Abstract
Cytochrome P450 (CYP)-dependent metabolites of arachidonic acid (AA) contribute to the regulation of cardiovascular function. CYP enzymes also accept EPA and DHA to yield more potent vasodilatory and potentially anti-arrhythmic metabolites, suggesting that the endogenous CYP-eicosanoid profile can be favorably shifted by dietary omega-3 fatty acids. To test this hypothesis, 20 healthy volunteers were treated with an EPA/DHA supplement and analyzed for concomitant changes in the circulatory and urinary levels of AA-, EPA-, and DHA-derived metabolites produced by the cyclooxygenase-, lipoxygenase (LOX)-, and CYP-dependent pathways. Raising the Omega-3 Index from about four to eight primarily resulted in a large increase of EPA-derived CYP-dependent epoxy-metabolites followed by increases of EPA- and DHA-derived LOX-dependent monohydroxy-metabolites including the precursors of the resolvin E and D families; resolvins themselves were not detected. The metabolite/precursor fatty acid ratios indicated that CYP epoxygenases metabolized EPA with an 8.6-fold higher efficiency and DHA with a 2.2-fold higher efficiency than AA. Effects on leukotriene, prostaglandin E, prostacyclin, and thromboxane formation remained rather weak. We propose that CYP-dependent epoxy-metabolites of EPA and DHA may function as mediators of the vasodilatory and cardioprotective effects of omega-3 fatty acids and could serve as biomarkers in clinical studies investigating the cardiovascular effects of EPA/DHA supplementation.
Keywords: cytochrome P450, lipidomics, nutrition
Cytochrome P450 (CYP) enzymes catalyze the formation of biologically active epoxy- and hydroxy-metabolites of long-chain PUFAs (1). Traditionally, and in line with the prevalence of n-6 PUFAs in the “Western diet”, arachidonic acid (AA) (20:4 n-6) has been considered as the main precursor and the corresponding metabolites were categorized as a subclass of eicosanoids (2). CYP-eicosanoid formation is also known as the “third branch of the AA cascade,” complementary to the previously discovered cyclooxygenase (COX)- and lipoxygenase (LOX)-initiated pathways of prostanoid and leukotriene formation (3, 4).
Physiologically important AA-derived CYP-eicosanoids include a set of regio- and stereoisomeric epoxyeicosatrienoic acids (EETs) and 20-HETE (2, 5). EETs and 20-HETE play partially opposing roles in the regulation of vascular, renal, and cardiac function (6–9). The contribution of EETs to cardiovascular function is influenced by the soluble epoxide hydrolase (sEH) that metabolizes EETs to less potent dihydroxyeicosatrienoic acids (DHETs) (10). Imbalances in CYP-eicosanoid formation are linked to the development of endothelial dysfunction and hypertension; ischemia-induced injury of the heart, kidney and brain; inflammatory disorders; and atherosclerosis (11–17).
Recent studies demonstrated that the same CYP isoforms that epoxidize or hydroxylate AA, also efficiently metabolize fish oil n-3 PUFAs such as EPA (20:5 n-3) and DHA (22:6 n-3) (18–22). CYP2C and CYP2J isoforms convert AA to EETs, EPA to epoxyeicosatetraenoic acids (EEQs), and DHA to epoxydocosapentaenoic acids (EDPs). The ω-3 double bond distinguishing EPA and DHA from AA is the preferred site of attack of most of the epoxygenases, resulting in the formation of 17,18-EEQ and 19,20-EDP as main metabolites. CYP4A and CYP4F isoforms, hydroxylating AA to 20-HETE, metabolize EPA to 20-hydroxyeicosapentaenoic acid (HEPE) and DHA to 22-hydroxydocosahexaenoic acid (HDHA). CYP1A1, CYP2E1, and other isoforms that convert AA predominantly to 19-HETE show pronounced ω-3 epoxygenase activities with EPA and DHA. Like EETs, EEQs and EDPs can also be degraded to the corresponding vicinal diols [dihydroxyeicosatetraenoic acids (DHEQs) and dihydroxydocosapentaenoic acids (DHDPs)] by sEH-mediated hydrolysis (23).
The currently known biological activities of EPA- and DHA-derived CYP metabolites partially resemble those of their AA-derived counterparts, appear in part unique, or may even produce opposite effects (24). The epoxy-metabolites of all three PUFAs share vasodilatory properties, whereby the potencies of EEQs and EDPs may largely exceed those of EETs in some vascular beds (18, 25). Anti-inflammatory effects were first revealed for 11,12- and 14,15-EET but are also exerted by EPA-epoxides, as exemplified by 17,18-EEQ (26, 27). 17,18-EEQ and 19,20-EDP inhibit the Ca2+- and isoproterenol-induced increased contractility of neonatal cardiomyocytes, indicating that these metabolites may act as endogenous antiarrhythmic agents (22). Whereas certain EET regioisomers promote tumor angiogenesis and metastasis, 19,20-EDP and other regioisomeric DHA-epoxides inhibit these crucial events in cancerogenesis (28, 29). These findings suggest that CYP metabolites may serve as mediators in a variety of the beneficial effects attributed to fish oil n-3 PUFAs, such as protection against cardiovascular disease, sudden cardiac death, and tumor development (30, 31).
Based on the substrate specificity of recombinant CYP isoforms, we supposed that the profile of physiologically active CYP-eicosanoids is extensively modulated in vivo by changing the dietary n-6/n-3 PUFA ratio. We proved this hypothesis first in rats, where we found a clear shift from AA- to EPA- and DHA-derived epoxy- and ω-hydroxy-metabolites in all major organs and tissues upon dietary EPA/DHA supplementation (22). We have now expanded our studies to humans and analyzed the effect of EPA/DHA supplementation on the profile of circulating and urinary CYP-eicosanoids in a trial with 20 healthy volunteers. Simultaneously, we also determined the levels of various LOX- and COX-dependent metabolites to gain insight into the relative susceptibility of the three branches of the human AA cascade to changes in the dietary n-6/n-3 PUFA ratio.
MATERIALS AND METHODS
Participants of the study
Healthy volunteers (men and women) with an age between 18 and 60 years were included. Main exclusion criteria were: i) permanent medication (only thyroid hormone substitution and contraception allowed); ii) BMI ≥30; iii) more than one marine fish meal per week or Omega-3 Index ≥6 (32); and iv) prior diagnosis of dyslipidemia. Occasional intake of drugs including nonsteroidal anti-inflammatory drugs (NSAIDs) was documented at each visit. Only two participants reported the use of NSAIDs, taken shortly before (2 × 200 mg ibuprofen) or 1 week after starting EPA/DHA supplementation (1 × 200 mg ibuprofen). The study was performed in accordance with the Declaration of Helsinki, current institutional guidelines, and good clinical practice. Our Institutional Review Board approved the study and written informed consent was obtained from all participants before entry.
Study design
The “OMEICOS study” (EudraCT identifier: 2009-013458-33) was conducted at the Experimental and Clinical Research Center (ECRC), Berlin, Germany, between December 2009 and December 2010. The primary aim of OMEICOS was to analyze the effect of dietary omega-3 fatty acid supplementation on the profile of main CYP-, LOX-, and COX-dependent metabolites in blood plasma and urine. From the 38 volunteers initially screened, 20 fulfilled all criteria for study inclusion. After analysis of basal parameters, dietary omega-3 fatty acid supplementation was started. Study participants received one capsule OMACOR® (Abbott GmbH, Germany) per day (containing 460 mg EPA and 380 mg DHA as ethyl esters) from weeks 1 to 4, and two capsules OMACOR® per day (980 mg EPA and 760 mg DHA as ethyl esters) from weeks 5 to 8. Afterwards, omega-3 supplementation was stopped and the participants were followed-up for another 8 weeks (weeks 9–16). Study visits and assessment of clinical and biochemical parameters were done before (basal), after week 1, week 4, week 8, week 9, and week 16.
Assessment
Clinical standard parameters (body weight, blood pressure, ECG) and food intake were documented at each study visit in a standardized fashion. Blood samples (after overnight fasting) and 24 h-urine samples were taken at each study visit. Glucose, lipoproteins, and triglycerides were determined by standard methods in a certified clinical laboratory. Blood (EDTA-plasma) and urine samples were stored at −80°C for eicosanoid profiling.
Determination of eicosanoid profiles
Plasma samples (500 μl) were subjected to alkaline hydrolysis and subsequent solid phase extraction (SPE) was performed exactly as described previously (22). Urine samples (2 ml) were treated with 0.2 mg β-glucuronidase from Escherichia coli in 0.1 mol/l phosphate buffer (pH 6.8) containing 1 mg/ml BSA for 2 h at 37°C. After that, pH was adjusted to 6.0 with acetic acid and the metabolites were extracted using the same SPE procedure as with plasma. The capacity of blood cells to produce leukotrienes and other LOX metabolites, as well as thromboxanes, was determined after incubating fresh blood samples (4.5 ml) with 50 μM of the calcium ionophore A23187 for 30 min at 37°C as described previously (33). The free metabolites present after calcium ionophore stimulation were directly extracted via SPE without prior alkaline hydrolysis.
LC-MS/MS analysis of the extracted metabolites was performed using an Agilent 6460 Triple Quad mass spectrometer with JetStream ion source (Agilent Technolgies, Santa Clara, CA) coupled with an Agilent 1200 HPLC system (degasser, binary pump, well plate sampler, thermostated column compartment). The HPLC system was equipped with a Phenomenex Kinetex column (150 mm × 2.1 mm, 2.6 μm; Phenomenex, Aschaffenburg, Germany). Chromatography was done under gradient conditions with acetonitrile/0.1% formic acid in water as mobile phase. Gradient was started at 5% acetonitrile, increased to 55% after 0.5 min, to 69% after 14.5 min, and to 95% after 14.6 min. The flow rate was 0.3 ml/min during the run time of 20 min. The injection volume was 7.5 μl. Drying gas was adjusted at 250°C/10 l/min, sheath gas at 380°C/12 l/min. Capillary and nozzle voltage were optimized at −4,500 V and −1,500 V, respectively. A complete list of the metabolites analyzed, as well as the corresponding conditions for multiple reaction monitoring, is given in supplementary Table I. The internal standards added to the samples before extraction included 10 ng each of 20-HETE-d6, 14,15-EET-d8, 14,15-DHET-d11, prostaglandin E2 (PGE2)-d4, leukotriene B4 (LTB4)-d5, and 15-HETE-d8 (Cayman Chemical, Ann Arbor, MI) and served for the quantification of groups of similar metabolites. Calibration curves for the quantification of individual metabolites were established based on the changes in the relative peak area in response to different target compound/internal standard-concentration ratios. Linearity was r2 > 0.99 over a range from 1 to 20 ng absolute for any compound.
Determination of fatty acid profiles
Red blood cell (RBC) fatty acid compositions were analyzed according to the HS-Omega-3 Index methodology as described previously (32). Fatty acid methyl esters were generated by acid transesterification and analyzed by gas chromatography using a GC2010 gas chromatograph (Shimadzu, Duisburg, Germany) equipped with a SP2560 100 m column (Supelco, Bellefonte, PA) using hydrogen as carrier gas. Fatty acids were identified by comparison with a standard mixture of fatty acids characteristic of erythrocytes. The Omega-3 Index is given as EPA plus DHA expressed as a percentage of total identified fatty acids after response factor correction. The coefficient of variation for EPA plus DHA was 5%. Analyses were quality-controlled according to DIN ISO 15189.
Sample size and statistical analysis
Prior to data analysis, a frequency distribution of the Omega-3 Index, including all screened individuals, was performed (GraphPad Prism 5.0). Out of 38 volunteers, 20 subjects were selected for the study fulfilling the criterion of an Omega-3 Index below 6. The data from participant Omeicos26 was excluded from analysis due to noncompliance to the study protocol, giving a total of n = 19 for the subsequent per-protocol analysis.
Statistical analyses were performed with PASW Statistics 18, SPSS Inc. All data were tested for normal distribution and are given as mean ± SEM. To test for the differences in response to dietary intervention, we analyzed our data by a general linear model for repeated measurements followed by a post hoc test based on estimated marginal means with a Holm step-down Bonferroni procedure for correction of P values. To test for gender differences, a t-test for independent variables was applied. P < 0.05 was defined as statistically significant. Associations between parameters were determined using Pearson or Spearman Rho correlation analysis.
RESULTS
Basic characteristics of the participants
Ten healthy men and ten healthy women participated in this study. Their mean age was 32 ± 8 years (males) and 38 ± 6 years (females), and their BMI was 24.9 ± 2.7 kg/m2 (males) and 25.5 ± 3.8 kg/m2 (females); see Table 1 for further characteristics of the participants. These subjects were selected out of a total of 38 volunteers using an Omega-3 Index >6 as exclusion criterion, i.e., EPA + DHA comprised less than 6% of the total fatty acids in RBCs of the subjects included (Fig. 1A). If not specifically stated otherwise, we did not observe statistically significant differences in the response of males and females to EPA/DHA supplementation and thus report the data as means for the whole group of the participants.
TABLE 1.
Participant clinical parameters
| Supplementation | |||
| Parameters | Basal (week 0) | OM-3a (2 g) (week 8) | Follow-up (week 16) |
| Age (years) | |||
| Male | 31.3 ± 2.5 | — | — |
| Female | 38.0 ± 1.8 | — | — |
| Body weight (kg) | |||
| Male | 79.9 ± 5.6 | 80.8 ± 6.1 | 81.2 ± 6.2 |
| Female | 68.4 ± 4.6 | 68.2 ± 4.6 | 68.0 ± 4.6 |
| BMI (kg/m2) | |||
| Male | 24.9 ± 1.0 | 24.9 ± 1.0 | 24.9 ± 1.0 |
| Female | 25.5 ± 1.3 | 25.5 ± 1.3 | 25.5 ± 1.3 |
| Heart rate (bpm) | 64.4 ± 2.1 | 67.4 ± 2.1 | 67.9 ± 2.1 |
| Systolic blood pressure (mmHg) | 123.2 ± 2.9 | 121.3 ± 2.9 | 121.2 ± 2.9 |
| Diastolic blood pressure (mmHg) | 75.5 ± 1.7 | 72.0 ± 1.7 | 73.4 ± 1.7 |
| Total cholesterol (mmol/l) | 5.2 ± 0.2 | 5.6 ± 0.2 | 5.2 ± 0.2 |
| LDL (mmol/l) | 3.1 ± 0.2 | 3.4 ± 0.2 | 3.2 ± 0.2 |
| HDL (mmol/l) | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 |
| Triglycerides (mmol/l) | 1.5 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.2 |
All values are expressed as mean ± SEM.
OM-3, EPA/DHA-supplement as described in Materials and Methods.
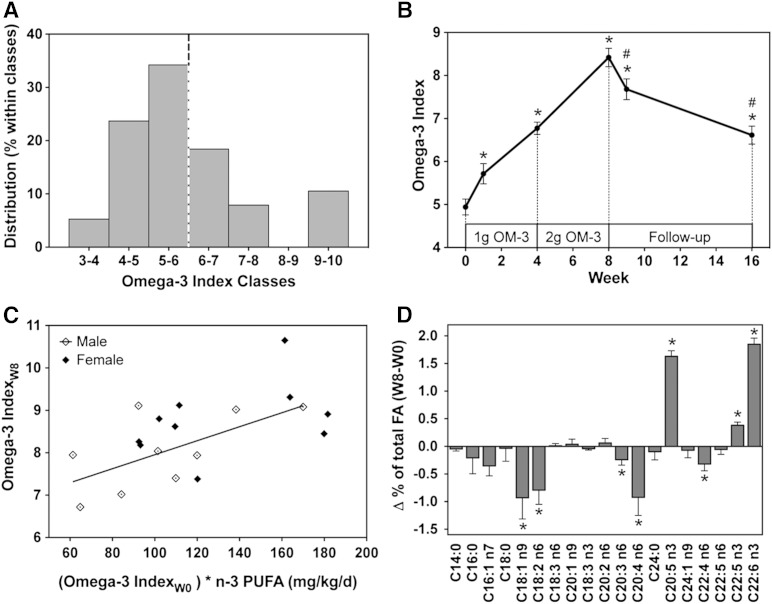
Fig. 1.
Effects of EPA/DHA supplementation on the Omega-3 Index and fatty acid composition in RBCs. A: Frequency distribution of the basal Omega-3 Index in the prescreened group of 38 healthy volunteers. Twenty subjects with an Omega-3 Index ≤6 (dashed line) were included into the study. B: Time- and dose-dependent changes of the Omega-3 Index during the whole course of the study. C: Correlation of the Omega-3 Index achieved after 8 weeks of EPA/DHA supplementation with the product of the baseline Omega-3 Index and the dose per kilogram of body weight. D: Changes in the abundance of individual fatty acids comparing their percentages of total fatty acid in RBCs at week 8 and week 0 of EPA/DHA supplementation. Data are given as mean ± SEM, n = 19. A general linear model for repeated measurements was used for analysis (B, D) and significant changes are indicated as: *P < 0.05 versus basal level [week 0 (W0)] and #P < 0.05 versus maximum treatment [week 8 (W8)]. For dosage dependency (C), a Pearson correlation was performed: r = 0.599, P < 0.01.
Changes in RBC fatty acid composition upon dietary EPA/DHA supplementation
EPA/DHA supplementation caused a time- and dose-dependent increase of the Omega-3 Index in all subjects, except one male who remained at baseline RBC (EPA + DHA) level for unknown reasons and was not included in the further analysis. The maximal increase of the Omega-3 Index was observed 8 weeks after starting EPA/DHA supplementation, i.e., after the participants ingested one Omacor® capsule (480 mg EPA + 360 mg DHA) daily for the first 4 weeks and two capsules daily in the subsequent 4 weeks (Fig. 1B). At this time point, the Omega-3 Index reached 8.4 ± 0.2 compared with 4.9 ± 0.2 at baseline. The individually achieved peak values ranged between 6.7 and 10.7 and strongly correlated with the product of the baseline Omega-3 Index and the dose per kg of body weight (Fig. 1C). Simultaneously with the increase in RBC EPA and DHA levels, the contents of AA (20:4 n-6), linoleic acid (C18:2 n-6), and oleic acid (C18:1 n-9) were significantly decreased (Fig. 1D). After discontinuing EPA/DHA supplementation, the Omega-3 Index immediately started to decline, but still remained significantly elevated for the following eight weeks (6.6 ± 0.2) compared with the low baseline values (Fig. 1B).
Over the whole study period, the relative ratio of AA, EPA, and DHA changed from 1:0.04:0.25 at baseline (week 0), to over 1:0.15:0.38 after maximal EPA/DHA supplementation (week 8), to 1:0.07:0.35 at the end of the posttreatment period (16 weeks); compare supplementary Table II for the complete RBC fatty acid compositions at these three stages of the study.
Changes in the CYP-eicosanoid profile upon dietary EPA/DHA supplementation
We introduced the “omega-3-epoxyeicosanoid index” as a parameter that reflects the relative abundance of CYP-epoxygenase-dependent metabolites derived from n-3 PUFAs (EPA + DHA) compared with those derived from AA. We included the regioisomeric epoxy-metabolites as well as the corresponding vicinal diols resulting from sEH-mediated hydrolysis of the primary epoxy-metabolites and calculated this parameter as: [(EEQs + DHEQs) + (EDPs + DHDPs)]/(EETs + DHETs); see supplementary Table III for a complete set of the primary data for the weeks 0, 8, and 16. At baseline, the participants showed a mean plasma omega-3-epoxyeicosanoid index of 0.6 ± 0.03, i.e., the levels of AA-derived metabolites exceeded those derived from (EPA + DHA) nearly twice. This ratio was rapidly and almost completely inversed upon dietary EPA/DHA supplementation (Fig. 2A). The omega-3-epoxyeicosanoid index reached a peak value of 2.1 ± 0.15 at week 8, and returned nearly to baseline after discontinuation of EPA/DHA supplementation.
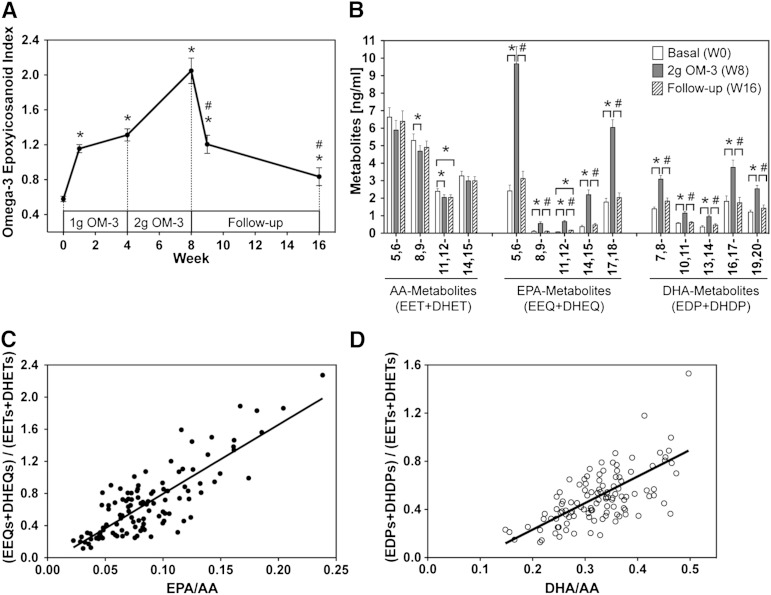
Fig. 2.
Effect of EPA/DHA supplementation on the profile of circulating CYP-epoxygenase metabolites. A: Time- and dose-dependent changes of the CYP-epoxyeicosanoid index during the whole course of the study. B: Regioisomeric composition of epoxygenase products derived from AA, EPA, and DHA at baseline [week 0 (W0)], after maximal EPA/DHA supplementation [week 8 (W8)], and after discontinuation of supplementation [week 16 (W16)]. C: Correlation of the relative plasma levels of EPA- and AA-derived metabolites with the EPA/AA precursor fatty acid ratio. D: Correlation of the relative plasma levels of DHA- and AA-derived metabolites with the DHA/AA precursor fatty acid ratio. Metabolite levels are given as the sum of the primary CYP-epoxygenase products and the corresponding vicinal diols produced by sEH-mediated hydrolysis of the primary epoxides. Total metabolite levels (free + esterified) were determined after alkaline hydrolysis. Data are given as mean ± SEM, n = 19. A general linear model for repeated measurements was used for analysis (A, B) and significant changes are indicated as: *P < 0.05 versus basal level (W0) and #P < 0.05 versus maximum treatment (W8). For relative efficiencies, a Pearson correlation was performed: y = 8.60x − 0.06; r = 0.826 with P < 0.001 (C) and y = 2.21x − 0.21; r = 0.587 with P < 0.001 (D).
Despite these massive changes in the relative abundance of AA-, EPA-, and DHA-derived metabolites, the principal regioisomeric composition within each of the metabolite groups remained largely constant (Fig. 2B). Among the AA-derived metabolites, the 5,6-, 8,9-, 11,12-, and 14,15-regioisomers contributed to the total (EET + DHET) levels with relative percentages of 38:30:14:18 at week 0, 38:30:13:19 at week 8, and 40:30:13:18 at week 16. The EPA-derived 5,6-, 8,9-, 11,12-, 14,15-, and 17,18-regioisomers (EEQs + DHEQs) occurred in ratios of 49:2:2:9:39 (week 0), 50:3:3:12:32 (week 8), and 51:2:3:9:36 (week 16). The regioisomeric composition of the DHA-derived 7,8-, 10,11-, 13,14-, 16,17-, and 19,20-regioisomers (EDPs + DHDPs) was 26:10:7:33:23 (week 0), 27:10:8:33:22 (week 8), and 31:11:8:30:26 (week 16). EPA/DHA supplementation for 8 weeks slightly, but significantly, decreased the AA-derived 5,6-, 8,9-, and 11,12-regioisomers, increased each of the regioisomeric EPA-derived metabolites almost 4-fold, and doubled the levels of all regioisomeric DHA-derived metabolites (Fig. 2B). Over the whole study, the relative plasma levels of EPA- and DHA-derived metabolites compared with AA-derived metabolites strongly correlated with the respective precursor fatty acid ratios (Figs. 2C, D).
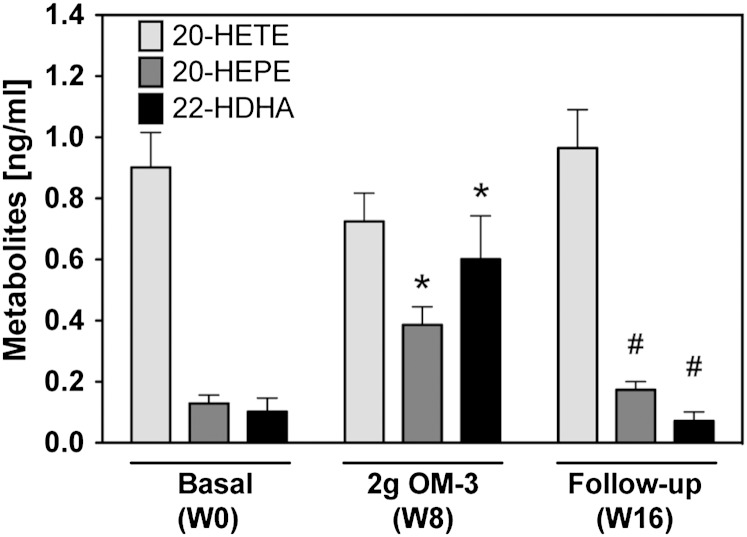
EPA/DHA supplementation also influenced the formation of ω-hydroxylase products (Fig. 3). At baseline, plasma 20-HETE levels exceeded those of 20-HEPE and 22-HDHA 7- to 9-fold. 20-HETE was detectable in all participants, ranged between 0.3 and 2.3 ng/ml, and remained largely unaffected over the whole study period. 20-HEPE was undetectable in 7 out of 19 participants at baseline, but was clearly formed in all subjects after EPA/DHA supplementation and reached maximal levels between 0.4 and 0.9 ng/ml at week 8. 22-HDHA was low at baseline (0.1 ± 0.04 ng/ml), increased almost 6-fold after maximal EPA/DHA supplementation, and declined to baseline levels during the follow-up period (Fig. 3).
Fig. 3.
Effect of EPA/DHA supplementation on the plasma levels of CYP-ω-hydroxylase metabolites derived from AA (20-HETE), EPA (20-HEPE), and DHA (22-HDHA). Metabolite levels were determined after alkaline hydrolysis and thus represent the sum of free and esterified metabolites. Bars show mean ± SEM, n = 19. A general linear model for repeated measurements was used for analysis and significant changes are indicated as: *P < 0.05 versus basal level [week 0 (W0)] and #P < 0.05 versus maximum treatment [week 8 (W8)].
Similar pronounced changes were also observed in the urinary metabolite profiles (supplementary Table IV). All CYP-epoxygenase products were predominantly excreted after being hydrolyzed to the corresponding vicinal diols (compare supplementary Table IV). At all stages of the study, 8,9-DHET was the predominant AA-derived metabolite in the urine followed by 14,15-DHET. Among the EPA-derived vicinal diols, 17,18-DHEQ followed by 5,6-DHEQ was most prominent, whereas 7,8- and 10,11-DHDP predominated among the DHA-derived regioisomers. EPA/DHA supplementation increased the total urinary concentration of EPA- and DHA-derived vicinal diols more than 2.5-fold at week 8 compared with baseline, without significantly reducing the excretion of the AA-derived metabolites (supplementary Table IV). Among the ω-hydroxylase products, 20-HETE excretion remained largely unaffected by EPA/DHA supplementation. The EPA-derived 20-HEPE increased about 3-fold from week 0 to week 8 and returned to baseline in the follow-up period (supplementary Table IV). 22-HDHA excretion was not significantly modulated by EPA/DHA supplementation.
Changes in the profile of LOX- and COX-dependent metabolites upon dietary EPA/DHA supplementation
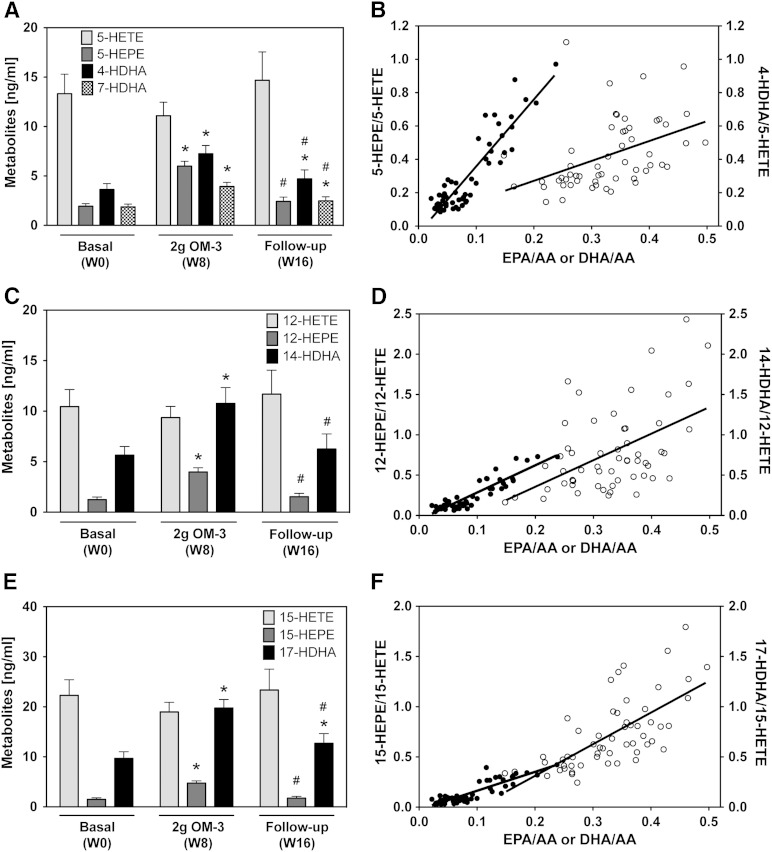
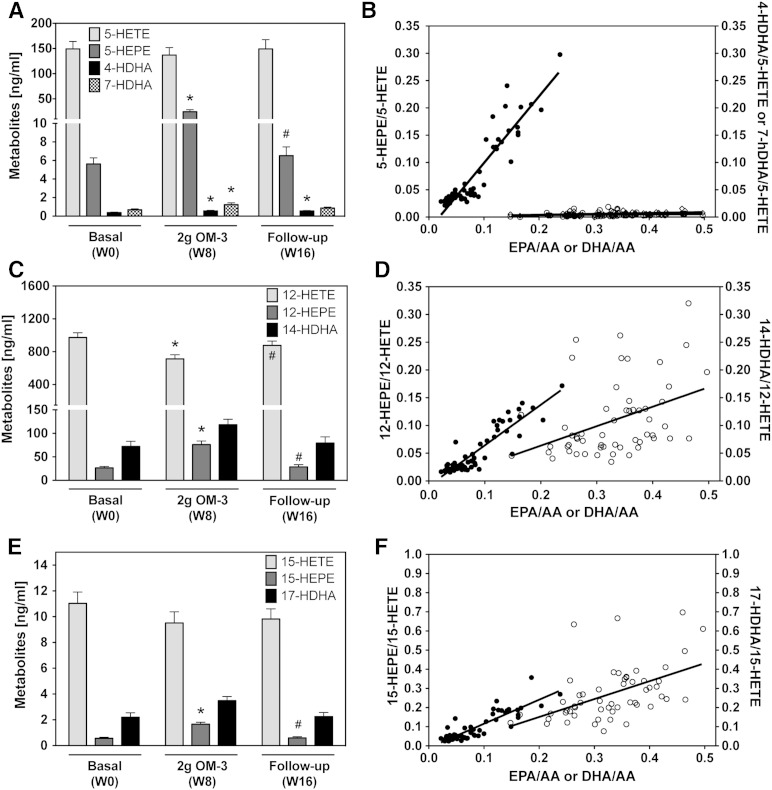
5-LOX-dependent monohydroxy-metabolites of EPA (5-HEPE) and DHA (4-HDHA and 7-HDHA) significantly increased from week 0 to week 8 and declined almost to baseline after the participants stopped taking the EPA/DHA supplement (Fig. 4A). The 5-HEPE/5-HETE ratio increased linearly with the EPA/AA ratio (Fig. 4B). Strong correlations were also observed for the 4-HDHA/5-HETE ratio (Fig. 4B) and for the 7-HDHA/5-HETE ratio (y = 1.00x − 0.01; r = 0.696 with P < 0.001, data not shown) when plotted against the DHA/AA ratio. The 12-LOX pathway that produces 12-HETE from AA, 12-HEPE from EPA, and 14-HDHA from DHA responded to EPA/DHA supplementation with significant increases in 12-HEPE and 14-HDHA formation (Fig. 4C). Whereas the 12-HEPE/12-HETE ratio strongly correlated with the EPA/AA ratio, only a weak correlation was detectable between 14-HDHA/12-HETE formation and the DHA/AA precursor ratio (Fig. 4D). Increased formation of the EPA metabolite, 15-HEPE, and of the DHA metabolite, 17-HDHA, were the characteristic features of the response of the 15-LOX pathway to EPA/DHA supplementation (Fig. 4E, F). Besides 17-HDHA, 18-HEPE, another precursor of the resolvin family, was also strongly increased upon EPA/DHA supplementation (Fig. 5).
Fig. 4.
Effect of EPA/DHA supplementation on the plasma levels of LOX-dependent monohydroxy-metabolites. A, B: Plasma levels of 5-LOX-dependent metabolites derived from AA (5-HETE), EPA (5-HEPE), and DHA (4-HDHA and 7-HDHA) and their relative abundance in correlation with the corresponding precursor fatty acid ratios (filled circles for EPA/AA; open circles for DHA/AA). C, D: Plasma levels of 12-LOX-dependent metabolites derived from AA (12-HETE), EPA (12-HEPE), and DHA (14-HDHA) and their relative abundance in correlation with the corresponding precursor fatty acid ratios (filled circles for EPA/AA; open circles for DHA/AA). E, F: Plasma levels of 15-LOX-dependent metabolites derived from AA (15-HETE), EPA (15-HEPE), and DHA (17-HDHA) and their relative abundance in correlation with the corresponding precursor fatty acid ratios (filled circles for EPA/AA; open circles for DHA/AA). Metabolite levels were determined after alkaline hydrolysis and thus represent the sum of free and esterified metabolites. Bars show mean ± SEM, n = 19. A general linear model for repeated measurements was used for analysis (A, C, E) and significant changes are indicated as: *P < 0.05 versus basal level [week 0 (W0)] and #P < 0.05 versus maximum treatment [week 8 (W8)]. For relative efficiencies a Pearson/Spearman Rho correlation was performed: 5-HEPE/5-HETE, y = 4.02x − 0.04, r = 0.790 with P < 0.001 and 4-HDHA/5-HETE, y = 1.89x − 0.20, r = 0.696 with P < 0.001 (B); 12-HEPE/12-HETE, y = 3.37x − 0.05, r = 0.821 with P < 0.001 and 14-HDHA/12-HETE, y = 3.30x − 0.30; r = 0.493 with P < 0.001 (D); and 15-HEPE/15-HETE, y = 1.87x − 0.02, r = 0.795 with P < 0.001 and 17-HDHA/15-HETE, y = 3.15x − 0.32, r = 0.688 with P < 0.001 (F).
Fig. 5.
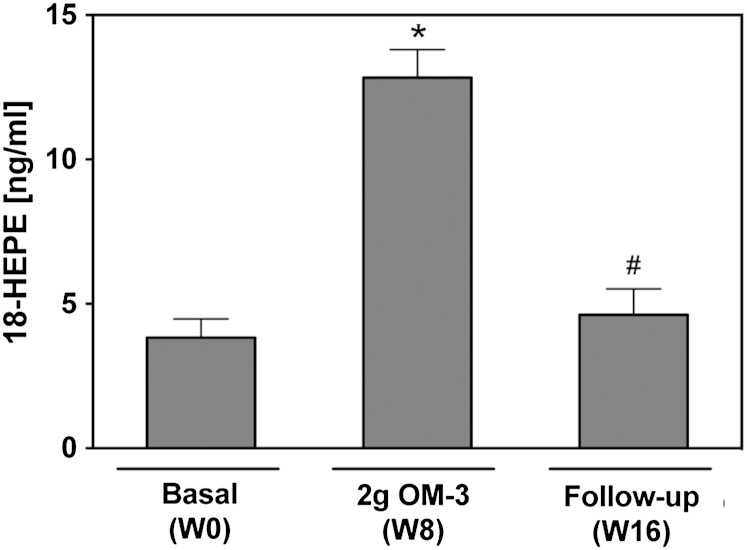
Effect of EPA/DHA supplementation on 18-HEPE formation. Total plasma levels of 18-HEPE were determined after alkaline hydrolysis. Data are given as mean ± SEM, n = 19. A general linear model for repeated measurements was used for analysis and significant changes are indicated as: *P < 0.05 versus basal level [week 0 (W0)] and #P < 0.05 versus maximum treatment [week 8 (W8)].
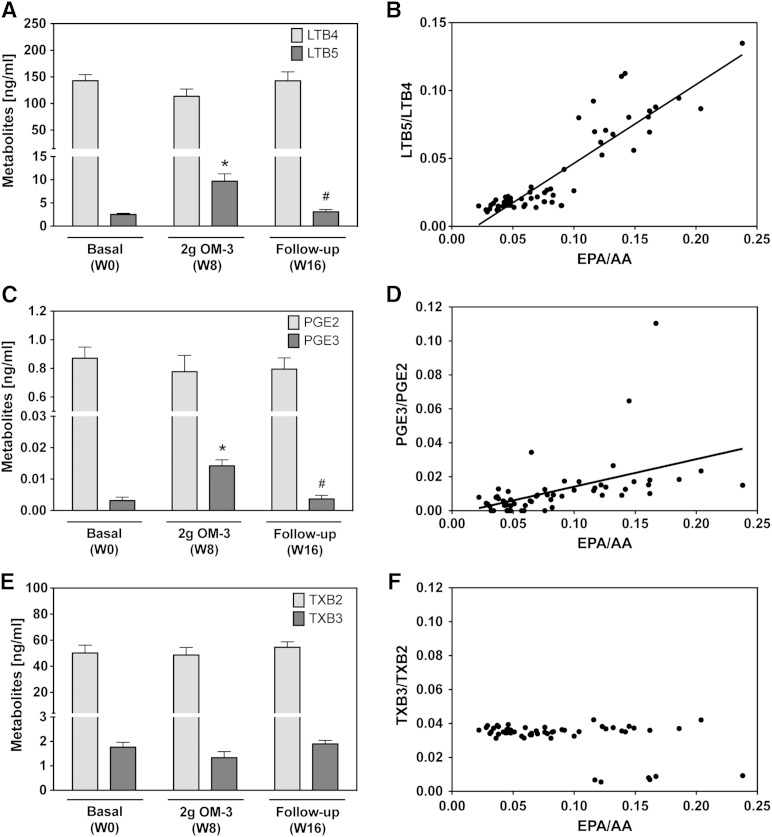
To study the effects of EPA/DHA supplementation on leukotriene and prostanoid formation, we stimulated the de novo synthesis of these metabolites by treating whole blood samples, freshly obtained from the participants, with the Ca2+ ionophore A23187. As shown in Fig. 6A, C, the capacity of blood cells to produce LTB5 and PGE3 from EPA significantly increased after maximal EPA/DHA supplementation and returned to baseline in the follow-up period. Nonetheless, the AA-derived LTB4 and PGE2 remained predominant at all stages of the study. The LTB5/LTB4 ratio ranged between 0.01 and 0.1 and strongly correlated with the EPA/AA ratio (Fig. 6B). Also, the relative formation of PGE3 and PGE2 was linearly correlated with the EPA/AA ratio (Fig. 6D); however, the formation of PGE2 exceeded that of PGE3 almost 20-fold at baseline and was still about 8-fold after maximal EPA/DHA supplementation. Ca2+ ionophore-stimulated thromboxane formation was largely unaffected by EPA/DHA supplementation in most of the participants, and the ratio between AA-derived thromboxane B2 (TXB2) and EPA-derived TXB3 remained unchanged at about 25:1 (Fig. 6E, F). However, in 5 females and 1 male out of the 19 participants, this ratio even increased to roughly 100:1 at week 8 and declined again to 26–31:1 at week 16.
Fig. 6.
Effect of EPA/DHA supplementation on leukotriene and prostanoid formation after calcium ionophore stimulation. The levels of free metabolites were determined after A23187-mediated stimulation of whole blood samples and thus reflect the metabolic capacity and substrate specificity of the corresponding enzymes expressed in blood cells. A, B: Generated levels of AA-derived LTB4 and EPA-derived LTB5 and the relative abundance of LTB5 and LTB4 in correlation with the EPA/AA precursor fatty acid ratio. C, D: Levels of AA-derived PGE2 and EPA-derived PGE3 and the relative abundance of PGE3 and PGE2 in correlation with the EPA/AA precursor fatty acid ratio. E, F: Levels of AA-derived TXB2 and EPA-derived TXB3 and the relative abundance of TXB3 and TXB2 in correlation with the EPA/AA precursor fatty acid ratio. Data are given as mean ± SEM, n = 19. A general linear model for repeated measurements was used for analysis (A, C, E) and significant changes are indicated as: *P < 0.05 versus basal level [week 0 (W0)] and #P < 0.05 versus maximum treatment [week 8 (W8)]. For relative efficiencies, a Pearson/Spearman Rho correlation was performed: LTB5/LTB4, y = 0.58x − 0.01, r = 0.864 with P < 0.001 (B); PGE3/PGE2, y = 0.16x − 0.002, r = 0.712 with P < 0.001 (D); TXB3/TXB2 no correlation (F).
In the urine samples of all participants, TXB2 and TXB3, as well as the corresponding 11-dehydro derivatives, were below the limit of quantification of our method (8 and 20 pg for the 11-dehydro derivatives and 1.5 and 2 pg/ml urine for TXB2 and TXB3, respectively). Urinary excretion of prostacyclin (PGI) derivatives was slightly modulated in response to EPA/DHA supplementation (supplementary Table IV). However, the ratio between the PGI3- and PGI2-derived hydrolysis products (Δ17 6-keto-PGF1α/6-keto-PGF1α) showed large inter-individual differences and did not correlate with the EPA/AA ratio (supplementary Table IV).
Ca2+-ionophore treatment did not only induce the formation of leukotrienes and prostanoids, but also specifically increased the levels of 5-LOX- and 12-LOX-dependent monohydroxy-metabolites. After Ca2+-ionophore stimulation, the concentrations of free 5-HETE and 5-HEPE were approximately 12- and 3-fold higher, respectively, compared with the normal circulating levels of these metabolites (compare Figs. 7A and 4A). The relative formation of 5-HEPE and 5-HETE almost perfectly followed the relative abundance of their precursor fatty acids (Fig. 7B). In contrast, DHA was obviously not metabolized via the 5-LOX pathway after Ca2+-ionophore stimulation, as indicated by the unchanged low levels of 4-HDHA and 7-HDHA (Fig. 7A vs. Fig. 4A). 12-LOX-dependent metabolites of AA and EPA, as well as DHA, largely increased upon Ca2+-ionophore stimulation (Fig. 7C vs. Fig. 4C). Compared with their normal circulating levels, 12-HETE increased 93-fold, 12-HEPE increased 21-fold, and 14-HDHA increased 13-fold. The 12-HEPE/12-HETE ratio strongly correlated with the relative abundance of EPA and AA, whereas there was only a weak correlation between the 14-HDHA/12-HETE and the DHA/AA ratios (Fig. 7D). In contrast to its marked effects on metabolite formation via the 5-LOX and 12-LOX pathways, Ca2+-ionophore treatment did not stimulate the generation of 15-LOX-dependent metabolites (Fig. 7E vs. Fig. 4E) and also did not increase the levels of 18-HEPE (data not shown).
Fig. 7.
Calcium ionophore stimulated monohydroxy-metabolite formation via LOX enzymes. Shown is the formation of free metabolites via 5-LOX (A), 12-LOX (C) and 15-LOX (E) enzymes in whole blood samples at baseline [week 0 (W0)], after maximal EPA/DHA-supplementation [week 8 (W8)], and after discontinuation of supplementation [week 16 (W16)]. Bars represent mean ± SEM, n = 19. A general linear model for repeated measurements was used for analysis (A, C, E) and significant changes are indicated as: *P < 0.05 versus basal level (W0) and #P < 0.05 versus maximum treatment (W8). For relative efficiencies a Pearson/Spearman Rho correlation was performed: 5-HEPE/5-HETE versus EPA/AA: y = 1.2x − 0.02, r = 0.884 with P < 0.001 and 4-HDHA/5-HETE versus DHA/AA, y = 0.01x − 0.00008, r = 0.351 with P < 0.001; 7-HDHA/5-HETE versus DHA/AA: y = 0.02x − 0.0008, r = 0.421 with P < 0.001 (B). 12-HEPE/12-HETE versus EPA/DHA: y = 0.73x − 0.01, r = 0.857 with P < 0.001 and 14-HDHA/12-HETE versus DHA/AA: y = x − 0.35 − 0.01, r = 0.444 with P < 0.001 (D). 15-HEPE/15-HETE versus EPA/DHA: y = 1.26x − 0.01, r = 0.834 with P < 0.001 and 17-HDHA/15-HETE versus DHA/AA: y = 0.93x − 0.03, r = 0.510 with P < 0.001 (F).
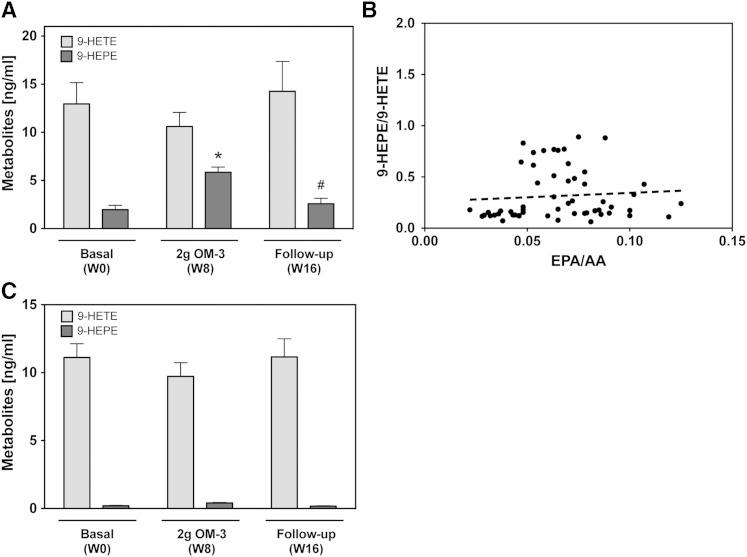
9-HETE and 9-HEPE, metabolites presumably produced by nonenzymatic oxidation of AA and EPA, were also detectable in the plasma samples. 9-HEPE levels increased almost 2-fold upon EPA/DHA supplementation (week 8) and returned to baseline in the follow-up period (Fig. 8A). The 9-HEPE/9-HETE ratio ranged between 0.1 and 0.9 and correlated poorly with the EPA/AA ratio (Fig. 8B). Over 90% of the total 9-HETE and 9-HEPE plasma levels were only detectable after alkaline hydrolysis (data not shown), indicating that these metabolites circulated primarily in an esterified form. The formation of free 9-HETE, but not free 9-HEPE, was Ca2+-ionophore inducible (Fig. 8C).
Fig. 8.
Effect of EPA/DHA supplementation on the formation of nonenzymatic oxidation products. A: Plasma levels of AA-derived 9-HETE and EPA-derived 9-HEPE at baseline [week 0 (W0)], after maximal EPA/DHA supplementation [week 8 (W8)], and after discontinuation of supplementation [week 16 (W16)]. A general linear model for repeated measurements was used for analysis (A, C) and significant changes are indicated as: *P < 0.05 versus basal level (W0) and #P < 0.05 versus maximum treatment (W8). B: Correlation of the plasma 9-HEPE/9-HETE ratio with the EPA/AA precursor ratio. Metabolite levels in (A) and (B) refer to the total amounts of 9-HETE and 9-HEPE as determined after alkaline hydrolysis. C: Presence of free 9-HETE and 9-HEPE in calcium ionophore treated blood samples.
Effects of EPA/DHA supplementation on clinical parameters
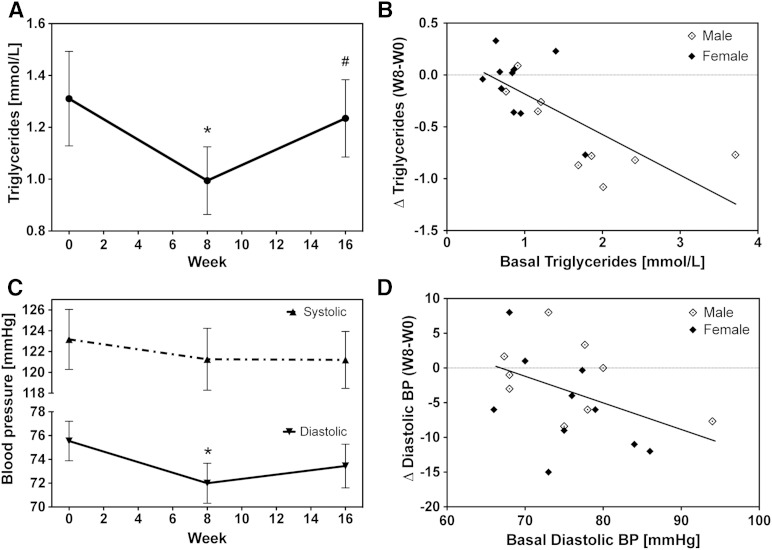
As summarized in Table 1, BMI as well as circulating lipoproteins and total cholesterol remained unchanged over the whole duration of the study. EPA/DHA supplementation caused a significant reduction in plasma triglyceride levels from an average of 1.31 ± 0.2 at week 0 to 0.99 ± 0.1 at week 8 (Fig. 9A). After discontinuation of the supplementation, the triglyceride levels returned almost to baseline values (1.23 ± 0.2). The triglyceride lowering effects achieved at week 8 strongly correlated with the basal triglyceride levels (Fig. 9B). Remarkably, triglyceride levels were almost cut in half in those participants who started with relatively high values of about 2 mmol/l. In contrast, no reduction occurred in participants having already low (<1 mmol/l) triglyceride levels at baseline (Fig. 9B).
Fig. 9.
Effect of EPA/DHA supplementation on clinical risk factors. Shown are the changes in triglyceride levels (A) and blood pressure (C) and their dependencies on the respective basal levels [(B) and (D), respectively]. Data are given as mean ± SEM, n = 19. A general linear model for repeated measurements was used for analysis (A, C) and significant changes are indicated as: *P < 0.05 versus basal level [week 0 (W0)] and #P < 0.05 versus maximum treatment [week 8 (W8)].
The diastolic blood pressure significantly decreased from 75.5 ± 1.7 at week 0 to 72.0 ± 1.7 at week 8 and increased again after discontinuation of EPA/DHA supplementation to 73.4 ± 1.8 at week 16 (Fig. 9C). Changes in systolic blood pressure apparently followed a similar pattern but did not reach significance (Fig. 9C). The diastolic blood pressure lowering effect showed large inter-individual differences and tended to be most pronounced in those participants who displayed relatively high diastolic blood pressure values at baseline (Fig. 9D).
DISCUSSION
The results of the present study demonstrate that the human CYP-eicosanoid pathway is highly susceptible to changes in the dietary supply of the fish oil omega-3 fatty acids, EPA and DHA. In particular, EPA/DHA supplementation caused a pronounced increase in the circulatory and urinary levels of EPA- and DHA-derived CYP-epoxygenase metabolites. These in vivo findings are in line with the results of previous in vitro studies on recombinant enzymes showing that various human AA-metabolizing CYP isoforms accept EPA and DHA as efficient alternative substrates (1, 19–22). Our results also indicate that CYP-eicosanoid formation is most responsive to dietary EPA/DHA supplementation compared with the LOX- and COX-initiated branches of the human AA cascade.
Numerous studies indicated that diets rich in long-chain n-3 PUFAs protect against the development of cardiovascular disease (30, 31, 34–37), although, based on recent meta-analysis of clinical trials, considerable controversy remains regarding the association of n-3 PUFAs and major cardiovascular end points (38, 39). EPA and DHA have anti-inflammatory, anti-platelet, vasodilatory, hypolipidemic, and anti-arrhythmic properties and may thus exert pleiotropic beneficial effects on cardiovascular function (30, 31). Uncertainty exists about the target n-3 PUFA levels to be adjusted by EPA/DHA supplementation for achieving the desired clinical effects. The antiplatelet, anti-inflammatory, and triglyceride-lowering effects apparently require relatively high doses (3–4 g/day), whereas antiarrhythmic effects and reduction of sudden cardiac death can be achieved at doses between 0.5 and 1 g/day (31, 37). In the GISSI-Prevenzione trial, the risk of sudden death was significantly reduced by treating patients after myocardial infarction with 1 g of Omacor® per day (40). In the present study, the omega-3 status of the participants was quantified using the Omega-3 Index. This index represents the percentage of EPA + DHA of total fatty acids in RBCs and strongly correlates with the content of these n-3 PUFAs in other tissues and organs including the heart (41). The incorporation and washout kinetics of EPA and DHA in RBCs proceeds on a time scale of weeks compared with the acute diet-induced changes in plasma phospholipids (days) and the very long-lasting effects in adipose tissues (42, 43). In the normal population, the Omega-3 Index shows a rather broad distribution and ranges from less than 4 to 10–12. We intentionally included only subjects with an Omega-3 Index <6 in our study. This prescreening allowed us to start EPA/DHA supplementation with a group of males and females that were rather uniform in terms of having low to median n-3 PUFA levels at baseline (the Omega-3 Index of the selected participants ranged from 3.6 to 5.9 and was on average 4.9 ± 0.2). Similarly, as reported in a recent study (44), we observed inter-individual differences in the response to EPA/DHA supplementation that strongly correlated with the product of the baseline Omega-3 Index and the dose per kilogram of body weight. According to this correlation, those participants that started with the lowest baseline values and had the lowest body weight showed the highest increase of the Omega-3 Index. Comparing the omega-3 indices achieved after maximal EPA/DHA supplementation, there was a tendency for an increased response of females, who reached an Omega-3 Index of 8.8 ± 0.3 at week 8, compared with 8.0 ± 0.3 in males. However, this apparent sex difference was obviously largely due to the fact that all participants received the same dose independent of their body weight that was on average lower in females than males.
The Omega-3 Index range covered in the present study corresponds to the range where EPA/DHA supplementation is most effective in protecting against fatal cardiac arrhythmia. Based on epidemiological and clinical studies, the Omega-3 Index is inversely correlated with the risk of sudden cardiac death and a 10-fold risk reduction may be achieved by raising the Omega-3 Index from below 4 to more than 8 (45). Searching for changes in eicosanoid formation that may mediate the antiarrhythmic effect of n-3 PUFAs, our study shows that raising the Omega-3 Index from 4 to 8 is accompanied by a strong increase of EPA- and DHA-derived CYP-epoxygenase metabolites. Actually, EEQs increased almost 4-fold and EDPs 2-fold when comparing the plasma levels of these metabolites at week 0 and week 8. Similar marked increases of EPA- and DHA-derived epoxides and vicinal diols were reported from studies that treated healthy volunteers for 4 weeks with 4 g of an EPA/DHA supplement (46) or asthmatic patients for three weeks with 4 g EPA + 2 g DHA per day (47). Even without dietary intervention, the individual differences in the serum concentrations of EPA-derived epoxy- and dihydroxy-metabolites correlated well with the EPA content in RBCs, as shown in a recent study comparing the metabolite profiles in hyper- and normolipidemic men (48). The levels determined in the present study represent the sum of free and esterified metabolites as accessible after alkaline hydrolysis of the plasma samples. We used this method because previous studies showed that greater than 90% of the plasma EETs and related metabolites are esterified to the phospholipids of circulating lipoproteins including LDLs, HDLs, and VLDLs (49, 50). Moreover, we considered both the primary epoxides and the corresponding vicinal diols formed by sEH-mediated hydrolysis as the products that reflect metabolite formation via the CYP-epoxygenase pathway. As tested with a subset of our plasma samples, more than 90% of the total epoxides derived from AA, EPA, and DHA, and 40–50% of the vicinal diols only became detectable after alkaline hydrolysis (data not shown), suggesting that the primary and secondary products of the CYP-epoxygenase pathways were incorporated to different degrees into lipoprotein lipids.
It is noteworthy that the diol/epoxide ratios observed in the human circulation did not follow the substrate preference of the sEH (23). For example, the 17,18-DHEQ/17,18-EEQ ratio was much higher than the 14,15-DHET/14,15-EET ratio in the human plasma samples, whereas the catalytic efficiency of the human recombinant sEH is three times higher with 14,15-EET than with 17,18-EEQ as substrate (23). The mechanisms of how epoxides and diols are released from the tissues and eventually become constituents of circulating lipoproteins are largely unknown, making it difficult to explain this finding. Cells preferentially release DHETs while storing the EETs (9), suggesting that certain diols might be overrepresented in the circulation compared with the epoxide/diol ratios in the tissues where these metabolites were primarily produced.
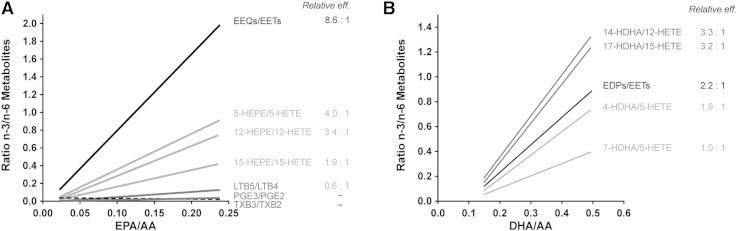
The changes in the metabolite profile produced by the CYP-epoxygenase pathway largely exceeded the diet-induced changes in the relative availability of the precursor fatty acids. This result suggests that EPA and DHA were efficiently metabolized by the CYP epoxygenases even when AA was still present as the predominant long-chain PUFA. The ratio between EPA- and AA-derived as well, as DHA- and AA-derived epoxy-metabolites, strongly correlated with the corresponding precursor PUFA ratios. The slopes of the correlation lines indicate that EPA was metabolized to EEQs with an 8.6-fold higher efficiency than AA to EETs, and DHA was metabolized to EDPs with a 2.2-fold higher efficiency than AA to EETs. Linear relationships were also obtained when the ratios of EPA-/AA-derived or DHA-/AA-derived metabolite pairs originating from the LOX and COX pathways were plotted against the corresponding precursor PUFA ratios. As summarized in Fig. 10A, the slopes of the correlation lines suggest that the susceptibility to EPA was by far the highest for the CYP-epoxygenase pathway followed by 5-, 12-, and 15-LOX-dependent formation of monohydroxy-metabolites. In addition, 18-HEPE, potentially arising from nonenzymatic oxidation or CYP-catalyzed monooxygenation (51), was among the EPA-derived metabolites showing the highest increase upon EPA/DHA supplementation. In comparison, 5-LOX/LTA4-hydrolase-dependent leukotriene and COX-dependent prostaglandin E formation were much less responsive. The AA-derived metabolites LTB4 and PGE2 remained clearly predominant and only a weak increase of the EPA-derived LTB5 and PGE3 occurred upon EPA/DHA supplementation. Least susceptible was thromboxane formation, where we could not detect any significant effect of EPA/DHA supplementation on the TXB3/TXB2 ratio produced in whole blood samples after Ca2+-ionophore stimulation. The different pathways also varied in their response to increased DHA availability (Fig. 10B). The over proportional increases of 14-HDHA and 17-HDHA suggest that DHA was preferred over AA by the 12-LOX and 15-LOX pathways.
Fig. 10.
Comparison of the susceptibilities of the three branches of the human AA-cascade to dietary EPA/DHA supplementation. This figure summarizes the linear correlations obtained for the corresponding metabolite precursor fatty acid pairs. The slopes of the correlation lines were taken as a measure for the relative efficiencies by which EPA and AA (A) or DHA and AA (B) were utilized by the different enzymatic pathways of eicosanoid formation.
The marked elevation of circulating 17-HDHA and 18-HEPE levels is of particular interest because these DHA- and EPA-derived metabolites are the precursors of the D- and E-series of resolvins. Resolvins are members of a novel class of lipid mediators that have highly potent anti-inflammatory and pro-resolution properties (52, 53). We also directly searched for the presence of resolvin D1 in plasma samples but, for unknown reasons, were unable to detect this metabolite (limit of detection 6 pg/ml), neither after extracting the plasma samples with or without prior alkaline hydrolysis nor in whole blood samples after Ca2+-ionophore stimulation.
The first studies investigating the effect of fish oil on eicosanoid formation were stimulated by the seminal observation in the 1970s of significantly lower myocardial infarction rates in Greenland Inuits, who traditionally live on EPA/DHA-rich seafood, compared with Danish controls (54). Subsequent world-wide epidemiological studies revealed the general existence of striking cardiovascular mortality differences between populations living on n-6 PUFA- versus n-3 PUFA-rich diets (55). EPA was shown to compete with AA to yield less pro-aggregatory (TXA3 vs. TXA2) and less pro-inflammatory eicosanoids (LTB5 vs. LTB4) via the COX- and LOX-dependent pathways (54, 56). In contrast, PGI3, formed from EPA in the endothelium, acts with the same potency as a vasodilator and inhibitor of platelet aggregation as its AA-derived counterpart PGI2. Indeed, a favorable shift of the thromboxane/PGI ratio to a more anti-aggregatory and vasodilatory state was shown in Inuits, as well as in persons after long-term intake of high amounts of EPA (10–15 g/day) (57, 58). In the present study, the extent of EPA/DHA supplementation was obviously not sufficient to cause pronounced changes in thromboxane and PGI formation. As reviewed recently, the (PGI2 + PGI3)/thromboxane ratio starts to increase significantly only after achieving EPA/AA ratios higher than 0.2:1 (59), and thus at EPA/AA ratios clearly above those maximally reached in the present study (0.15:1). EPA and AA interfere at the level of virtually all enzymes and receptors involved in prostanoid formation and signaling (60). In the present study, we analyzed only a few examples of all the 3-series/2-series pairs of prostanoids potentially formed from EPA/AA, and we looked only for the circulatory and urinary levels of these compounds or their stable metabolites. Thus, we cannot exclude that the competition between EPA and AA was more efficient in the production of other prostaglandin families. Moreover, we had no access to potentially highly important local changes such as the formation of 15d-PGJ3 in adipose tissue (61). A further limitation may come from the use of currently available surrogate parameters (stable prostaglandin degradation products) that only partially reflect the actual utilization of EPA by COX enzymes (62). However, based on the properties of the COXs, the capacity of COX-1 to metabolize EPA may be limited by the availability of endogenous hydroperoxides, and COX-2 preferentially oxygenates AA when EPA is simultaneously present (60). We also could not detect a major shift from LTB4 to LTB5 formation. The lack of this effect is in line with similar results of previous studies that also used n-3 PUFAs in a relatively low, but recommended, cardioprotective dose (63).
Our study included a group of relatively young and healthy volunteers. Thus, we did not expect major effects on clinical parameters that were all in the normal range at baseline. Surprisingly, however, we observed significant reductions of diastolic blood pressure and triglyceride levels upon EPA/DHA supplementation. These effects were mostly expressed in participants featuring the highest baseline values and became reversed after discontinuing the treatment. Anti-hypertensive and triglyceride-lowering effects of n-3 PUFAs are well-documented in patients, and the use of EPA/DHA supplements has been recommended for the treatment of hyperlipidemia (64–67). Triglyceride lowering by n-3 PUFAs involves changes in gene expression that coordinately suppress lipogenesis, promote lipolysis, and upregulate fatty acid β-oxidation (68). The blood pressure-lowering effect of n-3 PUFAs requires activation of Ca2+-dependent potassium (BK) channels in vascular smooth muscle cells, as indicated by a recent study showing that DHA decreases blood pressure in wild-type but not in BK channel knockout mice (69). BK channels are also the main effector of the vasodilatory action of CYP-epoxygenase metabolites (18, 25, 70–72), suggesting that the effects attributed to n-3 PUFAs might actually be mediated by the increased formation of EEQs and EDPs. In line with this hypothesis, pharmacological sEH inhibition increased the endogenous levels of EEQs and EDPs and enhanced the antihypertensive effect of EPA/DHA supplementation in angiotensin II-hypertensive mice (73). Moreover, Cyp1a1 knockout mice showed elevated blood pressure and reduced vasodilation to n-3 PUFAs, presumably due to reduced production of 17,18-EEQ and 19,20-EDP (74).
The molecular mechanisms mediating the beneficial cardiovascular effects of n-3 PUFAs are only partially understood and include changes in membrane structures and gene expression, direct interactions with ion channels, and alterations in eicosanoid biosynthesis (60, 75–80). The results of the present study expand and specify the general hypothesis that dietary EPA/DHA supplementation causes pronounced changes in the endogenous eicosanoid profile by providing alternative substrates for all three branches of the AA cascade. We focused on the effects of relatively low doses of EPA/DHA that were previously shown to mediate protection against heart failure and fetal ventricular arrhythmia. Specifically, our data show that raising the Omega-3 Index from about 4 to 8 in humans primarily results in a large increase of EPA-derived CYP-epoxygenase metabolites followed by increases of EPA- and DHA-derived hydroxy-metabolites including, in particular, the precursors of the resolvin family. In contrast, the classical effects on leukotriene and thromboxane formation remained rather weak or were not expressed at all within the Omega-3 Index range covered by the present study.
Thus far, it is unclear whether the alterations observed in the human circulatory and urinary eicosanoid profiles reflect corresponding changes in the heart or other organs and tissues. Our previous study in rats demonstrated that the shift from AA-derived CYP-epoxygenase metabolites to EPA- and DHA-derived CYP-epoxygenase metabolites occurred with largely identical efficiencies in the plasma, heart, kidney, liver, lung, and pancreas (22). Moreover, the circulating epoxy-metabolites that are predominantly esterified with lipoprotein lipids may themselves become biologically active in other tissues after being released by lipoprotein lipases (81). There is increasing evidence for a cardioprotective role of CYP-epoxygenase metabolites from studies in animal models of ischemia/reperfusion injury, maladaptive cardiac hypertrophy, and arrhythmia (12, 24, 82). We showed that 17,18-EEQ and 19,20-EDP are potential candidates for mediating the antiarrhythmic effect of n-3 PUFAs (22). Thus, the formation of these metabolites may provide a suitable biomarker for evaluating the outcome of clinical studies investigating the effects of EPA/DHA supplementation in patients after myocardial infarction or suffering from atrial fibrillation.
Supplementary Material
Acknowledgments
The authors thank Christel Andrée and Inci Dogan for excellent technical assistance.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- BK
- Ca2+ -activated potassium channel
- COX
- cyclooxygenase
- CYP
- cytochrome P450
- DHDP
- dihydroxydocosapentaenoic acid
- DHEQ
- dihydroxyeicosatetraenoic acid
- DHET
- dihydroxyeicosatrienoic acid
- EDP
- epoxydocosapentaenoic acid
- EEQ
- epoxyeicosatetraenoic acid
- EET
- epoxyeicosatrienoic acid
- HDHA
- hydroxydocosahexaenoic acid
- HEPE
- hydroxyeicosapentaenoic acid
- LOX
- lipoxygenase
- LT
- leukotriene
- PGE
- prostaglandin E
- PGI
- prostacyclin
- RBC
- red blood cell
- sEH
- soluble epoxide hydrolase
- SPE
- solid phase extraction
- TX
- thromboxane
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG): Schu822/5; FOR 1054. The authors do not have any financial conflicts of interest to disclose.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four tables.
REFERENCES
- 1.Konkel A., Schunck W. H. 2011. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim. Biophys. Acta. 1814: 210–222. [DOI] [PubMed] [Google Scholar]
- 2.Capdevila J. H., Falck J. R. 2002. Biochemical and molecular properties of the cytochrome P450 arachidonic acid monooxygenases. Prostaglandins Other Lipid Mediat. 68–69: 325–344. [DOI] [PubMed] [Google Scholar]
- 3.Funk C. D. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294: 1871–1875. [DOI] [PubMed] [Google Scholar]
- 4.Buczynski M. W., Dumlao D. S., Dennis E. A. 2009. Proteomics. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 50: 1015–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroetz D. L., Zeldin D. C. 2002. Cytochrome P450 pathways of arachidonic acid metabolism. Curr. Opin. Lipidol. 13: 273–283. [DOI] [PubMed] [Google Scholar]
- 6.Campbell W. B., Fleming I. 2010. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 459: 881–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGiff J. C., Quilley J. 1999. 20-HETE and the kidney: resolution of old problems and new beginnings. Am. J. Physiol. 277: R607–R623. [DOI] [PubMed] [Google Scholar]
- 8.Roman R. J. 2002. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 82: 131–185. [DOI] [PubMed] [Google Scholar]
- 9.Spector A. A., Norris A. W. 2007. Action of epoxyeicosatrienoic acids on cellular function. Am. J. Physiol. Cell Physiol. 292: C996–C1012. [DOI] [PubMed] [Google Scholar]
- 10.Harris T. R., Hammock B. D. 2013. Soluble epoxide hydrolase: gene structure, expression and deletion. Gene. 526: 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C. C., Gupta T., Garcia V., Ding Y., Schwartzman M. L. 2014. 20-HETE and blood pressure regulation: clinical implications. Cardiol Rev. 22: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seubert J. M., Zeldin D. C., Nithipatikom K., Gross G. J. 2007. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 82: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoff U., Lukitsch I., Chaykovska L., Ladwig M., Arnold C., Manthati V. L., Fuller T. F., Schneider W., Gollasch M., Muller D. N., et al. 2011. Inhibition of 20-HETE synthesis and action protects the kidney from ischemia/reperfusion injury. Kidney Int. 79: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regner K. R., Zuk A., Van Why S. K., Shames B. D., Ryan R. P., Falck J. R., Manthati V. L., McMullen M. E., Ledbetter S. R., Roman R. J. 2009. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int. 75: 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imig J. D., Simpkins A. N., Renic M., Harder D. R. 2011. Cytochrome P450 eicosanoids and cerebral vascular function. Expert Rev. Mol. Med. 13: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Y., Theken K. N., Lee C. R. 2010. Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J. Mol. Cell. Cardiol. 48: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imig J. D., Hammock B. D. 2009. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 8: 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauterbach B., Barbosa-Sicard E., Wang M. H., Honeck H., Kargel E., Theuer J., Schwartzman M. L., Haller H., Luft F. C., Gollasch M., et al. 2002. Cytochrome P450-dependent eicosapentaenoic acid metabolites are novel BK channel activators. Hypertension. 39: 609–613. [DOI] [PubMed] [Google Scholar]
- 19.Barbosa-Sicard E., Markovic M., Honeck H., Christ B., Muller D. N., Schunck W. H. 2005. Eicosapentaenoic acid metabolism by cytochrome P450 enzymes of the CYP2C subfamily. Biochem. Biophys. Res. Commun. 329: 1275–1281. [DOI] [PubMed] [Google Scholar]
- 20.Fer M., Dreano Y., Lucas D., Corcos L., Salaun J. P., Berthou F., Amet Y. 2008. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch. Biochem. Biophys. 471: 116–125. [DOI] [PubMed] [Google Scholar]
- 21.Fer M., Corcos L., Dreano Y., Plee-Gautier E., Salaun J. P., Berthou F., Amet Y. 2008. Cytochromes P450 from family 4 are the main omega hydroxylating enzymes in humans: CYP4F3B is the prominent player in PUFA metabolism. J. Lipid Res. 49: 2379–2389. [DOI] [PubMed] [Google Scholar]
- 22.Arnold C., Markovic M., Blossey K., Wallukat G., Fischer R., Dechend R., Konkel A., von Schacky C., Luft F. C., Muller D. N., et al. 2010. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of omega-3 fatty acids. J. Biol. Chem. 285: 32720–32733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morisseau C., Inceoglu B., Schmelzer K., Tsai H. J., Jinks S. L., Hegedus C. M., Hammock B. D. 2010. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J. Lipid Res. 51: 3481–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westphal C., Konkel A., Schunck W. H. 2011. CYP-eicosanoids–a new link between omega-3 fatty acids and cardiac disease? Prostaglandins Other Lipid Mediat. 96: 99–108. [DOI] [PubMed] [Google Scholar]
- 25.Ye D., Zhang D., Oltman C., Dellsperger K., Lee H. C., VanRollins M. 2002. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 303: 768–776. [DOI] [PubMed] [Google Scholar]
- 26.Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D. C., Liao J. K. 1999. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 285: 1276–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morin C., Sirois M., Echave V., Albadine R., Rousseau E. 2010. 17,18-epoxyeicosatetraenoic acid targets PPARγ and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: role of soluble epoxide hydrolase. Am. J. Respir. Cell Mol. Biol. 43: 564–575. [DOI] [PubMed] [Google Scholar]
- 28.Panigrahy D., Edin M. L., Lee C. R., Huang S., Bielenberg D. R., Butterfield C. E., Barnes C. M., Mammoto A., Mammoto T., Luria A., et al. 2012. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J. Clin. Invest. 122: 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G., Panigrahy D., Mahakian L. M., Yang J., Liu J. Y., Stephen Lee K. S., Wettersten H. I., Ulu A., Hu X., Tam S., et al. 2013. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc. Natl. Acad. Sci. USA. 110: 6530–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kris-Etherton P. M., Harris W. S., Appel L. J. 2002. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 106: 2747–2757. [DOI] [PubMed] [Google Scholar]
- 31.Lavie C. J., Milani R. V., Mehra M. R., Ventura H. O. 2009. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 54: 585–594. [DOI] [PubMed] [Google Scholar]
- 32.Harris W. S., Von Schacky C. 2004. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev. Med. 39: 212–220. [DOI] [PubMed] [Google Scholar]
- 33.Gomolka B., Siegert E., Blossey K., Schunck W. H., Rothe M., Weylandt K. H. 2011. Analysis of omega-3 and omega-6 fatty acid-derived lipid metabolite formation in human and mouse blood samples. Prostaglandins Other Lipid Mediat. 94: 81–87. [DOI] [PubMed] [Google Scholar]
- 34.Mozaffarian D. 2008. Fish and n-3 fatty acids for the prevention of fatal coronary heart disease and sudden cardiac death. Am. J. Clin. Nutr. 87: 1991S–1996S. [DOI] [PubMed] [Google Scholar]
- 35.Simopoulos A. P. 2008. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. (Maywood). 233: 674–688. [DOI] [PubMed] [Google Scholar]
- 36.Leaf A., Kang J. X., Xiao Y. F., Billman G. E. 2003. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 107: 2646–2652. [DOI] [PubMed] [Google Scholar]
- 37.De Caterina R. 2011. n-3 fatty acids in cardiovascular disease. N. Engl. J. Med. 364: 2439–2450. [DOI] [PubMed] [Google Scholar]
- 38.Hooper L., Thompson R. L., Harrison R. A., Summerbell C. D., Ness A. R., Moore H. J., Worthington H. V., Durrington P. N., Higgins J. P., Capps N. E., et al. 2006. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 332: 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizos E. C., Ntzani E. E., Bika E., Kostapanos M. S., Elisaf M. S. 2012. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 308: 1024–1033. [DOI] [PubMed] [Google Scholar]
- 40.Marchioli R., Barzi F., Bomba E., Chieffo C., Di Gregorio D., Di Mascio R., Franzosi M. G., Geraci E., Levantesi G., Maggioni A. P., et al. 2002. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 105: 1897–1903. [DOI] [PubMed] [Google Scholar]
- 41.Harris W. S., Sands S. A., Windsor S. L., Ali H. A., Stevens T. L., Magalski A., Porter C. B., Borkon A. M. 2004. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 110: 1645–1649. [DOI] [PubMed] [Google Scholar]
- 42.Katan M. B., Deslypere J. P., van Birgelen A. P., Penders M., Zegwaard M. 1997. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J. Lipid Res. 38: 2012–2022. [PubMed] [Google Scholar]
- 43.Arterburn L. M., Hall E. B., Oken H. 2006. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 83: 1467S–1476S. [DOI] [PubMed] [Google Scholar]
- 44.Keenan A. H., Pedersen T. L., Fillaus K., Larson M. K., Shearer G. C., Newman J. W. 2012. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. J. Lipid Res. 53: 1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Schacky C. 2009. Cardiovascular disease prevention and treatment. Prostaglandins Leukot. Essent. Fatty Acids. 81: 193–198. [DOI] [PubMed] [Google Scholar]
- 46.Shearer G. C., Harris W. S., Pedersen T. L., Newman J. W. 2010. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J. Lipid Res. 51: 2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundström S. L., Yang J., Brannan J. D., Haeggström J. Z., Hammock B. D., Nair P., O’Byrne P., Dahlén S. E., Wheelock C. E. 2013. Lipid mediator serum profiles in asthmatics significantly shift following dietary supplementation with omega-3 fatty acids. Mol. Nutr. Food Res. 57: 1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuchardt J. P., Schmidt S., Kressel G., Dong H., Willenberg I., Hammock B. D., Hahn A., Schebb N. H. 2013. Comparison of free serum oxylipin concentrations in hyper- vs. normolipidemic men. Prostaglandins Leukot. Essent. Fatty Acids. 89: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karara A., Wei S., Spady D., Swift L., Capdevila J. H., Falck J. R. 1992. Arachidonic acid epoxygenase: structural characterization and quantification of epoxyeicosatrienoates in plasma. Biochem. Biophys. Res. Commun. 182: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 50.Shearer G. C., Newman J. W. 2008. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins Leukot. Essent. Fatty Acids. 79: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weylandt K. H., Chiu C. Y., Gomolka B., Waechter S. F., Wiedenmann B. 2012. Omega-3 fatty acids and their lipid mediators: towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 97: 73–82. [DOI] [PubMed] [Google Scholar]
- 52.Serhan C. N., Chiang N., Van Dyke T. E. 2008. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serhan C. N., Yang R., Martinod K., Kasuga K., Pillai P. S., Porter T. F., Oh S. F., Spite M. 2009. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 206: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dyerberg J., Bang H. O., Stoffersen E., Moncada S., Vane J. R. 1978. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 2: 117–119. [DOI] [PubMed] [Google Scholar]
- 55.Lands W. E. 2005. Dietary fat and health: the evidence and the politics of prevention: careful use of dietary fats can improve life and prevent disease. Ann. N. Y. Acad. Sci. 1055: 179–192. [DOI] [PubMed] [Google Scholar]
- 56.Terano T., Salmon J. A., Moncada S. 1984. Biosynthesis and biological activity of leukotriene B5. Prostaglandins. 27: 217–232. [DOI] [PubMed] [Google Scholar]
- 57.Fischer S., Weber P. C., Dyerberg J. 1986. The prostacyclin/thromboxane balance is favourably shifted in Greenland Eskimos. Prostaglandins. 32: 235–241. [DOI] [PubMed] [Google Scholar]
- 58.von Schacky C., Fischer S., Weber P. C. 1985. Long-term effects of dietary marine omega-3 fatty acids upon plasma and cellular lipids, platelet function, and eicosanoid formation in humans. J. Clin. Invest. 76: 1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohnishi H., Saito Y. 2013. Eicosapentaenoic acid (EPA) reduces cardiovascular events: relationship with the EPA/arachidonic acid ratio. J. Atheroscler. Thromb. 20: 861–877. [DOI] [PubMed] [Google Scholar]
- 60.Wada M., DeLong C. J., Hong Y. H., Rieke C. J., Song I., Sidhu R. S., Yuan C., Warnock M., Schmaier A. H., Yokoyama C., et al. 2007. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J. Biol. Chem. 282: 22254–22266. [DOI] [PubMed] [Google Scholar]
- 61.Lefils-Lacourtablaise J., Socorro M., Geloen A., Daira P., Debard C., Loizon E., Guichardant M., Dominguez Z., Vidal H., Lagarde M., et al. 2013. The eicosapentaenoic acid metabolite 15-deoxy-δ(12,14)-prostaglandin J3 increases adiponectin secretion by adipocytes partly via a PPARγ-dependent mechanism. PLoS ONE. 8: e63997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuklev D. V., Hankin J. A., Uhlson C. L., Hong Y. H., Murphy R. C., Smith W. L. 2013. Major urinary metabolites of 6-keto-prostaglandin F2α in mice. J. Lipid Res. 54: 1906–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nielsen M. S., Gammelmark A., Madsen T., Obel T., Aardestrup I., Schmidt E. B. 2012. The effect of low-dose marine n-3 fatty acids on the biosynthesis of pro-inflammatory 5-lipoxygenase pathway metabolites in overweight subjects: a randomized controlled trial. Prostaglandins Leukot. Essent. Fatty Acids. 87: 43–48. [DOI] [PubMed] [Google Scholar]
- 64.Morris M. C., Sacks F., Rosner B. 1993. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 88: 523–533. [DOI] [PubMed] [Google Scholar]
- 65.Bays H. E., Tighe A. P., Sadovsky R., Davidson M. H. 2008. Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications. Expert Rev. Cardiovasc. Ther. 6: 391–409. [DOI] [PubMed] [Google Scholar]
- 66.Mozaffarian D., Wu J. H. 2011. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 58: 2047–2067. [DOI] [PubMed] [Google Scholar]
- 67.Cabo J., Alonso R., Mata P. 2012. Omega-3 fatty acids and blood pressure. Br. J. Nutr. 107(Suppl 2): S195–S200. [DOI] [PubMed] [Google Scholar]
- 68.Davidson M. H. 2006. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am. J. Cardiol. 98: 27i–33i. [DOI] [PubMed] [Google Scholar]
- 69.Hoshi T., Wissuwa B., Tian Y., Tajima N., Xu R., Bauer M., Heinemann S. H., Hou S. 2013. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca2+-dependent K+ channels. Proc. Natl. Acad. Sci. USA. 110: 4816–4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campbell W. B., Falck J. R. 2007. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 49: 590–596. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y., Oltman C. L., Lu T., Lee H. C., Dellsperger K. C., VanRollins M. 2001. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am. J. Physiol. Heart Circ. Physiol. 280: H2430–H2440. [DOI] [PubMed] [Google Scholar]
- 72.Hercule H. C., Salanova B., Essin K., Honeck H., Falck J. R., Sausbier M., Ruth P., Schunck W. H., Luft F. C., Gollasch M. 2007. The vasodilator 17,18-epoxyeicosatetraenoic acid targets the pore-forming BK alpha channel subunit in rodents. Exp. Physiol. 92: 1067–1076. [DOI] [PubMed] [Google Scholar]
- 73.Ulu A., Harris T. R., Morisseau C., Miyabe C., Inoue H., Schuster G., Dong H., Iosif A. M., Liu J. Y., Weiss R. H., et al. 2013. Anti-inflammatory effects of omega-3 polyunsaturated fatty acids and soluble epoxide hydrolase inhibitors in angiotensin-II-dependent hypertension. J. Cardiovasc. Pharmacol. 62: 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agbor L. N., Walsh M. T., Boberg J. R., Walker M. K. 2012. Elevated blood pressure in cytochrome P4501A1 knockout mice is associated with reduced vasodilation to omega-3 polyunsaturated fatty acids. Toxicol. Appl. Pharmacol. 264: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chapkin R. S., McMurray D. N., Davidson L. A., Patil B. S., Fan Y. Y., Lupton J. R. 2008. Bioactive dietary long-chain fatty acids: emerging mechanisms of action. Br. J. Nutr. 100: 1152–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jump D. B. 2002. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 277: 8755–8758. [DOI] [PubMed] [Google Scholar]
- 77.Deckelbaum R. J., Worgall T. S., Seo T. 2006. n-3 fatty acids and gene expression. Am. J. Clin. Nutr. 83: 1520S–1525S. [DOI] [PubMed] [Google Scholar]
- 78.Xiao Y. F., Sigg D. C., Leaf A. 2005. The antiarrhythmic effect of n-3 polyunsaturated fatty acids: modulation of cardiac ion channels as a potential mechanism. J. Membr. Biol. 206: 141–154. [DOI] [PubMed] [Google Scholar]
- 79.Schmitz G., Ecker J. 2008. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 47: 147–155. [DOI] [PubMed] [Google Scholar]
- 80.Calder P. C. 2006. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 83: 1505S–1519S. [DOI] [PubMed] [Google Scholar]
- 81.Shearer G. C., Newman J. W. 2009. Impact of circulating esterified eicosanoids and other oxylipins on endothelial function. Curr. Atheroscler. Rep. 11: 403–410. [DOI] [PubMed] [Google Scholar]
- 82.Westphal C., Spallek B., Konkel A., Marko L., Qadri F., Degraff L. M., Schubert C., Bradbury J. A., Regitz-Zagrosek V., Falck J. R., et al. 2013. CYP2J2 overexpression protects against arrhythmia susceptibility in cardiac hypertrophy. PLoS ONE. 8: e73490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.