Dear Editor,
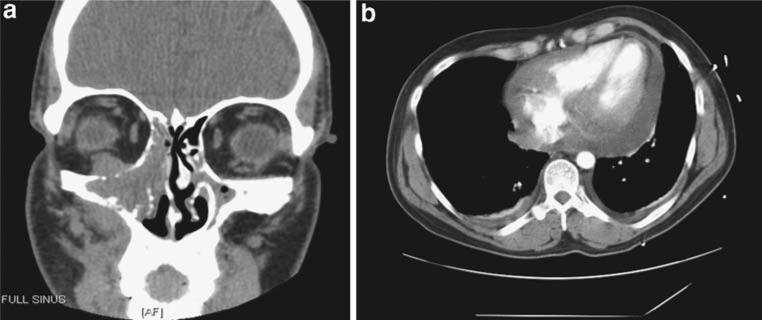
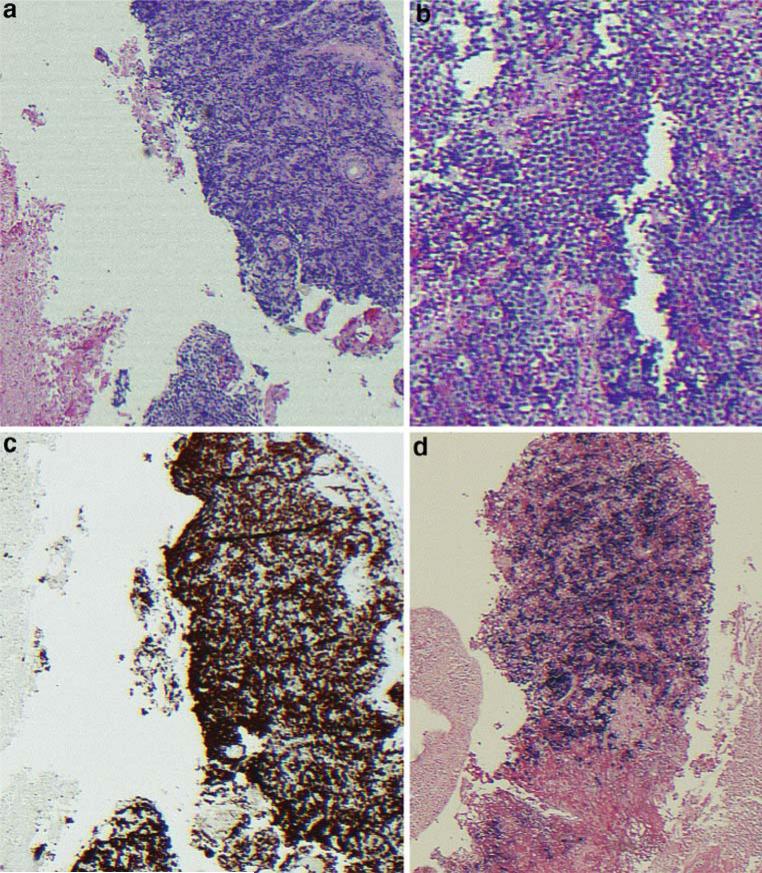
A 42-year-old man was transferred to our hospital after presenting with syncope. Approximately 3 and 6 weeks before admission, the patient had been involved in two separate fights resulting in significant facial injury and swelling. The patient did not seek immediate medical attention but persistent pain prompted evaluation at our hospital 10 days before the relevant admission. At that time, a computed tomography (CT) of the head and facial bones demonstrated facial fractures and an expansile opacification of the right maxillary sinus with erosive change of the adjacent osseous structures and contiguous abnormal soft tissue extending into the periantral fat and extraconal orbit (Fig. 1a). The radiographic differential was an infected mucocele with concomitant osteomyelitis versus a neoplastic process, and thus, biopsy was recommended. The patient was taken to the operating room, and a biopsy of the mass was performed. He was given antibiotics for a presumed infected mucocele with osteomyelitis and discharged with plans to follow-up in 1 week as an outpatient for the pathology results. Before his follow-up appointment, he presented to an outside hospital with complaints of syncope with pre-syncopal dizziness. By this time, the pathology results from the initial maxillary biopsy had revealed sheets of tumor composed of primitive cells with round hyperchromatic nuclei and prominent nucleoli (Fig. 2). Numerous large cells with clear cytoplasm were scattered through the tumor in a starry-sky-type pattern. The tumor appeared to be proliferating rapidly with frequent mitoses and geographic areas of necrosis. Immunohistochemical staining indicated B-cell lineage with aberrant CD10 co-expression and an extremely high proliferative index. The in situ hybridization study for Epstein Barr virus (EBV) genome was positive. Together, the findings were consistent with a diagnosis of Burkitt's lymphoma. He was transferred to our hospital for further management.
Fig. 1.
a Coronal section of head CT demonstrating soft tissue mass eroding the right maxillary sinus. b Chest CT image demonstrating complex pericardial effusion
Fig. 2.
a, b 10×, 20× magnification of H&E staining of R maxillary sinus mass biopsy. c Immunohistochemical staining for Ki67 proliferative index and d in situ hybridization study for EBV genome
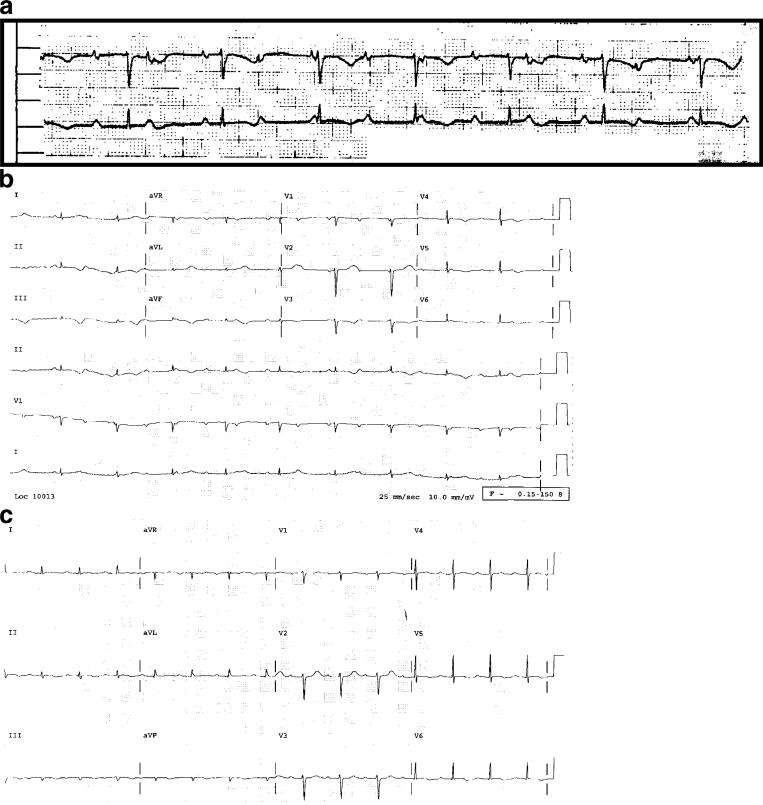
At the time of transfer, the patient was disheveled but in no acute distress. Before the diagnosis of the facial fracture, his medical history had been unremarkable other than surgical repair of an inguinal hernia. He was not taking medications other than acetaminophen and hydrocodone for pain and amoxicillin/clavulanate since his recent discharge. He had a complicated social history. He had been previously married but was divorced and was currently homeless. In the 1 to 3 years before admission, he had unprotected sexual intercourse with both men and women. He denied injection drug use but admitted to heavy alcohol use, as well as recreational cocaine and marijuana. He had smoked one to two packs per day for 27 years. His blood pressure was 123/67 mmHg, and his pulse was bradycardic in the 50 s. His physical exam was notable for a left-eye periorbital ecchymosis and right temple edema without ecchymosis, status post surgical incisions. He had bilateral mild axillary lymphadenopathy but no other evidence of lymph node enlargement. Cardiovascular exam was notable for a regular, bradycardic rhythm without murmur, gallop, or rub. His lungs were decreased at the bases with dullness to percussion, and otherwise clear. His abdomen was soft, non-tender, non-distended without palpable mass or organomegaly. He was neurologically intact. Admission labs at this time were notable for a WBC of 27.4 k/ul with 91% neutrophils (greater than 20% bands), normal hemoglobin and platelets, a normal liver function panel (expect for an albumin of 2.6 g/dl), normal renal function, a uric acid of 6.1 and a lactic dehydrogenase (LDH) of 591. A test for HIV was ordered. An initial rhythm strip (Fig. 3a) prompted an admission electrocardiogram (EKG; Fig. 3b) that was notable for third-degree atrioventricular block with a ventricular escape rate of 58, as well as abnormal T waves in the inferior leads. Transthoracic echocardiography (TTE) showed a moderate size pericardial effusion, normal left ventricular (LV) systolic function and no evidence of tamponade. The patient was admitted to the cardiac care unit for further management, and a transvenous pacemaker was placed.
Fig. 3.
a Initial rhythm strip demonstrating complete heart block. b EKG on admission demonstrating complete heart block. c EKG on day 3 of chemotherapy demonstrating sinus rhythm with first-degree AV block
On hospital day 2, his HIV test returned positive and his CD4 count was measured at 104/ul with a viral load of 280 copies/ml. An urgent oncology consult was called. A CT scan of the chest, abdomen, and pelvis confirmed the moderate complex pericardial effusion, suggesting possible lymphomatous involvement of the pericardium (Fig. 1b), and also revealed small bilateral pleural effusions, bilateral axillary and para-aortic lymphadenopathy. The patient refused bone marrow biopsy. Colony stimulation factor (CSF) studies were negative for malignant cells but the CSF polymerase chain reaction (PCR) was positive for EBV. He was clinically staged as having 4A disease. A peripherally inserted central catheter (PICC) line was placed, and aggressive chemotherapy was initiated with an rituximab plus EPOCH (R-EPOCH) regimen dose adjusted for his low CD4 count consisting of rituximab (375 mg/m2 on day 1 of the cycle), etoposide (50 mg/m2 on days 1–4 by continuous infusion), prednisone (60 mg/m2 on days 1–5), vincristine (0.4 mg/m2 on days 1–4 by continuous infusion), cyclophosphamide (375 mg/m2 IV on day 5), and doxorubicin (10 mg/m2 by continuous infusion on days 1–4) [1]. The patient also received a single dose of intrathecal methotrexate 12.5 mg on day 1 of the chemotherapy cycle, as well as granulocyte (G)-CSF support after each cycle of treatment. The infectious disease service recommended initiating pneumocystis carinii pneumonia (PCP) prophylaxis with trimethoprim/sulfamethozazole.
The patient tolerated the chemotherapy without complications. His transvenous pacer was not triggered. Over the first 2 days of chemotherapy, his blood pressure remained stable and his EKGs began to demonstrate intermittent type 1 second-degree AV block (Wenckebach), also with a ventricular rate in the 50 s. However, by the completion of the third day of chemotherapy, the patient was noted to have returned to normal sinus rhythm (NSR) with first-degree AV block at a normal ventricular rate (Fig. 2c). NSR was maintained until his discharge 8 days after initiating chemotherapy. Follow-up TTE revealed a complete resolution of the complex pericardial effusion.
Highly active antiretroviral therapy (HAART) was initiated as an outpatient with good clinical response. The CD4 count improved to 487/ul, and his viral load eventually became undetectable. The patient received a total of six cycles of R-EPOCH chemotherapy and had an excellent partial response. Follow-up positron emission tomography (PET) CT studies demonstrated a marked reduction in the size of the right maxillary sinus mass, as well as the intrathoracic lymphadenopathy. Unfortunately, his lymphoma relapsed 6 months after initially starting chemotherapy. He received four cycles of R-ESHAP chemotherapy but again relapsed almost immediately. Rescue therapy was attempted with methotrexate and cytarabine coupled with cranial radiation therapy but his disease was refractory to treatment, and he passed away approximately 1 year after initial diagnosis. He did not have recurrent problems with third-degree AV block.
Heart block is a rare complication of lymphoma. Many causes can affect the atrioventricular conduction system; the most common among these include ischemic heart disease or sclerodegenerative changes. However, other etiologies including drug-related toxicities, congenital disease, viral myocarditis, and infiltrative processes such as amyloidosis, sarcoidosis, and malignancies should be considered, especially in younger patients. Although viral myocarditis from either HIV and/or associated opportunistic infection were also possibilities with our patient, the findings on the CT scan of his chest, echocardiogram, and the rapid reversibility after initiation of chemotherapy suggests that the patient's complete heart block was due to infiltrative Burkitt's lymphoma.
This case serves as an illustration that malignant infiltration causing complete heart block may, in some cases, be reversible if identified quickly with rapid initiation of therapy, thus, avoiding permanent pacemaker placement. Burkitt's lymphoma is classified as a high-grade, aggressive lymphoma, and typically is responsive to chemotherapy. There are several case reports of both primary cardiac lymphoma and metastatic infiltrative lymphoma causing complete heart block that have been reversible with rapid initiation of chemotherapy [2–9]. Most of these cases were intermediate-grade to high-grade B-cell lymphomas.
Standard therapy for Burkitt's lymphoma at our institution includes combination systemic and intrathecal chemotherapy with a modified Magrath regimen plus conventional dose rituximab [10], except for those patients with a single site of <10 cm of disease and normal LDH. However, for those patients with HIV, particularly those with any significant cytopenia or opportunistic infections at the initiation of therapy, the safety of such a regimen has not been tested, and such patients are generally treated with a CD4 cell count dose-adjusted R-EPOCH as described above.
This case is among a few reported cases specifically of Burkitt's lymphoma leading to complete heart block in a limited literature review [11]. In this case, only 5 days had passed between the time a mass was identified on imaging and the time the patient developed complete heart block. This serves as a reminder of the very aggressive nature of this tumor, and the importance of urgent oncology referral for treatment.
Acknowledgment
The authors would like to acknowledge Dr. Prista Charuworn, Dr. Jenny Schneider, and Dr. Ron Witteles for their help with this case. Joshua W. Knowles is supported by a post-doctoral fellowship award from the American Heart Association, Western States Affiliate, as well as by a Dean's Fellowship from Stanford University.
Contributor Information
Joshua W. Knowles, Division of Cardiovascular Medicine, Stanford University Medical Center, Falk CVRC, 300 Pasteur Drive, Stanford, CA 94305, USA knowlej@stanford.edu
Amy B. Elliott, Department of Internal Medicine, Stanford University Medical Center, Stanford, CA, USA
Joshua Brody, Division of Oncology, Stanford University Medical Center, Stanford, CA, USA.
References
- 1.Little RF, Pittaluga S, Grant N, et al. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101:4653–4659. doi: 10.1182/blood-2002-11-3589. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell-Heggs P, Calvert H. Intrathoracic lymphoma associated with atrial arrhythmias and artrioventricular conduction defects. Br J Dis Chest. 1978;72:71–73. doi: 10.1016/0007-0971(78)90010-4. [DOI] [PubMed] [Google Scholar]
- 3.Otsuji Y, Arima N, Fujiwara H, Saito K, Kisanuki A, Tanaka H. Reversible complete atrioventricular block due to malignant lymphoma. Eur Heart J. 1994;15:407–408. doi: 10.1093/oxfordjournals.eurheartj.a060514. [DOI] [PubMed] [Google Scholar]
- 4.Giudici MC, Sadler RL, Robken JA, Ahearn MA, Sekharan R, Kovach G. Complete atrioventricular block due to large cell lymphoma: resolution with chemotherapy. Clin Cardiol. 1996;19:262–264. doi: 10.1002/clc.4960190326. [DOI] [PubMed] [Google Scholar]
- 5.Nakayama Y, Uchimoto S, Tsumura K, Morii H. Primary cardiac lymphoma with infiltration of the atrioventricular node: remission with reversal of the atrioventricular block induced by chemotherapy. Cardiology. 1997;88:613–616. doi: 10.1159/000177436. [DOI] [PubMed] [Google Scholar]
- 6.Nagano M, Uike N, Suzumiya J, et al. Successful treatment of a patient with cardiac lymphoma who presented with a complete atrioventricular block. Am J Hematol. 1998;59:171–174. doi: 10.1002/(sici)1096-8652(199810)59:2<171::aid-ajh12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo T, Nishiura R, Tsumori Y, et al. Disappearance of complete atrioventricular block after chemotherapy for malignant lymphoma: a case report. J Cardiol. 1999;34:345–349. [PubMed] [Google Scholar]
- 8.Clifford SM, Guerra SM, Mangion JR. Massive metastatic intracardiac lymphoma presenting with complete heart block with resolution following chemotherapy. Echocardiography. 2003;20:201–202. doi: 10.1046/j.1540-8175.2003.03001.x. [DOI] [PubMed] [Google Scholar]
- 9.Takenaka S, Mitsudo K, Inoue K, et al. Successful treatment of primary cardiac lymphoma with atrioventricular nodal block. Int Heart J. 2005;46:927–931. doi: 10.1536/ihj.46.927. [DOI] [PubMed] [Google Scholar]
- 10.Lacasce A, Howard O, Lib S, et al. Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased toxicity. Leuk Lymphoma. 2004;45:761–767. doi: 10.1080/1042819031000141301. [DOI] [PubMed] [Google Scholar]
- 11.Cole TO, Attah EB, Onyemelukwe GC. Burkitt's lymphoma presenting with heart block. Br Heart J. 1975;37:94–97. doi: 10.1136/hrt.37.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]