Abstract
A recent surge in newly described inborn errors of immune function-related genes that result in susceptibility to fungal disease has greatly enhanced our understanding of the cellular and molecular basis of antifungal immune responses. Characterization of single-gene defects that predispose to various combinations of superficial and deep-seated infections caused by yeasts, molds, and dimorphic fungi has unmasked the critical role of novel molecules and signaling pathways in mucosal and systemic antifungal host defense. These experiments of nature offer a unique opportunity for developing new knowledge in immunological research and form the foundation for devising immune-based therapeutic approaches for patients infected with fungal pathogens.
Several dozen genetic defects that predispose to various fungal infections have been identified. Their characterization has improved our understanding of the cellular and molecular basis of antifungal immune responses.
Fungal infections have emerged as significant causes of morbidity and mortality over the past few decades (Brown et al. 2012a). For example, mucosal yeast infections carry a substantial global disease burden as >90% AIDS patients will develop oral thrush and ∼75% of women worldwide will develop vulvovaginal candidiasis (Sobel 2007). In addition, systemic mycoses caused by molds, yeasts, and dimorphic fungi are a major cause of mortality in patients with cancer, transplantation, and AIDS (Brown et al. 2012b). No fungal vaccines are yet available to prevent disease, and, despite the advent of potent antifungal therapies, mortality of infected patients still exceeds 40%–50%. Therefore, better understanding of the cellular and molecular basis of mammalian antifungal immunity is fundamental to improve patient outcomes. To that end, the study of primary immunodeficiencies (PIDs) provides important insights into immunological perturbations that lead to mucosal and systemic fungal disease.
Different constituents of the immune system mediate fungus-specific and site-specific antifungal immune responses. On the one hand, professional phagocytes are crucial for host defense during deep-seated fungal infection. Specifically, tissue-resident macrophages, recruited monocytes, and neutrophils mediate uptake and killing of yeasts and inhaled molds, principally via oxidative cytotoxic mechanisms (Fig. 1) (LeibundGut-Landmann et al. 2012; Lionakis et al. 2013). For intracellular dimorphic fungi, macrophages are key effector cells via interleukin (IL)-12/interferon (IFN)-γ production, which enhances antigen presentation, T-lymphocyte activation and intracellular killing (Fig. 1) (Rosenzweig and Holland 2005). On the other hand, T lymphocytes of the Th17 differentiation program and epithelial cells are important for controlling mucosal yeast infections (Puel et al. 2010). In brief, dectin-1-mediated fungal recognition and downstream Syk-CARD9–induced production of IL-6 and IL-23, which orchestrate STAT3-dependent Th17 differentiation, promotes secretion of IL-17-related cytokines that instruct the production of antifungal antimicrobial peptides and neutrophil-recruiting chemoattractants by epithelial cells, thus conferring mucosal antifungal protection (Fig. 2) (Hernández-Santos and Gaffen 2012).
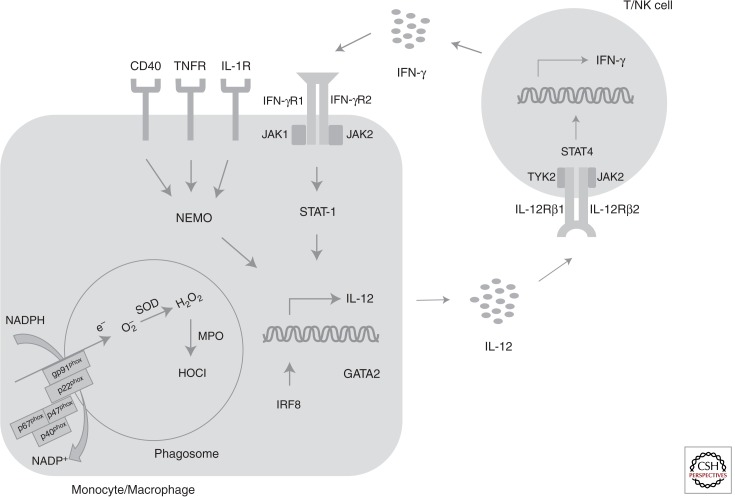
Figure 1.
Disorders associated with systemic fungal disease. The generation of superoxide by the NADPH oxidase complex and of hypochlorous acid by myeloperixodase within phagocytes is critical for the killing of filamentous molds and yeasts. The interaction between monocytes/macrophages and T/NK lymphocytes is important for control of infections by intracellular dimorphic fungi. Specifically, interleukin-12 is released by monocytes/macrophages in response to fungal ingestion and binds to its cognate receptor on T/NK cells. This, in turn, results in STAT4-dependent release of interferon-γ, which acts on monocytes/macrophages to enhance fungal killing via activation of STAT1. IRF8 is critical for myeloid cell differentiation and interleukin-12 production, and GATA2 plays a significant role in monocyte, dendritic cell and NK cell maintenance and effector function. Activation of NEMO, downstream from CD40, interleukin-1 receptor, and TNF-receptor signaling, is important for control of Pneumocystis infection.
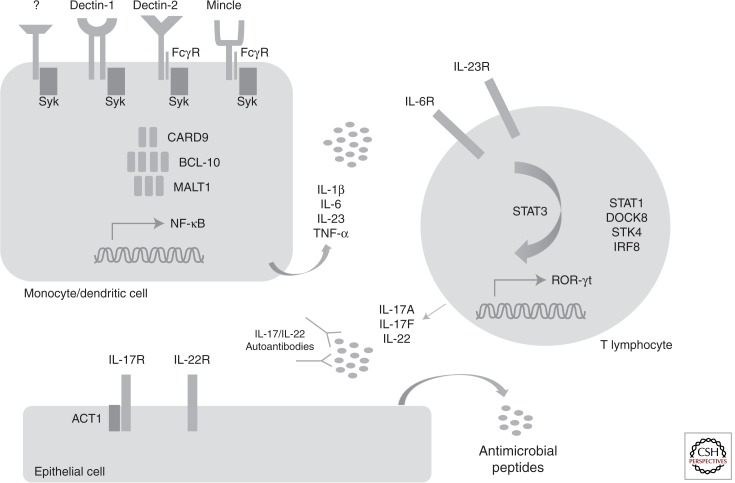
Figure 2.
Disorders associated with mucocutaneous fungal disease. Candida recognition by myeloid cell C-type lectin receptors such as dectin-1 results in activation of the CARD9/BCL-10/MALT1 signaling complex. This in turn orchestrates the production of proinflammatory cytokines that direct T-lymphocyte differentiation toward the Th17 program, a STAT3-dependent process. DOCK8 and IRF8 also contribute to Th17 differentiation, and gain-of-function STAT1 mutations that lead to STAT1 hyperphosphorylation create a cytokine milieu that inhibits the generation of Th17 cells. STK4 is crucial for T-cell survival. Th17 cells produce interleukin-17 and interleukin-22, which recruit phagocytes to the site of fungal infection and induce the generation of potent antifungal antimicrobial peptides by epithelial cells. Mucocutaneous candidiasis is seen in patients with mutations in interleukin-17F, interleukin-17RA, and the adaptor protein ACT1, which impair interleukin-17-dependent signaling, and in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy who have neutralizing autoantibodies against interleukin-17 and interleukin-22.
Genetic defects that adversely affect the aforementioned effector cells and molecular pathways result in varying combinations of systemic and/or mucosal fungal infections (Table 1). Here we present the PIDs that result in fungal infection susceptibility and we review the recent advances in our understanding of genetic and immunological perturbations that result in heightened susceptibility to fungal disease.
Table 1.
Clinical and immunological characteristics of genetic defects associated with development of mucosal and systemic fungal disease
| Gene (chromosome) | Clinical syndrome | Mode of inheritance | Mucosal versus systemic fungal disease | Fungal pathogens | Nonfungal infection susceptibility | Noninfectious manifestations | Immunological defects accounting for fungal susceptibility |
|---|---|---|---|---|---|---|---|
| A. Disorders of phagocyte oxidative machinery | |||||||
| CYBB (Xp) CYBA (16q) NCF-1 (7q) NCF-2 (1q) NCF-4 (22q) |
CGD | X linked AR AR AR AR |
Systemic | Aspergillosis (including A. nidulans) Other molds (Paecilomyces, Neosartorya) Systemic candidiasis |
Staphylococcus Serratia Nocardia Burkholderia |
Inflammatory bowel disease | Lack of superoxide generation |
| MPO (17q) | MPO deficiency | AR | Systemic | Systemic candidiasis | None | None | Lack of hypochlorous acid production |
| B. Disorders of cytokine signaling | |||||||
| IFNGR1 (6q) | MSMD | AD | Systemic | Coccidioidomycosis Histoplasmosis | NTM Salmonella | None | Impaired IFN-γ cellular responses |
| IL12RB1 (19p) | MSMD | AD or AR | Both | Coccidioidomycosis Paracoccidioidomycosis Histoplasmosis Cryptococcosis CMC |
NTM Salmonella |
None | Impaired IL-12/ IL-23-dependent IFN-γ production Impaired IL-12Rβ1-dependent Th17 differentiation |
| IL-17RA (22q) | AR | Mucosal | CMC |
Staphylococcus URIs |
Atopic dermatitis | Lack of IL-17 cellular responses | |
| IL-17F (6p) | AD | Mucosal | CMC |
Staphylococcus URIs |
Asthma | Impaired IL-17F receptor binding and bioactivity | |
| ACT1 (6q) | AR | Mucosal | CMC | Staphylococcus | Atopic dermatitis | Impaired ACT1 interactions with IL-17 receptors Impaired IL-17 cellular responses |
|
| IL2RG (Xq) | SCID | X linked | Both | PCP CMC |
URIs Bacteria Viruses |
Failure to thrive Diarrhea Graft versus host disease |
Severe lymphocytopenia |
| IL7RA (5p) CD45 (11q) ADA (20q) AK2 (1p) RAG1 (11p) RAG2 (11p) JAK3 (19p) ARTEMIS (10p) |
SCID | AR | Both | PCP CMC |
URIs Bacteria Viruses |
Failure to thrive Diarrhea Graft versus host disease |
Severe lymphocytopenia |
| C. Disorders of transcription factors | |||||||
| STAT3 (17q) | HIES (Job’s syndrome) |
AD | Both | CMC Nail dermatophytosis Aspergillosis Scedosporiosis PCP Cryptococcosis Histoplasmosis Coccidioidomycosis |
Bacteria (skin, lungs) | Eczema Pneumatoceles Characteristic facial features Scoliosis Coronary artery aneurysms Tooth development abnormalities Bone fractures |
Impaired Th17 differentiation Decreased proportion of IL-17/IL-22+-producing T cells Decreased IL-17 production by mononuclear cells Impaired production of antimicrobial peptides by keratinocytes Impaired candidacidal activity of saliva Decreased levels of IL-17-induced candidacidal antimicrobial peptides in saliva |
| STAT1 (2q) | AD | Both | Coccidioidomycosis Histoplasmosis Fusariosis (skin) CMC |
CMV Bacteria |
Inflammatory bowel disease Hypothyroidism Squamous cell carcinoma |
Decreased IL-17 production by mononuclear cells Enhanced cellular responses to IFN-α/β, IFN-γ, and IL-27, which inhibit Th17 differentiation Defective IL-12R/IL-23R signaling |
|
| GATA2 (3q) | MonoMAC syndrome | AD | Systemic | Aspergillosis Histoplasmosis Cryptococcosis |
NTM Warts |
MDS/AML Lymphedema PAP |
Monocytopenia Decreased circulating and tissue-resident dendritic cells Neutrophil abnormalities |
| AIRE (21q) | APECED | AR | Mucosal | CMC | None | Autoimmune endocrinopathies Autoimmune hepatitis Malabsorption Dental enamel abnormalities Oral/esophageal carcinoma Vitiligo |
Neutralizing autoantibodies to IL-17A, IL-17F, and IL-22 Decreased production of IL-17/IL-22 and other proinflammatory cytokines by mononuclear cells Impaired candidacidal activity of saliva Decreased levels of candidacidal antimicrobial peptides in saliva |
| IRF8 (16q) | AR | Mucosal | CMC | NTM | None | Monocytopenia Decreased circulating and tissue-resident dendritic cells Decreased IL-17 production by mononuclear cells Impaired antigen presentation |
|
| D. Disorders of other signaling molecules | |||||||
| CARD9 (9q) | AR | Both |
Candida meningitis Invasive dermatophytosis Subcutaneous phaeohyphomycosis CMC Nail dermatophytosis |
None | None | Impaired IL-17 and proinflammatory cytokine cellular responses Decreased proportion of IL-17/IL-22+-producing T cells Impaired neutrophil Candida killing |
|
| DECTIN-1 (12p) | AR | Mucosal | Vaginal candidiasis Nail dermatophytosis |
None | None | Decreased IL-17 production by mononuclear cells | |
| DOCK8 (9p) | HIES | AR | Mucosal | CMC Nail dermatophytosis |
Viruses (skin) | Eczema Vasculitis Hematological malignancy Squamous cell carcinoma |
Decreased IL-17 production by mononuclear cells Impaired Th17 differentiation |
| TYK2 (19p) | HIES MSMD |
AR | Mucosal | CMC | Bacteria NTM Viruses |
Atopic dermatitis | Unknown |
| NEMO/IKBKG (Xq) | EDA-ID HIGM |
X-linked | Both | PCP CMC |
NTM Bacteria Viruses |
Anhidrotic ectodermal dysplasia | Severe lymphocytopenia |
| IKBA (14q) | EDA-ID HIGM |
AD | Both | PCP CMC |
NTM Bacteria |
Anhidrotic ectodermal dysplasia Colitis |
Severe lymphocytopenia Decreased proportion of IL-17+-producing T cells |
| CD40L (Xq) | HIGM | X-linked | Systemic | PCP | NTM Bacteria Cryptosporidia |
Inflammatory bowel disease | Impaired T-cell responses |
| STK4 (20q) | AR | Mucosal | CMC | Bacteria HSV Viruses (skin) |
EBV-driven lymphoproliferation Structural heart abnormalities |
Impaired T-cell survival and proliferation | |
AD, autosomal dominant; APECED, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome; AR, autosomal recessive; CGD, chronic granulomatous disease; CMC, chronic mucocutaneous candidiasis; CMV, cytomegalovirus; EBV, Epstein–Barr virus; EDA-ID, anhidrotic ectodermal dysplasia with immune deficiency; HIES, hyper-IgE syndrome; HIGM, hyper-IgM syndrome; HSV, herpes simplex virus; MDS/AML, myelodysplastic syndrome/acute myelogenous leukemia; MPO, myeloperoxidase; MSMD, Mendelian susceptibility to mycobacterial disease; NTM, nontuberculous mycobacteria; PAP, pulmonary alveolar proteinosis; PCP, Pneumocystis pneumonia; SCID, severe combined immunodeficiency; URIs, upper respiratory infections.
DISORDERS OF THE PHAGOCYTE OXIDATIVE BURST MACHINERY
Chronic Granulomatous Disease (CGD)
CGD is a rare PID (frequency, ∼1/200,000) caused by defects in any of the five subunits of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex (Segal et al. 2000). The majority of cases (65%) are X-linked recessive because of mutations in CYBB encoding subunit gp91phox. The remaining cases (35%) are autosomal recessive caused by mutations in CYBA (5%), NCF-1 (25%), NCF-2 (5%), and NCF-4 (one reported case), encoding subunits p22phox, p47phox, p67phox, and p40phox, respectively (Holland 2013). No autosomal dominant cases have been reported.
NADPH oxidase is critical for generation of superoxide within phagocytes. On fungal ingestion, the cytosolic subunits p47phox and p67phox become phosphorylated and bind together. Then, the cytochrome b558 complex consisting of the membrane-bound gp91phox and p22phox within secondary granules fuses with the phagolysosome, followed by fusion of the primary granules, which contain the antimicrobial peptides cathepsin G and neutrophil elastase. Then, the cytosolic p47phox-p67phox complex together with p40phox and RAC2 combine with cytochrome b558 to form the intact NADPH oxidase complex, which catalyzes the transfer of an electron from NADPH to molecular oxygen within the phagolysosome to form superoxide (Segal et al. 2000). Superoxide dismutase then converts superoxide to hydrogen peroxide, which in the presence of myeloperoxidase (MPO) is converted to hypohalous acid (Fig. 1). Therefore, CGD phagocytes are defective in superoxide generation and exhibit impaired oxygen-dependent microbiocidal activity (Brown 2011). In fact, the level of residual reactive oxygen intermediate production determines overall survival in CGD patients (Kuhns et al. 2010). Besides the direct fungicidal effects of oxidative products, superoxide generation is important for fungicidal activity via K+ flux-mediated activation of granule proteases within the phagolysosome (Reeves et al. 2002). In addition, the formation of neutrophil extracellular traps, which can ensnare and kill fungi, may be NADPH-dependent; gene therapy in CGD patients has been reported to reconstitute extracellular trap formation and neutrophil antifungal activity (Bianchi et al. 2009).
Invasive aspergillosis (IA) is by far the most common mycosis and accounts for more than one-third of all infections in CGD; indeed, in the absence of iatrogenic risk factors, IA occurs almost exclusively in CGD, typically before the age of 20 in patients without underlying structural lung disease. Staphylococcus aureus, Serratia marsescens, Burkholderia cepacia complex, and Nocardia species and are the other CGD “signature” pathogens in North America (Winkelstein et al. 2000). Besides impaired phagocyte oxidative killing, exuberant inflammatory responses to fungal particles have also been implicated in the pathogenesis of IA in CGD (Morgenstern et al. 1997; Romani et al. 2008). In fact, inhalation of aerosolized decayed organic matter in mulch or hay can cause fulminant Aspergillus pneumonitis, known as “mulch pneumonitis,” a combined hypersensitivity and IA syndrome, for which prompt corticosteroid and antifungal drug administration is imperative for good clinical response (Siddiqui et al. 2007). Similar to immunosuppressed patients at risk for IA, Aspergillus fumigatus is the most common species, but Aspergillus nidulans is encountered almost exclusively in CGD, for reasons that remain unknown, and is distinctive because of its resistance to antifungals and its propensity to invade contiguous anatomical planes (Segal et al. 1998).
In addition to A. nidulans, other CGD-characteristic molds include Paecilomyces variotii, Paecilomyces lilacinus and Neosartorya udagawae. In contrast, mucormycosis is uncommon in CGD and occurs in the setting of iatrogenic immunosuppression or infants with CGD (Vinh et al. 2009a). Similarly, systemic candidiasis is infrequent in CGD (Winkelstein et al. 2000). This fungus-specific difference in infection susceptibility may relate to the greater phagocyte dependence on nonoxidative mechanisms for killing Candida and Rhizopus over Aspergillus (Diamond and Clark 1982). Yet, nonoxidative killing mechanisms are also operational against Aspergillus (Zarember et al. 2007), explaining why 65% of CGD patients never develop IA despite the ubiquitous exposure to environmental Aspergillus conidia during their lifetime. Not surprisingly, consonant with the lack of heightened susceptibility of neutropenic patients to mucosal yeast infections, cryptococcosis and dimorphic fungi, these infections do not occur in CGD. IA was the leading cause of mortality in CGD, causing more than one-third of all deaths. However, the recent introduction of new-generation azole drugs for prophylaxis and treatment has dramatically decreased IA-related mortality (Segal et al. 2005).
MPO Deficiency
MPO deficiency is the most common inherited phagocytic disorder with an estimated frequency of ∼1/2000; the disease is autosomal recessive. MPO converts hydrogen peroxide into hypochlorous acid during the respiratory burst (Fig. 1). MPO deficiency manifests with significantly decreased Candida killing in vitro but minimally impaired ability to kill bacteria (Lehrer and Cline 1969). Affected patients may have complete or partial MPO deficiency associated with absence or ∼50% of normal MPO levels detectable within phagocytes, respectively (Nauseef et al. 1983). Nonetheless, reemphasizing the important immunological differences between mice and humans, although MPO-deficient mice are susceptible to systemic candidiasis (Aratani et al. 1999), only <5% of patients with MPO deficiency develop the infection and the vast majority are asymptomatic; in fact, systemic candidiasis occurs almost exclusively in those with complete MPO deficiency who also suffer from diabetes, which independently impairs neutrophil candidacidal function (Cech et al. 1979).
DISORDERS IN CYTOKINE SIGNALING
IL-12/IFN-γ Signaling
Engulfment of intracellular pathogens by tissue-resident macrophages results in production of IL-12p70, which stimulates T and NK cells via its cognate receptor to secrete IFN-γ. IFN-γ then acts via its receptor on macrophages to activate STAT1, which translocates to the nucleus and up-regulates the transcription of IFN-γ–related genes, facilitating intracellular pathogen clearance (Fig. 1) (O’Shea et al. 2013). Not unexpectedly, mutations in the IL-12/IFN-γ signaling cascade cause Mendelian susceptibility to mycobacterial disease (MSMD). The severity of the clinical phenotype in MSMD correlates with the extent of impairment in production or response to IFN-γ; hence, early-onset life-threatening infections by nontuberculous mycobacteria (NTM), Salmonella and viruses occur in patients with homozygous null mutations in IFN-γR1 or IFN-γR2 who lack IFN-γ cellular responses, whereas mild mycobacterial disease is seen in patients with homozygous null mutations in IL-12Rβ1 or IL-12p40, or heterozygous hypomorphic IFN-γR1 and IFN-γR2 mutations (Rosenzweig and Holland 2005).
Patients with mutations in the IL-12/IFN-γ axis also develop disseminated infections by intracellular dimorphic fungi. Specifically, the autosomal dominant form of IFN-γR1 deficiency causes partial receptor deficiency and predisposes to coccidioidomycosis and histoplasmosis (Zerbe and Holland 2005; Vinh et al. 2009b). The defect is a result of an interstitial deletion that creates a signal-impaired but cell-surface-persisting molecule that exerts a dominant-negative effect on IFN-γ cellular responses of the wild-type receptor. Furthermore, coccidioidomycosis, histoplasmosis, paracoccidioidomycosis and cryptococcosis have been reported in patients with missense homozygous or heterozygous mutations in the β1 subunit of IL-12R (Moraes-Vasconcelos et al. 2005; Rezai et al. 2008; de Beaucoudrey et al. 2010; Vinh et al. 2011). Although cellular responses to IFN-γ are unaffected in these patients, IL-12-dependent IFN-γ production is impaired. Severe disseminated coccidioidomycosis has also been identified in a patient with heterozygous mutation of IL-12Rβ (SM Holland, unpubl.). Thus far, blastomycosis, sporotrichosis and penicilliosis have not been reported in patients with mutations in the IL-12/IFN-γ axis; whether the dimorphic fungus-specific susceptibility in these patients reflects differential dependence on IL-12/IFN-γ signaling for host defense merits further investigation. Of note, ∼20% of patients with IL-12Rβ1 mutations reportedly develop mild chronic mucocutaneous candidiasis (CMC) (de Beaucoudrey et al. 2010), possibly a result of the secondary role of IL-12Rβ1 in Th17 cell differentiation and expansion (de Beaucoudrey et al. 2008).
IL-17 Signaling
The nonredundant role of IL-17 signaling in mucosal antifungal immunity was directly demonstrated in two kindreds with CMC (Puel et al. 2011). In a consanguineous family from Morocco, a homozygous c.850C>T, p.Q284X nonsense mutation in IL-17RA led to absence of IL-17RA expression and was the cause of autosomal recessive CMC associated with skin staphylococcal infections, atopic dermatitis, and early-life upper respiratory infections. The patient exhibited normal proportions of circulating IL-17/IL-22-producing cells but lacked cellular responses to IL-17A and IL-17F in leukocytes and fibroblasts.
In a second kindred from Argentina, a heterozygous c.284C>T, p.S65L missense mutation in IL-17F caused autosomal dominant CMC associated with upper respiratory infections, asthma, and furunculosis. The hypomorphic mutant IL-17F allele exerted a dominant-negative effect and exhibited incomplete clinical penetrance, as two family members with the mutation did not develop CMC. The patients had normal proportions of circulating IL-17/IL-22-producing cells and normal production of IL-17F homodimers and IL-17A/IL-17F heterodimers. Nonetheless, the mutant S65L protein displayed impaired binding to IL-17RA and resulted in decreased cytokine production in leukocytes, fibroblasts, and keratinocytes.
Additional direct evidence for the importance of IL-17 signaling in mucosal antifungal immunity was the report of two siblings from a consanguineous Algerian family, in which a homozygous c.1607C>T, p.T536I missense mutation in the SEFIR domain of ACT1 caused autosomal recessive CMC associated with staphylococcal blepharitis and transient atopic dermatitis during infancy (Boisson et al. 2013). ACT1 is a cytoplasmic protein that forms direct interactions with IL-17 receptors for downstream IL-17-dependent signaling to occur (Fig. 2) (Gaffen 2011). Hence, the mutant T536I protein abolished the homotypic interaction of ACT1 with IL-17 receptors and resulted in impaired cytokine responses to IL-17 cytokines in leukocytes and fibroblasts.
IL-2 Common γ-Chain Signaling
The common γ-chain (γc) or interleukin-2 receptor subunit γ (IL-2RG) is shared by the receptor complexes needed for optimal IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 signaling, which are critical for normal lymphocyte development and differentiation. More than 250 mutations (i.e., nonsense, missense, splice, deletions, insertions) have been described in IL2RG, which result in X-linked severe combined immunodeficiency syndrome (SCID), the most common form of SCID. X-SCID is characterized by cellular immunodeficiency resulting from T and NK lymphocytopenia and humoral immunodeficiency because of nonfunctional B lymphocytes (Notarangelo 2010). SCID presents with a severe phenotype in the first months of life because of bacterial, viral, and fungal infections, and may be fatal without hematopoietic stem cell transplantation or corrective gene therapy. Mucosal candidiasis and Pneumocystis jirovecii pneumonia (PCP) are the two mycoses that develop. Besides X-linked SCID, several autosomal recessive forms of SCID have been described caused by mutations in IL7RA, CD45, ADA, AK2, RAG1, RAG2, JAK3, ARTEMIS, and other genes (Notarangelo 2010); these mutations also confer susceptibility to CMC and PCP but manifest mutation-specific varying degrees of T, B, and NK cell impairments.
TRANSCRIPTION FACTOR DISORDERS
STAT3 Mutations
Autosomal dominant hyper-IgE syndrome (AD-HIES), initially termed Job’s syndrome (Davis et al. 1966), is caused by heterozygous missense mutations or in-frame deletions in the DNA or SH2-binding domains of the transcription factor STAT3 (Holland et al. 2007; Minegishi et al. 2007). A few sporadic cases caused by de novo mutations have also been described. The mutant STAT3 alleles have complete clinical penetrance and exert a dominant-negative effect on wild-type STAT3 function by decreasing homodimer activity to ∼25% of normal (Minegishi et al. 2007).
AD-HIES is characterized by staphylococcal pulmonary infections and skin “cold” abscesses, eczema, and elevated IgE (Grimbacher et al. 1999). In addition, AD-HIES patients manifest characteristic facial features, scoliosis, joint hyperextensibility, delayed primary teeth exfoliation, and coronary artery aneurysms, consonant with the central role of STAT3 in modulating a multitude of immunological and nonimmunological biological processes.
Approximately 80% of patients with AD-HIES develop CMC and less often superficial dermatophytosis (Grimbacher et al. 1999). AD-HIES was the first PID in which impaired IL-17 immunity was associated with CMC susceptibility. Specifically, AD-HIES CD4+ T lymphocytes are unable to induce ROR-γt for differentiation into Th17 cells (Fig. 2) (Milner et al. 2008). In agreement, AD-HIES patients have very reduced circulating IL-17-producing T lymphocytes. Furthermore, AD-HIES T cells produce less IL-17A and IL-22 after Candida stimulation, suggesting an intrinsic T-cell defect (Milner et al. 2008). As a result of impaired IL-17/IL-22 secretion, AD-HIES T cells fail to prime keratinocytes to produce IL-17-dependent β-defensins and neutrophil-recruiting chemoattractants (Fig. 2) (Minegishi et al. 2009). Moreover, AD-HIES patient saliva has diminished candidacidal activity associated with decreased levels of β-defensin-2 and histatin (Conti et al. 2011), implying that STAT3 is critical for the orchestration of mucosal immune responses for Candida clearance. Nonetheless, the report of patients with AD-HIES caused by somatic mosaicism who developed CMC despite normal numbers of peripheral Th17 cells suggests that CMC in some patients may occur because of STAT3-dependent, IL-17-independent immune defects or that the percent of peripheral Th17 cells may not be an accurate surrogate marker to portray the integrity of mucosal IL-17-dependent immunity in CMC (Hsu et al. 2013b).
Besides mucosal yeast infections, AD-HIES patients develop pulmonary mold disease (Vinh et al. 2010b). However, this susceptibility does not appear to be solely derived from defects in the innate immune function of professional phagocytes, unlike CGD. Instead, AD-HIES patients develop invasive pulmonary mold infections later in life, often after age 30, as a result of bronchiectasis and pneumatoceles that develop because of recurrent bacterial pneumonias. These structural lung abnormalities form the substrate for secondary mold colonization and infection. In a recent report, 28% of AD-HIES patients developed mold infections with IA being most common followed by scedosporiosis. Despite therapy, these infections have considerable mortality (∼20%) (Vinh et al. 2010). AD-HIES patients also develop PCP, even before development of structural lung disease for reasons that remain unknown (Freeman et al. 2006); similarly, the mechanisms by which STAT3 mutations occasionally predispose to cryptococcosis, histoplasmosis, and coccidioidiomycosis of the gastrointestinal tract are poorly understood (Hsu et al. 2010).
STAT1 Mutations
STAT1 is a critical transcription factor downstream from IFN-α/β and IFN-γ signaling. Null autosomal recessive STAT1 mutations cause life-threatening NTM, viral, and bacterial infections, whereas heterozygous hypomorphic STAT1 mutations are associated with a milder phenotype characterized by NTM infections; these loss-of-function STAT1 mutations do not cause apparent fungal infection susceptibility (Rosenzweig and Holland 2005).
In contrast, autosomal dominant gain-of-function STAT1 missense mutations in the DNA-binding and coiled-coil domains were recently identified in several kindreds with CMC (Liu et al. 2011; van de Veerdonk et al 2011). The patients had decreased circulating IL-17+ and IL-22+ T lymphocytes and their mononuclear cells were defective in secretion of IL-17 and IL-22. Although unclear how hypermorphic STAT1 mutations impair IL-17 immunity in vivo, hyperphosphorylation of STAT1 is a constant molecular feature (Liu et al. 2011; Smeekens et al. 2011). It has been postulated that this is because of impaired nuclear dephosphorylation of activated STAT1, leading to (a) defective IL-12R/IL-23R signaling, and (b) enhanced STAT1-mediated responses to IFN-α/β, IFN-γ, and IL-27, which are known collectively to inhibit Th17 development (Liu et al. 2011; Smeekens et al. 2011). One of the mutations reported by Liu and colleagues (c.604A>G; p.M202V) was the cause of refractory extensive cutaneous fusariosis without CMC in a Chinese child (Wang et al. 2013a). How STAT1 hypermorphic mutations confer susceptibility against molds is still unknown.
Notably, gain-of-function STAT1 mutations in the DNA-binding and coiled-coil domains also predispose to severe disseminated infections by dimorphic fungi, such as coccidioidomycosis and histoplasmosis, with or without CMC (Sampaio et al. 2013; Uzel et al. 2013). In these patients, enhanced STAT1 phosphorylation, DNA binding, and transactivation results in initial increased IFN-γ-induced gene expression but subsequent impaired responses to IFN-γ re-stimulation, implicating IFN-γ tachyphylaxis, but not impaired IL-17 immunity, as the underlying mechanism accounting for susceptibility to systemic fungal disease in STAT1 gain-of-function mutations. Of interest, a patient with gain-of-function STAT1 mutation and CMC was reported to respond clinically to G-CSF administration with associated restoration of Th17 responses (Wildbaum et al. 2013). Future studies are warranted to explore whether this effect is reproducible in more patients and whether the G-CSF-mediated protective effects are also seen in patients with disseminated infections by dimorphic fungi. Importantly, the phenotypic variation in patients with gain-of-function STAT1 mutations reminds us that different germline mutations in the same gene can lead to differential pathogen susceptibility via different cellular and molecular immunological mechanisms.
GATA2 Mutations
Systemic fungal disease caused by molds, yeasts, and dimorphic fungi also occurs in the syndrome of monocytopenia and susceptibility to mycobacteria, papillomaviruses, fungi, and myelodysplasia (MonoMAC) (Vinh et al. 2010a). MonoMAC is caused by sporadic or autosomal dominant mutations in the transcription factor GATA2. Both missense mutations and deletions in the zinc finger domain that result in translation of abnormal GATA2, full gene deletions, and early stop and frame shift mutations that cause nonsense-induced decay of GATA2 mRNA have been reported (Hsu et al. 2011). GATA2 deficiency also develops as a result of mutations within conserved intronic regions that adversely affect GATA2 transcription (Hsu et al. 2013a); collectively, MonoMAC is the result of GATA2 haploinsufficiency. The same GATA2 haploinsufficiency is also the underlying cause of the syndromes originally named dendritic cell myeloid and NK cell deficiency (DCML) (Bigley et al. 2011; Dickinson et al. 2011), Emberger syndrome (Ostergaard et al. 2011), familial MDS/AML (Hahn et al. 2011), and classical NK cell deficiency (Biron et al. 1989).
More than 80% of GATA2-deficient patients develop disseminated warts and NTM infections, whereas up to one-half develop myelodysplasia and/or acute leukemia (Spinner et al. 2014). Hematopoietic stem cell transplantation is curative (Cuellar-Rodriguez et al. 2011). Other features of GATA2 deficiency include lymphedema and pulmonary alveolar proteinosis, indicative of the central role of GATA2 in hematopoiesis, immunity, and vascular development. Systemic but not mucosal fungal disease occurs in ∼30% of MonoMAC patients. Histoplasmosis and IA are most common; cryptococcosis is infrequently seen (Vinh et al. 2010a). GATA2-deficient patients exhibit profound circulating monocytopenia, B and NK lymphocytopenia, and decreased circulating and tissue-resident dendritic cells. Intriguingly, despite cytopenias, patients have tissue-resident macrophages, plasma cells, and NK cells at sites of infection. Neutrophils are variably affected with decreased granularity, dysplasia, and impaired surface antigen expression (Vinh et al. 2010a). Future research will be needed to elucidate which GATA2-dependent immunological defects account for increased fungus-specific susceptibility seen in only some patients.
Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy (APECED)
APECED is a rare autosomal recessive syndrome caused by AIRE mutations (Mathis and Benoist 2009). It occurs worldwide but is most prevalent among Finnish, Sardinians, and Iranian Jews with estimated lifetime incidences of ∼1/25000, ∼1/14500, and ∼1/9000, respectively. More than 60 different AIRE mutations have been reported so far; the p.R257X nonsense mutation is most prevalent in Finland (>80%), the p.R139X nonsense mutation is most prevalent in Sardinia (>90%), the 13kb deletion c.967–979del13bp is most common in North American and British patients, and the p.Y85C mutation is unique among Iranian Jews (Björses et al. 1996; Heino et al. 1999; Meloni et al. 2012).
AIRE is a transcriptional regulator expressed by medullary thymic epithelial cells, where it regulates the expression of peripheral tissue-specific self-antigens and promotes central tolerance via deletion of self-reactive T cells (Anderson et al. 2002). AIRE has recently been implicated in induction of peripheral tolerance; specifically, antigen-presenting extrathymic AIRE-expressing cells within secondary lymphoid organs can functionally inactivate CD4+ T cells via regulatory T-cell-independent mechanisms (Gardner et al. 2013). Not surprisingly, AIRE deficiency results in escape of self-reactive T cells in the periphery and development of self-reactive autoantibodies, which are both considered responsible for the autoimmune manifestations of the syndrome, principal of which are hypoparathyroidism and adrenal insufficiency (Ahonen et al. 1990).
Besides autoimmunity, APECED patients almost universally develop CMC, the sole consistent infectious disease phenotype. Interestingly, genotype-specific CMC penetrance has been reported; while CMC develops in >90% of patients with the R257X and R139X mutations, it is seen in <20% of Iranian Jews with the Y85C mutation (Zlotogora and Shapiro 1992). CMC usually manifests within the first two years of life and is the initial disease feature in the majority of APECED patients. In ∼10% of adult patients, chronic oral candidiasis is associated with development of oral carcinoma (Rautemaa et al. 2007).
Although the immunological mechanisms accounting for AIRE-mediated mucosal anti-Candida immune responses are poorly understood, APECED patients have neutralizing autoantibodies against IL-17F and IL-22, but not against other proinflammatory cytokines (Fig. 2) (Kisand et al. 2010; Puel et al. 2010). Similar autoantibodies were identified in thymoma patients who also developed CMC (Tarr et al. 2001; Kisand et al. 2010), suggesting that CMC susceptibility in APECED may have an autoimmune basis. Based on that, B-cell-depleting treatment with rituximab, which was effective in amelioration of autoimmunity in Aire-deficient mice (Gavanescu et al. 2008), has been used in APECED patients with varying success (Popler et al. 2012). In addition, immunosuppression with tacrolimus and other lymphocyte immunomodulatory agents has been used to treat patients, with reported improvement of CMC (Ulinski et al. 2006). Yet, in addition to autoantibodies targeting IL-17 immunity, AIRE-deficient mononuclear cells exhibit reduced secretion of IL-17 and other proinflammatory cytokines on polyclonal stimulation (Kisand et al. 2010), and AIRE-deficient saliva exhibited defective candidacidal activity and decreased levels of the anti-Candida protein cystatin SA1 (Lindh et al. 2013), implying that yet uncharacterized intrinsic leukocyte and/or epithelial cells defects may also contribute to CMC susceptibility.
Interferon Regulatory Factor 8 (IRF8) Mutations
IRF8 is a critical transcription factor for myeloid progenitor cell differentiation into monocytes. A 10-week-old infant with a homozygous missense IRF8 variant (p.K108E) developed disseminated BCG infection after vaccination and oral candidiasis (Hambleton et al. 2011). The infant exhibited complete absence of CD14+ and CD16+ monocytes and CD11c+ myeloid and CD123+ plasmacytoid dendritic cells in blood and profound decrease of tissue dendritic cells with variable deficits in tissue macrophages. The K108E variant resulted in impaired DNA-binding and transactivation potential of IRF8 with resultant reduced binding to target gene promoter regions. The patient’s cells had impaired production of IL-12 and IFN-γ (Fig. 1), which likely contributed to susceptibility to mycobacterial disease. In addition, IRF8-deficient T cells were defective in IL-17 secretion (Fig. 2) suggesting that impaired Th17 differentiation coupled with impaired IL-12 responses and absence of tissue antigen-presenting cells may account for heightened susceptibility to mucosal candidiasis. Conversely, patients with a heterozygous dominant-negative IRF8 variant (p.T80A) within the DNA-binding domain that suppressed the transactivation potential of wild-type IRF8 had selective susceptibility to NTM but no mucosal candidiasis (Hambleton et al. 2011). These patients exhibited marked specific loss of IL-12-producing CD11c+CD1c+ dendritic cells but maintained normal monocyte and other dendritic cell subsets.
DISORDERS IN OTHER SIGNALING MOLECULES
Dectin-1–CARD9 Signaling
Fungal recognition by pattern recognition receptors (PRRs) is the first step in mounting effective antifungal immunity. Among PRRs, Toll-like receptors (TLRs) and C-type lectin receptors (CLRs) recognize fungi; mouse data suggest that both are important for antifungal immune responses (Netea et al. 2008). In humans, however, CLR and not TLR signaling appears critical for antifungal host defense. Hence, patients with mutations in MYD88, the adaptor molecule downstream from TLRs do not develop fungal disease (von Bernuth et al. 2008); conversely, patients with mutations in CARD9, the adaptor molecule downstream from CLRs, which forms a complex with BCL-10 and MALT1 for optimal signaling, are susceptible to both mucosal and systemic mycoses (Fig. 2) (Glocker et al. 2009; Drewniak et al. 2013; Lanternier et al. 2013; Wang et al. 2013b).
Specifically, an initial report described members of a consanguineous Iranian family with CMC, superficial dermatophytosis, and Candida meningitis caused by a homozygous CARD9 point mutation (p.Q295X) associated with decreased proportions of circulating IL-17+ T lymphocytes (Glocker et al. 2009), implying a role for human CARD9 in Th17 differentiation, consonant with a similar role of CARD9 in mice (LeibundGut-Landmann et al. 2007). In agreement, CARD9-deficient monocytes from a compound heterozygous patient for two other CARD9 mutations (p.G72S and p.R373P) who also developed CMC and Candida meningitis exhibited diminished production of IL-1β and IL-6, which are essential for priming Th17 cell differentiation (Drewniak et al. 2013). These IL-17-related defects likely contribute to development of CMC; in contrast, susceptibility to systemic candidiasis appears to result from a Candida-specific, nonoxidative-dependent killing defect of CARD9-deficient neutrophils. More recently, two novel homozygous CARD9 mutations (p.Q289X and p.R101C) were reported in 17 unrelated patients from Morocco, Tunisia, and Algeria who developed severe deep-seated dermatophytoses (Lanternier et al. 2013). However, the impact of CARD9 deficiency on specific antidermatophyte immune responses was not tested. More recently, four patients from China were reported to develop severe subcutaneous phaeohyphomycosis with Phialophora verrucosa as a result of novel point and frameshift CARD9 mutations, which abolished P. verrucosa-induced mononuclear cellular responses (Wang et al. 2013b). No candidiasis was seen in these patients (Wang et al. 2013b) nor in the majority of the patients with p.Q289X and p.R101C mutations (Lanternier et al. 2013), implying that different CARD9 mutations may confer different fungal infection susceptibilities at different anatomical sites. Remarkably, although Card9 is indispensable for control of IA, tuberculosis, and listeriosis in mice (Hsu et al. 2007; Dorhoi et al. 2010; Jhingran et al. 2012), no infections by molds, intracellular bacteria or mycobacteria have thus far been reported in CARD9-deficient humans. Of note, MALT1 mutations were reported recently to result in an autosomal recessive SCID phenotype with severe bacterial infections and associated mucosal candidiasis of the gastrointestinal tract but no systemic fungal disease (Jabara et al. 2013). Identification of more patients with MALT1 mutations and of patients with BCL-10 mutations will be required to draw firm conclusions with regard to the differential role of CARD9, BCL-10, and MALT1 in mediating mucosal and systemic antifungal immune responses.
Intriguingly, the CLR(s) that promote antifungal immune responses upstream of CARD9 in humans remain(s) to be elucidated. Because the severity and spectrum of fungal infections is much greater in CARD9-deficient patients than in patients with dectin-1 deficiency (Ferwerda et al. 2009; Glocker et al. 2009), CLRs such as dectin-2, dectin-3, mincle, or others may play that role (Drummond et al. 2011; Zhu et al. 2013). Alternatively, the redundant nature of CLRs may account for the mild phenotype in individuals defective in a single CLR. Specifically, three siblings with the homozygous loss-of-expression and loss-of-function dectin-1 allele p.Y238X developed recurrent vulvovaginal candidiasis and superficial dermatophytosis associated with impaired IL-17 production by mononuclear cells, but no severe CMC, systemic candidiasis, deep-seated dermatophytosis or subcutaneous phaeohyphomycosis as was seen in CARD9 deficiency (Ferwerda et al. 2009). In addition, as opposed to the rarity of CARD9 deficiency, the c.714T>G, p.Y238X dectin-1 allele is a common single nucleotide polymorphism with a frequency of ∼7% in Europe and up to 40% in the San population in South Africa. It is thus rational to propose that although dectin-1 deficiency represents an immunological defect in the recognition of β-glucans, it clinically presents more like a genetic polymorphism rather than a bona fide PID. Therefore, more research is required to elucidate the contribution of various CLRs in fungus-specific and site-specific human antifungal immune responses.
DOCK8 and TYK2 Deficiencies (Autosomal Recessive HIES)
Although the majority of HIES patients have autosomal dominant STAT3 mutations, several kindreds were found to have a combined immune deficiency with elevated IgE and eosinophilia with an autosomal recessive mode of inheritance because of DOCK8 deficiency (Renner et al. 2004; Zhang et al. 2009). DOCK8 deficiency is a distinct clinical entity, as these patients lack the somatic features of autosomal dominant STAT3 deficiency, do not develop pneumatoceles, and thus are not at risk for late-onset mold superinfections. However, they are susceptible to cutaneous viral infections and malignancies (Renner et al. 2004; Grimbacher et al. 2005; Zhang et al. 2009). DOCK8 is a Cdc42-specific guanine nucleotide exchange factor involved in cytoskeletal rearrangement during cell adhesion, migration, and growth (Ruusala and Aspenström 2004). Therefore, DOCK8 deficiency leads to impaired Th17 differentiation and T-cell priming, the latter because of defective dendritic cell migration to lymph node (Harada et al. 2012). Similar to autosomal dominant STAT3 deficiency, CMC develops in DOCK8 deficiency, but with lower frequency and severity (Chu et al. 2012). Defects in T lymphocyte IL-17 production also occurs in DOCK8 deficiency (Fig. 2), but unlike STAT3 mutations, which impair the initial steps in Th17 differentiation, DOCK8-deficient patients have defects in Th17 cell terminal differentiation and persistence (Khatib et al. 2009). DOCK8 deficiency is caused by homozygous or compound heterozygous point mutations as well as large deletions in DOCK8 with resultant reduced or absent protein (Engelhardt et al. 2009; Zhang et al. 2009).
A Japanese patient with a homozygous deletion in TYK2 was reported to have a combined immune deficiency with IgE elevation (Minegishi et al. 2006). The patient had susceptibility to bacillus Calmette–Guérin (BCG), which is atypical of STAT3 deficiency and mild CMC, although IL-17 immune parameters were not evaluated. However, a second Turkish patient with a different homozygous deletion in TYK2 also developed disseminated BCG infection, but did not have CMC nor marked IgE elevation (Kilic et al. 2012). No other kindreds have been found with TYK2 mutations so far (Woellner et al. 2007). Therefore, the frequency of TYK2 mutations is likely low and the role of TYK2 in antifungal immunity remains unclear.
Anhidrotic Ectodermal Dysplasia with Immunodeficiency (EDA-ID)
EDA-ID features anhidrotic ectodermal dysplasia (i.e., presence of conical teeth and lack of sweating) because of defective ectodysplasin receptor signaling with a combined immunodeficiency characterized by compromised B- and T-cell function resulting from impaired NF-κB activation (Picard et al. 2011). X-linked recessive EDA-ID is caused by hypomorphic mutations in the regulatory subunit of the IKK complex, NEMO/IKBKG, which impair NF-κB nuclear translocation (Döffinger et al. 2001; Jain et al. 2001) (Fig. 1). Autosomal dominant EDA-ID is caused by hypermorphic heterozygous mutations in IKBA, which impair Iκ-Bα phosphorylation and degradation, and result in incomplete NF-κB nuclear translocation (Courtois et al. 2003). Because NF-κB nuclear activation is downstream from several immune receptor families, such as the TCR, BCR, TLR, IL-1R, IL-18R, and TNF-R, Iκ-Bα-deficient patients have severe impairment in TCR signaling and NEMO-deficient patients have varying degrees of impairment in these pathways. Patients with EDA-ID as a result of either NEMO or IKBA mutations develop invasive pyogenic bacterial infections. With regard to fungal disease, patients with IKBA mutations are universally affected by CMC and 60% of them develop PCP (Picard et al. 2011); decreased numbers of IL-17+ T cells have been reported in these patients (Schimke et al. 2013). Instead, CMC and PCP are far less common in NEMO-deficient patients (<10%) (Salt et al. 2008; Picard et al. 2011). Both EDA-ID syndromes belong in the heterogeneous group of disorders termed hyper-IgM syndromes (HIGM), characterized by elevated IgM despite hypo-γ-globulinemia as a result of defects in immunoglobulin class switch recombination. The most common HIGM syndrome is caused by mutations in the X-linked gene CD40L (Fig. 1). These patients have a relatively high rate of PCP, because this molecule is important for effective cross talk between T lymphocytes and mononuclear phagocytes (Aruffo et al. 1993).
Serine-Threonine Protein Kinase 4 (STK4/MST1) Mutations
Autosomal recessive nonsense mutations in STK4 result in a PID characterized by bacterial infections, herpetic viral infections, cutaneous viral infections, EBV-driven lymphoproliferation, and structural heart abnormalities (Abdollahpour et al. 2012; Nehme et al. 2012). CMC was also reported in a portion of patients (Abdollahpour et al. 2012). STK4/MST1 encodes the ubiquitously expressed MST1, the mammalian homolog of the highly conserved Drosophila Hpo protein, which modulates apoptosis, cell growth, and tumorigenesis (Wu et al. 2003). Because of increased FAS-induced apoptosis and impaired proliferation, STK4-deficient patients have CD4+ T-cell lymphocytopenia with markedly reduced naïve T cells, decreased central memory T cells, and preserved effector memory T cells (Abdollahpour et al. 2012; Nehme et al. 2012). These patients also exhibit intermittent neutropenia with normal bone marrow neutrophil maturation. The mechanism by which STK4 deficiency enhances susceptibility to CMC in some patients likely relates to the low numbers of T lymphocytes; however, whether STK4 is important for IL-17 signaling merits investigation.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
The explosion in the discovery of inborn errors of immunologic and somatic factors that protect humans from infection by ubiquitous fungi continues. However, despite the significant progress of the last years, several challenges still remain for the future. First, although novel signaling pathways and immune effector molecules have been revealed, many patients with a clear phenotype of PID and associated fungal infections still lack a genetic diagnosis. Second, the clinical phenotype of patients with a well-defined genetic defect is often variable; hence, understanding the modulatory factors underlying these differences will be critical for enhancing our understanding of the disease. From this perspective, genetic modulators in the form of secondary mutations or polymorphisms, but also external factors such as colonizing microbiota may prove important (Oh et al. 2013; Smeekens et al. 2013). Finally, and most importantly, by synthesizing the knowledge provided by these experiments of nature, we should be able to develop a detailed mechanistic understanding of how our immune system handles different fungi at different sites, which in turn should aide in devising better strategies for risk assessment, treatment and prognostication of patients with fungal disease.
ACKNOWLEDGMENTS
This work is supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. M.G.N. is supported by an ERC Consolidator Grant (No. 310372).
Footnotes
Editors: Arturo Casadevall, Aaron P. Mitchell, Judith Berman, Kyung J. Kwon-Chung, John R. Perfect, and Joseph Heitman
Additional Perspectives on Human Fungal Pathogens available at www.perspectivesinmedicine.org
REFERENCES
- Abdollahpour H, Appaswamy G, Kotlarz D, Diestelhorst J, Beier R, Schäffer AA, Gertz EM, Schambach A, Kreipe HH, Pfeifer D, et al. 2012. The phenotype of human STK4 deficiency. Blood 119: 3450–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahonen P, Myllärniemi S, Sipilä I, Perheentupa J 1990. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med 322: 1829–1836 [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, et al. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science 298: 1395–1401 [DOI] [PubMed] [Google Scholar]
- Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, Maeda N 1999. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun 67: 1828–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A, Farrington M, Hollenbaugh D, Li X, Milatovich A, Nonoyama S, Bajorath J, Grosmaire LS, Stenkamp R, Neubauer M, et al. 1993. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell 72: 291–300 [DOI] [PubMed] [Google Scholar]
- Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, Reichenbach J 2009. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114: 2619–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigley V, Haniffa M, Doulatov S, Wang XN, Dickinson R, McGovern N, Jardine L, Pagan S, Dimmick I, Chua I, et al. 2011. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J Exp Med 208: 227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Byron KS, Sullivan JL 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med 320: 1731–1735 [DOI] [PubMed] [Google Scholar]
- Björses P, Aaltonen J, Vikman A, Perheentupa J, Ben-Zion G, Chiumello G, Dahl N, Heideman P, Hoorweg-Nijman JJ, Mathivon L, et al. 1996. Genetic homogeneity of autoimmune polyglandular disease type I. Am J Hum Genet 59: 879–886 [PMC free article] [PubMed] [Google Scholar]
- Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, Belkadi A, Picard C, Abel L, Fieschi C, et al. 2013. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity 39: 676–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD 2011. Innate antifungal immunity: The key role of phagocytes. Annu Rev Immunol 29: 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Levitz SM 2012a. Tackling human fungal infections. Science 336: 647. [DOI] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC 2012b. Hidden killers: Human fungal infections. Sci Transl Med 4: p165rv13. [DOI] [PubMed] [Google Scholar]
- Cech P, Papathanassiou A, Boreux G, Roth P, Miescher PA 1979. Hereditary myeloperoxidase deficiency. Blood 53: 403–411 [PubMed] [Google Scholar]
- Chu EY, Freeman AF, Jing H, Cowen EW, Davis J, Su HC, Holland SM, Turner ML 2012. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol 148: 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, Edgerton M, Gaffen SL 2011. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol 4: 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G, Smahi A, Reichenbach J, Döffinger R, Cancrini C, Bonnet M, Puel A, Chable-Bessia C, Yamaoka S, Feinberg J, et al. 2003. A hypermorphic Iκ-Bα mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T-cell immunodeficiency. J Clin Invest 112: 1108–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar-Rodriguez J, Gea-Banacloche J, Freeman AF, Hsu AP, Zerbe CS, Calvo KR, Wilder J, Kurlander R, Olivier KN, Holland SM, et al. 2011. Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood 118: 3715–3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SD, Schaller J, Wedgwood RJ 1966. Job’s syndrome. Recurrent, “cold,” staphylococcal abscesses. Lancet 1: 1013–1015 [DOI] [PubMed] [Google Scholar]
- de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Jannière L, et al. 2008. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med 205: 1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaucoudrey L, Samarina A, Bustamante J, Cobat A, Boisson-Dupuis S, Feinberg J, Al-Muhsen S, Jannière L, Rose Y, de Suremain M, et al. 2010. Revisiting human IL-12Rβ1 deficiency: A survey of 141 patients from 30 countries. Medicine (Baltimore) 89: 381–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond RD, Clark RA 1982. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect Immun 38: 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, Lakey JH, Rahman T, Wang XN, McGovern N, et al. 2011. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood 118: 2656–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis-Girod S, Blanche S, et al. 2001. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-κB signaling. Nat Genet 27: 277–285 [DOI] [PubMed] [Google Scholar]
- Dorhoi A, Desel C, Yeremeev V, Pradl L, Brinkmann V, Mollenkopf HJ, Hanke K, Gross O, Ruland J, Kaufmann SH 2010. The adaptor molecule CARD9 is essential for tuberculosis control. J Exp Med 207: 777–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, van Hamme JL, van Leeuwen EM, Roos D, Scalais E, de Beaufort C, et al. 2013. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 121: 2385–2392 [DOI] [PubMed] [Google Scholar]
- Drummond RA, Saijo S, Iwakura Y, Brown GD 2011. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur J Immunol 41: 276–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, Chen A, Kim HS, Lloret MG, Schulze I, et al. 2009. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol 124: 1289–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, et al. 2009. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 361: 1760–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AF, Davis J, Anderson VL, Barson W, Darnell DN, Puck JM, Holland SM 2006. Pneumocystis jiroveci infection in patients with hyper-immunoglobulin E syndrome. Pediatrics 118: e1271–1275 [DOI] [PubMed] [Google Scholar]
- Gaffen SL 2011. Recent advances in the IL-17 cytokine family. Curr Opin Immunol 23: 613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JM, Metzger TC, McMahon EJ, Au-Yeung BB, Krawisz AK, Lu W, Price JD, Johannes KP, Satpathy AT, Murphy KM, et al. 2013. Extrathymic Aire-expressing cells are a distinct bone marrow-derived population that induce functional inactivation of CD4+ T cells. Immunity 39: 560–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavanescu I, Benoist C, Mathis D 2008. B cells are required for Aire-deficient mice to develop multi-organ autoinflammation: A therapeutic approach for APECED patients. Proc Natl Acad Sci 105: 13009–13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker EO, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, et al. 2009. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 361: 1727–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, Miller JA, O'Connell AC, Puck JM 1999. Hyper-IgE syndrome with recurrent infections—An autosomal dominant multisystem disorder. N Engl J Med 340: 692–702 [DOI] [PubMed] [Google Scholar]
- Grimbacher B, Holland SM, Puck JM 2005. Hyper-IgE syndromes. Immunol Rev 203: 244–250 [DOI] [PubMed] [Google Scholar]
- Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, Babic M, Lin M, Carmagnac A, Lee YK, et al. 2011. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet 43: 1012–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, et al. 2011. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med 365: 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, Hanawa-Suetsugu K, Kukimoto-Niino M, Nishizaki T, Shirouzu M, et al. 2012. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood 119: 4451–4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino M, Scott HS, Chen Q, Peterson P, Mäebpää U, Papasavvas MP, Mittaz L, Barras C, Rossier C, Chrousos GP, et al. 1999. Mutation analyses of North American APS-1 patients. Hum Mutat 13: 69–74 [DOI] [PubMed] [Google Scholar]
- Hernández-Santos N, Gaffen SL 2012. Th17 cells in immunity to Candida albicans. Cell Host Microbe 11: 425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SM 2013. Chronic granulomatous disease. Hematol Oncol Clin North Am 27: 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, et al. 2007. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med 357: 1608–1619 [DOI] [PubMed] [Google Scholar]
- Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, Qin XF, Dong C, Lin X 2007. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol 8: 198–205 [DOI] [PubMed] [Google Scholar]
- Hsu AP, Davis J, Puck JM, Holland SM, Freeman AF 2010. Autosomal dominant hyper IgE syndrome. In GeneReviews (ed. Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K). University of Washington, Seattle [Google Scholar]
- Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, Frucht DM, Vinh DC, Auth RD, Freeman AF, et al. 2011. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood 118: 2653–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AP, Johnson KD, Falcone EL, Sanalkumar R, Sanchez L, Hickstein DD, Cuellar-Rodriguez J, Lemieux JE, Zerbe CS, Bresnick EH, et al. 2013a. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. Blood 121: 3830–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AP, Sowerwine KJ, Lawrence MG, Davis J, Henderson CJ, Zarember KA, Garofalo M, Gallin JI, Kuhns DB, Heller T, et al. 2013b. Intermediate phenotypes in patients with autosomal dominant hyper-IgE syndrome caused by somatic mosaicism. J Allergy Clin Immunol 131: 1586–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabara HH, Ohsumi T, Chou J, Massaad MJ, Benson H, Megarbane A, Chouery E, Mikhael R, Gorka O, Gewies A, et al. 2013. A homozygous mucosa-associated lymphoid tissue 1 (MALT1) mutation in a family with combined immunodeficiency. J Allergy Clin Immunol 132: 151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Ma CA, Liu S, Brown M, Cohen J, Strober W 2001. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol 2: 223–228 [DOI] [PubMed] [Google Scholar]
- Jhingran A, Mar KB, Kumasaka DK, Knoblaugh SE, Ngo LY, Segal BH, Iwakura Y, Lowell CA, Hamerman JA, Lin X, et al. 2012. Tracing conidial fate and measuring host cell antifungal activity using a reporter of microbial viability in the lung. Cell Rep 2: 1762–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib S, Keles S, Garcia-Lloret M, Karakoc-Aydiner E, Reisli I, Artac H, Camcioglu Y, Cokugras H, Somer A, Kutukculer N, et al. 2009. Defects along the TH17 differentiation pathway underlie genetically distinct forms of the hyper IgE syndrome. J Allergy Clin Immunol 124: 342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic SS, Hacimustafaoglu M, Boisson-Dupuis S, Kreins AY, Grant AV, Abel L, Casanova JL 2012. A patient with tyrosine kinase 2 deficiency without hyper-IgE syndrome. J Pediatr 160: 1055–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisand K, Bøe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, et al. 2010. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 207: 299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, Uzel G, DeRavin SS, Priel DA, Soule BP, et al. 2010. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med 363: 2600–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C, Migaud M, Taibi L, Ammar-Khodja A, Boudghene Stambouli O, et al. 2013. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med 369: 1704–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer RI, Cline MJ 1969. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: The role of myeloperoxidase in resistance to Candida infection. J Clin Invest 48: 1478–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, et al. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 8: 630–638 [DOI] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Wüthrich M, Hohl TM 2012. Immunity to fungi. Curr Opin Immunol 24: 449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh E, Brännström J, Jones P, Wermeling F, Hässler S, Betterle C, Garty BZ, Stridsberg M, Herrmann B, Karlsson MC, et al. 2013. Autoimmunity and cystatin SA1 deficiency behind chronic mucocutaneous candidiasis in autoimmune polyendocrine syndrome type 1. J Autoimmun 42: 1–6 [DOI] [PubMed] [Google Scholar]
- Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A, Weigert R, Mikelis C, et al. 2013. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest 123: 5035–5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, et al. 2011. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med 208: 1635–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D, Benoist C 2009. Aire. Annu Rev Immunol 27: 287–312 [DOI] [PubMed] [Google Scholar]
- Meloni A, Willcox N, Meager A, Atzeni M, Wolff AS, Husebye ES, Furcas M, Rosatelli MC, Cao A, Congia M 2012. Autoimmune polyendocrine syndrome type 1: An extensive longitudinal study in Sardinian patients. J Clin Endocrinol Metab 97: 1114–1124 [DOI] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. 2008. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452: 773–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, Takada H, Hara T, Kawamura N, Ariga T, et al. 2006. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity 25: 745–755 [DOI] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, et al. 2007. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448: 1058–1062 [DOI] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, Yamada M, Kawamura N, Ariga T, et al. 2009. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med 206: 1291–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes-Vasconcelos D, Grumach AS, Yamaguti A, Andrade ME, Fieschi C, de Beaucoudrey L, Casanova JL, Duarte AJ 2005. Paracoccidioides brasiliensis disseminated disease in a patient with inherited deficiency in the β1 subunit of the interleukin (IL)-12/IL-23 receptor. Clin Infect Dis 41: 31–37 [DOI] [PubMed] [Google Scholar]
- Morgenstern DE, Gifford MA, Li LL, Doerschuk CM, Dinauer MC 1997. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med 185: 207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauseef WM, Root RK, Malech HL 1983. Biochemical and immunologic analysis of hereditary myeloperoxidase deficiency. J Clin Invest 71: 1297–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme NT, Pachlopnik Schmid J, Debeurme F, André-Schmutz I, Lim A, Nitschke P, Rieux-Laucat F, Lutz P, Picard C, Mahlaoui N, et al. 2012. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood 119: 3458–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Brown GD, Kullberg BJ, Gow NA 2008. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 6: 67–78 [DOI] [PubMed] [Google Scholar]
- Notarangelo LD 2010. Primary immunodeficiencies. J Allergy Clin Immunol 125: S182–194 [DOI] [PubMed] [Google Scholar]
- Oh J, Freeman AF, NISC Comparative Sequencing Program, Park M, Sokolic R, Candotti F, Holland SM, Segre JA, Kong HH 2013. The altered landscape of the human skin microbiome in patients with primary immunodeficiencies. Genome Res 23: 2103–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ, Holland SM, Staudt LM 2013. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med 368: 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, Dafou D, Kilo T, Smithson S, Lunt P, et al. 2011. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet 43: 929–931 [DOI] [PubMed] [Google Scholar]
- Picard C, Casanova JL, Puel A 2011. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or Iκ-Bα deficiency. Clin Microbiol Rev 24: 490–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popler J, Alimohammadi M, Kämpe O, Dalin F, Dishop MK, Barker JM, Moriarty-Kelsey M, Soep JB, Deterding RR 2012. Autoimmune polyendocrine syndrome type 1: Utility of KCNRG autoantibodies as a marker of active pulmonary disease and successful treatment with rituximab. Pediatr Pulmonol 47: 84–87 [DOI] [PubMed] [Google Scholar]
- Puel A, Döffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachée-Chardin M, Toulon A, Bustamante J, et al. 2010. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 207: 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, et al. 2011. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332: 65–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautemaa R, Hietanen J, Niissalo S, Pirinen S, Perheentupa J 2007. Oral and oesophageal squamous cell carcinoma—A complication or component of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED, APS-I). Oral Oncol 43: 607–613 [DOI] [PubMed] [Google Scholar]
- Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416: 291–297 [DOI] [PubMed] [Google Scholar]
- Renner ED, Puck JM, Holland SM, Schmitt M, Weiss M, Frosch M, Bergmann M, Davis J, Belohradsky BH, Grimbacher B 2004. Autosomal recessive hyperimmunoglobulin E syndrome: A distinct disease entity. J Pediatr 144: 93–99 [DOI] [PubMed] [Google Scholar]
- Rezai MS, Khotael G, Kheirkhah M, Hedayat T, Geramishoar M, Mahjoub F 2008. Cryptococcosis and deficiency of interleukin-12r. Pediatr Infect Dis J 27: 673. [DOI] [PubMed] [Google Scholar]
- Romani L, Fallarino F, De Luca A, Montagnoli C, D’Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, et al. 2008. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 451: 211–215 [DOI] [PubMed] [Google Scholar]
- Rosenzweig SD, Holland SM 2005. Defects in the interferon-γ and interleukin-12 pathways. Immunol Rev 203: 38–47 [DOI] [PubMed] [Google Scholar]
- Ruusala A, Aspenström P 2004. Isolation and characterisation of DOCK8, a member of the DOCK180-related regulators of cell morphology. FEBS Lett 572: 159–166 [DOI] [PubMed] [Google Scholar]
- Salt BH, Niemela JE, Pandey R, Hanson EP, Deering RP, Quinones R, Jain A, Orange JS, Gelfand EW 2008. IKBKG (nuclear factor-κB essential modulator) mutation can be associated with opportunistic infection without impairing Toll-like receptor function. J Allergy Clin Immunol 121: 976–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio EP, Hsu AP, Pechacek J, Bax HI, Dias DL, Paulson ML, Chandrasekaran P, Rosen LB, Carvalho DS, Ding L, et al. 2013. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol 131: 1624–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke LF, Rieber N, Rylaarsdam S, Cabral-Marques O, Hubbard N, Puel A, Kallmann L, Sombke SA, Notheis G, Schwarz HP, et al. 2013. A novel gain-of-function IKBA mutation underlies ectodermal dysplasia with immunodeficiency and polyendocrinopathy. J Clin Immunol 33: 1088–1099 [DOI] [PubMed] [Google Scholar]
- Segal BH, DeCarlo ES, Kwon-Chung KJ, Malech HL, Gallin JI, Holland SM 1998. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore) 77: 345–354 [DOI] [PubMed] [Google Scholar]
- Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM 2000. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 79: 170–200 [DOI] [PubMed] [Google Scholar]
- Segal BH, Barnhart LA, Anderson VL, Walsh TJ, Malech HL, Holland SM 2005. Posaconazole as salvage therapy in patients with chronic granulomatous disease and invasive filamentous fungal infection. Clin Infect Dis 40: 1684–1688 [DOI] [PubMed] [Google Scholar]
- Siddiqui S, Anderson VL, Hilligoss DM, Abinun M, Kuijpers TW, Masur H, Witebsky FG, Shea YR, Gallin JI, Malech HL, et al. 2007. Fulminant mulch pneumonitis: An emergency presentation of chronic granulomatous disease. Clin Infect Dis 45: 673–681 [DOI] [PubMed] [Google Scholar]
- Smeekens SP, Plantinga TS, van de Veerdonk FL, Heinhuis B, Hoischen A, Joosten LA, Arkwright PD, Gennery A, Kullberg BJ, Veltman JA, et al. 2011. STAT1 hyperphosphorylation and defective IL12R/IL23R signaling underlie defective immunity in autosomal dominant chronic mucocutaneous candidiasis. PLoS ONE 6: e29248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens SP, Huttenhower C, Riza A, van de Veerdonk FL, Zeeuwen PL, Schalkwijk J, van der Meer JW, Xavier RJ, Netea MG, Gevers D 2013. Skin microbiome imbalance in patients with STAT1/STAT3 defects impairs innate host defense responses. J Innate Immun 6: 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel JD 2007. Vulvovaginal candidosis. Lancet 369: 1961–1971 [DOI] [PubMed] [Google Scholar]
- Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, Arthur DC, Gu W, Gould CM, Brewer CC, et al. 2014. GATA2 deficiency: A protean disorder of hematopoiesis, lymphatics and immunity. Blood 123: 809–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr PE, Sneller MC, Mechanic LJ, Economides A, Eger CM, Strober W, Cunningham-Rundles C, Lucey DR 2001. Infections in patients with immunodeficiency with thymoma (Good syndrome). Report of 5 cases and review of the literature. Medicine (Baltimore) 80: 123–133 [DOI] [PubMed] [Google Scholar]
- Ulinski T, Perrin L, Morris M, Houang M, Cabrol S, Grapin C, Chabbert-Buffet N, Bensman A, Deschênes G, Giurgea I 2006. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome with renal failure: Impact of posttransplant immunosuppression on disease activity. J Clin Endocrinol Metab 91: 192–195 [DOI] [PubMed] [Google Scholar]
- Uzel G, Sampaio EP, Lawrence MG, Hsu AP, Hackett M, Dorsey MJ, Noel RJ, Verbsky JW, Freeman AF, Janssen E, et al. 2013. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol 131: 1611–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Plantinga TS, Smeekens SP, Joosten LA, Gilissen C, Arts P, Rosentul DC, Carmichael AJ, Smits-van der Graaf CA, Kullberg BJ, et al. 2011. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med 365: 54–61 [DOI] [PubMed] [Google Scholar]
- Vinh DC, Freeman AF, Shea YR, Malech HL, Abinun M, Weinberg GA, Holland SM 2009a. Mucormycosis in chronic granulomatous disease: Association with iatrogenic immunosuppression. J Allergy Clin Immunol 123: 1411–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh DC, Masannat F, Dzioba RB, Galgiani JN, Holland SM 2009b. Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-γ receptor 1 deficiency. Clin Infect Dis 49: 62–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, Spalding C, Hughes S, Pittaluga S, Raffeld M, et al. 2010a. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood 115: 1519–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh DC, Sugui JA, Hsu AP, Freeman AF, Holland SM 2010b. Invasive fungal disease in autosomal-dominant hyper-IgE syndrome. J Allergy Clin Immunol 125: 1389–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh DC, Schwartz B, Hsu AP, Miranda DJ, Valdez PA, Fink D, Lau KP, Long-Priel D, Kuhns DB, Uzel G, et al. 2011. Interleukin-12 receptor β1 deficiency predisposing to disseminated coccidioidomycosis. Clin Infect Dis 52: 99–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, et al. 2008. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321: 691–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lin Z, Gao L, Wang A, Wan Z, Chen W, Yang Y, Li R 2013a. Exome sequencing reveals a signal transducer and activator of transcription 1 (STAT1) mutation in a child with recalcitrant cutaneous fusariosis. J Allergy Clin Immunol 131: 1242–1243 [DOI] [PubMed] [Google Scholar]
- Wang X, Wang W, Lin Z, Wang X, Li T, Yu J, Liu W, Tong Z, Xu Y, Zhang J, et al. 2013b. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol in press [DOI] [PubMed] [Google Scholar]
- Wildbaum G, Shahar E, Katz R, Karin N, Etzioni A, Pollack S 2013. Continuous G-CSF therapy for isolated chronic mucocutaneous candidiasis: Complete clinical remission with restoration of IL-17 secretion. J Allergy Clin Immunol 132: 761–764 [DOI] [PubMed] [Google Scholar]
- Winkelstein JA, Marino MC, Johnston RB Jr, Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, et al. 2000. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 79: 155–169 [DOI] [PubMed] [Google Scholar]
- Woellner C, Schäffer AA, Puck JM, Renner ED, Knebel C, Holland SM, Plebani A, Grimbacher B 2007. The hyper IgE syndrome and mutations in TYK2. Immunity 26: 535. [DOI] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D 2003. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114: 445–456 [DOI] [PubMed] [Google Scholar]
- Zarember KA, Sugui JA, Chang YC, Kwon-Chung KJ, Gallin JI 2007. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J Immunol 178: 6367–6373 [DOI] [PubMed] [Google Scholar]
- Zerbe CS, Holland SM 2005. Disseminated histoplasmosis in persons with interferon-γ receptor 1 deficiency. Clin Infect Dis 41: 38–41 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, Matthews HF, Davis J, Turner ML, Uzel G, et al. 2009. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med 361: 2046–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, Jia XM, Lin X 2013. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 39: 324–334 [DOI] [PubMed] [Google Scholar]
- Zlotogora J, Shapiro MS 1992. Polyglandular autoimmune syndrome type I among Iranian Jews. J Med Genet 29: 824–826 [DOI] [PMC free article] [PubMed] [Google Scholar]