Abstract
The rate of noncatalyzed transfer of cholesterol (Chol) among lipoproteins and cells in the blood is of fundamental importance as a baseline to assess the role of active transport mechanisms, but remains unknown. Here we address this gap by characterizing the association of the Chol analog, ergosta-5,7,9(11),22-tetraen-3β-ol (DHE), with the lipoproteins VLDL, LDL, HDL2, and HDL3. Combining these results with data for the association of DHE with liposomes, we elaborated a kinetic model for the noncatalyzed exchange of free Chol among blood compartments. The computational results are in good agreement with experimental values. The small deviations are explained by the nonequilibrium distribution of unesterified Chol in vivo, due to esterification and entry of new unesterified Chol, and eventual effects introduced by incubations at low temperatures. The kinetic profile of the homeostasis of unesterified Chol in the blood predicted by the model developed in this work is in good agreement with the observations in vivo, highlighting the importance of passive processes.
Keywords: cholesterol homeostasis, dehydroergosterol, lipoproteins, low density lipoprotein, high density lipoprotein
The overall homeostasis of cholesterol (Chol) in humans is very complex (1, 2). Whereas dietary Chol is absorbed only in the intestine, endogenous Chol is synthesized in several tissues, the most important being the liver. The liver is also the central clearing-house in Chol homeostasis. Lipoprotein nanoparticles play a key role in this process. They are grouped according to their densities and/or protein content as chylomicrons, VLDLs, LDLs, and HDLs, each group having several subdivisions depending on particle size and chemical composition.
Chylomicrons transport Chol that is absorbed in the intestine and esterified with fatty acids to the liver. VLDLs are produced in the liver by packing esterified Chol together with triglycerides and transporting these lipids to peripheral tissues through the blood. Most of the VLDL triglycerides are taken up by muscle and adipose tissues, leaving particles with a cholesteryl ester-rich core (LDLs) that are internalized by peripheral cells via endocytosis. Cells get rid of excess free Chol by transport across their plasma membranes using transporters of the ATP-binding cassette protein family. Members of this family of transporters, in addition to ejecting free Chol from the plasma membrane, also bind lipid-poor apo-A1 particles (nascent HDL) facilitating direct transfer of the ejected Chol to these particles (1, 3, 4). Free Chol in discoid HDL particles is esterified by a serum protein, LCAT. Accumulation of cholesteryl esters in the HDL particles converts these to spherical HDL particles with a nonpolar cholesteryl ester core. The cholesteryl esters in the HDL may undergo an exchange [catalyzed by cholesteryl ester transfer protein (CETP)] for triglycerides with other lipoproteins in circulation, or removal from circulation by the liver. Chol that reaches the liver is either reprocessed and circulated or converted to bile salts that are eliminated together with some amount of free Chol into the intestinal lumen, whence they may be reabsorbed or excreted. The involvement of specific transporters in the transfer of Chol between blood compartments and cell membranes is well-established. However, the basal rate due to passive exchange processes is unknown, precluding the evaluation of the relative importance of passive and catalyzed processes in Chol homeostasis.
The seminal work of Phillips and colleagues (5, 6) and Steck and colleagues (7) has shown that the kinetics of unesterified Chol exchange between lipoproteins and erythrocytes (Erys) in vitro is slow and fully compatible with diffusion of Chol through the aqueous compartment. In contrast, when cyclodextrin is used as donor or acceptor of Chol, the exchange rate is much faster and proceeds via an activation-collision mechanism (8). The distribution of unesterified Chol among the various compartments in the blood was characterized in vivo by Schwartz et al. (9). Chol introduced in the blood associated with lipoproteins, equilibrated in less than one hour between the distinct lipoprotein classes, and it takes several hours for this cholesterol to equilibrate with the unesterified Chol pool in the Erys. The comparison between the results obtained in vitro for the rate of passive exchange between the different blood compartments and the in vivo studies could in principle allow the quantitative evaluation of the role of passive processes on the overall exchange of unesterified Chol among the various blood compartments. However, this has not yet been possible because some of the relevant rate and equilibrium constants were not known.
In this work, we are concerned with the noncatalyzed partitioning of free Chol in the blood from a kinetic and thermodynamic perspective. Because free Chol lacks spectroscopic signals that might enable the characterization of its association with relevant molecules and aggregates, we have used the fluorescent analog ergosta-5,7,9(11),22-tetraen-3β-ol [dehydroergosterol (DHE)] in our kinetic studies. This Chol analog has been used extensively to simulate Chol in cellular trafficking (10). Previously (11), we characterized the kinetics and thermodynamics of DHE interaction with serum albumin (Alb) and lipid bilayers that simulate the chemical composition of cell membranes. We now report the relevant kinetic and thermodynamic parameters for association of DHE with the various lipoprotein subclasses and proceed to use this information and our previous results to simulate its equilibrium distribution among all binding agents present in the blood: Alb, lipoproteins, and Ery membranes. We also use the kinetic information to simulate the time course of DHE distribution in the blood assuming that a bolus of free DHE is introduced directly into the blood compartment. We compare both these simulations with the distribution of free Chol in the blood observed in vivo.
MATERIALS AND METHODS
BSA, essentially free of fatty acids, and DHE were obtained from Sigma-Aldrich Química (Sintra, Portugal) and 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) was obtained from Avanti Polar Lipids Inc. (Alabaster, Alabama).
BSA concentrations were determined using the method of Lowry et al. (12) or by their absorbance at 279 nm using an extinction coefficient of 0.667 mg−1 ml cm−1 (13), and DHE concentration in acetone was determined by spectrophotometry using a molar extinction coefficient of 8,100 M−1 cm−1 at 340 nm. Absorption spectra were recorded on a Unicam UV530 UV/Vis spectrophotometer, and fluorescence measurements were performed on a Cary Eclipse fluorescence spectrophotometer equipped with a thermostated multi-cell holder accessory (Varian, Cary, NC). The samples were stirred continuously during measurements.
Lipoprotein fractions were obtained upon informed consent from a 26-year-old healthy male volunteer of normal weight after a 14 h fasting period, as described elsewhere (14, 15). Briefly, 50 ml of blood were collected by venous puncture into heparinized tubes and immediately centrifuged at 2,200 g for 10 min at room temperature. The plasma was collected and its density adjusted to 1.21 g/ml with solid KBr; 2.8 ml were deposited on the bottom of centrifuge tubes and gently covered by 6.8 ml of nitrogen-saturated PBS (d = 1.006 g/ml). The tubes were centrifuged at 65,000 rpm in a Beckman L80 ultracentrifuge with a fixed-angle 70.1 Ti rotor for 3 h at 15°C. The VLDL (d < 1.006 g/ml) and LDL fractions, located in the top and the yellowish band in the middle part of the tubes respectively, were extracted by aspiration and dialyzed against PBS in a nitrogen atmosphere using 50,000 Da cut-off polyethersulfone membranes. Further purification of the HDL fraction was based on methodologies described previously (16, 17) with some modifications. The density of the fraction containing the HDLs, located at the bottom of the tubes, was adjusted to 1.22 g/ml and centrifuged for 18 h at 65,000 rpm at 15°C to isolate HDLs from other plasma proteins. The floating HDL fraction was extracted by aspiration, its density adjusted to 1.125 g/ml by dilution with PBS, and centrifuged for 28 h at 65,000 rpm at 15°C. The yellow bands corresponding to the HDL2 (top) and HDL3 (bottom) fractions were extracted by aspiration and dialyzed as previously described.
Five collections were performed over a period of 6 months. After isolation of the lipoprotein fractions, the protein content of the samples was estimated by the method of Lowry et al. (12), and the lipoprotein concentration was estimated from this by assuming the reported mean aggregate masses and protein contents for each of the lipoprotein fractions (18), as previously described (14). The relevant characteristics of the lipoprotein fractions necessary for the present work are given in the supplementary information (supplementary Table I). The purity of the lipoprotein fractions was verified by 0.5% agarose gel electrophoresis (15).
Kinetic and equilibrium parameters for the association of DHE with BSA have been described elsewhere (11). Briefly, the equilibrium binding constant was obtained from the fluorescence increase of DHE when bound to BSA and the rate constants for the association, kB and k−B, were obtained with a methodology previously described by us (19), using 2,4-dinitrobenzene covalently attached to the BSA as a resonance energy acceptor of DHE fluorescence. The binding was characterized by the equilibrium constant KB ∼ 5 × 104 M−1 (at 35°C), and characteristic times, τB ∼ 4 s, under conditions where the binding step was pseudo-first order with respect to BSA (11).
Pre-association of DHE with BSA was done by squirting an acetone solution of DHE into an aqueous buffered solution of BSA [20 mM sodium phosphate, 110 mM NaCl, 1 mM EDTA, 0.02% NaN3 (pH 7.4)] at the desired concentration while vortexing the latter. Vortexing was carried on for a further 5 min. The solution was then allowed to attain equilibrium over 0.5–1 h at the desired temperature (15–35°C). The final concentration of acetone was always 1% (v/v).
The kinetics of the association of DHE with VLDLs and LDLs were studied by addition of the desired amount of lipoprotein (1.4 × 10−9 to 1.7 × 10−8 M and 7.0 × 10−9 to 6.8 × 10−8 M, respectively) to a solution of DHE (∼2 × 10−7 M), which had been previously equilibrated with BSA (∼2.7 × 10−4 M), as described above. The kinetics of association of DHE with HDL2 and HDL3 were studied by its exchange between the lipoproteins and large unilamellar vesicles (LUV) of POPC. Typically, DHE in acetone was added to an aqueous suspension of the lipoproteins and the mixture was allowed to reach equilibrium (0.5–1 h). The final DHE and lipoprotein concentrations were ∼2 × 10−7 M and ∼10−7 M, respectively, and the final acetone concentration was 1% (v/v). The desired amount of POPC LUV (2 × 10−10 to 4 × 10−9 M) was then added and DHE fluorescence was followed over time (λexc = 324 nm, λem = 374 nm). Data were analyzed using Excel® and Solver® (Microsoft, Seattle, WA) using the generalized reduced gradient algorithm, by minimizing the sum of squared deviation.
RESULTS
Kinetic and equilibrium parameters for the interaction of DHE with the lipoproteins
We have developed a methodology (19) that allows working with hydrophobic amphiphiles at concentrations in the micromolar range without self aggregation in the aqueous phase. The strategy is based on the use of a binding agent, such as BSA, that maintains the aqueous solution of amphiphile below the critical aggregation concentration. Accordingly, for studies with LDLs and VLDLs, DHE was first equilibrated with BSA and the transfer to lipoproteins was followed via variations in the fluorescence emission due to differences in the fluorescence quantum yield of DHE when associated with the various binding agents. However, BSA was unsuitable for the determination of the kinetic parameters for association of DHE with HDL2 and HDL3, because this was very fast and the fluorescence intensity variation was small, leading to a low signal-to-noise ratio. Thus, we obtained these parameters via the transfer of DHE from HDL2 and HDL3 to POPC LUVs, for which the association parameters are known (11). The use of these two distinct approaches did not influence the rate constants obtained because no direct interaction between the binding agents was expected. This assumption is further supported by the good fit of the model to the dependence of the exchange rate constant with the concentration of acceptor.
Distribution of DHE between the donor and acceptor binding agents is described by the kinetic scheme presented in equation 1:
| (Eq. 1) |
where S is the sterol (DHE) in the aqueous phase, BD and BA are the donor and acceptor binding agents, SBD and SBA represent the sterol associated with the donor and acceptor binding agents, respectively. The superscripts in the rate constants indicate the binding agent considered and the subscripts, + and −, refer to the association/dissociation reaction steps, respectively. The differential equations that describe the temporal evolution of the concentrations of all species in equation 1 were solved after the following simplifying assumptions. i) The association of DHE with the binding agents does not reduce their capacity to accommodate more DHE, and consequently the association step is pseudo-first order with rate constant . This approximation is valid because the concentration of BSA is much higher than that of DHE, and the amount of DHE associated with the lipoproteins or POPC LUVs is always much lower than the total lipid concentration. ii) The concentration of DHE in the aqueous media is at steady state. iii) DHE can only associate with the lipid monolayer at the surface of the lipoproteins. iv) DHE translocation between the outer and inner leaflets of the POPC bilayer in the LUVs is faster than the characteristic time for transfer between the donor and acceptor binding agents (11, 20–22). The temporal evolution of the concentration of DHE in all compartments considered is then given by equation 2, see supplementary information for details (section S2):
| (Eq. 2) |
where and fi is the fraction of DHE associated with the binding agent i that may dissociate into the aqueous phase outside the binding agent in a single step. For BSA and lipoproteins fi = 1, whereas for POPC LUVs fi = 0.5 because only DHE located in the outer monolayer may desorb into the aqueous phase outside the LUVs in a single step.
The transfer of DHE among the binding agents leads to a variation in the total fluorescence intensity according to equation 3:
| (Eq. 3) |
where Fi is the proportionality constant that relates the concentration of species i to its fluorescence intensity. The fluorescence intensity of DHE in the aqueous phase is negligible, compared with that of DHE associated with the binding agent, and therefore it was removed from the final equation used.
From equations 2 and 3 we obtain the expression for the evolution of the transfer of DHE between the donor and acceptor binding agents over time.
| (Eq. 4) |
Knowledge of the initial (F(0)) and equilibrium (F(∞)) fluorescence intensities and the time-dependence of the fluorescence intensity change, from which the rate constant for transfer (β) is directly obtained (equation 4), allows determination of all the kinetic and equilibrium parameters. In this work, we have focused on the time dependence of the fluorescence intensity because at the wavelengths of DHE fluorescence emission, the contribution from scattering by the binding agents may be significant; the equilibrium parameters are then computed from the ratio of the association and dissociation rate constants. It should be stressed that the characteristic rate constant for exchange between binding agents is dependent on the fraction of solute exchanged, but not on the magnitude of the variation in the observed variable. Therefore, larger variations in the fluorescence quantum yield of the solute in the donor and acceptor binding agents improve the method sensitivity but do not affect the characteristic rate constant of exchange.
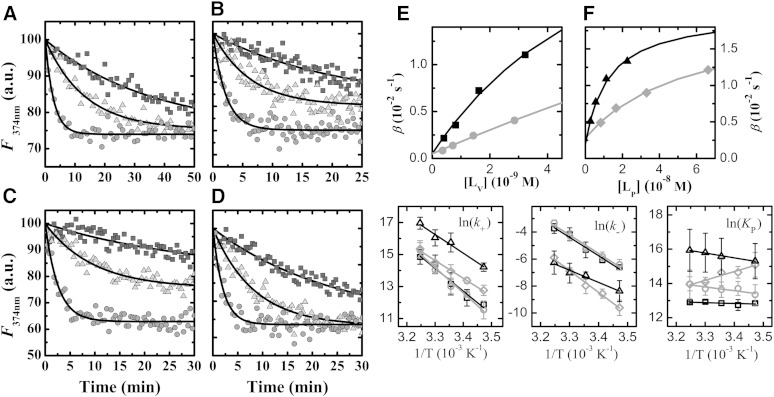
Typical results obtained for the transfer of DHE between BSA, or POPC LUVs, and lipoproteins are shown in. The best fit of equation 4 to these experimental results permitted obtaining the values of the transfer rate constants, β. The rate constants for association with, and dissociation from, the lipoproteins were obtained from the best fit of equation 2 to the dependence of β on the total concentration of acceptor (Fig. 1E, F).
Fig. 1.
Experimental results obtained for the exchange of DHE among the various blood compartments. A–D: Time dependence of DHE fluorescence intensity (2 × 10−7 M) due to exchange between binding agents at pH = 7.4 and 15°C (black box), 25°C (gray triangle), and 35°C (gray circle). A: For 2.5 × 10−4 M of BSA as donor and 2.8 × 10−8 M LDL as acceptor. B: For 2.5 × 10−4 M of BSA as donor and 6.8 × 10−9 M VLDL as acceptor. C: For 8.9 × 10−8 M HDL2 as donor and 2 × 10−9 M POPC LUV as acceptor. D: For 2.4 × 10−7 M HDL3 as donor and 2.6 × 10−9 M POPC LUV as acceptor. The lines are the best fit of equation 4. E, F: Dependence of the characteristic exchange rate constant (B) with the concentration of acceptor for the transfer of DHE from HDL2 (black square) or HDL3 (gray circle) to POPC LUVs (E) and from BSA to LDL (gray diamond) or to VLDL (black triangle) (F), at 35°C. The lines are the best fit of equation 2 with the parameters: = 5.1 × 107 M−1 s−1, = 1.0 × 10−3 s−1; = 8.5 × 102 M−1 s−1, = 2.0 × 10−2 s−1; = 3.9 × 106 M−1 s−1, = 3.6 × 10−2 s−1; = 1.0 × 107 M−1 s−1, = 6.6 × 10−2 s−1; = 5.0 × 106 M−1 s−1, = 2.8 × 10−3 s−1; = 2.1 × 107 M−1 s−1, = 1.9 × 10−3 s−1; and , . The temperature dependence of the rate constant for insertion (k+) and desorption (k−), as well as for the partition coefficient (KP) are shown in the lower panels on the right (average and standard deviation of three to six independent experiments) together with the best fit according to the absolute rate theory (23) (k+ and k−) or to the van’t Hoff equation (KP) with the parameters given in Table 1 for HDL2 (black open box), HDL3 (gray open circle), LDL (gray open diamond), and VLDL (black open triangle).
In contrast to what was observed for the transfer of DHE between BSA and POPC vesicles (11), the characteristic exchange rate constant β is a nonlinear function of the concentration of acceptor. This indicates that the rate of dissociation from the donor is comparable to the rate of dissociation from the acceptor and allows the calculation of both rate constants. The analysis of the equation for β defined in equation 2 shows that at low (high) acceptor concentrations β approaches the rate constant for dissociation from the acceptor (donor). For intermediate acceptor concentrations, β changes from one extreme situation to the other, and this allows the computation of the relative values of the rate constants for association with the donor or acceptor. The kinetic parameters for association with POPC LUVs were fixed at the values obtained previously, and those for association with BSA were allowed to change within 20% due to the large uncertainty associated with these parameters (11).
The rate constants obtained for the interaction of DHE with the various lipoproteins at 35°C are shown in Table 1. The values of (Table 1) are more than three orders of magnitude smaller than the diffusion-controlled rate constant, kdiff, indicating that the process is clearly not diffusion controlled. The same was observed for the interaction of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-amino-1,2-dimyristoylphosphatidyl ethanolamine (NBD-DMPE) with these lipoproteins (14). As discussed in (14), the values of and obtained for lipoproteins of different size cannot be directly compared. This can be circumvented by considering that insertion proceeds via the formation of an encounter complex between both reactants, as an intermediate between the reactants in the bulk aqueous phase and amphiphile associated with the binding agent (23), allowing the computation of the unimolecular insertion rate constant (kin). This parameter, and the rate of desorption of DHE from the lipoproteins , increases as their curvature increases reflecting the higher free volume and packing defects in the surface lipid monolayer of the smallest lipoproteins. The lower desorption rates of the amphiphile from LDLs may also be related to their composition. Being enriched in SM and Chol (24, 25), the surface monolayers of LDLs are expected to be more ordered than the monolayers of both HDLs and VLDLs. However, this is not reflected in a significantly higher partition coefficient for LDLs that might have been expected from the preferential partition of DHE into ℓo phases of SM-Chol at 35°C (11). In fact, the relative parameters obtained for the interaction of DHE with lipoproteins and POPC LUVs are similar to those obtained for NBD-DMPE (14, 26), except for a lower relative partition of DHE into LDLs and HDL2. This suggests that the equilibrium association of amphiphilic molecules with the lipoproteins is not strongly dependent on the structure of the amphiphile, at least at 35°C (see below).
TABLE 1.
Rate and equilibrium constants, as well as thermodynamic parameters (in kJ mol−1), for the interaction of DHE with the lipoproteins at 35°C. The parameters for DHE interaction with lipid vesicles were taken from literature (11) and are also shown.
| HDL3 | HDL2 | LDL | VLDL | POPC:Chol 1:1 | SM:Chol 6:4 | |
| kdiff (1010 M−1 s−1) | 2.1 | 2.9 | 5.6 | 14 | 25 | 25 |
| k−diff (106 s−1) | 143 | 66 | 16 | 2.5 | 0.77 | 0.77 |
| k+ (106 M−1 s−1) | 3.4 ± 1.2 | 3.0 ± 1.5 | 4.1 ± 1.2 | 21 ± 8 | 64 ± 6 | 13 ± 1 |
| kin (102 s−1) | 234 ± 84 | 67 ± 34 | 12 ± 4 | 3.9 ± 1.5 | 2.0 ± 0.2 | 0.40 ± 0.03 |
| k− (10−3 s−1) | 30 ± 8 | 25 ± 9 | 2.5 ± 1.1 | 1.7 ± 0.9 | 0.6 ± 0.2 | 0.07 ± 0.02 |
| (105)a | 11 ± 5 | 4.2 ± 0.9 | 11 ± 6 | 12 ± 10 | 25 ± 8 | 47 ± 15 |
| ΔH°(partition) | 19 ± 9 | 4 ± 5 | −42 ± 13 | 24 ± 30 | −13 ± 10 | 53 ± 7 |
| ΤΔS°(partition) | 54 ± 10 | 37 ± 5 | −6 ± 13 | 59 ± 30 | 23 ± 10b | 90 ± 7b |
| ΔH‡°(insertion)c | 130 ± 10 | 116 ± 14 | 90 ± 7 | 95 ± 15 | 83 ± 7 | 110 ± 14 |
| ΤΔS‡°(insertion)c | 93 ± 10 | 79 ± 15 | 54 ± 7 | 63 ± 15 | 53 ± 7 | 77 ± 15 |
| ΔH‡°(desorption)c | 111 ± 7 | 111 ± 10 | 132 ± 9 | 72 ± 21 | 95 ± 12 | 57 ± 16 |
| ΤΔS‡°(desorption)c | 27 ± 7 | 26 ± 11 | 41 ± 9 | −20 ± 20 | 1 ± 12 | −43 ± 16 |
The partition coefficient between the aqueous phase and the lipid monolayer at the lipoproteins surface was calculated from the respective equilibrium binding constant as previously described (14).
This entropy variation is different from the values given in the original reference because it is calculated from the temperature dependence of KP (instead of ) to allow comparison between the parameters obtained for binding agents with very distinct sizes.c ‡ refers to the thermodynamic parameters of the transition state in the kinetic steps.
The temperature dependence of the kinetic and equilibrium parameters may be used to calculate the thermodynamic parameters associated with the formation of the transition state, using the absolute rate theory (23), and the van’t Hoff enthalpy and entropy variations associated with partition between the aqueous phase and the lipoproteins (Fig. 1, Table 1).
Association of DHE with all lipoproteins has a large entropic contribution, indicating that the hydrophobic effect is the major driving force. An exception is noted for the association of DHE with LDLs, which is characterized by a significant favorable enthalpy contribution. This was not expected given the high percentage of SM in LDLs as compared with the other lipoproteins (24, 25), and the results obtained for SM:Chol (6:4) bilayers (11). Those results indicate a relatively more specific interaction of DHE with LDLs, leading to an increase in the organization of the system. This could be due to interactions between DHE and the lipoprotein surface components, such as apoB-100 (27), or with the lipoprotein core (enriched in cholesteryl esters).
Another, at first unexpected, observation is the high enthalpy variation associated with the formation of the transition state in the process of insertion of DHE into HDL3 and HDL2. The high curvature of these small lipoproteins generates a significant free volume at the lipid monolayer surface. The observation that the work required to open a cavity large enough to accommodate DHE is higher than for the larger lipoproteins reflects the fact that this cavity must extend deeply into the lipid monolayer where the high curvature imposes a larger lipid density. A similar behavior was previously observed for the insertion of NBD-DMPE into these lipoproteins (14).
DISCUSSION
Modeling the distribution of free Chol in the blood
Based on the results above and on those obtained previously (11), we developed a kinetic model for the passive distribution of Chol in the blood. We consider the following donors/acceptors of free Chol: VLDLs, LDLs, HDL2, HDL3, serum Alb, and Erys. In turn, we neglected Chol binding by the apical portion of the endothelial cells’ membrane because this membrane pool has similar properties to that of Erys and is much smaller [<10% (28)].
The parameters for the interaction with Erys were derived from the results obtained for the association of DHE with vesicles (11) taking into account the asymmetry of the Ery membrane and the difference in size between the Erys and the lipid vesicles. The Ery membrane has large amounts of SM, phophatidylcholine, and Chol with significant amounts of phosphatidylethanolamine, phosphatidylserine, and phosphatidylinositol (29–35). Most SM is located in the outer leaflet (30–32), while Chol is distributed in both leaflets (31, 36, 37). The acyl chains of the inner leaflet lipids have higher unsaturation levels (34), this monolayer being in a more fluid state (32). Based on this information (see section S1.2 in the supplementary information for details), we have modeled the outer leaflet of the Ery membrane by a SM:Chol (6:4) bilayer and the inner leaflet by a POPC:Chol (1:1) bilayer.
We have previously shown that the insertion of DHE into lipid LUVs in not diffusion controlled (11), and therefore the amount of sterol associated with the lipid phase at equilibrium is only dependent on the partition coefficient and on the volume of the lipid phase. Additionally, the rate of insertion/desorption depends only on the total surface between the lipidic and aqueous phases, although the rate constant for insertion must be converted from M−1 s−1 (rate constant per LUV particle) to the units of a permeability coefficient (dm s−1). The rate constant for desorption could be used directly because, being a first order process, it is not dependent on the fragmentation of the phase. However, for consistency within the model this rate constant was also converted into a permeability coefficient. The equations used to calculate the partition coefficient between the aqueous phase and the Ery membrane and the rate constants for insertion into and desorption from the outer leaflet of the Ery membrane are given below, see section S3 of the supplementary information for details.
| (Eq. 5) |
| (Eq. 6) |
Where and are the volumes of the outer and inner leaflet of the Ery membrane per liter of plasma being liters; is the molar surface area of the LUVs accessible to the external aqueous phase, being given by 4πr2NA and equal to 1.89 × 1012 dm2 mol−1 for r = 50 nm; and h is the thickness of the outer monolayer (taken as 2 nm for all monolayers considered in this model). The equilibrium and kinetic parameters considered were calculated from those obtained experimentally for SM:Chol (6:4) and POPC:Chol (1:1) LUVs (, respectively).
The equilibrium distribution of Chol among the various binding agents in the blood is given by equation 7, and the time dependence of the concentration of Chol in each compartment is given by equation 8, where is the surface area of the Erys’ outer leaflet (7.28 × 104 dm2 per liter of plasma).
Structural properties of the lipoproteins, Erys, and relevant lipid bilayers, as well as the concentration of binding agents and number of Chol molecules per binding agent, were taken from the literature (18, 24, 25, 29, 38–42) and are given in the supplementary information (supplementary Table I). Briefly, the number of unesterified Chol molecules per lipoprotein were 3,539, 475, 50, and 13 (39), and the concentration of each lipoprotein in the serum was 0.08, 1.5, 4, and 30 μM (38) for VLDLs, LDLs, HDL2, and HDL3, respectively. This leads to 1.6 mM total concentration of free Chol in the plasma, in accordance to the values reported in the literature for plasma that is considered to have a “normal” Chol concentration (1.4–1.6) (39, 43, 44). Adding the unesterified Chol present in the Erys (2.7 mM) yields 4.3 mM for the total concentration of free Chol in the blood ([Ch]T).
| (Eq. 7) |
| (Eq. 8) |
where is the fraction of Chol associated with the Erys that is in the outer leaflet, being equal to .
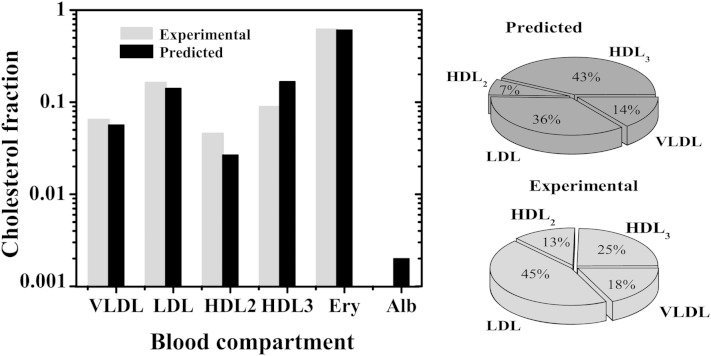
The equilibrium distribution of free Chol obtained from equation 7 was calculated from the rate and equilibrium constants obtained for DHE and is shown in Fig. 2, black bars. The agreement with the distribution expected from the literature (light gray bars) is very good for all the compartments considered. The estimated Chol content in the Ery is only slightly lower than the experimental reference value (a factor of 0.93), which is a very good agreement given the differences between the LUVs and Erys. The predicted Chol contents of VLDLs are also within the experimental range.
Fig. 2.
Equilibrium distribution of unesterified Chol in the various blood compartments considered in the model. Reference values in vivo (light gray bar) and equilibrium values predicted from the model using equation 7 and the parameters obtained in this work for DHE (black bar). Left panel: whole blood (a logarithm scale was used to allow comparison for all blood compartments, dominated by the Erys if a linear scale was used). Right panel: lipoprotein fraction.
In turn, the model overestimates the free Chol content in HDL3 and underestimates that in HDL2. However, there is better agreement between predictions and observations as regards the Chol contents of HDLs as a whole. This suggests that the discrepancies observed for HDL2 and HDL3 reflect the difficulty in experimentally distinguishing HDL subclasses. Indeed, the percentage of HDL2 particles within HDLs ranges from 12 to 40% depending on the reference considered (38, 45, 46). The discrepancy between the results obtained for LDL and those reported in the literature may be related to the strong temperature dependence of the association predicted for Chol (Table 1), whose equilibrium constant increases as temperature decreases. To experimentally quantify the amount of Chol in each blood compartment, the various lipoproteins must first be isolated and some exchange of Chol between compartments may occur. The extent of this effect depends on the rate of Chol equilibration among the various compartments, and on the duration and temperature during the sample processing.
It could be argued that the relative equilibrium constants obtained for the interaction between DHE and the different binding agents in the blood are not representative of the interactions observed for Chol. The absence of data in the literature for Chol precludes an unequivocal answer to this problem. However, the similar relative equilibrium constants obtained for DHE and the structurally unrelated phospholipid NBD-DMPE (14) give support to the adequacy of DHE to model the equilibrium distribution of Chol, which is structurally very similar.
Another explanation for the deviations encountered between the equilibrium distribution predicted and the amount of unesterified Chol experimentally measured in each lipoprotein pool may reflect a nonequilibrium distribution in vivo. This will be explored in sections below.
Kinetics of free Chol distribution among the blood compartments, modeled as DHE
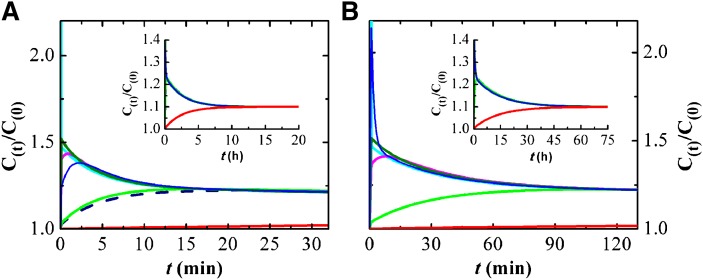
The rate constants obtained in this study and those previously reported by us for the interaction of DHE with lipid bilayers (11) allow the calculation of the rate of exchange of free Chol, modeled as DHE, among blood compartments. We have assessed this process by examining the temporal evolution of the concentration of free Chol in each blood compartment after addition of a bolus of 10% of total free Chol to a given compartment. Addition of the bolus to the aqueous phase (Fig. 3A) is the most biologically relevant situation, as it reflects the entry of Chol into the blood, associated with Chol binding proteins or nascent HDLs. It also presents the fastest exchange kinetics, generating transient increases in the Chol concentration of all blood compartments except Erys (the slowest one).
Fig. 3.
Simulation results for the kinetics of Chol redistribution in the blood at 37°C after a bolus of 10% total unesterified Chol in the aqueous compartment. The rate constants used in the kinetic model were those obtained in this work for DHE (A) or taken from literature for Chol (5, 48) (B). Time evolution of the concentration of unesterified Chol in the distinct blood compartments: aqueous phase (light blue line), HDL3 (dark green line), HDL2 (pink line), Alb (blue line), LDL (light green line), VLDL (dark blue dashed line), and Erys (red line). The y axis is the concentration at time t, C(t), divided by the equilibrium concentration before the bolus, C(0). The insets show the results obtained at longer simulation times, with the transfer of Chol from the lipoproteins to the Erys.
The instantly imposed excess Chol in the aqueous compartment equilibrates among the various blood compartments to reach a new equilibrium, with 10% more Chol in each compartment, over several hours. The excess unesterified Chol first equilibrates with the binding agents with a faster rate of association (HDL3, HDL2, and Alb) followed by redistribution from those pools into the lipoproteins with slower exchange rates (LDLs and VLDLs). Finally, the excess Chol is redistributed between those binding agents and the Erys, the binding agent with the slowest association kinetics (Fig. 3A, inset). The maximal excess Chol transiently accumulated is largest for the pools with the fastest kinetics (HDL3 > HDL2 > Alb > LDL > VLDL) and the time at which the concentration peak occurs follows the reverse order. No excess accumulation is observed for the pool with the slowest association kinetics (Erys). The amount of Chol in this pool undergoes a mono-exponential increase with a characteristic time of 150 min.
Importantly, these results indicate that the transient accumulation of Chol in HDL2 and HDL3 is much larger than that observed for LDLs and VLDLs. Thus, the amount of Chol in the small lipoprotein pool is more sensitive to variations in the rates of Chol entry into and removal from the blood, while LDLs and VLDLs are more robust regarding fluctuations in the total concentration of Chol.
Once the maximum concentration of Chol in a compartment is attained, it slowly decreases toward the value at the new equilibrium condition. For compartments with relatively slow exchange kinetics (LDLs and VLDLs), this decrease is approximately exponential with a characteristic time similar to that for equilibration with Erys (τ ≅ 150 min; corresponding to 100–130 min for 50% dissipation of the excess Chol, t1/2). The situation is different for the compartments with fast kinetics (HDL2, HDL3, and Alb). These show two characteristic times, a faster process due to equilibration with the compartments with intermediate kinetics (τ ≅ 5 min) followed by the final redistribution of Chol with the slowest compartment with a characteristic time constant around 150 min. This larger characteristic time is similar to that observed for the exchange of DHE between lipoproteins and cells (47).
Kinetics of free sterol distribution among the blood compartments: Chol versus DHE
In the section above, we have modeled the temporal evolution of unesterified Chol among the blood compartments, after a bolus in a given compartment, using the parameters obtained for DHE. The structure of both sterols is very similar and DHE is commonly used as a model of Chol (10). However, it can be argued that the parameters obtained for DHE are not equal to those of Chol. In fact, the rates of dissociation of [3H]Chol or [4-14C]Chol from lipoproteins and Erys have been reported in the literature and are significantly different from those obtained in this work for DHE: = 4.1 × 10−3 s−1, = 2.7 × 10−3 s−1, = 2.7 × 10−4 s−1 (5), and = 1.3 × 10−5 s−1 (48). To model Chol homeostasis, the association rate constants are also needed (as well as the corresponding equilibrium association constants), but those parameters are not available. To overcome this difficulty, we have assumed that the equilibrium association constants of Chol are equal to those obtained for DHE. This approximation is acceptable because the amphiphyle’s molecular properties tend to affect the rate constants of association and dissociation in the same direction (26, 49–51), with the effects in the equilibrium constants being much less sensitive to those properties. In particular, the relative equilibrium constants for association with different binding agents show very little dependence on the amphiphile’s molecular properties for structurally related solutes (11, 26, 50, 51). For this reason, in the following simulations we assumed that the equilibrium association of Chol is equal to that obtained for DHE and calculated the association rate constants from the relation k+ = Keqk−. To further support this assumption, we have performed complementary simulations considering equilibrium constants up to 10 times larger and verified that the results obtained are not significantly affected (less than 1% variation in all output variables considered in this work).
No data are available in the literature for the interaction of Chol with the VLDL lipoproteins and with Alb. We have considered that the kinetic and equilibrium parameters for the interaction of Chol with the VLDL lipoproteins are equal to those observed for association with LDLs, given that the results obtained with DHE are very similar for both lipoprotein classes, and that the parameters for association of Chol with Alb are equal to those obtained for DHE.
The results obtained for the temporal evolution of the concentration of free Chol in each blood compartment, after addition of a bolus of 10% of total free Chol to the aqueous phase, are given in Fig. 3B. As observed for DHE (Fig. 3A), the excess Chol in the aqueous phase is transiently accumulated in the compartments with fast exchange kinetics (Alb and HDLs) followed by an equilibration with the compartments having intermediate kinetics (LDLs and VLDLs) and, at longer times, with the compartment showing the slowest exchange kinetics (Erys). A larger transient accumulation is now observed for Chol associated with Alb because the kinetics considered for this pool are significantly faster than those of the remaining blood compartments.
Despite the very good qualitative agreement between the results obtained for the distribution of DHE and Chol among the blood compartments, the results show that equilibration of Chol directly predicted from the available parameters (Fig. 3B) is significantly slower than that observed for DHE (Fig. 3A).
Steady state distribution of Chol among the blood compartments: LCAT effect
The small deviations observed between the concentrations of Chol in the blood compartments predicted by the equilibrium parameters obtained in this study and those observed in vivo may have a physiological underpinning. In fact, the Chol distribution among blood components is not expected to be at equilibrium because there is entrance of Chol in the system as well as consumption by LCAT in HDL2 and HDL3 particles. The differential equation 8 was modified as follows so as to account for these processes: i) a term accounting for entry of Chol through the aqueous phase was added (v+), representing any route not considered in the model that may release Chol with fast kinetics, such as Chol binding proteins or nascent HDL particles; and ii) terms accounting for Chol consumption in HDL2 and HDL3 (kLCAT). The differential equations for HDL2, HDL3, and aqueous Chol in equation 8 were therefore substituted by:
| (Eq. 9) |
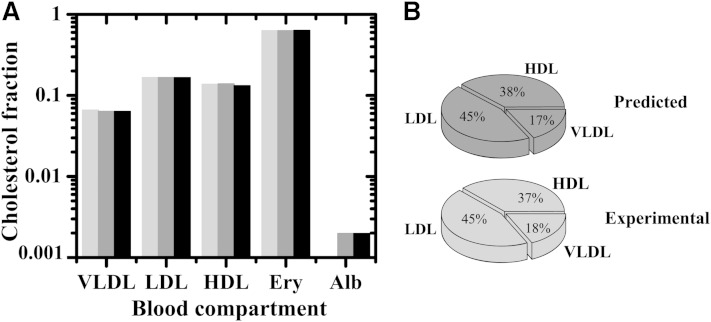
We solved the system for steady state and adjusted the rate of Chol influx (v+) such that the Chol concentration in LDLs matched the experimental value. The agreement between the computed steady state Chol distribution and the experimentally found distribution is remarkable, both when Chol distribution is modeled by DHE and when the literature data for the dissociation of Chol from the blood compartments is considered (Fig. 4).
Fig. 4.
Distribution of unesterified Chol in the various blood compartments considered in the model. Reference values in vivo (light gray bar) and steady state values predicted from the model using equation 9 for Chol in the aqueous phase and in HDL, and equation 8 for the remaining compartments, considering the rate constants obtained in this work for DHE (dark gray bar) and those taken from literature for Chol (5, 48) (black bar). The results for the whole blood are shown in the left panel and the distribution of Chol among the lipoproteins is given in the right panel. The steady-state distribution in the lipoproteins obtained from the kinetic data of Chol deviate less than 1% from those measured experimentally (lower panel) or calculated from DHE rate constants (upper panel). The steady state is attained with v+ = 7.5 × 10−6 M s−1, kLCAT = 1.3 × 10−2 s−1 and v+ = 9.9 × 10−7 M s−1, kLCAT = 1.8 × 10−3 s−1, when the dissociation rate constants of DHE and Chol are used.
In spite of the good agreement obtained, the rate of Chol turnover in the blood required to meet the imposed condition was unrealistically high: v+ = 7.5 × 10−6 M s−1 and kLCAT = 1.3 × 10−2 s−1 when the kinetic parameters obtained with DHE are considered, and v+ = 9.9 × 10−7 M s−1 and kLCAT = 1.8 × 10−3 s−1 considering the dissociation rate constants for Chol taken from literature (5, 48), whereas the usually accepted value is v+ = 1.1 × 10−8 M s−1 (52). This indicates that this process cannot be the main explanation for the differences observed. The turnover of unesterified Chol in the blood at the physiological rate causes only modest (<10%) changes relative to the equilibrium distribution. It decreases the discrepancies observed between the predicted and experimentally found fractions of Chol in the various lipoproteins, but does not eliminate them.
Chol homeostasis in the blood: passive versus active transport
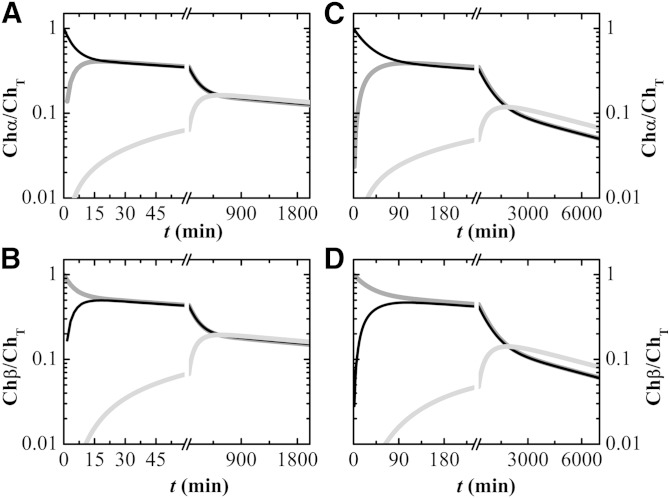
The homeostasis of unesterified Chol in vivo has been studied by Schwartz et al. (9) and the comparison between those results and the ones predicted by the kinetic model developed in this work may elucidate the relative importance of passive and active processes. The initial concentrations of Chol considered for each blood compartment were given by the steady state solution of the set of differential equations (equation 9) with the physiologic rate of Chol entry in the system, v+ = 1.1 × 10−8 M s−1 (52), and the corresponding rate constant for LCAT activity, kLCAT = 1.4 × 10−5 s−1. To simulate the conditions followed in the in vivo study, we then defined three distinct subsets of unesterified Chol (Ch, Chα, and Chβ, with ChT being the sum of all subsets of Chol). The Chol associated with HDLs was identified as Chα, that associated with LDLs and VLDLs was identified as Chβ, and the remaining Chol was maintained as Ch. The simulation was started and the exchange of Ch, Chα, and Chβ among the distinct blood compartments was followed. The results obtained for the enrichment of Chα and Chβ in the distinct blood compartments is shown in Fig. 5, using the kinetic parameters obtained in this work for DHE (Fig. 5A, B) and those taken from literature for Chol (Fig. 5C, D).
Fig. 5.
Simulation of the homeostasis of unesterified Chol in the blood at 37°C due to passive processes. The rate constants obtained in this work for DHE are used in (A) and (B), while desorption rate constants taken from literature for Chol (5, 48) are used in (C) and (D). The concentrations of unesterified Chol in the distinct pools were obtained from the steady state solution of the kinetic scheme developed in this work, equations 8 and 9, for a rate of Chol entry in the blood equal to 1.1 × 10−8 M s−1. At t = 0, the unesterified Chol in the HDL fraction was replaced by Chα and that in the LDL and VLDL fractions was replaced by Chβ. The relative enrichment in Chα (A, C) and Chβ (B, D) in the distinct blood compartments was followed over time for HDL (black line), LDL and VLDL (dark gray line), and the Erys (light gray line).
As observed in the in vivo study (9), unesterified Chol associated with the lipoproteins equilibrates rapidly: after 90 min the enrichment of Chol within the lipoprotein classes is undistinguishable and independent of the compartment where Chol was located at the beginning of the simulation (Fig. 5C, D). This equilibration time is reduced to 15 min when the rate constants predicted from DHE are used (Fig. 5A, B). The equilibration time within the lipoprotein pool was estimated as 30 min in the in vivo study, clearly showing that passive processes have a very significant contribution for the overall rate of exchange.
A similar situation is observed for the exchange of Chol between the lipoproteins and the Erys. The in vivo study shows that the enrichment of Chol in the Erys and in the lipoproteins converges at around 400–600 min, while the passive processes described by this kinetic model predict convergence at 1,500 min (450 min for predictions based on DHE, Fig. 5A, B). This kinetic model is also able to distinguish between the sources of unesterified Chol in the beginning of the equilibration with the Erys. The time at which 1% enrichment of Chα (from HDLs) in the Erys is attained is half the time required for 1% enrichment in Chβ (from LDLs and VLDLs). A faster partial equilibration between Chol from the HDL pool and the Erys was also observed in the in vivo study, as well as when Alb was used as the Chol donor. This clearly indicates that passive exchange processes mediated by Chol in the aqueous phase (the enrichment of which dependents directly on the dissociation rate constant from the donor pool) are significant even for the exchange between the lipoproteins and the Erys.
Limitations of this study
This work highlights important features of Chol homeostasis in the blood and is a significant contribution to this topic. It has, however, several limitations that should be understood to guide and improve future work. One should first consider the use of a single individual as donor of the lipoprotein fractions. This was a necessary condition in this work because the characteristics of the distinct lipoprotein fractions had to be maintained throughout the work. Further advancement in this subject should consider distinct donors and preferably individuals with distinct relative levels of HDLs and LDLs, as well as total Chol. It should be noted that all the kinetic and equilibrium parameters must be obtained for each donor to allow the establishment of eventual dependencies. Failure to do so will only increase the uncertainly associated with the parameters.
Another limitation is the use of DHE as a reporter of Chol. This was partially overcome with the use of kinetic data for Chol exchange taken from literature. However, the parameters were obtained with distinct methodologies and blood donors, which reduces the precision and consistency of the parameters. Furthermore, some of the required parameters could not be obtained from literature and those obtained in this work for DHE had to be used.
Future work on this subject should characterize quantitatively the exchange of Chol among the distinct blood compartments of a large number of individuals, both with similar and distinct Chol and lipoprotein levels.
Supplementary Material
Footnotes
Abbreviations:
- Alb
- albumin
- Chol
- cholesterol
- DHE
- dehydroergosterol (ergosta-5,7,9(11),22-tetraen-3β-ol)
- Ery
- erythrocyte
- LUV
- large unilamelar vesicles
- NBD-DMPE
- N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-amino-1,2-dimyristoylphosphatidyl ethanolamine
- POPC
- 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
This work was supported by the Fundação para a Ciência e a Tecnologia (FCT) of the Portuguese Ministry for Higher Education and Scientific Research through the POCTI program. L.M.B.B.E. and H.L.A.F. acknowledge support from FCT through grants SFRH/BD/6746/2001 and SFRH/BD/65375/2009.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure, one table, and text.
REFERENCES
- 1.Maxfield F. R., Tabas I. 2005. Role of cholesterol and lipid organization in disease. Nature. 438: 612–621. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein J. L., Brown M. S. 2001. Molecular medicine. The cholesterol quartet. Science. 292: 1310–1312. [DOI] [PubMed] [Google Scholar]
- 3.Rothblat G. H., Phillips M. C. 2010. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr. Opin. Lipidol. 21: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Eckardstein A., Nofer J. R., Assmann G. 2001. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 21: 13–27. [DOI] [PubMed] [Google Scholar]
- 5.Lund-Katz S., Hammerschlag B., Phillips M. C. 1982. Kinetics and mechanism of free cholesterol exchange between human serum high- and low-density lipoproteins. Biochemistry. 21: 2964–2969. [DOI] [PubMed] [Google Scholar]
- 6.Phillips M. C., Johnson W. J., Rothblat G. H. 1987. Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim. Biophys. Acta. 906: 223–276. [DOI] [PubMed] [Google Scholar]
- 7.Lange Y., Molinaro A. L., Chauncey T. R., Steck T. L. 1983. On the mechanism of transfer of cholesterol between human erythrocytes and plasma. J. Biol. Chem. 258: 6920–6926. [PubMed] [Google Scholar]
- 8.Steck T. L., Ye J., Lange Y. 2002. Probing red cell membrane cholesterol movement with cyclodextrin. Biophys. J. 83: 2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz C. C., Zech L. A., Vandenbroek J. M., Cooper P. S. 1993. Cholesterol kinetics in subjects with bile fistula. Positive relationship between size of the bile acid precursor pool and bile acid synthetic rate. J. Clin. Invest. 91: 923–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntosh A. L., Atshaves B. P., Huang H., Gallegos A. M., Kier A. B., Schroeder F. 2008. Fluorescence techniques using dehydroergosterol to study cholesterol trafficking. Lipids. 43: 1185–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estronca L. M. B. B., Moreno M. J., Vaz W. L. C. 2007. Kinetics and thermodynamics of the association of dehydroergosterol with lipid bilayer membranes. Biophys. J. 93: 4244–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 13.Peters T. 1996. All About Albumin: Biochemistry, Genetics, and Medical Applications. Academic Press, New York. [Google Scholar]
- 14.Estronca L. M. B. B., Moreno M. J., Laranjinha J. A. N., Almeida L. M., Vaz W. L. C. 2005. Kinetics and thermodynamics of lipid amphiphile exchange between lipoproteins and albumin in serum. Biophys. J. 88: 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vieira O. V., Laranjinha J. A. N., Madeira V. M. C., Almeida L. M. 1996. Rapid isolation of low density lipoproteins in a concentrated fraction free from water-soluble plasma antioxidants. J. Lipid Res. 37: 2715–2721. [PubMed] [Google Scholar]
- 16.Kostner G., Alaupovi P. 1972. Studies of composition and structure of plasma lipoproteins. Separation and quantification of lipoprotein families occurring in high density lipoproteins of human plasma. Biochemistry. 11: 3419–3428. [DOI] [PubMed] [Google Scholar]
- 17.Schumaker V. N., Puppione D. L. 1986. Sequential flotation ultracentrifugation. Methods Enzymol. 128: 155–170. [DOI] [PubMed] [Google Scholar]
- 18.Gotto A. M., Pownall H. J., Havel R. J. 1986. Introduction to the plasma lipoproteins. Methods Enzymol. 128: 3–41. [DOI] [PubMed] [Google Scholar]
- 19.Abreu M. S. C., Estronca L. M. B. B., Moreno M. J., Vaz W. L. C. 2003. Binding of a fluorescent lipid amphiphile to albumin and its transfer to lipid bilayer membranes. Biophys. J. 84: 386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett W. F. D., MacCallum J. L., Hinner M. J., Marrink S. J., Tieleman D. P. 2009. Molecular view of cholesterol flip-flop and chemical potential in different membrane environments. J. Am. Chem. Soc. 131: 12714–12720. [DOI] [PubMed] [Google Scholar]
- 21.Müller P., Herrmann A. 2002. Rapid transbilayer movement of spin-labeled steroids in human erythrocytes and in liposomes. Biophys. J. 82: 1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange Y., Dolde J., Steck T. L. 1981. The rate of transmembrane movement of cholesterol in the human erythrocyte. J. Biol. Chem. 256: 5321–5323. [PubMed] [Google Scholar]
- 23.Steinfeld J. I., Francisco J. S., Hase W. L. 1999. Chemical Kinetics and Dynamics. 2nd ed. Prentice Hall, Upper Saddle River, NJ. [Google Scholar]
- 24.Kuksis A., Myher J. J., Geher K., Jones G. J. L., Shepherd J., Packard C. J., Morrisett J. D., Taunton O. D., Gotto A. M. 1982. Effect of saturated and unsaturated fat diets on lipid profiles of plasma lipoproteins. Atherosclerosis. 41: 221–240. [DOI] [PubMed] [Google Scholar]
- 25.Chapman M. J. 1986. Comparative analysis of mammalian plasma lipoproteins. Methods Enzymol. 128: 70–143. [DOI] [PubMed] [Google Scholar]
- 26.Abreu M. S. C., Moreno M. J., Vaz W. L. C. 2004. Kinetics and Thermodynamics of association of a phospholipid derivative with lipid bilayers in liquid-disordered and liquid-ordered phases. Biophys. J. 87: 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murtola T., Vuorela T. A., Hyvonen M. T., Marrink S. J., Karttunen M., Vattulainen I. 2011. Low density lipoprotein: structure, dynamics, and interactions of apoB-100 with lipids. Soft Matter. 7: 8135–8141. [Google Scholar]
- 28.Wolinsky H. 1980. A proposal linking clearance of circulating lipoproteins to tissue metabolic activity as a basis for understanding atherogenesis. Circ. Res. 47: 301–311. [DOI] [PubMed] [Google Scholar]
- 29.Dodge J. T., Phillips G. B. 1967. Composition of phospholipids and of phospholipid fatty acids and aldehydes in human red cells. J. Lipid Res. 8: 667–675. [PubMed] [Google Scholar]
- 30.Rothman J. E., Lenard J. 1977. Membrane asymmetry. Science. 195: 743–753. [DOI] [PubMed] [Google Scholar]
- 31.Op den Kamp J. A. 1979. Lipid asymmetry in membranes. Annu. Rev. Biochem. 48: 47–71. [DOI] [PubMed] [Google Scholar]
- 32.Devaux P. F. 1992. Protein involvement in transmembrane lipid asymmetry. Annu. Rev. Biophys. Biomol. Struct. 21: 417–439. [DOI] [PubMed] [Google Scholar]
- 33.Owen J. S., Bruckdorfer K. R., Day R. C., McIntyre N. 1982. Decreased eryrhrocyte membrane fluidity and altered lipid composition in human-liver disease. J. Lipid Res. 23: 124–132. [PubMed] [Google Scholar]
- 34.Leidl K., Liebisch G., Richter D., Schmitz G. 2008. Mass spectrometric analysis of lipid species of human circulating blood cells. Biochim. Biophys. Acta. 1781: 655–664. [DOI] [PubMed] [Google Scholar]
- 35.Barceló F., Perona J. S., Prades J., Funari S. S., Gomez-Gracia E., Conde M., Estruch R., Ruiz-Gutiérrez V. 2009. Mediterranean-style diet effect on the structural properties of the erythrocyte cell membrane of hypertensive patients: the Prevencion con Dieta Mediterranea Study. Hypertension. 54: 1143–1150. [DOI] [PubMed] [Google Scholar]
- 36.Fisher K. A. 1976. Analysis of membrane halves: cholesterol. Proc. Natl. Acad. Sci. USA. 73: 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeagle P. L. 1985. Cholesterol and the cell membrane. Biochim. Biophys. Acta. 822: 267–287. [DOI] [PubMed] [Google Scholar]
- 38.Freedman D. S., Otvos J. D., Jeyarajah E. J., Shalaurova I., Cupples L. A., Parise H., D’Agostino R. B., Wilson P. W. F., Schaefer E. J. 2004. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham study. Clin. Chem. 50: 1189–1200. [DOI] [PubMed] [Google Scholar]
- 39.Shen B. W., Scanu A. M., Kezdy F. J. 1977. Structure of human serum lipoproteins inferred from compositional analysis. Proc. Natl. Acad. Sci. USA. 74: 837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans E., Fung Y. C. 1972. Improved measurements of erythrocyte geometry. Microvasc. Res. 4: 335–347. [DOI] [PubMed] [Google Scholar]
- 41.Lund-Katz S., Laboda H. M., McLean L. R., Phillips M. C. 1988. Influence of molecular packing and phospholipid type on rates of cholesterol exchange. Biochemistry. 27: 3416–3423. [DOI] [PubMed] [Google Scholar]
- 42.Smaby J. M., Brockman H. L., Brown R. E. 1994. Cholesterol’s interfacial interactions with sphingomyelins and phosphatidylcholines: hydrocarbon chain structure determines the magnitude of condensation. Biochemistry. 33: 9135–9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Löfgren L., Ståhlman M., Forsberg G. B., Saarinen S., Nilsson R., Hansson G. I. 2012. The BUME method: a novel automated chloroform-free 96-well total lipid extraction method for blood plasma. J. Lipid Res. 53: 1690–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bagdade J. D., Ritter M. C., Davidson M., Subbaiah P. V. 1992. Effect of marine lipids on cholesterol ester transfer and lipoprotein composition in patients with hypercholesterolemia. Arterioscler. Thromb. 12: 1146–1152. [DOI] [PubMed] [Google Scholar]
- 45.Colhoun H. M., Otvos J. D., Rubens M. B., Taskinen M. R., Underwood S. R., Fuller J. H. 2002. Lipoprotein subclasses and particle sizes and their relationship with coronary artery calcification in men and women with and without type 1 diabetes. Diabetes. 51: 1949–1956. [DOI] [PubMed] [Google Scholar]
- 46.de Grooth G. J., Kuivenhoven J. A., Stalenhoef A. F. H., de Graaf J., Zwinderman A. H., Posma J. L., van Tol A., Kastelein J. J. P. 2002. Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans: a randomized phase II dose-response study. Circulation. 105: 2159–2165. [DOI] [PubMed] [Google Scholar]
- 47.Storey S. M., Gallegos A. M., Atshaves B. P., McIntosh A. L., Martin G. G., Parr R. D., Landrock K. K., Mer A. B., Ball J. M., Schroeder F. 2007. Selective cholesterol dynamics between lipoproteins and caveolae/lipid rafts. Biochemistry. 46: 13891–13906. [DOI] [PubMed] [Google Scholar]
- 48.Lange Y., Dalessandro J. S., Small D. M. 1979. Affinity of cholesterol for phosphatidylcholine and sphingomyelin. Biochim. Biophys. Acta. 556: 388–398. [DOI] [PubMed] [Google Scholar]
- 49.Cardoso R. M. S., Martins P. A. T., Gomes F., Doktorovova S., Vaz W. L. C., Moreno M. J. 2011. Chain-length dependence of insertion, desorption, and translocation of a homologous series of 7-nitrobenz-2-oxa-1,3-diazol-4-yl-labeled aliphatic amines in membranes. J. Phys. Chem. B. 115: 10098–10108. [DOI] [PubMed] [Google Scholar]
- 50.Cardoso R. M. S., Filipe H. A. L., Gomes F., Moreira N. D., Vaz W. L. C., Moreno M. J. 2010. Chain length effect on the binding of amphiphiles to serum albumin and to POPC bilayers. J. Phys. Chem. B. 114: 16337–16346. [DOI] [PubMed] [Google Scholar]
- 51.Sampaio J. L., Moreno M. J., Vaz W. L. C. 2005. Kinetics and thermodynamics of association of a fluorescent lysophospholipid derivative with lipid bilayers in liquid-ordered and liquid-disordered phases. Biophys. J. 88: 4064–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nestel P. J., Whyte H. M., Goodman D. S. 1969. Distribution and turnover of cholesterol in humans. J. Clin. Invest. 48: 982–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.