Abstract
Morphine administration elicits pronounced effects on the immune system, including decreases in natural killer (NK) cell activity and lymphocyte mitogenic responsiveness. These immune alterations can become conditioned to environmental stimuli that predict morphine as a result of Pavlovian conditioning processes. Prior work in our laboratory has shown that acute morphine exposure produces dopamine-dependent reductions of NK cell activity that are mediated peripherally by neuropeptide Y Y1 receptors. The present study examined the involvement of dopamine D1 and neuropeptide Y Y1 receptors in the conditioned immunomodulatory effects of morphine. Rats received two conditioning sessions during which an injection of morphine was paired with a distinctive environment which served as the conditioned stimulus (CS). The results show that systemic administration of the D1 antagonist SCH-23390 prior to CS re-exposure prevented the conditioned suppression of splenic NK activity but did not alter conditioned decreases in mitogen-induced lymphocyte proliferation. Furthermore, bilateral microinjections of SCH-23390 directly into the nucleus accumbens shell fully blocked conditioned changes in NK activity. In a subsequent manipulation, subcutaneous injection of the Y1 receptor antagonist BIBP3226 prior to CS re-exposure was also shown to prevent conditioned effects on NK activity. Collectively, these findings provide evidence that the nucleus accumbens shell plays an important role in conditioned immunomodulation and further suggest that the conditioned and unconditioned immunomodulatory effects of opioids involve similar receptor mechanisms.
Keywords: opioids, conditioning, immunomodulation, NK cell activity, lymphocytes, spleen, nucleus accumbens, dopamine, NPY
1. Introduction
Pavlovian (or classical) conditioning of immune responses is one of the most intriguing examples of neural regulation of the immune system. The basic conditioning paradigm involves the temporal pairing of a neutral conditioned stimulus (CS), such as a novel taste, odor, or context, with a stimulus that actively evokes an immunomodulatory response, termed the unconditioned stimulus (UCS). Following the CS-UCS pairing, re-exposure to the CS alone alters immune functioning in a manner which mimics the normal or unconditioned effect of the UCS. Ader and Cohen (1975) provided one of the earliest demonstrations of conditioned immunomodulation by showing that a gustatory stimulus which has been paired with the immunosuppressive drug cyclophosphamide can acquire immunosuppressive properties in mice. Subsequent investigations have demonstrated that many immune functions are susceptible to Pavlovian conditioning, including both innate and adaptive responses (for reviews see Ader and Cohen, 2001; Kusnecov et al., 1989). Furthermore, conditioned immune alterations have been shown to modify disease progression in numerous models of clinical diseases, indicating that conditioned effects on the immune system are biologically significant (Ader and Cohen, 1982; Exton et al., 1998; Klosterhalfen and Klosterhalfen, 1983; Lysle et al., 1992b).
Although Pavlovian conditioning of immune responses is now well established, the neural mechanisms underlying this phenomenon are poorly understood. While there is undoubtedly a myriad of receptors and signaling molecules involved in the mediation of complex processes such as Pavlovian conditioning of immunity, there has been considerable interest in the role of the endogenous opioid system. Numerous studies have shown that many of the behavioral and physiological effects of opioid drugs can be conditioned, suggesting that the opioid system is particularly integral to several conditioning phenomena. For instance, the involvement of opioid receptors has been established in morphine-conditioned analgesia (Miller et al., 1990), place preference (Piepponen et al., 1997), taste aversion (Leblanc and Cappell, 1975), and hyperthermia (Lal et al., 1976). Notably, opioid receptors have also been shown to mediate a number of conditioned immunomodulatory effects. Much of the impetus for investigating the role of endogenous opioids in conditioned immunomodulation was derived from early studies showing that the opioid antagonist naltrexone blocked stressor-induced reductions in natural killer (NK) cell activity produced by exposure to inescapable electric shock in rats (Cunnick et al., 1988; Shavit et al., 1984). Further studies demonstrated that these stress-induced, opioid-mediated immune alterations could be conditioned to the environmental context (the CS) associated with the stressful or aversive stimulus. For example, the presentation of a CS which has previously been paired with aversive electric shock produces naltrexone-reversible reductions in NK cell activity and lymphocyte mitogenic responses (Lysle et al., 1992a). These conditioned immune alterations were subsequently shown to be mediated specifically by μ-opioid receptors in the central nervous system (CNS), providing direct evidence for the involvement of the central opioid system in conditioned immunomodulation (Perez and Lysle, 1997).
Given the important role of the endogenous opioid system in immunomodulation, it is not surprising that exogenously administered opioids are also capable of modulating immunity. Morphine administration induces a host of immunomodulatory effects which are mediated by μ-opioid receptors in the CNS (Carr et al., 1993; Fecho et al., 1996a; Shavit et al., 1986). These pharmacological effects of morphine on immune status can also be conditioned to environmental stimuli. For example, when rats are re-exposed to a distinctive environment in which they have previously received morphine, immunological alterations occur that are similar to those produced by the drug alone, including decreased mitogen responsiveness of blood and splenic lymphocytes, reduced interleukin-2 production, and decreased NK cell activity in the spleen (Coussons et al., 1992). These conditioned effects are specifically associated with re-exposure to the CS, as extensive control procedures have shown that the immune alterations are not related to ancillary effects of the conditioning procedure. Thus, alterations of immune status can be induced by stimuli associated with opioid administration, indicating that any detrimental health consequences of opioid use may also be conditioned to environmental stimuli and not solely a pharmacological property of the drug.
There is accumulating evidence suggesting that morphine conditioned stimuli induce immune alterations by activating the same neural pathways responsible for morphine's unconditioned effects. For example, both conditioned and unconditioned immunomodulatory effects of morphine are initiated by the activation of central opioid receptors and involve increased activity of the sympathetic nervous system (Coussons-Read et al., 1994a; Coussons-Read et al., 1994b; Fecho et al., 1996b). The sympathetic nervous system provides a “hardwired” neuroimmune communication pathway as sympathetic fibers directly innervate lymphoid organs and form synaptic-like contacts with splenic lymphocytes (Felten et al., 1985; Felten and Olschowka, 1987). Thus, activation of sympathetic nerves directly modulates immune status via the release of catecholamines and neuropeptide Y (NPY), which interact with immunocyte cell surface receptors. Sympathetic efferent nerves appear to be the major neuroimmune pathway responsible for immune alterations induced by morphine and morphine conditioned stimuli. For instance, administration of the peripherally acting β-adrenoceptor antagonist nadolol was shown to block both the conditioned and unconditioned effects of morphine on splenocyte proliferative responses to T and B cell mitogens (Coussons-Read et al., 1994b; Fecho et al., 1993). However, these studies reported that neither the conditioned nor unconditioned effects of morphine on splenic NK cell activity are attenuated by antagonism of β-adrenoceptors.
Recent findings have demonstrated that the suppression of NK cell activity is mediated by mechanisms distinct from those which govern morphine's effect on lymphocyte proliferative responses. Morphine administration markedly increases extracellular dopamine levels in the nucleus accumbens, and it appears that this effect is critically involved in the modulation of NK cell responses. Specifically, the activation of dopamine D1 receptors in the nucleus accumbens shell, but not core, was shown to be necessary for morphine-induced suppression of splenic NK activity (Saurer et al., 2006a). The nucleus accumbens is intimately associated with areas involved in autonomic regulation, suggesting that the nucleus accumbens may be involved in the facilitation of sympathetic nervous system activity that results in morphine-induced immune alterations. Our laboratory has recently provided evidence that peripheral NPY Y1 receptors mediate the dopamine-dependent effects of morphine on NK activity (Saurer et al., 2006b). This suggests that morphine-induced increases in nucleus accumbens D1 receptor activation modulate splenic NK activity by increasing the activity of NPY-releasing sympathetic nerves. However, whether similar mechanisms play a role in conditioned immunomodulation is unknown. The goal of the present study was to determine whether exposure to a CS previously associated with morphine would elicit conditioned immunomodulatory effects by acting via similar dopamine and NPY receptor-dependent mechanisms. To address this issue, we evaluated the effects of the dopamine D1 antagonist SCH-23390 and the NPY Y1 antagonist BIBP3226 on the expression of conditioned immune alterations which were induced by environmental stimuli previously paired with the administration of morphine.
2. Materials and methods
2.1. Animals
Adult male Lewis rats weighing approximately 200-250 grams were purchased from Charles River Laboratories (Raleigh, NC). Upon arrival, animals were individually housed and maintained on a reverse 12-hr light/dark cycle. Animals were habituated to handling and the colony room environment for two weeks prior to any experimental manipulation. Food and water were available ad libitum throughout the experiment.
2.2. Drugs
Morphine sulfate (NIDA, Bethesda, MD) and R(+)-SCH-23390 hydrochloride (Sigma-Aldrich, St. Louis, MO) were dissolved in sterile 0.9% saline. For all experiments, morphine was administered subcutaneously at a dose of 15 mg/kg in a 1.0 ml/kg volume. This dose was selected based on previous studies from our laboratory showing that the effects of morphine are dose-dependent and that the effects were blocked by naltrexone, indicating the involvement of opioid receptors (Lysle et al., 1993). BIBP3226 (Sigma) was dissolved in sterile water.
2.3 Conditioning Procedures
The conditioning apparatus consisted of standard rodent conditioning chambers individually contained within sound-attenuating cubicles. The conditioning chambers provided distinctive visual (stainless steel and plexiglass walls), tactile (wire grid floors), auditory (white noise), and olfactory (cedar chips) cues to distinguish this environment from the home cage.
The first experiment examined the effect of systemic administration of the dopamine D1 antagonist SCH-23390 on the expression of conditioned morphine-induced immune alterations. All animals received two conditioning sessions separated by 48 hours, during which an injection of morphine was paired with a distinctive environment, the conditioning chamber. Thus, the injection of morphine served as the unconditioned stimulus (US), and the distinctive environment served as the conditioned stimulus (CS). During each session, rats were administered morphine immediately prior to being placed into the conditioning chambers for 1 hour. Animals were returned to their home cages following each conditioning session. This training phase was separated from the test day by a 12-day recovery period during which the animals received only handling. On the test day, rats were assigned to one of three groups (n = 8) in which they were administered saline or SCH-23390 (0.05, or 0.5 mg/kg, s.c.). Thirty minutes following SCH-23390 administration, half of the animals in each group were re-exposed to the CS and the other half remained in the home cage. Thus, there were six treatment groups (n = 4) in this experiment. A previous study using extensive control manipulations showed that the immune alterations observed following exposure to the conditioning chamber are the result of conditioning processes (Coussons et al., 1992). Immediately following the test session (one hour after the onset of CS re-exposure) animals were sacrificed by cervical dislocation and spleens were collected for immunological assessment. Animals that remained in the home cages on the test day were sacrificed concurrently with those that were exposed to the CS.
The second experiment examined the effect of SCH-23390 administration into the nucleus accumbens shell prior to CS re-exposure on the conditioned immunomodulatory effects of morphine. The conditioning procedures were identical to those described in the first experiment. On the test day, rats received a microinjection of saline or SCH-23390 (0.15 μg/side) into the nucleus accumbens shell. Thirty minutes following microinjection, animals were either re-exposed to the conditioning chamber or remained in the home cage. One hour later, animals were sacrificed to assess immune status. This experiment was replicated, yielding an experimental design consisting of four groups (n = 10 per group).
A third experiment was performed to control for the possibility that the effect of SCH-23390 was due to diffusion of the drug into other dopamine terminal regions near the injection site. In this experiment, the effect of bilateral SCH-23390 administration into the nucleus accumbens core was assessed using the same procedures as described above. Animals were assigned to one of four groups (n = 5) in which they received saline or SCH-23390 (0.15 μg/side) into the nucleus accumbens core. Thirty minutes following microinjection, animals were either re-exposed to the conditioning chamber or remained in the home cage. One hour later, animals were sacrificed to assess immune status.
In the fourth experiment, the effect of subcutaneous injections of the NPY Y1 antagonist BIBP3226 was examined. Rats received two conditioning sessions and subsequent re-exposure to the CS exactly as described in the procedures of the first experiment, but in this study animals received a subcutaneous injection of vehicle or BIBP3226 (0.1 or 1.0 mg/kg) on the test day. Thirty minutes following injection, half of the animals in each dose group were re-exposed to the CS and the other half remained in the home cages. Animals were sacrificed one hour later. This experiment was replicated, giving an experimental design comprised of six treatment groups (n = 4 - 6 per group).
2.4. Surgery and microinjection procedures
For the intra-accumbens microinjections, stereotaxic surgeries were performed under anesthesia induced with a 0.2 ml intramuscular injection of a 1:1 (vol/vol) mixture of ketamine (100 mg/ml) and xylazine (20 mg/ml). Animals were implanted with bilateral 26-gauge guide cannulae (Plastics One, Roanoke, VA) directed toward the nucleus accumbens shell (AP +1.7, ML ±0.8, DV –5.4) or the nucleus accumbens core (AP +1.7, ML ±1.5, DV –4.8). Coordinates are expressed as millimeters from bregma (Paxinos and Watson, 1986). Animals were given a one-week recovery period prior to the start of the conditioning trials. On the testing day, animals received a bilateral injection of saline or SCH-23390 (0.15 μg/ side). SCH-23390 was administered in a 0.5 μl volume per side via a 33-gauge injector that protruded 2 mm beyond the tip of the guide cannula (final coordinates: AP +1.7, ML ±0.8, DV –7.4 for the shell; or AP +1.7, ML ±1.5, DV –6.8 for the core). Intracranial drug microinjections were performed over a 30 second period using a microsyringe pump (Harvard Apparatus, Holliston, MA) and the injector was left in place for one minute to allow diffusion of the drug into the injection site. Immediately following the injection, animals were returned to the home cages.
Following sacrifice, Alcian blue dye was injected via the cannula and brains were removed and post-fixed in 4% paraformaldehyde. Brains were then transferred to a 30% sucrose solution for cryoprotection, frozen, and stored at −80°C for subsequent analysis. Accurate cannulae placements were verified by examination of unstained 50 μm tissue sections under a dissecting microscope. Only animals with placements within the targeted region were included in the analysis.
2.5. Tissue Collection
Following sacrifice, spleens were removed and placed in 7 ml of supplemented RPMI media (RPMI-1640 tissue culture media supplemented with 10 mM HEPES, 2 mM glutamine, and 50 μg/ml gentamicin; GIBCO, Grand Island, NY). Each spleen was prepared as a single-cell suspension by gently pressing the tissue between two sterile, frosted microscope slides in complete RPMI media (supplemented RPMI enriched with 10% fetal bovine serum; GIBCO). Splenic leukocytes were counted using a Hemavet 850 cell analyzer (CDC Technologies Inc., Oxford, CT), and cell suspensions were adjusted to 5 × 106 leukocytes/ml by diluting with complete RPMI.
2.6. Natural Killer Cell Assay
Splenic NK cell activity was assessed using a standard chromium release assay. Adjusted splenocyte suspensions were co-incubated with the murine T-cell lymphoma, YAC-1. The YAC-1 target cells were labeled by incubation for 70 min with 200 μCi of sodium chromate-51 [51Cr]. YAC-1 cells were then washed three times with complete RPMI to remove exogenous [51Cr]. Splenic leukocytes were used as effectors and were plated in triplicate at 10, 5, 2.5, and 1.25 × 105 cells/well of a 96 well plate. Labeled targets were diluted and plated at 1 × 104 cells/well to give effector/target (E:T) ratios of 100:1, 50:1, 25:1 and 12.5:1. Following 5 hour incubation at 37°C in a humidified CO2 incubator, the amount of [51Cr] released into the supernatant was determined using an LKB gamma counter (model 1272 CliniGamma). Percent specific lysis at all E:T ratios were used to calculate lytic units (Pross and Maroun, 1984). Results are reported as the number of lytic units per 107 effector cells. A lytic unit was defined as the number of splenic leukocytes necessary to lyse 20% of the target cells.
2.7. Splenocyte Proliferation Assay
Mitogen stimulation assays were completed using adjusted splenocyte suspensions. Splenic T- and B-lymphocyte proliferation was induced with the mitogens Con-A and LPS (Sigma-Aldrich), respectively. One hundred microliters of the adjusted cell suspensions were pipetted in triplicate into microtiter plate wells containing final concentrations of 0, 0.5, and 5.0 μg/ml Con-A and 0.5 and 5.0 μg/ml LPS. Splenocyte cultures were then incubated for 48 hours at 37°C in a humidified CO2 incubator. Each culture well was pulsed with 1 μCi of [3H] thymidine during the last 5 hours of the incubation period. Cultures were then harvested onto glass fiber filter paper using a Tomtec automatic 96-well cell harvester. The amount of [3H]-thymidine incorporated into the DNA of proliferating cells was measured using a liquid scintillation counter (Wallac, Model 1205) and is expressed as the mean of the triplicate disintegrations per minute (DPM) for the samples from each rat.
2.8. Statistics
Data analysis for each experiment was performed using two-way analysis of variance (ANOVA) to assess the impact of drug antagonist treatment on conditioned immune alterations. For the two-way analysis, the first factor was drug dosage and the second factor was the type of treatment on the test day, i.e., re-exposure to the CS or home cage. Experimental replication was entered into the model as a covariate to control for inter-assay variability in the measured parameters for both the second and fourth experiments. For all data sets, planned contrasts were performed in accordance with a priori hypotheses that SCH-23390 and BIBP3226 would antagonize the effect of the CS. Planned contrasts in each analysis consisted of pairwise comparisons of means between CS re-exposed groups and home cage groups at each level of the factor “drug dosage.” All analyses were performed with the alpha-level of significance set at p < 0.05.
3. Results
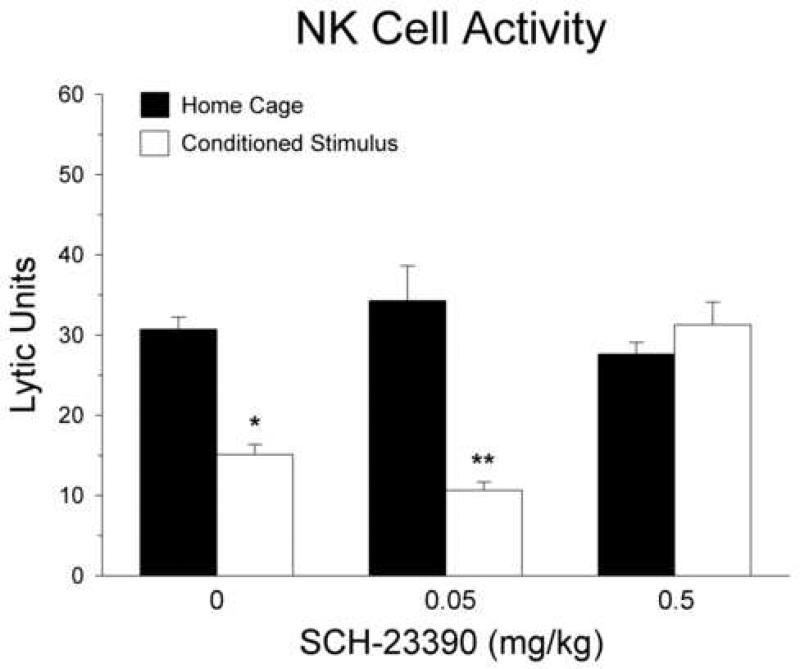
The first study examined the effect of systemic SCH-23390 administration on alterations of immune status induced by exposure to a distinctive environment (the CS) that had been previously paired with morphine administration. The results of the NK cell assay are shown in figure 1. One animal was excluded from this analysis due to technical difficulties. Analysis of variance indicated a significant main effect of CS re-exposure [F(1, 17) = 26.89; p < 0.001]. More importantly, the analysis revealed a significant interaction between SCH-23390 dosage and CS re-exposure [F(2, 17) = 12.54; p < 0.001], indicating that the effect of CS re-exposure was dependent on the dose of SCH-23390. Planned contrasts indicated that the NK activity was significantly suppressed in the CS re-exposed group compared to the corresponding home cage control group among animals that received saline [F(1,17) = 13.32; p < 0.01] or 0.05 mg/kg of SCH-23390 [F(1,17) = 35.64; p < 0.0001]. However, at the high dose of SCH-23390 (0.5 mg/kg), planned contrasts showed that there was no significant difference between the groups exposed to the CS or the home cage on the test day [F(1,17) = 0.85; p > 0.05], indicating that this dose of SCH-23390 fully blocked the conditioned suppression of NK activity.
Fig. 1.
Effect of systemic dopamine D1 receptor antagonism on conditioned suppression of splenic NK cell activity. Subcutaneous administration of SCH-23390 at a dose of 0.5 mg/kg reversed the inhibition of splenic NK activity induced by CS re-exposure. Data are expressed as lytic units (mean ± S.E.). Solid bars indicate rats that remained in their home cages on the test day and open bars represent groups re-exposed to the CS on the test day. *p < 0.01; **p < 0.001 compared with the home cage group that received the same dose of SCH-23390.
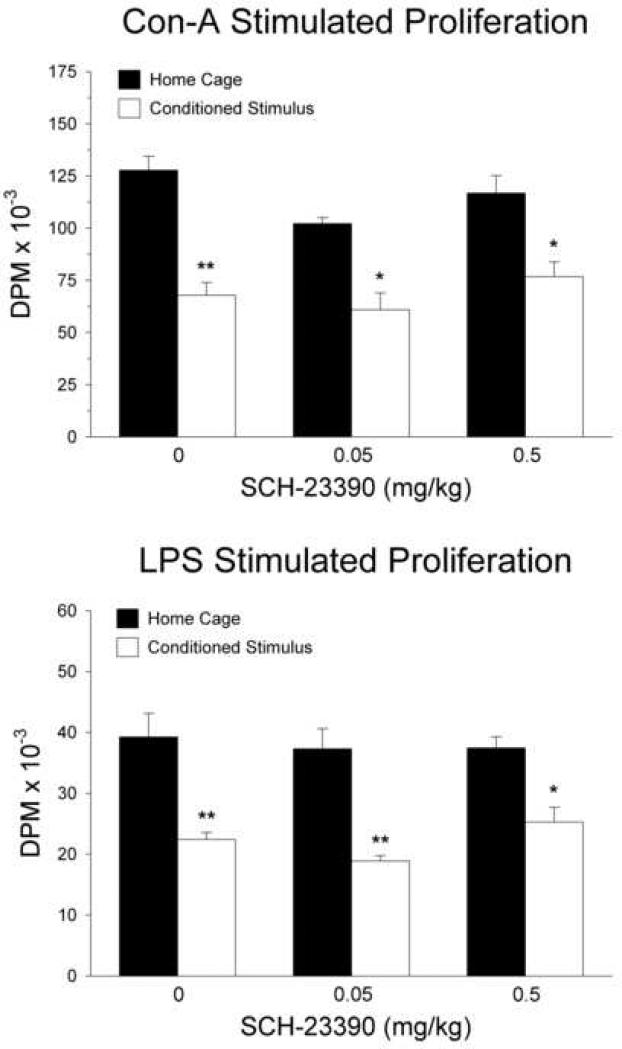
Figure 2 shows the results of the mitogen stimulation assays from the first experiment. Re-exposure to the CS produced a significant decrease in splenocyte proliferation induced by Con-A (top panel) as revealed by a significant main effect of CS re-exposure [F(1, 18) = 54.23; p < 0.0001]. Splenocyte proliferative responses to LPS (figure 2, bottom panel) were also significantly reduced in animals re-exposed to the CS as indicated by a significant main effect of CS re-exposure [F(1, 18) = 45.14; p < 0.0001]. There was no main effect of SCH-23390 dosage on the proliferative responses to Con-A [F(1, 18) = 2.71; p > 0.05] or LPS [F(1, 18) = 0.73; p > 0.05], nor was there a significant interaction between SCH-23390 dosage and CS re-exposure on responses to Con-A [F(2, 18) = 1.01; p > 0.05] or LPS [F(2, 18) = 0.64; p > 0.05]. Furthermore, SCH-23390 administration did not antagonize the suppressive effect of CS re-exposure on responses to Con-A or LPS at any dose tested, as planned comparisons indicated that all groups re-exposed to the conditioning chamber on the test day displayed significantly lower proliferation compared to the corresponding home cage group which received the same dose of SCH-23390 or saline (ps < 0.01).
Fig. 2.
Effect of systemic D1 receptor antagonism on conditioned suppression of lymphocyte proliferation. Subcutaneous SCH-23390 administration did not attenuate the inhibitory effect of CS re-exposure on splenocyte mitogenic responses to Con-A (5.0 μg/ml) or LPS (5.0 μg/ml). Data are expressed as disintegrations per minute (mean ± S.E.). Solid bars indicate rats that remained in their home cages on the test day and open bars represent groups re-exposed to the CS on the test day. *p < 0.01; **p < 0.001 compared with the home cage group that received the same dose of SCH-23390.
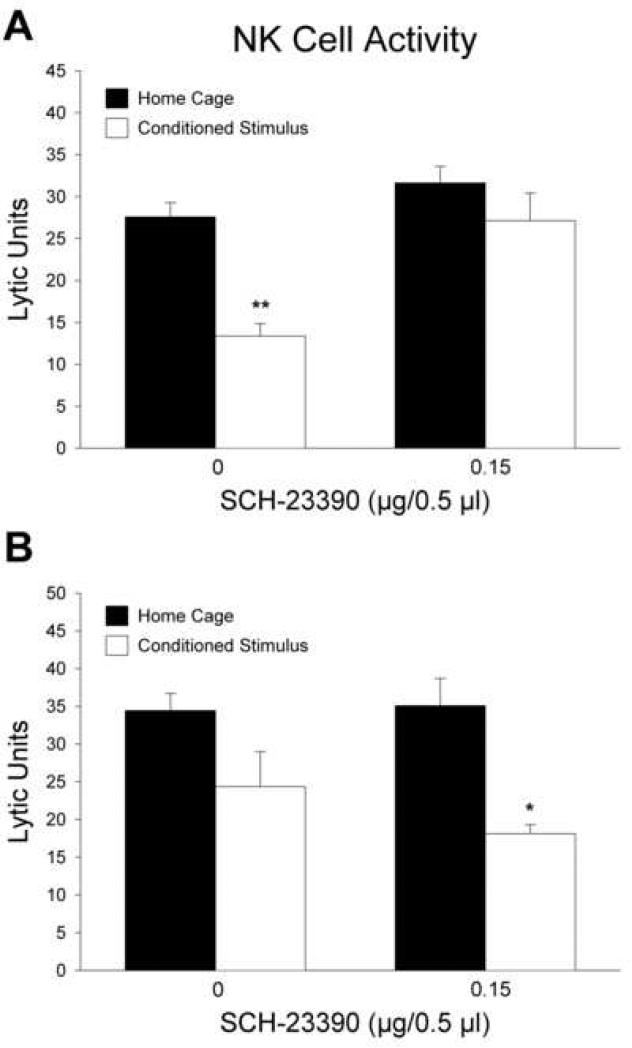
The second experiment was conducted to investigate the effect of bilateral SCH-23390 microinjections into the nucleus accumbens shell on the conditioned suppression of NK cell activity. The results of the NK cell activity assay are displayed in figure 3A. Analysis of variance showed a significant main effect of CS re-exposure [F(1, 32) = 18.34; p < 0.001]. More importantly, the analysis revealed a significant interaction between SCH-23390 administration and CS re-exposure [F(1, 32) = 4.92; p < 0.05], indicating that the effect of CS re-exposure on NK activity was altered by intra-accumbens shell SCH-23390 injections. Planned contrasts revealed that among saline treated animals, re-exposure to the CS induced a suppression of NK activity as evidenced by a significant difference between the CS re-exposed group and the home cage group [F(1, 32) = 20.01; p < 0.001]. However, in rats that received SCH-23390 microinjections, planned contrasts showed that there was no difference between the groups exposed to the CS or the home cage on the test day [F(1, 32) = 1.89; p > 0.05]. These findings show that bilateral administration of SCH-23390 at a dose of 0.15 μg/ side is sufficient to fully antagonize the conditioned suppression of splenic NK activity.
Fig. 3.
Effect of D1 receptor antagonism in the nucleus accumbens shell (A) or core (B) on conditioned suppression of NK cell activity. A, Bilateral injections of SCH-23390 into the nucleus accumbens shell blocked the inhibitory effect of CS re-exposure on NK activity. B, Administration of SCH-23390 into the nucleus accumbens core did not prevent the effect of CS re-exposure. Solid bars indicate rats that remained in their home cages on the test day and open bars represent groups re-exposed to the CS on the test day. Data are expressed as lytic units (mean ± S.E.). *p < 0.01; **p < 0.001 compared with the home cage group that received the same dose of SCH-23390.
To control for the possibility of drug diffusion away from the injection site in the nucleus accumbens shell, the effect of SCH-23390 administration into the nucleus accumbens core was examined. The results of the NK cell activity assay from this experiment are shown in figure 3B. Analysis of variance revealed a significant main effect of CS re-exposure [F(1, 15) = 13.31; p < 0.01], indicating that NK activity was significantly suppressed by CS re-exposure. Planned contrasts indicated that NK activity in CS re-exposed animals was significantly suppressed compared to the home cage group in animals which received SCH-23390 injections into the nucleus accumbens core [F(1, 15) = 9.31; p < 0.01]. Planned contrasts did not reveal a significant difference between the CS re-exposed group and home cage group among animals that received saline injections [F(1, 15) = 3.70; p = 0.07]. However, there was no main effect of SCH-23390 treatment [F(1, 15) = 0.57; p > 0.05], nor was there a significant SCH-23390 by morphine dose interaction [F(1, 15) = 0.86; p > 0.05]. Collectively, these findings suggest that D1 receptors located in the nucleus accumbens core are not involved in the conditioned suppression of NK activity and provide additional support for the specificity of microinjections in the shell.
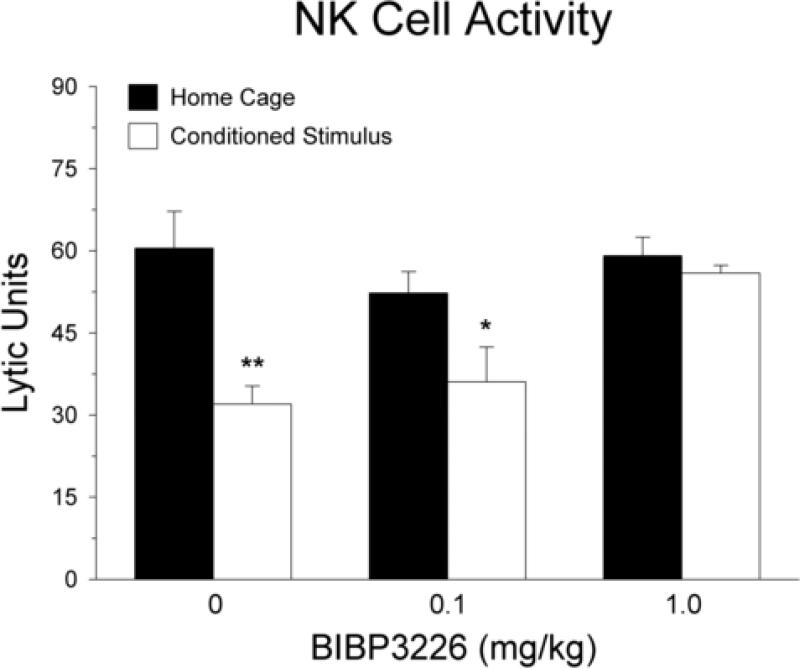
Figure 4 displays the effect of subcutaneous BIBP3226 administration on the conditioned suppression of NK activity. Analysis of variance showed a significant main effect of CS re-exposure [F(1, 22) = 19.04; p < 0.001]. The presence of a significant interaction between CS re-exposure and BIBP3226 dosage [F(2, 22) = 4.01; p < 0.05] indicates that the effect of CS re-exposure on NK activity was dependent on the dosage of BIBP3226. Planned contrasts showed that the CS re-exposed group differed from the corresponding home cage group among animals that received vehicle [F(1, 22) = 16.89; p < 0.001] or 0.1 mg/kg of BIBP3226 [F(1, 22) = 4.37; p < 0.05]. However, in animals that received the high dose of BIBP3226 (1.0 mg/kg), planned contrasts showed that there was no difference between the home cage and CS re-exposed groups [F(1, 22) = 0.22; p > 0.05]. These findings indicate that the 1.0 mg/kg dose of BIBP3226 fully prevents the reduction in NK activity induced by re-exposure to the CS. It should be noted that the baseline level of NK activity was higher in this experiment than the other experiments. Although the reason for the increase in baseline cytolytic activity is not immediately clear, the magnitude of the conditioned effect in the vehicle control groups was similar across all experiments.
Fig. 4.
Effect of NPY Y1 receptor antagonism on conditioned reductions of NK cell activity. Subcutaneous administration of BIBP3226 at a dose of 1.0 mg/kg blocked the inhibition of splenic NK activity induced by CS re-exposure. Solid bars indicate rats that remained in their home cages on the test day and open bars represent groups re-exposed to the CS on the test day. *p < 0.05; **p < 0.001 compared with the home cage group that received the same dose of BIBP3226.
4. Discussion
Exposure to environmental stimuli previously paired with morphine induces conditioned immune alterations which mimic morphine's pharmacological effects. Previous studies have established that these conditioned effects are due to the learned psychological state induced by the CS, as development of the conditioned immunomodulatory response requires the explicit pairing of morphine administration with the CS and is not attributable to ancillary influences of the conditioning procedure (Coussons et al., 1992). Furthermore, it has recently been shown that the conditioned immunomodulatory effects of heroin, an opioid drug closely related to morphine, are susceptible to extinction and latent inhibition, indicating that opioid-conditioned immune alterations conform to major principles of associative learning (Szczytkowski and Lysle, 2007).
The present study shows that the conditioned suppression of NK activity is blocked by systemic administration of the D1 antagonist SCH-23390 prior to CS re-exposure, suggesting that morphine conditioned stimuli modulate NK activity through increased D1 receptor-mediated signaling. In contrast, SCH-23390 did not attenuate the proliferative response of splenic lymphocytes to Con-A or LPS. This finding is notable because it shows that the learned association between morphine and the CS is not disrupted by D1 antagonist treatment and furthermore indicates that neuroimmune efferent communication is not impaired in a nonspecific manner. Moreover, the selective role of D1 receptors in mediating conditioned effects on NK activity mirrors our previous findings with morphine administration in which SCH-23390 was shown to block the effect of morphine on NK activity (Saurer et al., 2006a) but not lymphocyte proliferation (unpublished observations).
The current findings further demonstrate that microinjection of SCH-23390 into the nucleus accumbens shell prior to CS re-exposure completely blocks the suppression of NK activity. To control for the possibility that drug diffusion into neighboring brain regions was responsible for the observed effects, SCH-23390 was also administered into the nucleus accumbens core. This region was selected as the control injection site based on literature documenting that the nucleus accumbens core shows a conditioned dopaminergic response to a variety of stimuli (Cheng et al., 2003; Pezze et al., 2001). However in the present study, microinjection of SCH-23390 into the nucleus accumbens core did not disrupt the conditioned effect. Thus, these data indicate that the activation of D1 receptors in the nucleus accumbens shell is necessary for the expression of the conditioned effect on splenic NK activity. Interestingly, increased dopamine transmission in the nucleus accumbens shell has also been widely implicated in the acquisition of associative (Pavlovian) learning (Di Chiara et al., 2004; Pezze and Feldon, 2004). An important issue for future investigations is to address whether the nucleus accumbens also plays a role in the development of associative learning processes which establish conditioned effects on immunity.
Although further studies are necessary to elucidate the precise role of the nucleus accumbens in conditioned immune alterations, the involvement of the accumbens may simply represent a more fundamental efferent neuroimmunoregulatory mechanism rather than being specifically involved in conditioned effects per se. For instance, morphine's pharmacological effect on NK activity similarly requires D1 activation in the nucleus accumbens shell (Saurer et al., 2006a). Furthermore, prior investigations have shown that the pharmacological stimulation of accumbens D1 receptors alone is sufficient suppress NK activity comparably to morphine, indicating that the nucleus accumbens is intimately involved in the modulation of peripheral immune parameters (Saurer et al., 2006b; Saurer et al., 2006a). The precise neuroimmune efferent pathway through which accumbens D1 receptor activation elicits an inhibition of splenic NK cell activity remains to be fully delineated, although several lines of evidence suggest that it may involve the modulation of sympathetic nerve outflow. Neural control of splenic NK cell activity is a well documented phenomenon which occurs primarily via the sympathetic nervous system (Irwin et al., 1990; Katafuchi et al., 1993), and the nucleus accumbens shell has major efferent projections to hypothalamic autonomic regulatory centers (Heimer et al., 1991; Usuda et al., 1998). Moreover, hypothalamic nuclei which receive direct afferent projections from the nucleus accumbens shell have been shown to modulate NK cell activity in the spleen (Katafuchi et al., 1993; Wenner et al., 1996; Wrona and Trojniar, 2003; Wrona and Trojniar, 2005). Thus, one possibility is that dopamine transmission in the nucleus accumbens regulates splenic NK activity by modulating sympathetic outflow.
Prior evidence from our laboratory has shown that NPY – a peptide transmitter released from sympathetic fibers – mediates the dopamine-dependent modulation of splenic NK activity. Specifically, both morphine and intra-accumbens D1 agonist administration produce a suppression of NK activity that is prevented by blocking the activation of NPY Y1 receptors with the Y1 antagonist BIBP3226. In the present study, we show that administration of BIBP3226 prior to CS re-exposure blocks the conditioned suppression of NK cell activity. Because BIBP3226 reportedly does not penetrate the blood-brain barrier when administered systemically, these findings implicate the involvement of peripheral Y1 receptors specifically (Doods et al., 1996). Given the results of our previous investigations which indicate that Y1 receptors mediate the dopamine-dependent effects of morphine on immune status, the current findings suggest that conditioned increases in nucleus accumbens dopamine transmission modulates splenic NK activity by facilitating the release of NPY from sympathetic nerves. NPY may directly interact with NK cells to inhibit cytolysis, as splenic lymphocytes express functional Y1 receptors (Bedoui et al., 2002; Petitto et al., 1994) and NPY produces direct and dose-dependent suppressive effects on NK activity in vitro (Nair et al., 1993).
The involvement of NPY provides further evidence for the role of the sympathetic nervous system in conditioned immune alterations. Although early theories suggested that conditioned immunomodulation was simply a nonspecific stress reaction involving activation of the hypothalamic-pituitary-adrenal axis and the release of adrenocortical steroids, this explanation does not adequately account for many, if not most, conditioned effects on the immune system (e.g., Ader et al., 1979; Roudebush and Bryant, 1991). For example, the conditioned immunosuppressive effects of cyclosporine A on T-cell proliferation and cytokine production have been shown to be mediated solely via the sympathetic innervation of the spleen (Exton et al., 1998). Additionally, prior studies from our laboratory have shown that peripheral β-adrenoceptor activity mediates the conditioned effects of both aversive stimuli and morphine on the immune system (Coussons-Read et al., 1994b; Luecken and Lysle, 1992). Thus, the sympathetic nervous system may represent the principal neuroimmune pathway through which a variety of conditioned stimuli induce immune alterations, as sympathetic efferent mechanisms have been established in several paradigms.
In conclusion, the present study demonstrates that the expression of morphine's conditioned effects on splenic NK cell activity requires the activation of dopamine D1 receptors in the nucleus accumbens shell. Furthermore, the current results show that antagonism of NPY Y1 receptors with BIBP3226 also prevents the conditioned suppression of NK activity. Taken together with the results of previous investigations, the present findings provide additional support for the hypothesis that the conditioned and unconditioned effects of morphine involve similar mechanisms by demonstrating the involvement of similar dopamine and NPY receptor mechanisms. Specifically, the present data suggest that conditioned increases in the activation of nucleus accumbens D1 receptors may induce reductions in splenic NK activity by stimulating the release of NPY. Lastly, the present study adds to our current knowledge regarding the neural mechanisms responsible for conditioned modulation of immunity by providing the first demonstration for the role of the nucleus accumbens in Pavlovian conditioned immunomodulation.
Acknowledgments
This research was funded by United States Public Service Grant DA13371 and Research Scientist Award DA00334 from the National Institute on Drug Abuse. Timothy Saurer was supported by a National Institutes of Health predoctoral Ruth L. Kirschstein fellowship (DA019323). The authors wish to acknowledge the expert technical assistance of Jennifer Szczytkowski, Jay Elliott, and Ryan Lanier.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosom. Med. 1975;37:333–340. doi: 10.1097/00006842-197507000-00007. [DOI] [PubMed] [Google Scholar]
- Ader R, Cohen N. Behaviorally conditioned immunosuppression and murine systemic lupus erythematosus. Science. 1982;215:1534–1536. doi: 10.1126/science.7063864. [DOI] [PubMed] [Google Scholar]
- Ader R, Cohen N. Conditioning and immunity. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. Academic Press; San Diego: 2001. pp. 3–34. [Google Scholar]
- Ader R, Cohen N, Grota LJ. Adrenal involvement in conditioned immunosuppression. Int. J. Immunopharmacol. 1979;1:141–145. doi: 10.1016/0192-0561(79)90017-1. [DOI] [PubMed] [Google Scholar]
- Bedoui S, Lechner S, Gebhardt T, Nave H, Beck-Sickinger AG, Straub RH, Pabst R, von HS. NPY modulates epinephrine-induced leukocytosis via Y-1 and Y-5 receptor activation in vivo: sympathetic co-transmission during leukocyte mobilization. J. Neuroimmunol. 2002;132:25–33. doi: 10.1016/s0165-5728(02)00278-3. [DOI] [PubMed] [Google Scholar]
- Carr DJ, Gebhardt BM, Paul D. Alpha adrenergic and mu-2 opioid receptors are involved in morphine-induced suppression of splenocyte natural killer activity. J. Pharmacol. Exp. Ther. 1993;264:1179–1186. [PubMed] [Google Scholar]
- Cheng JJ, de Bruin JP, Feenstra MG. Dopamine efflux in nucleus accumbens shell and core in response to appetitive classical conditioning. Eur. J. Neurosci. 2003;18:1306–1314. doi: 10.1046/j.1460-9568.2003.02849.x. [DOI] [PubMed] [Google Scholar]
- Coussons ME, Dykstra LA, Lysle DT. Pavlovian conditioning of morphine-induced alterations of immune status. J. Neuroimmunol. 1992;39:219–230. doi: 10.1016/0165-5728(92)90256-k. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Dykstra LA, Lysle DT. Pavlovian conditioning of morphine-induced alterations of immune status: evidence for opioid receptor involvement. J. Neuroimmunol. 1994a;55:135–142. doi: 10.1016/0165-5728(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Dykstra LA, Lysle DT. Pavlovian conditioning of morphine-induced alterations of immune status: evidence for peripheral beta-adrenergic receptor involvement. Brain Behav. Immun. 1994b;8:204–217. doi: 10.1006/brbi.1994.1019. [DOI] [PubMed] [Google Scholar]
- Cunnick JE, Lysle DT, Armfield A, Rabin BS. Shock-induced modulation of lymphocyte responsiveness and natural killer activity: differential mechanisms of induction. Brain Behav. Immun. 1988;2:102–113. doi: 10.1016/0889-1591(88)90011-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Doods HN, Wieland HA, Engel W, Eberlein W, Willim KD, Entzeroth M, Wienen W, Rudolf K. BIBP 3226, the first selective neuropeptide Y1 receptor antagonist: a review of its pharmacological properties. Regul. Pept. 1996;65:71–77. doi: 10.1016/0167-0115(96)00074-2. [DOI] [PubMed] [Google Scholar]
- Exton MS, von HS, Schult M, Voge J, Strubel T, Donath S, Steinmuller C, Seeliger H, Nagel E, Westermann J, Schedlowski M. Behaviorally conditioned immunosuppression using cyclosporine A: central nervous system reduces IL-2 production via splenic innervation. J. Neuroimmunol. 1998;88:182–191. doi: 10.1016/s0165-5728(98)00122-2. [DOI] [PubMed] [Google Scholar]
- Fecho K, Dykstra LA, Lysle DT. Evidence for Beta-Adrenergic-Receptor Involvement in the Immunomodulatory Effects of Morphine. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1079–1087. [PubMed] [Google Scholar]
- Fecho K, Maslonek KA, Dykstra LA, Lysle DT. Assessment of the involvement of central nervous system and peripheral opioid receptors in the immunomodulatory effects of acute morphine treatment in rats. Journal of Pharmacology and Experimental Therapeutics. 1996a;276:626–636. [PubMed] [Google Scholar]
- Fecho K, Maslonek KA, Dykstra LA, Lysle DT. Evidence for sympathetic and adrenal involvement in the immunomodulatory effects of acute morphine treatment in rats. Journal of Pharmacology and Experimental Therapeutics. 1996b;277:633–645. [PubMed] [Google Scholar]
- Felten DL, Felten SY, Carlson SL, Olschowka JA, Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J. Immunol. 1985;135:755s–765s. [PubMed] [Google Scholar]
- Felten SY, Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J. Neurosci. Res. 1987;18:37–48. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Irwin M, Hauger RL, Jones L, Provencio M, Britton KT. Sympathetic nervous system mediates central corticotropin-releasing factor induced suppression of natural killer cytotoxicity. J. Pharmacol. Exp. Ther. 1990;255:101–107. [PubMed] [Google Scholar]
- Katafuchi T, Ichijo T, Take S, Hori T. Hypothalamic modulation of splenic natural killer cell activity in rats. J. Physiol. 1993;471:209–221. doi: 10.1113/jphysiol.1993.sp019898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosterhalfen W, Klosterhalfen S. Pavlovian conditioning of immunosuppression modifies adjuvant arthritis in rats. Behav. Neurosci. 1983;97:663–666. doi: 10.1037//0735-7044.97.4.663. [DOI] [PubMed] [Google Scholar]
- Kusnecov A, King MG, Husband AJ. Immunomodulation by behavioural conditioning. Biol. Psychol. 1989;28:25–39. doi: 10.1016/0301-0511(89)90109-9. [DOI] [PubMed] [Google Scholar]
- Lal H, Miksic S, Smith N. Naloxone antagonism of conditioned hyperthermia: an evidence for release of endogenous opioid. Life Sci. 1976;18:971–975. doi: 10.1016/0024-3205(76)90417-3. [DOI] [PubMed] [Google Scholar]
- Leblanc AE, Cappell H. Antagonism of morphine-induced aversive conditioning by naloxone. Pharmacol. Biochem. Behav. 1975;3:185–188. doi: 10.1016/0091-3057(75)90146-x. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Lysle DT. Evidence for the involvement of beta-adrenergic receptors in conditioned immunomodulation. J. Neuroimmunol. 1992;38:209–219. doi: 10.1016/0165-5728(92)90014-c. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Coussons ME, Watts VJ, Bennett EH, Dykstra LA. Morphine-Induced Alterations of Immune Status - Dose Dependency, Compartment Specificity and Antagonism by Naltrexone. Journal of Pharmacology and Experimental Therapeutics. 1993;265:1071–1078. [PubMed] [Google Scholar]
- Lysle DT, Luecken LJ, Maslonek KA. Modulation of immune status by a conditioned aversive stimulus: evidence for the involvement of endogenous opioids. Brain Behav. Immun. 1992a;6:179–188. doi: 10.1016/0889-1591(92)90017-i. [DOI] [PubMed] [Google Scholar]
- Lysle DT, Luecken LJ, Maslonek KA. Suppression of the development of adjuvant arthritis by a conditioned aversive stimulus. Brain Behav. Immun. 1992b;6:64–73. doi: 10.1016/0889-1591(92)90060-2. [DOI] [PubMed] [Google Scholar]
- Miller JS, Kelly KS, Neisewander JL, McCoy DF, Bardo MT. Conditioning of morphine-induced taste aversion and analgesia. Psychopharmacology (Berl) 1990;101:472–480. doi: 10.1007/BF02244224. [DOI] [PubMed] [Google Scholar]
- Nair MP, Schwartz SA, Wu K, Kronfol Z. Effect of neuropeptide Y on natural killer activity of normal human lymphocytes. Brain Behav. Immun. 1993;7:70–78. doi: 10.1006/brbi.1993.1007. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1986. [Google Scholar]
- Perez L, Lysle DT. Conditioned immunomodulation: investigations of the role of endogenous activity at mu, kappa, and delta opioid receptor subtypes. J. Neuroimmunol. 1997;79:101–112. doi: 10.1016/s0165-5728(97)00106-9. [DOI] [PubMed] [Google Scholar]
- Petitto JM, Huang Z, McCarthy DB. Molecular cloning of NPY-Y1 receptor cDNA from rat splenic lymphocytes: evidence of low levels of mRNA expression and [125I]NPY binding sites. J. Neuroimmunol. 1994;54:81–86. doi: 10.1016/0165-5728(94)90234-8. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog. Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Heidbreder CA, Feldon J, Murphy CA. Selective responding of nucleus accumbens core and shell dopamine to aversively conditioned contextual and discrete stimuli. Neuroscience. 2001;108:91–102. doi: 10.1016/s0306-4522(01)00403-1. [DOI] [PubMed] [Google Scholar]
- Piepponen TP, Kivastik T, Katajamaki J, Zharkovsky A, Ahtee L. Involvement of opioid mu 1 receptors in morphine-induced conditioned place preference in rats. Pharmacol. Biochem. Behav. 1997;58:275–279. doi: 10.1016/s0091-3057(96)00567-9. [DOI] [PubMed] [Google Scholar]
- Pross HF, Maroun JA. The Standardization of Nk Cell Assays for Use in Studies of Biological Response Modifiers. Journal of Immunological Methods. 1984;68:235–249. doi: 10.1016/0022-1759(84)90154-6. [DOI] [PubMed] [Google Scholar]
- Roudebush RE, Bryant HU. Conditioned immunosuppression of a murine delayed type hypersensitivity response: dissociation from corticosterone elevation. Brain Behav. Immun. 1991;5:308–317. doi: 10.1016/0889-1591(91)90025-6. [DOI] [PubMed] [Google Scholar]
- Saurer TB, Carrigan KA, Ijames SG, Lysle DT. Suppression of natural killer cell activity by morphine is mediated by the nucleus accumbens shell. J. Neuroimmunol. 2006a;173:3–11. doi: 10.1016/j.jneuroim.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Saurer TB, Ijames SG, Lysle DT. Neuropeptide Y Y1 receptors mediate morphine-induced reductions of natural killer cell activity. J. Neuroimmunol. 2006b;177:18–26. doi: 10.1016/j.jneuroim.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Shavit Y, Depaulis A, Martin FC, Terman GW, Pechnick RN, Zane CJ, Gale RP, Liebeskind JC. Involvement of brain opiate receptors in the immune-suppressive effect of morphine. Proc. Natl. Acad. Sci. U. S. A. 1986;83:7114–7117. doi: 10.1073/pnas.83.18.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavit Y, Lewis JW, Terman GW, Gale RP, Liebeskind JC. Opioid peptides mediate the suppressive effect of stress on natural killer cell cytotoxicity. Science. 1984;223:188–190. doi: 10.1126/science.6691146. [DOI] [PubMed] [Google Scholar]
- Szczytkowski JL, Lysle DT. Conditioned effects of heroin on the expression of inducible nitric oxide synthase in the rat are susceptible to extinction and latent inhibition. Psychopharmacology (Berl) 2007 doi: 10.1007/s00213-006-0673-z. [DOI] [PubMed] [Google Scholar]
- Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- Wenner M, Kawamura N, Miyazawa H, Ago Y, Ishikawa T, Yamamoto H. Acute electrical stimulation of lateral hypothalamus increases natural killer cell activity in rats. J. Neuroimmunol. 1996;67:67–70. doi: 10.1016/0165-5728(96)00040-9. [DOI] [PubMed] [Google Scholar]
- Wrona D, Trojniar W. Chronic electrical stimulation of the lateral hypothalamus increases natural killer cell cytotoxicity in rats. J. Neuroimmunol. 2003;141:20–29. doi: 10.1016/s0165-5728(03)00214-5. [DOI] [PubMed] [Google Scholar]
- Wrona D, Trojniar W. Suppression of natural killer cell cytotoxicity following chronic electrical stimulation of the ventromedial hypothalamic nucleus in rats. J. Neuroimmunol. 2005;163:40–52. doi: 10.1016/j.jneuroim.2005.02.017. [DOI] [PubMed] [Google Scholar]