Abstract
The CSF-1 receptor (CSF-1R) is activated by the homodimeric growth factors colony-stimulating factor-1 (CSF-1) and interleukin-34 (IL-34). It plays important roles in development and in innate immunity by regulating the development of most tissue macrophages and osteoclasts, of Langerhans cells of the skin, of Paneth cells of the small intestine, and of brain microglia. It also regulates the differentiation of neural progenitor cells and controls functions of oocytes and trophoblastic cells in the female reproductive tract. Owing to this broad tissue expression pattern, it plays a central role in neoplastic, inflammatory, and neurological diseases. In this review we summarize the evolution, structure, and regulation of expression of the CSF-1R gene. We review, the structures of CSF-1, IL-34, and the CSF-1R and the mechanism of ligand binding to and activation of the receptor. We further describe the pathways regulating macrophage survival, proliferation, differentiation, and chemotaxis downstream from the CSF-1R.
The CSF-1 receptor is a broadly expressed cell-surface protein, playing important roles in macrophage development and in diseases such as leukemias and lymphomas. It transduces signals by binding growth factors CSF-1 or IL-34.
The glycoprotein, colony-stimulating factor-1 (CSF-1), also known as macrophage-CSF (M-CSF), was the first of the CSFs to be purified (Stanley and Heard 1977) and was shown to stimulate the formation of colonies of macrophages (Stanley et al. 1978). This led to the identification (Guilbert and Stanley 1980) and purification (Yeung et al. 1987) of the CSF-1 receptor (CSF-1R) and the demonstration that it possessed intrinsic tyrosine kinase activity (Yeung et al. 1987). It was subsequently shown to be identical to the c-fms proto-oncoprotein (Sherr et al. 1985) previously studied by Sherr and colleagues (Rettenmier et al. 1985). The c-fms cDNA was cloned and shown to encode a typical class III receptor tyrosine kinase (RTK) (Coussens et al. 1986).
The CSF-1R plays a central role in many diseases. Dominant inactivating mutations in the CSF-1R lead to adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (Rademakers et al. 2011; Nicholson et al. 2013). Inappropriate expression of the CSF-1R contributes to the development of leukemias and lymphomas, and autocrine and paracrine regulation of the CSF-1R enhances the progression and metastasis of solid tumors (reviewed in Pollard 2009; Chitu and Stanley 2014). In addition, regulation through the CSF-1R contributes to chronic inflammatory diseases (reviewed in Chitu and Stanley 2006; Chitu et al. 2012). This review focuses on the CSF-1R regulation and signaling in cells of the myeloid lineage.
THE CSF-1R AND LIGANDS
The CSF-1R and Its Oncogenic Derivatives
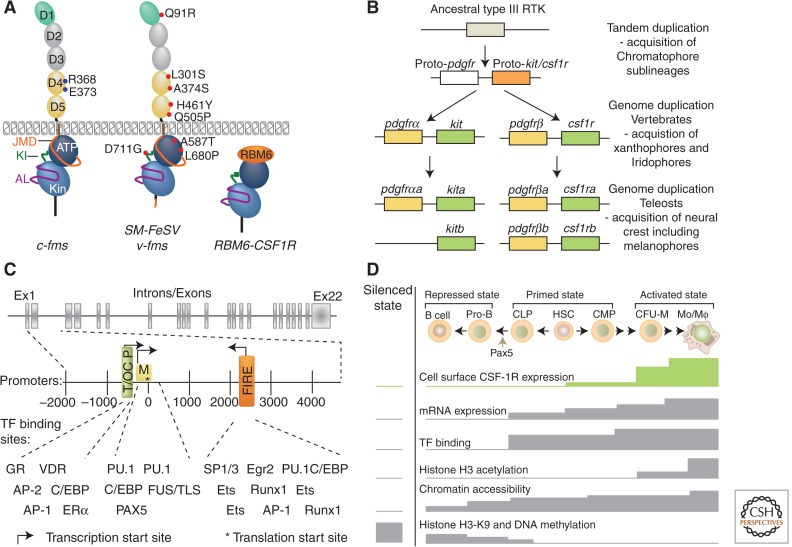
The CSF-1R belongs to the platelet-derived growth factor (PDGF) family. Similar to other family members, it possesses a highly glycosylated extracellular region comprised of five immunoglobulin domains (D1–D5, 498 amino acids), a transmembrane domain (21 amino acids), and an intracellular domain comprised of a juxtamembrane domain (JMD) (36 amino acids) and an intracellular tyrosine kinase domain (398 amino acids) that is interrupted by a kinase insert domain (73 amino acids) (Fig. 1A) (Coussens et al. 1986; Rothwell and Rohrschneider 1987; Hampe et al. 1989).
Figure 1.
Structure of the CSF-1R and regulation of Csf1r gene expression. (A) Structures of CSF-1R and oncogenic derivatives. (Left) The c-fms proto-oncogene, (middle) the v-fms oncogene, encoded by the Susan McDonough strain of feline sarcoma virus (SM-FeSV), and (right) the CSF-1R–RBL6 oncogenic fusion protein. Ovals D1–D5 represent the five extracellular Ig-like domains of CSF-1R. The ligand-binding domains are gray. The blue dots in D4 represent the ionic pairs that have been implicated in receptor homotypic contacts. The intracellular domain is shown as the juxtamembrane domain (JMD, orange), kinase N lobe (ATP, dark blue), kinase insert (KI, green), kinase C lobe (Kin, light blue), activation loop (AL, purple), and carboxy-terminal tail (black). All amino acid substitutions in v-fms are shown as annotated red dots and the carboxy-terminal amino acid sequence that is unrelated to c-fms is red (Woolford et al. 1988). (B) Evolution of closely related type III RTK genes by gene and genome duplications (based on data from Braasch et al. 2006). (C) (Top) Exon-intron structure of mouse Csf1r gene and (bottom) expanded promoter structure and transcription factor (TF) binding sites (based on data from Bonifer and Hume 2008; Ovchinnikov et al. 2010). (D) Regulation of Csf1r expression in hematopoiesis (based on data from Bonifer and Hume 2008). The silenced state levels for each parameter were measured in T cells and fibroblasts that do not express the CSF-1R. Mo, monocyte; Mϕ, macrophage.
Comparison of the sequences of cat c-fms and a v-fms retroviral oncogene derived from it revealed that a carboxy-terminal truncation, together with two point mutations (L301S and A374S) in the extracellular D4 domain, subsequently shown to contain the dimerization interface (Elegheert et al. 2011; Ma et al. 2012), are critical changes required for the full transforming activity of the oncogene (Fig. 1A) (Woolford et al. 1988). Another oncogenic derivative is the product of a t(3;5)(p21;q33) translocation, RBM6-CSF-1R, a constitutively activated CSF-1R fusion protein comprised of the amino-terminal 36 amino acids of RNA-binding motif 6 (RBM6), joined to the carboxy-terminal 399 amino acids of the CSF-1R, which leads to an acute megakaryoblastic leukemia (Fig. 1A).
CSF-1R Expression
The CSF-1R is expressed at low levels on hematopoietic stem cells (HSCs) (Sarrazin et al. 2009; Mossadegh-Keller et al. 2013), at higher levels on monocytes and tissue macrophages (Guilbert and Stanley 1980; Byrne et al. 1981), osteoclasts, myeloid dendritic cells (MacDonald et al. 2005), microglia (Nandi et al. 2012), and Paneth cells (Huynh et al. 2009) and controls the development of these cell types. It is also expressed on oocytes and preimplantation embryos, decidual and trophoblastic cells (reviewed in Pollard and Stanley 1996), neural progenitor cells and other neuronal cells (Wang et al. 1999a; Nandi et al. 2012; Luo et al. 2013), renal proximal tubule epithelial cells, and colonic epithelial cells (reviewed in Chitu and Stanley 2014). The broad pattern of expression of CSF-1R is consistent with its pleiotropic actions in embryonic development, adult physiology, innate immunity, inflammation, tissue repair, and in the tumor microenvironment (reviewed in Chitu and Stanley 2014).
The CSF-1R Ligands CSF-1 and IL-34
The known ligands for the CSF-1R are CSF-1 and IL-34 (Lin et al. 2008). Both in vitro and when expressed in vivo under the control of the CSF-1 promoter, the biological activities of homodimeric glycoprotein interleukin-34 (IL-34) resemble those of the secreted glycoprotein isoform of CSF-1 (Wei et al. 2010). Although there are significant differences in their signaling through the CSF-1R (Chihara et al. 2010), it is primarily the differential expression of IL-34 and CSF-1 (Wei et al. 2010; Greter et al. 2012; Nandi et al. 2012; Wang et al. 2012) that results in their differential spatiotemporal regulation through the CSF-1R in vivo (Wei et al. 2010; Nandi et al. 2012). The transmembrane and proteoglycan CSF-1 isoforms act locally (Wiktor-Jedrzejczak et al. 1991; Sundquist et al. 1995; Van Nguyen and Pollard 2002; Dai et al. 2004; Nandi et al. 2006). However, circulating CSF-1 (Stanley 1979; Janowska-Wieczorek et al. 1991) shows humoral regulation (Cecchini et al. 1994; Pollard and Stanley 1996; Dai et al. 2004). In contrast, IL-34 is not detectable in the circulation (Hwang et al. 2012; Tian et al. 2013) and thus IL-34 actions are likely to be restricted to the local microenvironments in which they are expressed. Through their different spatiotemporal expression, the two ligands play complementary roles in regulating the development, maintenance, and activity of specific macrophage populations, Langerhans cells, neuronal progenitors (Wei et al. 2010; Greter et al. 2012; Nandi et al. 2012; Wang et al. 2012), as well as osteoclasts (Dai et al. 2002) and Paneth cells (Huynh et al. 2009) and the regulation of cells of the female reproductive tract (Wei et al. 2010). Because all of the CSF-1 deficiency phenotypes are also shared with CSF-1R-deficient mice (Dai et al. 2002), the CSF-1R appears to be the only receptor for CSF-1, whereas IL-34 has recently been shown to act via an additional receptor, receptor-type protein tyrosine phosphatase-ζ (PTP-ζ) (Nandi et al. 2013).
THE CSF-1R GENE—EVOLUTION, STRUCTURE, AND REGULATION
CSF-1R Gene Evolution
The ancestral PDGF/VEGF-related RTKIII family expanded substantially during vertebrate evolution by gene and genome duplications (Rousset et al. 1995; Gu and Gu 2003; Leveugle et al. 2004; Braasch et al. 2006). In zebrafish, kit and csf1r play critical roles in the development of different neural crest–derived pigment cell types—kit for melanocytes (Parichy et al. 1999) and csf1r for xanthophores (Parichy et al. 2000; Parichy and Turner 2003)—and it is likely that the gene duplications were driven by selection for pigment cell innovations important for speciation (Fig. 1B) (Braasch et al. 2006; Salzburger et al. 2006).
CSF-1R Gene Structure and Regulation in Myeloid Cells
The Csf1r gene is located on human chromosome 5 (5q32) and in a syntenic region on mouse chromosome 18 (18D) (Le Beau et al. 1986; Hoggan et al. 1988; Bonifer and Hume 2008), juxtaposed head-to-tail as the 3′ neighbor of the PDGFR-β gene (Yarden et al. 1986; Roberts et al. 1988). Both Csf1r genes have 21 introns and 22 exons (Hampe et al. 1989; reviewed in Sherr 1990). The human intron 1 is ∼26 kb and transcription is initiated upstream of exon 1 in trophoblasts and immediately upstream of exon 2 in macrophages (Visvader and Verma 1989; Roberts et al. 1992). Regulation of Csf1r expression has been most studied in mouse, in which intron 1 is only 102 bp and trophoblast and macrophage transcripts are, respectively, initiated 500 bp–300 bp and within 300 bp upstream of the start codon in exon 2 (Sasmono et al. 2003). There are two separate promoter regions: the trophoblast/osteoclast (T/OC) promoter that drives expression in trophoblasts and osteoclasts and contains regulatory elements that increase expression during macrophage differentiation (Bonifer and Hume 2008; Ovchinnikov et al. 2010), and the more proximal macrophage Csf1r promoter (M) (Fig. 1C). Maximal CSF-1R expression in differentiated monocytes/macrophages requires a highly conserved 330 bp sequence enhancer element located in the 3′ end of intron 2, known as Fms-intronic regulatory element (FIRE), which also has reverse promoter activity (Himes et al. 2001; Sasmono et al. 2003; Laslo et al. 2006; Sauter et al. 2013). FIRE encodes an antisense CSF-1R transcript that may contribute to its ability to overcome repression by uncharacterized repressive elements within intron 2 (Bonifer and Hume 2008; Sauter et al. 2013). None of the Csf1r promoters has a TATA box, and transcription initiates at multiple sites for each. For macrophage expression, it is hypothesized that two TATA-associated factors, Ewing sarcoma (EWS) and FUS/TLS, which bind a loose repeat of CAG or CAA immediately adjacent to the dominant start site cluster, substitute for TATA-binding protein (Krysinska et al. 2007; Bonifer and Hume 2008). Transcription factors regulating Csf1r expression that bind sites within the T/OC, M, and FIRE regulatory regions are shown in Figure 1C.
CSF-1R expression is low on HSC (Sarrazin et al. 2009), increases by ∼10-fold on macrophage progenitors (colony-forming unit-macrophage, CFU-M) and is further increased gradually as CFU-M differentiate (monoblast → promonocyte → monocyte → macrophage) (Fig. 1D) (Tushinski et al. 1982; Bartelmez and Stanley 1985; Bartelmez et al. 1989). Low, HSC-equivalent levels of expression of Csf1r mRNA are found on common myeloid (CMP) and common lymphoid (CLP) progenitor cells (Tagoh et al. 2002, 2004). During CMP differentiation to macrophages, up-regulation of the CSF-1R occurs in two stages. The first stage involves transcription factor assembly (PU.1, Runx1, and C/EBP binding) and chromatin remodeling at the macrophage promoter (Walsh et al. 2002; Krysinska et al. 2007). The second stage involves factor assembly and chromatin remodeling at FIRE (Laslo et al. 2006). This two-step mechanism ensures that high levels of CSF-1R are only expressed in the more differentiated cells that respond to CSF-1 alone and not in multipotent cells that express lower levels and require synergistic growth factors, such as IL-3 or SCF (Bartelmez et al. 1989; Williams et al. 1992). In contrast, during B lymphocyte development from CLP, PAX5 acts as a repressor, silencing the Csf1r gene by binding directly to a site overlapping the main Csf1r transcriptional start sites recognized by EWS and Fus/TLS (Fig. 1D) (Tagoh et al. 2006; Bonifer and Hume 2008).
STRUCTURE OF CSF-1R AND LIGANDS
CSF-1 and IL-34
The CSF-1R is the only RTK activated by two ligands of unrelated sequence, CSF-1 and IL-34. CSF-1 differs from IL-34 in that, owing to alternative splicing and differential proteolysis of a longer precursor in the secretory vesicle, it is expressed in three functionally separable isoforms: a secreted glycoprotein, a secreted chondroitin sulfate proteoglycan, and a membrane-spanning, cell-surface glycoprotein (Rettenmier et al. 1987; Price et al. 1992; Suzu et al. 1992; Dai et al. 2004; Nandi et al. 2006). All three isoforms are dimeric and contain the same amino-terminal 150 amino acids of CSF-1 required for biological activity, but have distinct, yet overlapping activities (Dai et al. 2004; Nandi et al. 2006; Chitu and Stanley 2014) determined by the remaining carboxy-terminal sequence. In contrast, IL-34 is synthesized as a secreted glycoprotein possessing one biologically active isoform of lower activity in which the codon encoding Glu81 has been spliced out (Lin et al. 2008; Wei et al. 2010).
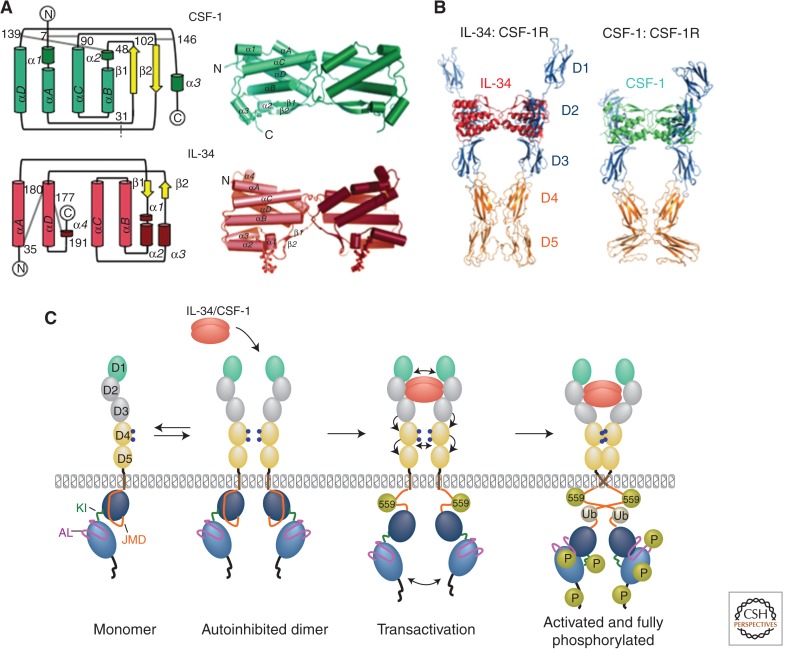
Despite sharing low sequence similarity, the biologically active regions of IL-34 and CSF-1 have similar four helical bundle (cytokine) folds (Fig. 2A). Both are head-to-head dimers, IL-34 noncovalently associated, whereas CSF-1 is linked through interchain disulfide bonds (Pandit et al. 1992; Liu et al. 2012; Ma et al. 2012). The N-linked oligosaccharides are critical for the stability of IL-34, but not of CSF-1 (Liu et al. 2012).
Figure 2.
Structure of CSF-1, IL-34, and CSF-1R ligand–receptor complexes. (A) Topological diagrams of monomeric (left) and ribbon representations of dimeric CSF-1 (top) and IL-34 (bottom) (based on data from Pandit et al. 1992; Liu et al. 2012; Ma et al. 2012). Gray lines in topological diagrams represent intramolecular disulfide bridges; dotted gray line in CSF-1 indicates position of the intermolecular disulfide bond. (B) Structure of CSF-1R ectodomain complex with IL-34 and CSF-1 (based on data from Felix et al. 2013). (C) Model of early events in CSF-1R activation (based on data from Verstraete and Savvides 2012). Gold spheres, phosphotyrosines (P), gray spheres, ubiquitination (Ub). Note that phosphorylation of CSF-1R tyrosine residue 559 and intracellular domain ubiquitination are important for full receptor activation and tyrosine phosphorylation.
CSF-1/CSF-1R AND IL-34/CSF-1R COMPLEX STRUCTURES
Studies of the interaction of 125I-CSF-1 with macrophages, together with cross-linking studies showed the existence of a single class of high-affinity binding sites through which all of the biological effects of CSF-1 were mediated (Guilbert and Stanley 1980, 1986; Morgan and Stanley 1984; Guilbert et al. 1986). Cell-based studies indicated that the unliganded CSF-1R may undergo rapid dimer-monomer transitions (Li and Stanley 1991), that the CSF-1-binding site was contained in CSF-1R domains D1–D3 (Wang et al. 1993), and that D4 was involved in ligand-induced oligomerization (Carlberg and Rohrschneider 1994). Structural studies, using a combination of electron microscopy and small-angle X-ray scattering, provided evidence for CSF-1R predimerization (Elegheert et al. 2011). Binding of CSF-1 to the CSF-1R is exclusively via the D2 and D3 domains, the D4 domains mediating CSF-1R homotypic interactions, via a broad interaction interface, whereas the D1 and D5 domains point away from the complex (Fig. 2B) (Chen et al. 2008; Elegheert et al. 2011; Ma et al. 2012; Felix et al. 2013). D4 shares a dimerization domain sequence fingerprint that has been identified in other closely related RTK III receptors, Kit and PDGFR (Yuzawa et al. 2007; Yang et al. 2008), and the presence of domains D4 and D5 in the CSF-1:CSF-1RD1–D5 significantly decreases the Kd of interaction (Chen et al. 2008; Elegheert et al. 2011; Ma et al. 2012) owing to CSF-1R homotypic interactions. However, the Kd for the CSF-1:CSF-1RD1–D5 interaction at 37°C (∼20 nm, human and mouse [Elegheert et al. 2011]) was still higher than the dissociation constants reported for the binding of mouse (0.4 nm [Guilbert and Stanley 1986]) or human (0.1 nm [Roussel et al. 1988]) CSF-1 to their cognate receptors on cells, suggesting a significant contribution of the transmembrane domain and spatial confinement of the membrane to affinity (Fig. 2C).
Consistent with the cross-competition of CSF-1 and IL-34 for binding to the CSF-1R (Chihara et al. 2010; Wei et al. 2010), both ligands bind to a concave surface formed by the CSF-1R D2 and D3 domains (Fig. 2B) (Chen et al. 2008; Elegheert et al. 2011; Liu et al. 2012; Ma et al. 2012; Felix et al. 2013). Despite their similarities, the IL-34:CSF-1R complex differs from the CSF-1:CSF-1R complex because: (1) there is a ∼20° rotation difference of their D3 domains when their D2 domains are superimposed, resulting in an elongated pose that differs from the kinked configuration of the CSF-1R:CSF-1 complex; (2) CSF-1 is clamped deeper by the CSF-1R D2-D3 junction than IL-34, so that overlapping, yet different CSF-1R segments are used by each ligand; (3) to compensate for loss of some D2 interactions, IL-34 adopts amino-terminal and carboxy-terminal extensions to contact D3 (Liu et al. 2012; Ma et al. 2012); and (4) the apparent rigidity of the IL-34 structure (Liu et al. 2012; Ma et al. 2012) differs from the more plastic CSF-1 structure, which undergoes local structural rearrangements for receptor binding (Chen et al. 2008).
Studies of mouse IL-34:CSF-1R interactions indicate that the interactions of IL-34 with D2 and D3 are not functionally equivalent. Mutation of individual hydrophilic residues involved in IL-34 interactions with CSF-1R D2 failed to affect IL-34 biological activity, whereas mutations of residues interacting with D3 substantially reduced IL-34 activity suggesting that charge interactions with D2 may capture IL-34 for subsequent interaction with D3 (Liu et al. 2012). Despite the differences between the IL-34:CSF-1R and CSF-1:CSF-1R complexes, the distance between the two D3-D4 junctions important for critical homotypic D4 interactions (Elegheert et al. 2011; Felix et al. 2013) is equivalent (62 Å and 60 Å, respectively) (Ma et al. 2012).
CSF-1R Kinase Structure and Activation
The structure of the inactive human CSF-1R kinase domain is two-lobed, similar to those of cKIT and FLT3 RTK IIIs (Schubert et al. 2007; Walter et al. 2007). The N lobe comprises a five-stranded, antiparallel β sheet (β1–β5) and a single α helix (αC), and is joined to the carboxy-terminal lobe by the kinase insert domain and a hinge region. The C lobe has seven α helices and two β strands. ATP binding, in a deep cleft between the N and C lobes, involves the N lobe and hinge regions, which also provide some catalytic residues. The C lobe mediates substrate binding and catalysis. In its inactive conformation, the CSF-1R kinase domain activation loop (AL) is folded back onto the ATP-binding cleft, with the sole AL tyrosine 809 acting as a pseudosubstrate, blocking substrate binding. Also, Asp-796 of the invariant DFG motif, necessary for Mg2+ coordination of ATP, is in a “DFG-out” conformation, owing to its displacement from the active site. The activation of CSF-1R therefore requires flipping of the DFG motif to a “DFG-in” conformation and reorganization of the AL. Interestingly, in other receptor kinases, this can involve phosphorylation of the AL tyrosine, but the data do not support a role for AL tyrosine phosphorylation in the early activation of the CSF-1R (Yu et al. 2012), or of cKIT (DiNitto et al. 2010). In the inactive CSF-1R kinase, the JMD (Q542-K574, between the transmembrane domain and the N lobe), mediates a critical autoinhibitory mechanism by blocking αC, preventing the AL from adopting an active conformation and restricting interlobe plasticity (Walter et al. 2007). Inhibition is relieved by phosphorylation of Tyr-561 (Tyr-559 in mouse). This tyrosine, the first residue to be phosphorylated in response to ligand binding, acts as a switch that is off in the absence of ligand and turned on by phosphorylation in response to ligand (Rohde et al. 2004; Yu et al. 2008; Xiong et al. 2011; Yu et al. 2012). Although the structure of the activated CSF-1R kinase domain has not been reported, the changes that take place on activation can be visualized by superimposing the AL of activated cKIT onto inactive CSF-1R structure (Schubert et al. 2007; Chitu and Stanley 2014). A schematic of ligand-induced CSF-1R activation is shown in Figure 2C.
CSF-1R SIGNAL TRANSDUCTION IN MYELOID CELLS
Early Events and Role of CSF-1R Tyrosine Phosphorylation
As undifferentiated progenitor cells are rare, CSF-1R signaling has been primarily studied in macrophages (Yu et al. 2008), osteoclasts (Faccio et al. 2007), or in transduced myeloid progenitor cell lines that normally do not express the receptor (Bourette et al. 1995; Csar et al. 2001). The approaches taken have been proteomic (identifying and analyzing the function of proteins tyrosine phosphorylated or activated in the response) (Yeung and Stanley 2003) and genetic (analysis of the effects of CSF-1R mutations) (Yu et al. 2012). CSF-1R signaling in all lineages has recently been reviewed in detail elsewhere (Chitu and Stanley 2014).
Kinetics of Early Responses in Macrophages
Studies of CSF-1-induced changes in mouse macrophages at 4°C and 37°C have permitted early steps in this process to be resolved. Before CSF-1 addition, CSF-1Rs are clustered, or are undergoing a rapid dimer-monomer transition (Li and Stanley 1991). CSF-1 binding initially leads to rapid dimerization, a first wave of tyrosine phosphorylation of the CSF-1R, and formation of CSF-1R complexes with Grb2/Sos and with SFK, Cbl, the regulatory subunit of PI-3 kinase (PI3K) (p85), Grb2, and other signaling molecules, many of which become tyrosine phosphorylated (Baccarini et al. 1991; Li and Stanley 1991; Li et al. 1991; Kanagasundaram et al. 1996; Wang et al. 1996, 1999b; Husson et al. 1997). The tyrosine phosphorylated proteins, representing 0.02% of the total cellular protein, are mainly in the membrane fraction (Yeung et al. 1998). The CSF-1R/Sos/Grb2 complexes are more transient than those involving the CSF-1R, Cbl, Shc, p85, and Grb2 (Wang et al. 1999b). Sos/Grb2 dissociates from the CSF-1R, which undergoes a second wave of tyrosine phosphorylation with serine phosphorylation, which is temporally associated with the Cbl-dependent CSF-1R ubiquitination and Cbl ubiquitination (Baccarini et al. 1991; Li and Stanley 1991; Wang et al. 1996, 1999b; Lee et al. 1999). There follows internalization of the CSF-1/CSF-1R complex, its entry into multivesicular bodies, and thence into the lysosomal system, in which both ligand (Guilbert and Stanley 1986) and receptor (Lee et al. 1999) are degraded. In contrast, the ubiquitinated Cbl ubiquitin ligase is not degraded, but recovered in deubiquitinated form in the cytosol 3–10 min after stimulation (Wang et al. 1996, 1999b; Lee et al. 1999) (reviewed in Yeung and Stanley 2003). Tyrosine phosphorylation and ubiquitination of the cell-surface CSF-1R dimers are stoichiometric (Li and Stanley 1991; Li et al. 1991; Wang et al. 1999b), although ubiquitination may be asymmetric and restricted to only one monomer of the ubiquitinated CSF-1R dimer (Xiong et al. 2011). Signaling may also occur from internalized receptors (Huynh et al. 2012). Many of the proteins in complexes with the rapidly tyrosine phosphorylated cellular proteins are cytoskeletal proteins (Yeung et al. 1998; Yeung and Stanley 2003) and the short-term responses include extensive cytoskeletal remodeling (Boocock et al. 1989; Pixley et al. 2001; Chitu et al. 2005; Sampaio et al. 2011; Pixley 2012). Increased protein synthesis is detected as early as 15 min following CSF-1R stimulation and plateaus at 2 h (Tushinski and Stanley 1983).
Later responses include increased motility and chemotaxis (Pixley et al. 2001; Chitu et al. 2005; Sampaio et al. 2011; Pixley 2012), altered gene expression leading to the entry of cells into S phase at ∼12 h (Tushinski and Stanley 1985), during which time cell-surface CSF-1R levels are down-regulated, but cell-surface CSF-1Rs are turning over rapidly and presumably signaling (Guilbert and Stanley 1986; Yu et al. 2012; reviewed in Sherr 1991; Yeung and Stanley 2003). The expression of CD11b (Yu et al. 2008) and of chemokines, cytokines, and cell-surface markers of M2 polarization state, are also increased (Fleetwood et al. 2007; Foucher et al. 2013).
Role of Individual CSF-1R Intracellular Domain Phosphotyrosines in Regulation of Macrophage Functions
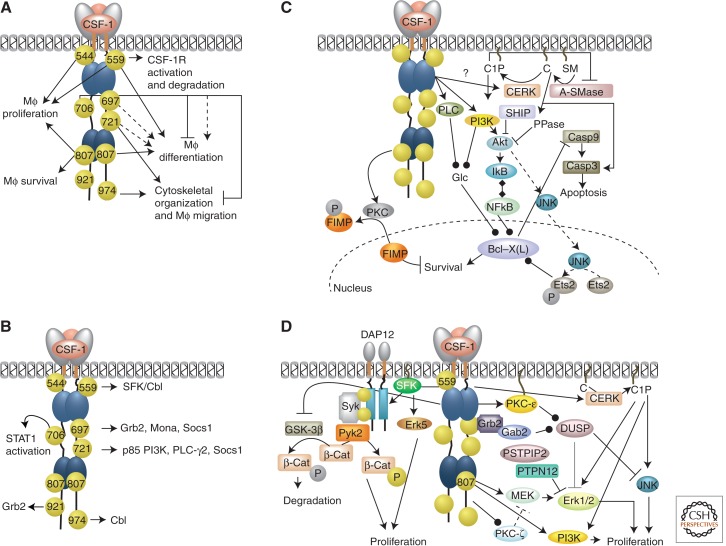
The functions of the eight tyrosines known to be phosphorylated in the activated mouse CSF-1R proto-oncoprotein/oncoprotein have been investigated in macrophages by their mutation to phenylalanine (to establish/investigate necessity) or by adding them back to a receptor backbone in which all eight tyrosines are mutated to phenylalanine (to establish/investigate sufficiency) (Fig. 3A). Add-back of AL Tyr-807, JMD Tyr-559, and JMD Tyr-544 is sufficient to restore full in vitro CSF-1R kinase activity (Yu et al. 2012). AL Tyr-807 alone confers constitutive activation of CSF-1R-regulated proliferation. JMD Tyr-559 is the first tyrosine phosphorylated in response to ligand (Yu et al. 2008; Xiong et al. 2011). Tyr-559 keeps CSF-1R kinase “off” in the absence of ligand and its phosphorylation relieves this autoinhibition, so that Tyr-559 controls CSF-1R responsiveness to ligand (Rohde et al. 2004; Takeshita et al. 2007; Yu et al. 2012). JMD Tyr-544, phosphorylated in the oncogenic receptor (Joos et al. 1996), is required for full kinase activation (Yu et al. 2008). Add-back of all three tyrosines fully restores CSF-1-regulated CSF-1R kinase activation, bulk cellular protein tyrosine phosphorylation, and the proliferative response (Yu et al. 2012). Tyr-559 is both necessary and sufficient for the recruitment of SFK that, in turn, associate with and activate c-Cbl. Cbl activation leads to CSF-1R multiubiquitination, conformational changes, increased phosphorylation, and internalization of receptor–ligand complexes (Baccarini et al. 1991; Rohde et al. 2004; Xiong et al. 2011). Tyr-721 is necessary and sufficient on the YEF.Y544,559,807AB background, for mediating macrophage chemotaxis to CSF-1 through the PI3K pathway (Sampaio et al. 2011). Mutations of KI tyrosines 706 or 721, or carboxy-terminal Tyr-974, alter morphological responses (Yu et al. 2008). AL Tyr-807 is required for multipotent progenitor cell differentiation to macrophages, which KI tyrosines 697, 706, and 721 augment (Rohrschneider et al. 1997), although Tyr-706 negatively regulates expression of CD11b in macrophages (Yu et al. 2008). Phosphorylation of individual CSF-1R tyrosines creates docking sites for several signaling molecules (Fig. 3B) (reviewed in Pixley and Stanley 2004).
Figure 3.
CSF-1R signaling in macrophage survival and proliferation. (A) Biological functions regulated by individual CSF-1R tyrosine residues. (B) Protein interactions and signaling events triggered by individual CSF-1R phosphotyrosine residues (based on data from Pixley and Stanley 2004). (C) CSF-1R signaling for macrophage survival. (D) Pathways mediating CSF-1R proliferative responses in macrophages. Arrows indicate activation; black line-capped arrows, inhibition; gray line-capped arrows, late-phase inhibition; round-capped arrows, increased expression or concentration; diamond-capped arrows, dissociation; dotted arrows, partial contribution; gold spheres, phosphotyrosines; and silver spheres, serine/threonine phosphorylation. Numbered gold spheres indicate the mouse CSF-1R tyrosine residues required for activation of specific pathways. Those without numbers indicate that the CSF-1R phosphotyrosyl residue triggering the response is not known.
Downstream Signaling Pathways in the Macrophage Lineage
Survival
Low CSF-1 concentrations stimulate macrophage survival that is associated with inhibition of total protein degradation (Tushinski et al. 1982; Tushinski and Stanley 1983). The PI3K/Akt pathway has a central role in CSF-1-mediated macrophage survival (Fig. 3C) (Kelley et al. 1999; Murray et al. 2000; Golden and Insogna 2004; Chang et al. 2009). In macrophages, Akt can be activated directly through the CSF-1R pTyr721/PI3K pathway (Lee and States 2000; Sampaio et al. 2011) and indirectly by ceramide-1-phosphate (C1P) (Gómez-Muñoz et al. 2004, 2005, 2010; Steinbrecher et al. 2004), or the Gab2/PI3K pathway (Lee and States 2000; Lee et al. 2011; Yu et al. 2012), which is counterbalanced by a CSF-1R pTyr559/Lyn/SHIP-1 pathway (Baran et al. 2003).
PI3K-independent pathways of CSF-1R-mediated survival involve phospholipase C (PLC) (Xu et al. 1993) and Fms-interacting protein (FIMP) (Mancini et al. 2004). PI3K and PLC independently enhance survival by controlling glucose uptake (Chang et al. 2009). CSF-1R-regulated PKC-dependent serine phosphorylation translocates FIMP from the nucleus, where it inhibits CSF-1R-mediated signaling, to the cytosol, thereby enhancing macrophage survival and differentiation (Fig. 3C) (Mancini et al. 2004).
Proliferation
Macrophage proliferation is associated with a CSF-1 dose-dependent increase in protein synthetic rate (Tushinski and Stanley 1983). CSF-1R pTyr-807 signaling activates both the MEK and PI3K pathways that independently contribute to macrophage proliferation (Munugalavadla et al. 2005; Yu et al. 2012). A CSF-1R pTyr-559/SFK-dependent pathway also contributes to macrophage proliferation (Takeshita et al. 2007; Yu et al. 2012). In addition, CSF-1 increases C1P production and C1P stimulates proliferation through activation of PI3K/Akt, JNK, and ERK1/2 pathways (Fig. 3D) (Gangoiti et al. 2008).
Multiple ERKs may be involved in the control of macrophage proliferation, as their involvement has been inferred by MEK inhibition. ERK5 is activated in a SFK-dependent manner by CSF-1 and is necessary for optimal proliferation (Rovida et al. 2008). ERK1/2 phosphorylation may act as a sensor of CSF-1 concentration (Rovida et al. 2002). Via activation of membrane-associated PKC-ε, the CSF-1R mediates increases in the expression of dual specificity phosphatase-1 (DUSP-1) that suppresses prolonged Erk1/2 activation, which would lead to cell-cycle arrest (Valledor et al. 1999). In myeloblasts, increased PKC-ζ activity regulates Erk1/2 activation and proliferation in a developmental stage-specific manner (Lee 2011). Overexpression of PKC-ζ in myeloblasts increased the intensity and duration of Erk1/2 phosphorylation and the proliferative response to CSF-1 (Fig. 4B). In contrast, in macrophages, PKC-ζ activates a negative regulatory step upstream of MEK (Fig. 3D) (Lee 2011).
Figure 4.
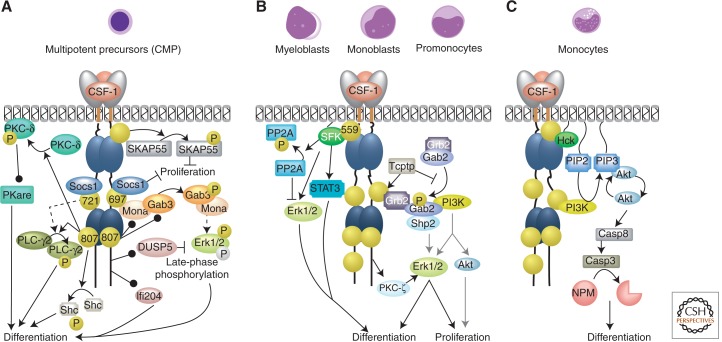
CSF-1R signaling in macrophage differentiation. (Top) Morphology of May-Grünwald-Giemsa-stained macrophage precursors. Gray arrows (middle panel) indicate that Gab2 significantly contributes to these pathways in monoblasts, but not in pro-monocytes. Symbols are as described in the legend for Figure 3.
The transmembrane adaptor protein DAP12 mediates CSF-1R proliferative signals through a MAPK- and Akt-independent pathway. The cytoplasmic domain of DAP12 contains immunoreceptor tyrosine-based activation motifs (ITAMs), which become phosphorylated in response to CSF-1R activation, recruiting and activating the cytosolic tyrosine kinase Syk, which activates the Pyk2 tyrosine kinase that phosphorylates β-catenin, triggering its nuclear translocation and activation of cell-cycle genes. The response is enhanced by DAP12-independent CSF-1R inhibition of β-catenin degradation (Otero et al. 2009). DAP12 deficiency leads to impaired CSF-1R-mediated proliferation and survival, but does not affect differentiation (Nataf et al. 2005; Otero et al. 2009).
Proline serine threonine phosphatase interacting protein 2 (PSTPIP2) is a membrane–cytoskeletal adaptor highly expressed in myeloid cells (Chitu and Stanley 2007, 2009). PSTPIP2 deficiency increases Erk1/2 phosphorylation and CSF-1-induced myeloid precursor proliferation, whereas overexpression in macrophages inhibits Erk1/2 phosphorylation and growth (Chitu et al. 2009). PSTPIP2 interacts with PEST-family tyrosine phosphatases PTPN12 (Chitu et al. 2012) and PTPN18 (Wu et al. 1998) and, as suggested by studies with PSTPIP1 (Yang and Reinherz 2006), it is possible that PSTPIP2 acts by recruiting PTP-PEST to signaling complexes upstream of Erk1/2 (Fig. 3D).
Differentiation
CSF-1R activation directly induces monocytic cell fate in HSCs through up-regulation of the myeloid transcription factor PU.1 (Mossadegh-Keller et al. 2013). It also instructs granulocyte/macrophage progenitors (GMP) to differentiate into macrophages (Rieger et al. 2009).
Studies with multipotent precursor cell lines (Fig. 4A) indicate that CSF-1R Tyr-807 and Tyr-721 promote macrophage differentiation via the PLC-γ2 pathway (Bourette et al. 1997). Tyr-807 is also required for the tyrosine phosphorylation of p46/52 Shc (Csar et al. 2001) and the tyrosine phosphorylation, activation, and membrane translocation of PKC-δ leading to increased expression of PKA-related protein kinase (Pkare), all of which contribute to monocytic differentiation (Junttila et al. 2003).
The Erk1/2 pathway has a central role in CSF-1R-regulated myeloid differentiation. CSF-1 induces early (peaking at ∼5 min) and persistent (starting at 1 h) waves of MEK/Erk1/2 phosphorylation. However, only the late wave, which is independent of Grb2/Sos assembly or PI 3-kinase activity, is required for macrophage differentiation (Gobert Gosse et al. 2005). Mona, an adaptor protein that increases late Erk1/2 phosphorylation (Bourgin et al. 2002) and Gab3 are coinduced during monocytic differentiation in a CSF-1R Tyr-807-dependent manner (Fig. 4A). Mona interacts with Gab3 and with the CSF-1R pTyr-697 site. This site is also important for Gab3 tyrosine phosphorylation, induction of Mona expression, and macrophage differentiation (Bourgin et al. 2002). Gab proteins interact with Shp2 and mediate Erk1/2 activation downstream from growth factor receptors (Nishida et al. 1999; Meng et al. 2005; Lee et al. 2011). The Gab3/Mona complex, but not Mona alone, enhances macrophage differentiation in cell cultures (Bourgin et al. 2000; Wolf et al. 2002). However, as macrophage development is normal in Gab3-deficient mice (Seiffert et al. 2003) it appears that CSF-1R/Mona/Gab3/Erk1/2 pathway is not essential for steady-state macrophage development in vivo.
CSF-1R also induces the expression/activation of several other regulators of multipotent progenitor proliferation/differentiation (Fig. 4A). These include DUSP5, a negative-feedback regulator of Erk1/2, which inhibits macrophage differentiation and favors granulocytic differentiation (Grasset et al. 2010), interferon-inducible gene 204 (Ifi204), which suppresses proliferation and favors differentiation (Dauffy et al. 2006), and the adaptor proteins suppressor of cytokine signaling 1 (Socs1) and SKAP55-related (SKAP55R). Socs1 associates with CSF-1R pTyr-697 and pTyr721 binding sites to inhibit proliferation by an unknown mechanism (Bourette et al. 2001). CSF-1 induces SKAP55R tyrosine phosphorylation and actin association. SKAP55R overexpression decreases CSF-1R-induced proliferation, but does not affect differentiation (Bourette et al. 2005).
Studies with myeloblasts/monoblasts/promonocytes (Fig. 4B) confirm the central role of the Erk1/2 pathway in CSF-1-induced differentiation (Wilson et al. 2005). In myeloblasts, a CSF-1R pTyr-559/SFK pathway initiates the activation of STAT3 (Marks et al. 1999) and Erk1/2 and the inactivation of PP2A, which plays a significant role in enhancing Erk1/2-mediated macrophage differentiation (McMahon et al. 2001). In multipotent cells, via a SFK-independent pathway, Gab2 becomes tyrosine phosphorylated, associating with signaling molecules, including Grb2, Shp2, and p85, to activate Erk1/2 and differentiation (Liu et al. 2001). T-cell protein-tyrosine phosphatase (Tcptp) dephosphorylates the activated CSF-1R, negatively regulating differentiation by suppressing both CSF-1R association with the Grb2/Gab2/Shp2 complex and activation of Erk1/2 (Simoncic et al. 2006). Gab2 deficiency in mice leads to a decreased frequency and proliferation of CFU-M, which retain the capacity to differentiate to macrophages (Lee et al. 2011). Studies with Gab2 mutants unable to interact with Shp2 or PI3K show that both interactions are required for restoration of CFU-M frequency and proliferation. As constitutively active Akt only rescues CFU-M size, but not frequency, the Gab2/PI3K/Akt axis predominantly promotes the proliferation of committed macrophage progenitors. Gab2 is required for Akt activation only in monoblasts and regulates Erk1/2 activity in a developmental stage-specific manner, increasing activation in monoblasts and promonocytes and decreasing activation in macrophages (Lee et al. 2011).
In primary human monocytes (Fig. 4C), CSF-1 triggers a cyclic activation of the PI3K and Erk1/2 pathways that is correlated with successive rounds of CSF-1R Tyr-723 phosphorylation and dephosphorylation. Successive waves of Akt activation, increasing in amplitude and duration, are required for caspase activation, which, via cleavage of nucleophosmin, enhances macrophage differentiation (Jacquel et al. 2009) toward a trophic, M2-like phenotype (Guery et al. 2011). Erk1/2 was activated with coordinated kinetics, but was not essential for nucleophosmin cleavage, and its role remains to be defined. In contrast, the SFKs, Hck, and, to a lesser extent, Lyn, but not Fyn or Src, mediated nucleophosmin cleavage downstream from CSF-1R (Jacquel et al. 2009).
Chemotaxis
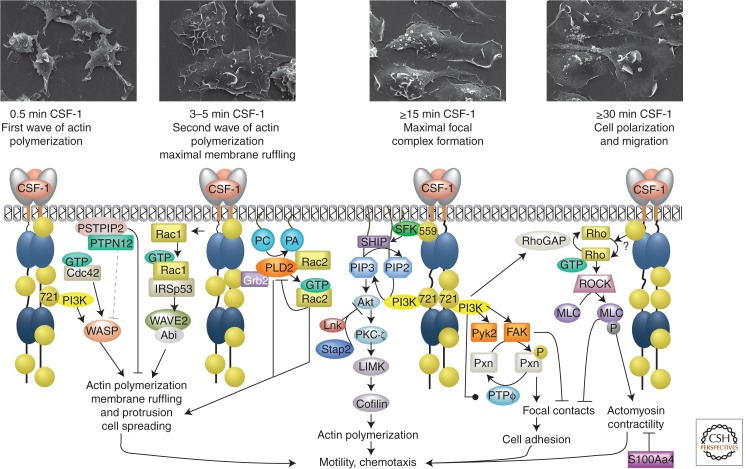
CSF-1 triggers a rapid membrane ruffling response followed by cell spreading and polarization (Boocock et al. 1989; Webb et al. 1996; Chitu et al. 2005), processes involving a dynamic reorganization of the actin cytoskeleton (reviewed in Park et al. 2011; Pixley 2012) and focal adhesions (Fig. 5) (Pixley et al. 2001; Sampaio et al. 2011). CSF-1-stimulates a biphasic actin polymerization response that initially peaks at 30 sec of stimulation and is followed by a longer-lasting wave, peaking at 5–6 min (Sampaio et al. 2011; Ishihara et al. 2012). The small GTPases, Cdc42, Rac, and Rho and their downstream effectors, Wiskott-Aldrich syndrome protein (WASP) and WASP-family verprolin homologous 2 (WAVE 2) actin nucleators, regulate actin polymerization and CSF-1R-induced chemotaxis (Kheir et al. 2005; Ridley 2008; Cammer et al. 2009; Dovas et al. 2009; Ishihara et al. 2012).
Figure 5.
CSF-1R signaling in macrophage migration and chemotaxis. (Top panels) Scanning electron microscopic images of macrophages stimulated with CSF-1 for the indicated times. The dotted gray arrow indicates a hypothetical mechanism. Symbols are as described in the legend for Figure 3.
The first wave of actin polymerization is initiated by the Cdc42- and CSF-1R pTyr-721/PI3K p110δ-dependent activation of WASP (Papakonstanti et al. 2008; Cammer et al. 2009; Sampaio et al. 2011; Mouchemore et al. 2013). CSF-1R also induces SFK-dependent tyrosine phosphorylation of WASP Tyr-291, which, although not necessary for WASP activation (Cammer et al. 2009), is necessary for macrophage chemotaxis to CSF-1 (Dovas et al. 2009). PSTPIP2 is tyrosine phosphorylated within 30 sec and inhibits actin polymerization and the ruffling response (Chitu et al. 2005). By competing with other F-BAR family proteins for phosphatidylinositol 4,5-bisphosphate (PIP2)-rich membrane-binding sites and recruiting PTPN12 to those sites, PSTPIP2 may mediate the local dephosphorylation and partial inactivation of WASP (Cote et al. 2002; Tsujita et al. 2013).
The second wave of actin polymerization leading to membrane ruffling involves Rac1/IRSp53 activation of the WAVE2/Abi complex (Kheir et al. 2005; Abou-Kheir et al. 2008) and is pTyr-721/PI3K independent (Sampaio et al. 2011). CSF-1R-regulated membrane ruffling also requires PLD2 lipase and Rac2 GEF activities (Mahankali et al. 2011a,b). Rac2 initially increases, but subsequently inhibits PLD2 activity, by preventing PLD interaction with PIP2 at the plasma membrane, thus leading to cell immobilization (Peng et al. 2011). Although the above studies in macrophage cell lines indicate that Rac1 and Rac2 are required for chemotaxis (Allen et al. 1998; Abou-Kheir et al. 2008), the findings are in conflict with those in primary macrophages, in which absence of Rac1, Rac2, or both, did not affect CSF-1-induced chemotaxis (Wells et al. 2004; Wheeler et al. 2006). An additional conflict relates to the mechanism of Rac1 activation by CSF-1R. Although studies in SHIP−/− macrophages implicate Vav proteins (Vedham et al. 2005), studies in single or triple-deficient Vav1/2/3−/− primary macrophages indicate that they are not required (Wells et al. 2005; Bhavsar et al. 2009). In contrast, the PI3K/Akt pathway plays a central role in CSF-1R-induced chemotaxis (Sampaio et al. 2011), via phosphorylation of PKC-ζ and LIMK/Cofilin (Zhang et al. 2009). Two adaptor proteins, Lnk and STAP-2, suppress Akt activation and inhibit CSF-1-induced macrophage migration (Ikeda et al. 2007; Gueller et al. 2010). The CSF-1R pTyr-721/PI3K pathway also regulates cell adhesion by controlling paxillin phosphorylation (Owen et al. 2007; Sampaio et al. 2011) and PTP-ϕ expression (Pixley et al. 2001; Sampaio et al. 2011).
During cell migration, the formation of adhesion structures is locally disrupted by inhibitory proteins (e.g., PTP-ϕ), and actomyosin-dependent contractility is necessary for retracting the trailing edge. S100A4 is a Ca2+ and myosin IIA-binding protein that inhibits actomyosin assembly downstream from the CSF-1R (Li et al. 2003, 2010). S100A4 deficiency leads to a defective CSF-1 chemotactic response, owing to reduced persistence and size of membrane protrusions associated with persistent and enhanced actomyosin-IIA assembly and hyperphosphorylation and mislocalization of paxillin (Li et al. 2010). The small GTPase Rho is required for CSF-1-mediated macrophage chemotaxis (Jones et al. 1998) and promotes tail retraction by controlling myosin activity (Hanley et al. 2010). In macrophages stimulated with CSF-1, Rho undergoes cycles of activation and deactivation. Although the mechanism of activation is unclear, RhoA deactivation is mediated by the CSF-1R/PI3K p110δ/p190RhoGAP axis (Papakonstanti et al. 2007).
PERSPECTIVE
CSF-1R signaling promotes myeloid differentiation, monocytic commitment, and the survival, proliferation, and chemotaxis of macrophages by regulating the tyrosine phosphorylation, activation, or expression of multiple proteins. Several of these proteins (e.g., Gab2, PKC-ζ) regulate downstream signaling pathways in a developmental stage-specific manner. Some conflicting results, in primary macrophages compared with macrophage cell lines, may result from the utilization of different effector isoforms by the activated CSF-1R (Papakonstanti et al. 2007). Thus caution should be taken in extrapolating results to different developmental stages, or types of macrophages. The existence of a new CSF-1R ligand, IL-34, that also interacts with PTP-ζ, which is coexpressed with the CSF-1R in several cell types, including HSC (Sarrazin et al. 2009; Himburg et al. 2012) and neural progenitors (von Holst et al. 2006; Nandi et al. 2013), may provide additional mechanisms for fine-tuning CSF-1R signaling in development, immunity, and disease. The discovery that Epstein–Barr virus encodes BamHI-A rightward frame-1 (BARF1), a secreted hexameric protein that binds the CSF-1 dimer interface with picomolar affinity and conformationally renders the cytokine unable to interact with the CSF-1R (Strockbine et al. 1998; Elegheert et al. 2012; Shim et al. 2012), explains how Epstein–Barr virus eludes the immune response and offers a starting point for therapeutic targeting of both CSF-1 and BARF1.
ACKNOWLEDGMENTS
This work is supported by National Institutes of Health grants PO1 CA100324 and CA 32551 (to E.R.S.), K01AR 054486 (to V.C.), and 5P30-CA13330 (a cancer center grant to the Albert Einstein College of Medicine).
Footnotes
Editors: Joseph Schlessinger and Mark A. Lemmon
Additional Perspectives on Receptor Tyrosine Kinases available at www.cshperspectives.org
REFERENCES
- Abou-Kheir W, Isaac B, Yamaguchi H, Cox D 2008. Membrane targeting of WAVE2 is not sufficient for WAVE2-dependent actin polymerization: A role for IRSp53 in mediating the interaction between Rac and WAVE2. J Cell Sci 121: 379–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen WE, Zicha D, Ridley AJ, Jones GE 1998. A role for Cdc42 in macrophage chemotaxis. J Cell Biol 141: 1147–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini M, Li W, Dello Sbarba P, Stanley ER 1991. Increased phosphorylation of the colony stimulating factor-1 receptor following transmembrane signaling. Receptor 1: 243–259 [PubMed] [Google Scholar]
- Baran CP, Tridandapani S, Helgason CD, Humphries RK, Krystal G, Marsh CB 2003. The inositol 5′-phosphatase SHIP-1 and the Src kinase Lyn negatively regulate macrophage colony-stimulating factor-induced Akt activity. J Biol Chem 278: 38628–38636 [DOI] [PubMed] [Google Scholar]
- Bartelmez SH, Stanley ER 1985. Synergism between hemopoietic growth factors (HGFs) detected by their effects on cells bearing receptors for a lineage specific HGF: Assay of hemopoietin-1. J Cell Physiol 122: 370–378 [DOI] [PubMed] [Google Scholar]
- Bartelmez SH, Bradley TR, Bertoncello I, Mochizuki DY, Tushinski RJ, Stanley ER, Hapel AJ, Young IG, Kriegler AB, Hodgson GS 1989. Interleukin 1 plus interleukin 3 plus colony-stimulating factor 1 are essential for clonal proliferation of primitive myeloid bone marrow cells. Exp Hematol 17: 240–245 [PubMed] [Google Scholar]
- Bhavsar PJ, Vigorito E, Turner M, Ridley AJ 2009. Vav GEFs regulate macrophage morphology and adhesion-induced Rac and Rho activation. Exp Cell Res 315: 3345–3358 [DOI] [PubMed] [Google Scholar]
- Bonifer C, Hume DA 2008. The transcriptional regulation of the colony-stimulating factor 1 receptor (csf1r) gene during hematopoiesis. Front Biosci 13: 549–560 [DOI] [PubMed] [Google Scholar]
- Boocock CA, Jones GE, Stanley ER, Pollard JW 1989. Colony-stimulating factor-1 induces rapid behavioural responses in the mouse macrophage cell line, BAC1.2F5. J Cell Sci 93: 447–456 [DOI] [PubMed] [Google Scholar]
- Bourette RP, Myles GM, Carlberg K, Chen AR, Rohrschneider LR 1995. Uncoupling of the proliferation and differentiation signals mediated by the murine macrophage colony-stimulating factor receptor expressed in myeloid FDC-P1 cells. Cell Growth Differ 6: 631–645 [PubMed] [Google Scholar]
- Bourette RP, Myles GM, Choi JL, Rohrschneider LR 1997. Sequential activation of phosphatidylinositol 3-kinase and phospholipase C-g2 by the M-CSF receptor is necessary for differentiation signaling. EMBO J 16: 5880–5893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourette RP, De Sepulveda P, Arnaud S, Dubreuil P, Rottapel R, Mouchiroud G 2001. Suppressor of cytokine signaling 1 interacts with the macrophage colony-stimulating factor receptor and negatively regulates its proliferation signal. J Biol Chem 276: 22133–22139 [DOI] [PubMed] [Google Scholar]
- Bourette RP, Therier J, Mouchiroud G 2005. Macrophage colony-stimulating factor receptor induces tyrosine phosphorylation of SKAP55R adaptor and its association with actin. Cell Signal 17: 941–949 [DOI] [PubMed] [Google Scholar]
- Bourgin C, Bourette R, Mouchiroud G, Arnaud S 2000. Expression of Mona (monocytic adapter) in myeloid progenitor cells results in increased and prolonged MAP kinase activation upon macrophage colony-stimulating factor stimulation. FEBS Lett 480: 113–117 [DOI] [PubMed] [Google Scholar]
- Bourgin C, Bourette RP, Arnaud S, Liu Y, Rohrschneider LR, Mouchiroud G 2002. Induced expression and association of the Mona/Gads adapter and Gab3 scaffolding protein during monocyte/macrophage differentiation. Mol Cell Biol 22: 3744–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch I, Salzburger W, Meyer A 2006. Asymmetric evolution in two fish-specifically duplicated receptor tyrosine kinase paralogons involved in teleost coloration. Mol Biol Evol 23: 1192–1202 [DOI] [PubMed] [Google Scholar]
- Byrne PV, Guilbert LJ, Stanley ER 1981. Distribution of cells bearing receptors for a colony-stimulating factor (CSF) in murine tissues. J Cell Biol 91: 848–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammer M, Gevrey JC, Lorenz M, Dovas A, Condeelis J, Cox D 2009. The mechanism of CSF-1-induced Wiskott-Aldrich syndrome protein activation in vivo: A role for phosphatidylinositol 3-kinase and Cdc42. J Biol Chem 284: 23302–23311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg K, Rohrschneider LR 1994. The effect of activating mutations on dimerization, tyrosine phosphorylation and internalization of the macrophage colony stimulating factor receptor. Mol Cell Biol 5: 81–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini MG, Dominguez MG, Mocci S, Wetterwald A, Felix R, Fleisch H, Chisholm O, Hofstetter W, Pollard JW, Stanley ER 1994. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development 120: 1357–1372 [DOI] [PubMed] [Google Scholar]
- Chang M, Hamilton JA, Scholz GM, Masendycz P, Macaulay SL, Elsegood CL 2009. Phosphatidylinostitol-3 kinase and phospholipase C enhance CSF-1-dependent macrophage survival by controlling glucose uptake. Cell Signal 21: 1361–1369 [DOI] [PubMed] [Google Scholar]
- Chen X, Liu H, Focia PJ, Shim AH, He X 2008. Structure of macrophage colony stimulating factor bound to FMS: Diverse signaling assemblies of class III receptor tyrosine kinases. Proc Natl Acad Sci 105: 18267–18272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara T, Suzu S, Hassan R, Chutiwitoonchai N, Hiyoshi M, Motoyoshi K, Kimura F, Okada S 2010. IL-34 and M-CSF share the receptor Fms but are not identical in biological activity and signal activation. Cell Death Differ 17: 1917–1927 [DOI] [PubMed] [Google Scholar]
- Chitu V, Stanley ER 2006. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol 18: 39–48 [DOI] [PubMed] [Google Scholar]
- Chitu V, Stanley ER 2007. Pombe Cdc15 homology (PCH) proteins: Coordinators of membrane-cytoskeletal interactions. Trends Cell Biol 17: 145–156 [DOI] [PubMed] [Google Scholar]
- Chitu V, Stanley ER 2009. PSTPIP1 and PSTPIP2/MAYP. In The Pombe Cdc15 homology proteins (ed. Aspenstrom P), pp. 49–61 Landes Bioscience, Austin, Texas [Google Scholar]
- Chitu V, Stanley ER 2014. CSF-1 receptor. In The receptor tyrosine kinase handbook (ed. Wheeler DL, Yarden Y). Springer, New York: (in press) [Google Scholar]
- Chitu V, Pixley FJ, Macaluso F, Larson DR, Condeelis J, Yeung YG, Stanley ER 2005. The PCH family member MAYP/PSTPIP2 directly regulates F-actin bundling and enhances filopodia formation and motility in macrophages. Mol Biol Cell 16: 2947–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitu V, Ferguson PJ, de Bruijn R, Schlueter AJ, Ochoa LA, Waldschmidt TJ, Yeung YG, Stanley ER 2009. Primed innate immunity leads to autoinflammatory disease in PSTPIP2-deficient cmo mice. Blood 114: 2497–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitu V, Nacu V, Charles JF, Henne WM, McMahon HT, Nandi S, Ketchum H, Harris R, Nakamura MC, Stanley ER 2012. PSTPIP2 deficiency in mice causes osteopenia and increased differentiation of multipotent myeloid precursors into osteoclasts. Blood 120: 3126–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote JF, Chung PL, Theberge JF, Halle M, Spencer S, Lasky LA, Tremblay ML 2002. PSTPIP is a substrate of PTP-PEST and serves as a scaffold guiding PTP-PEST toward a specific dephosphorylation of WASP. J Biol Chem 277: 2973–2986 [DOI] [PubMed] [Google Scholar]
- Coussens L, Van Beveren C, Smith D, Chen E, Mitchell RL, Isacke CM, Verma IM, Ullrich A 1986. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxy-terminus. Nature 32: 277–280 [DOI] [PubMed] [Google Scholar]
- Csar XF, Wilson NJ, McMahon KA, Marks DC, Beecroft TL, Ward AC, Whitty GA, Kanangasundarum V, Hamilton JA 2001. Proteomic analysis of macrophage differentiation. p46/52(Shc) Tyrosine phosphorylation is required for CSF-1-mediated macrophage differentiation. J Biol Chem 276: 26211–26217 [DOI] [PubMed] [Google Scholar]
- Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER 2002. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99: 111–120 [DOI] [PubMed] [Google Scholar]
- Dai XM, Zong XH, Sylvestre V, Stanley ER 2004. Incomplete restoration of colony-stimulating factor 1 (CSF-1) function in CSF-1-deficient Csf1op/Csf1op mice by transgenic expression of cell surface CSF-1. Blood 103: 1114–1123 [DOI] [PubMed] [Google Scholar]
- Dauffy J, Mouchiroud G, Bourette RP 2006. The interferon-inducible gene, Ifi204, is transcriptionally activated in response to M-CSF, and its expression favors macrophage differentiation in myeloid progenitor cells. J Leukoc Biol 79: 173–183 [DOI] [PubMed] [Google Scholar]
- DiNitto JP, Deshmukh GD, Zhang Y, Jacques SL, Coli R, Worrall JW, Diehl W, English JM, Wu JC 2010. Function of activation loop tyrosine phosphorylation in the mechanism of c-Kit auto-activation and its implication in sunitinib resistance. J Biochem 147: 601–609 [DOI] [PubMed] [Google Scholar]
- Dovas A, Gevrey JC, Grossi A, Park H, Abou-Kheir W, Cox D 2009. Regulation of podosome dynamics by WASp phosphorylation: Implication in matrix degradation and chemotaxis in macrophages. J Cell Sci 122: 3872–3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elegheert J, Desfosses A, Shkumatov AV, Wu X, Bracke N, Verstraete K, Van Craenenbroeck K, Brooks BR, Svergun DI, Vergauwen B, et al. 2011. Extracellular complexes of the hematopoietic human and mouse CSF-1 receptor are driven by common assembly principles. Structure 19: 1762–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elegheert J, Bracke N, Pouliot P, Gutsche I, Shkumatov AV, Tarbouriech N, Verstraete K, Bekaert A, Burmeister WP, Svergun DI, et al. 2012. Allosteric competitive inactivation of hematopoietic CSF-1 signaling by the viral decoy receptor BARF1. Nat Struct Mol Biol 19: 938–947 [DOI] [PubMed] [Google Scholar]
- Faccio R, Takeshita S, Colaianni G, Chappel J, Zallone A, Teitelbaum SL, Ross FP 2007. M-CSF regulates the cytoskeleton via recruitment of a multimeric signaling complex to c-Fms Tyr-559/697/721. J Biol Chem 282: 18991–18999 [DOI] [PubMed] [Google Scholar]
- Felix J, Elegheert J, Gutsche I, Shkumatov AV, Wen YR, Bracke N, Pannecoucke E, Vandenberghe I, Devreese B, Svergun DI, et al. 2013. Human IL-34 and CSF-1 establish structurally similar extracellular assemblies with their common hematopoietic receptor. Structure 21: 528–539 [DOI] [PubMed] [Google Scholar]
- Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD 2007. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: Implications for CSF blockade in inflammation. J Immunol 178: 5245–5252 [DOI] [PubMed] [Google Scholar]
- Foucher ED, Blanchard S, Preisser L, Garo E, Ifrah N, Guardiola P, Delneste Y, Jeannin P 2013. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. Antagonistic effects of GM-CSF and IFNγ. PLoS ONE 8: e56045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangoiti P, Granado MH, Wang SW, Kong JY, Steinbrecher UP, Gómez-Muñoz A 2008. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell Signal 20: 726–736 [DOI] [PubMed] [Google Scholar]
- Gobert Gosse S, Bourgin C, Liu WQ, Garbay C, Mouchiroud G 2005. M-CSF stimulated differentiation requires persistent MEK activity and MAPK phosphorylation independent of Grb2-Sos association and phosphatidylinositol 3-kinase activity. Cell Signal 17: 1352–1362 [DOI] [PubMed] [Google Scholar]
- Golden LH, Insogna KL 2004. The expanding role of PI3-kinase in bone. Bone 34: 3–12 [DOI] [PubMed] [Google Scholar]
- Gómez-Muñoz A, Kong JY, Salh B, Steinbrecher UP 2004. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J Lipid Res 45: 99–105 [DOI] [PubMed] [Google Scholar]
- Gómez-Muñoz A, Kong JY, Parhar K, Wang SW, Gangoiti P, González M, Eivemark S, Salh B, Duronio V, Steinbrecher UP 2005. Ceramide-1-phosphate promotes cell survival through activation of the phosphatidylinositol 3-kinase/protein kinase B pathway. FEBS Lett 579: 3744–3750 [DOI] [PubMed] [Google Scholar]
- Gómez-Muñoz A, Gangoiti P, Granado MH, Arana L, Ouro A 2010. Ceramide-1-phosphate in cell survival and inflammatory signaling. Adv Exp Med Biol 688: 118–130 [DOI] [PubMed] [Google Scholar]
- Grasset MF, Gobert-Gosse S, Mouchiroud G, Bourette RP 2010. Macrophage differentiation of myeloid progenitor cells in response to M-CSF is regulated by the dual-specificity phosphatase DUSP5. J Leukoc 87: 127–135 [DOI] [PubMed] [Google Scholar]
- Greter M, Lelios I, Pelczar P, Hoeffel G, Price J, Leboeuf M, Kundig TM, Frei K, Ginhoux F, Merad M, et al. 2012. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity 37: 1050–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Gu X 2003. Natural history and functional divergence of protein tyrosine kinases. Gene 317: 49–57 [DOI] [PubMed] [Google Scholar]
- Gueller S, Goodridge HS, Niebuhr B, Xing H, Koren-Michowitz M, Serve H, Underhill DM, Brandts CH, Koeffler HP 2010. Adaptor protein Lnk inhibits c-Fms-mediated macrophage function. J Leukoc Biol 88: 699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guery L, Benikhlef N, Gautier T, Paul C, Jego G, Dufour E, Jacquel A, Cally R, Manoury B, Vanden Berghe T, et al. 2011. Fine-tuning nucleophosmin in macrophage differentiation and activation. Blood 118: 4694–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert LJ, Stanley ER 1980. Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J Cell Biol 85: 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert LJ, Stanley ER 1986. The interaction of 125I-colony stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem 261: 4024–4032 [PubMed] [Google Scholar]
- Guilbert LJ, Tynan PW, Stanley ER 1986. Uptake and destruction of 125I-CSF-1 by peritoneal exudate macrophages. J Cell Biochem 31: 203–216 [DOI] [PubMed] [Google Scholar]
- Hampe A, Shamoon BM, Gobet M, Sherr CJ, Galibert F 1989. Nucleotide sequence and structural organization of the human FMS proto-oncogene. Oncogene Res 4: 9–17 [PubMed] [Google Scholar]
- Hanley PJ, Xu Y, Kronlage M, Grobe K, Schon P, Song J, Sorokin L, Schwab A, Bahler M 2010. Motorized RhoGAP myosin IXb (Myo9b) controls cell shape and motility. Proc Natl Acad Sci 107: 12145–12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg HA, Harris JR, Ito T, Daher P, Russell JL, Quarmyne M, Doan PL, Helms K, Nakamura M, Fixsen E, et al. 2012. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep 2: 964–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes SR, Tagoh H, Goonetilleke N, Sasmono T, Oceandy D, Clark R, Bonifer C, Hume DA 2001. A highly conserved c-fms gene intronic element controls macrophage-specific and regulated expression. J Leukoc Biol 70: 812–820 [PubMed] [Google Scholar]
- Hoggan MD, Halden NF, Buckler CE, Kozak CA 1988. Genetic mapping of the mouse c-fms proto-oncogene to chromosome 18. J Virol 62: 1055–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson H, Mograbi B, Schmid-Antomarchi H, Fischer S, Rossi B 1997. CSF-1 stimulation induces the formation of a multiprotein complex including CSF-1 receptor, c-Cbl, PI 3-kinase, Crk-II and Grb2. Oncogene 14: 2331–2338 [DOI] [PubMed] [Google Scholar]
- Huynh D, Dai XM, Nandi S, Lightowler S, Trivett M, Chan CK, Bertoncello I, Ramsay RG, Stanley ER 2009. Colony stimulating factor-1 dependence of paneth cell development in the mouse small intestine. Gastroenterology 137: 136–144, 144 e131–e133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh J, Kwa MQ, Cook AD, Hamilton JA, Scholz GM 2012. CSF-1 receptor signalling from endosomes mediates the sustained activation of Erk1/2 and Akt in macrophages. Cell Signal 24: 1753–1761 [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Choi B, Kang SS, Chang JH, Kim YG, Chung YH, Sohn DH, So MW, Lee CK, Robinson WH, et al. 2012. Interleukin-34 produced by human fibroblast-like synovial cells in rheumatoid arthritis supports osteoclastogenesis. Arthritis Res Ther 14: R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda O, Sekine Y, Kakisaka M, Tsuji S, Muromoto R, Ohbayashi N, Oritani K, Yoshimura A, Matsuda T 2007. STAP-2 regulates c-Fms/M-CSF receptor signaling in murine macrophage Raw 264.7 cells. Biochem Biophys Res Commun 358: 931–937 [DOI] [PubMed] [Google Scholar]
- Ishihara D, Dovas A, Park H, Isaac BM, Cox D 2012. The chemotactic defect in Wiskott-Aldrich syndrome macrophages is due to the reduced persistence of directional protrusions. PLoS ONE 7: e30033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquel A, Benikhlef N, Paggetti J, Lalaoui N, Guery L, Dufour EK, Ciudad M, Racoeur C, Micheau O, Delva L, et al. 2009. Colony-stimulating factor-1-induced oscillations in phosphatidylinositol-3 kinase/AKT are required for caspase activation in monocytes undergoing differentiation into macrophages. Blood 114: 3633–3641 [DOI] [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Belch AR, Jacobs A, Bowen D, Padua RA, Paietta E, Stanley ER 1991. Increased circulating colony-stimulating factor-1 in patients with preleukemia, leukemia, and lymphoid malignancies. Blood 77: 1796–1803 [PubMed] [Google Scholar]
- Jones GE, Allen WE, Ridley AJ 1998. The Rho GTPases in macrophage motility and chemotaxis. Cell Adhes Commun 6: 237–245 [DOI] [PubMed] [Google Scholar]
- Joos H, Trouliaris S, Helftenbein G, Niemann H, Tamura T 1996. Tyrosine phosphorylation of the juxtamembrane domain of the v-Fms oncogene product is required for its association with a 55-kDa protein. J Biol Chem 271: 24476–24481 [DOI] [PubMed] [Google Scholar]
- Junttila I, Bourette RP, Rohrschneider LR, Silvennoinen O 2003. M-CSF induced differentiation of myeloid precursor cells involves activation of PKC-δ and expression of Pkare. J Leukoc Biol 73: 281–288 [DOI] [PubMed] [Google Scholar]
- Kanagasundaram V, Jaworowski A, Hamilton JA 1996. Association between phosphatidylinositol-3 kinase, Cbl and other tyrosine phosphorylated proteins in colony-stimulating factor-1- stimulated macrophages. Biochem J 320: 69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley TW, Graham MM, Doseff AI, Pomerantz RW, Lau SM, Ostrowski MC, Franke TF, Marsh CB 1999. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J Biol Chem 274: 26393–26398 [DOI] [PubMed] [Google Scholar]
- Kheir WA, Gevrey JC, Yamaguchi H, Isaac B, Cox D 2005. A WAVE2-Abi1 complex mediates CSF-1-induced F-actin-rich membrane protrusions and migration in macrophages. J Cell Sci 118: 5369–5379 [DOI] [PubMed] [Google Scholar]
- Krysinska H, Hoogenkamp M, Ingram R, Wilson N, Tagoh H, Laslo P, Singh H, Bonifer C 2007. A two-step, PU.1-dependent mechanism for developmentally regulated chromatin remodeling and transcription of the c-fms gene. Mol Cell Biol 27: 878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laslo P, Spooner CJ, Warmflash A, Lancki DW, Lee HJ, Sciammas R, Gantner BN, Dinner AR, Singh H 2006. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell 126: 755–766 [DOI] [PubMed] [Google Scholar]
- Le Beau MM, Pettenati MJ, Lemons RS, Diaz MO, Westbrook CA, Larson RA, Sherr CJ, Rowley JD 1986. Assignment of the GM-CSF, CSF-1, and FMS genes to human chromosome 5 provides evidence for linkage of a family of genes regulating hematopoiesis and for their involvement in the deletion (5q) in myeloid disorders. Cold Spring Harb Symp Quant Biol 51: 899–909 [DOI] [PubMed] [Google Scholar]
- Lee AW 2011. The role of atypical protein kinase C in CSF-1-dependent Erk activation and proliferation in myeloid progenitors and macrophages. PLoS ONE 6: e25580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AW, States DJ 2000. Both src-dependent and -independent mechanisms mediate phosphatidylinositol 3-kinase regulation of colony-stimulating factor 1-activated mitogen-activated protein kinases in myeloid progenitors. Mol Cell Biol 20: 6779–6798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PS, Wang Y, Dominguez MG, Yeung YG, Murphy MA, Bowtell DD, Stanley ER 1999. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J 18: 3616–3628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AW, Mao Y, Penninger JM, Yu S 2011. Gab2 promotes colony-stimulating factor 1-regulated macrophage expansion via alternate effectors at different stages of development. Mol Cell Biol 31: 4563–4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveugle M, Prat K, Popovici C, Birnbaum D, Coulier F 2004. Phylogenetic analysis of Ciona intestinalis gene superfamilies supports the hypothesis of successive gene expansions. J Mol Evol 58: 168–181 [DOI] [PubMed] [Google Scholar]
- Li W, Stanley ER 1991. Role of dimerization and modification of the CSF-1 receptor in its activation and internalization during the CSF-1 response. EMBO J 10: 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Yeung YG, Stanley ER 1991. Tyrosine phosphorylation of a common 57-kDa protein in growth factor-stimulated and -transformed cells. J Biol Chem 266: 6808–6814 [PubMed] [Google Scholar]
- Li ZH, Spektor A, Varlamova O, Bresnick AR 2003. Mts1 regulates the assembly of nonmuscle myosin-IIA. Biochemistry 42: 14258–14266 [DOI] [PubMed] [Google Scholar]
- Li ZH, Dulyaninova NG, House RP, Almo SC, Bresnick AR 2010. S100A4 regulates macrophage chemotaxis. Mol Biol Cell 21: 2598–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, et al. 2008. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science 320: 807–811 [DOI] [PubMed] [Google Scholar]
- Liu Y, Jenkins B, Shin JL, Rohrschneider LR 2001. Scaffolding protein Gab2 mediates differentiation signaling downstream of Fms receptor tyrosine kinase. Mol Cell Biol 21: 3047–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Leo C, Chen X, Wong BR, Williams LT, Lin H, He X 2012. The mechanism of shared but distinct CSF-1R signaling by the non-homologous cytokines IL-34 and CSF-1. Biochim Biophys Acta 1824: 938–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Elwood F, Britschgi M, Villeda S, Zhang H, Ding Z, Zhu L, Alabsi H, Getachew R, Narasimhan R, et al. 2013. Colony-stimulating factor 1 receptor (CSF1R) signaling in injured neurons facilitates protection and survival. J Exp Med 210: 157–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Lin WY, Chen Y, Stawicki S, Mukhyala K, Wu Y, Martin F, Bazan JF, Starovasnik MA 2012. Structural basis for the dual recognition of helical cytokines IL-34 and CSF-1 by CSF-1R. Structure 20: 676–687 [DOI] [PubMed] [Google Scholar]
- MacDonald KP, Rowe V, Bofinger HM, Thomas R, Sasmono T, Hume DA, Hill GR 2005. The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J Immunol 175: 1399–1405 [DOI] [PubMed] [Google Scholar]
- Mahankali M, Peng HJ, Cox D, Gomez-Cambronero J 2011a. The mechanism of cell membrane ruffling relies on a phospholipase D2 (PLD2), Grb2 and Rac2 association. Cell Signal 23: 1291–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahankali M, Peng HJ, Henkels KM, Dinauer MC, Gomez-Cambronero J 2011b. Phospholipase D2 (PLD2) is a guanine nucleotide exchange factor (GEF) for the GTPase Rac2. Proc Natl Acad Sci 108: 19617–19622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini A, Koch A, Whetton AD, Tamura T 2004. The M-CSF receptor substrate and interacting protein FMIP is governed in its subcellular localization by protein kinase C-mediated phosphorylation, and thereby potentiates M-CSF-mediated differentiation. Oncogene 23: 6581–6589 [DOI] [PubMed] [Google Scholar]
- Marks DC, Csar XF, Wilson NJ, Novak U, Ward AC, Kanagasundarum V, Hoffmann BW, Hamilton JA 1999. Expression of a Y559F mutant CSF-1 receptor in M1 myeloid cells: A role for Src kinases in CSF-1 receptor-mediated differentiation. Mol Cell Biol Res Commun 1: 144–152 [DOI] [PubMed] [Google Scholar]
- McMahon KA, Wilson NJ, Marks DC, Beecroft TL, Whitty GA, Hamilton JA, Csar XF 2001. Colony-stimulating factor-1 (CSF-1) receptor-mediated macrophage differentiation in myeloid cells: A role for tyrosine 559-dependent protein phosphatase 2A (PP2A) activity. Biochemical J 358: 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S, Chen Z, Munoz-Antonia T, Wu J 2005. Participation of both Gab1 and Gab2 in the activation of the ERK/MAPK pathway by epidermal growth factor. Biochem J 391: 143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CJ, Stanley ER 1984. Chemical crosslinking of the mononuclear phagocyte specific growth factor CSF-1 to its receptor at the cell surface. Biochem Biophys Res Commun 119: 35–41 [DOI] [PubMed] [Google Scholar]
- Mossadegh-Keller N, Sarrazin S, Prashanth KK, Espinoza L, Stanley ER, Nutt SL, Moore J, Sieweke MH 2013. M-CSF instructs myeloid lineage fate in single hematopoietic stem cells. Nature 497: 239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchemore KA, Sampaio NG, Murrey MW, Stanley ER, Lannutti BJ, Pixley FJ 2013. Specific inhibition of PI3K p110δ inhibits CSF-1-induced macrophage spreading and invasive capacity. FEBS J 280: 5228–5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munugalavadla V, Borneo J, Ingram DA, Kapur R 2005. p85α subunit of class IA PI-3 kinase is crucial for macrophage growth and migration. Blood 106: 103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JT, Craggs G, Wilson L, Kellie S 2000. Mechanism of phosphatidylinositol 3-kinase-dependent increases in BAC1.2F5 macrophage-like cell density in response to M-CSF: Phosphatidylinositol 3-kinase inhibitors increase the rate of apoptosis rather than inhibit DNA synthesis. Inflamm Res 49: 610–618 [DOI] [PubMed] [Google Scholar]
- Nandi S, Akhter MP, Seifert MF, Dai XM, Stanley ER 2006. Developmental and functional significance of the CSF-1 proteoglycan chondroitin sulfate chain. Blood 107: 786–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, Lin H, Mehler MF, Stanley ER 2012. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol 367: 100–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi S, Cioce M, Yeung YG, Nieves E, Tesfa L, Lin H, Hsu AW, Halenbeck R, Cheng HY, Gokhan S, et al. 2013. Receptor-type protein-tyrosine phosphatase ζ is a functional receptor for interleukin-34. J Biol Chem 288: 21972–21986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataf S, Anginot A, Vuaillat C, Malaval L, Fodil N, Chereul E, Langlois JB, Dumontel C, Cavillon G, Confavreux C, et al. 2005. Brain and bone damage in KARAP/DAP12 loss-of-function mice correlate with alterations in microglia and osteoclast lineages. Am J Pathol 166: 275–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AM, Baker MC, Finch NA, Rutherford NJ, Wider C, Graff-Radford NR, Nelson PT, Clark HB, Wszolek ZK, Dickson DW, et al. 2013. CSF1R mutations link POLD and HDLS as a single disease entity. Neurology 80: 1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Yoshida Y, Itoh M, Fukada T, Ohtani T, Shirogane T, Atsumi T, Takahashi-Tezuka M, Ishihara K, Hibi M, et al. 1999. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood 93: 1809–1816 [PubMed] [Google Scholar]
- Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, Tassi I, Takai T, Stanley SL, Miller M, et al. 2009. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and β-catenin. Nat Immunol 10: 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov DA, DeBats CE, Sester DP, Sweet MJ, Hume DA 2010. A conserved distal segment of the mouse CSF-1 receptor promoter is required for maximal expression of a reporter gene in macrophages and osteoclasts of transgenic mice. J Leukoc Biol 87: 815–822 [DOI] [PubMed] [Google Scholar]
- Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, Parsons JT, Beggs HE, Stanley ER, Bouton AH 2007. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J Cell Biol 179: 1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit J, Bohm A, Jancarik J, Halenbeck R, Koths K, Kim S-H 1992. Three-dimensional structure of dimeric human recombinant macrophage colony-stimulating factor. Science 258: 1358–1362 [DOI] [PubMed] [Google Scholar]
- Papakonstanti EA, Ridley AJ, Vanhaesebroeck B 2007. The p110δ isoform of PI 3-kinase negatively controls RhoA and PTEN. EMBO J 26: 3050–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakonstanti EA, Zwaenepoel O, Bilancio A, Burns E, Nock GE, Houseman B, Shokat K, Ridley AJ, Vanhaesebroeck B 2008. Distinct roles of class IA PI3K isoforms in primary and immortalised macrophages. J Cell Sci 121: 4124–4133 [DOI] [PubMed] [Google Scholar]
- Parichy DM, Turner JM 2003. Temporal and cellular requirements for Fms signaling during zebrafish adult pigment pattern development. Development 130: 817–833 [DOI] [PubMed] [Google Scholar]
- Parichy DM, Rawls JF, Pratt SJ, Whitfield TT, Johnson SL 1999. Zebrafish sparse corresponds to an orthologue of c-kit and is required for the morphogenesis of a subpopulation of melanocytes, but is not essential for hematopoiesis or primordial germ cell development. Development 126: 3425–3436 [DOI] [PubMed] [Google Scholar]
- Parichy DM, Ransom DG, Paw B, Zon LI, Johnson SL 2000. An orthologue of the kit-related gene fms is required for development of neural crest-derived xanthophores and a subpopulation of adult melanocytes in the zebrafish, Danio rerio. Development 127: 3031–3044 [DOI] [PubMed] [Google Scholar]
- Park H, Ishihara D, Cox D 2011. Regulation of tyrosine phosphorylation in macrophage phagocytosis and chemotaxis. Arch Biochem Biophys 510: 101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HJ, Henkels KM, Mahankali M, Marchal C, Bubulya P, Dinauer MC, Gomez-Cambronero J 2011. The dual effect of Rac2 on phospholipase D2 regulation that explains both the onset and termination of chemotaxis. Mol Cell Biol 31: 2227–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pixley FJ 2012. Macrophage migration and its regulation by CSF-1. Int J Cell Biol 2012: 501962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pixley FJ, Stanley ER 2004. CSF-1 regulation of the wandering macrophage: Complexity in action. Trends Cell Biol 14: 628–638 [DOI] [PubMed] [Google Scholar]
- Pixley FJ, Lee PS, Condeelis JS, Stanley ER 2001. Protein tyrosine phosphatase φ regulates paxillin tyrosine phosphorylation and mediates colony-stimulating factor 1-induced morphological changes in macrophages. Mol Cell Biol 21: 1795–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW 2009. Trophic macrophages in development and disease. Nat Rev Immunol 9: 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW, Stanley ER 1996. Pleiotropic roles for CSF-1 in development defined by the mouse mutation osteopetrotic. Adv Dev Biochem 4: 153–193 [Google Scholar]
- Price LKH, Choi HU, Rosenberg L, Stanley ER 1992. The predominant form of secreted colony stimulating factor-1 is a proteoglycan. J Biol Chem 267: 2190–2199 [PubMed] [Google Scholar]
- Rademakers R, Baker M, Nicholson AM, Rutherford NJ, Finch N, Soto-Ortolaza A, Lash J, Wider C, Wojtas A, DeJesus-Hernandez M, et al. 2011. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet 44: 200–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier CW, Chen JH, Roussel MF, Sherr CJ 1985. The product of the c-fms proto-oncogene: A glycoprotein with associated tyrosine kinase activity. Science 228: 320–322 [DOI] [PubMed] [Google Scholar]
- Rettenmier CW, Roussel MF, Ashmun RA, Ralph P, Price K, Sherr CJ 1987. Synthesis of membrane-bound colony-stimulating factor 1 (CSF-1) and downmodulation of CSF-1 receptors in NIH 3T3 cells transformed by cotransfection of the human CSF-1 and c-fms (CSF-1 receptor) genes. Mol Cell Biol 7: 2378–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ 2008. Regulation of macrophage adhesion and migration by Rho GTP-binding proteins. J Microsc 231: 518–523 [DOI] [PubMed] [Google Scholar]
- Rieger MA, Hoppe PS, Smejkal BM, Eitelhuber AC, Schroeder T 2009. Hematopoietic cytokines can instruct lineage choice. Science 325: 217–218 [DOI] [PubMed] [Google Scholar]
- Roberts WM, Look AT, Roussel MF, Sherr CJ 1988. Tandem linkage of human CSF-1 receptor (c-fms) and PDGF receptor genes. Cell 55: 655–661 [DOI] [PubMed] [Google Scholar]
- Roberts WM, Shapiro LH, Ashmun RA, Look AT 1992. Transcription of the human colony-stimulating factor-1 receptor gene is regulated by separate tissue-specific promoters. Blood 79: 586–593 [PubMed] [Google Scholar]
- Rohde CM, Schrum J, Lee AW 2004. A juxtamembrane tyrosine in the colony stimulating factor-1 receptor regulates ligand-induced Src association, receptor kinase function, and down-regulation. J Biol Chem 279: 43448–43461 [DOI] [PubMed] [Google Scholar]
- Rohrschneider LR, Bourette RP, Lioubin MN, Algate PA, Myles GM, Carlberg K 1997. Growth and differentiation signals regulated by the M-CSF receptor. Mol Reprod Dev 46: 96–103 [DOI] [PubMed] [Google Scholar]
- Rothwell VM, Rohrschneider LR 1987. Murine c-fms cDNA: Cloning, sequence analysis and retroviral expression. Oncogene Res 1: 311–324 [PubMed] [Google Scholar]
- Roussel MF, Downing JR, Rettenmier CW, Sherr CJ 1988. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell 55: 979–988 [DOI] [PubMed] [Google Scholar]
- Rousset D, Agnes F, Lachaume P, Andre C, Galibert F 1995. Molecular evolution of the genes encoding receptor tyrosine kinase with immunoglobulinlike domains. J Mol Evol 41: 421–429 [DOI] [PubMed] [Google Scholar]
- Rovida E, Baccarini M, Olivotto M, Sbarba PD 2002. Opposite effects of different doses of MCSF on ERK phosphorylation and cell proliferation in macrophages. Oncogene 21: 3670–3676 [DOI] [PubMed] [Google Scholar]
- Rovida E, Spinelli E, Sdelci S, Barbetti V, Morandi A, Giuntoli S, Dello Sbarba P 2008. ERK5/BMK1 is indispensable for optimal colony-stimulating factor 1 (CSF-1)-induced proliferation in macrophages in a Src-dependent fashion. J Immunol 180: 4166–4172 [DOI] [PubMed] [Google Scholar]
- Salzburger W, Niederstatter H, Brandstatter A, Berger B, Parson W, Snoeks J, Sturmbauer C 2006. Colour-assortative mating among populations of Tropheus moorii, a cichlid fish from Lake Tanganyika, East Africa. Proc Biol Sci 273: 257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio NG, Yu W, Cox D, Wyckoff J, Condeelis J, Stanley ER, Pixley FJ 2011. Phosphorylation of CSF-1R Y721 mediates its association with PI3K to regulate macrophage motility and enhancement of tumor cell invasion. J Cell Sci 124: 2021–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin S, Mossadegh-Keller N, Fukao T, Aziz A, Mourcin F, Vanhille L, Kelly Modis L, Kastner P, Chan S, Duprez E, et al. 2009. MafB restricts M-CSF-dependent myeloid commitment divisions of hematopoietic stem cells. Cell 138: 300–313 [DOI] [PubMed] [Google Scholar]
- Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA 2003. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101: 1155–1163 [DOI] [PubMed] [Google Scholar]
- Sauter KA, Bouhlel MA, O’Neal J, Sester DP, Tagoh H, Ingram RM, Pridans C, Bonifer C, Hume DA 2013. The function of the conserved regulatory element within the second intron of the mammalian Csf1r locus. PLoS ONE 8: e54935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert C, Schalk-Hihi C, Struble GT, Ma HC, Petrounia IP, Brandt B, Deckman IC, Patch RJ, Player MR, Spurlino JC, et al. 2007. Crystal structure of the tyrosine kinase domain of colony-stimulating factor-1 receptor (cFMS) in complex with two inhibitors. J Biol Chem 282: 4094–4101 [DOI] [PubMed] [Google Scholar]
- Seiffert M, Custodio JM, Wolf I, Harkey M, Liu Y, Blattman JN, Greenberg PD, Rohrschneider LR 2003. Gab3-deficient mice exhibit normal development and hematopoiesis and are immunocompetent. Mol Cell Biol 23: 2415–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ 1990. Colony-stimulating factor-1 receptor. Blood 75: 1–12 [PubMed] [Google Scholar]
- Sherr CJ 1991. Mitogenic response to colony-stimulating factor 1. Trends Genet 7: 398–402 [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Rettenmier CW, Sacca R, Roussel MF, Look AT, Stanley ER 1985. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell 41: 665–676 [DOI] [PubMed] [Google Scholar]
- Shim AH, Chang RA, Chen X, Longnecker R, He X 2012. Multipronged attenuation of macrophage-colony stimulating factor signaling by Epstein–Barr virus BARF1. Proc Natl Acad Sci 109: 12962–12967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncic PD, Bourdeau A, Lee-Loy A, Rohrschneider LR, Tremblay ML, Stanley ER, McGlade CJ 2006. T-cell protein tyrosine phosphatase (Tcptp) is a negative regulator of colony-stimulating factor 1 signaling and macrophage differentiation. Mol Cell Biol 26: 4149–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley ER 1979. Colony-stimulating factor (CSF) radioimmunoassay: Detection of a CSF subclass stimulating macrophage production. Proc Natl Acad Sci 76: 2969–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley ER, Heard PM 1977. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem 252: 4305–4312 [PubMed] [Google Scholar]
- Stanley ER, Chen DM, Lin H-S 1978. Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature 274: 168–170 [DOI] [PubMed] [Google Scholar]
- Steinbrecher UP, Gómez-Muñoz A, Duronio V 2004. Acid sphingomyelinase in macrophage apoptosis. Curr Opin Lipidol 15: 531–537 [DOI] [PubMed] [Google Scholar]
- Strockbine LD, Cohen JI, Farrah T, Lyman SD, Wagener F, DuBose RF, Armitage RJ, Spriggs MK 1998. The Epstein–Barr virus BARF1 gene encodes a novel, soluble colony- stimulating factor-1 receptor. J Virol 72: 4015–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist KT, Cecchini MG, Marks SC Jr, 1995. Colony-stimulating factor-1 injections improve but do not cure skeletal sclerosis in osteopetrotic (op) mice. Bone 16: 39. [PubMed] [Google Scholar]
- Suzu S, Ohtsuki T, Yanai N, Takatsu Z, Kawashima T, Takaku F, Nagata N, Motoyoshi K 1992. Identification of a high molecular weight macrophage colony-stimulating factor as a glycosaminoglycan-containing species. J Biol Chem 267: 4345–4348 [PubMed] [Google Scholar]
- Tagoh H, Himes R, Clarke D, Leenen PJ, Riggs AD, Hume D, Bonifer C 2002. Transcription factor complex formation and chromatin fine structure alterations at the murine c-fms (CSF-1 receptor) locus during maturation of myeloid precursor cells. Genes Dev 16: 1721–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagoh H, Schebesta A, Lefevre P, Wilson N, Hume D, Busslinger M, Bonifer C 2004. Epigenetic silencing of the c-fms locus during B-lymphopoiesis occurs in discrete steps and is reversible. EMBO J 23: 4275–4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagoh H, Ingram R, Wilson N, Salvagiotto G, Warren AJ, Clarke D, Busslinger M, Bonifer C 2006. The mechanism of repression of the myeloid-specific c-fms gene by Pax5 during B lineage restriction. EMBO J 25: 1070–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S, Faccio R, Chappel J, Zheng L, Feng X, Weber JD, Teitelbaum SL, Ross FP 2007. c-Fms tyrosine 559 is a major mediator of M-CSF-induced proliferation of primary macrophages. J Biol Chem 282: 18980–18990 [DOI] [PubMed] [Google Scholar]
- Tian Y, Shen H, Xia L, Lu J 2013. Elevated serum and synovial fluid levels of Interleukin-34 in rheumatoid arthritis: Possible association with disease progression via interleukin-17 production. J Interferon Cytokine Res 33: 398–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujita K, Kondo A, Kurisu S, Hasegawa J, Itoh T, Takenawa T 2013. Antagonistic regulation of F-BAR protein assemblies controls actin polymerization during podosome formation. J Cell Sci 126: 2267–2278 [DOI] [PubMed] [Google Scholar]
- Tushinski RJ, Stanley ER 1983. The regulation of macrophage protein turnover by a colony stimulating factor (CSF-1). J Cell Physiol 116: 67–75 [DOI] [PubMed] [Google Scholar]
- Tushinski RJ, Stanley ER 1985. The regulation of mononuclear phagocyte entry into S phase by the colony stimulating factor CSF-1. J Cell Physiol 122: 221–228 [DOI] [PubMed] [Google Scholar]
- Tushinski RJ, Oliver IT, Guilbert LJ, Tynan PW, Warner JR, Stanley ER 1982. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell 28: 71–81 [DOI] [PubMed] [Google Scholar]
- Valledor AF, Xaus J, Marques L, Celada A 1999. Macrophage colony-stimulating factor induces the expression of mitogen-activated protein kinase phosphatase-1 through a protein kinase C-dependent pathway. J Immunol 163: 2452–2462 [PubMed] [Google Scholar]
- Van Nguyen A, Pollard JW 2002. Colony stimulating factor-1 is required to recruit macrophages into the mammary gland to facilitate mammary ductal outgrowth. Dev Biol 247: 11–25 [DOI] [PubMed] [Google Scholar]
- Vedham V, Phee H, Coggeshall KM 2005. Vav activation and function as a rac guanine nucleotide exchange factor in macrophage colony-stimulating factor-induced macrophage chemotaxis. Mol Cell Biol 25: 4211–4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraete K, Savvides SN 2012. Extracellular assembly and activation principles of oncogenic class III receptor tyrosine kinases. Nat Rev Cancer 12: 753–766 [DOI] [PubMed] [Google Scholar]
- Visvader J, Verma IM 1989. Differential transcription of exon 1 of the human c-fms gene in placental trophoblasts and monocytes. Mol Cell Biol 9: 1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holst A, Sirko S, Faissner A 2006. The unique 473HD-Chondroitinsulfate epitope is expressed by radial glia and involved in neural precursor cell proliferation. J Neurosci 26: 4082–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JC, DeKoter RP, Lee HJ, Smith ED, Lancki DW, Gurish MF, Friend DS, Stevens RL, Anastasi J, Singh H 2002. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity 17: 665–676 [DOI] [PubMed] [Google Scholar]
- Walter M, Lucet IS, Patel O, Broughton SE, Bamert R, Williams NK, Fantino E, Wilks AF, Rossjohn J 2007. The 2.7 Å crystal structure of the autoinhibited human c-Fms kinase domain. J Mol Biol 367: 839–847 [DOI] [PubMed] [Google Scholar]
- Wang ZE, Myles GM, Brandt CS, Lioubin MN, Rohrschneider L 1993. Identification of the ligand-binding regions in the macrophage colony–stimulating factor receptor extracellular domain. Mol Cell Biol 13: 5348–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yeung YG, Langdon WY, Stanley ER 1996. c-Cbl is transiently tyrosine-phosphorylated, ubiquitinated, and membrane-targeted following CSF-1 stimulation of macrophages. J Biol Chem 271: 17–20 [DOI] [PubMed] [Google Scholar]
- Wang Y, Berezovska O, Fedoroff S 1999a. Expression of colony stimulating factor-1 receptor (CSF-1R) by CNS neurons in mice. J Neurosci Res 57: 616–632 [PubMed] [Google Scholar]
- Wang Y, Yeung YG, Stanley ER 1999b. CSF-1 stimulated multiubiquitination of the CSF-1 receptor and of Cbl follows their tyrosine phosphorylation and association with other signaling proteins. J Cell Biochem 72: 119–134 [PubMed] [Google Scholar]
- Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M 2012. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol 13: 753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SE, Pollard JW, Jones GE 1996. Direct observation and quantification of macrophage chemoattraction to the growth factor CSF-1. J Cell Sci 109: 793–803 [DOI] [PubMed] [Google Scholar]
- Wei S, Nandi S, Chitu V, Yeung YG, Yu W, Huang M, Williams LT, Lin H, Stanley ER 2010. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J Leukoc Biol 88: 495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CM, Walmsley M, Ooi S, Tybulewicz V, Ridley AJ 2004. Rac1-deficient macrophages exhibit defects in cell spreading and membrane ruffling but not migration. J Cell Sci 117: 1259–1268 [DOI] [PubMed] [Google Scholar]
- Wells CM, Bhavsar PJ, Evans IR, Vigorito E, Turner M, Tybulewicz V, Ridley AJ 2005. Vav1 and Vav2 play different roles in macrophage migration and cytoskeletal organization. Exp Cell Res 310: 303–310 [DOI] [PubMed] [Google Scholar]
- Wheeler AP, Wells CM, Smith SD, Vega FM, Henderson RB, Tybulewicz VL, Ridley AJ 2006. Rac1 and Rac2 regulate macrophage morphology but are not essential for migration. J Cell Sci 119: 2749–2757 [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W, Urbanowska E, Aukerman SL, Pollard JW, Stanley ER, Ralph P, Ansari AA, Sell KW, Szperl M 1991. Correction by CSF-1 of defects in the osteopetrotic op/op mouse suggests local, developmental, and humoral requirements for this growth factor. Exp Hematol 19: 1049–1054 [PubMed] [Google Scholar]
- Williams N, Bertoncello I, Kavnoudias H, Zsebo K, McNiece I 1992. Recombinant rat stem cell factor stimulates the amplification and differentiation of fractionated mouse stem cell populations. Blood 79: 58–64 [PubMed] [Google Scholar]
- Wilson NJ, Cross M, Nguyen T, Hamilton JA 2005. cAMP inhibits CSF-1-stimulated tyrosine phosphorylation but augments CSF-1R-mediated macrophage differentiation and ERK activation. Febs J 272: 4141–4152 [DOI] [PubMed] [Google Scholar]
- Wolf I, Jenkins BJ, Liu Y, Seiffert M, Custodio JM, Young P, Rohrschneider LR 2002. Gab3, a new DOS/Gab family member, facilitates macrophage differentiation. Mol Cell Biol 22: 231–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford J, McAuliffe A, Rohrschneider LR 1988. Activation of the feline c-fms proto-oncogene: Multiple alterations are required to generate a fully transformed phenotype. Cell 55: 965–977 [DOI] [PubMed] [Google Scholar]
- Wu Y, Dowbenko D, Lasky LK 1998. PSTPIP 2, a second tyrosine phosphorylated, cytoskeletal associated protein that binds a PEST-type protein-tyrosine phosphatase. J Biol Chem 273: 30487–30496 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Song D, Cai Y, Yu W, Yeung YG, Stanley ER 2011. A CSF-1 receptor phosphotyrosine 559 signaling pathway regulates receptor ubiquitination and tyrosine phosphorylation. J Biol Chem 286: 952–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X-X, Tessner TG, Rock CO, Jackowski S 1993. Phosphatidylcholine hydrolysis and c-myc expression are in collaborating mitogenic pathways activated by colony-stimulating factor 1. Mol Cell Biol 13: 1522–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Reinherz EL 2006. CD2BP1 modulates CD2-dependent T cell activation via linkage to protein tyrosine phosphatase (PTP)-PEST. J Immunol 176: 5898–5907 [DOI] [PubMed] [Google Scholar]
- Yang Y, Yuzawa S, Schlessinger J 2008. Contacts between membrane proximal regions of the PDGF receptor ectodomain are required for receptor activation but not for receptor dimerization. Proc Natl Acad Sci 105: 7681–7686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Escobedo JA, Kuang WJ, Yang-Fang TL, Daniel TO, Tremble PM, Chen EY, Ando ME, Harkins RN, Francke U, et al. 1986. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature 323: 226–232 [DOI] [PubMed] [Google Scholar]
- Yeung YG, Stanley ER 2003. Proteomic approaches to the analysis of early events in CSF-1 signal transduction. Mol Cell Proteomics 2: 1143–1155 [DOI] [PubMed] [Google Scholar]
- Yeung Y-G, Jubinsky PT, Sengupta A, Yeung DC-Y, Stanley ER 1987. Purification of the colony-stimulating factor 1 receptor and demonstration of its tyrosine kinase activity. Proc Natl Acad Sci 84: 1268–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung Y-G, Wang Y, Einstein DB, Lee PSW, Stanley ER 1998. Colony-stimulating factor-1 stimulates the formation of multimeric cytosolic complexes of signaling proteins and cytoskeletal components in macrophages. J Biol Chem 273: 17128–17137 [DOI] [PubMed] [Google Scholar]
- Yu W, Chen J, Xiong Y, Pixley FJ, Dai XM, Yeung YG, Stanley ER 2008. CSF-1 receptor structure/function in MacCsf1r−/− macrophages: Regulation of proliferation, differentiation, and morphology. J Leukoc Biol 84: 852–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Chen J, Xiong Y, Pixley FJ, Yeung YG, Stanley ER 2012. Macrophage proliferation is regulated through CSF-1 receptor tyrosines 544, 559, and 807. J Biol Chem 287: 13694–13704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzawa S, Opatowsky Y, Zhang Z, Mandiyan V, Lax I, Schlessinger J 2007. Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell 130: 323–334 [DOI] [PubMed] [Google Scholar]
- Zhang B, Ma Y, Guo H, Sun B, Niu R, Ying G, Zhang N 2009. Akt2 is required for macrophage chemotaxis. Eur J Immunol 39: 894–901 [DOI] [PubMed] [Google Scholar]