Abstract
Glaucoma is a neurodegenerative disorder in which degenerating retinal ganglion cells (RGC) produce significant visual disability. Clinically, glaucoma refers to an array of conditions associated with variably elevated intraocular pressure (IOP) that contributes to RGC loss via mechanical stress, vascular abnormalities, and other mechanisms, such as immune phenomena. The clinical diagnosis of glaucoma requires assessment of the ocular anterior segment with slit lamp biomicroscopy, which allows the clinician to recognize signs of conditions that can produce elevated IOP. After measurement of IOP, a specialized prismatic lens called a gonioscope is used to determine whether the angle is physically open or closed. The structural manifestation of RGC loss is optic nerve head atrophy and excavation of the neuroretinal rim tissue. Treatment is guided by addressing secondary causes for elevated IOP (such as inflammation, infection, and ischemia) whenever possible. Subsequently, a variety of medical, laser, and surgical options are used to achieve a target IOP.
Glaucoma is the most common optic neuropathy. Intraocular pressure remains the only modifiable parameter that alters its clinical course.
Glaucoma refers to a heterogeneous group of diseases whose common clinical denominator is an excavation of neuroretinal rim tissue located in the intrascleral portion of the optic nerve. The optic nerve is a white matter tract with intrascleral, retrobulbar, intracanalicular, and intracranial segments. Only the intrascleral portion of the optic nerve is available for direct clinical inspection. Glaucomatous changes in the intrascleral portion of the optic nerve were appreciated soon after Hermann von Hemholtz invented the ophthalmoscope in 1850 and these changes seemed intuitively related to elevated intraocular pressure (IOP).
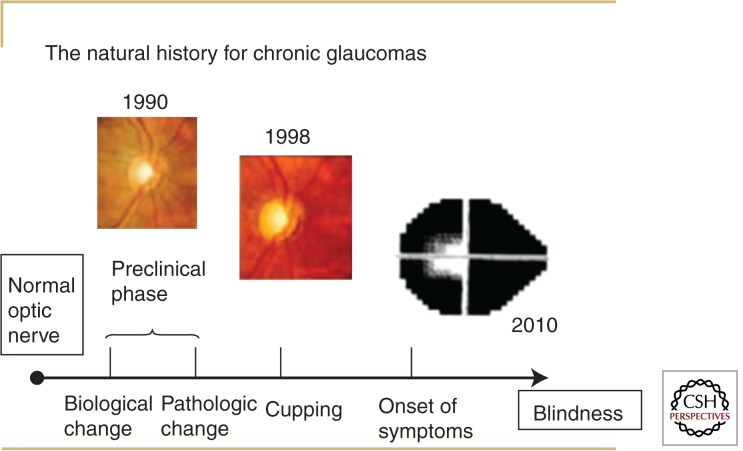
The typical course of chronic glaucoma progresses insidiously over decades (Fig. 1). Unless glaucoma is associated with markedly elevated IOP, it is not associated with pain; furthermore, visual symptoms do not develop until the disease is advanced. For most patients the disease is insidious in onset with a long and poorly defined preclinical phase. Visual symptoms attributable to glaucomatous optic neuropathy, such as difficulty reading a menu in a dimly lit restaurant, usually signify advanced disease. This symptom relates to impaired contrast sensitivity, which depends on the integrity of the optic nerve and deteriorates as the disease progresses (Abe et al. 1987). Glaucoma classically does not affect central vision first, but even when it does, the seeing portion of the fellow eye covers for the affected eye, and most patients are oblivious of the change.
Figure 1.
Natural history for chronic glaucomas. In the natural history of chronic glaucoma, there is a poorly defined preclinical phase that manifests here as enlargement of the vertical cup–disc ratio from 1990 to 1998. Symptoms typically develop late in the disease course, as depicted by the patient with advanced visual field loss in 2010.
Glaucoma can occur at any age, with developmental anomalies predominating as etiologic entities for young children. Trauma and inflammatory conditions produce the lion’s share of glaucoma in young- and middle-aged adults. In the elderly, glaucoma usually presents as a chronic, complex disease of unclear etiology. It is estimated there will be 5.9 million people bilaterally blinded by open angle glaucoma (OAG) and 5.3 million people bilaterally blinded by angle closure glaucoma (ACG) by the year 2020 (Quigley and Broman 2006). The latter figure stems from the high prevalence of angle closure glaucoma in Asia. OAG and ACG have IOP-related optic neuropathy as a common endpoint, but in the former, the site of aqueous humor egress from the eye is physically open, whereas in the latter, it is mostly sealed off owing to a variety of pathologies. The two most common forms of OAG in the elderly are primary open angle glaucoma (POAG) and exfoliation glaucoma (XFG). In POAG, the anterior segment exam does not reveal any particular cause for elevated IOP. In XFG, on the other hand, the filtration apparatus remains physically open but is rendered dysfunctional by deposits of grayish-white material and pigment.
Etiologic causes for glaucoma include trauma, infection, inflammation, retinal ischemia, and intraocular tumors; these etiologies, along with illustrative examples and mechanisms, are summarized in Table 1, which is by no means an exhaustive list. Many drugs can trigger angle closure in patients who are anatomically predisposed to this condition, and a handful of drugs can produce bilateral angle closure glaucoma even if the patient does not have preexisting narrow outlets in the filtration apparatus. Aqueous humor continuously circulates from its site of production (the ciliary body) behind the iris around the pupil. This aqueous humor leaves the eye via a microporous structure housed within the filtration apparatus called the trabecular meshwork. Steroids might produce OAG by altering the hydraulic conductivity in the trabecular meshwork (Clark et al. 2005). Generally speaking, lifestyle and diet seem to play a minor role in glaucoma, although the discovery of the full complement of genes involved in the disease process may clarify roles for lifestyle factors in this disorder.
Table 1.
Etiological entities known to produce glaucoma
| Etiology | An examplea | A mechanisma |
|---|---|---|
| Tumorigenic | Iris melanoma | Tumor seeding drainage angle |
| Traumatic | Angle recession | Direct angle contusion |
| Infectious | Herpes simplex | Trabeculitis |
| Inflammatory | Juvenile idiopathic arthritis | Scarring and inflammation in the outflow pathway |
| Degenerative | Primary open angle glaucoma | Multifactorial |
| Developmental | Congenital glaucoma | Incomplete angle development |
| Vascular | Neovascular glaucoma after diabetic retinopathy | Reduced outflow triggered by vascular endothelial growth factor diffusion into the anterior segment |
aThere are many examples and more than one mechanism involved in each etiological category.
CLINICAL FEATURES OF GLAUCOMA
Patient History
Because early to moderate glaucoma is generally asymptomatic, the initial question is whether there is a family history of glaucoma. A positive family history of glaucoma increases one’s risk of disease by three- to fourfold (Tielsch et al. 1994; Wolfs et al. 1998; Kang et al. 2007).
For patients with known glaucoma who might present for a second opinion, it is best to ascertain when the glaucoma was first diagnosed from the patient’s perspective. Patients who present with mild-stage glaucoma but claim a longer duration of disease may ultimately have a good prognosis, whereas patients with advanced disease (perhaps close to the symptomatic phase illustrated in Fig. 1) for a shorter duration may be less likely to retain vision. Acquire information regarding the highest known IOP to get a sense of the optic nerve’s vulnerability to degeneration. Patients with advanced damage for whom the highest known IOP was measured in their mid-teens (which is normal for most patients) likely have optic nerves that are quite sensitive to damage. Finally, ask patients about prior intolerance to medical treatments for the glaucoma, so that patients are not rechallenged with medicines that previously caused allergy or significant side effects.
A systematic review of a patient’s medical history is useful for determining the optimal IOP-lowering strategy in glaucoma. For example, it is best to avoid topical β-blocker in patients with asthma, as this treatment could exacerbate bronchospasm. Knowledge of specific medical conditions such as diabetes mellitus is important as well; when diabetic retinopathy leads to profound retinal ischemia, neovascular glaucoma can result.
Past ocular history might yield clues about potential causes of high IOP. A history of unilateral blunt ocular trauma may explain why IOP is high only in one eye. Prior ocular surgeries can occasionally lead to secondary damage to the filtration apparatus. For example, a retinal detachment repair requiring intraocular gas tamponade could result in forward rotation of the iris–lens diaphragm and closure of the trabecular meshwork. Thus, it is helpful to document prior ocular surgery and any known complications that ensued.
Some systemic medications used to treat nonophthalmic conditions elevate IOP whereas others may lower IOP and mask higher spontaneous ocular tension. Steroids administered by any route, including by dermatological application (Aggarwal et al. 1993) or nasal inhalation (Opatowsky et al. 1995), can lead to elevated IOP. On the other hand, systemic β-blockers used to treat systemic hypertension (Borthne 1976) and oral carbonic anhydrase inhibitors used to treat elevated intracranial pressure or seizure disorder can lower IOP and mask an otherwise higher spontaneous IOP. Of course, for patients with known glaucoma, it is important to document all topical ocular hypotensive medications currently in use.
Ophthalmic Examination
In glaucoma, Snellen acuity is preserved until the disease is advanced. In advanced disease, patients will read the chart more slowly or move their head in an attempt to see around the defect in the visual field. These details are not often documented when Snellen acuity is recorded. Red–green color vision, as recorded with Ishihara plates, is also well preserved until the disease is advanced. On the other hand, blue–yellow color vision, which is rarely recorded outside clinical research circles, has been reported to be depressed early in the disease process (Drance et al. 1981).
The optic nerve represents the afferent limb of the pupillary light reflex, whereas the efferent limb is carried by sympathetic and parasympathetic fibers to the pupil. This reflex is consensual, meaning that if the reflex is triggered in one eye, both pupils constrict owing to cross wiring of the pupillary light reflex in the midbrain. Thus unilateral or markedly asymmetric glaucoma does not alter pupil size but it will produce an afferent pupillary defect on a swinging flashlight test. (Schiefer et al. 2012; Chang et al. 2013). When light is placed in front of the healthier eye, both pupils constrict, but when the light swings over to the eye with more optic nerve disease, afferent conduction is slowed and the pupil exhibits a paradoxical dilatation. The relative pupillary light reflex should always be assessed during an evaluation for glaucoma.
An accurate diagnosis of glaucoma subtype requires meticulous assessment of the ocular anterior segment for pathological signs that are either a consequence of or an explanation for elevated IOP. Examples of a pathological change in the anterior segment resulting from elevated IOP are atrophy and blunting of the iris crypts caused by ischemic damage to the underlying longitudinal iris dilator muscle. An example of a sign that contributes to elevated IOP is the presence of exfoliation material in the ocular anterior segment. There are a myriad of anterior segment signs associated with the various forms of glaucoma. A detailed discussion of these signs is beyond the scope of this chapter, but some selected signs are summarized in Table 2.
Table 2.
Selected anterior segment signs associated with glaucoma
| Ocular region | Sign | Glaucoma diagnosis |
|---|---|---|
| Lids/adnexa | Nevus flameus | Sturge Weber Syndrome |
| Conjunctiva | Chemosis | Topiramate-induced, bilateral secondary angle closure glaucoma |
| Cornea | Haabs striae | Congenital glaucoma |
| Anterior chamber | Pigment release after pupil dilation | Pigmentary glaucoma |
| Iris | Melt holes and stretch holes | Iridocorneal endotheliopathy |
| Lens | Subluxation into the anterior chamber | Pupillary block glaucoma |
Gonioscopy is an essential technique for stratifying the glaucomas into open-angle and closed-angle types. The “angle” refers to the tissues located at the internal junction of the peripheral cornea and peripheral iris. This junction houses the trabecular meshwork, which serves as the conduit of aqueous humor egress from the eye. The trabecular meshwork is not available for direct inspection because light emanating from this structure is internally reflected owing to refractive index differences between aqueous humor and air. A special prism applied to the anesthetized ocular surface, aided by slit lamp biomicroscopic magnification, couples the tear film to the cornea and affords a view of the angle structures. There is a myriad of pathologies that can be discovered by inspecting this region of the eye, as is beautifully illustrated at http://gonioscopy.org. If the angle is physically closed (i.e., the aqueous humor does not have access to the trabecular meshwork for egress), the IOP can be quite high. Measures must be taken to understand why this physical blockage exists so that therapeutic strategies can be used to open it. Classically, a closed angle may result when there is abnormally high resistance to aqueous humor movement around the pupil and into the anterior chamber, a condition referred to as primary angle closure glaucoma. If this pupillary block is not responsible for physical closure of the angle, there are other mechanisms that include posterior pushing processes (for example, intraoperative choroidal hemorrhage from rupture of a posterior ciliary artery) or anterior pulling mechanisms (for example, epithelial downgrowth, a condition in which the epithelium of the ocular surface gains access to the intraocular space, proliferates and pulls the peripheral iris up against the trabecular meshwork). If the angle is open but the IOP is high, there may be clues that explain this phenomenon such as accumulation of excess pigment or angle contusion deformities from trauma. IOP can also be elevated when the trabecular meshwork appears normal. This is common in open angle glaucoma and suggests that subtle, as yet unknown biochemical and/or ultrastructural changes in the trabecular meshwork account for high IOP in these cases.
The Ocular Hypertension Treatment Study (OHTS) and European Glaucoma Prevention Study (EGPS) (Gordon et al. 2007) have shown that central corneal thickness (CCT) is an independent risk factor for the conversion from ocular hypertension to POAG. Thus measurement of CCT is an integral part of the glaucoma evaluation. The mean CCT is ∼550 µm, but values between 420 µm and 640 µm can be measured in large populations. People of African descent have thinner CCT values than do their Caucasian counterparts (La Rosa et al. 2001). There is a positive correlation between CCT and IOP measured by Goldmann applanation tonometry (IOP measurements are discussed below) (Emara et al. 1998). Interestingly, in one tertiary glaucoma practice, glaucoma patients with thin CCT tended to have more advanced disease than did patients with thicker CCT. Furthermore, glaucoma patients with a known maximum IOP closer to the normal range had a thinner CCT compared to open angle glaucoma patients with a history of higher IOP (Kniestedt et al. 2006). Collectively, these data suggest that true IOP in eyes with thin CCT is higher than that measured with Goldman tonometry. Nonetheless, using different formulae to adjust IOP measured with Goldmann Tonometry for CCT did not improve prediction of risk for developing POAG, compared to the model in which nonadjusted IOP scores and CCT measurements were entered (Brandt et al. 2012). Furthermore, no nomograms are available that accurately correct Goldman-measured readings for CCT, when compared to gold-standard manometric IOP measurements.
Measurement of IOP is of central importance in glaucoma. All clinical methods to measure IOP are based on quantifying a force needed to deform the globe in some way. In Goldman tonometry, the standard method to assess IOP, the number of grams needed to flatten the 3.06 mm2 of central corneal tissue is multiplied by 10 to arrive at the IOP in mm Hg. Thus, if 1.5 grams of force is needed to flatten the central corneal tissue, then the IOP is 15 mm Hg. When considering IOP levels the focus should not be on whether the value is inside or outside the normal range; rather, the IOP should be interpreted in the context of the CCT and overall health of the optic nerve. For example an IOP of 16 mm Hg, which may represent the statistical mean for a population, may be unacceptably high for an individual with a thin CCT (<500 µm) and advanced glaucomatous optic nerve degeneration. On the other hand, an IOP of 24 mm Hg may be entirely acceptable and not require treatment if the CCT is 600 µm and the optic nerve is healthy with no corresponding glaucomatous visual deficit. One must also be cognizant that any IOP measurement is a point estimate that is subject to considerable diurnal fluctuation. Thus, for some glaucoma patients who demonstrate rapid visual field deterioration, it can be helpful to obtain multiple IOP measurements throughout the course of a day.
Assessment of the optic nerve is critical in a glaucoma evaluation. The intrascleral portion of the optic nerve measures ∼2.69 mm2 (Jonas et al. 1988) and houses the confluence of ∼1 million retinal ganglion cell (RGC) axons. Generally speaking, the optic nerve has only two clinically visible responses to pathologic insults: swelling and atrophic change. In many instances, optic swelling is followed by atrophy. Glaucoma produces a specialized form of optic nerve atrophy that can be appreciated on neuroimaging (Kitsos et al. 2009) that results in excavation or undermining of the neuroretinal rim tissue. Evaluation of the optic nerve requires optical methods that produce optimum magnification and stereopsis so that excavation and erosion of the neuroretinal rim tissue can be observed. The direct ophthalmoscope provides 15× magnification but no stereopsis. Among the various methods that provide stereopsis, viewing the optic nerve with slit lamp biomicroscopy and a 60-diopter lens provides 11.5× magnification in a workable field of view.
Although the average optic nerve has a disc area of 2.69 mm2, areas can range from 1 mm2 to 5 mm2 (Jonas et al. 1988). Smaller optic nerves will appear more crowded with a smaller central depression referred to as the cup. Larger optic nerves will have larger cups. We refer to the ratio of the central depression size to the total disc size as the cup–disc ratio (CDR). The most common way to quantify this value involves comparing the vertical extent of the cup to the vertical extent of the disc itself, and is termed the vertical CDR. Because glaucomatous optic nerve degeneration manifests as an erosion of the superior and inferior poles of the optic nerves, the vertical CDR can quickly convey the structural integrity of the optic nerve. RGC loss in glaucoma produces an increase in cup area that translates into an increased vertical CDR as illustrated in Figure 2a. Compared to the fellow eye illustrated in Figure 2b, there is erosion of the inferior neuroretinal rim tissue in the glaucomatous optic nerve. As the RGC axons disappear there are also attendant changes in the nerve fiber layer near its exit from the eye (the peripapillary nerve fiber layer). Thinning of the peripapillary nerve fiber layer results in increased visibility of the retinal arterial walls and the underlying retinal pigment epithelium; furthermore, choroidal tissue adjacent to the disc becomes more visible. Another important sign associated with glaucoma is the presence of disc hemorrhage. Disc hemorrhage was an independent risk factor for disease progression in three randomized clinical trials: OHTS (Budenz et al. 2006), the Early Manifest Glaucoma Trial (EMGT) (Leske et al. 2004) and the Collaborative Normal Tension Glaucoma Study (CNTGS) (Drance et al. 2001). These trials suggest that the development of disc hemorrhage represents an IOP-independent mechanism of optic nerve deterioration in glaucoma. Understanding why disc hemorrhages develop represents fertile ground for future research in this field. The various optic nerve findings that occur in glaucoma are described at http://www.gone-project.com.
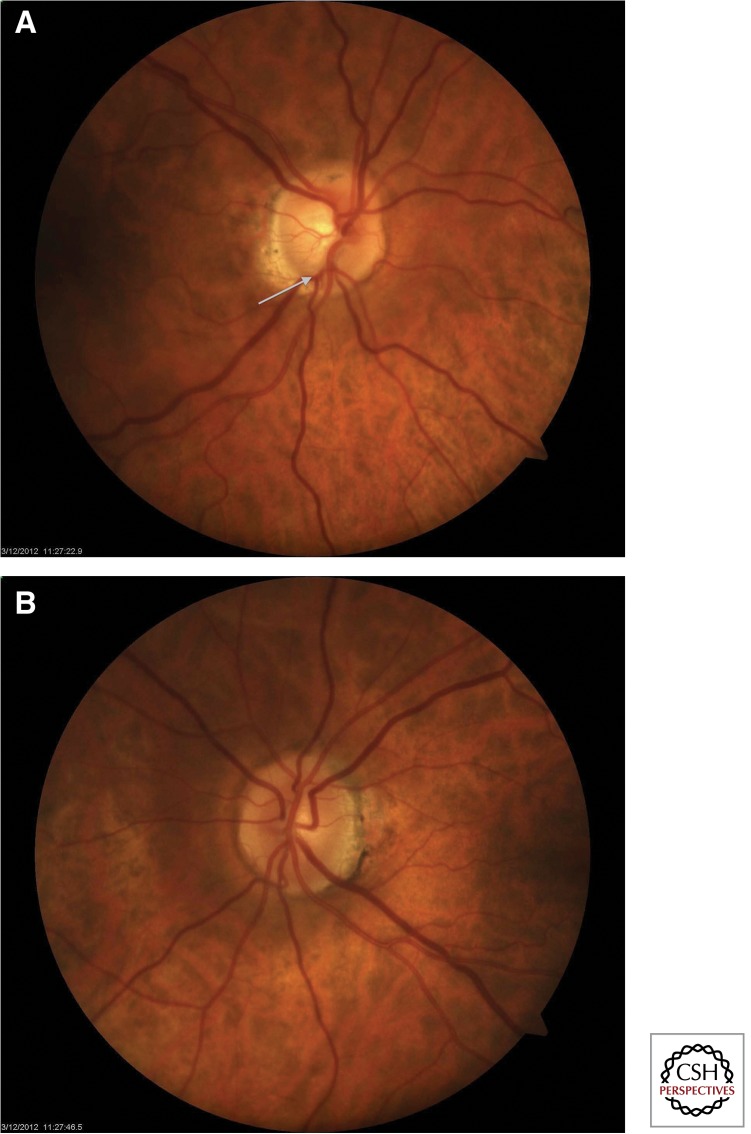
Figure 2.
Erosion of rim tissue in glaucomatous optic nerve. (A) The fundus photo of this glaucomatous right eye shows vertical extension of the cup to the inferior margin of the disc (white arrow). The vertical cup–disc ratio is 0.7. (B) The fellow eye shows a disc with intact neuroretinal rims and a cup–disc ratio of 0.3.
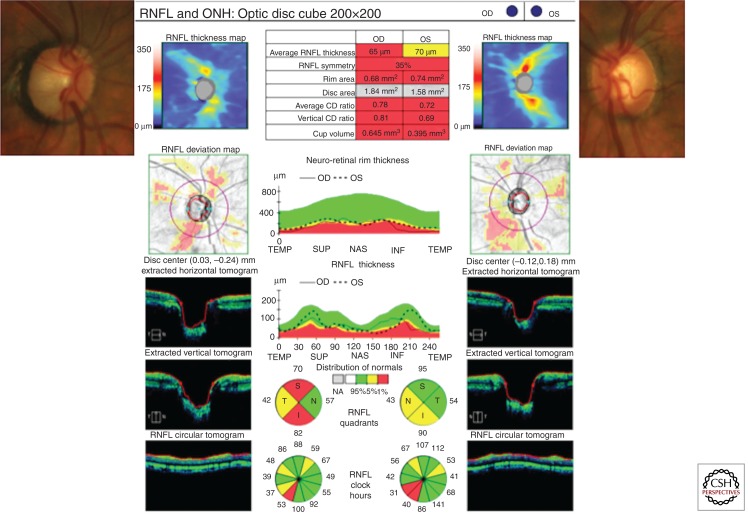
Because the intrascleral portion of the optic nerve represents a critical convergence of visual fibers into a small space, computerized optic nerve imaging is potentially useful to quantify structural features of the optic nerve such as disc size, CDR, and peripapillary nerve fiber layer thickness. Spectral domain optical coherence tomography (SD-OCT) has emerged as a modality that can provide quantitative structural information about the optic nerve and attendant nerve fiber layer tissue (Fig. 3). SD-OCT shows promise to reliably demonstrate disease progression (Wessel et al. 2013) before similar functional changes are manifest on visual field testing (Leung et al. 2012).
Figure 3.
The insets on the upper right and left illustrate optic nerves with pathologic cupping. The spectral domain optical coherence tomogram (SD-OCT) assigned a vertical cup–disc ratio (CDR) of 0.81 for the right optic nerve and 0.69 for the left optic nerve. The SD-OCT also measures other optic nerve structural parameters such as rim area and cup volume. The rim area is abnormally low and the cup volume is abnormally large for both eyes compared to an age-matched control; hence they are highlighted in red. These abnormal values reflect loss of retinal ganglion cell axons. The SD-OCT also measures average retinal nerve fiber layer (RNFL) thickness in a swath of tissue around the optic nerve. It is significantly thinner in the right eye and borderline thinner in the left eye compared to an age-matched control. The nerve fiber layer thickness map assigns areas of thicker nerve fiber layer tissue with red and yellow colors and assigns areas where RNFL is thin with blue colors. Normally the nerve fiber layer is thickest at the superior and inferior pole and thinnest at 3 o’clock and 9 o’clock. Abnormal blue color adjacent to the inferior pole of the right eye corresponds to extension of the inferior cup to the neuroretinal rim on the optic nerve photo. The nerve fiber layer deviation map highlights regional areas where the nerve fiber layer is thin compared to an age matched control. Extracted horizontal and vertical tomograms provide cross-sectional views of the optic nerves, illustrating the abnormally large cup volume in both eyes. There is also an anatomical reconstruction of a circular swath of tissue around the optic nerve corresponding to the red circle drawn on the nerve fiber layer deviation map. The middle tile provides regional inter-eye comparisons as well as comparisons to a normative database for neuroretinal rim thickness and nerve fiber layer thickness. These graphs illustrate that nerve fiber layer loss is greater in the right optic nerve where the CDR is larger.
Other abbreviations used: ONH, optic nerve head; T, temporal; N, nasal; S, superior; I, inferior; TEMP, temporal; SUP, superior; NAS, nasal; INF, inferior.
Although pathological cupping is a central sign in glaucoma, it is not pathognomonic for this condition. Other conditions that do not produce elevated IOP can also produce cupping, such as tumors that compress the anterior visual pathways (Bianchi-Marzoli et al. 1995). The cupping produced by chiasmal lesions can look similar to glaucomatous cupping, but these lesions also result in early onset red–green color vision deficits and ultimately visual field loss that respect the vertical meridian (in contrast, glaucomatous visual field loss respects the horizontal meridian). Other causes of nonglaucomatous cupping include arteritic anterior ischemic optic neuropathy, direct optic nerve trauma, methyl alcohol poisoning, and dominant optic atrophy. Careful history and physical exam that involves optic nerve head evaluation and visual field testing can readily differentiate these conditions from glaucoma.
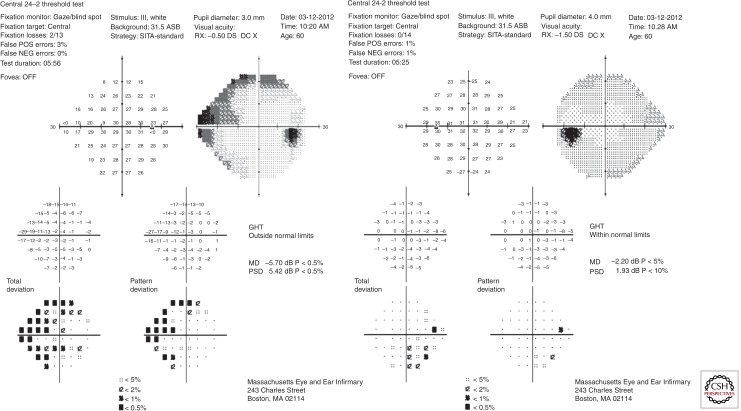
Mapping of the island of vision is performed with manual or automated visual field tests. These tests provide an overview of the function of the entire visual pathway from the tear film to the occipital lobes of the brain. Defects on the visual field localize to one of 4 territories: anterior segment/retina/choroid, optic nerve, chiasm, or postchiasmal regions. The pattern of visual field loss for each region is distinct, although it may not be readily apparent when the defects are incomplete. Glaucoma produces visual field defects in the optic nerve territory that conform to the topology of the nerve fiber layer bundles. The fibers respect the horizontal raphe in the temporal retina and thus visual field defects tend to respect the horizontal meridian (Fig. 4). Visual field loss or progression has been an important outcome in several randomized clinical trials, for glaucoma, although structural changes in the optic nerve typically precede functional loss manifested on visual field tests in early glaucoma (Kerrigan-Baumrind et al. 2000). Nonetheless, as there are no parameters for IOP or optic nerve structure that define glaucoma in a highly sensitive and specific way, reproducible visual field loss on reliable tests represents the best way to definitively confirm glaucoma.
Figure 4.
A Humphrey visual field for the patient illustrated in Figure 3 shows acceptable reliability parameters in both eyes (fixation loss ≤33%; false positive and false negative rates ≤20%). Raw retinal sensitivity data in decibels (converted from an apostilb scale ranging from 0 to 10,000 candela/m2) are provided. The grayscale representation illustrates the blind spots corresponding to the optic nerves, where there are no light-sensitive cells. These raw data are compared to a normative database to generate a Total Deviation plot. For each point, the probability that the sensitivity difference versus an age-matched control is statistically different is provided. In the Pattern Deviation plot the general reduction in retinal sensitivity is factored out to yield focal defects in the visual field. The right eye shows superior and inferior nasal defects in the peripheral visual field. Interestingly the left eye, which showed structural loss, has minimal loss of visual field. Other abbreviations used: GHT, glaucoma hemifield test; MD, mean deviation; PSD, pattern standard deviation.
TREATMENT OF GLAUCOMA
In this section, we mostly discuss treatment of open angle glaucoma. A discussion of treatment for angle closure glaucoma is beyond the scope of this chapter.
No medical or surgical therapy to date stops or reverses optic nerve damage in glaucoma. Treatment strategies for OAG focus on delaying the disease progression by controlling IOP. One treatment strategy involves establishing a target IOP based on patient age, level of IOP that was associated RGC loss and disease severity. Because the target IOP treatment strategy is somewhat arbitrary, periodic reassessment of this goal is needed based on serial assessment of structural and functional optic nerve parameters. Lowering IOP with ocular hypotensive medicines, and/or surgical outflow procedures the main options for delaying disease progression.
Lowering of IOP in Different Patient Groups
In the OHTS study, patients with high IOP (IOP 24–32 mm Hg) experienced a 60% risk reduction for optic disc degeneration and/or visual field loss attributable to glaucoma when assigned to the treatment arm, which consisted of a ≥20% lowering of IOP (Kass et al. 2002). In a meta-analysis of randomized clinical trials, treating high IOP was associated with a significant reduction in glaucoma development (0.56 hazards ratio) for patients at high-risk of converting to POAG. Patients in the treatment arm of these trials did not progress to glaucoma diagnoses in 63%–91% of the cases (Maier et al. 2005). Overall, the results of OHTS and other trials suggest that IOP-lowering agents are effective in reducing disease progression for high IOP patients with moderate to high risk for developing POAG. Although the benefit of IOP lowering is well established for IOP >24 mm Hg associated with high-risk characteristics, treatment benefits remains unclear for patients with borderline pressure (21–24 mm Hg).
Lowering of IOP is known to delay disease progression for patients newly diagnosed with glaucoma. In the Early Manifest Glaucoma Trial (EMGT), lowering of IOP by 25% combined with betaxolol (a β-blocker) and argon laser trabeculoplasty (ALT) resulted in less disease progression (45% with treatment vs. 62% without treatment; p < 0.007) over a median follow-up period of 6 yr. Visual field or optic disc criteria defined disease progression. There was less benefit of IOP lowering for open-angle glaucoma with IOP <21 mm Hg versus IOP ≥21 mm Hg (Heijl et al. 2002).
The treatment benefits from lowering of IOP for normal-tension glaucoma patients are less clear-cut. Normal-tension glaucoma is defined by glaucomatous optic nerve changes developing in the context of normal anterior segment findings, open angles, and untreated IOP close to the statistical norm for the population and typically not higher than 21 mm Hg. In the CNTGS, there was no difference in visual field changes for normal-tension glaucoma patients in the treatment arm, although IOP was lowered by 25%–30% compared to the untreated arm. When patients with cataracts were excluded, however, lowering of IOP had a favorable effect on visual field changes in the treatment arm, with 60% of patients experiencing visual field changes at 3 yr compared to 80% of patients in the control (Anderson 2003).
The Low-Pressure Glaucoma Treatment Study (LoPGTS) compared two twice-daily topical therapies, brimonidine to timolol, for normal-tension glaucoma patients. Although the IOP-lowering effect was similar at all time points between the two treatment arms, there was significantly less progressive visual field loss in the brimonidine arm, with 9.1% of brimonidine-treated patients experiencing progressive visual field loss versus 39.2% of patients using timolol (Krupin et al. 2011). This result suggests that two drugs with equal efficacy in lowering IOP may have different effects on glaucoma progression.
Medical Therapies to Lower IOP
Five broad drug classes are commonly used to lower IOP in patients with glaucoma (Table 3): prostaglandin analogues, β-blockers, α-agonists, carbonic anhydrase inhibitors and cholinergic agents.
Table 3.
Summary of drugs used to treat glaucoma
| Drug class | Mechanism | Clinical use | Ocular side effects | Systemic side effects |
|---|---|---|---|---|
| Prostaglandin analogues –Latanoprost –Travoprost –Bimatoprost –Tafluprost |
Increase aqueous humor outflow | Preferred first-line therapy (lowering of IOP by 6–7 mm Hg) Superior lowering of IOP; proof of neuroprotection pending |
Blurred vision Lid changes Dry eyes Heterochromia Hypertrichosis Hyperemia |
Uncommon |
| β-blockers –Timolol –Betaxolol –Levobunolol |
Decrease aqueous humor production | Acceptable first line therapy (lowering of IOP by 5–6 mm Hg) Proof of neuroprotection (Epstein et al. 1989) |
Burning/stinging | Broncho-spasm Worsening heart failure Bradycardia Heart block Depression |
| α-agonists –Brimonidine |
Increase aqueous humor outflow, decrease aqueous humor production | Appropriate first-line therapy (lowering of IOP by 3–4 mm Hg) Proof of neuroprotection (LoPGTS) |
Hyperemia Allergic conjunctivitis |
Somnolence (more common in children) |
| Carbonic anhydrase inhibitors –Dorzolamide –Brinzolamide |
Decrease aqueous humor production | Appropriate first line therapy (lowering of IOP by 3–4 mm Hg) No proof of neuroprotection (EGPS 2005) |
Burning Hyperemia Allergic conjunctivitis |
Allergic reaction Angioedema (rare) |
The goal of all therapy is to protect the optic nerve, yet only a few studies have been designed to determine whether pharmacological agents are neuroprotective. Those that investigated the efficacy of a single agent and used strict endpoints include the EGPS and the LoPGTS. The EGPS showed reductions in risk of glaucoma progression in moderate risk OHTN patients of 15% for 6 mo and 22% for 5 yr when using dorzolamide to lower IOP; however, compared to placebo, this result was not statistically significant (Miglior et al. 2005). The LoPGTS discussed above found brimonidine to be superior to timolol in terms of reducing progressive visual field loss in normal-tension glaucoma. Another study compared timolol to no timolol and found timolol to be significantly neuroprotective, with an adjusted risk ratio of 0.38 in OHTN patients (Epstein et al. 1989). More recently, the United Kingdom Glaucoma Prevention Study (UKGTS) assessed prostaglandins versus placebo using visual field loss over a 2-yr period as the primary end point (Garway-Heath et al. 2013); as of this writing, the results are pending.
Most clinical trials use lowering of IOP as a proxy for efficacy of glaucoma treatment. Head-to-head trials between β-blockers and prostaglandin analogues indicate that prostaglandin analogues are more effective than β-blockers in lowering IOP. In a pooled analysis of three randomized clinical trials, latanoprost, a prostaglandin analogue, lowered mean diurnal IOP by 1.1 mm Hg more than timolol, a β-blocker. Among the prostaglandin analogues, bimatoprost was more effective than travoprost (Noecker et al. 2003) or latanoprost (Konstas et al. 2005, 2007) in some trials, although the effect differences were small and perhaps not clinically meaningful. Generic and branded latanoprost were equivalent in lowering IOP by 6–7 mm Hg (Hedman et al. 2003).
A meta-analysis of 28 randomized clinical trials assessed eight drugs compared to placebos. All of the drugs were more effective in lowering IOP than was the placebo. The rank order of effectiveness for lowering of peak IOP, from highest to lowest, was bimatoprost, travoprost and latanoprost, brimonidine, timolol, dorzolamide, betaxolol, followed by brinzolamide. For lowering of trough IOP, prostaglandin analogues remained the most effective and brimonidine dropped to the least effective with rankings as follows: bimatoprost, latanoprost, travoprost, timolol, betaxolol, dorzolamide, brinzolamide, brimonidine (van der Valk et al. 2009). Combined therapies (i.e., latanoprost/timolol and dorzolamide/timolol) were as efficacious and in some studies more potent in lowering IOP than were the individual components. However, physicians prefer to initiate treatment with monotherapy to avoid excessive side effects (Higginbotham et al. 2010).
A panel of 10 ophthalmologists reached consensus that prostaglandin analogues were the preferred first-line therapy for medical management of glaucoma (Singh et al. 2008). The Marshfield Eye Clinic in Wisconsin noted that β blockers were prescribed for a majority of patients until 2000, when prostaglandin analogues became more commonly used (McCarty et al. 2008). Although prostaglandin analogues are cited to be preferable for their lack of systemic side effects, the ocular side effects are significant. A cross-sectional observational study associated prostaglandin analogue use to an increased likelihood of upper lid ptosis, deepening of the upper lid sulcus, lower lid retraction, loss of periorbital fat, and levator muscle dysfunction (Shah et al. 2013).
β-Blockers are associated with systemic side effects including exacerbation of airway constriction, bradycardia, and heart failure. Thus these agents are contraindicated in asthmatics and patients with decompensated heart failure. α-Agonists are highly associated with allergic conjunctivitis, as are topical carbonic anhydrase inhibitors. When patients cannot tolerate these medications or fail medical therapy, lowering of IOP must be achieved with laser trabeculoplasty or incisional therapies.
Laser Therapies to Lower IOP
In laser trabeculoplasty, laser energy is delivered to the trabecular meshwork, typically using either an argon laser or frequency-doubled Q-switched Nd:YAG laser, with the goal of achieving lower IOP. The Glaucoma Laser Trial (GLT) established the long-term efficacy of argon laser trabeculoplasty (ALT). Initial IOP was lowered by 9 mm Hg versus 7 mm Hg in the laser and medical (timolol) groups, respectively. In the laser group, 34% experienced transient IOP increases and 30% developed peripheral anterior synechiae, although neither of these outcomes affected long-term visual acuity. This study used lowering of IOP as its endpoint and found ALT to be as effective as medical therapy in lowering IOP. There was less evidence to support repeat laser therapy; at one yr, repeat ALT had reported success rates of 21% to 70%. This effect decreased with longer follow-up, with success rates of 11% at 24 mo and 5% at 48 mo (The Glaucoma Research Group 1990).
Compared to ALT, selective laser trabeculoplasty targets melanin granules in the trabecular meshwork, which theoretically creates less collateral damage. Most trials suggest the two laser treatment modalities are equally effective in lowering IOP at 6 mo and 1 yr of follow-up. In a review of laser treatments, patients with persistently elevated IOP > 20 mm Hg after initial laser trabeculoplasty experienced greater improvement in IOP with selective laser trabeculoplasty than with ALT (6.24 mm Hg and 4.65 mm Hg, respectively). Diode laser, ALT, and selective laser trabeculoplasty had equivalent efficacy in most trials, and adverse events were equally likely for all types of laser treatment. The most common adverse event was a transient increase in IOP. The incidence of this event was 12% for an increase in IOP of >10 mm Hg and 34% for an increase in IOP of >5 mm Hg. Other adverse events included a low-grade iritis (Samples et al. 2011).
Surgical Treatments to Lower IOP
In trabeculectomy surgery, aqueous humor egress from the eye is facilitated via a partial thickness sclerostomy. Clinical trials from the Moorfields Eye Hospital indicated surgical trabeculectomy was the most effective IOP-lowering treatment. Trabeculectomy lowered IOP the most (by 60%), compared to laser trabeculoplasty (decrease of 38%) and medical therapy groups (decrease of 49%), but optic nerve integrity was not fully studied. Nonetheless, the nonsurgical groups had more deterioration in visual fields than did the trabeculectomy group (Rolim de Moura et al. 2007). In the 1990s, the Collaborative Initial Glaucoma Treatment Study (CIGTS) randomized newly diagnosed OAG patients to initial medical or surgical management. Both groups experienced successful lowering of IOP, although the average IOP for the surgical group was 2–3 mm Hg lower than that for the medical group. Ultimately, visual field and visual acuity outcomes were similar in both groups. Surgery was overall found to be more cataractogenic. On secondary analysis, patients with moderate disease had less visual field loss when treated with surgery first (Musch et al. 2011). Collectively, the findings of these surgical trials have not impacted glaucoma management.
The Advanced Glaucoma Intervention Study (AGIS) compared initial laser trabeculoplasty to trabeculectomy for patients with advanced glaucoma who failed medical therapy. Overall results indicated that either modality was effective as initial treatment. Subgroup analysis results varied by race; African Americans experienced more success with initial ALT whereas Caucasians had better results with trabeculectomy as the first treatment. In both cases, failure rate was 50% by 10 yr of follow-up (Ederer et al. 2004). Approximately 50% of glaucoma doctors surveyed agree or strongly agree that this study has made race an important consideration when making treatment decisions (Panarelli et al. 2013).
A systematic review has demonstrated that surgical trabeculectomy decreases IOP more than do nonpenetrating surgeries. Furthermore, adjunctive use of the antimetabolites mitomycin C and 5-fluoruracil prevented scar tissue formation and helped lower IOP to a greater extent (by 4.5–5.5 mm Hg) than did trabeculectomy alone (Boland et al. 2013). In an early head-to-head study of eyes at high risk for surgical failure, mitomycin C was superior to 5-fluoruracil in terms of IOP-lowering ability (final IOP outcomes: 10.9 mm Hg and 14.2 mm Hg, respectively) and reduced corneal toxicity. Patients treated with mitomycin C also required fewer ocular hypotensive agents postsurgery (Skuta et al. 1992).
Prosthetic devices to enhance aqueous humor outflow during glaucoma filtration surgery experienced increased utilization in the 1990s (Ramulu et al. 2007). In the multicenter Tube versus Trabeculectomy (TVT) study, tube-shunt surgery had improved success at 3- and 5-yr follow-up periods compared to trabeculectomy with mitomycin C. Although tube-shunt surgery was associated with more adjunctive medical therapy and trabeculectomy associated with lower IOP in the short term, the two were equivalent in these measures at 3 yr. Trabeculectomy was associated with a greater number of postoperative complications, but visual field loss and cataract development were equal at 3 yr (Gedde et al. 2009). At the 5-yr follow-up, both modalities were associated with IOP in the 12–14 mm Hg range, but failure rates were lower in the tube group (29.8%) compared to the trabeculectomy group (46.9%). This resulted in 9% and 29% reoperation rates, respectively (Gedde et al. 2012). Trabeculectomy appears to be the most effective means to lower IOP when compared to laser and tube-shunt surgery, but the long-term results are less clear, and the side effects and failure rate of surgical trabeculectomy are significant.
Summary of Major Glaucoma Treatment Trials
All randomized clinical trials do not have equal impact (Table 4). In a survey of 894 American Glaucoma Society members, OHTS was ranked as the most impactful glaucoma study to date. Not only did it suggest the possibility of glaucoma prevention for high risk patients, it also demonstrated the importance of assessing CCT in evaluating when to start treatment. Of the eight major glaucoma treatment trials addressed in this survey, those with results showing no difference in treatment outcomes (EGPS, GLT, CIGTS) had less reported impact than did those showing superiority of one treatment over another (OHTS, AGIS) (Panarelli et al. 2013).
Table 4.
Summary of the randomized clinical trials in glaucoma along with their Likert impact scores
| Study name (Likert score: 0–5)a |
Study population | Treatment and control groups | Outcome | Clinical impact |
|---|---|---|---|---|
| OHTS (4.47) | Patients with ocular hypertension (no glaucoma) | T: IOP lowered by 20% (using any means) C: Observation |
Neuroprotection when IOP was lowered (>24 mm Hg) in OHTN | Prophylactic lowering of IOP for OHTN patients Importance of CCT |
| EMGT (3.48) | Patients newly diagnosed with open-angle glaucoma | T: IOP lowered by 30% (using a β-blocker and ALT) C: Observation |
A decrease in progression of glaucoma by 50% when IOP was lowered | Importance of IOP lowering in slowing disease progression |
| CNTGS (4.13) | Normal-tension glaucoma patients | T: IOP lowered by 30% C: Observation |
No significant difference in glaucoma progression except when excluding cataract patients | Lowering of IOP not proven effective in normal-tension glaucoma patients |
| LoPGTSb | Normal-tension glaucoma patients | T: Brimonidine C: Timolol |
Similar IOP lowering-results, but with brimonidine-treated subjects less likely to progress | Superior results with brimonidine in normal-tension glaucoma patients |
| EGPS (2.69) | Patients with moderate risk for glaucoma | T: Dorzolamide C: Placebo |
No difference in glaucoma progression | Clinical impact lowest |
| GLT (3.39) | Newly diagnosed open-angle glaucoma patients | T: ALT (argon laser trabeculectomy) C: Timolol |
No difference in IOP-lowering effect at 1yr | Established long-term efficacy of ALT |
| AGIS (3.78) | Advanced glaucoma patients with failed medical therapy | T: ATT (argon laser trabeculoplasty, trabeculectomy, trabeculectomy) C: TAT (trabeculectomy, argon laser trabeculoplasty, trabeculectomy) |
Decreased failure rate (repeat intervention) for African Americans in the ATT arm | Trabeculectomy not necessarily superior to laser trabeculoplasty in advanced glaucoma |
| CIGTS (3.44) | Newly diagnosed open-angle glaucoma patients | T: Medical therapy C: Surgical trabeculectomy |
Effective lowering of IOP for both, with more adverse effects associated with surgery | Confirmed conservative approach to surgical management |
aThe Likert scale is a psychosomatic scale that gauges responses to questionnaire data and quantifies the clinical impact of each randomized clinical trial. Abbreviations used: T, treatment arm; C, control arm; OHTS, Ocular Hypertension Treatment Study; EMGT, Early Manifest Glaucoma Trial; CNTGS, Collaborative Normal Tension Glaucoma Study; LoPGTS, Low-Pressure Glaucoma Treatment Study; EGPS, European Glaucoma Prevention Study; GLT, Glaucoma Laser Trial; AGIS, Advanced Glaucoma Intervention Study; CIGTS, Collaborative Initial Treatment Study; OHTN, ocular hypertension; CCT, central corneal thickness.
bLikert score not available.
CONCLUDING REMARKS
There are considerable differences in the pathophysiologies of the glaucoma subtypes and very few of these types are well understood at a molecular level. Nevertheless, advances in various bioinformatics disciplines are poised to increase this knowledge, as discussed elsewhere in this volume. A better understanding of glaucoma at the molecular level, will translate into more cost-effective treatments.
Footnotes
Editors: Eric A. Pierce, Richard H. Masland, and Joan W. Miller
Additional Perspectives on Retinal Disorders: Genetic Approaches to Diagnosis and Treatment available at www.perspectivesinmedicine.org
REFERENCES
- Abe H, Hasegawa S, Iwata K 1987. Contrast sensitivity and pattern visual evoked potential in patients with glaucoma. Doc Ophthalmol 65: 65–70 [DOI] [PubMed] [Google Scholar]
- Aggarwal RK, Potamitis T, Chong NH, Guarro M, Shah P, Kheterpal S 1993. Extensive visual loss with topical facial steroids. Eye (Lond) 7: 664–666 [DOI] [PubMed] [Google Scholar]
- Anderson DR 2003. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol 14: 86–90 [DOI] [PubMed] [Google Scholar]
- Bianchi-Marzoli S, Rizzo JF III, Brancato R, Lessell S 1995. Quantitative analysis of optic disc cupping in compressive optic neuropathy. Ophthalmology 102: 436–440 [DOI] [PubMed] [Google Scholar]
- Boland MV, Ervin AM, Friedman DS, Jampel HD, Hawkins BS, Vollenweider D, Chelladurai Y, Ward D, Suarez-Cuervo C, Robinson KA 2013. Comparative effectiveness of treatments for open-angle glaucoma: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 158: 271–279 [DOI] [PubMed] [Google Scholar]
- Borthne A 1976. The treatment of glaucoma with propranolol (Inderal). A clinical trial. Acta Ophthalmol 54: 291–300 [DOI] [PubMed] [Google Scholar]
- Brandt JD, Gordon MO, Gao F, Beiser JA, Miller JP, Kass MA 2012. Adjusting intraocular pressure for central corneal thickness does not improve prediction models for primary open-angle glaucoma. Ophthalmology 119: 437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budenz DL, Anderson DR, Feuer WJ, Beiser JA, Schiffman J, Parrish RK II, Piltz-Seymour JR, Gordon MO, Kass MA 2006. Detection and prognostic significance of optic disc hemorrhages during the ocular hypertension treatment study. Ophthalmology 113: 2137–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DS, Xu L, Boland MV, Friedman DS 2013. Accuracy of pupil assessment for the detection of glaucoma: A systematic review and meta-analysis. Ophthalmology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AF, Brotchie D, Read AT, Hellberg P, English-Wright S, Pang IH, Ethier CR, Grierson I 2005. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton 60: 83–95 [DOI] [PubMed] [Google Scholar]
- Drance SM, Lakowski R, Schulzer M, Douglas GR 1981. Acquired color vision changes in glaucoma. Use of 100-hue test and Pickford anomaloscope as predictors of glaucomatous field change. Arch Ophthalmol 99: 829–831 [DOI] [PubMed] [Google Scholar]
- Drance S, Anderson DR, Schulzer M 2001. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol 131: 699–708 [DOI] [PubMed] [Google Scholar]
- Ederer F, Gaasterland DA, Dally LG, Kim J, VanVeldhuisen PC, Blackwell B, Prum B, Shafranov G, Allen RC, Beck A 2004. The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10-year results. Ophthalmology 111: 651–664 [DOI] [PubMed] [Google Scholar]
- Emara B, Probst LE, Tingey DP, Kennedy DW, Willms LJ, Machat J 1998. Correlation of intraocular pressure and central corneal thickness in normal myopic eyes and after laser in situ keratomileusis. J Cataract Refract Surg 24: 1320–1325 [DOI] [PubMed] [Google Scholar]
- Epstein DL, Krug JH Jr, Hertzmark E, Remis LL, Edelstein DJ 1989. A long-term clinical trial of timolol therapy versus no treatment in the management of glaucoma suspects. Ophthalmology 96: 1460–1467 [DOI] [PubMed] [Google Scholar]
- Garway-Heath DF, Lascaratos G, Bunce C, Crabb DP, Russell RA, Shah A 2013. The United Kingdom Glaucoma Treatment Study: A multicenter, randomized, placebo-controlled clinical trial: Design and methodology. Ophthalmology 120: 68–76 [DOI] [PubMed] [Google Scholar]
- Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL 2009. Three-year follow-up of the tube versus trabeculectomy study. Am J Ophthalmol 148: 670–684 [DOI] [PubMed] [Google Scholar]
- Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL 2012. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol 153: 789–803, e782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MO, Torri V, Miglior S, Beiser JA, Floriani I, Miller JP, Gao F, Adamsons I, Poli D, D’Agostino RB, et al. 2007. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology 114: 10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman K, Alm A, Gross RL 2003. Pooled-data analysis of three randomized, double-masked, six-month studies comparing intraocular pressure-reducing effects of latanoprost and timolol in patients with ocular hypertension. J Glaucoma 12: 463–465 [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M 2002. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 120: 1268–1279 [DOI] [PubMed] [Google Scholar]
- Higginbotham EJ, Olander KW, Kim EE, Grunden JW, Kwok KK, Tressler CS 2010. Fixed combination of latanoprost and timolol vs individual components for primary open-angle glaucoma or ocular hypertension: A randomized, double-masked study. Arch Ophthalmol 128: 165–172 [DOI] [PubMed] [Google Scholar]
- Jonas JB, Gusek GC, Naumann GO 1988. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci 29: 1151–1158 [PubMed] [Google Scholar]
- Kang JH, Willett WC, Rosner BA, Hankinson SE, Pasquale LR 2007. Prospective study of alcohol consumption and the risk of primary open-angle glaucoma. Ophthalmic Epidemiol 14: 141–147 [DOI] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK II, Wilson MR, Gordon MO 2002. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 120: 701–713; discussion 829–730 [DOI] [PubMed] [Google Scholar]
- Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS 2000. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci 41: 741–748 [PubMed] [Google Scholar]
- Kitsos G, Zikou AK, Bagli E, Kosta P, Argyropoulou MI 2009. Conventional MRI and magnetisation transfer imaging of the brain and optic pathway in primary open-angle glaucoma. Br J Radiol 82: 896–900 [DOI] [PubMed] [Google Scholar]
- Kniestedt C, Lin S, Choe J, Nee M, Bostrom A, Sturmer J, Stamper RL 2006. Correlation between intraocular pressure, central corneal thickness, stage of glaucoma, and demographic patient data: Prospective analysis of biophysical parameters in tertiary glaucoma practice populations. J Glaucoma 15: 91–97 [DOI] [PubMed] [Google Scholar]
- Konstas AG, Katsimbris JM, Lallos N, Boukaras GP, Jenkins JN, Stewart WC 2005. Latanoprost 0.005% versus bimatoprost 0.03% in primary open-angle glaucoma patients. Ophthalmology 112: 262–266 [DOI] [PubMed] [Google Scholar]
- Konstas AG, Kozobolis VP, Katsimpris IE, Boboridis K, Koukoula S, Jenkins JN, Stewart WC 2007. Efficacy and safety of latanoprost versus travoprost in exfoliative glaucoma patients. Ophthalmology 114: 653–657 [DOI] [PubMed] [Google Scholar]
- Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S 2011. A randomized trial of brimonidine versus timolol in preserving visual function: Results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol 151: 671–681 [DOI] [PubMed] [Google Scholar]
- La Rosa FA, Gross RL, Orengo-Nania S 2001. Central corneal thickness of Caucasians and African Americans in glaucomatous and nonglaucomatous populations. Arch Ophthalmol 119: 23–27 [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hyman L, Bengtsson B, Komaroff E 2004. Factors for progression and glaucoma treatment: The Early Manifest Glaucoma Trial. Curr Opin Ophthalmol 15: 102–106 [DOI] [PubMed] [Google Scholar]
- Leung CK, Yu M, Weinreb RN, Lai G, Xu G, Lam DS 2012. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: Patterns of retinal nerve fiber layer progression. Ophthalmology 119: 1858–1866 [DOI] [PubMed] [Google Scholar]
- Maier PC, Funk J, Schwarzer G, Antes G, Falck-Ytter YT 2005. Treatment of ocular hypertension and open angle glaucoma: Meta-analysis of randomised controlled trials. BMJ 331: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty CA, Mukesh BN, Kitchner TE, Hubbard WC, Wilke RA, Burmester JK, Patchett RB 2008. Intraocular pressure response to medication in a clinical setting: The Marshfield Clinic Personalized Medicine Research Project. J Glaucoma 17: 372–377 [DOI] [PubMed] [Google Scholar]
- Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I 2005. Results of the European Glaucoma Prevention Study. Ophthalmology 112: 366–375 [DOI] [PubMed] [Google Scholar]
- Musch DC, Gillespie BW, Niziol LM, Lichter PR, Varma R 2011. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology 118: 1766–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noecker RJ, Earl ML, Mundorf T, Peace J, Williams RD 2003. Bimatoprost 0.03% versus travoprost 0.004% in black Americans with glaucoma or ocular hypertension. Adv Ther 20: 121–128 [DOI] [PubMed] [Google Scholar]
- Opatowsky I, Feldman RM, Gross R, Feldman ST 1995. Intraocular pressure elevation associated with inhalation and nasal corticosteroids. Ophthalmology 102: 177–179 [DOI] [PubMed] [Google Scholar]
- Panarelli JF, Banitt MR, Sidoti PA, Budenz DL, Singh K 2013. Clinical impact of 8 prospective, randomized, multicenter glaucoma trials. J Glaucoma. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT 2006. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90: 262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramulu PY, Corcoran KJ, Corcoran SL, Robin AL 2007. Utilization of various glaucoma surgeries and procedures in Medicare beneficiaries from 1995 to 2004. Ophthalmology 114: 2265–2270 [DOI] [PubMed] [Google Scholar]
- Rolim de Moura C, Paranhos A Jr, Wormald R 2007. Laser trabeculoplasty for open angle glaucoma. Cochrane Database Syst Rev: CD003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samples JR, Singh K, Lin SC, Francis BA, Hodapp E, Jampel HD, Smith SD 2011. Laser trabeculoplasty for open-angle glaucoma: A report by the American academy of ophthalmology. Ophthalmology 118: 2296–2302 [DOI] [PubMed] [Google Scholar]
- Schiefer U, Dietzsch J, Dietz K, Wilhelm B, Bruckmann A, Wilhelm H, Kitiratschky V, Januschowski K 2012. Associating the magnitude of relative afferent pupillary defect (RAPD) with visual field indices in glaucoma patients. Br J Ophthalmol 96: 629–633 [DOI] [PubMed] [Google Scholar]
- Shah M, Lee G, Lefebvre DR, Kronberg B, Loomis S, Brauner SC, Turalba A, Rhee DJ, Freitag SK, Pasquale LR 2013. A cross-sectional survey of the association between bilateral topical prostaglandin analogue use and ocular adnexal features. PLoS ONE 8: e61638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Lee BL, Wilson MR 2008. A panel assessment of glaucoma management: Modification of existing RAND-like methodology for consensus in ophthalmology. Part II: Results and interpretation. Am J Ophthalmol 145: 575–581 [DOI] [PubMed] [Google Scholar]
- Skuta GL, Beeson CC, Higginbotham EJ, Lichter PR, Musch DC, Bergstrom TJ, Klein TB, Falck FY Jr 1992. Intraoperative mitomycin versus postoperative 5-fluorouracil in high-risk glaucoma filtering surgery. Ophthalmology 99: 438–444 [DOI] [PubMed] [Google Scholar]
- Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC 1994. Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch Ophthalmol 112: 69–73 [DOI] [PubMed] [Google Scholar]
- The Glaucoma Research Group. 1990. The Glaucoma Laser Trial (GLT). 2. Results of argon laser trabeculoplasty versus topical medicines. Ophthalmology 97: 1403–1413 [PubMed] [Google Scholar]
- van der Valk R, Webers CA, Lumley T, Hendrikse F, Prins MH, Schouten JS 2009. A network meta-analysis combined direct and indirect comparisons between glaucoma drugs to rank effectiveness in lowering intraocular pressure. J Clin Epidemiol 62: 1279–1283 [DOI] [PubMed] [Google Scholar]
- Wessel JM, Horn FK, Tornow RP, Schmid M, Mardin CY, Kruse FE, Juenemann AG, Laemmer R 2013. Longitudinal analysis of progression in glaucoma using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 54: 3613–3620 [DOI] [PubMed] [Google Scholar]
- Wolfs RC, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT 1998. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch Ophthalmol 116: 1640–1645 [DOI] [PubMed] [Google Scholar]