Abstract
Objective
To find new bioactive natural products, the chemical composition and to sudy the antibacterial activity of essential oil components extracted from the aerial parts of the Algerian aromatic plant Pinus halepensis Miller (P. halepensis) (needles, twigs and buds).
Methods
The essential oil used in this study was isolated by hydrodistillation using a Clevenger-type apparatus according to the European Pharmacopoeia. The chemical composition was investigated using GC-retention indices (RI) and GC-MS.
Results
Forty-nine compounds, representing 97.9% of the total collective oil, were identified. Essential oil was dominated by hydrocarbon compounds (80.6%) especially monoterpenes (65.5%). The major compounds from ten oils stations were: myrcene (15.2%-32.0%), α-pinene (12.2%-24.5%), E-β-caryophyllene (7.0%-17.1%), terpinolene (1.8%-13.3%), 2-phenyl ethyl isovalerate (4.8%-10.9%), terpinene-4-ol (1.0%-8.2 %) and sabinene (1.5%-6.3%). The intra-species variations of the chemical compositions of P. halepensis aerial parts essential oils from ten Algerian sample locations were investigated using statistical analysis. Essential oil samples were clustered in 2 groups by hierarchical cluster analysis, according to their chemical composition. The essential oil revealed an interesting antimicrobial effect against Lysteria monocytogenes, Enterococcus faecalis, Pseudomonas aeruginosa, Acinetobacter baumanii, Citrobacter freundii and Klebsiella pneumoniae.
Conclusions
These results suggest that the essential oil from P. halepensis may be a new potential source as natural antimicrobial applied in pharmaceutical and food industries.
Keywords: Pinus halepensis Miller, Essential oils, GC/MS, Chemical variability, Antimicrobial activity
1. Introduction
The genus Pinus belongs to the family Pinaceae and comprises about 250 species. It is the largest genus of conifers occurring naturally in the northern hemisphere, especially in the Mediterranean region, Caribbean area, Asia, Europe, North and Central American. The genus Pinus has been planted in the temperate regions of the southern hemisphere. They are evergreen and resinous trees growing to 3-80 m tall with needle-like gray-green leaves that grow in pairs[1]–[3].
The medicinal and aromatic properties of the chemical compounds (e.g., turpentine, resins and essential oil….) of pine make it one of the most popular plants throughout all civilization. Pine is also still widely used in traditional therapeutic practice in world and has economic importance[4],[5]. In the Northern Mediterranean basin, Pinus halepensis Miller (P. halepensis) is a pioneer and expansionist species that colonizes abandoned agricultural lands characterized by high biodiversity. Owing to its richness of secondary metabolites, P. halepensis may play an important role in plant succession through several processes. For example, secondary compounds (terpenoids and/or phenolic compounds) can affect root symbionts and site quality, by interfering with decomposition, mineralization, and humification[6],[7]. P. halepensis may inhibit seedling establishment of various species in pine stands, suggesting the allelopathic nature of litter, leaf leachates, and/or root exudates[8],[9].
P. halepensis seeds are traditionally used throughout Tunisia and other Arabic countries, for preparing a sweet pudding of group pine seeds, called “Assida-Zgougou”. Recently, it has been employed as an ingredient in ice-creams and candies[10]. Essential oils from Pinus species have been reported to have various therapeutic properties. They are also used as fragrances in cosmetics, flavoring additives for food and beverages, scenting agents in a variety of household products and intermediates in the synthesis of perfume chemicals[1],[2],[4],[11]. Several phytochemical analyses of P. halepensis have been published on terpenes[12],[13], turpentine[14] and phenolic compounds[15]. The literature reports some works on the chemical composition of P. halepensis essential oil from Italy[6],[16], Algeria[5],[17]–[19], Greece[20], Morocco[21] and Turkey[22],[23]. Various compositions have been reported.
The first aim of this study was to elucidate the composition of P. halepensis essential oil using a combination of GC and GC/MS. The second aim was to characterize the intra-species variation in essential oil composition in natural populations using 10 oil samples from different locations of Algeria and to evaluate the antibacterial activity of essential oil.
2. Materials and methods
2.1. Plant Material
The aerial parts of P. halepensis (needles, twigs and buds) were collected in January 2012 from 10 locations from Tlemcen. The plant material was botanically identified by Prof. Noury Benabadji (Laboratory of Ecology and Ecosystem Management of University of Tlemcen, Algeria). Voucher specimens were deposited in the herbarium of the University of Tlemcen. Each fresh aerial part (400-500 g) was submitted to hydrodistillation for 5 h using a Clevenger-type apparatus according to the European Pharmacopoeia[24].
2.2. GC analysis
GC analyses were carried out using a Perkin Elmer Clarus 600 GC apparatus equipped with a dual flame ionization detection system and two fused-silica capillary columns (60 m×0.22 mm I.D., film thickness 0.25 µm), Rtx-1 (polydimethylsiloxane) and Rtx-Wax (polyethylenglycol). The oven temperature was programmed from 60 °C to 230 °C at 2 °C/min and then held isothermally at 230 °C for 35 min. Injector and detector temperatures were maintained at 280 °C. Samples were injected in the split mode (1/50), using helium as the carrier gas (1 mL/min); the injection volume was 0.2 µL. Retention indices (RI) of the compounds were determined from a software from Perkin-Elmer. Component relative concentrations were calculated based on GC peak areas without using correction factors.
2.3. GC-MS analysis
Samples were analyzed with a Perkin-Elmer Turbo mass detector (quadrupole), coupled to a Perkin-Elmer Autosystem XL, equipped with the fused-silica capillary columns Rtx-1 and Rtx-Wax (ion source temperature 150 °C; energy ionization 70 eV). EI mass spectra were acquired over the mass range 35-350 Da (scan time: 1 second). Other GC conditions were the same as described under GC except split 1/80.
2.4. Component identification
Identification of the components was based (i) on the comparison of their GC retention indices (RI) on non polar and polar columns, determined relative to the retention time of a series of n-alkanes with linear interpolation, with those of authentic compounds or literature data[25],[26] and (ii) on computer matching with commercial mass spectral libraries[27]–[29] and comparison of spectra with those of our laboratory-made library.
2.5. Statistical analysis
Data analyses were performed using principal component analysis (PCA) and cluster analysis (CA)[30]. Both methods aim at reducing the multivariate space in which objects (oil samples) are distributed but are complementary in their ability to present results[31]. Indeed, PCA provides the data for diagrams in which both objects (oil samples) and variables (oil components) are plotted while canonical analysis informs a classification tree in which objects (sample locations) are gathered. PCA was carried out using function ‘PCA’ from the statistical R software.
The variables (volatile components) have been selected using function from the statistical software. The cluster analysis produced a dendrogram (tree) using the Ward's method of hierarchical clustering, based on the Euclidean distance between pairs of oil samples.
2.6. Antimicrobial activity
2.6.1. Test microorganisms
Antibacterial activity of P. halepensis essential oil was tested against 11 strains of bacteria: Gram-positive bacteria: Staphylococcus aureus ATCC 25923 (S. aureus), Bacillus cereus ATCC 10876 (B. cereus), Enterococcus faecalis ATCC 49452 (E. faecalis), Lysteria monocytogenes ATCC 15313 (L. monocytogenes) and Gram-negative bacteria: Pseudomonas aeruginosa ATCC 27853 (P. aeruginosa), Escherichia coli ATCC 25922 (E. coli), Salmonella typhimurium ATCC 13311, Acinetobacter baumanii ATCC 19606, Citrobacter freundii ATCC 8090, Proteus mirabilis ATCC 35659, Klebsiella pneumoniae ATCC 700603 (K. pneumoniae). The microorganisms were obtained from Pasteur Institute of Paris.
2.6.2. Paper-disc diffusion method
Antibacterial activity was tested by the agar-well diffusion method[32],[33]. All bacterial cultures were first grown on MHI agar (Muller-Hinton infusion) plates at 37 °C for 18-24 h prior to inoculation onto the nutrient agar. One or several colonies of similar morphology of the respective bacteria were transferred into API suspension medium (Biomérieux) and adjusted to 0.5 McFarland turbidity standard with a Densimat (Biomérieux). The inoculums of the respective bacteria were streaked onto MHI agar plates using a sterile swab. A sterile filter disc (diameter 6 mm, Whatman paper No.3) was placed. The disc was impregnated by the tested essential oils (10 µL/disc). The treated Petri dishes were placed at 4 °C for 1-2 h and then incubated at 37 °C for 24 h. Antibacterial activity was evaluated by measuring the zone of growth inhibition around the discs after 24 h of incubation at 37 °C. The diameter of the zones of inhibition around each of the discs was taken as measure of the antibacterial activity. Each experiment was carried out in triplicate and the mean diameter of the inhibition zone was recorded. The scale of measurement was as follows[34] (disc diameter included): ≥ 8 mm: good activity; 7.5-7.9 mm: average activity; 7-7.4 mm: moderate activity; 6.5-6.9: low activity; ≤ 6.4 mm: no activity.
3. Results
3.1. Sample location and oil yields
We regrouped in Table 1 the major components of P. halepensis essential oils reported in literature. Various compositions have been reported, characterized by the occurrence of monoterpenes, sesquiterpenes and phenylpropanoids compounds.
Table 1. Main components of the essential oils of P. halepensis from different origins previously reported.
| Plant origin Sites Extraction modes |
Algeria[5],[17]–[19] |
Greece[20] |
Italy[6],[16] |
Morocco[21] |
Turkey[22],[23] | ||||||||||||
| Ghazaouet | Saïda | Sidi Feradj | Tissemsilt | Djelfa | HD | HD | HD | HD | HD | HD | HD | ||||||
| HD | HD | HD | HD | HD | |||||||||||||
| No | Compounds | % | % | % | % | % | % | % | % | % | % | % | % | ||||
| 2 | α-Pinene | nd | 6.4 | 1.2 | 6.7 | 17.6 | 13.4 | 18.1 | 8.5 | 23.3 | 47.1 | 18.4 | 16.4 | ||||
| 4 | Sabinene | nd | 0.7 | 1.2 | 7 | 2.6 | 1.3 | 9.4 | 6.1 | 3.7 | nd | 0.1 | 0.6 | ||||
| 5 | β-Pinene | nd | 5.6 | 0.2 | 2.0 | 1.6 | 1.1 | 2.0 | 1.1 | 3.1 | 2.8 | 46.8 | 18.7 | ||||
| 6 | Myrcene | nd | 0.5 | 3.1 | 8.7 | 3.2 | 6.6 | 27.9 | 12.5 | 16.3 | 6.3 | 1.3 | 3.8 | ||||
| 8 | 3-Carene | nd | 0.4 | 0.2 | 0.1 | 1.9 | 6.9 | 1.7 | 1 | nd | 1.7 | 0.9 | 16.3 | ||||
| 10 | p-Cymene | nd | nd | nd | 0.3 | 3 | nd | 1.1 | 11.4 | 0.7 | 0.4 | tr | 0.1 | ||||
| 12 | Limonene | nd | 0.1 | tr | 0.8 | 0.1 | 5.0 | 1.1 | 1 | 1.3 | 0.8 | 2.3 | 18.7 | ||||
| 16 | Terpinolene | nd | 2.4 | nd | nd | nd | nd | nd | nd | nd | nd | 0.3 | 1.8 | ||||
| 21 | Terpinen-4-ol | 0.4 | 0.6 | tr | nd | 0.6 | 0.7 | nd | nd | 3.8 | nd | nd | nd | ||||
| 22 | α-Terpinolene | nd | nd | 0.1 | 0.2 | tr | 3.1 | 9.9 | nd | 10.1 | 1 | nd | nd | ||||
| 28 | E-β-Caryophyllene | 3 | nd | nd | 7.1 | 2.7 | nd | nd | nd | nd | 11.2 | 9.2 | 9.5 | ||||
| 29 | α-Humulene | 0.74 | 10.5 | 7.9 | 2.8 | 1.4 | 3.4 | 2.9 | nd | 3.2 | 2.7 | 1.8 | 1.8 | ||||
| 30 | 2-Phenylethyl isovalerate | nd | nd | nd | 7.4 | 8.4 | nd | nd | nd | nd | nd | nd | nd | ||||
| 31 | Germacrene D | nd | 0.8 | 0.5 | 0.2 | tr | 0.5 | 0.1 | nd | nd | tr | 8.8 | 1.5 | ||||
| 37 | Caryophyllene oxide | 48.2 | nd | nd | nd | nd | nd | 0.1 | nd | 1.2 | 7.8 | 0.4 | 0.4 | ||||
| 44 | Bulnesol | nd | nd | nd | nd | nd | 7.6 | nd | nd | nd | nd | nd | nd | ||||
| Z-β-Caryophyllene | nd | 25 | 40.31 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |||||
| Aromadendrene | nd | 5.4 | 7.1 | nd | nd | nd | nd | nd | nd | nd | nd | nd | |||||
| Humulene oxide | 6.7 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | |||||
| Thumbergol | 8.3 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | |||||
Only the main components were reported; main components are classed by number corresponding to the Table 1; only one sample was studied. Extraction mode: HD: Hydrodistillation; tr: trace (<0.05%); nd: compounds not detetcted.
In our study, the aerial parts of P. halepensis were collected from 10 locations in West Northern of Algeria. Some information concerning the 10 harvest areas (origins, latitudes, longitudes and essential oil yield) were tabulated in Table 2.
Table 2. Essential oils yields, origins, latitudes and longitudes of ten Algerian P. halepensis.
| Areas | Samples | Regions | Latitudes | Longitudes | Altitudes | Essential oil yields (%) | |
| Littoral | Area 1 | S1 | Nedroma | 34° 52′ 38″ | 1°14′ 25″ | 650 m | 0.14 |
| S2 | Hwanet | 34° 58′ 13″ | 1°48′ 10″ | 650 m | 0.20 | ||
| S3 | Oued Tlala | 35° 03′ 51″ | 1°44′ 59″ | 197 m | 0.40 | ||
| S4 | Bab Taza | 34° 58′ 13″ | 1° 45′ 16″ | 662 m | 0.22 | ||
| S5 | Bab El Assa | 34° 58′ 08″ | 2° 01′ 40″ | 537 m | 0.63 | ||
| S6 | Sidi youchaa | 35° 06′ 55″ | 1° 46′ 47″ | 111 m | 0.30 | ||
| S10 | Ghazaouet | 35° 05′ 59″ | 1° 50′ 59″ | 118 m | 0.40 | ||
| Mountain | Area 2 | S7 | Agadir | 34° 53′ 21″ | 1° 18′ 06″ | 914 m | 0.13 |
| S8 | Amieur | 35° 01′ 59″ | 1° 15′ 20″ | 706 m | 0.14 | ||
| S9 | Mansourah | 34° 52′ 27″ | 1° 19′ 11″ | 983 m | 0.35 | ||
The sample locations were distributed in two areas. Area 1 was considered as littoral zone near to the Mediterranean Sea, while area 2 was a Mountain zone with altitudes up to 700 m. Area 2 has a warm and sub-humid climate while the soil of area 1 is red fersiallitic with vertic character. The yield of essential oils obtained from fresh aerial part in the ten locations of P. halepensis ranged from 0.13% to 0.63% and more precisely it is noticeable that higher yields (0.20% to 0.63%) were linked to sample oils from area 1 while lower yields (0.13% to 0.35%) were linked to sample oils from area 2 (Table 2). Volatile oil yield of P. halepensis in different parts from Algeria had similar results. Values of 0.3%, 0.52%, 0.8% and 0.9% (in dry weight basis) were found in the Ghazaouet, Saida, Djelfa and Sidi Fredj, respectively[5],[17]–[19].
3.2. Chemical analysis of P. halepensis essential oils
Chemical composition of P. halepensis oils from 10 samples were studied using GC and GC/MS (Table 3). Fourty nine compounds, which accounted for 97.9% of oil, were isolated.
Table 3. Chemical composition of P. halepensis essential oils from West Northern of Algeria.
| Compounds | IRa LIT | IRb app | IRc pol | Collective Oild Simple Oilse |
Identification | ||
| % | %Min | %Max | |||||
| α-Thujene | 922 | 923 | 1021 | 0.7 | 0.4 | 1.0 | RI, MS |
| α-Pinene | 931 | 932 | 1023 | 16.8 | 12.2 | 24.5 | RI, MS |
| Camphene | 943 | 944 | 1066 | 0.2 | 0.1 | 0.3 | RI, MS |
| Sabinene | 964 | 966 | 1118 | 4.2 | 1.5 | 6.3 | RI, MS |
| β-Pinene | 970 | 971 | 1108 | 1.9 | 1.7 | 2.2 | RI, MS |
| Myrcene | 970 | 983 | 1159 | 25.2 | 15.2 | 32.0 | RI, MS |
| α-Phelandrene | 997 | 998 | 1157 | 0.1 | nd | 0.2 | RI, MS |
| 3-Carene | 1005 | 1006 | 1147 | 1.6 | 0.6 | 5.5 | RI, MS |
| α-Terpinene | 1008 | 1010 | 1175 | 0.9 | 0.1 | 1.8 | RI, MS |
| p-Cymene | 1010 | 1012 | 1259 | 0.6 | 0.2 | 1.8 | RI, MS |
| β-Phelandrene | 1021 | 1021 | 1204 | 0.4 | 0.7 | 1.4 | RI, MS |
| Limonene | 1020 | 1021 | 1195 | 0.9 | 0.6 | 1.4 | RI, MS |
| Z-β-Ocimene | 1024 | 1025 | 1225 | 0.4 | nd | 1.4 | RI, MS |
| E-β-ocimene | 1034 | 1036 | 1241 | 1.4 | 0.52 | 3.4 | RI, MS |
| γ-Terpinene | 1047 | 1049 | 1237 | 1.4 | 0.2 | 2.6 | RI, MS |
| Terpinolene | 1078 | 1082 | 1247 | 8.3 | 1.8 | 13.8 | RI, MS |
| Linalool | 1080 | 1084 | 1529 | 0.4 | 0.1 | 0.8 | RI, MS |
| Perillene | 1090 | 1099 | 1414 | 0.1 | tr | 0.1 | RI, MS |
| Cis-p-menth-2-en-1-ol | 1108 | 1107 | 1600 | 0.2 | 0.1 | 0.3 | RI, MS |
| Trans-p-menth-2-en-1-ol | 1113 | 1117 | 1612 | 0.1 | tr | 0.2 | RI, MS |
| Terpinene-4-ol | 1161 | 1164 | 1583 | 4.2 | 1.0 | 8.2 | RI, MS |
| α-Terpinolene | 1179 | 1175 | 1688 | 0.4 | tr | 0.7 | RI, MS |
| Bornyl acetate | 1269 | 1268 | 1475 | 0.1 | tr | 0.3 | RI, MS |
| Citronellyl acetate | 1331 | 1333 | 1645 | 0.1 | tr | 0.1 | RI, MS |
| Neryl acetate | 1342 | 1342 | 1409 | 0.1 | 0.1 | 0.2 | RI, MS |
| Geranyl acetate | 1361 | 1360 | 1740 | 0.2 | tr | 0.5 | RI, MS |
| α-Copaene | 1379 | 1373 | 1475 | 0.2 | 0.1 | 0.3 | RI, MS |
| E-β-Caryophyllene | 1424 | 1418 | 1583 | 10.9 | 7.0 | 17.1 | RI, MS |
| α-Humulene | 1456 | 1449 | 1651 | 2.1 | 1.3 | 3.4 | RI, MS |
| 2-Phenylethyl isovanerate | 1463 | 1468 | 1973 | 7.7 | 4.8 | 10.9 | RI, MS |
| Germacrene D | 1480 | 1474 | 1692 | 0.2 | 0.1 | 0.2 | RI, MS |
| α-muurolene | 1496 | 1492 | 1709 | 0.2 | tr | 0.6 | RI, MS |
| δ-Cadinene | 1516 | 1513 | 1738 | 0.3 | tr | 0.5 | RI, MS |
| E-α-Bisabolene | 1532 | 1532 | 1740 | 0.2 | 0.1 | 0.3 | RI, MS |
| Phenylethyl Tiglate E | 1547 | 1546 | 2141 | 0.1 | nd | 0.2 | RI, MS |
| Phenylethyl Tiglate Z | 1559 | 1568 | 2145 | 0.8 | tr | 3.3 | RI, MS |
| Caryophyllene oxide | 1576 | 1583 | 1898 | 0.8 | 0.2 | 2.2 | RI, MS |
| Guaiol | 1591 | 1592 | 2070 | 0.2 | 0.1 | 0.5 | RI, MS |
| Humulene epoxyde | 1601 | 1613 | 2035 | 0.1 | tr | 0.1 | RI, MS |
| Epi-Cubenol | 1624 | 1625 | 2043 | 0.1 | 0.1 | 0.2 | RI, MS |
| Tau-Cadinol | 1632 | 1633 | 2163 | 0.2 | tr | 0.1 | RI, MS |
| T-Muurolol | 1634 | 1638 | 2141 | 0.1 | 0.1 | 0.3 | RI, MS |
| α-Cadinol | 1645 | 1640 | 2163 | 0.2 | tr | 0.3 | RI, MS |
| Bulnesol | 1659 | 1666 | 2195 | 0.1 | nd | 0.1 | RI, MS |
| Cembrene | 1938 | 1940 | 2185 | 0.4 | 0.1 | 1.6 | RI, MS, Ref. |
| m-Camphorene | 1947 | 1939 | 2234 | 0.2 | nd | 0.4 | RI, MS, Ref. |
| Cembrene A | 1962 | 1951 | 2227 | 0.1 | nd | 0.3 | RI, MS, Ref. |
| p-Camphorene | 1980 | 1974 | 1987 | 0.3 | nd | 1.1 | RI, MS, Ref. |
| Geranyl Linalool | 2037 | 2037 | 2540 | 1.5 | nd | 3.0 | RI, MS |
| Total Identification % | 97.9 | ||||||
| Yields % (w/w) | 0.26 | 0.10 | 0.63 | ||||
| Hydrocarbon compounds | 80.6 | ||||||
| Monoterpene hydrocarbons | 65.5 | ||||||
| Sesquiterpene hydrocarbons | 14.1 | ||||||
| diterpenic hydrocarbons | 1.0 | ||||||
| Oxygenated compounds | 17.3 | ||||||
| Oxygenated monoterpenes | 5.4 | ||||||
| Oxygenated sesquiterpenes | 1.8 | ||||||
| Oxygenated diterpenes | 1.5 | ||||||
| Non-terpenic oxygenated compounds | 8.6 | ||||||
a: Retention indices of literature on the apolar column (/RILit) reported from König et al., 2001; b: Retention indices on the apolar Rtx-1 column; c: Retention indices on the polar Rtx-Wax column; d: Normalized percentages abundance are given on the apolar column except for components with identical RI (percentages are given on the polar column), tr=trace (<0.05%). e: Minimum and maximum normalized percentages abundance from simple oils; RI: Retention indices; MS: Mass spectra in electronic impact mode; Ref.: compounds identified from literature data: König et al., 2001).
3.3. Chemical variation of P. halepensis essential oils
To identify possible relationships between volatile compound abundances and geographical origins, PCA and CA were applied to a matrix linking essential oil compositions to sample locations. The data mentioned in Table 4 and presented in Figures 1 and 2 were obtained from the correlation matrix and the standardized matrix.
Table 4. Clustering of P. halepensis oils samples by statistical analysis.
| Components | Group I (S2-S6, S10) |
Group II (S1-S7-9) |
|||
| Rangeb | Averageb | Rangeb | Averageb | ||
| Monoterpene hydrocarbons | 54.39 | 54.55 | |||
| α-Pinene | 12.2-14.4 | 13.4 | 18.0-24.5 | 21.8 | |
| Sabinene | 3.5-6.3 | 5.52 | 1.5-3.0 | 2.20 | |
| Myrcene | 15.2-28.5 | 23.94 | 24.1-32.0 | 27.15 | |
| Terpinolene | 9.4-13.8 | 11.53 | 1.8-5.8 | 3.42 | |
| Oxygenated monoterpenes | 5.78 | 1.85 | |||
| Terpinene-4-ol | 3.9-8.2 | 5.78 | 1.0-2.5 | 1.85 | |
| Monoterpene sesquiterpenes | 8.78 | ||||
| E-β-Caryophyllene | 7.0-11.1 | 8.78 | 11.0-17.1 | 14.15 | |
| Non-terpenic compounds | 10.1 | 5.76 | |||
| 2-Phenylethyl isovanerate | 8.7-10.9 | 10.1 | 4.8-7.0 | 5.76 | |
b Normalized % abundances.
Figure 1. PCA of chemical compositions of P. halepensis oils from Algeria.
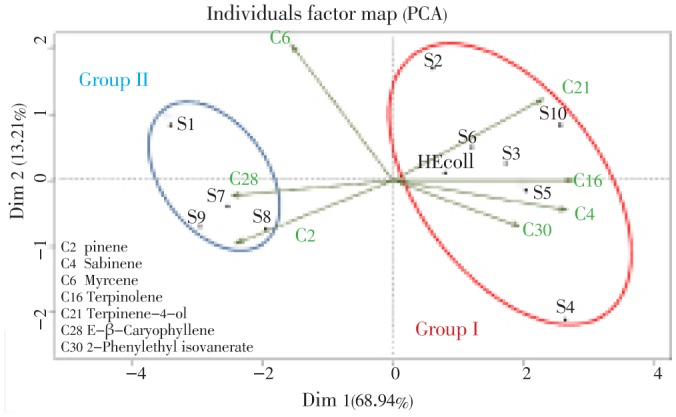
Figure 2. Cluster Analysis of chemical compositions of P. halepensis from Algeria.

3.4. Antimicrobial activity
The antibacterial activity of P. halepensis essential oil originating from the West Northern of Algeria was evaluated by paper disc diffusion method against 11 bacteria. Table 5 showed that oil has a variable antibacterial activity (8-10 mm) against tested strains. The maximum zone of inhibition was recorded against L. monocytogenes (10 mm), K. pneumoniae (10 mm), E. faecalis (9 mm) and Acinetobacter baumanii (9.5 mm). Other hand, the oil was ineffective against S. aureus, B. cereus, E. coli, Salmonella typhimurium and Proteus mirabilis. According to Sheng-Hsien[34], the essential oil of P. halepensis showed good inhibitory effects on some tested microorganisms.
Table 5. Antibacterial activity of P. halepensis essential oils from the West Northern of Algeria.
| Microorganisms | Diameters of inhibition (mm) |
| Gram-positive bacteria | |
| S. aureus | n.a |
| B. cereus | n.a |
| E. faecalis | 9.0 |
| L. monocytogenes | 10.0 |
| Gram-negative bacteria | |
| P. aeruginosa | 8.0 |
| E. coli | n.a |
| Salmonella typhimurium | n.a |
| Acinetobacter baumanii | 9.5 |
| Citrobacter freundii | 8.0 |
| Proteus mirabilis | n.a |
| Klebsiella pneumoniae | 10.0 |
Essential oil (10 µL/disc) of aerial part of P. halepensis; n.a: not active.
4. Discussion
The chromatographic profile of essential oil from P. halepensis showed that oils are constituted of 26 monoterpenes, 16 sesquiterpenes, 4 diterpenes and 3 non-terpenic compounds. The oils are mainly composed by hydrocarbon compounds that accounted for 80.6%. The main components were myrcene (25.2%), α-pinene (16.8%), E-β-caryophyllene (10.9%) and terpinoplene (8.3%). However, the oxygenated compounds have the lowest percentage (17.3%), most of them being non-terpenic (8.6%) and monoterpenes oxygenated (5.4%) represented by 2-phenylethyl isovanerate (7.7%) and terpinene-4-ol (4.2%). From a chemotaxonomic viewpoint, it should be noted that P. halepensis essential oils are qualitatively similar to those of literature but differ in the amounts of the major components. Indeed, several reports on the composition of oils of other Pinus species revealed that monoterpene hydrocarbons were the major constituent in the most of the oils; they often constituted 50% or more of the oil[6],[21].
Although the 10 essential oils contained similar types of compounds, there were significant differences in the concentrations of the major components. For instance, the concentrations of α-pinene (C2), sabinene (C4), myrcene (C6), terpinolene (C16), terpinene-4-ol (C21), E-β-caryophyllene (C28) and 2-phenylethyl isovanerate (C30) ranged from 12.2% to 24.5% of oil, from 15.2% to 32.0% of oil, from 1.8% to 13.8% of oil, from 1.0% to 8.2% of oil, from 7.0% to 17.1% of oil and from 4.8% to 10.9% of oil, respectively. The principal factorial plane accounts for 93.56% of the chemical essential oils variance. The F1 axis (68.94%) are positively correlated with oxygenated sesquiterpenes (C4, C16, C21 and C30) and negatively correlated with E-β-caryophyllene (C28) and 2-phenylethyl isovanerate (C30). The plot established using the first two axes suggests that there are two main groups of P. halepensis oils. The first group (I) includes oil samples from 6 localities (S2-S6, S10), characterized by more high levels of myrcene C6 (15.2-28.5% of oil), terpinolene C16 (9.4%-13.8% of oil), 2-phenylethyl isovanerate C30 (8.7%-10.9% of oil), sabinene C4 (3.5%-6.3% of oil) and terpinene-4-ol C21 (3.9%-8.2% of oil). The group II includes 4 oil samples (S1, S7-9) was characterized by a high content of α-pinene C2 (18.0%-24.5% of oil), myrcene C6 (24.1%-32.0% of oil) and E-β-caryophyllene C28 (11.0%-17.1% of oil). However, statistical analysis clustered the essential oil samples into two distinct groups linked to the origin of harvest. Group I consisted of oils rich in α-pinene, myrcene, terpinolene and 2-phenylethyl isovanerate, originated from littoral zone (Area 1) and group II consisted of oils rich in α-pinene, myrcene and E-β-caryophyllene, originated from mountains of Tlemcen (Area 2). These results suggested that variation in the compositions of essential oils among populations can be attributed to the growing conditions and environmental factors.
The essential oils of P. halepensis showed good inhibitory effects on some tested microorganisms. It would be related to their oxygenated monoterpenes components which constitute more than 16.2% of the oil. The antibacterial activity of essential oil of P. halepensis from Ghazaouet (West Northern of Algeria) was evaluated against four strains of bacteria: S. aureus, P. aeruginosa, E. coli and B. cereus, using disc diffusion method. The essential oil showed a strong activity against S. aureus and B. cereus. Contrary, the oil was ineffective against P. aeruginosa and E. coli[35]. However, it is difficult to attribute the activity of a complex mixture to a single or particular constituent. Secondly there is some evidence that minor components have a critical part to play in antibacterial activity, possibly by producing a synergistic effect between other components[36],[37]. The variation in chemical composition of essential oil might be responsible for the different antibacterial activities.
In conclusion, the comparison of our results with literature shows considerable qualitative and quantitative difference in yields and composition of P. halepensis oils. The variability in oil composition is present even in P. halepensis and these variations, sufficient to allow the distinction of different chemotypes, are the results of an adaptive process to particular ecologic conditions (geographical regions, climate conditions, altitude), period of collection of the plant, studied parts of plant, state of plant (fresh or dry) and method of extraction of the essential oil. Bioassay screening of oil showed an activity against L. monocytogenes and K. pneumoniae. The results of the current study have shown that essential oil of P. halepensis is potentially a good source of antimicrobial compounds.
Acknowledgments
The authors are thankful to Professor Noury Benabadji of the Botanical Laboratory, Biology Department, Abou Bekr Belkaïd University for the identification of the vegetable matter. This study was supported by the University of Tlemcen and the Ministry of Higher Education and Scientific Research of the Algerian People's Democratic Republic for the granting of financial assistance under the project CNEPRU (Ref. E02020100067/2011-2013).
Comments
Background
Plant-derived essential oils have long served as flavoring agents in foods and beverages, and due to their versatile content of antimicrobial compounds, they possess potential as natural agents for food preservation. The antimicrobial activity of essential oils is assigned to a number of small terpenoid and phenolic compounds, which also in pure form have been shown to exhibit antibacterial activity. Essential oils are known to be active against a wide variety of microorganisms, including Gram-positive and Gram-negative bacteria. This study was conducted to determine the antibacterial activity of P. halepensis essential oils against bacteria.
Research frontiers
The data obtained from the present experiments show a new application of essential oil from aerial parts of P. halepensis which is in agreement with its use in traditional medicine.
Related reports
In this present investigation, the authors have followed standard protocols to assess the antimicrobial activity of essential oil from P. halepensis growing in Algeria. The results suggest that the essential oil from P. halepensis may be a new potential source as natural antimicrobial applied in pharmaceutical and food industries.
Innovations & breakthroughs
To my knowledge, there is no work for antimicrobial activity of essential oil from aerial parts of P. halepensis growing in West Northern of Algeria. The present report serves as the first hand information on the fact that this plant is potentially a good source of antimicrobial compounds.
Applications
There is strong interest in the use of naturally occurring compounds which have antibacterial activity for preservation of minimally processed foods. Plant essential oils are a potential source of antimicrobials of natural origin. Essential oils have been evaluated for their effects on the growth of food spoilage and foodborne pathogenic microorganisms, including Gram-positive and Gram-negative bacteria. Possible use of essential oils as food preservatives has been studied.
Peer review
Essential oils from P. halepensis growing in Algeria have been reported to have various therapeutic properties. They are also used as fragrances in cosmetics, flavoring additives for food and beverages. In this study, the authors provide evidence of its main antimicrobial activity.
Footnotes
Foundation Project: Supported by the University of Tlemcen and the Ministry of Higher Education and Scientific Research of the Algerian People's Democratic Republic for the granting of financial assistance under the project CNEPRU (Ref. E02020100067/2011-2013).
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- 1.Fuentes JL, Vernhe M, Cuetava EB, Sanchez-Lamar A, Santana JL, Llagostera M. Tannins from barks of Pinus caribaea protect Escherichia coli cells against DNA damage induced by gamma-rays. Fitoterapia. 2006;77(2):116–120. doi: 10.1016/j.fitote.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Kozan E, Kupeli E, Yesilda E. Evaluation of some plants used in Turkish folk medicine against parasitic infections for their in vivo anthelmintic activity. J Ethnopharmacol. 2006;108(2):211–216. doi: 10.1016/j.jep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Quézel P, Santa S. New flora of Algeria and the Southern disert regions. Volume I. Paris, France: CNRS; 1963. pp. 39–40. [Google Scholar]
- 4.Baba Aissa F. Medicinal plants in Algeria. Identification, description of active ingredient properties and traditional use of common plants in Algeria. Algiers: Bouchene and Ad. Diwan; 1991. p. 181. [Google Scholar]
- 5.Dob T, Berramdane T, Chelghoum C. Essential oil composition of Pinus halepensis Mill. from three different regions of Algeria. J Essent Oil Res. 2007;19:40–43. [Google Scholar]
- 6.Macchioni F, Cioni PL, Flamini G, Morelli I, Maccioni S, Ansaldi M. Chemical composition of essential oils from needles, branches and cones of Pinus pinea, P. halepensis, P. pinaster and P. nigra from central Italy. Flavour Frag J. 2003;18:139–143. [Google Scholar]
- 7.Kainulainen P, Holopainen JK. Concentrations of secondary compounds in Scots pine needles at different stages of decomposition. Soil Biol Biochem. 2002;34:37–42. [Google Scholar]
- 8.Fernandez C, Voiriot S, Mévy JP, Vila B, Ormeño E, Dupouyet S, et al. Regeneration failure of Pinus halepensis Mill.: the role of autotoxicity and some abiotic environnemental parameters. Forest Ecol Manag. 2008;255(7):2928–2936. [Google Scholar]
- 9.Navarro-Cano JA, Barbera GG, Ruiz-Navarro A, Castillo VM. Pine plantation bands limit seedling recruitment of a perennial grass under semiarid conditions. J Arid Environ. 2009;73(1):120–126. [Google Scholar]
- 10.Cheikh-Rouhou S, Hentati B, Besbes S, Blecker C, Deroanne C, Attia H. Chemical composition and lipid fraction characteristics of Alleppo pine (Pinus halepensis Mill.) seeds cultivated in Tunisia. Food Sci Tech Int. 2006;12(5):407–415. [Google Scholar]
- 11.Kubeczka KH, Schultze W. Biology and chemistry of conifer oils. Flavour Frag J. 1987;2:137–148. [Google Scholar]
- 12.Asensio D, Owen SM, Llusia J, Penũelas J. The distribution of volatile isoprenoids in the soil horizons around Pinus halepensis trees. Soil Biol Biochem. 2008;40:2937–2947. [Google Scholar]
- 13.Ormeño E, Baldy V, Ballini C, Fernandez C. Production and diversity of volatile terpenes from plants on calcareous and siliceous soils: Effect of soil nutrients. J Chem Ecol. 2008;34(9):1219–1229. doi: 10.1007/s10886-008-9515-2. [DOI] [PubMed] [Google Scholar]
- 14.Mirov NT, Iloff PM., Jr Composition of gum turpentines of pines. XXIII. A report on three mediterranean species: Pinus pinea (cultivated in California), P. halepensis (from Israel) and P. brutia (from Cyprus) J Am Pharm Asso. 1955;44(3):186–189. doi: 10.1002/jps.3030440317. [DOI] [PubMed] [Google Scholar]
- 15.Pasqualini V, Robles C, Garzino S, Greff S, Bousquet-Melou A, Bonin G. Phenolic compounds content in Pinus halepensis Mill. needles: a bioindicator of air pollution. Chemosphere. 2003;52(1):239–248. doi: 10.1016/S0045-6535(03)00268-6. [DOI] [PubMed] [Google Scholar]
- 16.Vidrich V, Mechelozzi M, Fusi P, Heimler D. Grassi G, Delmon B, Molle JF, Zibetta H, editors. Essential oils of vegetables species of the Mediterranean and Alpine temperate climate areas. 4th EC Conference on Biomass for Energy and Industry. 1988.
- 17.Tazerouti F, Badjah-Hadj-Ahmed AY, Meklati BY, Favre-Bonvin J, Bobenrieth MJ. Analysis of essential oils from needles of Pinus halepensis Mill. by gas chromatography and mass spectrometry. Plantes Medicinales et Phytotherapie. 1993;26(3):161–176. [Google Scholar]
- 18.Dob T, Berramdane T, Chelghoum C. Chemical composition of essential oil of Pinus halepensis Miller growing in Algeria. Comptes Rendus Chimie. 2005;8(11-12):1939–1945. [Google Scholar]
- 19.Abi-Ayad M, Abi-Ayad FZ, Lazzouni HA, Rebiahi SA, Ziani-Cherif C, Bessiere JM. Chemical composition and antifungal activity of Aleppo pine essential oil. J Med Plant Res. 2011;5(22):5433–5436. [Google Scholar]
- 20.Roussis V, Petrakis PV, Ortiz A, Mazomenos EB. Volatile constituents of needles of five Pinus species grown in Greece. Phytochem. 1995;39(2):357–361. [Google Scholar]
- 21.Hmamouchi M, Hamamouchi J, Zouhdi M, Bessiere JM. Chemical and antimicrobial properties of essential oils of five Moroccan Pinaceae. J Essent Oil Res. 2001;13(4):298–302. [Google Scholar]
- 22.Tumen I, Hafizoglu Kilic A, Dönmez IE, Sivrikaya H, Reunanen M. Yields and constituents of essential oil from cones of Pinaceae spp. natively grown in Turkey. Molecules. 2010;15:5797–5806. doi: 10.3390/molecules15085797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ustun O, Sezer Senol F, Kurkcuoglu M, Erdogan Orhan I, Kartal M, Husnu Can Baser K. Investigation on chemical composition, anticholinesterase and antioxidant activities of extracts and essential oils of Turkish Pinus species and pycnogenol. Ind Corp Prod. 2012;38:115–123. [Google Scholar]
- 24.Council of Europe . European Pharmacopoeia. 1st ed. Strasbourg: Council of Europe; 1997. [Google Scholar]
- 25.Jennings W, Shibamoto T. Qualitative analysis of flavour and fragrance volatiles by glass-capillary gas chromatography. New York: Academic Press; 1980. [Google Scholar]
- 26.König WA, Hochmuth DH, Joulain D. Terpenoids and related constituents of essential oils. Hamburg, Germany: Library of Mass Finder 2.1 University of Hamburg, Institute of Organic Chemistry. 2001. [Online] Available from: http://massfinder.com/wiki/Terpenoids_Library [Assessed on 16 July, 2013].
- 27.Mc Lafferty FW, Stauffer DB. The Wiley/NBS registry of mass spectral data. New York: Wiley-interscience; 1989. [Google Scholar]
- 28.Mc Lafferty FW, Stauffer DB. Mass Spectrometry Library Search System Bench-Top/PBM version 3.10d, Palisade, Newfield. 6 1994. Wiley registry of mass spectral data.
- 29.NIST: National Institute of Standards and Technology PC Version 1.7 of the NIST/EPA/NIH Mass Spectral Library Perkin Elmer Corporation, Norwalk, CT. 1999.
- 30.Brereton RG. Chemometrics: Data analysis for the laboratory and chemical plant. New-York: Wiley Interscience; 2003. [Google Scholar]
- 31.Massart DL. Chemometrics: A textbook. New York: Elsevier Sciences Ltd; 1998. [Google Scholar]
- 32.Bagamboula CF, Uyttendaele M, Candan F, Daferera D, Unli GV, Polissiou M, et al. Antimicrobial and antioxidative activities of the essential oils and methanol extracts. Food Chem. 2004;84:519–552. [Google Scholar]
- 33.Mighri H, Hadjlaoui H, Akrout A, Najjaa H, Neffati M. Antimicrobial and antioxidant activities of Artemisia herba-alba essential oil cultivated in Tunisian arid zone. Comptes Rendus Chimie. 2010;13:380–386. [Google Scholar]
- 34.Sheng-Hsien L, Ku-Shang C, Min-Sheng S, Yung-Sheng H, Hung-Der J. Effects of some Chinese medicinal plant extracts on five different fungi. Food Control. 2007;18:1547–1554. [Google Scholar]
- 35.Abi-Ayad M, Abi-Ayad FZ, Lazzouni HA, Rebiahi SA. Antibacterial activity of Pinus halepensis essential oil from Algeria (Tlemcen) J Nat Prod Plant Resour. 2011b;1(1):33–36. [Google Scholar]
- 36.Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil) J Appl Microbiol. 2000;88:170–175. doi: 10.1046/j.1365-2672.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 37.Zouari S, Zouari N, Fakhfakh N, Bougatef A, Ayadi MA, Neffati M. Chemical composition and biological activities of a new essential oil chemotype of Tunisian Artemisia herba alba Asso. J Med Plant Res. 2010;4(10):871–880. [Google Scholar]


