in Al(OH)2 matrix |
|
Lettuce |
0.013% to 0.066% (w/w) |
No effect on germination, improved shoot/root ratio |
[13] |
| Silicon |
10 |
Zucchini |
1,000 mg L-1 |
Completely inhibited germination |
[46] |
| Silver |
20 |
Flax |
20, 40, 60, 80 and 100 mg L-1 |
No effect on germination |
[48] |
| 2 |
Cucumber, lettuce |
62, 100 and 116 mg L-1 |
Low to zero toxicity |
[47] |
|
| 20.6 ± 3.1 |
Clover |
0.01 mg kg-1 |
Reduced aboveground biomass |
[49] |
|
| 0.1 mg kg-1 |
No effect on biomass |
[49] |
|||
| 1 mg kg-1 |
No effect on biomass |
[49] |
|||
| 10 |
Wheat |
0.5, 1.5, 2.5, 3.5 and 5.0 mg kg-1 |
Reduced shoot and root length |
[50] |
|
| 5 |
Barley |
10 mg L-1 |
Reduced germination |
[48] |
|
| |
Flax, barley |
10 mg L-1 |
Reduced shoot length |
[48] |
|
| 20 |
Barley |
10 mg L-1 |
Reduced germination |
[48] |
|
| |
Barley |
10 mg L-1 |
Reduced shoot length |
[48] |
|
| |
Barley, ryegrass |
20 mg L-1 |
Reduced shoot length |
[48] |
|
| 100 |
Zucchini |
100, 500 and 1,000 mg L-1 |
Reduced transpiration |
[46] |
|
| 100 |
Zucchini |
500 and 1,000 mg L-1 |
Reduced biomass |
[46] |
|
| <100 |
Onion |
100 mg L-1 |
Decreased mitosis, disturbed metaphase, sticky chromosome, cell wall disintegration and breaks |
[51] |
|
| Silver colloidal form |
0.6 to 2 |
Ryegrass |
10 mg L-1 |
Reduced germination |
[48] |
| |
Ryegrass |
20 mg L-1 |
Reduced germination |
[48] |
|
| |
Flax, ryegrass |
10 mg L-1 |
Reduced shoot length |
[48] |
|
| |
Barley, flax, ryegrass |
20 mg L-1 |
Reduced shoot length |
[48] |
|
| Zinc | Corn, cucumber, lettuce, radish, rapeseed, ryegrass | 2,000 mg L-1 | Reduced root growth and elongation | [44] |
The toxic metals like Cd, Hg, Pb and Tl will always produce toxic nanoparticles which may produce adverse effect in both plants and animals whether aquatic or terrestrial. However, several positive effects of engineered metal nanoparticles have been practically proved. Zn is known to be an essential element for both plants and animals. Since it is an essential constituent of over 30 enzymes, the activity of such metalloenzymes is lost during deficiency of the metal. It has always positive effect in the human system, provided it does not exceed the permissible limit. A suspension of 200 mg Zn L-1 showed phytotoxicity in certain vegetable plants [44], although such concentration is seldom attained in nature. It is clear that a concentration of up to 1 to 4 mg Zn L-1 does not exhibit any phytotoxicity which means that such results can be obtained only under experimental conditions. The phytotoxicity causes retardation in growth to the extent of plant being stunted. This effect can successfully be used in growing bonsai and ornamental plants on large scale. The effect that is produced after years of pruning the plants can be achieved in few months. Further, most frequently used engineered metal nanoparticles are discussed in the forthcoming sections.
Silver nanoparticles
Silver nanoparticles may be used in cosmetics, food and medicine. The Ag nanocrystals or even the silver metal is known to possess antibacterial, antifungal and antioxidant properties [52-58]. They may also be useful in catalysis, although no specific reaction is known where Ag metal may have been used as a catalyst. The Ag nanoparticles or even silver nitrate is used in ointments to cure injury and burns as it prevents infection from spreading over the wound, increasing the surface area [59]. Unlike zinc oxide, silver has the inherent tendency to kill the bacteria without interacting deep into the cell wall of the microorganism. Zinc oxide, on the other hand, interacts with the enzyme present in the body cell which prevents further multiplication of microbes. Although the synthesis of nanoparticles using a variety of chemicals has become a focal theme in the recent time, biosynthesis of nanocrystals of varying shapes and sizes using plant extracts containing redox chemicals is prevalent. Such technologies need attention perhaps because they are environment friendly and prevent from further pollution by unwanted chemicals.
Antioxidant activity of a substance is defined as the removal of free radical before it causes oxidative damage to the living system. Ag nanoparticle is believed to be capped with the oxidized form of the functional groups present in the compounds in plant extract, thereby acting as antioxidant. It has been observed that the antioxidant action of capped Ag nanoparticles containing plant extract is higher than that of the plant extract alone [50,54]. Enhanced antimicrobial activity of Ag nanoparticles prepared from Mimusops elengi was reported against multi-drug resistant clinical isolates [60]. Ag nanoparticles synthesized from Artemisia nilagirica [61] and Pongamia pinnata [62] have also been found to be active against several microorganisms. Ag nanoparticles synthesized from Morinda citrifolia root extract have also exhibited cytotoxic effect on HeLa cell lines [63].
It is quite obvious that the plant extract certainly contains substantial quantity of benign chemicals which reduce the metal salt into nanocrystals. It has been practically determined that the quantity of Cinnamomum camphora, as reductant, is responsible for the size of nanocrystals of AgNO3. When 50 mL solution of 1 mM AgNO3 is exposed to as little as 0.1 g of biomass of C. camphora at 30°C, the nanoparticles are produced within 1 h, although completion of the reaction occurs in 118 h [64]. The absorption spectrum of the reduced product containing different quantities of the leaf extract has revealed that there are two absorption peaks, a strong peak at 440 nm due to particles of one shape in abundance and a weak peak at 360 nm owing to some scattered particles of different shape. It is apparent from the scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of silver nanoparticles that the morphology of the crystals are slightly different, although their size ranges between 55- and 80 nm. The nanocrystals produced from small quantity of the biomass are scattered and are of better quality. When the quantity of biomass is increased, the time of formation of nanocrystals is drastically reduced from 118 h for 0.5 g biomass to 24 h for 1.0 g [64]. However, in such cases, the nanoparticles are aggregated, while with low quantity of the biomass, they remain segregated. It has also been observed that with increasing biomass the shape of nanocrystals also changes. The different absorption maxima correspond to different types of the nanocrystals formed. It has been reported by Huang et al. [64] that C. camphora leaf contains alkaloids, hydroxybenzenes, anthracene, steroids, terpenoids, coumarins, lactones, linalools, polysaccharides, amino acids and proteins. The silver and gold nanocrystals have been produced from the dried biomass of leaves. The study of the Fourier transform infrared (FTIR) spectrum of the dried leaf biomass before and after reduction of Ag+ and Au3+ shows changes in the functional groups of biomolecules [64]. There appear absorption bands at 1,109, 1,631 and 1,726 cm-1 which are attributed to CO, C = C and C = O stretching frequencies, respectively, in the free leaf powder. The IR spectrum of the leaf biomass recorded after the reduction of silver/gold ions shows the disappearance of the band at 1,109 cm-1 assigned to polyols present in the leaf biomass. It has been concluded that polyols are mainly responsible for the bioreduction of metal ions leaving behind RCO, which in turn, may react with the solvent to give a neutral species. The decoction of the leaf is a mixture of many compounds which cannot be identified; nevertheless, some of the frequencies remained unaltered which is believed to be due to C = C or ring vibrations. Huang et al. [64] have suggested that the shape of nanocrystals is mainly due to the protective and reductive biomolecules in the suspension. This idea of protective and reductive biomolecules is conceptually vague because when the nanocrystals are separated and dried they do not contain biomolecules to stabilize them. The biomolecules in our opinion react with other species to stay as neutral molecules after the nanocrystals have been isolated from the solvent.
Development and regeneration of root/shoot can occur in IBA-mediated adventitious root in the presence of 100 to 250 μm Na2S2O3 in agar gel [65]. The authors claimed that the potential of Na2S2O3 in facilitating culture development has not been recognized prior to this report. Many experiments were performed with different agar gels where precipitation of silver ions occurs. Generally, the incubated plant tissue culture produce ethylene and accumulation of hormone occurs which does not favour the culture growth. Addition of Ag+ ions inhibits the ethylene action. Though no one has commented on the mechanism of action of Ag+ with ethylene, it is for sure that ethylene reacts with Ag+ to give stable complex. The evolution of ethylene is not inhibited rather ethylene forms silver complex as (C2H4) Ag. Merril et al. [66] and Costa-Coquelard et al. [67] have suggested that Ag+ is precipitated as colloidal AgCl which changes colour when exposed to sunlight. Further, they have suggested that the change in colour of AgCl is a function of nanoparticle size and chemical composition. It should be viewed with caution that the composition of AgCl does not vary and being aggregate it settles at the bottom of the container. This is true that reduction of Ag+ ion is hindered unless there is some reducing agent in that medium. The effect of AgNO3 and Ag2S2O3 on shoot and root growth is comparable, although in this work [65], Ag2S2O3 has not been directly used. Na2S2O3 was added to AgNO3 as a consequence of which Ag2S2O3 would have been formed according to the following equation:
The authors have examined the effect of thiosulfate ion on the root/shoot development but simultaneously ignored the effect of the nitrate ion and did not perform any experiment with free ion to exclude its impact. Many workers have quoted that [68-70] Ag+ ions react with polysaccharide, amino acids, protein, RNA and DNA to form nanoparticles. It is not always true because Ag+ may form complex with such electron donors, and for reduction, a reducing agent is required which in turn will be oxidized.
Precipitation of Ag+ as AgCl in agar gel medium occurs due to the presence of HCl as a contaminant. If an excess of AgNO3 is added to this broth, only then free Ag+ ion will be available which may be reduced to nanosized particles. However, contrary to the present report, both the AgNO3 and Ag2S2O3 will furnish Ag+ ions which will have the same influence on the root growth, if the effect of and ions is ignored [71]. In this work [65], the Ag2S2O3 was prepared by mixing 0.1 M solutions of AgNO3 and Na2S2O3 in 1:4 M ratio at ambient temperature. Since, according to the simple metathetical reaction as given below, the two components react in 2:1 M ratio, there is always an excess of Na2S2O3 in this preparation.
Silver nanoparticles may be present with large crystal (three to five times) of Na2S2O3 and hence the influence of ions on the shoot growth may be ignored. The development of root by Ag+ ion (obtained from AgNO3) in the presence of Cl- ion is shown, which was obtained from Ag2S2O3 [65]. It is to be made clear that if the chloride ion is present in the solution, the entire AgNO3 will be precipitated and no free Ag+ ion will be available to exhibit its influence on root growth. If AgNO3 is in large excess and there is only little Cl- ion available, some of it will be available as free ions.
The silver ions may be available for interaction with other molecules. However, it is important to note that when AgNO3 is taken in the presence of Na2S2O3, the Ag2S2O3 thus formed remains dissolved, and both the Ag+ and ions are available.
The cumulative effect of both the Ag+ and ions on root development may be encountered. To eliminate the effect of ion, similar experiment, only with Na2S2O3 mediated with IBA showed that the concentration of Na2S2O3 above 100 μm was most effective [65].
Song and Kim [21] have reported the synthesis of silver nanoparticles using the leaf extract of five different plants, namely pine, persimmon, ginkgo, magnolia and platanus. Of all the five leaf extracts, magnolia leaf broth was found to be the most effective reductant for silver nitrate to silver nanoparticles. The process of production of nanoparticles was so fast that nearly 90% of Ag+ ion was converted to silver metal in about 11 min at 95°C. The average particle size ranges between 15- and 500 nm. The authors have observed that the size of the particles can be monitored by (i) changing the temperature and (ii) the concentration of AgNO3 and (iii) that of the leaf extract. It has already been studied that the particle size of the nanocrystal decreases with the increase in reaction temperature. Song and Kim [21] have hypothesized that with increasing temperature the rate of reduction of Ag+ ion to Ag also increases, stopping the secondary reduction process on the surface. It is worth observing that there is no secondary reduction process as the Ag+ ion requires only one electron (Ag+ + e- → Ag) for reduction to metal. The secondary reduction will mean capturing one more electron by silver atom to become Ag- which is impossible because it cannot hold an extra electron into its orbit.
There are some vascular plants which store crystal metal and are called metallophytes, for instance, Brassica juncea, Medicago sativa, etc. They accumulate metal up to 13.6% weight in 72 h when it is available for absorption in the form of salt, like AgNO3 [72]. It is quite obvious that reduction of AgNO3 is followed by absorption which means that the plant contains some compounds which reduce Ag+ to Ag nanoparticles of approximately 50 nm size. It has been demonstrated that the metals thus stored in the plants as nanocrystals are analytically pure to the lowest limit of detection by any instrument like AAS. The sequestering of metal by plant from a large heap of sand, sediments and non-essential non-metals is a process that saves time and manpower. If bacteria and small plants are grown in such mining areas where a large heap of nanocrystal of metal ions is available, they can be easily taken up by them and harvested. The extraction of metal by conventional method is a tedious task as it takes a long span of time; even then, it is not as pure as sequestered by plants. It has been reported by Blaylock et al. [73] that the addition of a chelating agent like ethylene diamine tetraacetate (EDTA) to the soil increases the bioavailability of the metal. It is true that EDTA forms a soluble complex with metal ions available but not the metal. The EDTA therefore acts as a carrier, not as a reductant. Since EDTA is not a selective chelating agent, it may hook up all metal ions regardless of their useful/harmful effect. If the metal remains bound to a chelating agent, it is not available even to the plants and hence may cause a deficiency of certain essential trace metals in them.
Haverkamp and Marshall [74] have studied the uptake of AgNO3, Na3Ag(S2O3)2, Ag(NH3)2NO3 and their reduction to nanoparticles by B. juncea. Quantitative determination of Ag by AAS and XANES has been done. The reduction of metal depends on the chemicals present in the plant and the concentration of metal salts in the solution. Gold [75-77], silver [78,79], copper [80] and gold-silver-copper alloy [81] nanoparticles have been reported to be present in the plants. Besides the plants, some microorganisms also induce the metal ions which are accumulated and translocated in different parts of the plants. Ni, Cu, Cd, Pb and Cr have not been exclusively found to yield nanoparticles, perhaps these are also not common metals required by the plants for their growth. The uptake and distribution of metal ion/metal itself in the plant is a matter of debate. It is not clear whether nanocrystals are formed outside of the plants and then transported through the membrane into various parts or if the nanoparticles are formed within the plant by the reduction of the metal salt. Some workers [76,77] believe that nanoparticles may be formed on the root tips and then transported into plant. If the root exudes some organic molecules which may reduce the metal salts, only then metal nanoparticles may be formed and transported. Since the root absorbs the minerals dissolved in water by osmotic pressure or capillary action, the metal salts ascend in ionic form and subsequently reduced to elemental form as nanoparticles [82]. The rate of growth of silver nanoparticle is independent of the concentration of salt but mobility is dependent on the size of ion. If the Na3Ag(S2O3)2 and AgNO3 are taken, the availability of Ag+ ion in AgNO3 will be larger than the ion. The authors suggest that three forms of Ag appear to be present (Ag+, AgNO3 and Ag2O). It is not the form of Ag but the anion in equilibrium with the cation, . However, the rate of deposition of Ag nanoparticle from AgNO3 containing small anion is faster than that with large anion like .
Gold nanoparticles
Biosynthesis of gold nanoparticles depends on the (i) concentration of plant extract or biomass, (ii) concentration of metal salt, (iii) temperature and (iv) pH of the solution. It has been observed during the synthesis of gold nanoparticles by Avena sativa biomass that several types of nanoparticles are produced with different structures [83]. The face centred cubic, tetrahedral, hexagonal, decahedral, icosahedral and irregular rod-shaped gold nanoparticles were produced. The yield was highest at pH 3. At higher pH, the nanoparticles of small size are produced. However, rod-shaped nanoparticles were produced at all pH which have been reported to be formed mainly by electrodeposition. In the present case, KAuCl4 was taken as the source which on dissolution in water gives anion. It ought to be bonded to carboxylic groups which are already protonated at low pH. The oat biomass shows the ability to bind and its subsequent reduction to gold nanoparticles. They have been produced from dead and live tissue of alfalfa [76,84-86], hops [87], fungus [88,89] and algae [90-92]. The basic idea behind the formation of nanoparticles is the reduction of metal ion to elemental metal. The plant biomass or even the extract of green leaves must, therefore, contain such chemicals so as to reduce the metal ion. As mentioned earlier, the plants which have aroma contain flavonoids, reducing sugars or alcohols/phenols which act as reductant leading to the formation of nanoparticles. The focal point of our attention must therefore be directed towards all species and smelling leaves, flowers and plants for the synthesis of nanoparticles because they all contain such chemicals which reduce the metal ion to metal nanoparticles. The FTIR spectra of leaf extract or dried leaf biomass, before and after the formation of nanoparticles, reveal the changes in the functional groups. It shows the presence of OCH3 group in Phyllanthin extract [93] eugenol in clove extract [94] and polyol in C. camphora leaf [64]. Geranium leaf, neem leaf [95,96], lemon grass, etc. have been used to produce gold nanoparticles [97]. As the progress is made in nanotechnology, biosynthesis is made easy. Instead of using the aqueous extract of plant leaf by boiling, only sun-dried leaf powder in water at ambient temperature is now used. In such procedure, a moderator and accelerator like ammonia is not needed, but the concentration of leaf extract is the rate-determining step. It is a significant step in bioreduction of chloroaurate ions [AuCl4]- that biomolecules of molecular weight less than 3 kDa can cause its reduction.
The metals can be sequestered from a mixture of several metals in different forms such as oxides, halides, carbonates, nitrates, sulphates, acetate, etc. Zhan et al. [98] have reported the biosynthesis of gold nanoparticles by Cacumen platycladi leaf extract. They have made a simulation of the active components and prepared a mixture of several known chemical substances on the basis of FTIR spectral data of C. platycladi leaf extract before and after the biosynthesis of nanoparticles. They were characterized by UV-visible (UV-vis) spectroscopy, thermogravimetric analysis (TGA), X-ray diffractometry (XRD), SEM and TEM. The structure, shape, temperature, pH and distribution of nanoparticles were studied. The extract was found to contain polysaccharide, reducing sugar, flavonoid and protein. The addition of C. platycladi leaf extract to aqueous solution of HAuCl4 showed a change in colour from pale yellow to brownish red in a span of 5 min. Its UV-vis spectrum exhibited λ max at 530 nm, the intensity of which increased with time and attained a maximum after 90 min showing the completion of the reaction. Surprisingly, the average nanoparticle size is fairly small, of the order of 15.3 nm. The FTIR spectrum after nanoparticle formation showed a reduction in the intensity of some prominent bands. The IR spectrum of purified nanoparticles showed the reduction of peaks at 3,448, 1,610 and 1,384 cm-1 which means that some of the leaf biomass remains stuck to nanoparticles; otherwise, elemental gold would not show any peak in the IR spectrum. The TGA and differential thermal analysis (DTA) results of the gold nanoparticles after thorough washing were recorded. It starts decomposing after 100°C and completes at 525°C; thereafter, a plateau appears which remains stable even at 800°C. The metal thus left as residue is actually gold oxide because the TGA was done in open where oxidation of metal may not be avoided. The authors have not clarified whether the end product is pure metal or metal oxide. The DTA of course shows two distinct changes in temperature (234°C and 507°C) indicating volatilization of organic components from leaf extract which may have acted as stabilizer or protective substance. Phenols, in fact, act as reducing agent and they themselves get oxidized to quinone. This property should have been discussed at length.
Random use of any metal nanoparticles in plants or food crops may not produce desired vegetative growth or enhance the yield of food crops. It must be known which trace elements are useful for the plant under experiment so that the same nanoparticles are used to increase the yield. The B. juncea seedlings on treatment with gold nanoparticles in the field (foliar spray) showed changes both in growth and yield of seed [99]. Like CuO nanoparticle in wheat [100], gold nanoparticle was also accumulated in Brassica [99]. The percentage of germination increased when B. juncea seedling were sprayed/inoculated with 25-ppm gold nanoparticles. However, as the concentration of gold nanoparticles increases, the rate of germination is slowed down. The authors have suggested that the antagonistic effect of gold nanoparticles slows down the effect of ethylene; as a result of which, an increase in the number of leaves of B. juncea occurs. In fact, it is not the antagonism of gold nanoparticles but the complexation of ethylene with gold or adsorption of ethylene on gold nanoparticles. An average 19% increase in the seed of B. juncea was noted after treating the plant with about 10-ppm gold nanoparticles. However, it is not economically feasible as the cost of gold nanoparticles (10 mg L-1) sprayed seems greater than the yield of the crop nevertheless; it is an attempt towards a bright future for increased food crop produced with engineered gold nanoparticles.
Nickel, platinum and palladium nanoparticles
Bali et al. [101] have studied the formation of platinum nanoparticles from Pt(II) by M. sativa and B. juncea plant biomass. The conversion of Pt(II) to metallic platinum was studied in acidic medium between pH 2 and 3. However, such high pH amongst plant kingdom is never achieved. This process can be used to extract metals from clinical disposal sites to prevent recycling in the soil. Generally, the metals in the soil or at mining sites exist in the form of salts rather than a co-reduction compound. The platinum metal concentration in this study showed the accumulation of platinum between 0.77 and 36.83 mg of platinum per gram of dry biomass of M. sativa. Spherical-shaped palladium nanoparticles have also been obtained using peel extract of Annona squamosa [102]. It is a useful study of platinum metal uptake by plants which can be extended to other metal ions of this group of metals, viz. Ni, Pt and Pd. Both the living and dead organisms are equally useful in producing nanosized crystal of metal [103]. Reduction of Pd(II) to elemental palladium has been achieved by formate or hydrogen [104].
Beneficial and adverse effects of metal nanoparticles
Nanoparticles of specific size are capable of penetrating and migrating to different regions of plant cells [105]. These nanoparticles can be stopped at certain point or their movement may be accelerated by the use of small magnets provided that the nanoparticle is magnetic in nature as the non-transition metal ions are not attracted towards a magnet. Some experiments using carbon-coated nanoparticles of iron have been shown to have some unusual influence on the plant growth and yield of the grain or fruit. The nanoparticle movement may be directed to certain parts of the plant or certain specific organ in microbes/animals. The disease in plant or animal may thus be effectively treated with nanoparticles [106]. Corredor et al. [105] have shown the application of carbon-coated iron nanoparticle to pumpkin plant for the dissected release of chemicals into the specific part of the plant prone to infection by pathogens. The nanoparticles enter the living cells and are distributed over the entire part, the mechanism of which is yet to be understood. The nanoparticles were applied in different modes, namely by injection and spraying. Though a very small quantity of nanoparticles is required for injection, it is practically not possible on large scale and hence, generally, spraying is done. Sometimes, small magnets are inserted at certain points of the plant so that immobilized nanoparticles are accelerated at target point. The dark precipitate deposited in the inner surface of the pith cavity is visible even with the naked eye (Figure 5). The presence of nanoparticles was confirmed by SEM and TEM images. These nanoparticles appeared as intracellular aggregates and have also been observed in the cytoplasm of epidermal cells. Plant cells respond to a high density of nanoparticles by changing their subcellular organizations. The number of nanoparticles and cytotoxicity are related to each other. Nanoparticles when sprayed normally penetrate through the stomata and so are used for pathogens of different species. They may therefore be killed by nanoparticles preventing the plant/fruit from further damage.
Figure 5.
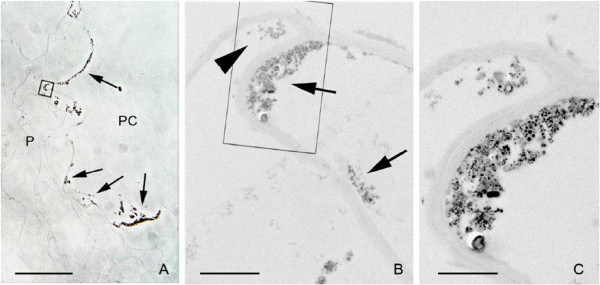
Penetration of nanoparticles into the first cell layer surrounding the pith cavity. (A) Phase contrast image of the parenchymatic cells (P) closer to the pith cavity (PC). The nanoparticle aggregates on the application surface appear as an optically dense material (arrows). (B) Transmission electron micrograph of the region squared in (A). Nanoparticle aggregates appear in the cell wall facing the pith cavity (arrows) and into the cytoplasm of the first cell layer (arrow head). (C) High magnification of the region squared in (B). The intracellular aggregate is smaller than the extracellular one in the pith cavity. Bar in (A) = 40 μm, (B) = 2 μm, (C) = 1 μm [105].
Of the various nanoparticles, gold nanoparticle has assumed more importance due to its application in almost all areas of medicine [107-109] and technology. Recently, the gold nanoparticles synthesized from Gnidia glauca flower extract has been used as chemocatalytic agent in the reduction of 4-nitrophenol to 4-aminophenol in the presence of sodium borohydride [110].
 |
The formation of nanoparticles may be followed spectrophotometrically by the change in colour from yellow to dark red in the visible region of the spectrum between 450- and 600 nm (Figure 6). The UV-vis spectrum of gold nanoparticles as a function of time shows that the reaction is completed within 20 min. It has been shown that the formation of gold nanoparticles starts 2 min after the interaction of plant extract with HAuCl4 [110]. The current method [110] of gold nanoparticle synthesis is faster and efficient than that reported earlier by Vankar and Bajpai [111] which took approximately 2 h for the completion of reaction. At concentration as low as 0.7 mM, the synthesis was optimum, and above this concentration, the formation of gold nanoparticles ceases to continue (Figure 6). The rate of synthesis of gold nanoparticles from G. glauca flower extract increases with increasing temperature and attains maximum between 40°C and 50°C. A similar pattern was found to follow when gold nanoparticle was synthesized from Nyctanthes arbortristis flower extract [112]. In this case, the particles are spherical in size ranging between 5- and 20 nm [113,114]. Polydispersed gold nanoparticles can be obtained from Rosa hybrida petal extract [115]. When the concentration of HAuCl4 is low, gold nanoparticles of smaller size are produced, although they are often covered with larger particles as aggregates [114]. The FTIR spectra of dried G. glauca flower [110] extract before and after the synthesis of nanoparticles revealed a decrease in all stretching frequencies of the probable functional groups of the phenols, flavonoids and amines present in the extract. It suggests a decrease in the concentration of the functional groups after the synthesis of gold nanoparticles, which is obvious. During the phytosynthesis of metal nanoparticles, all alcohol, aldehyde and phenol present in the plant extract are oxidized (as shown below), and the metal ions are reduced to metal nanoparticles:
Figure 6.
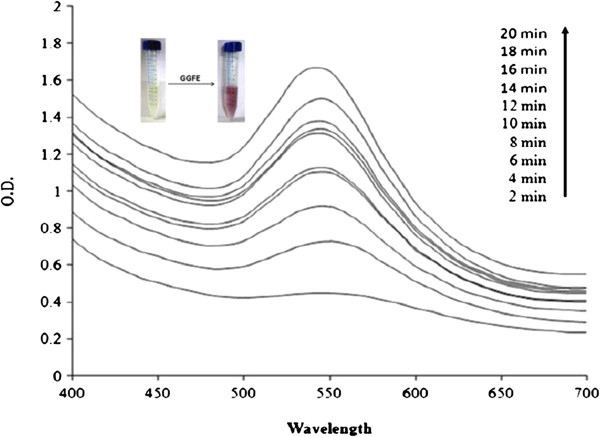
Time course of gold nanoparticle formation. As obtained with different concentrations of chloroauric acid using Gnidia glauca flower extract at 40°C [110].
Alcohol → Aldehyde
Aldehyde → Carboxylic acid
Phenol → Ketone
Flavonoids → Flavone
These nanoparticles may be used as chemocatalytic agent in the reduction and degradation of organic compounds. Photocatalytic degradation of methylene blue was done under sunlight by the silver nanoparticles synthesized from Morinda tinctoria leaf extract. The deep blue colour of the dye starts fading after 1 h with the above experimental conditions under sunlight. The maximum absorbance for methylene is at 660 nm. The colour of methylene blue turned light green after 1 h and finally became colourless after 72 h showing its degradation up to a maximum of 95%. This demonstrates the photocatalytic activity of silver nanoparticles for methylene blue which may be exploited for the benign treatment of dye stuffs [116]. Ganaie et al. [117] have explored the catalytic degradation of dye such as Alizarin Red S and Remazol Brilliant Blue R by silver nanoparticles in the presence of hydrogen peroxide and sodium borohydride, respectively. The degradation of dye, as pollutant, was found to increase rapidly which was monitored spectrophotometrically by the decrease in absorbance. A variety of methods have been developed to synthesize the metal nanoparticles, although the shape and size vary greatly with the concentration of precursor metal and the reductant. The silver nanoparticles prepared from Citrullus colocynthis extract were found to be spherical in shape and approximately of 31 nm with different morphology [118]. The use of silver nanoparticles in medicine prompts scientists to explore more application in this area [119]. Biological methods for the synthesis of nanoparticles such as using microorganism [120], enzymes [121] or plant extract [21] are eco-friendly and efficient. Satyavani et al. [118] have recently studied the cytotoxicity of silver nanoparticles against cancer cell lines in vitro. The nanoparticles showed a decrease in viability of the HEp2 cells. The effect is time and concentration dependent. When the cancer cells were exposed to 50 nM concentration of silver nanoparticles for 5 h, their viability was reduced to 50% which is considered as IC50. The longer the exposure time, the greater the toxicity. The silver nanoparticles possess angiogenic properties [121], and therefore, it can be tested against various types of cancer cells. The effect of silver nanoparticles on osteoblast cancer cells has also been studied. It has been shown that a single dose of as little as 3.42 μg mL-1 of IC50 is more effective than the toxic heavy metals [122]. The replication of cancer cells under experimental conditions is inhibited regardless of the method of synthesis of silver nanoparticles. The release of lactate dehydrogenase is a marker of the effect of silver nanoparticles on cancer cell, which is significantly increased compared to untreated cells. It has been nicely demonstrated that silver nanoparticles caused death of cells through apoptosis which was also shown by cellular DNA fragmentation. The HEp2 cells treated with silver nanoparticles showed the cleavage of double strand of DNA fragment. It was observed that silver nanoparticles are manifold more effective against HEp2 cancer cells than silver ion [118], although the mobility of silver ion is obviously greater than the silver atom. The cytotoxicity of silver nanoparticle is mainly due to its interaction with the functional groups of the proteins within the cancer cell and nitrogen bases in DNA. It has been reported that green tea and decaffeinated green tea also inhibit activity of H1299 human lung carcinoma cell line. It is believed that its activity is synergized by polyphenols. Since such metal nanoparticles are not selective, they may equally damage the living cells. The living cells have the ability to repair themselves even though they may also be prevented from damage by such metals while treating for cancer.
In a study, Patil et al. [123] have synthesized silver nanoparticles from Pergularia daemia latex. They characterized and studied its toxic effect on some mosquitoes and non-target fish. Such studies are not common [123,124] even though an attempt has been made to see the toxicity of metal nanoparticles. The importance of such studies lies in its benign effect on the environment. Silver nanoparticles are also synthesized by dry and fresh latex of P. daemia, but the yield of nanoparticles by fresh latex was larger than that synthesized by dry latex. A comparison of both types of silver nanoparticles was made; an absorption spectrum showed a peak at 520 nm which is generally the characteristic of silver nanoparticles formed along with some of the biomolecules present in the latex or extract. Richardson et al. [125] have shown that plant extract containing carbohydrates and proteins serve as reducing agent for silver ions. Quercetin, a flavone derivative, was shown to be involved in the formation of silver nanoparticles [126], perhaps by catalysing the reaction through dissolved oxygen in the solutions. Jatropha curcas latex is known to reduce Ag+ to very small size nanoparticles of the order 20- to 30 nm. This plant is known to contain a peptide called curcacycline A and B which is involved in the reduction and stabilization of silver nanoparticles [127]. In the case of P. daemia latex, the protein part seems to be responsible for the synthesis of silver nanoparticles. The nanoparticles laced with latex are toxic to mosquito larvae, and in short-term experiment, it may be useful. However, contradictory report has also appeared that silver nanoparticles induce embryonic injuries and reduce survival of zebra fish [128]. The ability of silver nanoparticles as toxic material to reduce pathogens without disturbing the benign microbes and fish should be viewed with caution. Long-term study can only prove if it may be safely used without disturbing the ecosystem.
Metal oxide nanoparticles
Numerous positive effects of engineered metal oxide nanoparticles have been practically proved (Table 2). It has been observed that SiO2 and TiO2 nanoparticles in appropriate ratio increase nitrate reductase activity in soybean, increase its capacity to absorb fertilizer and eventually reduce the time for germination [129]. They also enhance the rate of photosynthesis in spinach [130,131]. It is worth noting that nano-Al2O3 inhibits the root growth in maize and cucumbers. This seems as if the nanoparticles of certain elements may have adverse effect on plants or even in man [132]. The effect of silver and titanium dioxide nanoparticles on the growth inhibition of aquatic plants has been studied by Kim et al. [133]. Since the size and structure of nanoparticles have different properties from their salt or bulk material, they drastically alter or modify the physicochemical properties [134,135]. Natural availability of Ag and TiO2 nanoparticles makes them prominent. Major work has been done with TiO2 as it is a naturally occurring mineral which is capable of existing as rutile, anatase, brookite and amorphous forms [136]. TiO2 can generate potential reactive oxygen species (ROS) at its surface, in the presence of UV light [137], though ROS activity has been shown even in the absence of light [138]. Lethal effect of silver nanoparticles on bacteria [139] and yeast [52] are known [53,140]. Photocatalytic degradation of indigo carmine by TiO2-strewn sheet under UV light as a function of time has been studied. It has also been investigated spectrophotometrically. The concentration of indigo carmine dye after photodegradation was analysed at its absorption maximum at 610 nm. The intensity of this peak decreases with the passage of time eventually reaching the baseline indicating the complete degradation after about 5 h [141]. Since metal oxide nanoparticles, such as ZnO, MgO, TiO2 and SiO2, are also known to possess antimicrobial activities, they can be exploited in the treatment of common bacterial infection and in the sterilization of surgical instruments, but their toxicity to biological systems may be overlooked [142]. Enhanced antibacterial activity of Argemone mexicana treated with iron oxide nanoparticles was also reported against Proteus mirabilis and Escherichia coli [143]. The silver ions are also effective against these microbes, but the efficiency depends on its microlevel concentration [144]. It was found that Lemna paucicostata (7-day-old) grown in the presence of different concentrations of Ag and TiO2 nanoparticles inhibited its growth [133]. At ≥1 ppm, silver nanoparticles showed significant decrease in L. paucicostata growth, but with nanoparticles ≤100 ppm, the growth is completely inhibited. On the contrary, the growth inhibition by TiO2 nanoparticles is effective only at 500-ppm level. These nanoparticles may be used to eradicate the unwanted aquatic weed and plants, but the damage to other plants and aquatic animals may not be prevented. It can work in isolated system, but in ponds, it may cause havoc by destroying the non-target plants and animals like fish, etc. Crop yield and grain quality may be improved by the use of manufactured nanomaterial. The method of application and absorption may vary; the manufactured nanomaterial may be sprayed or mixed with the soil. Experiment with nano-CeO and nano-ZnO on soybean showed an increase in quality and yield of crops. The ZnO nanoparticle was taken up by the plant and distributed uniformly throughout the plant tissues. All manufactured nanomaterials may not be equally effective for all crops. In this case [145], the soybean treated with CeO2 gave unexpected result. The nano-CeO2-treated plants had decreased leaf counts irrespective of its concentration. Even the lowest concentration showed retarded growth in the harvested plant. The stunted plants may be grown with CeO2 nanoparticles, but any increase in crop yield has not been recorded.
Table 2.
Effects of engineered metal oxide nanoparticles on plants
| Nanoparticle | Size (nm) | Plant | Concentration | Effect | References |
|---|---|---|---|---|---|
| Al2O3 |
|
Corn, cucumber, lettuce, radish, rapeseed, ryegrass |
2,000 mg L-1 |
No effect on germination |
[44] |
| 13 |
Carrots, cabbage, cucumber, maize |
2,000 mg L-1 |
Reduced root growth |
[146] |
|
| |
Corn |
2,000 mg L-1 |
Reduced root length |
[44] |
|
| CeO2 |
7 |
Alfalfa |
1,000 and 2,000 mg L-1 |
Slightly reduced shoot growth |
[147] |
| |
Tomato |
2,000 mg L-1 |
Reduced shoot growth |
[147] |
|
| |
Cucumber |
2,000 mg L-1 |
Reduced shoot growth |
[147] |
|
| |
Maize |
500, 1,000 and 2,000 mg L-1 |
Reduced shoot growth |
[147] |
|
| |
Alfalfa |
500 mg L-1 |
Reduced biomass |
[147] |
|
| |
Maize |
500 to 2,000 mg L-1 |
Reduced germination |
[147] |
|
| |
Maize |
4,000 mg L-1 |
Reduced root growth |
[147] |
|
| |
Tomato, cucumber |
2,000 mg L-1 |
Reduced germination |
[147] |
|
| |
Tomato |
1,000 to 2,000 mg L-1 |
Reduced root growth |
[147] |
|
| |
Alfalfa |
2,000 to 4,000 mg L-1 |
Reduced root growth |
[147] |
|
| |
Soybean |
2,000 mg L-1 |
Reduced germination |
[147] |
|
| 7 |
Alfalfa, corn, soybean |
500, 1,000, 2,000 and 4,000 mg L-1 |
Increased root and stem growth |
[147] |
|
| <25 |
Wheat |
100 mg L-1 |
[148] |
||
| 8.0 ± 1.0 |
Coriander |
125 mg kg-1 |
Increased shoot and root length, increased biomass, increased catalase activity in shoots and increased ascorbate peroxidise activity in roots |
[149] |
|
| 231 ± 16 |
Rice |
62.50 and 125 mg L-1 |
Reduced H2O2 generation in shoots and roots |
[150] |
|
| 500 mg L-1 |
Increased electrolyte leakage and lipid peroxidation in shoots |
[150] |
|||
| FeO |
10.2 ± 2.6 |
Clover |
3.2 mg kg-1 |
Reduced aboveground and belowground biomass |
[49] |
| Fe3O4 |
20 |
Pumpkin |
500 mg L-1 |
No toxic effect |
[29] |
| 7 |
Cucumber, lettuce |
62, 100 and 116 mg L-1 |
Low to zero toxicity |
[47] |
|
| Magnetite (iron oxide) |
|
Soybean |
0.2, 0.4, 1.0 and 2.0 mg L-1 |
Increased chlorophyll levels |
[151] |
| Mixture of SiO2/TiO2 |
|
Soybean |
|
Increased germination and shoot growth, increased nitrate reductase activity, increased absorption and utilization of water/fertilizer and enhanced antioxidant system |
[129] |
| Ni(OH)2 |
8.7 |
Mesquite |
2 mg L-1 |
No effect |
[152] |
| Nanosized TiO2 |
21 |
Wheat |
10 ppm |
Reduced germination |
[153] |
| |
|
|
2 and 10 ppm |
Increased shoot and seedling lengths |
|
| |
|
|
100 and 500 ppm |
Reduced shoot and seedling lengths |
|
| |
|
|
100 ppm |
Increased root dry matter production |
|
| Nanoanatase (TiO2) |
4 to 6 |
Spinach |
0.25% |
Enhanced rca mRNA expressions, protein levels, activity of Rubisco activase, Rubisco carboxylation, the rate of photosynthetic carbon reaction, single plant dry weight and chlorophyll content |
[154] |
| 5 |
Spinach |
0.25% |
Improved spinach growth related to N2 fixation by TiO2 |
[155] |
|
| 5 |
Spinach |
0.25% |
Improved light absorbance, transformation from light energy to electron energy, and active chemical energy and promoted carbon dioxide assimilation |
[156] |
|
| Rutile (TiO2) |
|
Spinach (naturally aged) |
0.25% to 4% |
Increased germination and germination and vigour indices, plant dry weight, chlorophyll formation, ribulose bisphosphate carboxylase/oxygenase activity and photosynthetic rate |
[10] |
| |
Spinach |
0.25% to 4% |
Promoted photosynthesis, the rate of evolution of oxygen in the chloroplasts was accelerated |
[130] |
|
| TiO2/inorganic bentonite clay |
30/1 to 60 |
Maize |
300 and 1,000 mg L-1 |
Inhibited hydraulic conductivity, leaf growth and transpiration |
[157] |
| ZnO |
8 |
Soybean |
500 mg L-1 |
Increased root growth |
[147] |
| 9 to 37 (mean 19 ± 7) |
Ryegrass |
1,000 mg L-1 |
Reduced biomass, shrank root tips, epidermis and root cap were broken, highly vacuolated and collapsed cortical cells |
[44] |
|
| |
Corn |
2,000 mg L-1 |
Reduced germination |
[44] |
|
| |
Corn, cucumber, lettuce, radish, rapeseed, ryegrass |
2,000 mg L-1 |
Reduced root growth and elongation |
[44] |
|
| 5 |
Zucchini |
1,000 mg L-1 |
Reduced biomass |
[46] |
|
| 8 |
Soybean |
2,000 and 4,000 mg L-1 |
Decreased root growth |
[147] |
|
| 3-Amino-functionalized SiO2 | Lettuce | 0.013% to 0.066% (w/w) | No effect on germination, improved shoot/root ratio | [13] |
Beneficial and adverse effects of metal oxide nanoparticles
Bulk and nanosized TiO2 particles have different impacts on plants and microorganisms. Concentrations of bulk and nanoparticles ranging from 1 to 500 ppm have been tried on wheat germination and seedling growth. The Ti compounds showed the following improvements after the crop or seedlings were treated with it [158]:
(i) The enhancement of yield of various crops, 10% to 20%
(ii) An improvement of some essential element contents in plants
(iii) An increase in enzyme activity like peroxide, catalase and nitrate reductase activity in plant tissue
(iv) Enhancement of chlorophyll pigment
TiO2 nanoparticles have also been demonstrated to increase the rate of germination and growth of spinach (Spinacia oleracea) [10]. It is believed that such nanoparticles influence the plant growth due to their antimicrobial properties. However, it is one of the several factors but not the consequence of antimicrobial properties that is responsible for the growth of plants. Nanosized TiO2 particles can promote nitrogen metabolism in the plant leading to growth as a whole. On the other hand, alumina nanoparticles affected adversely the elongation of corn, cucumber, soybean, cabbage and carrot [146]. Besides TiO2, other metal nanoparticles have also been shown to influence the crop production and their vegetative growth (Table 2). In almost all studies, the size of nanoparticles appears to be the critical factor. As the concentration of metal or metal oxide nanoparticles increases, the growth increases and reaches an optimum value after which either it becomes constant or retardation in growth occurs. In such instances, the enzyme activity is either lost or the nanoparticles block the passage of other nutrients as a consequence of accumulation. The germination time of seed with TiO2 was reduced to 0.89 days; shoot and seedling length was also increased after treatment of wheat seeds with TiO2 nanoparticles at 2- and 10-ppm concentration. When the concentration was raised to 100 ppm, no improvement was observed [10]. The effect of TiO2 nanoparticles on seed growth and germination is size and concentration dependent, because the small particles can easily penetrate the cell wall of the plant and move to various other parts. TiO2 nanoparticles coupled with SiO2 increase the nitrate reductase enzyme in soybean [129], increase the capacity to absorb water and fertilizer and promote its antioxidant activity, the germination of seed and growth of the plant.
Zhou et al. [100] have made a distinction between adsorption and absorption. Adsorption is a surface phenomenon, while absorption depends on the concentration, size factors and temperature. Both adsorption and absorption may occur simultaneously in plants [159]. The uptake of nanoparticles may be checked in plants, but adsorption is the accumulation of nanoparticles that remains on the surface of the plants. The adsorbed CuO nanoparticles on the root surface were checked in the presence of complexing agents such as Na4EDTA and NaOAC. It is however very interesting to believe that EDTA dissolves CuO nanoparticles by forming complex with released Cu2+.
According to this metathesis, free Cu2+ will not be available for subsequent reaction with EDTA, rather Na+ is replaced by Cu2+ ions leading to the formation of Cu2(EDTA). The equilibrium between CuO nanoparticles and Cu2(EDTA) depends on the quantum of Na4EDTA added and that of CuO nanoparticles present. Since the authors insist that the equilibrium between CuO nanoparticles and Cu2+ is lost, the dissolution of CuO nanoparticles is enhanced. It is not true because the number of moles of EDTA-Cu complex produced will correspond to the number of moles of EDTA added. The speculation that Cu nanoparticles adhered to the root is only due to complex formation may not be true, as there must be some complexing agent exuded by the root hairs. The adsorption of CuO nanoparticles by wheat root is concentration dependent. The authors have unnecessarily compared the adsorption with the uptake of nanoparticles [100]. The amount of nanoparticles adsorbed is actually retained on the surface due to electrostatic force, and fewer particles are absorbed into the plant system. When CuO nanoparticles are adhered to the outer surface of the root, they may not be transported to the cells unless they are absorbed. The absorption and uptake are synonymous in the present context because wherever it is absorbed it is in fact taken up by the plant. The authors have concluded that Na4EDTA increases the solubility of CuO nanoparticles, if it is the case, a mixture of CuO nanoparticles and Na4EDTA should be administered to the plant instead of taking the troublesome route of adherence of nanoparticles and their subsequent dissolution by Na4EDTA for absorption.
Contradictory reports have been received on the application of CuO nanoparticles on plants. While CuO nanoparticles have been shown to absorb in wheat, it has been reported to produce adverse effect on maize plants [160]. It has been reported that CuO nanoparticles have apparently no effect on the germination of maize seeds; nevertheless, it increased chlorosis and inhibited the growth of maize seedlings when exposed to 100 mg L-1 CuO nanoparticles. The transportation of nanoparticles is supposed to pass through the epidermis and cortex and finally to stele of the plant. It has been reported, perhaps for the first time, that the CuO nanoparticles were transported to the shoots and translocated back to the roots via phloem. It has also been shown that during the process of transportation of CuO nanoparticles to shoot via xylem and back to root via phloem, some of the Cu(II) in CuO is reduced to Cu(I). If this assumption is true, it may follow the reaction:
Since the authors have observed a blue colour after the addition [95] of Na4EDTA to CuO nanoparticles, it confirms the presence of Cu2+ rather than Cu+1 because Cu+1 having d10 configuration is colourless. This also confirms that the above hypothesis may not be true as it is not supplemented by experimental evidences. Root development of maize was inhibited by CuO nanoparticles followed by reduced biomass of the plant. The nanoparticles were distributed all over the plant parts which have adverse effect on them.
In an experiment with nanoparticles of different metal oxides on Arabidopsis thaliana, Lee et al. [161] have shown that all Al2O3, SiO2, Fe3O4 and ZnO are toxic. Seed germination, root elongation and leaf count were examined when seed or plants were exposed to concentrations of nanoparticles ranging from 400 to 4,000 mg L-1. The toxicity of metal oxide nanoparticles follows the order:
The solubility of ZnO nanoparticles is 33 times lower than the corresponding ZnCl2 in aqueous medium. It is surprising that while Zn2+ is a major constituent of over 30 enzymes in the human system, the ZnO-NP is toxic to A. thaliana even in very low concentration. Not all metal nanoparticles are useful to plants/animals, but some may be useful in some cases while others produce toxic effect. The seed germination was nearly inhibited but the leaves and roots did not grow at all in the presence of ZnO nanoparticles, while Fe3O4, SiO2 and Al2O3 nanoparticles had no marked influence at low concentration. It is stated by many workers that the toxicity of metal oxide nanoparticles may be caused by their dissolution and then the release of toxic metal ions [44,132,162]. However, it may happen only when known toxic metal nanoparticles such as Cd, Hg, Pd, As and Tl are taken. The innocuous types of metal oxide nanoparticles or metal nanoparticles in low concentration are not expected to produce adverse effect. It is also true that Zn being the most useful in mammalian system in low concentration may be toxic in higher concentration. A chemical in low concentration may act as medicine, but it may become poison when taken in bulk. Zn concentration up to 250 mg L-1 does not affect seed germination [161] which suggests that the phytotoxicity of metal oxide nanoparticles may be used to enhance or inhibit the plant growth (of certain type only). The influence of TiO2 and ZnO nanoparticles on seed germination, root length and number of roots of rice plant has been studied [163]. Although, there is no reduction in the percentage of germination of rice seed in both the cases, ZnO nanoparticles cause complete growth inhibition of the root. It cautions both agriculturist and environmentalist that dumping of waste disposal on the agricultural land may cause damage to the crops. As low as 400 mg L-1 ZnO nanoparticles inhibit root germination, and therefore, waste disposal at such places may be hazardous.
The toxic effect of CuO, NiO, TiO2, Fe2O3 and Co3O4 nanoparticles on germination, root elongation and growth of common edible plants such as lettuce, radish and cucumber has been done [164]. CuO and NiO nanoparticles at 12.9 and 27.9 mg L-1 concentration, respectively, are toxic to the above plants, while the other nanoparticles at such concentration are ineffective. The common trend of toxicity follows the order:
In some cases, TiO2 and SiO2 nanoparticles were found to enhance both the germination and growth of Glycine max seeds [129]. Carbon nanotubes (CNT) were found to enhance germination and root elongation of tomato seed [165] and produced two times more flowers and fruit [166]. Likewise, Al nanoparticles were found to be useful in augmenting the root of radish and rape seedlings [44]. Such effect depends on the concentration of nanoparticles and plant species under question. The CuO nanoparticle is not as much effective as free Cu2+ ions obtained from CuCl2. It is obvious that the quantity of Cu2+ ions released from CuO nanoparticles will be too small to be effective for germination of seeds.
The interaction of metal oxide nanoparticles with seed or plant tissue is poor comparative to free metal ions. The hypothesis that smaller nanoparticles can penetrate easily in plant cells and interact with biomolecules may not hold as the mobility of the particle may be the key factor. The small-sized nanoparticles will have higher degree of freedom for movement, and hence, they would be more efficiently absorbed by the plant.
Al2O3 nanoparticle has been shown to affect the plant growth and crop production. Phytotoxicity of Al2O3 nanoparticles was tested against five plant species [146]. When the same experiment was also run with Al2O3 loaded with phenanthrene (which is one of the hydrocarbons found in the atmosphere), it was found to be less toxic (root growth inhibition) than pure Al2O3. It suggests that Al2O3 nanoparticles may induce toxic effects on seedling root growth. However, submicron alumina particles loaded or unloaded with phenanthrene did not show any significant effect on seedling root growth. The decreased toxic effect of Al2O3 phenanthrene may be ascribed to size effect. Here, the nanoparticles accumulated and further accelerated due to phenanthrene which may have reduced the phytotoxicity of these particles. The FTIR spectrum of the particles showed bands in 850 to 1,050 cm-1 region which are assigned to vibrational modes of alumina [167].
Since Al2O3 was taken in aqueous medium (either loaded or unloaded with phenanthrene), it may immediately react with water to give Al(OH)3. The FTIR spectrum will therefore, exhibit peak for Al-OH and not due to loss of hydroxyl group (Figure 7). The OH group may be lost if Al(OH)3 is heated in open according to
Figure 7.
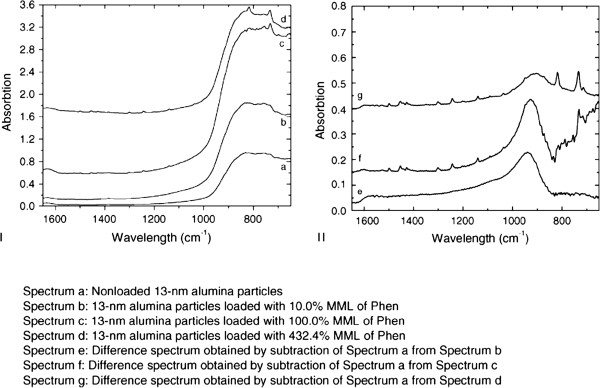
FTIR spectra. I: loaded particles (a); particles loaded with 10.0% (b), 100.0% (c) and 432.4% (d) monomolecular layer of phenanthrene. II: spectra obtained by subtraction of spectrum a from b, c and d, resulting in e, f and g, respectively. The band near 950 cm-1 is related to the surface characteristics of alumina nanoparticles [167]. The absorbance of phenanthrene can be distinguished in both spectra, f and g [146].
Pure Al2O3 may exhibit a peak due to Al-O. This assignment, on the basis of IR spectral data, may not be true. The authors [146] claim that dimethyl sulphoxide (DMSO) used in their experiment is a hydroxyl radical scavenger, and in aqueous medium, it removes the OH radical as shown below [168,169]:
The last equation is wrong in the above reactions. It should produce CH3OH not CH2OH. Generally, free radicals combine with another species to give a molecule.
The effect of two fluorescent nanoparticles, fluorescein isothiocyanate (FITC)-silica nanoparticles and quantum dots (QD), on germination of rice seeds has been studied [170]. In addition, the uptake capacity of photostable CdSe QD and FITC-labelled silica nanoparticles (SNP) has also been studied. It was observed that germination in the presence of FITC-labelled SNP was enhanced while it was arrested with QD. Since the QD contain Cd as one of the known toxic metal ions, it may have reversibly acted on germination of rice seeds. However, transport of both fluorescent nanoparticles has been observed in rice seedlings. The FITC-SNP appears to be useful to plants and has shown good fluorescence in rice seedlings. It is therefore suggested that it may be used for bioimaging in plant tissues because of the photostability of SNP. Bioimaging can be done only with the help of fluorescent materials especially in vivo. Since very limited study has been done in this direction [171], the exact nature and mechanism of transport of nanoparticles is not well understood. It can equally be used in mammals, but the toxicity of such nanoparticles in biological system must be checked prior to its use. Conflicting reports have been received about the toxicity of QD [172,173] in mammals even though CdSe QD is known to arrest the root growth of rice seedlings.
The useful application of metal or/and metal oxide nanoparticles is still a matter of controversy. In some cases, it has been found to be useful, while in many other instances, it appears to be phytotoxic [9-13]. The ZnO nanoparticles in this context have been used as growth promoter for Cicer arietinum and Vigna radiata seedlings [174]. They were monodispersed and their spherical shape was confirmed by SAED pattern (Figure 8). It was observed that in the case of V. radiata the root elongation occurs at 1-ppm level of ZnO nanoparticle while the shoot is almost unaffected when seedlings were exposed for 60 h. When the dose exceeds 1 to 20 ppm of ZnO, a sudden decrease in the shoot and root of V. radiata and C. arietinum seedlings occurs which is suggested to be the toxic level. From the analysis of ZnO nanoparticles in various parts of plant, it is found that the nanoparticles are absorbed and transported to other parts. Dispersion of epidermis, cortex and vascular cylinder was observed after higher concentration was administered (Figure 9). The adsorption and aggregation of ZnO nanoparticles in the root and damage to the architecture of the root were noted when a quantity above the optimum dose was given.
Figure 8.
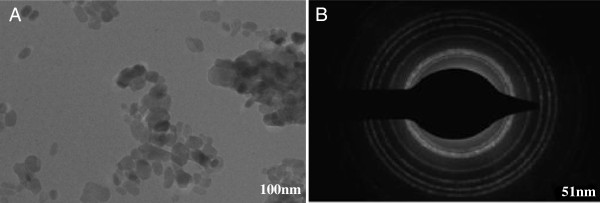
TEM image (A) and SAED pattern (B) of nano-ZnO particles [174].
Figure 9.
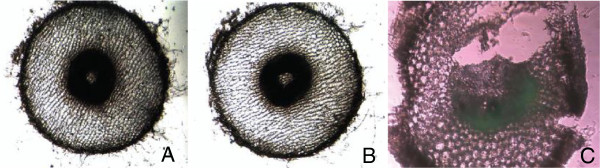
Transverse section of Cicer arietinum seedling roots. (A) Control, (B) at 1 ppm and (C) at 2,000 ppm of nano-ZnO treatment [174].
Carbon nanomaterials and its beneficial and adverse effects
Carbon nanomaterials have received greater attention because of unique physical and chemical properties that enable the synthesis and manipulation to a degree not yet matched by inorganic nanostructures [175,176]. The effect of carbon nanomaterials of varying sizes and concentrations on different parts of a variety of plants has been studied [44,46,148,166,177-182]. Multi-walled carbon nanotubes (MWCNTs) enhanced alfalfa and wheat germination and root elongation, but the particle uptake and translocation was insignificant [183]. Increased root growth in response to carbon nanotubes was reported for onion, cucumber [177] and ryegrass [44]. MWCNTs have increased the growth of tobacco cells and tomato plants by affecting expression genes that are essential for cell division and plant development [166,184,185]. In addition to these, a number of other investigators have demonstrated toxicity of carbon nanomaterials to a range of plant species [46,186].
In an experiment, Mondal et al. [25] have shown that MWCNTs of approximately 30 nm diameter enhance the rate of germination and growth of B. juncea. Likewise, TiO2 nanoparticles have also been reported to enhance the rate of germination and strength of spinach seedlings [10]. Later, it was found in [165] that such nanoparticles increase the moisture contents of the seeds. The same is true with MWCNT which facilitates the reduction of water by adsorption and subsequent penetration into the seed coat and root of mustard plant. The oxidized CNT had better effect on the seed germination than the CNT alone, although the concentration of the oxidized CNT was much lower. Quite good results were obtained with oxidized MWCNT (2.3 × 10-3 mg mL-1), but when the concentration exceeds 46 × 10-3 mg mL-1, both MWCNT and oxidized MWCNT inhibit the germination of mustard seeds. It indicated that the rate of growth is concentration dependent. This technique may, therefore, be applied to increase the rate of germination of crop plants which reduces the time. It has been suggested that electrical conductivity of a solution increases when the plant tissues are immersed in it. This is correct up to a limit above which the conductance becomes constant because, as the concentration [187] of leached salts, amino acids, potassium, phosphate, sugar, carbohydrates, etc. increases, the freedom of movement of these molecules and ions decreases. Aquaporins are water channels that not only selectively allow water molecules to flow in and out of the tissue but also reject certain substances in order to maintain the equilibrium. It is concluded that pre-soaking of seeds with very low concentration of oxidized MWCNT have positive effect on seed germination. Exploitation of nanoparticles in different areas has become a fashionable trait even though their inadvertent use may create an imbalance in the ecosystem. For instance, Oberdörster [188] showed for the first time that the fullerenes, C60, cause lipid peroxidation in fish brain tissue, an example of adverse effect of nanoparticles in aquatic animals. Furthermore, fullerene (C60) is known for its multifunctional use such as imaging probe, antioxidant and drug carrier [189], but it has been shown to exhibit genotoxicity and cytotoxicity and also to induce ROS in rat/fish cell lines [190-192]. C60 can cause damage to E. coli but not to the extent of being used as a drug. On the other hand, an attempt to exploit it in other areas without knowing its properties may be hazardous. Wang et al. [193] studied the effect of gold, silver, iron and C60 nanoparticles on the growth of E. coli, Bacillus subtilis and Agrobacterium tumefaciens. It was observed that silver nanoparticle is most effective against all the above bacteria, while the other two nanoparticles have little or no influence on their growth. Perhaps, the silver nanoparticles easily penetrate the cell wall and interact with the pathogens inhibiting their further replication. The Au, Fe and C60 are regarded to be ineffective because they may be essential ingredients of these microbes. As little as 1 μg mL-1 silver nanoparticles are effective against the above bacterial strains. Approximately 5 μg mL-1 silver nanoparticles cause 100% mortality. It is clear from the SEM images that the cell wall of E. coli is damaged preventing further growth (Figure 10). In an experiment, Liu et al. [194] subjected human cell lines to silver nanoparticles of different sizes and demonstrated that smaller particles enter the cell more easily than the larger ones. Only penetration of nanoparticles into the cell wall is not the reason for their toxicity. It is concluded from a study that the toxicity of silver nanoparticles is due to their interaction with essential sulfhydryl group of the respiratory enzyme present in the bacterial cells [195].
Figure 10.
Images of E. coli taken by SEM after exposure to nano-Ag. (A) Control and (B) 1 μg mL-1 nano-Ag. Magnifications and plotting scales are marked out in each picture [193].
The other assumption is that there is a formation of the free radical which induces membrane damage. The free radical is obviously very reactive, but the production of free radical requires homolytic fission of a species which may be linked to the protein. ROS may also be produced and may cause damage to the cell. The mechanism of action of silver nanoparticles with different cell lines is not yet clear, but it appears as if they adhere to the surface of bacterial cells leading to their mortality.
Conclusions
The currently available information on nanomaterials suggests that it has great potential application in agri-food sectors, cosmetics (TiO2, ZnO, fullerene, Fe2O3 Cu, Ag, Au) catalyst (NiO, Pt, Pd) lubricants, fuel additives (CeO2, Pt, MoS3), paints and coatings (TiO2, SiO2, Ag, CdSe), agro-chemicals (SiO2), food packaging (Ag, TiO2. ZnO, TiN, nanoclay) nanomedicine and nanocarriers (Ag, Fe, magnetic materials). Nanotechnology offers a new range of benefits to food chain and human health by increasing the taste and flavour and reducing the amount of salt intake and fat thereby increasing the absorption and bioavailability of nutrients/supplements. Over 200 companies are conducting R&D into the application of nanotechnology in almost all areas. It has been estimated that about 150 applications of nanotechnology in food are at developmental stages and over 500 patents are in the pipeline. It is therefore anticipated that the use of nanotechnology will brighten the future prospect and enhance our knowledge with drastic reduction in the cost of nano-based food and medicines.
In conclusion, emphasis had been given to the phytosynthesis of nanoparticles from plant extract and their application in agriculture for substantial increase in biomass, fruit and crop yield especially in edible plants and vegetables such as cucumber, spinach, cabbage, radish, carrot, bitter melon and tomato. Many precious metals are also used as nanocatalyst to increase the production and decrease the cost. The drug delivery by nanomaterials is more important as the drug is quickly transported to the target cell without damaging the normal cells. Many nanomaterials are also essential plant nutrients and may therefore be absorbed to supplement deficiency in living system. Since with the minimum quantity of nanomaterial maximum yield is obtained, the disposal of nanomaterials will not create an environmental problem. This review is relevant in the present day scenario when there is an urgent need of enhanced food grain production to overcome its scarcity and to treat fatal diseases like cancer and AIDS.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AH gathered the research data. AH and KSS analysed these data findings and wrote this review paper. Both authors read and approved the final manuscript.
Contributor Information
Azamal Husen, Email: adroot92@yahoo.co.in.
Khwaja Salahuddin Siddiqi, Email: ks_siddiqi@yahoo.co.in.
Acknowledgements
The authors are thankful to the publishers for the permission to adopt their figures for this review.
References
- Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdörster G, Philbert MA, Ryan J, Seaton A, Stone V, Tinkle SS, Tran L, Walker NJ, Warheit DB. Safe handling of nanotechnology. Nature. 2006;9:267–269. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- Rejeski D, Lekas D. Nanotechnology field observations: scouting the new industrial west. J Cleaner Prod. 2008;9:1014–1017. [Google Scholar]
- Dawson NG. Sweating the small stuff, environmental risk and nanotechnology. Bio Sci. 2008;9:690. [Google Scholar]
- FAO/WHO [Food and Agriculture Organization of the United Nations/World Health Organization] FAO/WHO Expert meeting on the application of nanotechnologies in the food and agriculture sectors: potential food safety implications. Rome: Meeting report; 2010. [Google Scholar]
- Roco MC, Bainbridge WS. Societal Implications of Nanoscience and Nanotechnology. Boston: Kluwer; 2001. pp. 3–4. [Google Scholar]
- Brooks RR, Chambers MF, Nicks LJ, Robinson BH. Phytomining. Trends Plant Sci. 1998;9:359–362. [Google Scholar]
- McGrath SP, Zhao FJ. Phytoextraction of metals and metalloids from contaminated soils. Curr Opin Biotechnol. 2003;9:277–282. doi: 10.1016/s0958-1669(03)00060-0. [DOI] [PubMed] [Google Scholar]
- Jabeen R, Ahmad A, Iqbal M. Phytoremediation of heavy metals: physiological and molecular aspects. Bot Rev. 2009;9:339–364. [Google Scholar]
- Zhang WX. Nanoscale iron particles for environmental remediation: an overview. J Nano Res. 2003;9:323–332. [Google Scholar]
- Zheng L, Hong F, Lu S, Liu C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Element Res. 2005;9:83–91. doi: 10.1385/BTER:104:1:083. [DOI] [PubMed] [Google Scholar]
- Galbraith DW. Nanobiotechnology: silica breaks through in plants. Nature Nanotechno. 2007;9:272–273. doi: 10.1038/nnano.2007.118. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim SH, Kim HJ, Choi SH. A new composition of nanosized silica-silver for control of various plant diseases. Plant Patho. 2007;9:295–302. [Google Scholar]
- Shah V, Belozerova I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009;9:143–148. [Google Scholar]
- Rico CM, Majumdar S, Duarte-Gardea M, Peralta-Videa JR, Gardea-Torresdey JL. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J Agric Food Chem. 2011;9:3485–3498. doi: 10.1021/jf104517j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS. Nanoparticulate material delivery to plants. Plant Sci. 2010;9:154–163. [Google Scholar]
- Zhang L, Fang M. Nanomaterials in pollution trace detection and environmental improvement. Nano Today. 2010;9:128–142. [Google Scholar]
- Liu F, Wen LX, Li ZZ, Yu W, Sun HY, Chen JF. Porous hollow silica nanoparticles as controlled delivery system for water-soluble pesticide. Mat Res Bull. 2006;9:2268–2275. [Google Scholar]
- Kumar R, Roopan SM, Prabhakarn A, Khanna VG, Chakroborty S. Agricultural waste Annona squamosa peel extract: biosynthesis of silver nanoparticles. Spectro Acta A Mol Biomol Spectrosc. 2012;9:173–176. doi: 10.1016/j.saa.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Roopan SM, Bharathi A, Prabhakarn A, Rahuman AA, Velayutham K, Rajakumar G, Padmaja RD, Lekshmi M, Madhumitha G. Efficient phyto-synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectro Acta A Mol Biomol Spectrosc. 2012;9:86–90. doi: 10.1016/j.saa.2012.08.055. [DOI] [PubMed] [Google Scholar]
- Nisha SN, Aysha OS, Rahaman JSN, Kumar PV, Valli S, Nirmala P, Reena A. Lemon peels mediated synthesis of silver nanoparticles and its antidermatophytic activity. Spectro Acta A Mol Biomol Spectrosc. 2014;9:194–198. doi: 10.1016/j.saa.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009;9:79–84. doi: 10.1007/s00449-008-0224-6. [DOI] [PubMed] [Google Scholar]
- Husen A, Worku N, Nega B, Birhanu A. Genetically modified crops/genetically modified organisms, prospects and problems. Focus Chro. 2001;9:283–300. [Google Scholar]
- Biswas P, Wu CY. Critical review, nanoparticles and the environment. J Air Waste Manag Assoc. 2005;9:708–746. doi: 10.1080/10473289.2005.10464656. [DOI] [PubMed] [Google Scholar]
- Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol. 2008;9:8959–8964. doi: 10.1021/es801785m. [DOI] [PubMed] [Google Scholar]
- Mondal A, Basu R, Das S, Nandy P. Beneficial role of carbon nanotubes on mustard plant growth, an agricultural prospect. J Nanopart Res. 2011;9:4519–4528. [Google Scholar]
- Monica RC, Cremonini R. Nanoparticles and higher plants. Caryologia. 2009;9:161–165. [Google Scholar]
- US EPA (US Environmental Protection Agency) Ecological Effects Test Guidelines. Seed Germination/Root Elongation Toxicity Test. OPPTS 850.4200. Washington, D.C: US EPA; 1996. [Google Scholar]
- Battke F, Leopold K, Maier M, Schidhalter U, Schuster M. Palladium exposure of barley uptake and effects. Plant Biol. 2008;9:272–276. doi: 10.1111/j.1438-8677.2007.00017.x. [DOI] [PubMed] [Google Scholar]
- Zhu H, Han J, Xiao JQ, Jin Y. Uptake, translocation, and accumulation of manufactured iron oxide by pumpkin plants. J Environ Monit. 2008;9:713–717. doi: 10.1039/b805998e. [DOI] [PubMed] [Google Scholar]
- Lee WM, An YJ, Yoon H, Kwbon HS. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestrivum): plant agar test for water-insoluble nanoparticles. Environ Toxico Chem. 2008;9:1915–1921. doi: 10.1897/07-481.1. [DOI] [PubMed] [Google Scholar]
- Brooks RR, Robinson BH. In: Plants that Hyperaccumulate Heavy Metals. Brooks RR, editor. New York: CAB International; 1998. The potential use of hyperaccumulators and other plants for phytomining; pp. 327–356. [Google Scholar]
- Anderson CWN, Brooks RR, Chiarucci A, LaCoste CJ, Leblanc M, Robinson BH, Simcock R, Stewart RB. Phytomining for nickel, thallium and gold. J Geochem Explor. 1999;9:407–415. [Google Scholar]
- An J, Zhang M, Wang S, Tang J. Physical, chemical and microbiological changes in stored green asparagus spears as affected by coating of silver nanoparticles-PVP. LWT-Food Sci Technol. 2008;9:1100–1107. [Google Scholar]
- Roghayyeh SMS, Mehdi TS, Rauf SS. Effects of nano-iron oxide particles on agronomic traits of soybean. Notulae Sci Biol. 2010;9:112–113. [Google Scholar]
- Miao AJ, Quigg A, Schwehr K, Xu C, Santschi P. 2nd International Conference on the Environmental Effects of Nanoparticles and Nanomaterials: Sept 24–25. London; 2007. Engineered silver nanoparticles (ESNs) in coastal marine environments, bioavailability and toxic effects to the phytoplankton Thalassiosira weissflogii. [Google Scholar]
- Musante C, White JC. Toxicity of silver and copper to Cucurbita pepo, differential effects of nano and bulk-size particles. Environ Toxic. 2010;9:510–517. doi: 10.1002/tox.20667. [DOI] [PubMed] [Google Scholar]
- Husen A, Mishra VK. Effect of IBA and NAA on vegetative propagation of Vitex negundo L. through leafy stem cuttings from hedged shoots during rainy season. Ind Perf. 2001;9:83–87. [Google Scholar]
- Husen A. Adventitious root formations of shoot cuttings of Datura innoxia Mill. by IBA under intermittent mist. Ann For. 2002;9:280–283. [Google Scholar]
- Husen A. Effects of IBA and NAA treatments on rooting of Rauvolfia serpentina Benth. ex Kurz shoot cuttings. Ann For. 2003;9:88–93. [Google Scholar]
- Husen A. Changes of soluble sugars and enzymatic activities during adventitious rooting in cuttings of Grewia optiva as affected by age of donor plants and auxin treatments. Am J Plant Physiolo. 2012;9:1–16. [Google Scholar]
- Husen A. Clonal propagation of Dalbergia sissoo Roxb. and associated metabolic changes during adventitious root primordium development. New Forest. 2008;9:13–27. [Google Scholar]
- Husen A. Clonal Propagation of Teak (Tectona grandis Linn. f.) - Adventitious Root Formation: Influence of Physiological and Chemical Factors. Saarbrücken: LAP LAMBERT Academic Publishing; 2012. pp. 1–461. [Google Scholar]
- Burris JN, Lenaghan SC, Zhang M, Stewart CN. Nanoparticle biofabrication using English ivy (Hedera helix) J Nanobiotech. 2012;9:41. doi: 10.1186/1477-3155-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut. 2007;9:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Doshi R, Braida W, Christodoulatos C, Wazne M, O'Connor G. Nano-aluminum, transport through sand columns and environmental effects on plants and soil communities. Environ Res. 2008;9:296–303. doi: 10.1016/j.envres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Stampoulis D, Sinha SK, White JC. Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol. 2009;9:9473–9479. doi: 10.1021/es901695c. [DOI] [PubMed] [Google Scholar]
- Barrena R, Casals E, Colon J, Font X, Sanchez A, Puntes V. Evaluation of the ecotoxicity of model nanoparticles. Chemo. 2009;9:850–857. doi: 10.1016/j.chemosphere.2009.01.078. [DOI] [PubMed] [Google Scholar]
- El-Temsah YS, Joner EJ. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol. 2012;9:42–49. doi: 10.1002/tox.20610. [DOI] [PubMed] [Google Scholar]
- Feng Y, Cui X, He S, Dong G, Chen M, Wang J, Lin X. The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth. Environ Sci Technol. 2013;9:9496–9504. doi: 10.1021/es402109n. [DOI] [PubMed] [Google Scholar]
- Dimkpa CO, McLean JE, Martineau N, Britt DW, Haverkamp R, Anderson AJ. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ Sci Technol. 2013;9:1082–1090. doi: 10.1021/es302973y. [DOI] [PubMed] [Google Scholar]
- Kumari M, Mukherjee A, Chadrasekaran N. Genotoxicity of silver nanoparticle in Allium cepa. Sci Total Environ. 2009;9:5243–5246. doi: 10.1016/j.scitotenv.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, Kim YK, Lee YS, Jeong DH, Cho MH. Antimicrobial effects of silver nanoparticles. Nanomed Nanotechno Biol Med. 2007;9:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Raffin M, Hussain F, Bhatti TM, Akhter JI, Hameed A, Hasan MM. Antibacterial characterization of silver nanoparticles against E. Coli ATCC-15224. J Mater Sci Technol. 2008;9:192–196. [Google Scholar]
- Abdel-Aziz MS, Shaheen MS, El-Nekeety AA, Abdel-Wahhab MA. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J Saudi Chem Soc. 2013. http://dx.doi.org/10.1016/j.jscs.2013.09.011.
- Abou El-nour KM, Eftaiha A, Al-Warthan A, Ammar RA. Synthesis and applications of silver nanoparticles. Arab J Chem. 2010;9:135–140. [Google Scholar]
- Priyadarshini S, Gopinath V, Priyadharsshini NM, MubarakAli D, Velusamy P. Synthesis of anisotropic silver nanoparticles using novel strain, Bacillus flexus and its biomedical application. Coll Surf B. 2013;9:232–237. doi: 10.1016/j.colsurfb.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Mittal AK, Kaler A, Banerjee UC. Free radical scavenging and antioxidant activity of silver nanoparticles synthesized from flower extract of Rhododendron dauricum. Nano Biomed Eng. 2012;9:118–124. [Google Scholar]
- Jeeva K, Thiyagarajan M, Elangovan V, Geetha N, Venkatachalam P. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind Crop Prod. 2014;9:714–720. [Google Scholar]
- Becker RO. Silver ions in the treatment of local infections. Met Based Drugs. 1999;9:297–300. doi: 10.1155/MBD.1999.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash P, Gnanaprakasam P, Emmanuel R, Arokiyaraj S, Saravanan M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Coll Surf B. 2013;9:255–259. doi: 10.1016/j.colsurfb.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Vijayakumar M, Priya K, Nancy FT, Noorlidah A, Ahmed ABA. Biosynthesis, characterisation and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind Crop Prod. 2013;9:235–240. [Google Scholar]
- Raut RW, Kolekar NS, Lakkakula JR, Mendhulkar VD, Kashid SB. Extracellular synthesis of silver nanoparticles using dried leaves of Pongamia pinnata (L) Pierre. Nano-Micro Lett. 2010;9:106–113. [Google Scholar]
- Suman TY, Rajasree SRR, Kanchana A, Elizabeth SB. Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Coll Surf B. 2013;9:74–78. doi: 10.1016/j.colsurfb.2013.01.037. [DOI] [PubMed] [Google Scholar]
- Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Wang Y, Shao W, He N, Hong J, Chen C. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechno. 2007;9:105104. [Google Scholar]
- Steinitz B, Barr N, Tabib Y, Vaknin Y, Bernstein N. Control of in vitro rooting and plant development in Corymbia maculata by silver nitrate, silver thiosulfate and thiosulfate ion. Plant Cell Rep. 2010;9:1315–1323. doi: 10.1007/s00299-010-0918-5. [DOI] [PubMed] [Google Scholar]
- Merril CR, Bisher ME, Harrington M, Steven AC. Coloration of silver-stained protein bands in polyacrylamide gels is caused by light-scattering from silver grains of characteristic sizes. Proc Natl Acad Sci U S A. 1988;9:453–457. doi: 10.1073/pnas.85.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Coquelard C, Schaming D, Lampre I, Ruhlmann L. Photocatalytic reduction of Ag2SO4 by the Dawson anion [alpha]-[P2W18O62]6- and tetracobalt sandwich complexes. Appl Catal B Environ. 2008;9:835–842. [Google Scholar]
- Tsai CM, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;9:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;9:93–99. [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem. 1996;9:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Eapen S, George L. Plant regeneration from peduncle segments of oil seed Brassica species: influence of silver nitrate and silver thiosulfate. Plant Cell Tissue Organ Cult. 1997;9:229–232. [Google Scholar]
- Harris AT, Bali R. On the formation and extent of uptake of silver nanoparticles by live plants. J Nanopart Res. 2008;9:691–695. [Google Scholar]
- Blaylock MJ, Salt DE, Dushenkov S, Zakharova O, Gussman C, Kapulnik Y, Ensley BD, Raskin I. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ Sci Technol. 1997;9:860–865. [Google Scholar]
- Haverkamp RG, Marshall AT. The mechanism of metal nanoparticle formation in plants: limits on accumulation. J Nanopart Res. 2009;9:1453–1463. [Google Scholar]
- Anderson CWN, Brooks RR, Stewart RB, Simcock R. Harvesting a crop of gold in plants. Nature. 1998;9:553–554. [Google Scholar]
- Gardea-Torresdey J, Parsons J, Gomez E, Peralta-Videa J, Troiani H, Santiago P, Yacaman M. Formation of Au nanoparticle inside live alfalfa plants. Nano Lett. 2002;9:397–401. [Google Scholar]
- Sharma NC, Sahi SV, Nath S, Parsons JG, Gardea-Torresdey JL, Pal T. Synthesis of plant-mediated gold nanoparticles and catalytic role of biomatrix-embedded nanomaterials. Environ Sci Technol. 2007;9:5137–5142. doi: 10.1021/es062929a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WV, Mollenhauer H, Johnson C. An electron microscope study of silver nitrate reduction in leaf cells. Am J Bot. 1962;9:57–63. [Google Scholar]
- Vijay Kumar PPN, Pammi SVN, Kollu P, Satyanarayana KVV, Shameem U. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti bacterial activity. Ind Crop Prod. 2014;9:562–566. [Google Scholar]
- Manceau A, Nagy KL, Marcus MA, Lanson M, Geoffroy N, Jacquet T, Kirpichtchikova T. Formation of metallic copper nanoparticles at the soil–root interface. Environ Sci Technol. 2008;9:1766–1772. doi: 10.1021/es072017o. [DOI] [PubMed] [Google Scholar]
- Haverkamp RG, Marshall AT, van Agterveld D. Pick your carats: nanoparticles of gold–silver–copper alloy produced in vivo. J Nanopart Res. 2007;9:697–700. [Google Scholar]
- Gardea-Torresdey J, Rodriguez E, Parsons JG, Peralta-Videa JR, Meitzner G, Cruz-Jimenez G. Use of ICP and XAS to determine the enhancement of gold phytoextraction by Chilopsis linearis using thiocyanate as a complexing agent. Anal Bioanal Chem. 2005;9:347–352. doi: 10.1007/s00216-004-2966-6. [DOI] [PubMed] [Google Scholar]
- Armendariz V, Herrera I, Peralta-Videa JR, Jose-Yacaman M, Troiani H, Santiago P, Gardea-Torresdey JL. Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. J Nano Res. 2004;9:377–382. [Google Scholar]
- Gardea-Torresdey JL, Tiemann KJ, Gamez G, Dokken K, Tehuacamanero S, Jose-Yacaman M. Gold nanoparticles obtained by bio-precipitation from gold(III) solutions. J Nanopart Res. 1999;9:397–404. [Google Scholar]
- Gardea-Torresdey JL, Tiemann KJ, Parsons JG, Gamez G, Yaccaman MJ. Characterization of trace level Au(III) binding to alfalfa biomass. Adv Environ Res. 2002;9:313–323. [Google Scholar]
- Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, Parsons JG, Troiani H, Jose-Yacaman M. Alfalfa sprouts, a natural source for the synthesis of silver nanoparticles. Langumir. 2003;9:1357–1361. [Google Scholar]
- Lopez ML, Gardea-Torresdey JL, Peralta-Videa JR, de la Rosa G, Armendariz V, Herrera I, Troiani H. Gold binding by native and chemically modified hop biomasses. Bioinorg Chem Appl. 2005;9:29–41. doi: 10.1155/BCA.2005.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Ramani R, Parischa R, Ajayakumar PV, Alam M, Sastry M, Kumar R. Bioreduction of AuCl4- ions by fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew Chem Int Ed Engl. 2001;9:3585–3588. doi: 10.1002/1521-3773(20011001)40:19<3585::aid-anie3585>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Senapati S, Mandal D, Ahmad A, Khan MI, Kumar R, Sastry M. Extracellular synthesis of gold nanoparticles by using Fusarium oxysporum. Chem Biochem. 2002;9:461–463. doi: 10.1002/1439-7633(20020503)3:5<461::AID-CBIC461>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Greene B, Hosea M, McPherson R, Henzi M, Alexander MD, Darnall DW. Interaction of gold(I) and gold(III) complexes with algal biomass. Environ Sci Technol. 1986;9:627–632. doi: 10.1021/es00148a014. [DOI] [PubMed] [Google Scholar]
- Hosea M, Greene B, McPherson R, Henzl M, Alexander MD, Darnall DW. Accumulation of elemental gold on the alga Chlorella vulgaris. Inorg Chem Acta. 1986;9:161–165. [Google Scholar]
- Kuyucak N, Volesky B. Accumulation of gold by algal biosorbent. Biorecovery. 1989;9:189–204. [Google Scholar]
- Kasthuri J, Kathiravan K, Rajendiran N. Phyllanthin assisted biosynthesis of silver and gold nanoparticles: a novel biological approach. J Nanopart Res. 2009;9:1075–1085. [Google Scholar]
- Singh AK, Talat M, Singh DP, Srivastava ON. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J Nanopart Res. 2010;9:1667–7165. [Google Scholar]
- Shankar SS, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog. 2003;9:1627–1631. doi: 10.1021/bp034070w. [DOI] [PubMed] [Google Scholar]
- Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Coll Inter Sci. 2004;9:496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M. Biological synthesis of triangular gold nanoprisms. Nat Mater. 2004;9:482–488. doi: 10.1038/nmat1152. [DOI] [PubMed] [Google Scholar]
- Zhan G, Huang J, Lin L, Lin W, Emmanuel K, Li Q. Synthesis of gold nanoparticles by Cacumen Platycladi leaf extract and its simulated solution: toward the plant-mediated biosynthetic mechanism. J Nanopart Res. 2011;9:4957–4968. [Google Scholar]
- Arora S, Sharma P, Kumar S, Nayan R, Khanna PK, Zaidi MGH. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012;9:303–310. [Google Scholar]
- Zhou D, Jin S, Li L, Wang Y, Weng N. Quantifying the adsorption and uptake of CuO nanoparticles by wheat root based on chemical extractions. J Environ Sci. 2011;9:1852–1857. doi: 10.1016/s1001-0742(10)60646-8. [DOI] [PubMed] [Google Scholar]
- Bali R, Siegele R, Harris AT. Biogenic Pt uptake and nanoparticle formation in Medicago sativa and Brassica juncea. J Nanopart Res. 2010;9:3087–3095. [Google Scholar]
- Roopan SM, Bharathi A, Kumar R, Khanna VG, Prabhakarn A. Acaricidal, insecticidal, and larvicidal efficacy of aqueous extract of Annona squamosa L peel as biomaterial for the reduction of palladium salts into nanoparticles. Coll Surf B. 2012;9:209–212. doi: 10.1016/j.colsurfb.2011.11.044. [DOI] [PubMed] [Google Scholar]
- Klaus T, Joerger R, Olsson E, Granqvist CG. Silver-based crystalline nanoparticles, microbially fabricated. Proc Natl Acad Sci U S A. 1999;9:13611–13614. doi: 10.1073/pnas.96.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong P, Rowson N, Farr JPG, Harris I, Macaskie L. Bioreduction and biocrystallization of palladium by Desulfovibrio desulfuricans NCIMB 8307. Biotechnol Bioeng. 2002;9:369–379. doi: 10.1002/bit.10369. [DOI] [PubMed] [Google Scholar]
- Corredor E, Testillano PS, Coronado MJ, González-Melendi P, Fernández-Pacheco R, Marquina C, Ibarra MR, de la Fuente JM, Rubiales D, Pérez-de-Luque A, Risueño MC. Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification. BMC Plant Biol. 2009;9:45. doi: 10.1186/1471-2229-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NJ, Fauquet CM. Microparticle bombardment as a tool in plant science and agricultural biotechnology. DNA Cell Biol. 2002;9:963–977. doi: 10.1089/104454902762053891. [DOI] [PubMed] [Google Scholar]
- BarathManiKanth S, Kalishwaralal K, Sriram M, Pandian SBRK, Youn H, Eom SH, Gurunathan S. Antioxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J Nanobiotech. 2010;9:16. doi: 10.1186/1477-3155-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2008;9:507–517. [Google Scholar]
- Wu H, Huang X, Gao M, Liao X, Shi B. Polyphenol-grafted collagen fiber as reductant and stabilizer for one-step synthesis of size-controlled gold nanoparticles and their catalytic application to 4-nitrophenol reduction. Green Chem. 2011;9:651–658. [Google Scholar]
- Ghosh S, Patil S, Ahire M, Kitture R, Gurav DD, Jabgunde AM, Kale S, Pardesi K, Shinde V, Bellare J, Dhavale DD, Chopade BA. Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J Nanobiotechno. 2012;9:17. doi: 10.1186/1477-3155-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankar PS, Bajpai D. Preparation of gold nanoparticles from Mirabilis jalapa flowers. Ind J Biochem Biophys. 2010;9:157–160. [PubMed] [Google Scholar]
- Das RK, Gogoi N, Bora U. Green synthesis of gold nanoparticles using Nyctanthes arbortristis flower extract. Bioprocess Biosyst Eng. 2011;9:615–619. doi: 10.1007/s00449-010-0510-y. [DOI] [PubMed] [Google Scholar]
- Smitha SL, Philip D, Gopchandrana KG. Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectro Acta A Mol Biomol Spectrosc. 2009;9:735–739. doi: 10.1016/j.saa.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Philip D. Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectro Acta A Mol Biomol Spectrosc. 2010;9:807–810. doi: 10.1016/j.saa.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Noruzi M, Zare D, Khoshnevisan K, Davoodi D. Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. Spectro Acta A Mol Biomol Spectrosc. 2011;9:1461–1465. doi: 10.1016/j.saa.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Vanaja M, Paulkumar K, Baburaja M, Rajeshkumar S, Gnanajobitha G, Malarkodi C, Sivakavinesan M, Annadurai G. Degradation of methylene blue using biologically synthesized silver nanoparticles. Bioinor Chem App. 2014;9:8. doi: 10.1155/2014/742346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganaie SU, Abbasi T, Anuradha J, Abbasi SA. Biomimetic synthesis of silver nanoparticles using the amphibious weed ipomoea and their application in pollution control. J King Saud Uni–Sci. 2014. http://dx.doi.org/10.1016/j.jksus.2014.02.004.
- Satyavani K, Gurudeeban S, Ramanathan T, Balasubramanian T. Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.) Schrad. J Nanobiotechno. 2011;9:43. doi: 10.1186/1477-3155-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S, Smith DR, Mock JJ, Schultz DA. Single-target molecule detection with non bleaching multicolor optical immunolabels. Proc Natio Acad Sci. 2000;9:996–1001. doi: 10.1073/pnas.97.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair B, Pradeep T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst Growth Des. 2002;9:293–298. [Google Scholar]
- Gurunathan S, Lee KJ, Kalimuthu K, Sheikpranbabu S, Vaidyanathan R, Eom SH. Anti angiogenic properties of silver nanoparticles. Biomaterials. 2009;9:6341–6350. doi: 10.1016/j.biomaterials.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Moaddab S, Ahari H, Shahbazzadeh D, Motallebi AA, Anvar AA, Rahman-Nya J, Shokrgozar MR. Toxicity study of nanosilver (Nanocid) on osteoblast cancer cell line. Int Nano Lett. 2011;9:11–16. [Google Scholar]
- Patil CD, Borase HP, Patil SV, Salunkhe RB, Salunke BK. Larvicidal activity of silver nanoparticles synthesized using Pergularia daemia plant latex against Aedes aegypti and Anopheles stephensi and nontarget fish Poecillia reticulate. Parasitol Res. 2012;9:555–562. doi: 10.1007/s00436-012-2867-0. [DOI] [PubMed] [Google Scholar]
- Salunkhe RB, Patil SV, Patil CD, Salunke BK. Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera, Culicidae) Parasitol Res. 2011;9:823–831. doi: 10.1007/s00436-011-2328-1. [DOI] [PubMed] [Google Scholar]
- Richardson A, Chan BC, Crouch RD, Janiec A, Chan BC, Crouch RD. Synthesis of silver nanoparticles: an undergraduate laboratory using green approach. Chem Educ. 2006;9:331–333. [Google Scholar]
- Kumar V, Yadav SK. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol. 2009;9:151–157. [Google Scholar]
- Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Coll Surf A Physicochem Eng Asp. 2009;9:134–139. [Google Scholar]
- Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem. 2008;9:1972–1978. doi: 10.1897/08-002.1. [DOI] [PubMed] [Google Scholar]
- Lu CM, Zhang CY, Wen JQ, Wu GR, Tao MX. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002;9:168–172. [Google Scholar]
- Hong F, Zhou J, Liu C, Yang F, Wu C, Zheng L, Yang P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem Res. 2005;9:269–279. doi: 10.1385/BTER:105:1-3:269. [DOI] [PubMed] [Google Scholar]
- Hong FS, Yang F, Liu C, Gao Q, Wan ZG, Gu FG, Wu C, Ma ZN, Zhou J, Yang P. Influences of nano-TiO2 on the chloroplast aging of spinach under light. Biol Trace Elem Res. 2005;9:249–260. doi: 10.1385/BTER:104:3:249. [DOI] [PubMed] [Google Scholar]
- Murashov V. Comments on “Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles” by Yang, L., Watts, D.J., Toxicology Letters, 2005, 158, 122–132. Toxicol Lett. 2006;9:185–187. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Kim E, Kim SH, Kim HC, Lee SG, Lee SJ, Jeong SW. Growth inhibition of aquatic plant caused by silver and titanium oxide nanoparticles. Toxicol Environ Health Sci. 2011;9:1–6. [Google Scholar]
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;9:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, Bruinink A, Stark WJ. In vitro cytotoxicity of oxide nanoparticle: comparison to asbestos, silica, and effect of particle solubility. Environ Sci Technol. 2006;9:4374–4381. doi: 10.1021/es052069i. [DOI] [PubMed] [Google Scholar]
- Reyes-Coronado D, Rodríguez-Gattorno G, Espinosa-Pesqueira ME, Cab C, de Coss R, Oskam G. Phase-pure TiO2 nanoparticles, anatase, brookite and rutile. Nanotechnol. 2008;9:10–19. doi: 10.1088/0957-4484/19/14/145605. [DOI] [PubMed] [Google Scholar]
- Armelao L, Barreca D, Bottaro G, Gasparotto A, Maccato C, Maragno C, Tondello E, Štangar UL, Bergant M, Mahne D. Photocatalytic and antibacterial activity of TiO2 and Au/TiO2 nanosystems. Nanotechnol. 2007;9:375709. [Google Scholar]
- Reeves JF, Davies SJ, Dodd NJF, Jha AN. Hydroxyl radicals (OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat Res. 2008;9:113–122. doi: 10.1016/j.mrfmmm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Coll Inter Sci. 2004;9:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Gade AK, Bonde PP, Ingle AP, Marcato PD, Duran N, Rai MK. Exploitation of Aspergillus niger for fabrication of silver nanoparticles. J Biobased Mater Bioenergy. 2008;9:243–247. [Google Scholar]
- Sriwong C, Wongnawa S, Patarapaiboolchai O. Rubber sheet strewn with TiO2 particles: photocatalytic activity and recyclability. J Environ Sci. 2012;9:464–472. doi: 10.1016/s1001-0742(11)60794-8. [DOI] [PubMed] [Google Scholar]
- Sobha K, Surendranath K, Meena V, Jwala KT, Swetha N, Latha KSM. Emerging trends in nanobiotechnology. J Biot Mol Biol Rev. 2010;9:1–12. [Google Scholar]
- Arokiyaraj S, Saravanan M, Udaya Prakash NK. Enhanced antibacterial activity of iron oxide magnetic nanoparticles treated with Argemone mexicana L. leaf extract: an in vitro study. Mat Res Bull. 2013;9:3323–3327. [Google Scholar]
- Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PK, Chiu JF, Che CM. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;9:916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- Priestera JH, Gea Y, Mielkea RE, Horsta AM, Moritzb SC, Espinosae K, Gelbf J, Walkerg SL, Nisbetb RM, Ani YJ, Schimelb JP, Palmere RG, Hernandez-Viezcasc JA, Zhaoc L, Gardea-Torresdeyc JL, Holdena PA. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc Natl Acad Sci U S A. 2012;9:14734–14735. doi: 10.1073/pnas.1205431109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Watts DJ. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxico Lett. 2005;9:122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Lopez-Moreno ML, De La Rosa G, Hernandez-Viezcas JA, Peralta-Videa JR, Gardea-Torresdey JL. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J Agric Food Chem. 2010;9:3689–3693. doi: 10.1021/jf904472e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild E, Jones KC. Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants. Environ Sci Techno. 2009;9:5290–5294. doi: 10.1021/es900065h. [DOI] [PubMed] [Google Scholar]
- Morales MI, Rico CM, Hernandez-Viezcas JA, Nunez JE, Barrios AC, Tafoya A, Flores-Marges JP, Peralta-Videa JR, Gardea-Torresdey JL. Toxicity assessment of cerium oxide nanoparticles in cilantro (Coriandrum sativum L.) plants grown in organic soil. J Agric Food Chem. 2013;9:6224–6230. doi: 10.1021/jf401628v. [DOI] [PubMed] [Google Scholar]
- Rico CM, Hong J, Morales MI, Zhao L, Barrios AC, Zhang JY, Peralta-Videa JR, Jorge L, Gardea-Torresdey JL. Effect of cerium oxide nanoparticles on rice: a study involving the antioxidant defense system and in vivo fluorescence imaging. Environ Sci Technol. 2013;9:5635–5642. doi: 10.1021/es401032m. [DOI] [PubMed] [Google Scholar]
- Ghafariyan MH, Malakouti MJ, Dadpour MR, Stroeve P, Mahmoudi M. Effects of magnetite nanoparticles on soybean chlorophyll. Environ Sci Technol. 2013;9:10645–10652. doi: 10.1021/es402249b. [DOI] [PubMed] [Google Scholar]
- Parsons JG, Lopez ML, Gonzalez CM, Peralta-Videa JR, Gardea-Torresdey JL. Toxicity and biotransformation of uncoated and coated nickel hydroxide nanoparticles on mesquite plants. Environ Toxicol Chem. 2010;9:1146–1154. doi: 10.1002/etc.146. [DOI] [PubMed] [Google Scholar]
- Feizi H, Moghaddam PR, Shahtahmassebi N, Fotovat A. Impact of bulk and nanosized titanium dioxide (TiO2) on wheat seed germination and seedling growth. Biol Trace Elem Res. 2012;9:101–106. doi: 10.1007/s12011-011-9222-7. [DOI] [PubMed] [Google Scholar]
- Gao F, Hong F, Liu C, Zheng L, Su M, Wu X, Yang F, Wu C, Yang P. Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach. Biol Trace Elem Res. 2006;9:239–253. doi: 10.1385/BTER:111:1:239. [DOI] [PubMed] [Google Scholar]
- Yang F, Liu C, Gao F, Su M, Wu X, Zheng L, Hong F, Yang P. The improvement of spinach growth by nano-anatase TiO2 treatment is related to nitrogen photoreduction. Biol Trace Elem Res. 2007;9:77–88. doi: 10.1007/s12011-007-0046-4. [DOI] [PubMed] [Google Scholar]
- Linglan M, Chao L, Chunxiang Q, Sitao Y, Jie L, Fengqing G, Fashui H. Rubisco activase mRNA expression in spinach: modulation by nanoanatase treatment. Biol Trace Elem Res. 2008;9:168–178. doi: 10.1007/s12011-007-8069-4. [DOI] [PubMed] [Google Scholar]
- Asli S, Neumann M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009;9:577–584. doi: 10.1111/j.1365-3040.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- Hruby M, Cigler P, Kuzel S. Contribution to understanding the mechanism of titanium action in plant. J Plant Nutr. 2002;9:577–598. [Google Scholar]
- Lin DH, Xing BS. Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Techno. 2008;9:5580–5585. doi: 10.1021/es800422x. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Xie XY, Zhao J, Liu XY, Feng WQ, White JC, Xing B. Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.) Environ Sci Technol. 2012;9:4434–4441. doi: 10.1021/es204212z. [DOI] [PubMed] [Google Scholar]
- Lee CW, Mahendra S, Zodrow K, Li D, Tsai YC, Braam J, Alvarez PJJ. Developmental phytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana. Environ Toxico Chem. 2010;9:669–675. doi: 10.1002/etc.58. [DOI] [PubMed] [Google Scholar]
- Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol. 2007;9:8484–8490. doi: 10.1021/es071445r. [DOI] [PubMed] [Google Scholar]
- Boonyanitipong P, Kositsup B, Kumar P, Baruah S, Dutta J. Toxicity of ZnO and TiO2 nanoparticles on germinating rice seed Oryza sativa L. Int J Biosci Biochem Bioinfor. 2011;9:282–285. [Google Scholar]
- Wu SG, Huang L, Head J, Chen DR, Kong IC, Tang YJ. Phytotoxicity of metal oxide nanoparticles is related to both dissolved metals ions and adsorption of particles on seed surfaces. Pet Environ Biotechnol. 2012;9:1000126. [Google Scholar]
- Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F, Biris AS. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano. 2009;9:3221–3227. doi: 10.1021/nn900887m. [DOI] [PubMed] [Google Scholar]
- Khodakovskaya MV, Kim BS, Kim JN, Alimohammadi M, Dervishi E, Mustafa T, Cernigla CE. Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community. Small. 2013;9:115–123. doi: 10.1002/smll.201201225. [DOI] [PubMed] [Google Scholar]
- Lavalley JC, Benaissa M. In: Adsorption and Catalysis on Oxide Surfaces. Che M, Bond GC, editor. Amsterdam: Elsevier; 1985. Infrared study of surface modes on alumina; pp. 251–261. [Google Scholar]
- Tai C, Gu X, Zou H, Guo Q. A new simple and sensitive fluorometric method for the determination of hydroxyl radical and its application. Talanta. 2002;9:661–667. doi: 10.1016/s0039-9140(02)00370-3. [DOI] [PubMed] [Google Scholar]
- Zhang L, Somasundaran P, Mielczarski J, Mielczarski E. Adsorption mechanism of n-dodecyl-β-D-maltoside on alumina. J Coll Inter Sci. 2002;9:16–22. [Google Scholar]
- Nair R, Poulose AC, Nagaoka Y, Yoshida Y, Maekawa T, Sakthi Kumar D. Uptake of FITC labeled silica nanoparticles and quantum dots by rice seedlings, effects on seed germination and their potential as biolabels for plants. J Fluoresc. 2011;9:2057–2068. doi: 10.1007/s10895-011-0904-5. [DOI] [PubMed] [Google Scholar]
- Hischemoller A, Nordmann J, Ptacek P, Mummenhoff K, Haase M. In-vivo imaging of the uptake of upconversion nanoparticles by plant roots. J Biomed Nanotech. 2009;9:278–284. doi: 10.1166/jbn.2009.1032. [DOI] [PubMed] [Google Scholar]
- Guo G, Liu W, Liang J, He Z, Xu H, Yang X. Probing the cytotoxicity of CdSe quantum dots with surface modification. Mater Lett. 2007;9:1641–1644. [Google Scholar]
- Gagne F, Auclair J, Turcotte P, Fournier M, Gagnon C, Sauve S, Blaise C. Ecotoxicity of CdTe quantum dots to freshwater mussels: impacts on immune system, oxidative stress and genotoxicity. Aquat Toxicol. 2008;9:333–340. doi: 10.1016/j.aquatox.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Mahajan P, Dhoke SK, Khanna AS. Effect of nano-ZnO particle suspension on growth of mung (Vigna radiata) and gram (Cicer arietinum) seedlings using plant agar method. J Nanotechno. 2011;9:7. [Google Scholar]
- Mauter MS, Elimelech M. Environmental applications of carbon-based nanomaterials. Environ Sci Technol. 2008;9:5843–5859. doi: 10.1021/es8006904. [DOI] [PubMed] [Google Scholar]
- Mota LC, Urena-Benavides EE, Yoon Y, Son A. Quantitative detection of single walled carbon nanotube in water using DNA and magnetic fluorescent spheres. Environ Sci Technol. 2013;9:493–501. doi: 10.1021/es303671u. [DOI] [PubMed] [Google Scholar]
- Cañas JE, Long M, Nations S, Vadan R, Dai L, Luo M, Ambikapathi R, Lee EH, Olszyk D. Effects of functionalized and nonfunctionalized single-walled carbon-nanotubes on root elongation of select crop species. Environ Toxicol Chem. 2008;9:1922–1931. doi: 10.1897/08-117.1. [DOI] [PubMed] [Google Scholar]
- Tan XM, Lin C, Fugetsu B. Studies on toxicity of multiwalled carbon nanotubes on suspension rice cells. Carbon. 2009;9:3479–3487. [Google Scholar]
- Lin S, Reppert J, Hu Q, Hudson JS, Reid ML, Ratnikova TA, Rao AM, Luo H, Ke PC. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small. 2009;9:1128–1132. doi: 10.1002/smll.200801556. [DOI] [PubMed] [Google Scholar]
- Torre-Roche RDL, Hawthorne J, Deng Y, Xing B, Cai W, Newman LA, Wang Q, Ma X, Hamdi H, White JC. Multiwalled carbon nanotubes and C60 fullerenes differentially impact the accumulation of weathered pesticides in four agricultural plants. Environ Sci Technol. 2013;9:12539–12547. doi: 10.1021/es4034809. [DOI] [PubMed] [Google Scholar]
- Kole C, Kole P, Randunu KM, Choudhary P, Podila R, Ke PC, Rao AM, Marcus RK. Nanobiotechnology can boost crop production and quality: first evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia) BMC Biotechno. 2013;9:37. doi: 10.1186/1472-6750-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husen A, Siddiqi KS. Carbon and fullerene nanomaterials in plant system. J Nanobiotechno. 2014;9:16. doi: 10.1186/1477-3155-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles P, Johnson E, Church TL, Harris AT. Multiwalled carbon nanotubes in alfalfa and wheat, toxicology and uptake. J R Soc Inter. 2012;9:3514–3527. doi: 10.1098/rsif.2012.0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodakovskaya MV, de Silva K, Nedosekin D, Dervishi E, Biris AS, Shashkov EV, Galanzha EI, Zharov VP. Complex genetic, photothermal, and photoacoustic analysis of nano particle plant interactions. Proc Natl Acad Sci U S A. 2011;9:1028–1033. doi: 10.1073/pnas.1008856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodakovskaya MV, de Silva K, Biris AS, Dervishi E, Villagarcia H. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano. 2012;9:2128–2135. doi: 10.1021/nn204643g. [DOI] [PubMed] [Google Scholar]
- Chen R, Ratnikova TA, Stone MB, Lin S, Lard M, Huang G, Hudson JS, Ke PC. Differential uptake of carbon nanoparticles by plant and mammalian cells. Small. 2010;9:612–617. doi: 10.1002/smll.200901911. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh M. Relationships between electrical conductivity of imbibed seeds leachate and subsequent seedling growth (viabiliy and vigour) in omid wheat. J Agric Set Technol. 2000;9:67–71. [Google Scholar]
- Oberdörster E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile large mouth bass. Environ Health Perspect. 2004;9:1058–1062. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi N, Hantgan RR, Lively MO, Carroll DL, Prasad GL. C60-fullerenes, detection of intracellular photoluminescence and lack of cytotoxic effects. J Nanobiotechn. 2006;9:14. doi: 10.1186/1477-3155-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Oberdorster E, Haasch ML. Toxicity of an engineered nanoparticle (fullerene, C60) in two aquatic species, Daphnia and fathead minnow. Mar Environ Res. 2006;9:S5–S9. doi: 10.1016/j.marenvres.2006.04.059. [DOI] [PubMed] [Google Scholar]
- Jacobsen NR, Pojana G, White P, Møller P, Cohn CA, Korsholm KS, Vogel U, Marcomini A, Loft S, Wallin H. Genotoxicity, cytotoxicity, and reactive oxygen species induced by single-walled carbon nanotubes and C60 fullerenes in the FE1-Muta™ mouse lung epithelial cells. Environ Mol Mutagen. 2008;9:476–487. doi: 10.1002/em.20406. [DOI] [PubMed] [Google Scholar]
- Folkmann JK, Risom L, Jacobsen NR, Wallin H, Loft S, Møller P. Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenes and single-walled carbon nanotubes. Environ Health Perspect. 2009;9:703–708. doi: 10.1289/ehp.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Wang L, Wang Y, Liang Y, Zhang J. Toxicity effects of four typical nanomaterials on the growth of Escherichia coli, Bacillus subtilis and Agrobacterium tumefaciens. Environ Earth Sci. 2012;9:1643–1649. [Google Scholar]
- Liu W, Wu Y, Wang C, Li HC, Wang T, Liao CY, Cui L, Zhou QF, Yan B, Jiang GB. Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxico. 2010;9:319–330. doi: 10.3109/17435390.2010.483745. [DOI] [PubMed] [Google Scholar]
- Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;9:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]


