Abstract
A new mechanism for formation of 7-ketocholesterol was recently described involving cytochrome P-450 (CYP)7A1-catalyzed conversion of 7-dehydrocholesterol into 7-ketocholesterol with cholesterol-7,8-epoxide as a side product. Some patients with cerebrotendinous xanthomatosis (CTX) and all patients with Smith-Lemli-Opitz syndrome (SLO) have markedly increased levels of 7-dehydrocholesterol in plasma and tissues. In addition, the former patients have markedly upregulated CYP7A1. We hypothesized that these patients may produce 7-ketocholesterol from 7-dehydrocholesterol with formation of cholesterol-7,8-epoxide as a side product. In accord with this hypothesis, two patients with CTX were found to have increased levels of 7-ketocholesterol and 7-dehydrocholesterol, as well as a significant level of cholesterol-7,8-epoxide. The latter steroid was not detectable in plasma from healthy volunteers. Downregulation of CYP7A1 activity by treatment with chenodeoxycholic acid reduced the levels of 7-ketocholesterol in parallel with decreased levels of 7-dehydrocholesterol and cholesterol-7,8-epoxide. Three patients with SLO were found to have markedly elevated levels of 7-ketocholesterol as well as high levels of cholesterol-7,8-epoxide. The results support the hypothesis that 7-dehydrocholesterol is a precursor to 7-ketocholesterol in SLO and some patients with CTX.
Keywords: cerebrotendinous xanthomatosis; Smith-Lemli-Opitz syndrome; cholesterol 7,8-epoxide; cytochrome P-450 7A1; liquid chromatography-mass spectrometryn
In 1986, one of us (I.B.) reported that patients with cerebrotendinous xanthomatosis (CTX) have elevated levels of 7-ketocholesterol (1). This was also later confirmed by another group (2). A possible explanation was presented by Jessup and Brown (3), who reported that 7-ketocholesterol is a substrate for sterol 27-hydroxylase [cytochrome P-450 (CYP)27A1] that is lacking in patients with CTX. The high levels of 7-ketocholesterol may thus be a consequence of a reduced metabolism.
Treating CTX patients with chenodeoxycholic acid suppresses the cholesterol 7α-hydroxylase (CYP7A1) and markedly reduces the production of 7α-hydroxylated products in the circulation, such as 7α-hydroxycholesterol and 7α-hydroxy-4-cholesten-3-one (4, 5). The latter oxysterol is a precursor to cholestanol, and the treatment reduces production of cholestanol as well as the size of the xanthomas [for a review, see (4)].
In order to confirm the diagnosis and to follow the effect of treatment with bile acids (chenodeoxycholic acid), we have analyzed serum samples from a number of patients with CTX. In some of the CTX patients treated with bile acids, we found that the treatment reduced not only the levels of 7α-hydroxycholesterol but also the levels of 7-ketocholesterol in the circulation (I. Björkhem, unpublished observation). This is not consistent with the hypothesis that high levels of 7-ketocholesterol in patients with CTX are due to the reduced metabolism only.
The possibility must be considered that 7-ketocholesterol is a metabolite of 7α-hydroxycholesterol that accumulates in patients with CTX due to the marked upregulation of the cholesterol 7α-hydroxylase (CYP7A1). The latter upregulation is a consequence of the reduced negative feedback inhibition of this enzyme by bile acids. While a significant in vivo interconversion between 7β-hydroxycholesterol and 7-ketocholesterol in humans has been documented (6), most probably mediated by the 11β-hydroxysteroid dehydrogenase (7), no evidence has been given for a conversion of 7α-hydroxycholesterol into 7-ketocholesterol in humans. The possibility has been clearly excluded that there is a conversion of 7-ketocholesterol into 7α-hydroxycholesterol in vivo in humans (6).
Very recently, two of us (F.P.G. and K.T.) described a new pathway from 7-dehydrocholesterol into 7-ketocholesterol with cholesterol-7,8-epoxide (cholest-5-en-7,8-epoxide) as a side product. In parallel with this there is a conversion of lathosterol into a mixture of 7-ketocholestanol and cholestanol-7,8-epoxide (8). Both conversions were found to be catalyzed by the human cholesterol 7α-hydroxylase (CYP7A1). If this mechanism is of importance under in vivo conditions, upregulation of CYP7A1 may be associated with increased formation of 7-ketocholesterol, provided that sufficient levels of 7-dehydrocholesterol are present. Furthermore, production of cholesterol-7,8-epoxide would also be expected. It should be emphasized that it has been clearly excluded that there is a direct production of 7-ketocholesterol from cholesterol by the CYP7A1 enzyme (9).
Given the marked upregulation of CYP7A1 in the liver of patients with untreated CTX (1, 10) and the resulting upregulation of cholesterol synthesis with increased levels of 7-dehydrocholesterol in at least some of these patients (11), we considered the possibility that at least part of the accumulation of 7-ketocholesterol in CTX is a consequence of CYP7A1-mediated conversion of 7-dehydrocholesterol.
Patients with Smith-Lemli-Opitz syndrome (SLO) have markedly increased levels of 7-dehydrocholesterol in the circulation and tissues as a consequence of a defect in 7-dehydrocholesterol 7-reductase (12). These patients have normal or low activity of CYP7A1 in the liver (13). We considered the possibility that these patients might also have increased levels of 7-ketocholesterol in the circulation. After completion of the present work, a study was published reporting that 7-ketocholesterol is present at elevated levels in the circulation of SLO patients (14). Interestingly, the levels of 7-ketocholesterol correlated with the severity score of the SLO. The possibility that this steroid is formed ex vivo from 7-dehydrocholesterol was clearly excluded and 7-ketocholesterol is not among the products formed from 7-dehydrocholesterol under conditions of lipid peroxidation (15), although it is from cholesterol by ex vivo oxidation. The possibility was discussed that the elevated levels of 7-ketocholesterol in patients with SLO may be formed by the above CYP7A1-mediated mechanism.
MATERIAL AND METHODS
Patients
CTX patient 1.
CTX patient 1 is a 32-year-old woman referred to the lipid clinic in Oslo due to large xanthomas on both Achilles tendons and normal plasma lipid values. The xanthomas developed during her teens. There had been a progressive cognitive decline from the she started school at the age of 7. She also had premature cataracts, which were surgically removed. These features of her medical history triggered the suspicion for CTX, a diagnosis that was verified by markedly reduced plasma levels of 27-hydroxycholesterol (Table 1). Cerebral MRI, echocardiography, Doppler of the carotid arteries, and stress ECG showed normal findings. Treatment was initiated with chenodeoxycholic acid (250 mg three times daily) in May 2009. Due to lack of sufficient effect, the dose was doubled after a year.
TABLE 1.
Levels of oxysterols in three patients with CTX before and after treatment with chenodeoxycholic acid
| Patient 1 | Patient 2 | Patient 3 | ||||
| Steroid | Before | Aftera | Before | Afterb | Before | Afterc |
| 27-Hydroxycholesterol (ng/ml) | <5 | <5 | <5 | <5 | 8 | 7 |
| 7α-Hydroxycholesterol (μg/ml) | 3.4 | 0.4 | 2.2 | 0.04 | 0.6 | 0.01 |
| 7β-Hydroxycholesterol (μg/ml) | 1.0 | 0.3 | 0.3 | 0.05 | 0.05 | 0.01. |
| 7-Ketocholesterol (μg/ml) | 2.1 | 0.3 | 0.6 | 0.1 | 0.03 | 0.03 |
Values were determined by GC-MS and are for the sum of esterified and nonesterified sterols. Normal levels of 27-hydroxycholesterol, 90–230 ng/ml; 7α-hydroxycholesterol, <0.12 μg/ml; 7β-hydroxycholesterol, <0.02 μg/ml; 7-ketocholesterol, <0.03 μg/ml (16).
The patients were treated with 750 mg/day of chenodeoxycholic acid for 9 months.
The patients were treated with 500 mg/day of chenodeoxycholic acid for 6 months.
The patients were treated with 1,000 mg/day of chenodeoxycholic acid for 12 months.
Plasma samples were obtained from the patient in May 2009, March 2012, and March 2013 (Table 1).
CTX patient 2.
CTX patient 2 is a woman diagnosed with CTX at the age of 38 years. In childhood, she had unspecific chronic diarrhea and in her teens she was operated on for bilateral cataracts. When she was about 15 years old, she developed problems with balance and concentration and had her first seizures. From the age of 30, the neurological problems progressed further with cerebellar ataxia, dysphagia, and decline in cognitive function. At age 37, cerebral MRI showed cerebellar atrophy. Swelling of the Achilles tendons due to xanthoma was noted and the diagnosis of CTX was confirmed by the demonstration of very low levels of 27-hydroxycholesterol in combination with high levels of 7α-hydroxycholesterol. The patient was treated with chenodeoxycholic acid (500 mg/day) (Table 1).
CTX patient 3.
CTX patient 3 is a female who developed Achilles xanthoma when she was 18–20 years of age. Initially, the condition was believed to be due to hypercholesterolemia, but treatment with statins was unsuccessful. At the age of 32, the diagnosis of CTX was established by the demonstration of low levels of 27-hydroxcholesterol and high excretion of 25-hydroxylated bile alcohols. Cerebral MRI showed normal findings. The patient was treated with chenodeoxycholic acid (750 mg/day) (Table 1). A detailed characterization of this patient with a very mild form of CTX has been published (16).
SLO patient 1.
SLO patient 1 is a 23-year-old woman in whom the diagnosis was suspected in infancy because of microcephalus, cleft hard palate, and feeding difficulties with pronounced vomiting. It was confirmed at the age of 9, when the cholesterol deficiency was discovered. She was then able to walk with a frame, had no speech, and was fed orally. She had a severe photosensitivity and started treatment with cholesterol and bile acids. Her light tolerance improved, which was verified by UVA-light tests. She now has a stable condition, is wheel-chair dependent, and has some autistic behavior in addition to severe mental retardation.
SLO patient 2.
SLO patient 2 is a 6-year-old girl born with typical features, cleft hard palate, and severe feeding difficulties. The SLO diagnosis was verified at the age of 3 months. She has had bilateral cataracts, has a hearing defect, and is fed by a gastrostomy. She has severe mental retardation, cannot sit unsupported, and has no speech. She has an increased susceptibility to infections and a moderate photosensitivity. She has been treated with cholesterol since the diagnosis was confirmed with little effect on her sterol pattern.
SLO patient 3.
SLO patient 3 is an 11-year-old girl in whom the diagnosis was obtained early. She has severe mental retardation and feeding difficulties, with frequent vomiting. At present she is fed by a gastrostomy. She was treated with cholesterol during the first years of life, but at present is not using any medications.
Healthy volunteers
Blood was collected from six healthy subjects (laboratory staff, four males and two females, age 31–82 years, mean age 64 years).
Ethical aspects
Informed consent to scientific use of blood analysis and clinical examinations was obtained from the three CTX patients and/or the legal guardian in accordance with ethical regulations for case reports. In the case of the analyses of serum from the three SLO subjects, serum had been collected for diagnostic purposes and ethical permission was obtained from the local ethical committee at Huddinge University Hospital to use the excess of this material for other measurements. Permission was also obtained from this committee to collect blood from healthy subjects for reference purposes.
Standards
Cholesterol-7,8-epoxide was synthesized as described (8). The deuterium-labeled 24-hydroxycholesterol used as internal standard was synthesized as described previously (17).
Analysis
GC-MS.
The oxysterols (7α- and 7β- hydroxycholesterol, 7-ketocholesterol, and cholesterol-7,8-epoxide were analyzed by combined GC-MS after hydrolysis, extraction, and silicic acid chromatography as described (17). The epoxide was stable during hydrolysis and appeared in the same chromatographic fraction as other oxysterols. The steroids were converted into trimethylsilyl ether derivatives prior to GC. Deuterium-labeled 24-hydroxycholesterol was added as internal standard, and the quantitation was performed with use of a standard curve obtained by analysis of mixtures of a fixed amount of the standard with increasing amounts of cholesterol-7,8-epoxide. The area of the peak obtained in the tracing of the ion m/z 364 (cholesterol-7,8-epoxide) and the peak obtained in the tracing of the ion m/z 416 (d3-24-hydroxycholesterol) were used in the quantitations. Cholesterol precursors, including 7-dehydrocholesterol and lathosterol, were assayed as described (18).
LC-MSn.
In contrast to the GC-MS analysis, where total oxysterols were measured (esterified and nonesterified), in the LC-MSn analysis, no hydrolysis was performed and nonesterified oxysterols were analyzed as described in (19). This experiment is qualitative in nature and was done to confirm the identity of the epoxide with use of a different method and with use of a high resolution instrument. Key aspects of the LC-MSn method were: i) separation of oxysterols from more hydrophobic sterols, including cholesterol and 7-dehydrocholesterol in an initial step; ii) charge-tagging of the oxysterols by exploiting oxidation of the 3β-hydroxy group to a 3-oxo group by cholesterol oxidase, and subsequent derivatization of the oxo group with the cationic Girard P reagent; iii) chromatographic separation of oxysterols on a C18 column; and iv) ESI-MSn analysis by exploiting high-resolution exact mass measurements and MS3 fragmentation ([M]+→[M-Py]+→), where [M-Py]+ corresponds to loss of pyridine from the molecular ion.
RESULTS AND DISCUSSION
As shown in Table 1, all three CTX patients had markedly reduced levels of 27-hydroxycholesterol. However, the level in CTX patient 3 was higher than in the other two cases, consistent with the very mild symptoms. Due to the reduced production of bile acids, cholesterol 7α-hydroxlase is upregulated in untreated CTX patients resulting in high levels of 7α-hydroxycholesterol in the circulation (4, 5). In accordance with this, all three patients had high levels of 7α-hydroxycholesterol in the untreated state. As could have been expected from above, CTX patient 3 had considerably lower levels of 7α-hydroxycholesterol than the other two patients. 7-Ketocholesterol was markedly increased in patients 1 and 2, but in the upper normal range in patient 3. 7β-Hydroxycholesterol was increased in all three untreated patients with the highest increase in patient 1.
Treatment with chenodeoxycholic acid resulted in a marked decrease in 7α-hydroxycholesterol in all three patients. In patients 2 and 3, the treatment normalized the plasma level of 7α-hydroxycholesterol. In patient 1, the level of 7α-hydroxycholesterol was still above normal after treatment with 750 mg chenodeoxycholic acid for 9 months. Further treatment of this patient with a higher dose of chenodeoxycholic acid normalized, however, the level of 7α-hydroxycholesterol (Table 2).
TABLE 2.
Levels of oxysterols, cholesterol precursors, and cholesterol-7,8-epoxide in the circulation of CTX patients 1 and 2 after treatment with chenodeoxycholic acid
| Patient 1 | Patient 2 | ||||
| Steroid | Before Treatment | Treatment for 9 Months | Treatment for 21 Monthsa | Before Treatment | Treatment for 6 Months |
| 7α-Hydroxycholesterol (ng/ml) | 3.4 | 0.4 | 0.09 | 2.2 | 0.04 |
| 7β-Hydroxycholesterol (μg/ml) | 1.0 | 0.3 | 0.05 | 0.3 | 0.05 |
| 7-Ketocholesterol (μg/ml) | 2.1 | 0.3 | 0.09 | 0.6 | 0.1 |
| 7-Dehydrocholesterol (μg/ml) | 55 | 1.5 | 0.3 | 1.2 | 0.5 |
| Lathosterol (μg/ml) | 12 | 1.3 | 1.1 | 3.2 | 0.6 |
| Cholesterol-7,8-epoxide (μg/ml) | 1.8 | 0.1 | <0.05 | 0.1 | 0.07 |
Values were determined by GC-MS and are for the sum of esterified and nonesterified sterols. Normal levels of 7α-hydroxycholesterol, <0.12 μg/ml; 7β-hydroxycholesterol, <0.02 μg/ml; 7-ketocholesterol, <0.03 μg/ml; 7-dehydrocholesterol, < 0.2 μg/ml; lathosterol, <4 μg/ml; 7,8 epoxide, <0.02 μg/ml.
During the last 12 months the dose of chenodeoxycholic acid was increased from 250 mg (×3) to 500 mg (×3).
Treatment of patients 1 and 2 with chenodeoxycholic acid decreased the level of 7-ketocholesterol (Tables 1, 2). The levels after the treatment were, however, still higher than normal. This may be a consequence of a need for the sterol 27-hydroxylase to metabolize 7-ketocholesterol (3). It is noteworthy that the levels of 7β-hydroxycholesterol were also higher than normal in patients 1 and 2 after treatment. The possibility must be considered that 7β-hydroxycholesterol may also require sterol 27-hydroxylase for its metabolism. The treatment had no effect on the relatively low level of 7-ketocholesterol in CTX patient 3.
In patients 1 and 2, we had the opportunity to measure 7-dehydrocholesterol and lathosterol in addition to the oxysterols. The results of these measurements are shown in Table 2.
If 7-ketocholesterol is a product of 7-dehydrocholesterol and CYP7A1, increased levels of 7-dehydrocholesterol would be expected in CTX patients 1 and 2, who had high levels of 7-ketocholesterol in the untreated state. As shown in Table 2, the level of 7-dehydrocholesterol was increased in both CTX patients 1 and 2, but considerably more in patient 1 than in patient 2. The cholesterol precursor, lathosterol, was also markedly increased in CTX patient 1 but not in CTX patient 2. The increase in the levels of the cholesterol precursors is likely to be a consequence of the high level of synthesis of cholesterol needed to compensate for the consumption of cholesterol, due to the increased activity of CYP7A1 in CTX.
After treatment with chenodeoxycholic acid, the levels of 7-dehydrocholesterol and lathosterol decreased in parallel with the decrease in the level of 7-ketocholesterol.
If the CYP7A1-mediated mechanism is active toward 7-dehydrocholesterol, the presence of cholesterol-7,8-epoxide would be expected in the circulation of the untreated CTX patients 1 and 2.
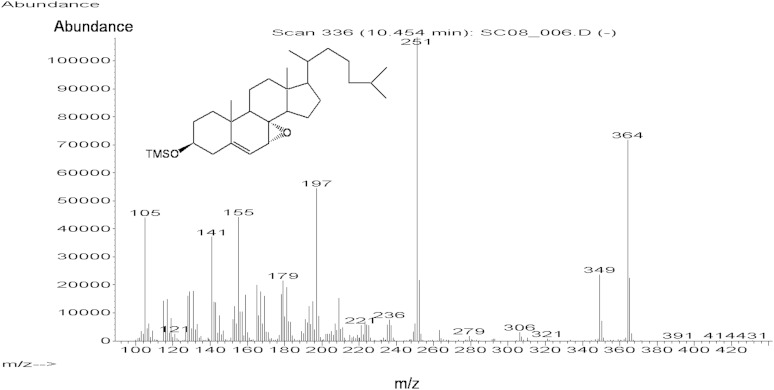
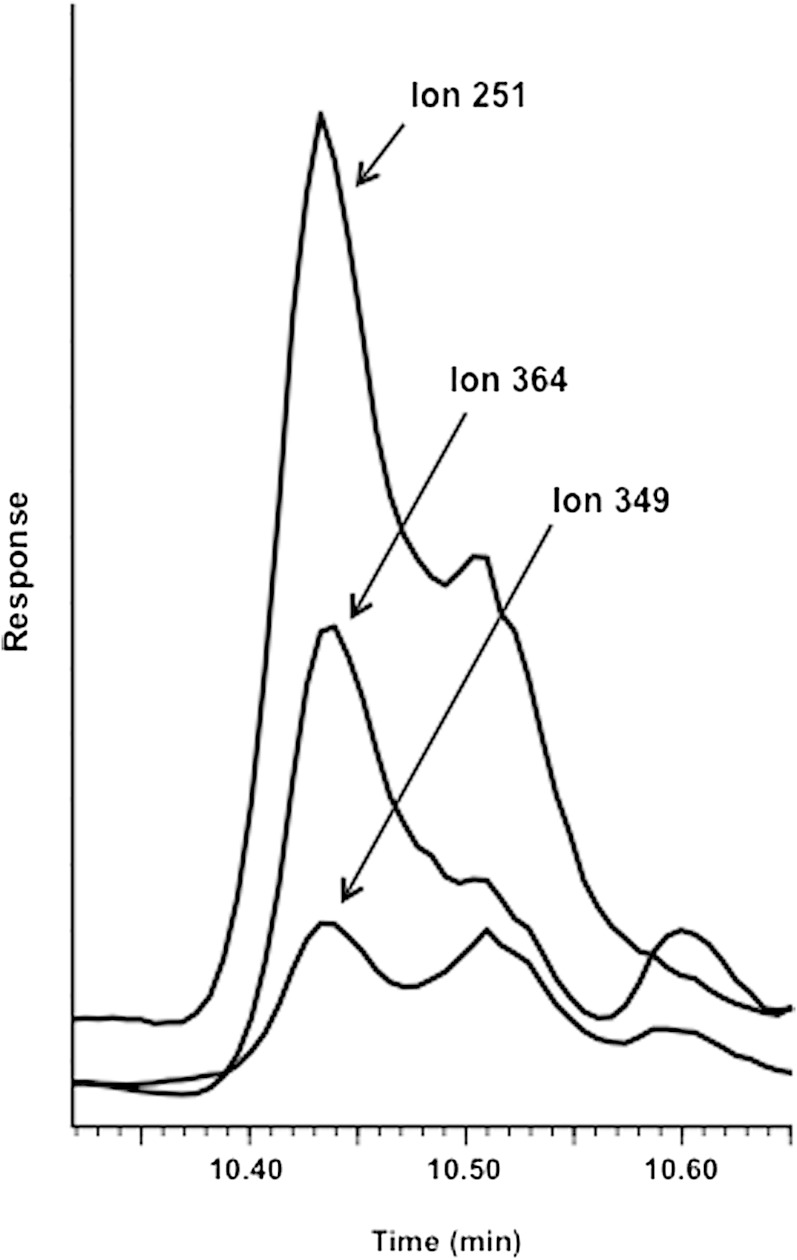
Figure 1 shows the GC-MS mass spectrum of the trimethylsilyl derivative of cholesterol-7,8-epoxide. The mass spectrum is consistent with the structure with dominant ions at m/z 364 (M-90-18), m/z 349 (M-90-18-15), and m/z 251 (steroid nucleus with four double bonds). Figure 2 shows the ion chromatogram obtained in the analysis of trimethylsilyl ether of the oxysterol fraction isolated from plasma of the untreated CTX patient 1. A peak was obtained with the same retention time as the trimethylsilyl ether of cholesterol-7,8-epoxide in the tracing of the ions m/z 364, m/z 349, and m/z 251. The presence of other contaminating steroids (possibly cholesterol precursors) with similar retention time prevented us from recording a clean mass spectrum. The ion intensity ratios for m/z 251, m/z 364, and m/z 349 were, however, almost identical to those obtained in the mass spectrum of the authentic compound.
Fig. 1.
GC-MS mass spectrum of the trimethylsilyl ether of cholesterol-7,8-epoxide.
Fig. 2.
Selected monitoring of the ions m/z 251, m/z 349, and m/z 364 in the GC-MS analysis of trimethylsilyl ether of the oxysterol fraction isolated from plasma of the untreated CTX patient. Under the chromatographic conditions employed, cholesterol-7,8-epoxide has a retention time of 10.43 min.
The amount of the 7,8-epoxide could be calculated with use of the deuterated internal standard and a standard curve constructed by analysis of mixtures of the 7,8-epoxide with a fixed amount of the deuterated standard. The concentration of the epoxide was found to be 1.8 μg/ml in the plasma sample from the untreated CTX patient 1. In contrast, the level of this epoxide was found to be <0.02 μg/ml in plasma from six healthy volunteers. The concentration of the 7,8-epoxide was considerably lower in CTX patient 2 then in CTX patient 1, most probably as a consequence of the much lower level of 7-dehydrocholesterol. As could be expected, the amount of the cholesterol-7,8-epoxide was markedly reduced after treatment of CTX patient 1 with chenodeoxycholic acid (Table 1). Also, in CTX patient 2, there was a decrease in the level of the epoxide after treatment with chenodeoxycholic acid.
All the above quantitations were made after hydrolysis. Using the same method as above, it was shown, however, that the epoxide could also be identified in an extract that had not been hydrolyzed.
The data given in Table 2 are consistent with the possibility that 7-dehydrocholesterol is a precursor to 7-ketocholesterol. It seems likely that a major part of the 7-ketocholesterol that accumulates in patients with CTX is produced by the newly described CYP7A1-mediated mechanism. It should be noted that the therapy with chenodeoxycholic acid did not result in complete normalization of the elevated levels of 7-ketocholesterol in CTX patients 1 and 2. Most probably this is due to a need for the sterol 27-hydroxylase for the normal metabolism of this steroid (3).
In addition to 7-dehydrocholesterol, the level of lathosterol was also increased in the circulation of CTX patient 1 in the untreated state. In view of this and the high degree of upregulation of CYP7A1, we considered the possibility that 7-ketocholestanol and 7,8-epoxycholestanol may be formed under these conditions. Attempts to identify these two compounds in the circulation of CTX patient 1 failed, however.
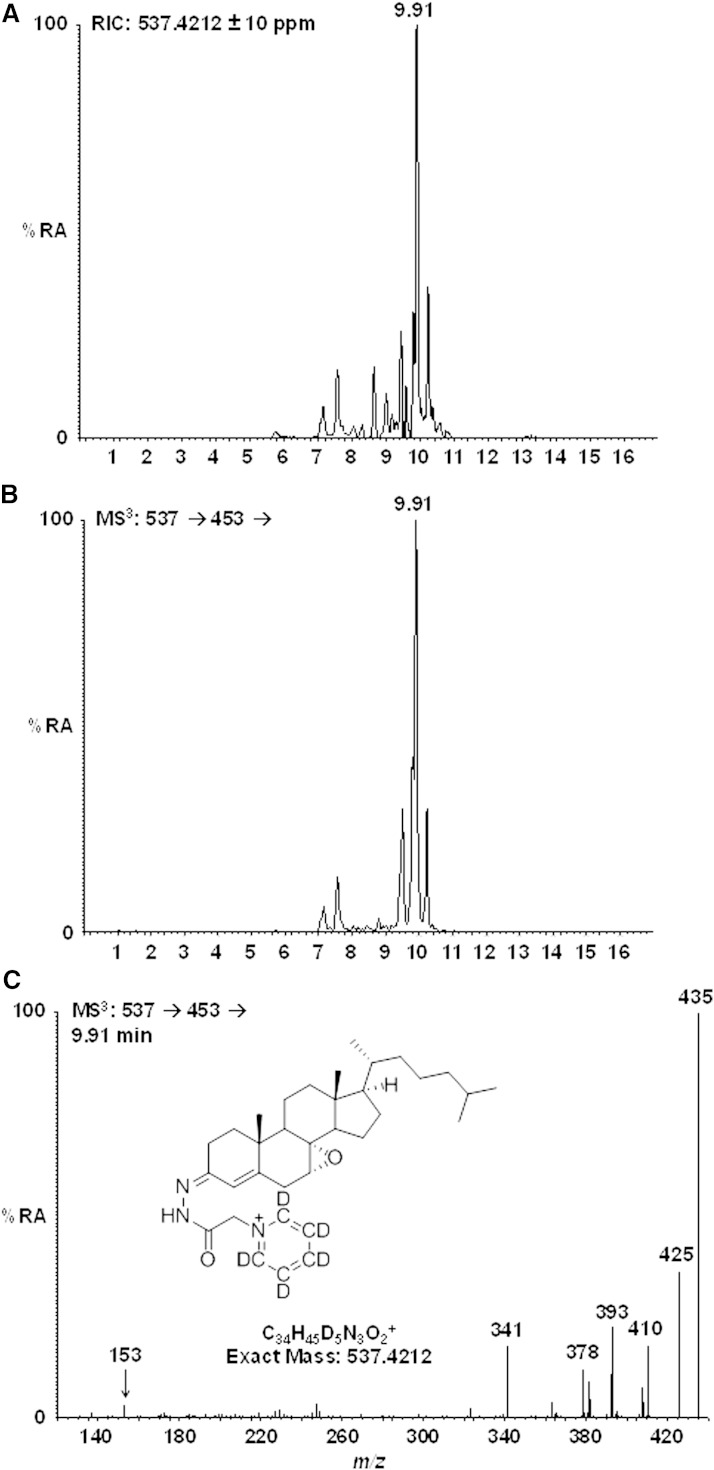
Patients with SLO have levels of 7-dehydrocholesterol even higher than those observed in CTX patients (12). Table 3 shows the results of GC-MS measurements of 7-ketocholesterol and 7-dehydrocholesterol in serum from three patients with SLO. In accordance with our expectation and the recent study by Liu et al. (14), high levels of 7-ketocholesterol were present in all three patients. It is noteworthy that the increased levels of 7-ketocholesterol were not associated with increased levels of 7α- or 7β-hydroxycholesterol, excluding the possibility that it had been formed by autoxidation of cholesterol (20). It has been reported that the activity of CYP7A1 is normal or reduced in the liver of patients with SLO (13). The high levels of 7-ketocholesterol were associated with high levels of the cholesterol-7,8-epoxide. In this case, the identity of the cholesterol-7,8-epoxide was ascertained by GC-MS, not only by ion chromatography but also by recording full mass spectra that were identical to those of the reference compound (results not shown). The identification of cholesterol-7,8-epoxide was further confirmed by LC-MSn using a high resolution mass spectrometer. Shown in Fig. 3 (upper trace) is the reconstructed ion chromatogram (±10 ppm) for m/z 537.4212, the m/z corresponding to the [M]+ ion of cholesterol-7,8-epoxide charge-tagged with d5-GP (Girard P derivative). The central panel shows the total ion chromatogram for the MS3 transition [M]+→[M-Py]+→ and the lower panel the MS3 spectrum [M]+→[M-Py]+→ for the peak eluting at 9.91 min. Authentic charge-tagged cholesterol-7,8-epoxide eluted at 9.91 min and gave an identical MS3 spectrum to that shown. No evidence of cholesterol-7,8-epoxide was found by LC-MSn analysis of a control sample (National Institute of Standards and Technology standard reference material).
TABLE 3.
Levels of oxysterols, 7-dehydrocholesterol, and cholesterol-7,8-epoxide in the circulation of three SLO patients
| Steroid | SLO Patient 1 | SLO Patient 2 | SLO Patient 3 |
| 7α-Hydroxycholesterol (μg/ml) | 0.03 | 0.03 | 0.02 |
| 7β-Hydroxycholesterol (μg)ml) | 0.03 | 0.03 | 0.03 |
| 7-Ketocholesterol (μg/ml) | 0.10 | 0.73 | 0.41 |
| 7-Dehydrocholesterol (μg/ml) | 228 | 200 | 282 |
| Cholesterol-7,8-epoxide (μg/ml) | 2.1 | 2.7 | 3.6 |
Values were determined by GC-MS and are for the sum of esterified and nonesterified sterols.
Fig. 3.
LC-MSn analysis of plasma from a SLO patient. Oxysterols were charge-tagged with the d5-GP reagent. Upper panel shows the reconstructed ion chromatogram for the [M]+ ion (537.4212 ± 10 ppm) of cholesterol-7,8-epoxide. Central panel shows the total ion chromatogram for the MS3 transition [M]+→[M-Py]+→. Lower panel shows the MS3 spectrum [M]+→[M-Py]+→ for the peak eluting at 9.91 min. Authentic cholesterol-7,8-epoxide elutes at 9.9 min and gives an MS3 spectrum identical to that shown in the lower panel.
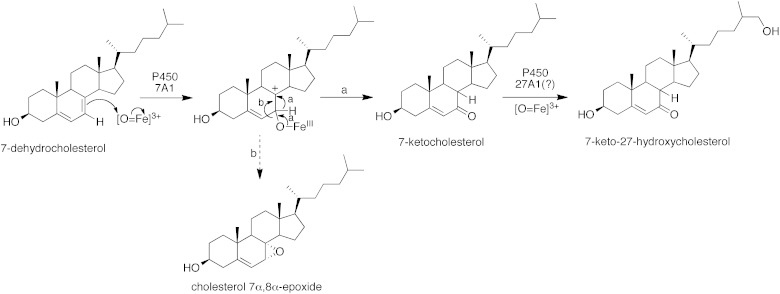
It seems likely that 7-dehydrocholesterol is a precursor to 7-ketocholesterol in the SLO patients also, in spite of a reported normal or low activity of CYP7A1 (13). Figure 4 summarizes the mechanism by which 7-ketocholesterol is formed from 7-dehydrocholesterol. The level of 7-ketocholesterol is dependent upon three factors: i) activity of CYP7A1; ii) level of 7-dehydrocholesterol; and iii) activity of sterol 27-hydroxylase. It should be emphasized that a high level of CYP7A1 will lead to increased consumption of cholesterol, which will be compensated for by increased cholesterol synthesis and thus increased levels of 7-dehydrocholesterol (21).
Fig. 4.
Mechanism of conversion of 7-dehydrocholesterol into 7-ketocholesterol and cholesterol-7,8-epoxide by CYP7A1 [modified from (8)]. The further metabolism of 7-ketocholesterol by CYP27 is also shown in the figure.
To summarize, the present results suggest that 7-dehydrocholesterol is likely to be a precursor to 7-ketocholesterol, not only under the in vitro conditions previously described (8) but also under in vivo conditions with high levels of 7-dehydrocholesterol. The high levels of 7-dehydrocholesterol must be combined with normal or high activity of the cholesterol 7α-hydroxylase. In addition to CTX, a formation of 7-ketocholesterol is likely to occur in other conditions also, with a marked upregulation of CYP7A1, e.g., due to bile acid malabsorption or treatment with resins.
Footnotes
Abbreviations:
- CTX
- cerebrotendinous xanthomatosis
- CYP
- cytochrome P-450
- SLO
- Smith-Lemli-Opitz syndrome
This work was supported by grants from the Swedish Science Council (Vetenskapsrådet) and from the Swedish Brain Power. Work in Swansea was supported by a Biotechnology and Biological Sciences Research Council Grant (BB/I001735/1), and in Nashville was supported in part by National Institutes of Health Grant R37 CA090426 and National Institute of Child Health and Human Development Grant R01HD064727 (to Ned Porter).
REFERENCES
- 1.Björkhem I. 1986. Assay of unesterified 7-oxocholesterol in human serum by isotope dilution mass spectrometry. Anal. Biochem. 154: 497–501. [DOI] [PubMed] [Google Scholar]
- 2.Burnett J. R., Moses E. A., Croft K. D., Brown A. J., Grainger K., Vasikaran S. D., Leitersdorf E., Watts G. F. 2001. Clinical and biochemical features, molecular diagnosis and long-term management of a case of cerebrotendinous xanthomatosis. Clin. Chim. Acta. 306: 63–69. [DOI] [PubMed] [Google Scholar]
- 3.Jessup W., Brown A. J. 2005. Novel routes for metabolism of 7-ketocholesterol. Rejuvenation Res. 8: 9–12. [DOI] [PubMed] [Google Scholar]
- 4.Björkhem I., Boberg K. M. 1995. Inborn errors in bile acid biosynthesis and storage of sterols other than cholesterol. In The Metabolic and Molecular Basis of Inherited Disease. C. R. Scriver, A. L. Beaudet, W. S. Sly, and D. Valle, editors. McGraw-Hill, New York. 2073–2099. [Google Scholar]
- 5.Koopman B. J., van der Molen J. C., Wolthers B. G., Vanderpass J. B. 1987. Determination of some hydroxycholesterols in human serum samples. J. Chromatogr. 416: 1–13. [DOI] [PubMed] [Google Scholar]
- 6.Larsson H., Böttiger Y., Iuliano L., Diczfalusy U. 2007. In vivo interconversion of 7β-hydroxycholesterol and 7-ketocholesterol, potential surrogate marker for oxidative stress. Free Radic. Biol. Med. 43: 695–701. [DOI] [PubMed] [Google Scholar]
- 7.Schweizer R. A. S., Zurcher M., Balazs Z., Dick B., Odermatt A. 2004. Rapid hepatic metabolism of 7-ketocholesterol by 11β-hydroxysteroid dehydrogenase type I. Species differences between the rat, human and hamster enzyme. J. Biol. Chem. 279: 18415–18424. [DOI] [PubMed] [Google Scholar]
- 8.Shinkyo R., Xu L., Tallman K. A., Cheng Q., Porter N. A., Guengerich F. P. 2011. Conversion of 7-dehydrocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J. Biol. Chem. 286: 33021–33028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogishima T., Deguchi S., Okuda K. 1987. Purification and characterization of cholesterol 7α-hydroxylase from rat liver microsomes. J. Biol. Chem. 262: 7646–7650. [PubMed] [Google Scholar]
- 10.Honda A., Salen G., Matsuzaki Y., Batta A. K., Xu G., Leitersdorf E., Tint G. S., Erickson S. K., Tanaka N., Shefer S. 2001. Side chain hydroxylation in bile acid biosynthesis catalyzed by CYP3A is markedly upregulated in Cyp27-/- mice but not in cerebrotendinous xanthomatosis. J. Biol. Chem. 276: 34579–34585. [DOI] [PubMed] [Google Scholar]
- 11.de Sain-van der Velden M. G., Verrips A., Prinsen B. H., de Barse M., Berger R., Visser G. 2008. Elevated cholesterol precursors other than cholestanol can also be a hallmark for CTX. J. Inherit. Metab. Dis. 31: S387–S393. [DOI] [PubMed] [Google Scholar]
- 12.Tint G. S., Batta A. K., Xu G., Shefer S., Honda A., Irons M., Elias E. R., Salen G. 1997. The Smith-Lemli-Opitz syndrome: a potentially fatal birth defect caused by a block in the last enzymatic step of cholesterol biosynthesis. In Subcellular Biochemistry. Vol. 28. R. Bittman, editor. Plenum Press, New York. 117–144. [DOI] [PubMed] [Google Scholar]
- 13.Honda A., Salen G., Shefer S., Batta A. K., Xu G., Tint G. S., Matsuzaki Y., Shoda J., Tanaka J. 1999. Bile acid synthesis in the Smith-Lemli-Opitz syndrome: effects of dehydrocholesterols on cholesterol 7 alpha hydroxylase and 27-hydroxylase activities in rat liver. J. Lipid Res. 40: 1520–1528. [PubMed] [Google Scholar]
- 14.Liu W., Xu L., Lamberson C. R., Merkens L. S., Steiner R. D., Elias E. R., Haas D., Porter N. A. 2013. Assays of plasma dehydrocholesteryl esters and oxysterols from Smith-Lemli-Opitz syndrome patients. J. Lipid Res. 54: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L., Korade Z., Rosado D. A., Mirnics K., Porter N. A. 2013. Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J. Lipid Res. 54: 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansson M., Olin M., Floren C. H., von Bahr S., van’t Hooft F., Meaney S., Eggertssen G., Björkhem I. 2007. Unique patient with cerebrotendinous xanthomatosis. Evidence for presence of a defect in a gene that is not identical to sterol 27-hydroxylase. J. Intern. Med. 261: 504–510. [DOI] [PubMed] [Google Scholar]
- 17.Dzeletovic S., Breuer O., Lund E., Diczfalusy U. 1995. Determination of cholesterol oxidation products in human plasma by isotope dilution mass spectrometry. Anal. Biochem. 225: 73–80. [DOI] [PubMed] [Google Scholar]
- 18.Acimovic J., Lövgren-Sandblom A., Monostiry K., Rozman D., Golicnik M., Lutjohann D., Björkhem I. 2009. Combined gas chromatographic/mass spectrometric analysis of cholesterol precursors and plant sterols in cultured cells. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 2081–2086. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths W. J., Crick P. J., Wang Y., Ogundare M., Tuschl K., Morris A. A., Bigger B. W., Clayton P. T., Wang Y. 2013. Analytical strategies for characterization of oxysterol lipidomes: liver X receptor ligands in plasma. Free Radic. Biol. Med. 59: 69–84. [DOI] [PubMed] [Google Scholar]
- 20.Schroepfer G. J., Jr 2000. Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 80: 361–554. [DOI] [PubMed] [Google Scholar]
- 21.Sudjana-Sugiaman E., Eggertsen G., Sjöblom P., Maeda Y., Rudling M., Okuda M., Björkhem I. 1994. Presence of cholesterol 7α-hydroxylase enzyme protein in COS cells leads to increased HMG CoA reductase activity. Biochem. Biophys. Res. Commun. 202: 896–901. [DOI] [PubMed] [Google Scholar]