Abstract
Inflammatory activity is evident in patients with chronic kidney disease with limited data available in autosomal dominant polycystic kidney disease (ADPKD). We hypothesized that inflammation is an upstream event in the pathogenesis of ADPKD and may be a contributing factor in the disease severity and progression. Serum samples from 61 HALT study A group patients were compared with samples from 49 patients from HALT study B group with moderately advanced disease. Targeted MS analysis of bioactive lipid mediators as markers of inflammation was performed and correlated with eGFR and total kidney volume (TKV) normalized to the body surface area (BSAR) to assess if these markers are predictive of ADPKD severity. ADPKD patients with eGFR >60 ml/min/1.73 m2 showed higher levels of 5- and 12/15-lipoxygenase (LOX) and cyclooxygenase, and generated higher levels of hydroxy-octadecadienoic acids 9-HODE and 13-HODE and HETEs 8-HETE, 11-HETE, 12-HETE, and 15-HETE as compared with healthy subjects. Linear regression of 9-HODE and 13-HODE revealed a significant relationship with eGFR and TKV, while 15-HETE significantly correlated with TKV/BSAR. Production of 20-HETE, a P450-produced metabolite of arachidonic acid, was higher in ADPKD patients as compared with healthy subjects and significantly correlated with eGFR and TKV/BSAR. Perturbation in fatty acid metabolism is evident early in ADPKD patients, even in those with preserved kidney function. The identified LOX pathways may be potential therapeutic targets for slowing down ADPKD progression.
Keywords: autosomal dominant polycystic kidney disease, arachidonic acid, bioactive lipid biomarkers, liquid chromatography-mass spectrometry
CVD is the leading cause of premature mortality in autosomal dominant polycystic kidney disease (ADPKD), with >80% of deaths attributable to coronary artery disease (1). Recent evidence has implicated systemic vascular dysfunction and vascular inflammation in the pathogenesis of atherosclerotic CVD (2, 3), left ventricular hypertrophy (4–6), hypertension (7), and renal demise (8, 9) in patients with chronic kidney disease.
ADPKD patients also have an elevated augmentation index, a measure of systemic vascular function and ventricular-vascular coupling (10). There is a strong link between systemic vascular dysfunction and inflammation (11, 12) and patients with ADPKD have an activated pro-inflammatory phenotype (13, 14).
A previous study that utilized samples collected during the HALT Progression of Polycystic Kidney Disease (HALT-PKD) trial showed significantly increased serum levels of vascular inflammatory markers: intercellular adhesion molecule-1, vascular cell adhesion molecule-1, P-selectin, E-selectin, and soluble Fas in normotensive ADPKD patients with preserved renal function as compared with healthy controls (15, 16). Another HALT-ancillary study showed increased serum levels of pro-inflammatory C-reactive protein and IL-8 with inflammation exhibiting a graded relationship with levels of kidney function (17).
The involvement of inflammation in ADPKD has also been shown in studies in which statin therapy was able to increase renal blood flow and GFR and improve the endothelium-dependent vasodilation (18, 19). Although the underlying mechanisms are not well understood, presumably these reno-protective effects were mediated by statin-induced inhibition of G-proteins resulting in improvement of endothelial function (20, 21) and reduced vascular inflammation (22, 23).
Bioactive lipids are shown to play an important role in systematic homeostasis, pathogenesis of atherosclerosis, and renal-vascular pathology (24–27). For example, cytochrome P450 products of fatty acids demonstrate anti-inflammatory effects by preventing the activation of nuclear factor κB (28). Others, such as 20-HETE, display multiple biological activities including regulation of blood pressure (BP) and cardioprotection (29–32), while lipoxygenase (LOX) metabolites such as 12-HETE, 15-HETE, and 13-hydroxy-octadecadienoic acid (HODE) exhibit additional effects on cell proliferation and regulation of apoptosis (33).
We hypothesized that higher bioactive lipid markers of inflammation would be present and correlate with ADPKD severity as indicated by lower estimated glomerular filtration rate (eGFR) or higher total kidney volume (TKV). In addition, we aimed to investigate a relationship between the change in bioactive lipids and the occurrence of left ventricular hypertrophy as indicated through the change in left ventricular mass index (LVMI). To test our hypothesis, we analyzed a panel of LOX-derived lipid mediators in serum samples from a homogenous group of 61 ADPKD HALT study A patients that was considered an early disease group with an eGFR >60 ml/min/1.73 m2. These cross-sectional results were compared with those of samples from 49 HALT study B patients with moderately advanced disease as defined by a eGFR of 25–60 ml/min/1.73 m2. All samples were collected at baseline.
METHODS
Study population
The ongoing HALT-PKD trial (ClinicalTrials.gov identifier NCT00283686) consists of two concurrent randomized clinical trials designed to evaluate the effects of renin-angiotensin-aldosterone system (RAAS) suppression on the progression of ADPKD (12). Study A recruited patients with an eGFR >60 ml/min/1.73 m2, and study B recruited patients with an eGFR of 25–60 ml/min/1.73 m2. Age limits were 15 to 49 years for study A and 18 to 64 years for study B. Major exclusion criteria for both studies included renal vascular disease, diabetes, and history of severe heart failure.
Here we utilized baseline samples from 61 study A and 49 study B subjects collected at the University of Colorado, Anschutz Medical Campus site of the HALT-PKD trial. In addition, serum samples from 15 healthy subjects were collected on site [8 male (median age 37 years) and 7 female (median age 30 years)]. The subjects were not on BP control medication and had no history of kidney or heart disease.
In ADPKD patients, GFR was estimated using the four-variable modification of diet in renal disease equation (34). A trained nurse assessed BP using an automated oscillometric monitor (GE Healthcare, Waukesha, WI) following standard guidelines. Measurements were made in patients in a seated position after 5–10 min of quiet rest. In addition, ADPKD patients from the study A group underwent measurements of TKV and LVMI. The HALT protocol at the University of Colorado, Anschutz Medical Campus site was approved by the Colorado Multi-Institutional Review Board (University of Colorado). Patients gave their written informed consent. The study was conducted in full compliance with the International Conference on Harmonization (ICH) Harmonized Tripartite Guidelines for Good Clinical Practice (1996), the Declaration of Helsinki (version 11, October 2000), and all other applicable regulatory guidances. The use of blood samples for biomarker assay establishment was Colorado Multi-Institutional Review Board exempt.
Measurement of biomarkers
Analysis of bioactive lipid mediators.
Bioactive lipid mediators were analyzed using a multi-analyte LC-MS/MS assay. This assay was a modification of a previously described method (35).
Briefly, to 200 μl of serum, 800 μl methanol/ZnSO4 (70:30, v/v) protein precipitation solution containing the internal standards (2 ng/ml mixture of internal standards, see below) were added. Samples were vortexed for 10 min, centrifuged for 10 min at 13,000 g, and transferred into HPLC vials.
Fifty microliters of the supernatants were injected onto a 4.6 × 12.5 mm guard column (Eclipse XDB-C18, 5 μm; Agilent Technologies, Palo Alto, CA) and then back-flushed with 100% acetonitrile onto a 1.0 × 250 mm analytical column (Luna C18(2), 3 μm, 100 Å; Phenomenex, Torrance, CA). For HPLC separation, the starting mobile phase consisted of 65% acetonitrile/0.1% formic acid (9:91, v/v) and 35% acetonitrile with a flow of 0.2 ml/min for the first minute. After 2 min, the gradient consisted of 82% acetonitrile and was increased to 95% acetonitrile within 8 min. Acetonitrile was then held at 95% for 2 min. The column was reequilibrated for 2 min to starting conditions.
The API5000 mass spectrometer (AB Sciex, Concord, ON, Canada) was run in the positive ESI in the multiple reaction monitoring mode. The following hydroxy-fatty acids were quantified: 9-HODE, 13-HODE, 5-hydroxy-eicosapentaenoic acid (HEPE), 8-HEPE, 18-HEPE, 9-HEPE, 15-HEPE, 12-HEPE, 5-HETE, 8-HETE, 11-HETE, 15-HETE, 12-HETE, 20-HETE, 9-HETE, and 17-hydroxy-docosahexaenoic acid (17S-HDHA). All compounds including the internal standards (5-HETE-d8, 12-HETE-d8, 20-HETE-d6, 9-HODE-d4, and 13-HODE-d4) were purchased from Cayman Chemicals (Ann Arbor, MI). No chiral analysis of hydroxylated fatty acids was performed, thus no information about the enzymatic source can be provided within this study.
Statistical analyses
Descriptive statistics were calculated for each of the study groups (healthy controls, ADPKD eGFR >60 ml/min /1.73 m2, and ADPKD eGFR 25–60 ml/min/1.73 m2) including percentages for categorical data for approximately normally distributed continuous variables and geometric mean and 95% confidence interval for skewed variables. Arithmetic means ± SD were used to present results in all figures and for the comparison of group characteristics. For the results presented in Tables 1–3, biomarkers were ln transformed for regression analyses since the distributions were skewed. Linear regression was used to evaluate the relationship of each biomarker with eGFR, LVMI, and TKV normalized to the body surface area (BSAR) across studies A and B (estimates/beta coefficients ± SE are presented). Three models were run for each marker: 1) unadjusted; 2) adjusted for age and sex; and 3) adjusted for age, sex, systolic BP, and ln serum creatinine. t-Tests were used to compare differences in biomarker levels between ADPKD patient study groups; for comparison between healthy subjects and both ADPKD patient study groups, one-way ANOVA with Tukey post hoc testing was used. SPSS (Chicago, IL) 21.0 version and SAS 9.3 (Cary, NC) were used for the analyses. P < 0.05 was considered significant.
TABLE 1.
Baseline characteristics of the study groups based on their eGFR
| eGFR >60 ml/min/1.73 m2 | eGFR 25–60 ml/min/1.73 m2 | |
| Age (years) | 37.0 ± 7.8 | 47.1 ± 8.0 |
| Male (%) | 60.7 | 50.0 |
| Systolic BP (mm Hg) | 127.5 ± 13.0 | 132.0 ± 12.8 |
| Diastolic BP (mm Hg) | 74.5 ± 13.2 | 78.0 ± 9.6 |
| eGFR (ml/min/1.73 m2) | 82.4 ± 17.7 | 48.2 ± 8.4 |
| Urine creatinine (mg/ml) | 0.7 ± 0.4 | 0.6 ± 0.3 |
| Serum creatinine (mg/dl) | 1.0 ± 0.2 | 1.5 ± 0.3 |
| Urine microalbumin (mg/day) | 43.1 ± 93.0 | 53.1 ± 69.3 |
| BSAR (m2) | 2.0 ± 0.3 | 2.0 ± 0.2 |
| LV mass (g) | 127.2 ± 29.7 | ND |
| LVMI (g/m2) | 64.0 ± 13.4 | ND |
| TKV (ml) | 1,365 ± 702 | ND |
| TKV/BSAR (ml/m2) | 674 ± 333 | ND |
Data are presented as mean ± SD. BSAR, body surface area; eGFR, estimated glomerular filtration rate; LV, left ventricular; LVMI, LV mass index; ND, not determined; TKV, total kidney volume.
TABLE 3.
Regression analysis of TKV/BSAR and serum markers of inflammation
| Variable | Model | Estimate ± SE | P |
| 9-HODE | Unadjusted | 156.68 ± 68.17 | 0.0256 |
| Model 1 | 153.12 ± 68.98 | 0.0310 | |
| Model 2 | 140.11 ± 70.72 | 0.0533 | |
| 13-HODE | Unadjusted | 166.24 ± 70.60 | 0.0224 |
| Model 1 | 164.62 ± 71.24 | 0.0250 | |
| Model 2 | 149.40 ± 73.09 | 0.0464 | |
| 5-HETE | Unadjusted | 99.10 ± 55.92 | 0.0822 |
| Model 1 | 91.78 ± 56.79 | 0.1124 | |
| Model 2 | 76.16 ± 57.70 | 0.1931 | |
| 8-HETE | Unadjusted | 93.69 ± 49.72 | 0.0652 |
| Model 1 | 81.22 ± 51.66 | 0.1223 | |
| Model 2 | 70.11 ± 52.37 | 0.1871 | |
| 9-HETE | Unadjusted | 121.11 ± 50.69 | 0.0207 |
| Model 1 | 113.47 ± 52.51 | 0.0357 | |
| Model 2 | 103.16 ± 52.38 | 0.0549 | |
| 11-HETE | Unadjusted | 83.75 ± 49.18 | 0.0946 |
| Model 1 | 74.35 ± 50.12 | 0.1444 | |
| Model 2 | 62.65 ± 51.29 | 0.2280 | |
| 12-HETE | Unadjusted | 134.10 ± 68.16 | 0.0546 |
| Model 1 | 124.63 ± 68.43 | 0.0747 | |
| Model 2 | 98.80 ± 69.76 | 0.1633 | |
| 15-HETE | Unadjusted | 109.56 ± 51.93 | 0.0399 |
| Model 1 | 96.64 ± 53.24 | 0.0758 | |
| Model 2 | 84.24 ± 53.65 | 0.1232 | |
| 20-HETE | Unadjusted | 256.46 ± 55.92 | <0.0001 |
| Model 1 | 275.18 ± 59.84 | <0.0001 | |
| Model 2 | 248.61 ± 64.01 | 0.0003 | |
| 5-HEPE | Unadjusted | 29.59 ± 62.05 | 0.6354 |
| Model 1 | 27.84 ± 62.25 | 0.6566 | |
| Model 2 | 21.14 ± 64.28 | 0.7437 | |
| 8-HEPE | Unadjusted | 26.87 ± 53.99 | 0.6209 |
| Model 1 | 19.43 ± 54.84 | 0.7246 | |
| Model 2 | 18.82 ± 55.49 | 0.7360 | |
| 9-HEPE | Unadjusted | 22.32 ±± 51.18 | 0.6646 |
| Model 1 | 14.07 ± 52.22 | 0.7887 | |
| Model 2 | 10.97 ± 53.28 | 0.8377 | |
| 12-HEPE | Unadjusted | −58.30 ± 51.70 | 0.2646 |
| Model 1 | −52.63 ± 52.21 | 0.3183 | |
| Model 2 | −71.88 ± 51.93 | 0.1727 | |
| 15-HEPE | Unadjusted | −48.80 ± 57.10 | 0.3967 |
| Model 1 | −52.37 ± 56.55 | 0.3589 | |
| Model 2 | −83.99 ± 55.84 | 0.1393 | |
| 18-HEPE | Unadjusted | 31.53 ± 54.59 | 0.5661 |
| Model 1 | 31.71 ± 55.32 | 0.5691 | |
| Model 2 | 28.77 ± 56.07 | 0.6103 | |
| 17S-HDHA | Unadjusted | 124.44 ± 56.49 | 0.0323 |
| Model 1 | 116.47 ± 57.81 | 0.0496 | |
| Model 2 | 102.41 ± 60.02 | 0.0947 |
The three models in this table were tested as unadjusted; model 1, adjusted for age and sex; model 2, model 1 + systolic BP and ln serum creatinine. Data are presented as estimates (log of beta) ± SE. P < 0.05 was considered significant and is shown in bold. The variables were ln transformed prior to analysis.
RESULTS
Baseline characteristics by study group
Patients were eligible for study A or study B based upon their eGFR (36, 37). The mean age of all subjects was 42 years and 56% were male. The mean systolic/diastolic BP was 128/74 mm Hg in the ADPKD eGFR >60 ml/min/1.73 m2 group and 132/78 mm Hg in the ADPKD eGFR 25–60 ml/min/1.73 m2 group, suggesting that BP was well controlled in these patients and did not increase significantly during the 2 week drug washout period. During the washout period patients were on labetalol and/or clonidine medication. Baseline characteristics and their distribution in the ADPKD study populations are presented in Table 1.
Patients with an eGFR >60 ml/min/1.73 m2 were further classified into following subgroups based on their TKV: 15 (24.5%) with TKV of <800 ml, 28 (46.0%) with TKV of 800–1,500 ml, and 18 (29.5%) with TKV >1,500 ml.
Linear regression analysis of serum biomarkers with eGFR, LVMI, and TKV normalized to the BSAR
In cross-sectional unadjusted analyses of ADPKD eGFR >60 and eGFR 25–60 ml/min/1.73 m2 patient groups, linear regression analysis of eGFR and bioactive lipid mediators revealed significant correlations of eGFR with 5-HEPE, 15-HEPE, 9-HODE, 13-HODE, and 20-HETE (Table 2). Adjustments for age, sex, systolic BP, and ln serum creatinine did not reduce the number of significant correlations between eGFR and the above listed markers, except for 13-HODE (Table 2).
TABLE 2.
Regression analysis of eGFR and serum markers of inflammation
| Variable | Model | Estimate ± SE | P |
| 9-HODE | Unadjusted | −0.11 ± 0.03 | 0.0019 |
| Model 1 | −0.08 ± 0.03 | 0.0066 | |
| Model 2 | −0.08 ± 0.03 | 0.0068 | |
| 13-HODE | Unadjusted | −0.08 ± 0.04 | 0.0496 |
| Model 1 | −0.06 ± 0.03 | 0.0539 | |
| Model 2 | −0.06 ± 0.03 | 0.0547 | |
| 5-HETE | Unadjusted | −0.02 ± 0.04 | 0.6126 |
| Model 1 | −0.02 ± 0.03 | 0.4761 | |
| Model 2 | −0.02 ± 0.03 | 0.4752 | |
| 8-HETE | Unadjusted | −0.00 ± 0.03 | 0.9745 |
| Model 1 | −0.02 ± 0.03 | 0.4065 | |
| Model 2 | −0.02 ± 0.03 | 0.4076 | |
| 9-HETE | Unadjusted | 0.01 ± 0.03 | 0.8722 |
| Model 1 | 0.01 ± 0.03 | 0.5884 | |
| Model 2 | 0.01 ± 0.03 | 0.5910 | |
| 11-HETE | Unadjusted | −0.01 ± 0.03 | 0.8091 |
| Model 1 | −0.01 ± 0.03 | 0.5820 | |
| Model 2 | −0.01 ± 0.03 | 0.5825 | |
| 12-HETE | Unadjusted | 0.01 ± 0.04 | 0.7826 |
| Model 1 | 0.01 ± 0.03 | 0.8678 | |
| Model 2 | 0.01 ± 0.03 | 0.8684 | |
| 15-HETE | Unadjusted | −0.07 ± 0.04 | 0.0890 |
| Model 1 | −0.04 ± 0.03 | 0.1658 | |
| Model 2 | −0.04 ± 0.03 | 0.1681 | |
| 20-HETE | Unadjusted | −0.25 ± 0.04 | <0.0001 |
| Model 1 | −0.17 ± 0.03 | <0.0001 | |
| Model 2 | −0.18 ± 0.03 | <0.0001 | |
| 5-HEPE | Unadjusted | 0.11 ± 0.03 | 0.0002 |
| Model 1 | 0.07 ± 0.02 | 0.0031 | |
| Model 2 | 0.08 ± 0.02 | 0.0031 | |
| 8-HEPE | Unadjusted | 0.07 ± 0.04 | 0.0639 |
| Model 1 | 0.05 ± 0.03 | 0.0863 | |
| Model 2 | 0.05 ± 0.03 | 0.0917 | |
| 9-HEPE | Unadjusted | 0.03 ± 0.04 | 0.4286 |
| Model 1 | 0.03 ± 0.03 | 0.3051 | |
| Model 2 | 0.03 ± 0.03 | 0.3193 | |
| 12-HEPE | Unadjusted | 0.05 ± 0.04 | 0.2318 |
| Model 1 | 0.01 ± 0.04 | 0.7934 | |
| Model 2 | 0.01 ± 0.04 | 0.7885 | |
| 15-HEPE | Unadjusted | 0.15 ± 0.02 | <0.0001 |
| Model 1 | 0.11 ± 0.02 | <0.0001 | |
| Model 2 | 0.11 ± 0.02 | <0.0001 | |
| 18-HEPE | Unadjusted | −0.03 ± 0.03 | 0.3805 |
| Model 1 | −0.00 ± 0.03 | 0.9104 | |
| Model 2 | −0.00 ± 0.03 | 0.8931 | |
| 17S-HDHA | Unadjusted | 0.03 ± 0.04 | 0.5075 |
| Model 1 | −0.00 ± 0.04 | 0.9386 | |
| Model 2 | −0.01 ± 0.04 | 0.8784 |
The three models in this table were tested as unadjusted; model 1, adjusted for age and sex; and model 2, model 1 + systolic BP and ln serum creatinine. Data are presented as estimates (log of beta) ± SE. P < 0.05 was considered significant and is shown in bold. The variables were ln transformed prior to analysis.
In regards to the LVMI as the regression parameter, only 5-HETE showed a significant relationship when adjusted for age (P = 0.0208) and when adjusted for sex and age, sex systolic BP, and ln serum creatinine (P = 0.0243).
Linear regression of TKV normalized to the BSAR showed a significant relationship with 9-HODE, 13-HODE, 9-HETE, 15-HETE, 20-HETE, and 17S-HDHA (unadjusted, Table 3). When adjusted for age, sex, systolic BP, and ln serum creatinine, 13-HODE and 20-HETE remained significantly correlated (Table 3).
Statistical comparison of serum markers that significantly correlated with eGFR, LVMI, and/or TKV/BSAR between the study groups: metabolic fate of fatty acids
Metabolism by LOXs and cyclooxygenases.
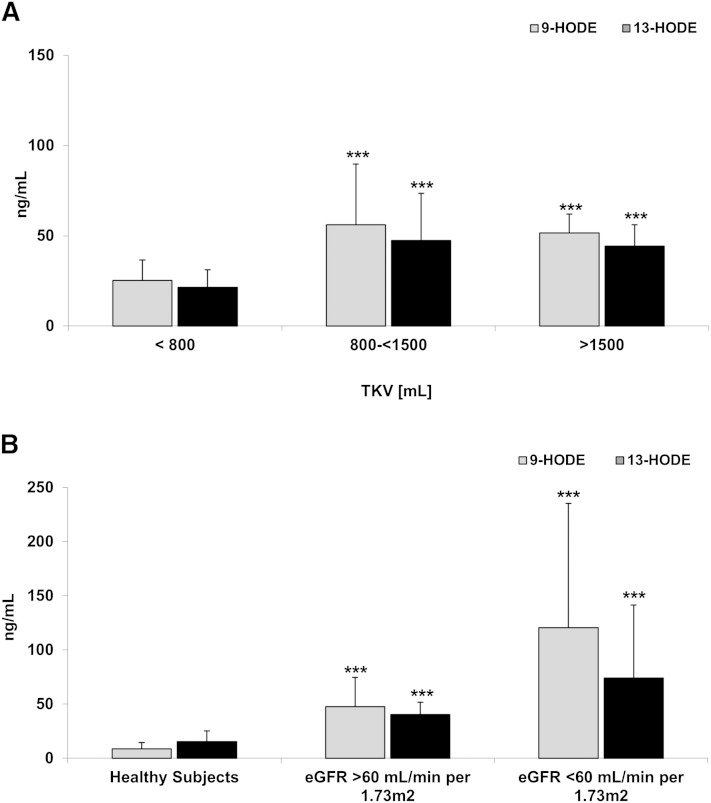
Concentrations of 9-HODE and 13-HODE, two linoleic acid (LA) metabolites generated by cyclooxygenase (COX) and 15-LOX/COX, respectively, were higher in patients with early ADPKD as compared with healthy subjects (Fig. 1B). In patients with more severe ADPKD, levels of 9-HODE and 13-HODE remained high, and even doubled in patients with an eGFR <60 ml/min/1.73 m2 (Fig. 1A).
Fig. 1.
Differences in 9-HODE and 13-HODE concentrations in serum of ADPKD adults with increasing TKV but normal eGFR >60 ml/min/1.73 m2 (A) and comparison between healthy subjects and ADPKD patients with normal and moderately reduced eGFR (B). Both markers were higher in patients with ADPKD as compared with healthy subjects. 9-HODE and 13-HODE significantly correlated with eGFR and TKV/BSAR. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 versus TKV <800 ml (A) and versus healthy subjects (B).
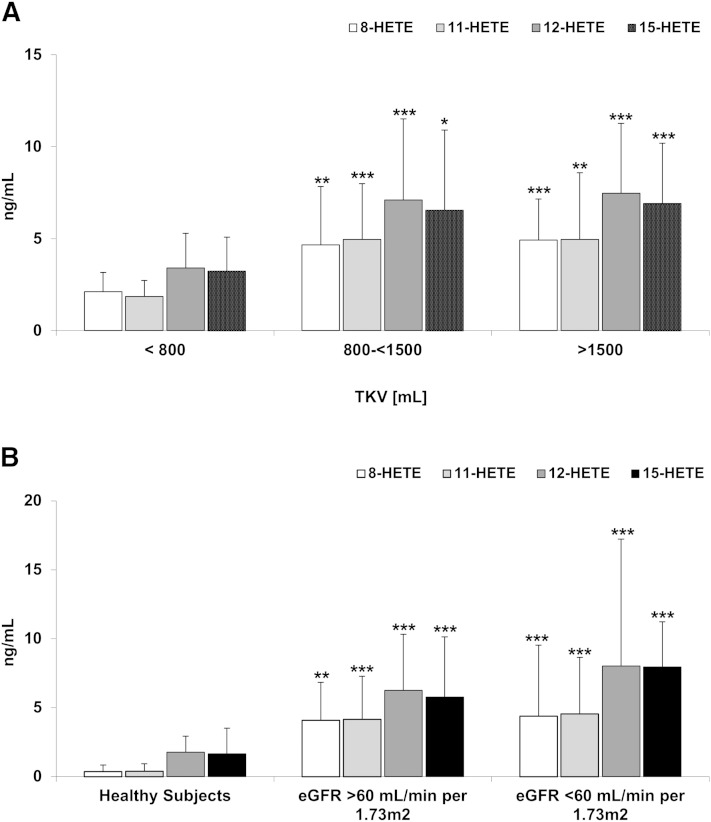
In terms of arachidonic acid (AA) metabolism, concentrations of 12/15-LOX metabolites 8-HETE, 12-HETE, and 15-HETE, as well as COX metabolite 11-HETE, were higher in patients with TKVs above 800 ml (and an eGFR >60 ml/min/1.73 m2), as well as in patients with an eGFR of 25–60 ml/min/1.73 m2 when compared with ADPKD patients with a TKV <800 ml (and preserved eGFR) (Fig. 2A). However, except for 15-HETE, no significant difference between the HALT study A and HALT study B patients were observed (Fig. 2B), suggesting an effect more dependent on cyst growth and less on kidney function. Surprisingly, while showing a significant relationship with LVMI, serum concentrations of 5-HETE, a 5-LOX metabolite, remained unchanged in patients with higher TKV and also did not change in patients with lower eGFR.
Fig. 2.
Differences in HETEs as products of COX (11-HETE) and 12/15-LOX-mediated metabolism of AA in serum of ADPKD adults with increasing TKV but normal eGFR >60 ml/min/1.73 m2 (A) and comparison between healthy subjects and ADPKD patients with normal and moderately reduced eGFR (B). 8-HETE, 11-HETE, 12-HETE, and 15-HETE were elevated in ADPKD patients with early disease as compared with healthy subjects. However, except for 15-HETE, there was no significant difference between the HALT study A and HALT study B patients suggesting a threshold effect independent of renal function. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 versus TKV <800 ml (A) and versus healthy subjects (B).
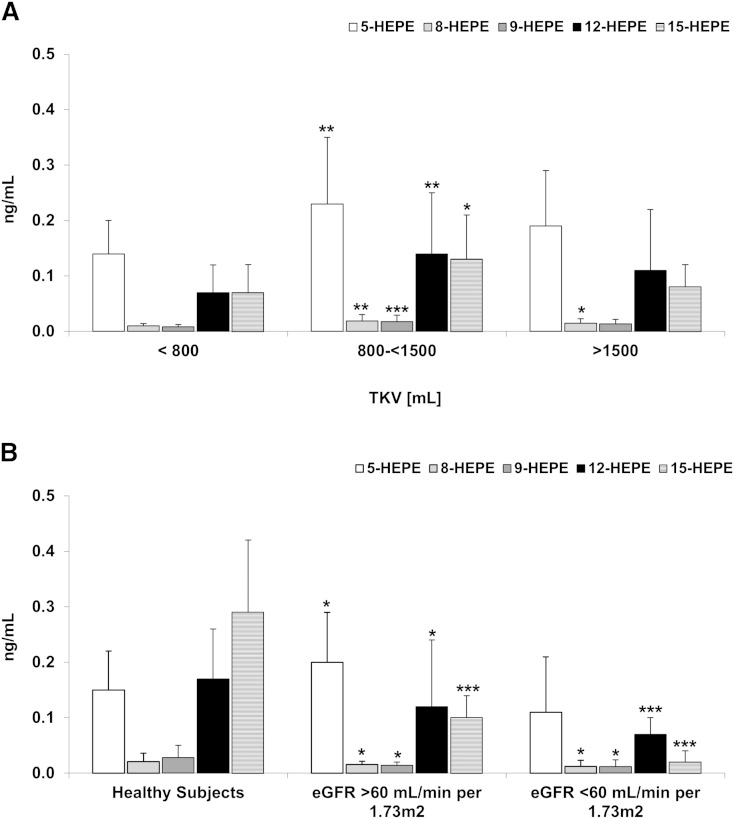
5-LOX and 12/15-LOX are responsible for metabolism of EPA to 5-HEPE, 12-HEPE, and 15-HEPE, respectively. Interestingly, the HEPE compounds were higher in patients with a TKV of 800–<1,500 ml compared with those with a TKV of <800 ml (Fig. 3A). However, as the TKV increased above 1,500 ml, the HEPE compounds returned to levels comparable to patients with a TKV <800 ml (Fig. 3A). This observed initial increase of the serum levels of HEPE compounds was also responsible for the observed difference between HALT study A and HALT study B patients, with 5-HEPE and 15-HEPE significantly lower in ADPKD patients with impaired renal function (eGFR of <60 ml/min/1.73 m2) (Fig. 3B, Table 4).
Fig. 3.
Differences in HEPEs as products of 12/15-LOXs in serum of ADPKD adults with increasing TKV but normal eGFR >60 ml/min/1.73 m2 (A) and comparison between healthy subjects and ADPKD patients with normal and moderately reduced eGFR (B). HEPEs with potential anti-inflammatory properties were initially higher in patients with higher TKV. However, as the disease progressed and the kidney function declined, so did the concentrations of 5-, 8-, 12-, and 15-HEPE. These were significantly lower in the patients with an eGFR <60 ml/min/1.73 m2 as compared with those with a normal eGFR of >60 ml/min/1.73 m2. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 versus TKV <800 ml (A) and versus healthy subjects (B).
TABLE 4.
Comparison of serum biomarker concentrations in HALT A and HALT B ADPKD patients
| Variable | HALT A (eGFR >60 ml/min/1.73 m2) | HALT B (eGFR <60 ml/min/1.73 m2) | P |
| 9-HODE (ng/ml) | 47.58 ± 27.07 | 120.47 ± 114.89 | <0.0001 |
| 13-HODE (ng/ml) | 40.41 ± 11.36 | 74.20 ± 67.36 | 0.0014 |
| 5-HETE (ng/ml) | 6.67 ± 3.66 | 7.50 ± 4.71 | 0.3459 |
| 8-HETE (ng/ml) | 4.09 ± 2.31 | 4.39 ± 5.16 | 0.7150 |
| 9-HETE (ng/ml) | 4.20 ± 2.46 | 4.74 ± 4.61 | 0.4951 |
| 11-HETE (ng/ml) | 4.16 ± 3.78 | 4.55 ± 4.10 | 0.5821 |
| 12-HETE (ng/ml) | 6.26 ± 3.98 | 8.04 ± 9.19 | 0.2147 |
| 15-HETE (ng/ml) | 5.78 ± 3.47 | 7.96 ± 5.87 | 0.0363 |
| 20-HETE (ng/ml) | 0.42 ± 0.25 | 0.90 ± 0.21 | <0.0001 |
| 5-HEPE (ng/ml) | 0.20 ± 0.09 | 0.11 ± 0.10 | <0.0001 |
| 8-HEPE (ng/ml) | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.0621 |
| 9-HEPE (ng/ml) | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.3980 |
| 12-HEPE (ng/ml) | 0.12 ± 0.12 | 0.07 ± 0.03 | 0.0009 |
| 15-HEPE (ng/ml) | 0.10 ± 0.04 | 0.02 ± 0.02 | <0.0001 |
| 18-HEPE (ng/ml) | 0.04 ± 0.02 | 0.06 ± 0.07 | 0.1943 |
| 17S-HDHA (ng/ml) | 0.40 ± 0.28 | 0.27 ± 0.14 | 0.0039 |
HALT A (eGFR >60 ml/min/1.73 m2 group, n = 61) and HALT B (eGFR 25–60 ml/min/1.73 m2, n = 50). Data are presented as mean ± SD. P < 0.05 was considered significant and is shown in bold.
DHA can be, via 17S-HDHA, converted to 17S-resolvins and protectins, while P450 enzymes contribute to resolvin E1 biosynthesis by converting EPA to 18-HEPE. Interestingly, in the ADPKD patients with normal kidney function, higher concentrations of 18-HEPE and 17S-HDHA were noted with higher TKV. 17S-HDHA was 0.22 ± 0.13 ng/ml in patients with TKV <800 ml, 0.48 ± 0.31 ng/ml (P < 0.001) in patients with TKV 800–1,500 ml, and 0.44 ± 0.26 ng/ml (P < 0.01) in patients with TKV >1,500 ml. However in the case of reduced eGFR, the levels of 17S-HDHA were also reduced to 0.27 ± 0.14 ng/ml in patients with eGFR of <60 ml/min/1.73 m2 (P < 0.005). 18-HEPE followed a similar pattern with higher levels in patients with higher TKV.
Metabolism by P450 enzymes.
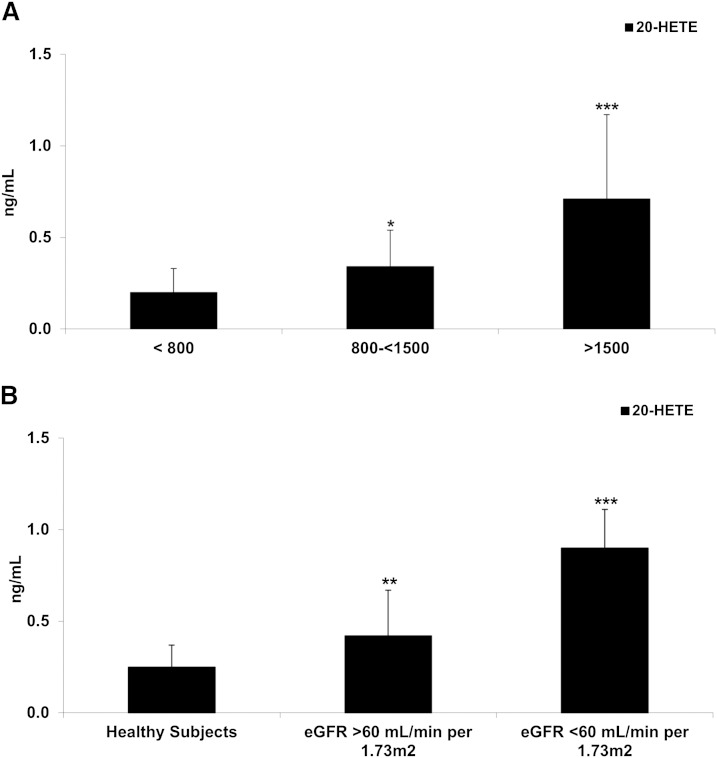
20-HETE, a product of AA metabolism by P450 enzymes, significantly correlated with both eGFR and TKV. The levels of 20-HETE were also already higher in the ADPKD patients with normal eGFR and TKV >1,500 ml (Fig. 4A) and further increased with diminishing kidney function (Fig. 4B).
Fig. 4.
Differences in 20-HETE concentration in serum of ADPKD adults with increasing TKV but normal eGFR >60 ml/min/1.73 m2 (A) and comparison between healthy subjects and ADPKD patients with normal and moderately reduced eGFR (B). 20-HETE is produced from AA by P450 enzymes, and was found higher in patients with high TKV and/or lower eGFR. Data are presented as mean ± SD. *P < 0.05 versus TKV <800 ml (A) and versus healthy subjects (B).
DISCUSSION
Phospholipases (PLA2) mediate the release of fatty acids from membrane phospholipids, including LA, AA, and EPA (38). These fatty acids can be further metabolized by LOX, COX, or P450 enzymes (39, 40). LA is oxidized by 15-LOX and COX into 13-HODE (41–43), and by COX into 9-HODE (41). AA can be metabolized by COX enzymes into prostaglandins (PGs) and 11-HETE; through 5-LOX and 12/15-LOX enzymes into HETE compounds: 5-HETE, 12-HETE, 15-HETE, and 8-HETE (44–47); and by P450 enzymes to 20-HETE (48, 49). Lastly, EPA is metabolized into HEPE compounds, mainly 12-HEPE and 15-HEPE (31, 50).
It is widely recognized that HODE/HETE/HEPE metabolites have important physiological as well as key pathological functions that modulate ion transport, renal and pulmonary functions, vascular tone and reactivity, and inflammatory and growth responses. Recent reviews have summarized the effects of the fatty acid metabolites in vascular homeostasis, renal vasculature, and tubule-glomerular feedback (48, 49, 51, 52). Due to the recognized importance of lipid signaling in renal diseases, we focused on the changes of lipid mediators as a result of severity of ADPKD.
In the serum samples from the same group of HALT patients, we could show that COX-mediated AA metabolites such as PGE2, PGF2α, and the AA-derivative 8-isoprostane increase in serum of ADPKD patients with increasing TKV and decreasing eGFR.
In the present study, we showed that the severity of ADPKD is accompanied with significant changes in the LOX-, COX-, and P450-mediated eicosanoid synthesis (Tables 4, 5). The results suggested higher activities of LA- and AA-converting LOXs and P450 enzymes in ADPKD patients with reduced eGFR and/or higher TKV, resulting in higher levels of 9-HODE, 13-HODE, 12-HETE, 15-HETE, and 20-HETE compounds. These lipids are pro-inflammatory and involved in the regulation of angiotensin II (Ang II), angiogenesis, and various cell signaling and cell proliferation pathways as briefly described below.
TABLE 5.
Summary of identified marker changes
| Marker | Directional Change in ADPKD | Signaling Pathway | Relationship With |
| 9-HODE and 13-HODE | Increase | LA metabolism by COXs (9-HODE) and LOXs | eGFR, TKV/BSAR |
| 8-HETE, 11-HETE, 12-HETE, and 15-HETE | Increase | AA metabolism by LOXs and COXs (11-HETE) | 15-HETE with TKV/BSAR |
| 20-HETE | Increase | AA metabolism by P450 enzymes | eGFR, TKV/BSAR |
| 5-HEPE and 15-HEPE | Decrease | EPA metabolism by lipoxygenases | eGFR |
| 17S-HDHA | Increase | Oxidation of docosahexaenoic acid in resolvin and protectin synthesis | TKV/BSAR |
Based on the results of the present study, a combinatorial biomarker is proposed that will require further clinical qualification as a prognostic tool for severity and progression of ADPKD.
Interestingly, the only eicosanoid that showed a significant relationship with LVMI was 5-HETE. With 5-LOX implicated in myocardial infarction and heart disease (53, 54), and with 5-HETE shown to increase under ischemic conditions in the heart (55), its clinical relevance in patients with ADPKD deserves further attention.
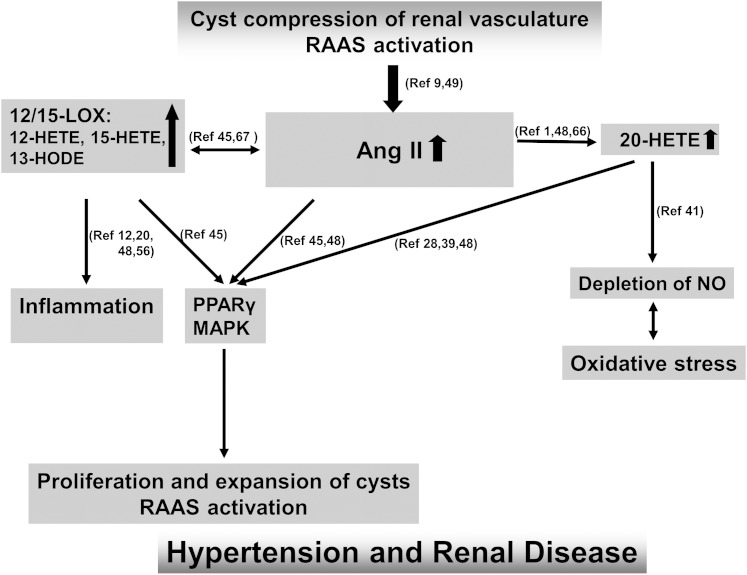
The role of RAAS as a major contributor to cyst growth in both animals and humans with ADPKD (56, 57) is well established, and thus our finding that ADPKD patients have higher serum levels of 12- and 15-LOX metabolites 13-HODE [in accordance with (58)], 12-HETE, and 15-HETE, is of potential clinical relevance. These LOX metabolites have been shown to directly function as key mediators of Ang II-induced renin inhibition (59, 60). Their increase in patients with ADPKD further accelerates Ang II-mediated oxidative stress, vascular inflammation, cell proliferation, and endothelial dysfunction (61–63), leading to an uncontrolled vicious cycle and progression of the disease (Fig. 5). In addition, 12-HETE has been shown to activate p38 MAPK (60), suggesting a novel interaction between 12-LOX and p38 MAPK that ultimately leads to mesangial cell matrix synthesis.
Fig. 5.
Mechanistic hypothesis based on the published data and the results of the present study. A vicious cycle is created in which renal cysts activate the RAAS, and the resulting increase in Ang II leads to oxidative stress, vascular inflammation, and endothelial dysfunction. AngII, angiotensin II; HODE, hydroxy-octadecadienoic acid; LOX, lipoxygenases; RAAS, renin-angiotensin-aldosterone system.
12-HETE and 15-HETE promote cancer development via their involvement in the stimulation of angiogenesis (64–66). Angiogenesis, and thus also 12/15-LOX, is an important potential therapeutic target in ADPKD, because this process may be necessary for the cysts to grow and may be responsible for increased vascular permeability facilitating fluid secretion into the cysts (67, 68). Recent findings from our group in children with ADPKD have identified the role angiogenesis plays in the development of the disease (68), thus providing a new therapeutic target in early stages of ADPKD, where therapy would be most beneficial.
5-LOX and 12/15-LOX can also metabolize the n-3 fatty acid EPA to 5-HEPE, 12-HEPE, and 15-HEPE, respectively. The EPA-derived lipid mediators have much less inflammatory potency than the corresponding AA-derived eicosanoids (69). The EPA-derived HEPE metabolites even have anti-inflammatory properties (70, 71). Interestingly, we observed a bell-shaped concentration distribution of HEPE compounds, with these compounds being higher in patients with TKVs of 800–1,500 ml than in those with TKVs <800 ml or TKVs above 1,500 ml. It can be speculated that HEPEs are involved in a compensatory effect in the early stages of the cyst growth and renal volume increase, and before a change in renal function occurs. However, this effect levels out as the disease progresses, with the levels of these potentially anti-inflammatory compounds, 5-HEPE and 15-HEPE, being lower in patients with reduced eGFR.
20-HETE was another AA-derived metabolite that was elevated in the serum of ADPKD patients and that correlated with TKV/BSAR as well as reduced eGFR. Acute and chronic elevations in circulating Ang II levels increase 20-HETE formation in the kidney and peripheral vasculature, and this may contribute to the pressor effect of Ang II (48, 72). As 20-HETE is a potent vasoconstrictor and PPAR activator (72, 73), it seems reasonable to expect that inhibition of its formation may provide an effective mechanism in reducing cyst cell proliferation while attenuating the Ang II production in ADPKD patients (74). In addition, inhibition of NO synthase by L-nitroarginine methylester augmented the conversion of AA to 20-HETE (75), indicating an involvement of 20-HETE in the regulation of NO production (76). Interestingly, in the same ADPKD patients that showed increased serum levels of 20-HETE, we also observed increased levels of asymmetric dimethylarginine, an endothelial dysfunction marker and NO synthase inhibitor.
DHA and EPA can be metabolized to resolvins and protectins via 17S-HDHA and 18-HEPE (70). Both compounds possess protective, pro-resolving, anti-inflammatory, and antifibrotic actions (77). Interestingly, an increase of both protective compounds in ADPKD patients with slightly reduced eGFR was noted in patients with larger TKV. While 18-HEPE returned to the initial levels, 17S-HDHA remained elevated in the patients with a TKV of >2,500 ml. In patients with lower eGFR, there was no change in 18-HEPE serum concentrations, whereas 17S-HDHA further decreased. These results suggest that growth of renal cysts in early ADPKD is accompanied with the rise of the pro-inflammatory as well as anti-inflammatory processes; however as the renal function diminishes, the pro-inflammatory processes seem to prevail.
Limitations
With 61 patients divided into three groups based on their TKV/BSAR, performing linear regression analysis with multiple adjustments leaves a risk of type I and type II statistical errors. All samples collected at the University of Colorado Denver site of the HALT-PKD clinical trial were utilized for the study, without any pre-selection process.
In summary, ADPKD patients have dysregulated metabolism of bioactive lipids (Table 5, Fig. 5). An increased synthesis of pro-inflammatory and angiogenic markers derived from metabolism of n-3 and n-6 acids by COX, LOX, and P450 enzymes was observed in patients with impaired eGFR as compared with those ADPKD patients with normal renal function. However, our study was designed as an exploratory biomarker discovery study and it cannot be completely excluded that our results are subject to residual confounders for which adjustments were not made in this study. The next step is to further qualify the markers identified in this study in statistically adequately powered prospective studies, as well as to establish the specificity of the markers by comparison with chronic kidney disease patients.
Our study, however, not only provides a critical proof-of-concept, but also provides important information for the design of future clinical intervention and biomarker qualification studies.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- ADPKD
- autosomal dominant polycystic kidney disease
- Ang
- angiotensin
- BP
- blood pressure
- BSAR
- body surface area
- COX
- cyclooxygenase
- eGFR
- estimated glomerular filtration rate
- HALT-PKD
- HALT Progression of Polycystic Kidney Disease trial
- HEPE
- hydroxy-eicosapentaenoic acid
- HODE
- hydroxy-octadecadienoic acid
- LA
- linoleic acid
- LOX
- lipoxygenase
- LVMI
- left ventricular mass index
- PG
- prostaglandin
- RAAS
- renin-angiotensin-aldosterone system
- 17S-HDHA
- 17-hydroxy-docosahexaenoic acid
- TKV
- total kidney volume
This study was funded by National Institutes of Health Grant U01 DK062402 and University of Colorado iC42 Clinical Research and Development Service Center. The study was also supported in part by National Institutes of Health/National Center for Research Resources Colorado CTSI Grant UL1 RR025780. The authors do not have any conflicting financial interests to disclose.
REFERENCES
- 1.Perrone R. D., Ruthazer R., Terrin N. C. 2001. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. Am. J. Kidney Dis. 38: 777–784. [DOI] [PubMed] [Google Scholar]
- 2.Filiopoulos V., Vlassopoulos D. 2009. Inflammatory syndrome in chronic kidney disease: pathogenesis and influence on outcomes. Inflamm. Allergy Drug Targets. 8: 369–382. [DOI] [PubMed] [Google Scholar]
- 3.Small D. M., Coombes J. S., Bennett N., Johnson D. W., Gobe G. C. 2012. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton). 17: 311–321. [DOI] [PubMed] [Google Scholar]
- 4.Perrone R. D., Abebe K. Z., Schrier R. W., Chapman A. B., Torres V. E., Bost J., Kaya D., Miskulin D. C., Steinman T. I., Braun W., et al. 2011. Cardiac magnetic resonance assessment of left ventricular mass in autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 6: 2508–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardají A., Martinez-Vea A., Valero A., Gutierrez C., Garcia C., Ridao C., Oliver J. A., Richart C. 2001. Cardiac involvement in autosomal-dominant polycystic kidney disease: a hypertensive heart disease. Clin. Nephrol. 56: 211–220. [PubMed] [Google Scholar]
- 6.Alam A., Perrone R. D. 2013. Left ventricular hypertrophy in ADPKD: changing demographics. Curr. Hypertens. Rev. 9: 27–31. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell G. F., Wang N., Palmisano J. N., Larson M. G., Hamburg N. M., Vita J. A., Levy D., Benjamin E. J., Vasan R. S. 2010. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 122: 1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomiyama H., Tanaka H., Hashimoto H., Matsumoto C., Odaira M., Yamada J., Yoshida M., Shiina K., Nagata M., Yamashina A. 2010. Arterial stiffness and declines in individuals with normal renal function/early chronic kidney disease. Atherosclerosis. 212: 345–350. [DOI] [PubMed] [Google Scholar]
- 9.Tonelli M., Sacks F., Pfeffer M., Jhangri G. S., Curhan G. 2005. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 68: 237–245. [DOI] [PubMed] [Google Scholar]
- 10.Borresen M. L., Wang D., Strandgaard S. 2007. Pulse wave reflection is amplified in normotensive patients with autosomal-dominant polycystic kidney disease and normal renal function. Am. J. Nephrol. 27: 240–246. [DOI] [PubMed] [Google Scholar]
- 11.Lieb W., Larson M. G., Benjamin E. J., Yin X., Tofler G. H., Selhub J., Jacques P. F., Wang T. J., Vita J. A., Levy D., et al. 2009. Multimarker approach to evaluate correlates of vascular stiffness: the Framingham Heart Study. Circulation. 119: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnabel R., Larson M. G., Dupuis J., Lunetta K. L., Lipinska I., Meigs J. B., Yin X., Rong J., Vita J. A., Newton-Cheh C., et al. 2008. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 51: 1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merta M., Tesar V., Zima T., Jirsa M., Rysava R., Zabka J. 1997. Cytokine profile in autosomal dominant polycystic kidney disease. Biochem. Mol. Biol. Int. 41: 619–624. [DOI] [PubMed] [Google Scholar]
- 14.Park E. Y., Seo M. J., Park J. H. 2010. Effects of specific genes activating RAGE on polycystic kidney disease. Am. J. Nephrol. 32: 169–178. [DOI] [PubMed] [Google Scholar]
- 15.Heffernan K. S., Patvardhan E. A., Hession M., Ruan J., Karas R. H., Kuvin J. T. 2010. Elevated augmentation index derived from peripheral arterial tonometry is associated with abnormal ventricular-vascular coupling. Clin. Physiol. Funct. Imaging. 30: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heffernan K. S., Kuvin J. T., Sarnak M. J., Perrone R. D., Miskulin D. C., Rudym D., Chandra P., Karas R. H., Menon V. 2011. Peripheral augmentation index and vascular inflammation in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 26: 2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menon V., Rudym D., Chandra P., Miskulin D., Perrone R., Sarnak M. 2011. Inflammation, oxidative stress, and insulin resistance in polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 6: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dijk M. A., Kamper A. M., van Veen S., Souverijn J. H., Blauw G. J. 2001. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 16: 2152–2157. [DOI] [PubMed] [Google Scholar]
- 19.Namli S., Oflaz H., Turgut F., Alisir S., Tufan F., Ucar A., Mercanoglu F., Ecder T. 2007. Improvement of endothelial dysfunction with simvastatin in patients with autosomal dominant polycystic kidney disease. Ren. Fail. 29: 55–59. [DOI] [PubMed] [Google Scholar]
- 20.Harris M. B., Blackstone M. A., Sood S. G., Li C., Goolsby J. M., Venema V. J., Kemp B. E., Venema R. C. 2004. Acute activation and phosphorylation of endothelial nitric oxide synthase by HMG-CoA reductase inhibitors. Am. J. Physiol. Heart Circ. Physiol. 287: H560–H566. [DOI] [PubMed] [Google Scholar]
- 21.Hernández-Perera O., Pérez-Sala D., Navarro-Antolín J., Sánchez-Pascuala R., Hernández G., Díaz C., Lamas S. 1998. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J. Clin. Invest. 101: 2711–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulhaupt F., Matter C. M., Kwak B. R., Pelli G., Veillard N. R., Burger F., Graber P., Luscher T. F., Mach F. 2003. Statins (HMG-CoA reductase inhibitors) reduce CD40 expression in human vascular cells. Cardiovasc. Res. 59: 755–766. [DOI] [PubMed] [Google Scholar]
- 23.Rezaie-Majd A., Prager G. W., Bucek R. A., Schernthaner G. H., Maca T., Kress H. G., Valent P., Binder B. R., Minar E., Baghestanian M. 2003. Simvastatin reduces the expression of adhesion molecules in circulating monocytes from hypercholesterolemic patients. Arterioscler. Thromb. Vasc. Biol. 23: 397–403. [DOI] [PubMed] [Google Scholar]
- 24.Dailey L. A., Imming P. 1999. 12-Lipoxygenase: classification, possible therapeutic benefits from inhibition, and inhibitors. Curr. Med. Chem. 6: 389–398. [PubMed] [Google Scholar]
- 25.Wu X. C., Richards N. T., Michael J., Johns E. 1994. Relative roles of nitric oxide and cyclo-oxygenase and lipoxygenase products of arachidonic acid in the contractile responses of rat renal arcuate arteries. Br. J. Pharmacol. 112: 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao L., Funk C. D. 2004. Lipoxygenase pathways in atherogenesis. Trends Cardiovasc. Med. 14: 191–195. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y. S., Xu Z. G., Reddy M. A., Li S. L., Lanting L., Sharma K., Adler S. G., Natarajan R. 2005. Novel interactions between TGF-{beta}1 actions and the 12/15-lipoxygenase pathway in mesangial cells. J. Am. Soc. Nephrol. 16: 352–362. [DOI] [PubMed] [Google Scholar]
- 28.Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D. C., Liao J. K. 1999. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 285: 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serhan C. N., Savill J. 2005. Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6: 1191–1197. [DOI] [PubMed] [Google Scholar]
- 30.Imig J. D. 2005. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am. J. Physiol. Renal Physiol. 289: F496–F503. [DOI] [PubMed] [Google Scholar]
- 31.Imig J. D. 2006. Eicosanoids and renal vascular function in diseases. Clin. Sci. (Lond.). 111: 21–34. [DOI] [PubMed] [Google Scholar]
- 32.Sarkis A., Lopez B., Roman R. J. 2004. Role of 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids in hypertension. Curr. Opin. Nephrol. Hypertens. 13: 205–214. [DOI] [PubMed] [Google Scholar]
- 33.Shureiqi I., Lippman S. M. 2001. Lipoxygenase modulation to reverse carcinogenesis. Cancer Res. 61: 6307–6312. [PubMed] [Google Scholar]
- 34.Levey A. S., Bosch J. P., Lewis J. B., Greene T., Rogers N., Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 35.Masoodi M., Mir A. A., Petasis N. A., Serhan C. N., Nicolaou A. 2008. Simultaneous lipidomic analysis of three families of bioactive lipid mediators leukotrienes, resolvins, protectins and related hydroxy-fatty acids by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 22: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman A. B., Torres V. E., Perrone R. D., Steinman T. I., Bae K. T., Miller J. P., Miskulin D. C., Rahbari Oskoui F., Masoumi A., Hogan M. C., et al. 2010. The HALT polycystic kidney disease trials: design and implementation. Clin. J. Am. Soc. Nephrol. 5: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres V. E., Chapman A. B., Perrone R. D., Bae K. T., Abebe K. Z., Bost J. E., Miskulin D. C., Steinman T. I., Braun W. E., Winklhofer F. T., et al. 2012. Analysis of baseline parameters in the HALT polycystic kidney disease trials. Kidney Int. 81: 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Funk C. D. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294: 1871–1875. [DOI] [PubMed] [Google Scholar]
- 39.Massey K. A., Nicolaou A. 2011. Lipidomics of polyunsaturated-fatty-acid-derived oxygenated metabolites. Biochem. Soc. Trans. 39: 1240–1246. [DOI] [PubMed] [Google Scholar]
- 40.Medhora M., Dhanasekaran A., Gruenloh S. K., Dunn L. K., Gabrilovich M., Falck J. R., Harder D. R., Jacobs E. R., Pratt P. F. 2007. Emerging mechanisms for growth and protection of the vasculature by cytochrome P450-derived products of arachidonic acid and other eicosanoids. Prostaglandins Other Lipid Mediat. 82: 19–29. [DOI] [PubMed] [Google Scholar]
- 41.Baer A. N., Costello P. B., Green F. A. 1991. Stereospecificity of the hydroxyeicosatetraenoic and hydroxyoctadecadienoic acids produced by cultured bovine endothelial cells. Biochim. Biophys. Acta. 1085: 45–52. [DOI] [PubMed] [Google Scholar]
- 42.Kühn H. 1996. Biosynthesis, metabolization and biological importance of the primary 15-lipoxygenase metabolites 15-hydro(pero)XY-5Z,8Z,11Z,13E-eicosatetraenoic acid and 13-hydro(pero)XY-9Z,11E-octadecadienoic acid. Prog. Lipid Res. 35: 203–226. [DOI] [PubMed] [Google Scholar]
- 43.Fang X., Kaduce T. L., Spector A. A. 1999. 13-(S)-hydroxyoctadecadienoic acid (13-HODE) incorporation and conversion to novel products by endothelial cells. J. Lipid Res. 40: 699–707. [PubMed] [Google Scholar]
- 44.Kuhn H. 2005. Biologic relevance of lipoxygenase isoforms in atherogenesis. Expert Rev. Cardiovasc. Ther. 3: 1099–1110. [DOI] [PubMed] [Google Scholar]
- 45.Dobrian A. D., Lieb D. C., Cole B. K., Taylor-Fishwick D. A., Chakrabarti S. K., Nadler J. L. 2011. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog. Lipid Res. 50: 115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camara N. O., Martins J. O., Landgraf R. G., Jancar S. 2009. Emerging roles for eicosanoids in renal diseases. Curr. Opin. Nephrol. Hypertens. 18: 21–27. [DOI] [PubMed] [Google Scholar]
- 47.Hao C. M., Breyer M. D. 2007. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 71: 1105–1115. [DOI] [PubMed] [Google Scholar]
- 48.Roman R. J. 2002. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 82: 131–185. [DOI] [PubMed] [Google Scholar]
- 49.Hoagland K. M., Maier K. G., Moreno C., Yu M., Roman R. J. 2001. Cytochrome P450 metabolites of arachidonic acid: novel regulators of renal function. Nephrol. Dial. Transplant. 16: 2283–2285. [DOI] [PubMed] [Google Scholar]
- 50.Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., Gronert K. 2000. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192: 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theken K. N., Schuck R. N., Edin M. L., Tran B., Ellis K., Bass A., Lih F. B., Tomer K. B., Poloyac S. M., Wu M. C., et al. 2012. Evaluation of cytochrome P450-derived eicosanoids in humans with stable atherosclerotic cardiovascular disease. Atherosclerosis. 222: 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleming I. 2001. Cytochrome p450 and vascular homeostasis. Circ. Res. 89: 753–762. [DOI] [PubMed] [Google Scholar]
- 53.Topol E. J., Smith J., Plow E. F., Wang Q. K. 2006. Genetic susceptibility to myocardial infarction and coronary artery disease. Hum. Mol. Genet. 15(Spec No 2): R117–R123. [DOI] [PubMed] [Google Scholar]
- 54.Listì F., Caruso M., Incalcaterra E., Hoffmann E., Caimi G., Balistreri C. R., Vasto S., Scafidi V., Caruso C., Candore G. 2008. Pro-inflammatory gene variants in myocardial infarction and longevity: implications for pharmacogenomics. Curr. Pharm. Des. 14: 2678–2685. [DOI] [PubMed] [Google Scholar]
- 55.Hughes H., Gentry D. L., McGuire G. M., Taylor A. A. 1991. Gas chromatographic-mass spectrometric analysis of lipoxygenase products in post-ischemic rabbit myocardium. Prostaglandins Leukot. Essent. Fatty Acids. 42: 225–231. [DOI] [PubMed] [Google Scholar]
- 56.Barrett B. J., Foley R., Morgan J., Hefferton D., Parfrey P. 1994. Differences in hormonal and renal vascular responses between normotensive patients with autosomal dominant polycystic kidney disease and unaffected family members. Kidney Int. 46: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 57.Chapman A. B., Johnson A., Gabow P. A., Schrier R. W. 1990. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N. Engl. J. Med. 323: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 58.Wang D., Strandgaard S., Borresen M. L., Luo Z., Connors S. G., Yan Q., Wilcox C. S. 2008. Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 51: 184–191. [DOI] [PubMed] [Google Scholar]
- 59.Xu Z. G., Yuan H., Lanting L., Li S. L., Wang M., Shanmugam N., Kato M., Adler S. G., Reddy M. A., Natarajan R. 2008. Products of 12/15-lipoxygenase upregulate the angiotensin II receptor. J. Am. Soc. Nephrol. 19: 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reddy M. A., Adler S. G., Kim Y. S., Lanting L., Rossi J., Kang S. W., Nadler J. L., Shahed A., Natarajan R. 2002. Interaction of MAPK and 12-lipoxygenase pathways in growth and matrix protein expression in mesangial cells. Am. J. Physiol. Renal Physiol. 283: F985–F994. [DOI] [PubMed] [Google Scholar]
- 61.Brunner H. R. 2001. Experimental and clinical evidence that angiotensin II is an independent risk factor for cardiovascular disease. Am. J. Cardiol. 87: 3C–9C. [DOI] [PubMed] [Google Scholar]
- 62.Laursen J. B., Rajagopalan S., Galis Z., Tarpey M., Freeman B. A., Harrison D. G. 1997. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 95: 588–593. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe T., Barker T. A., Berk B. C. 2005. Angiotensin II and the endothelium: diverse signals and effects. Hypertension. 45: 163–169. [DOI] [PubMed] [Google Scholar]
- 64.Kim G. Y., Lee J. W., Cho S. H., Seo J. M., Kim J. H. 2009. Role of the low-affinity leukotriene B4 receptor BLT2 in VEGF-induced angiogenesis. Arterioscler. Thromb. Vasc. Biol. 29: 915–920. [DOI] [PubMed] [Google Scholar]
- 65.Viita H., Markkanen J., Eriksson E., Nurminen M., Kinnunen K., Babu M., Heikura T., Turpeinen S., Laidinen S., Takalo T., et al. 2008. 15-lipoxygenase-1 prevents vascular endothelial growth factor A- and placental growth factor-induced angiogenic effects in rabbit skeletal muscles via reduction in growth factor mRNA levels, NO bioactivity, and downregulation of VEGF receptor 2 expression. Circ. Res. 102: 177–184. [DOI] [PubMed] [Google Scholar]
- 66.Shappell S. B., Olson S. J., Hannah S. E., Manning S., Roberts R. L., Masumori N., Jisaka M., Boeglin W. E., Vader V., Dave D. S., et al. 2003. Elevated expression of 12/15-lipoxygenase and cyclooxygenase-2 in a transgenic mouse model of prostate carcinoma. Cancer Res. 63: 2256–2267. [PubMed] [Google Scholar]
- 67.Bello-Reuss E., Holubec K., Rajaraman S. 2001. Angiogenesis in autosomal-dominant polycystic kidney disease. Kidney Int. 60: 37–45. [DOI] [PubMed] [Google Scholar]
- 68.Reed B. Y., Masoumi A., Elhassan E., McFann K., Cadnapaphornchai M. A., Maahs D. M., Snell-Bergeon J. K., Schrier R. W. 2011. Angiogenic growth factors correlate with disease severity in young patients with autosomal dominant polycystic kidney disease. Kidney Int. 79: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grimm H., Mayer K., Mayser P., Eigenbrodt E. 2002. Regulatory potential of n-3 fatty acids in immunological and inflammatory processes. Br. J. Nutr. 87(Suppl 1): S59–S67. [DOI] [PubMed] [Google Scholar]
- 70.Serhan C. N., Chiang N., Van Dyke T. E. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poulsen R. C., Gotlinger K. H., Serhan C. N., Kruger M. C. 2008. Identification of inflammatory and proresolving lipid mediators in bone marrow and their lipidomic profiles with ovariectomy and omega-3 intake. Am. J. Hematol. 83: 437–445. [DOI] [PubMed] [Google Scholar]
- 72.Alonso-Galicia M., Maier K. G., Greene A. S., Cowley A. W., Jr, Roman R. J. 2002. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283: R60–R68. [DOI] [PubMed] [Google Scholar]
- 73.Cheng J., Wu C. C., Gotlinger K. H., Zhang F., Falck J. R., Narsimhaswamy D., Schwartzman M. L. 2010. 20-hydroxy-5,8,11,14-eicosatetraenoic acid mediates endothelial dysfunction via IkappaB kinase-dependent endothelial nitric-oxide synthase uncoupling. J. Pharmacol. Exp. Ther. 332: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park F., Sweeney W. E., Jr, Jia G., Akbulut T., Mueller B., Falck J. R., Birudaraju S., Roman R. J., Avner E. D. 2009. Chronic blockade of 20-HETE synthesis reduces polycystic kidney disease in an orthologous rat model of ARPKD. Am. J. Physiol. Renal Physiol. 296: F575–F582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Escalante B. A., McGiff J. C., Oyekan A. O. 2002. Role of cytochrome P-450 arachidonate metabolites in endothelin signaling in rat proximal tubule. Am. J. Physiol. Renal Physiol. 282: F144–F150. [DOI] [PubMed] [Google Scholar]
- 76.Oyekan A. 2002. Nitric oxide inhibits renal cytochrome P450-dependent epoxygenases in the rat. Clin. Exp. Pharmacol. Physiol. 29: 990–995. [DOI] [PubMed] [Google Scholar]
- 77.Serhan C. N., Chiang N. 2008. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br. J. Pharmacol. 153(Suppl 1): S200–S215. [DOI] [PMC free article] [PubMed] [Google Scholar]