Abstract
LC/MS quantification of multiple plasma proteins that differ by several orders of magnitude in concentration from a single sample is challenging. We present a strategy that allows the simultaneous determination of the concentration and turnover kinetics of higher and lower abundant proteins from a single digestion mixture. Our attention was directed at a cluster of proteins that interact to affect the absorption and interorgan lipid trafficking. We demonstrate that apos involved in TG metabolism such as apoC2, C3, E, and A4 (micromolar concentration), and apoB48 and apoA5 (single-digit nanomolar concentration) can be quantified from a single digestion mixture. A high degree of correlation between LC/MS and immunobased measurements for apoC2, C3, E, and B48 was observed. Moreover, apoA5 fractional synthesis rate was measured in humans for the first time. Finally, the method can be directly applied to studies involving nonhuman primates because peptide sequences used in the method are conserved between humans and nonhuman primates.
Keywords: stable isotopes, mass spectrometry, kinetic biomarker, dyslipidemia
There is growing evidence to support an association between elevated TG levels and CVD (1). However, despite decades of research, the precise role of TG in CVD is still a subject of intense debate (2). The metabolism of TG involves both exogenous and endogenous pathways. The exogenous pathway begins with uptake of dietary TG FAs and cholesterol by the intestinal enterocytes where the FA and cholesterol are reesterified and packaged with apoB48, apoA1, apoA4, the C apos, phospholipids, and unesterified cholesterol into chylomicrons. The chylomicrons enter the bloodstream where, in adipose and muscle tissue, the core TG is hydrolyzed by LPL. The known activators of LPL include apoC2, apoA4, apoA5, and lipase maturation factor 1, whereas apoC3 and angiopoietin-like proteins 3 and 4 inhibit LPL (3, 4). Remnant chylomicron particles are formed by the removal of the TG core. These remnants are then taken up into the liver. The endogenous pathway of TG metabolism starts with TG synthesized by the liver. These TGs are then secreted into the circulation within the core of VLDL particles. At the cellular level, the synthesis and secretion of VLDL particles resembles that of chylomicrons, except that a different B apo (B100 instead of B48) is required, with apoE and C proteins possibly acting as modifiers. Once in the plasma, VLDL TG is hydrolyzed by LPL, generating smaller and denser VLDL and IDL particles, with the latter either taken up by the liver or further remodeled to become LDL (3).
Clearly, apos such as B48, B100, A4, A5, E, C2, and C3 play important roles in TG metabolism. Accurate determination of the concentrations and the turnover kinetics of these proteins in healthy and dyslipidemic individuals could shed new light on the regulation of plasma TG levels and, therefore, facilitate the development of better treatments. Because these proteins simultaneously interact with both the exogenous and endogenous pathways of TG metabolism, an ideal method would be one that allowed investigators to examine their interplay. Although good immunobased assays exist for the apos, they cannot meet all the demands of quantifying a broad panel of these proteins for both clinical and preclinical studies from limited sample volumes. Furthermore, most high-quality immunoassays are for human samples and have limited ability to be translated into preclinical species. Finally, multiplexed immunoassays frequently are not feasible because of the wide range of plasma concentrations of the apos of interest. By contrast, protein quantitation by LC/MS permits relatively quick development and can be easily multiplexed. In addition, an LC/MS platform can also be used to simultaneously measure the turnover kinetics of the apos on each lipoprotein, important information that cannot be provided by immunoassays.
Previously, our group and others have demonstrated the feasibility of MS to quantify apos (e.g., apoA1 and apoB100) in the micromolar concentration range from only several microliters of plasma (5). However, relatively lower abundant apos such as apoB48 and apoA5 are below the limit of quantification by the approaches taken previously. In the present study, we have implemented a workflow that incorporates efficient trypsin digestion for quantifying abundant apos and immunoenrichment using antipeptide antibodies for apoB48 and apoA5, which allows the simultaneous measurement of concentrations and turnover kinetics of several apos involved in TG metabolism from a single digestion mixture.
EXPERIMENTAL
Materials
Chemicals and reagents.
Stable-isotope-labeled peptides were purchased from New England Peptides (Gardner, MA). The stable isotopes 13C and 15N were incorporated into the carboxyl terminal of all peptide standards, giving a mass shift of 8 Da for lysine, 10 Da for arginine, and 7 Da for isoleucine. Purity for each peptide was >95%. Amino acid analysis was performed by the manufacturer for peptide concentration determination. These stable-isotope-labeled peptides were used as internal standards (ISTDs) for LC/MS experiments. An ISTD master mix was generated by diluting the concentrated peptide stocks (∼800–2,000 µM) to 8 µM for peptides representing apoE, C2, C3, and A4; 100 nM for apo B48; and 25 nM for apoA5 in 50 mM ammonium bicarbonate containing 25 µg/ml BSA.
Ammonium bicarbonate, DTT, iodoacetamide (IAA), formic acid, PBS, and sodium deoxycholate were purchased from Sigma-Aldrich (St. Louis, MO). Tris (2-carboxyethyl) phosphine hydrochloride was obtained from ThermoFisher Scientific. Trypsin used for plasma digestions was Promega Gold (Madison, WI). The 96-well nonbinding microtiter plates were purchased from Corning (Tewksburg, MA). Recombinant apos were purchased from Origene (Rockville, MD). Tosylactivated magnetic dynabeads M-280 were purchased from Life Technologies (Grand Island, NY). RIPA lysis buffer (10×) was obtained from Millipore (Billerica, MA, USA). RIPA lysis buffer (1×) was purchased from Teknova (Hollister, CA).
Antipeptide antibodies.
To confirm the apoB48 C-terminal peptide sequence, total apoB (i.e., B100 and B48) was immunoprecipitated out of pooled human plasma using an anti-apoB antibody (Meridian Life Sciences, Memphis, TN) and then separated using SDS-PAGE (see supplementary Experimental methods). Gel bands that migrated close to the size of apoB48 and apoB100 were cut, digested with trypsin, and analyzed using nanoAcquity (Waters Corporation, Milford, MA) and LTQ Orbitrap mass spectrometer (ThermoFisher Scientific, San Jose, CA). To select apoA5-derived peptides for antipeptide antibody generation, recombinant apoA5 protein was digested with trypsin and analyzed by LTQ Orbitrap.
Polyclonal antibodies were generated by immunizing New Zealand white rabbits with keyhole limpet hemocyanin-conjugated peptides H2N-C(dPEG4)LSQLQTYMI-OH for apoB48 and H2N-C(dPEG4)VQELQEQLR-OH for apoA5, respectively, at New England Peptides. Cysteine residues were added to the peptides to facilitate conjugation and were separated from the natural amino acid sequence by polyethylene glycol (PEG) spacers in order to ensure availability of the epitope. The rabbits were bled twice after three total biweekly immunizations, and the serum was affinity-purified against peptide columns prepared with SulfoLink gel (Thermo Fisher Scientific).
Acquisition of plasma samples.
Human EDTA plasma samples were obtained either from Bioreclamation (Hicksville, NY) or Merck volunteers (Kenilworth, NJ). Informed consent was obtained from all the donors. For the fed and fasted conditions, donors were allowed to eat ad libitum from a full cafeteria menu. Plasma samples were either collected in the morning upon overnight fasting or ∼1 h after the meal.
Methods
Biochemical sample processing.
Plasma samples were biochemically processed into peptides using Matrix 2 × 2 liquid handler (Thermo Fisher Scientific, Hudson, NH) following a procedure adapted from previous publication (5). Briefly, for the quantitation of high abundant proteins, 24 µl of plasma was diluted with reaction mixture containing 816 µl 50 mM ammonium bicarbonate (pH ∼8), 240 µl ISTD peptide mix (final concentration of 8 µM), 60 µl 10% sodium deoxycholate, and 12 µl 500 mM DTT and incubated at 60°C for 45 min to reduce the disulfide bonds. The sample mixture was then alkylated with 40.2 µl 10 mM IAA for 60 min at room temperature in the dark and digested overnight with 36 µl (0.5 µg/µl) trypsin at 37°C. An aliquot of 200 µl digested sample was taken out, and 10 µl 20% formic acid was then added to stop digestion and precipitate sodium deoxycholate. Samples were centrifuged for 15 min at 4200 rpm, and 160 µl of the supernatant was collected for LC/MS analysis. The rest of the digested sample was used in immunoenrichment of apoB48 and apoA5.
Immunoenrichment of apoB48 and apoA5.
Antibodies for apoB48 and apoA5 were conjugated to magnetic beads (tosylactivated M-280 Dynabeads) according to the manufacturer’s instruction. Antibody (2 mg) was used to conjugate on 50 mg of beads. Conjugated beads were resuspended at 20 mg/ml concentration in 0.1% PBS/Tween-20 solution. For the enrichment of apoA5 and apoB48 peptides, 100 µl 10× RIPA buffer (Millipore, Temecula, CA), 10 µl 100× protease inhibitor cocktail (Millipore), and antibody-conjugated beads (5 µl each) were added to 1 ml of plasma digest. The mixture was incubated at 4°C overnight with rotation. The next day, beads were washed once with 1 ml 1× RIPA buffer (Teknova) and 3 times with 1 ml of 20 mM ammonium bicarbonate (pH ∼8). Liquid handler Zephyer (PerkinElmer, Waltham, MA) was used in all washing steps for better precision. Peptides were eluted with 100 µl elution buffer (water solution containing 10% acetonitrile and 10% formic acid) at 37°C for 15 min with gentle rocking. The eluted samples were filtered through 0.45 µm polyvinylidene difluoride membrane (Millipore) and spun in a Speed-Vac (Thermo Fisher Scientific) with medium heat to dryness. For LC/MS analysis, the samples were resuspended in 20 µl solution containing 5% acetonitrile, 5% formic acid, and 500 nM tris (2-carboxyethyl) phosphine hydrochloride to minimize the oxidation of methionine in the apoB48 peptide.
LC/MS analysis.
For the quantification of apoE, C2, C3, and A4, an Acquity UPLC system coupled to a triple quadrupole mass spectrometer (TSQ Vantage, Thermo Scientific) was used. Chromatographic separation was performed on a Waters CSH C18 column (100 × 2.1 mm; 1.7 µm) maintained at 50°C. The gradient was 98% A (0.1% formic acid in water)/2% B (0.1% formic acid in acetonitrile); ramped incrementally to 93% at 1 min, 88% at 2.5 min, 85% at 2.55 min, and 75% at 3 min (to achieve better separation between elution points and allow for proper scans/peak); then ramped linearly to 5% A at 3.25 min and held to 3.75 min; and reequilibrated to initial conditions (total run time of 4.6 min running at a flow rate of 400 µl/min). The injection volume was 25 µl (equivalent to 0.5 µl neat plasma).
The column effluent was introduced into the mass spectrometer using a heated electrospray ionization source with multiple-reaction monitoring (MRM) and positive ion mode. The MS/MS experiments were performed using argon as collision gas (1.5 mTorr). Peak widths for Q1 and Q3 were set at 0.7 full width at half maximum. Product ion scan widths were set at 0.3 m/z. Scan time for each MRM transition was 0.05 s. The raw data were processed using Xcalibur Qual Browser. Protein concentration in plasma was determined using the following formula: Protein Concentration = Peak Area Ratio (Unknown/ISTD) × Concentration of ISTD × Dilution Factor (Final Digestion Volume/Plasma Input Volume).
To quantify apoB48 and apoA5, samples (5 µl) were partial-loop injected onto a TRIZAIC nanoTile (150 µm × 100 mm packed with ethylene bridged hybrid C18, 1.7 µm particle, prototype obtained from Waters) using a nanoAcquity UPLC (Waters Corp.) interfaced with a triple quadrupole mass spectrometer (Xevo TQS, Waters). The gradient was 97% A (0.1% formic acid in water)/3% B (0.1% formic acid in acetonitrile), ramped linearly to 10% A at 5 min, and then reequilibrated at initial condition (total run time: 8 min; flow rate: 3 µl/min). The nanoTile was maintained at 60°C throughout the chromatographic gradient elution. An infusion nanoTile (no packing material) was used to optimize emitter position, signal intensity, and MRM transitions.
Digestion time course, precision, and accuracy tests.
To evaluate the reproducibility of the protein digestion, 1 ml of pooled human plasma was digested in a total volume of 50 ml, which was conducted in triplicate. After the addition of trypsin, 1,200 µl aliquots were removed at different time intervals, heated at 95°C for 10 min to inactivate trypsin. The samples were cooled to room temperature and divided into aliquots containing 200 or 1,000 µl. The 200 µl aliquot was used for quantifying high abundance apos, whereas the 1,000 µl aliquot underwent immunoenrichment of apoB48 and apoA5.
To evaluate the assay precision, pooled plasma samples were digested and analyzed by LC/MS either in the same experiment done in triplicates for intra-assay precision or three times independently during a 2-week period of time for interassay precision. To evaluate the effect of freeze/thawing on the measurement of apos, fresh plasma samples were collected from human donors (Bioreclamation) and stored at 4°C overnight. Aliquots (500 µl) then underwent three cycles of freeze/thaw: freeze under −80°C and thaw under room temperature. Plasma samples (n = 3 replicates) from freeze/thaw, along with the fresh set, were digested and analyzed by LC/MS directly for high abundant proteins or immunoenriched prior to LC/MS analysis for low abundant protein quantitation.
Immunoturbidimetric and ELISA assays.
ApoC2, apoC3, and apoE from human plasma were measured on a Roche Hatachi Cobas clinical chemistry analyzer using immunoturbidimetrics method. ApoC2 reagents were from Randox Diagnostic Inc. ApoC3 and apoE reagents were from Kamiya Diagnostics. Human apoB48 ELISA kit was obtained from BioVendor Research and Diagnostic Products (Ashville, NC). Plasma samples were diluted 100 times using buffer solutions supplied with the assay kit, and the concentration of apoB48 was measured according to the manufacturer’s instruction.
Subjects and stable isotope tracer infusion protocol.
To establish proof of concept for the methodology, protein turnover kinetics were examined in plasma samples collected from an individual who participated in a previous turnover study. The study was a Merck-sponsored phase 2 trial examining the effects of a cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoprotein kinetics (unpublished data on file; Merck Sharp and Dohme Corp.). This individual did not receive anacetrapib treatment. The Columbia University Investigational Review Committee approved the study protocol. Informed consent was obtained from the participant before commencement of the study procedures.
A primed infusion of [5,5,5-2H3]l-leucine protocol was used as previously described (6). Briefly, while consuming a low-fat liquid diet every 2 h, the subject was given a bolus injection of [5,5,5-2H3]l-leucine (10 µmol/kg body weight) immediately followed by a constant infusion of [5,5,5-2H3]l-leucine (10 µmol/kg body weight/h) for 15 h. Blood samples were initially collected at 0, 20, and 40 min and then at 2, 4, 6, 8, 10, 12, and 15 h; samples were stored at –80 °C until analysis. VLDL apoB100 enrichment was performed previously in the anacetrapib study, and the same samples were used to determine the enrichment for apoC2, C3, E, B48, and apoA5. Fractional synthesis rate (FSR) was calculated using established formulas (7).
RESULTS
Workflow for multiplexed apo quantitation and kinetic analysis
The physiological levels of apos involved in the regulation of TG metabolism (apoB100, C2, C3, E, A4, A5, and B48) are from the single-digit nanomolar to double-digit micromolar range, spanning more than four orders of magnitude (8, 9). To quantify both the abundant apos (e.g., apoC2, C3, E, and A4) and less abundant proteins (e.g., apoB48 and A5) from the same aliquots of samples, we developed a working process to digest 24 µl of plasma samples in one tryptic digestion reaction. The digested plasma was then split into two aliquots: one was used directly for quantifying apoC2, C3, E, and A4 on a high-low LC/MS system; and the other underwent immunoenrichment of apoB48 and apoA5 and was then analyzed on a low-flow LC/MS system (Fig. 1).
Fig. 1.
Diagrammatic workflow showing that both high and low abundant plasma proteins can be quantified in one digestion reaction. Human plasma sample (24 µl) was digested with trypsin with a final volume of 1,200 µl, of which 200 µl was used for the analysis of high abundant plasma proteins. The rest was immunoenriched for the analysis of low abundant plasma proteins.
Peptide selection and MRM optimization
Selection of peptides that can be used to stoichiometrically represent the protein concentration is a critical first step in developing a targeted LC/MS assay. Typically, we started with the evaluation of tryptic peptides from recombinant protein digests. When recombinant proteins were not available, peptide candidates were selected based on in silico sequence analysis (10), literature reports, or known databases (e.g., the Global Proteome Machine) (11–13). In addition, the peptides should also be suitable for kinetic measurements (i.e., allowing for the quantification of tracer enrichment). For example, if D3-leucine is used in kinetic studies, then the peptide needs to contain at least one leucine residue. When there were multiple peptides providing similar quality for quantitation and kinetic analysis, the one that was conserved cross-species was selected to allow the method to be used in both human and preclinical studies.
Because the amino acid sequence of apoB48 is identical to 48% of N-terminal apoB100 (14), trypsin digestion of apoB48 will yield a set of peptides that are indistinguishable from those apoB100 tryptic peptides. One exception is the C-terminal apoB48. As illustrated in Fig. 2A, the C-terminal tryptic peptide is LSQLQTYMI for apoB48 because the protein ends, whereas tryptic peptide LSQLQTYMIQFDQYIK is for apoB100. Thus, peptide LSQLQTYMI can be used to distinguish apoB48 from apoB100. To confirm C-terminal apoB48 peptide sequence, we immunoprecipitated total apoB (B48 and B100) from pooled human plasma using an anti-apoB antibody and separated the pull-down mixture by SDS-PAGE. The two bands that corresponded to the size of apoB100 and B48 were cut, in-gel trypsin digested, and analyzed by high-resolution LC/MS. Fig. 2B shows an MS/MS spectrum of the C-terminal apoB48 peptide that was unequivocally detected in the apoB48 gel band, but not in the apoB100 band.
Fig. 2.
Confirmation of apoB48 C-terminal peptide sequence using SDS-PAGE and LC/MS/MS. ApoB (B100 and B48) was immunoprecipitated out of pooled human plasma and then separated by SDS-PAGE. Gel bands that migrated close to the size of apoB48 and apoB100 were cut, digested with trypsin, and analyzed by LC/MS/MS. A: Tryptic peptide sequence (underlined) that distinguishes apoB48 from apoB100. B: MS/MS spectra of peptide LSQLQTYMI detected from apoB48 gel band, but not in apoB100 band.
Stable-isotope-labeled (13C15N) forms of these peptide candidates were synthesized and infused into the mass spectrometer to identify the best MRM transitions for both quantification and kinetic measurements (Table 1). Normally, we selected two to three peptides from each protein, and two to three MRM transitions per peptide, except for apoB48 where only a single peptide was evaluated. After optimization of the MRM transitions and LC conditions for each peptide, pooled plasma and a panel of individual plasma samples were tested using these conditions to eliminate those peptides that were prone to interferences from plasma matrix.
TABLE 1.
Acquisition parameters used for quantifying protein concentration and turnover kinetics
| Transitions Monitored | |||||
| Protein | Peptide Sequence | Product Ion Sequence | M0 | M3 | ISTD |
| ApoC2 | TYLPAVDEK | PAVDEK | 518.3→658.3 | 519.8→658.3 | 522.3→666.3 |
| TYLPAVDE[K-13C615N2] | PAVDE[K-13C615N2] | ||||
| ApoC3 | GWVTDGFSSLK | TDGFSSLK | 598.8→854.5 | 600.3→857.5 | 602.8→862.5 |
| GWVTDGFSSL[K-13C615N2] | TDGFSSL[K-13C615N2] | ||||
| ApoE | LGPLVEQGR | VEQGR | 484.8→588.3 | 486.3→588.3 | 489.8→598.3 |
| LGPLVEQG[R-13C615N4] | VEQG[R-13C615N4] | ||||
| ApoA4 | SELTQQLNALFQDK | NALFQDK | 817.7→948.5 | 819.2→951.5 | 821.7→956.5 |
| SELTQQLNALFQD[K-13C615N2] | NALFQD[K-13C615N2] | ||||
| ApoB48 | LSQLQTYMI | LSQLQTYM | 548.8→965.5 | 550.3→968.5 | 552.3→965.5 |
| LSQLQTYM[I-13C615N] | |||||
| ApoA5 | VQELQEQLR | ELQEQLR | 571.8→915.5 | 573.3→918.5 | 576.8→925.5 |
| VQELQEQL[R-13C615N4] | ELQEQL[R-13C615N4] | ||||
Quantification of apoE, C2, C3, and A4 directly from plasma digests
One of the challenges of developing a multiplexed LC/MS quantification assay is to make sure that the digestion condition used is suitable for all proteins of interest. Previously, our laboratory showed that the addition of sodium deoxycholate (a water-soluble, ionic detergent) greatly enhanced the trypsin digestion efficiency for plasma apoB100 (5). At the end of digestion process, sodium deoxycholate was removed via acid precipitation; thus the addition of detergent did not interfere with LC/MS analysis. Here, we wanted to evaluate whether reproducible digestion could be achieved for other apos using the same digestion conditions. Therefore, experiments were performed to examine the impact of time course on the digestion (supplementary Fig. I). Peptide intensity for apoC2, C3, E, and A4 all reached plateau after overnight digestion (16 h), and the intensity remained stable up to 28 h, indicating that the standard conditions (e.g., 16–20 h) should result in stable peptide yields.
To ensure assay robustness, we also tested the effect of plasma freeze/thaw cycles on our ability to quantify these apos via LC/MS/MS. Plasma samples from 10 individual human subjects were collected and analyzed under the following conditions: fresh or after one to three cycles of freeze/thaw treatments. As shown in supplementary Fig. II, even after three cycles of freeze/thawing, the differences in peptide intensity between treatment conditions was not statistically significant. These results suggest that several cycles of freeze/thawing did not affect the assay performance, which is clearly beneficial when analyzing clinical samples where frequent freeze/thawing may be encountered.
We next estimated the linear range of the assay, including the lower limit of quantification (LLOQ) and assay precision. To this end, stable-isotope-labeled peptides with various known concentrations were spiked into pooled plasma digests, resulting in a final labeled peptide concentration between 0.2 nM and 2 µM. These samples were subsequently analyzed by LC/MS. The mass spectrometric response was plotted against the spiked-in concentration. The LLOQ for each peptide is listed in supplementary Table I and is defined as the lowest concentration value for which signal-to-noise ratio is ≥10, with a coefficient of variation (CV) of <20%. The LLOQ for peptides that represent apoC2, C3, and E was 3 nM, and 300 nM for apoA4 peptide. The linear ranges for the four peptides were three orders of magnitude, which is sufficient to cover biological and pathological relevant concentrations (11–13). Intra-assay and interassay precision for these peptides were mostly between 2% and 4% (supplementary Table I), which is in the range of high-performance immunoturbidimetric assays (<5%).
The accuracy of the LC/MS assay was assessed by comparing plasma samples obtained from 30 human donors using LC/MS measurements and commercially available immunoturbidimetric assays for apoC2, apoC3, and apoE. The results from LC/MS measurement for all three proteins showed a high degree of correlation to those obtained from immunoturbidimetric readout (Fig. 3). The correlation coefficient r2 was 0.93 for apoC2, 0.83 for apoC3, and 0.94 for apoE. Because these immunoturbidimetric assays are either approved for clinical use or represent high-performing research-grade assays developed by companies specializing in diagnostic tests, these results added confidence to the LC/MS method. As for the quantification of apoA4, because there was no commercially available immunoturbidimetric assay, we selected two peptides for the quantification of apoA4 in 30 human plasma samples. Good correlation between the concentrations measured from two peptides was observed (data not shown). Most of the protein concentrations determined by LC/MS were in the range of values reported in the literature (supplementary Table II).
Fig. 3.
Comparison between LC/MS measurements with immunoturbidimetric assays for high abundant plasma proteins. Plasma samples from 30 human donors were digested with trypsin. The concentrations of apoC2, C3, and E were measured by LC/MS and the immunoturbidimetric assay. Correlation between the two methods was illustrated in apoC2 (A), apoC3 (B), and apoE (C).
Quantification of apoB48 and apoA5 from immunoenriched plasma digests
ApoB48 and apoA5 plasma levels are at single-digit nanomolar range. To quantify these two proteins by LC/MS, we utilized antipeptide antibodies to enrich apoB48 and apoA5 peptides from digested plasma, namely the stable-isotope-labeled standards and capture by antipeptide antibody approach (15). In doing so, both the abundant proteins (apoC2, C3, E, and A4) and the less abundant ones (apoB48 and apoA5) can be quantified using the same plasma digests, thus eliminating additional digestion steps. For the immunoenrichment, we used magnetic beads with a covalently linked antibody. This setup permits an easy adaption to a well-plate format, including scale-up and automation, which is crucial for good assay precision and throughput.
The LLOQ and linearity range were assessed by spiking various known amounts of stable-isotope-labeled peptide standards into plasma digests, ranging from 0.3 pM to 10 nM, which is equivalent to 15 pM and 500 nM plasma protein concentration. These samples underwent immunoaffinity purification, and the eluent was analyzed by LC/MS. The peak area ratios of peptide standards to endogenous peptides were plotted against the concentration of spiked peptides (Fig. 4A, B). The LLOQ was ∼150 pM for apoB48 and below 15 pM for apoA5. The linear response range was at least three orders of magnitude for apoB48, and four orders of magnitude for apoA5, which is sufficient to cover biologically relevant ranges for both proteins (4, 14, 16).
Fig. 4.
Dynamic range of apoB48 and apoA5 LC/MS measurement. Various concentrations of stable-isotope-labeled peptides for apoB48 and apoA5 were spiked into a pooled human plasma digests. LC/MS/MS was used to measure the ratio of stable-isotope-labeled peptides and tryptic peptides released from endogenous proteins after immunoenrichment. The peak area ratios of labeled peptides to endogenous peptides were plotted against the concentration of spiked-in labeled peptides to determine the lower and upper limit of quantitation: apoB48 (A) and apoA5 (B). Each data point is the mean of three measurements. The arrow in A points to the LLOQ.
Trypsin digestion time-course experiments were performed to ensure reproducible digestion of apoB48 and apoA5. The standard overnight digestion duration (16–20 h) produced stable peptide yields for both proteins (supplementary Fig. III). The assay precision was evaluated using individual plasma samples with spiked-in stable-isotope-labeled peptide standards. Human plasma digests (n = 3) were subjected to replicate digestion and immunoenrichment of targeted peptides before analysis by LC/MS/MS. Table 2 lists the endogenous concentration for apoB48 and apoA5 measured from three subjects, along with intra- and interassay precision. The CV of intra- and interassay precision for both proteins was mostly below 10%. We also tested the effect of plasma freeze/thaw on the quantification of apoB48 and apoA5. No significant differences were observed up to three cycles of freeze/thaw treatment (supplementary Fig. IV).
TABLE 2.
Evaluation of assay precision for quantifying apoB48 and apoA5
| Protein | Subject | Mean Concentration (nM) | %CV for Intra-Assay Precision(n = 4 replicates) | %CV for Interassay Precision(n = 3 days) |
| ApoB48 | 1 | 1.8 | 6.8 | 6.0 |
| 2 | 2.4 | 7.5 | 6.4 | |
| 3 | 3.2 | 4.3 | 10.4 | |
| ApoA5 | 1 | 1.7 | 3.4 | 3.0 |
| 2 | 1.5 | 2.1 | 3.0 | |
| 3 | 1.2 | 2.4 | 2.4 |
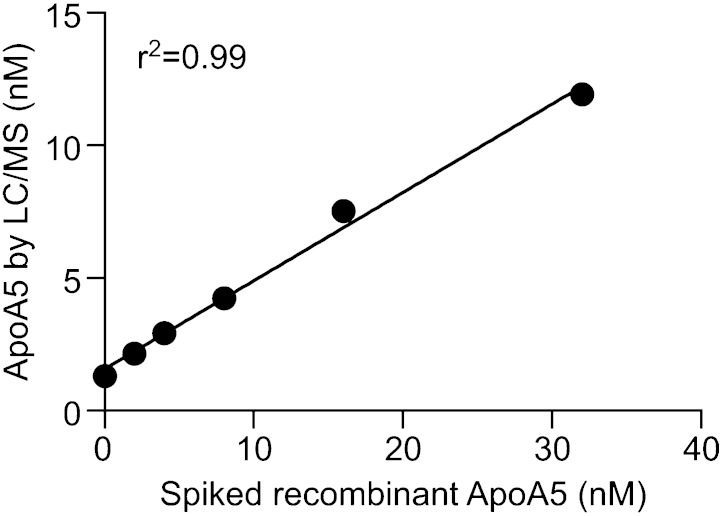
It is known that apoB48 concentration increases in plasma during the postprandial state. Therefore, as a way to increase the confidence of our LC/MS assay and to test its applicability, we used the method to quantify plasma apoB48 from human donors before and 1 h after a meal (i.e., fasted and fed conditions). As shown in Fig. 5A, four out of five individuals exhibited an increase in postprandial apoB48 level. For the one subject whose apoB48 level did not change postprandially, the total TG level also remained unaltered. This observation was probably due to the fact that the donors were allowed to eat ad libitum from a full cafeteria menu and the postprandial samples were collected at 1 h after the meal, which is not at its peak appearance in plasma (typically observed 4–5 h after a meal) (17). Fig. 5B, C illustrates the scatter plots between plasma TG and apoB48 level. Fasting plasma TG was largely from VLDL; thus the correlation between TG and apoB48 level is poor (Fig. 5B, r2 = 0.09). By contrast, postprandial TG was mainly from chylomicron, where TG level should correlate well with apoB48 level. Indeed, the correlation coefficient in the postprandial state (Fig. 5C) is 0.83. ApoB48 levels from the fasted/fed experiment were also determined using immunoassay. As shown in Fig. 5D, apoB48 concentration obtained from LC/MS correlated well with the ELISA measurement (r2 = 0.88). The data point at the highest concentration (∼80 nM) was out of the linear range of the ELISA (if excluded, the correlation coefficient r2 became 0.96). As for apoA5, because robust immunoassays for apoA5 are not commercially available, we used a spike and recovery experiment to confirm the specificity of the LC/MS assay. Recombinant human apoA5 protein was spiked into pooled human plasma from 2 to 32 nM. ApoA5 concentration was then measured by LC/MS. Fig. 6 illustrates the relationship between the peak area of apoA5 peptide and spiked apoA5 protein concentration (r2 = 0.99). Taken together, these data increased the confidence of the LC/MS method.
Fig. 5.
Measurement of apoB48 and total plasma TG concentration from fasted/fed human donors. Upon overnight fasting, plasma samples were collected from five volunteers before and ∼1 h after a standard meal. ApoB48 and total plasma TG were measured. A: ApoB48 concentration measured from fasted and fed plasma samples by LC/MS/MS after trypsin digestion and immunoenrichment. B: Scatter plot between apoB48 and TG in fasted plasma samples. C: Scatter plot between apoB48 and TG in fed plasma samples. D: ApoB48 concentration was also quantified using the ELISA method and plotted against the value obtained from LC/MS measurement.
Fig. 6.
Spike and recovery of human recombinant apoA5 in plasma. Recombinant human apoA5 was spiked into pooled human plasma and serial diluted using the same pool of plasma samples. These samples were digested, and the peptide for apoA5 was immunoenriched prior to LC/MS analysis. The concentration of apoA5 measured by LC/MS was plotted against the spiked recombinant apoA5.
Static and turnover kinetic measurements from an individual infused with D3-leucine
To demonstrate proof of concept, plasma samples from a human subject infused with D3-leucine were processed using the workflow described previously, that is, quantification of apoC2, C3, E, and A4 directly from plasma digest using high-flow LC/MS, and apoB48 and apoA5 from immunoenriched plasma digest using low-flow LC/MS. The incorporation of D3-leucine into a given protein was measured by the ratio of M3/M0 of the protein-derived peptide. Fig. 7 shows the M3/M0 ratios for the six proteins of interest. As expected, the M3/M0 ratio reached plateau within 15 h tracer infusion for relatively fast turnover proteins (e.g., apoA5, B48, and E). By contrast, the M3/M0 ratio increased linearly for proteins with relatively slower turnover rates (e.g., apoC2, C3, and A4). Table 3 lists the FSR for apoC2, C3, A4, E, B48, and apoA5, which was consistent with literature reports (6, 18–22). To our knowledge, the FSR of apoA5 has not been measured previously in humans. Our estimated FSR (∼19.82 pools/day) suggests that apoA5 turns over rapidly. The clearance of injected recombinant apoA5 in a mouse model was very fast (23), which supports our observation. It is worth pointing out that the actual D3-leucine enrichment is the M3/M0 ratio divided by the number of leucines in the precursor to product transition. Taking apoA5 as an example, although the precursor (VQELQEQLR, m/z 573.31, 2+) to product (ELQEQLR, m/z 918.49, 1+) transition was measured as the M3/M0 ratio, the actual enrichment for D3-leucine was the M3/M0 ratio divided by 2, taking into account the two leucine residues in the MRM transition.
Fig. 7.
Kinetic measurements for apos involved in TG metabolism from a subject that was infused with D3-leucine. A human volunteer was infused with D3-leucine, and plasma samples were collected from 0 to 15 h. The plasma samples were digested with trypsin, and the M3/M0 ratio in the target peptides was quantified along with its spiked ISTD for concentration measurement. M3/M0 ratio at each time point was illustrated in apoC2 (A), apoC3 (B), apoE (C), apoA4 (D), apoA5 (E), and apoB48 (F). All graphs were either linear fit or nonlinear with one-phase association using Prism software.
TABLE 3.
Fractional synthesis rate (pools/day) for apoC2, C3, A4, E, B48, and A5 from a human subject
| Protein | Concentration (nM) | Change in Isotope Labeling (slope/h) | Asymptotic Enrichment (%) | FSR | FSR Reported in Literature |
| ApoC2 | 1,019.9 | 0.152 | 0.61 | 0.70a | |
| ApoC3 | 3,246.8 | 0.154 | 0.62 | 0.65; 0.82b | |
| ApoA4 | 3,064.4 | 0.434 | 1.74 | 1.56c | |
| ApoE | 559.0 | 5.97 | 3.30 | 3.80; 4.76d | |
| ApoB48 | 25.2 | 5.93 | 4.52 | 4.39; 5.02e | |
| ApoA5 | 1.0 | 5.59 | 19.82 | Not previously reported |
DISCUSSION
It has been shown that cholesterol-lowering interventions (e.g., statin therapy) reduce the risk of first-time or recurrent coronary heart disease events only by about 35% compared with placebo (2). Hence, the identification of other potential risk-reducing therapeutic targets becomes important. One such target is hypertriglyceridemia. A large body of evidence has been accumulated on the association between high TG and CVD risk (2, 3). Because apos such as apoB48, A5, C2, C3, and E play important roles in TG metabolism, the ability to accurately determine the concentration and turnover kinetics for these proteins is of importance in understanding the physiological basis of hypertriglyceridemia and the effects of different therapeutic approaches.
Immunoassays are typically the method of choice for determining protein concentration in a high-throughput manner. However, one of the important advantages of an LC/MS method over a traditional immunobased assay is the ability to multiplex analyses (i.e., multiple proteins can be simultaneously quantified from a single sample). Moreover, information on protein kinetics (e.g., synthesis or clearance), a critical attribute to metabolism, can also be obtained at the same time if stable isotope tracers are given to the study subjects. Thus, a multiplexed LC/MS method not only saves time and resources because multiple end points are measured simultaneously, but also minimizes sample volumes required. To date, numerous LC/MS assays have been developed to quantify multiple proteins in a sample (11, 24, 25). For example, Kuzyk et al. (12) demonstrated that it is feasible to quantify 45 endogenous proteins in plasma. Domanski et al. (13) showed an MRM-based quantification of 67 CVD protein markers in human plasma. Here we present a strategy that incorporates the static and kinetic measurement of higher and lower abundant proteins from a single digestion mixture. This strategy can be expanded to the analysis of other proteins and stable isotope tracers. In addition, all peptides selected in the current method are fully conserved in nonhuman primates, permitting the usage of this method in studies involving certain preclinical models.
A high degree of correlation was observed between LC/MS and immunoturbidimetric assay for apoC2, apoC3, and apoE (Fig. 3). However, the slope of the scatter plots was biased toward the immunoturbidimetric measurements. The discrepancy in the absolute values between the two measurements could be due to a number of reasons. For example, the two assays used two different reference standards. LC/MS used stable-isotope-labeled peptides whose concentration was determined by amino acid analysis. By contrast, immunoturbidimetric assay used lyophilized serum as reference standards. The accuracy of these reference standards will affect the final readout. In addition, LC/MS measures the surrogate peptides derived from the protein of interest. Consequently, the accuracy of the LC/MS measurement depends on how well the protein is converted into peptides (i.e., enzyme digestion efficiency). Incomplete digestion of the protein of interest could also contribute to the discrepancy in the final value. Thus, we propose to use a set of plasma samples as reference material where the concentrations of apos are defined by immunobased assay to correct any bias in the LC/MS analysis.
In summary, the results reported herein describe a novel strategy for measuring the concentration and turnover kinetics of apos in a single digestion mixture. This strategy can be readily expanded to the analysis of many other proteins and adapted to the analysis of plasma samples from preclinical species.
Supplementary Material
Acknowledgments
The authors thank Dr. Harry Davis for his edits and Dr. Thomas McAvoy and Derek Chapelle from the Clinical Development Laboratory at Merck for helpful discussions on immunoenrichment procedures.
Footnotes
Abbreviations:
- CV
- coefficient of variation
- FSR
- fractional synthesis rate
- ISTD
- internal standard
- LLOQ
- lower limit of quantification
- MRM
- multiple-reaction monitoring
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of Experimental methods, two tables, and four figures.
REFERENCES
- 1.Do R., Willer C. J., Schmidt E. M., Sengupta S., Gao C., Peloso G. M., Gustafsson S., Kanoni S., Ganna A., Chen J., et al. 2013. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 45: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballantyne C. M., Grundy S. M., Oberman A., Kreisberg R. A., Havel R. J., Frost P. H., Haffner S. M. 2000. Hyperlipidemia: diagnostic and therapeutic perspectives. J. Clin. Endocrinol. Metab. 85: 2089–2112. [DOI] [PubMed] [Google Scholar]
- 3.Miller M., Stone N. J., Ballantyne C., Bittner V., Criqui M. H., Ginsberg H. N., Goldberg A. C., Howard W. J., Jacobson M. S., Kris-Etherton P. M., et al. 2011. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 123: 2292–2333. [DOI] [PubMed] [Google Scholar]
- 4.Otokozawa S., Ai M., Diffenderfer M. R., Asztalos B. F., Tanaka A., Lamon-Fava S., Schaefer E. J. 2009. Fasting and postprandial apolipoprotein B-48 levels in healthy, obese, and hyperlipidemic subjects. Metabolism. 58: 1536–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lassman M. E., McLaughlin T. M., Somers E. P., Stefanni A. C., Chen Z., Murphy B. A., Bierilo K. K., Flattery A. M., Wong K. K., Castro-Perez J. M., et al. 2012. A rapid method for cross-species quantitation of apolipoproteins A1, B48 and B100 in plasma by ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 26: 101–108. [DOI] [PubMed] [Google Scholar]
- 6.Millar J. S., Brousseau M. E., Diffenderfer M. R., Barrett P. H., Welty F. K., Cohn J. S., Wilson A., Wolfe M. L., Nartsupha C., Schaefer P. M., et al. 2008. Effects of the cholesteryl ester transfer protein inhibitor torcetrapib on VLDL apolipoprotein E metabolism. J. Lipid Res. 49: 543–549. [DOI] [PubMed] [Google Scholar]
- 7.Zhou H., Castro-Perez J., Lassman M. E., Thomas T., Li W., McLaughlin T., Dan X., Jumes P., Wagner J. A., Gutstein D. E., et al. 2013. Measurement of apo(a) kinetics in human subjects using a microfluidic device with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 27: 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson N. L. 2010. The clinical plasma proteome: a survey of clinical assays for proteins in plasma and serum. Clin. Chem. 56: 177–185. [DOI] [PubMed] [Google Scholar]
- 9.Hortin G. L., Sviridov D., Anderson N. L. 2008. High-abundance polypeptides of the human plasma proteome comprising the top 4 logs of polypeptide abundance. Clin. Chem. 54: 1608–1616. [DOI] [PubMed] [Google Scholar]
- 10.MacLean B., Tomazela D. M., Shulman N., Chambers M., Finney G. L., Frewen B., Kern R., Tabb D. L., Liebler D. C., MacCoss M. J. 2010. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 26: 966–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson L., Hunter C. L. 2006. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol. Cell. Proteomics. 5: 573–588. [DOI] [PubMed] [Google Scholar]
- 12.Kuzyk M. A., Smith D., Yang J., Cross T. J., Jackson A. M., Hardie D. B., Anderson N. L., Borchers C. H. 2009. Multiple reaction monitoring-based, multiplexed, absolute quantitation of 45 proteins in human plasma. Mol. Cell. Proteomics. 8: 1860–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domanski D., Percy A. J., Yang J., Chambers A. G., Hill J. S., Freue G. V., Borchers C. H. 2012. MRM-based multiplexed quantitation of 67 putative cardiovascular disease biomarkers in human plasma. Proteomics. 12: 1222–1243. [DOI] [PubMed] [Google Scholar]
- 14.Jackson K. G., Williams C. M. 2004. Apolipoprotein B-48: comparison of fasting concentrations measured in normolipidaemic individuals using SDS-PAGE, immunoblotting and ELISA. Atherosclerosis. 176: 207–217. [DOI] [PubMed] [Google Scholar]
- 15.Anderson N. L., Anderson N. G., Haines L. R., Hardie D. B., Olafson R. W., Pearson T. W. 2004. Mass spectrometric quantitation of peptides and proteins using Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA). J. Proteome Res. 3: 235–244. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien P. J., Alborn W. E., Sloan J. H., Ulmer M., Boodhoo A., Knierman M. D., Schultze A. E., Konrad R. J. 2005. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clin. Chem. 51: 351–359. [DOI] [PubMed] [Google Scholar]
- 17.Schrezenmeir J., Weber P., Probst R., Biesalski H. K., Luley C., Prellwitz W., Krause U., Beyer J. 1992. Postprandial pattern of triglyceride-rich lipoprotein in normal-weight humans after an oral lipid load: exaggerated triglycerides and altered insulin response in some subjects. Ann. Nutr. Metab. 36: 186–196. [DOI] [PubMed] [Google Scholar]
- 18.Batal R., Tremblay M., Barrett P. H., Jacques H., Fredenrich A., Mamer O., Davignon J., Cohn J. S. 2000. Plasma kinetics of apoC-III and apoE in normolipidemic and hypertriglyceridemic subjects. J. Lipid Res. 41: 706–718. [PubMed] [Google Scholar]
- 19.Ghiselli G., Krishnan S., Beigel Y., Gotto A. M., Jr 1986. Plasma metabolism of apolipoprotein A-IV in humans. J. Lipid Res. 27: 813–827. [PubMed] [Google Scholar]
- 20.Huff M. W., Fidge N. H., Nestel P. J., Billington T., Watson B. 1981. Metabolism of C-apolipoproteins: kinetics of C-II, C-III1 and C-III2, and VLDL-apolipoprotein B in normal and hyperlipoproteinemic subjects. J. Lipid Res. 22: 1235–1246. [PubMed] [Google Scholar]
- 21.Lichtenstein A. H., Hachey D. L., Millar J. S., Jenner J. L., Booth L., Ordovas J., Schaefer E. J. 1992. Measurement of human apolipoprotein B-48 and B-100 kinetics in triglyceride-rich lipoproteins using [5,5,5-2H3]leucine. J. Lipid Res. 33: 907–914. [PubMed] [Google Scholar]
- 22.Welty F. K., Lichtenstein A. H., Barrett P. H., Dolnikowski G. G., Schaefer E. J. 1999. Human apolipoprotein (Apo) B-48 and ApoB-100 kinetics with stable isotopes. Arterioscler. Thromb. Vasc. Biol. 19: 2966–2974. [DOI] [PubMed] [Google Scholar]
- 23.Shu X., Nelbach L., Weinstein M. M., Burgess B. L., Beckstead J. A., Young S. G., Ryan R. O., Forte T. M. 2010. Intravenous injection of apolipoprotein A-V reconstituted high-density lipoprotein decreases hypertriglyceridemia in apoav−/− mice and requires glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1. Arterioscler. Thromb. Vasc. Biol. 30: 2504–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiteaker J. R., Zhao L., Anderson L., Paulovich A. G. 2010. An automated and multiplexed method for high throughput peptide immunoaffinity enrichment and multiple reaction monitoring mass spectrometry-based quantification of protein biomarkers. Mol. Cell. Proteomics. 9: 184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whiteaker J. R., Zhao L., Abbatiello S. E., Burgess M., Kuhn E., Lin C., Pope M. E., Razavi M., Anderson N. L., Pearson T. W., et al. 2011. Evaluation of large scale quantitative proteomic assay development using peptide affinity-based mass spectrometry. Mol. Cell. Proteomics. 10: M110.005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.