Abstract
Memory T cells comprise the most abundant lymphocyte population in the body for the majority of one’s lifetime; however, our understanding of memory T cell generation, function and maintenance mainly derives from mouse studies, which cannot recapitulate the decades-long exposure to multiple pathogens that occurs in humans. Here, we review studies focused on human memory T cells that reveal key properties including subset heterogeneity and diverse tissue residence in multiple mucosal and lymphoid tissue sites. We also discuss how the function and adaptability of human memory T cells depend on spatial and temporal compartmentalization.
Introduction
The acquisition of antigen experience is marked by the generation and persistence of memory T cells which can provide life-long protection against pathogens. Studies of mouse models have demonstrated the robust generation of memory T cells in response to diverse pathogens, and their efficacious protective responses upon reinfection; however, the role of memory T cells in protecting and maintaining long-term health in humans is less clear.
In mouse models, it is well-documented that antigen-specific naive CD4+ or CD8+ T cells become activated upon antigen exposure, and subsequently undergo proliferative expansion and differentiation into effector T cells. It is generally thought that these activated, effector T cell populations contain the precursors of antigen-specific long-lived memory T cells, which persist in vivo as heterogeneous populations in multiple sites, and can coordinate protective immune responses upon pathogen re-exposure. Mouse memory CD8+ T cell-mediated protection has been demonstrated in the well-characterized lymphocytic choriomeningitis virus (LCMV) infection model and for additional mouse pathogens (for reviews, see1,2). Memory CD4+ T cells can similarly mediate protective immune responses in mice to influenza virus3–5, Mycobacterium tuberculosis6 and parasite7 infections.
While most of our current understanding of memory T cell generation, function and maintenance is based on results from mouse models, studies in mice cannot recapitulate the exposure to multiple pathogens that occurs in humans over decades. The duration of a mouse memory study is typically several months, with the lifespan of laboratory mice lasting 1–2 years, which constitutes only a small fraction of the decades-long duration of immunological memory in humans with an average lifespan of 75 years or longer. Additionally, humans are exposed to diverse pathogens through the aerodigestive tract, genital mucosa and skin, which are sites of extensive colonization with >2,000 species of commensal microorganisms8. By contrast, most experimental mice are maintained in highly stringent, pathogen-free conditions, thus limiting the breadth of microbial exposure. Therefore, the generation and maintenance of human memory T cells should be considered within the context of the unique human exposure to pathogenic and non-pathogenic microorganisms, and not only relative to mouse models in controlled in vivo settings.
Human T cell studies are generally limited in two respects: first, most studies sample only peripheral blood, though the vast majority of memory T cells reside in tissue sites, including lymphoid tissues, intestines, lungs and skin (see later). Second, most studies on human memory T cells use samples obtained from young- or middle-aged adults, although the majority of memory T cell responses are formed during childhood from primary infections. Recent conceptual and technological breakthroughs, however, are now enabling novel explorations of T cell responses in humans. In this Review, we integrate these new studies with previous findings on T cells from healthy and diseased patients for an analysis of the current knowledge of human memory T cells. We describe recent studies that are beginning to assess how memory is organized in human tissue sites, including their functional capacities and antigen specificities, and discuss their implications for promoting in situ immunity in response to vaccines through targeted therapies. We also discuss the accumulation of memory T cells over a lifetime and how compartmentalization and specificity of memory T cells is maintained through homeostasis.
Memory T cell accumulation throughout life
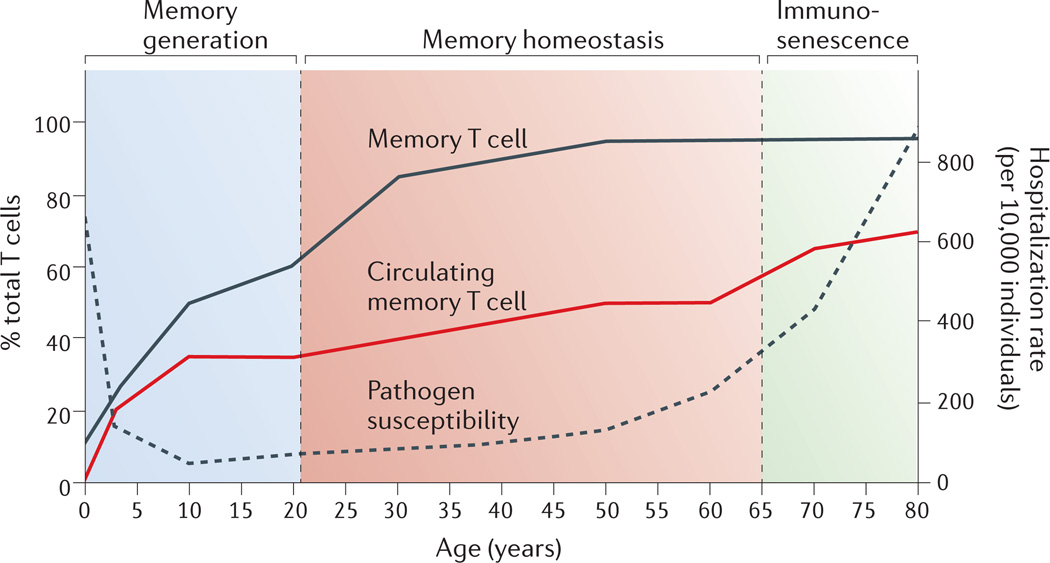
The frequency of memory T cells undergoes dynamic changes throughout an individual’s lifetime that can be divided into three phases: memory generation, memory homeostasis, and immunosenescence (Fig. 1). At birth, all T cells in peripheral blood are naïve, and memory T cells develop over time in response to diverse antigen exposure. A dramatic increase in the proportion of circulating memory T cells occurs in the first decade of life, and memory T cells comprise up to 35% of circulating T cells by the end of the second decade9. During this initial memory generation phase, particularly during infancy and early childhood, individuals exhibit the highest susceptibility to pathogens as measured by infectious disease hospitalization rates10.
Figure 1. Memory T cell frequency, pathogen susceptibility and mortality throughout human life.
Memory T cells pass through three distinct phases: memory generation, memory homeostasis and immunosenescence. Memory T cells are mostly generated following antigen exposure during infancy, youth and young adulthood (ages 0–20). Their levels subsequently plateau and are maintained through homeostasis throughout adulthood (ages 30–65), after which they enter the third stage and exhibit senescent changes (ages 65 and up). Previous studies have shown that there is an increase in the frequency of memory T cells in the blood (red line) over time9,12. In the whole body, which includes the blood, intestines, lungs, skin, liver, brain and lymphoid tissues, the overall frequency of memory T cells (black line) also increases with age11. The increase in memory T cell frequency throughout the body inversely correlates to a decrease pathogen susceptibility (dashed line) calculated from infectious disease hospitalization rates (per 10,000 people) recorded from 1998–2006 in the US based on 40,085,978 total hospitalizations10.
The second phase, termed memory homeostasis, occurs after age 20–25, when circulating memory T cell frequencies reach a plateau and remain stable throughout adulthood11,12 (Fig. 1). Thymic output gradually diminishes during this phase and T cell numbers are largely maintained through homeostatic cell turnover13. Individuals in these middle years are less susceptible to pathogens, as evidenced by the low hospitalization rate of infectious diseases10, and the immune response trends to homeostasis. After decades of stable frequencies, the proportion and functionality of memory T cells becomes altered during immunosenescence, starting at 65–70 years of age9,12,14. Immunosenescence also marks an increased susceptibility to pathogens due, in part, to age-associated immune dysregulation and non-immune-related physiological decline. As immunosenescence has recently been reviewed elsewhere14,15, we focus here on the first two phases: memory generation and homeostasis.
Memory T cell frequency in blood is a vast underestimate of the total frequency and numbers of memory T cells in the whole body. Estimates of the number of T cells in human tissues are 2×1010 in the skin16,17, 1×1010 in lungs18, 3×1010 in the intestines19, and 20×1010 in lymphoid tissues (that is, the spleen, lymph nodes and bone marrow)19. Therefore, peripheral blood T cells (5–10×109 in human blood) represent only 2–2.5% of the total T cell complement in the body19, and memory T cells represent the predominant T cell subset in mucosal sites, skin, spleen and bone marrow20. Early in infancy, T cells are observed to populate the intestines21, and lungs22, with 20% of these cells in the intestines exhibiting a memory phenotype in newborns21, perhaps due to antigens encountered in utero (see later). Recent studies in human tissues (described below) have demonstrated that by the end of puberty, lymphoid tissues, mucosal sites and the skin are populated predominantly by memory T cells, which persist through adult life and represent the most abundant lymphocyte population throughout the body11,23.
Memory T cell subsets and heterogeneity
Heterogeneity in peripheral blood
Memory T cells in humans are classically distinguished by the expression of the CD45RO isoform and by lack of CD45RA isoform expression (CD45RO+CD45RA−)24,25. CD45RO+CD45RA− T cells are now known to comprise heterogeneous populations of memory T cell subsets. Nearly 15 years ago, Lanzavecchia and colleagues first identified this heterogeneity in human peripheral blood based on the expression of the lymph node homing CC-chemokine receptor CCR726. Naïve T cells uniformly express CCR7, reflecting their predominant residence in lymphoid tissue, whereas memory T cells are subdivided into CD45RA−CCR7+ central memory T (TCM) cells, which traffic to lymphoid tissues, and CD45RACCR7− effector memory T (TEM) cells, which can migrate to multiple peripheral tissue sites. They further showed that TCM cells produced more interleukin-2 (IL-2) than TEM cells, which produced more effector cytokines, and they proposed a differentiation model with TCM cells being an intermediate stage in development of naive T cells into TEM cells in peripheral tissue sites27. The existence of TCM and TEM subsets in lymphoid and peripheral tissue sites was confirmed in mouse models28,29. The original model of functional memory T cell subsets has since been revised to include a further characterization of human peripheral blood memory T cell subsets in health and disease. Functionally, an effector capacity is not confined to TEM cells, as both TCM and TEM cell subsets produce effector cytokines in response to viruses, antigens and other stimuli30–33, although TCM cells exhibit a higher proliferative capacity30,34.
Recently, the expression of additional surface markers, including the death receptor CD95 (also known as FAS) and the memory-associated marker CD122 (also known as IL-2Rβ) in the context of a naïve-appearing T cells were found to delineate a new memory T cell subset in humans35 and mice36, designated T memory stem (TSCM) cells, which exhibit superior survival potential and efficiently engraft in allogeneic transplant models. In humans, TSCM cells resemble naïve T cells in that they are CD45RA+CD45RO− and they express high levels of the co-stimulatory receptors CD27 and CD28, IL-7 receptor α-chain (IL-7Rα), CD62L and CCR7. TSCM cells have high proliferative capacity and are both self-renewing and ‘multipotent’, in that they can further differentiate into other subsets including TCM and TEM cells35,37. These properties define their ‘stem cell-like’ capacities35,38.
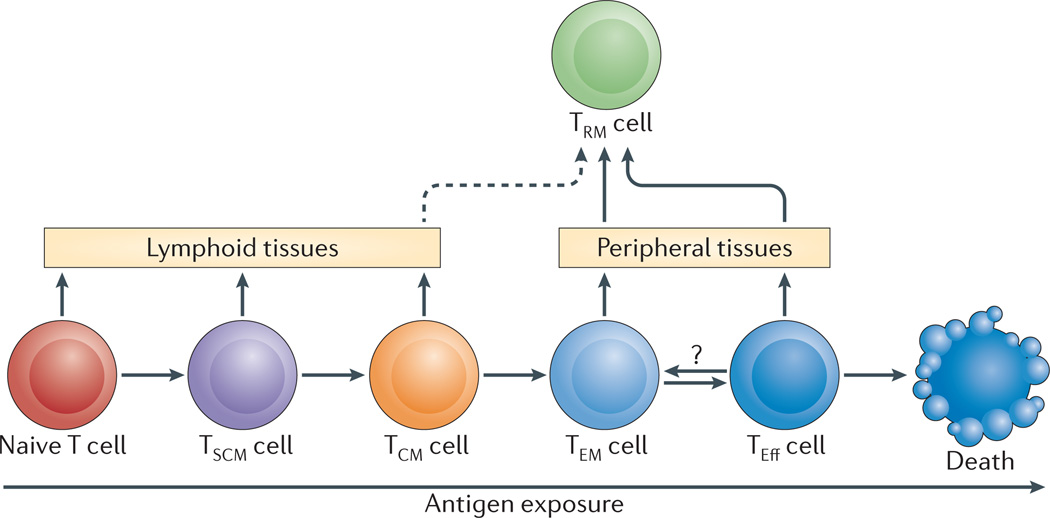
Elucidating the differentiation pathways for heterogeneous memory T cell subset development following naive T cell activation has been an active area of debate and investigation. Although the lineage relationship of human T cell subsets is difficult to assign, a progressive differentiation pathway based on signal strength and/or extent of activation, places naive, TSCM, TCM and TEM cells in a differentiation hierarchy, serving as precursors for effector T cells38–40 (Fig. 2). Another model proposes the TEM and TCM cell subsets derive from effector T cells with TEM cells giving rise to TCM cells, and a third model posits a divergent generation of effector, TCM and TEM cell subsets (reviewed in reference41). Recent studies of the fate of individual T cells in mice during infection provides evidence that a single naive T cell can give rise to heterogeneous memory T cell populations following activation42–44, and supports a progressive differentiation model (Fig. 2). Moreover, these individual fates in mouse T cells can be determined at early times after activation due to asymmetrical division45. The mechanisms and timing for human effector and memory T cell fate determination remain undefined.
Figure 2. Model for the generation of human memory T cell subsets.
A schematic model for differentiation of circulating and tissue-resident memory T cell subsets. Progressive differentiation of the three major circulating subsets — stem cell memory T cells (TSCM cells), central memory T cells (TCM cells) and effector memory T cells (TEM cells) — from activated naïve T cells is shown relative to the extent of antigen exposure. Effector T cells (TEff cells) represent terminally differentiated cells, and death is one outcome of increased antigen exposure and proliferation. Naïve, TSCM and TCM cells circulate and migrate to lymphoid tissue, whereas TEM and TEff cells are the subsets with the capacity to traffic to peripheral tissues. Tissueresident memory T cells (TRM cells) in peripheral tissue sites may derive from either TEM or TEff cells that migrate to these sites via tissue-specific influences. It is possible that TCM cells could develop into TRM cells in lymphoid sites (dotted line). TRM cells in peripheral compartments are likely terminally differentiated since they do not circulate or convert to other memory T cell subsets.
The heterogeneity of memory T cell subsets in peripheral blood reveals only a small fraction of the total complexity of memory T cell distribution throughout the body. Seminal mouse studies showed that antigen-specific memory CD4+ and CD8+ T cells can populate and persist in multiple tissue sites long after virus or antigen was cleared28,29. Early studies of surgical explants in humans showed that CCR7– TEM cells were the predominant memory T cell subset found in intestine and lungs, whereas tonsils contained both TCM and TEM cells46. This diverse tissue distribution of memory T cells raised the question of whether they had just circulated through these tissues, or had become resident at these sites as a consequence of further differentiation.
In the last several years, mouse studies have established the existence of a new ‘tissue-resident’ memory T cell (TRM cell) subset as a non-circulating subset that resides in peripheral tissue sites and, in some cases, elicits rapid in situ protective responses. Mouse CD4+ TRM cells can be generated in the lungs from adoptive transfer of activated (effector) T cells4 or following respiratory virus infection47, and are distinguished from splenic and circulating memory T cells by their upregulation of the early activation marker CD69, their tissue-specific retention in niches of the lung47, and their enhanced ability to mediate protection to influenza virus infection compared to circulating memory CD4+ T cells4. An analogous non-circulating CD4+ TRM cell subset has been identified in the bone marrow of mice following systemic virus infection that exhibits enhanced helper functions48.
Memory CD8+ T cells were initially found to persist as circulating and resident populations in lymphoid tissues, lungs and mucosal tissues in mice49. CD8+ TRM cells generated following infection have subsequently been identified in multiple mouse tissues including the skin50,51, vaginal mucosa52,53, intestine49,54,55, lungs47,56 and even brain57. CD8+ TRM cells are collectively distinguished from splenic and circulating memory CD8+ T cells by their increased expression of CD69 (similar to CD4+ TRM cells), and by expression of the epithelial cell-binding integrin αEβ7 (also known as CD103)20,52,58–60. Protection by CD8+ TRM cells has been demonstrated in the skin of mice in response to intradermal herpes simplex virus 2 (HSV2) infection61–63 and to HSV infection at the vaginal mucosa52,53. These mouse studies indicate that T cell-mediated memory responses, and in particular those with high protective capacity, are highly compartmentalized in tissue sites, necessitating the study of memory T cell populations in anatomic sites other than the blood.
Although TRM cells are less well characterized in humans than in mice, recent studies are beginning to define these populations by sampling tissues from organ donors11 and from surgical explants18,50. A whole body analysis of T cells in multiple lymphoid and mucosal sites revealed that memory CD4+ T cells predominate throughout the body, and persist as CCR7+ or CCR7− subsets localized to lymphoid tissues and mucosal sites, respectively, whereas memory CD8+ T cells persist as mainly CCR7− subsets in all sites, with low numbers of CD8+ TCM cells in lymphoid tissues and negligible numbers of these cells in other sites11. Importantly, most memory T cells in human mucosal, lymphoid and peripheral tissue sites such as skin express the putative TRM cell marker CD6914–16,20,58, whereas circulating blood memory T cells uniformly lack CD69 expression11. Together, these studies suggest that TRM cells expressing CD69 are present as majority populations in human mucosal and peripheral tissue sites, similar to their counterparts in mice. Unlike mouse lymphoid memory T cells, a significant proportion of TEM cells in human lymph nodes and spleen also express CD6911, suggesting that TRM may also be present in lymphoid tissue.
The pathway(s) and mechanisms for the generation of TRM cells remain unknown and is an important area for future studies. Mouse studies suggest that TRM cell generation occurs within tissue sites from either activated effector T cells and/or TEM cell precursors that migrate to tissue sites47,64 (Fig. 2). We propose that the pathway to human TRM cell development involves both migration and tissue-specific factors, as suggested by the examination of TRM cell distribution and properties11,16,18 (Fig. 3). Blood-borne memory TCM and TEM cell subsets can enter certain tissue sites such as spleen, lungs, lymph nodes and bone marrow at a low frequency, but are not significantly represented in the skin and intestine. (TSCM cells appear in lymph nodes in non-human primates65 but their distribution in humans is not known). CD4+ TRM cells (CCR7− CD69+) and CD8+ TRM cells expressing CD103 are the predominant T cell subsets in the lungs, intestine, skin and bone marrow11,20,66. Lymphoid tissues contain TCM cells and TEM cells, some of which also express CD69 but not CD10311. It is not known whether additional markers define lymphoid TRM and/or whether CD69+ TEM cells in lymphoid tissue are true resident memory cells.
Figure 3. Schematic of memory T cell heterogeneity in peripheral blood and tissues.
A Diagram shows the tissue distribution and migration patterns of the major human memory T cell subsets, including three major circulating populations —, stem cell memory T cells (TSCM cells), central memory T cells (TCM cells) and effector memory T cells (TEM cells) — and TRM cells in multiple sites, as well as CD8+ TRM cells that are defined by expression of CD103 and are associated with mucosal sites and skin. The individual sites are defined in terms of circulation (red), lymphoid origin (grey) or peripheral tissues (yellow). Circulating TSCM, TCM and TEM cell subsets migrate from the blood and circulate through the spleen and lungs, where they can be primed to migrate to intestines71. They also migrate via the lymphatics and efferent vessels to lymph nodes. TRM cells predominate in skin, lungs, bone marrow and intestines, but may also be present within the CD69+TEM subsets in the spleen and lymph nodes. Mucosal sites and the skin also contain specific CD103+ TRM cells. The expression of certain chemokine receptors and/or integrins is associated with T cell migration and/or residence in lymph nodes (CCR7), skin (CCR4, CLA and CCR10), intestines (CCR9 and integrin α4β7), lungs (CCR6) and bone marrow (integrin α2β1). B Key phenotypic and functional properties of circulating and resident subsets are shown, with CD45RA and CCR7 distinguishing circulating memory T cell subsets and CD69 (and CD103) expression delineating TRM cells from circulating subsets. Memory subsets can produce similar types of recall cytokines, such as interleukin-2 (IL-2), interferon-γ (IFNγ) and tumour necrosis factor (TNF), but differ in the extent and quality of these responses.
Human TRM cells also exhibit tissue-specific properties (Fig. 3), suggesting in situ influences. TRM cells in skin express cutaneous lymphocyte antigen (CLA) and the skin-homing-associated chemokine receptors CCR4 and CCR1016,67,68; memory T cells in the small intestine and colon express the gut-homing receptor CCR969 and the integrin α4β770, and memory T cells in the lungs upregulate CCR6 expression18. There is also evidence for crosstalk between mucosal sites such as lung and intestines71. Migration of TEM cells to the bone marrow in mice requires expression of integrin α2β1 (also known as VLA2)64, but it is not known whether human bone marrow TRM cells have a similar requirement. This differential chemokine receptor and/or integrin expression may derive from activated or effector populations that enter the site72,73, or alternately be upregulated during homeostasis of naive or memory T cells74. This identification of human TRM cells with tissue-specific signatures suggests anatomic control of memory T cell generation in humans.
Functional capacity of heterogeneous subsets
The extensive phenotypic and tissue complexity among heterogeneous memory T cell subsets suggests a corresponding functional heterogeneity. Mouse and human CD4+ T cells of a non-regulatory lineage are subdivided into functional subsets — the most prevalent being Th1 cells that produce interferon-γ (IFNγ), IL-2 and tumour necrosis factor (TNF), Th2 cells secreting IL-4, IL-5, IL-10 and IL-13, and Th17 cells producing IL-17. CD8+ T cells are not typically subdivided into functional subsets, and generally produce IFNγ and TNF, and express cytolytic markers such as perforin and CD107. In the peripheral blood of healthy individuals, most circulating memory CD4+ and CD8+ T cells produce IFNγ, IL-2 and/or TNF following shortterm stimulation, and only low numbers of IL-4-, IL-10- and IL-17-producing memory CD4+ T cells are observed35,75. Human peripheral blood TEM, TCM and TSCM subsets differ in the relative proportion of cells producing IL-2, IFN-γ and/or TNF: TEM cells have the highest proportion of IFNγ- and TNF-producers and lowest proportion of IL-2-producing cells, TSCM cells have the lowest proportion of IFNγ-producers, with more IL-2-producing cells compared to TEM, while TCM cells have the highest frequency of IL-2-producers and frequencies of IFN-γ and TNF intermediate between TSCM and TEM cells35,38 (Fig. 3). Variations in expression of chemokine receptors have also been associated with different functional capacities of human effector T cells. For example, in vitro polarized Th1 effector cells were found to express CXC receptor 3, CCR5 and CCR2, while Th2-polarized effector cells expressed CCR4 and CCR376. However, expression of these chemokine receptors can be transient, depend on the microenvironment77, and does not consistently delineate functional subsets among resting memory T cells78.
The functional capacities of human TRM cells are beginning to be defined. Overall, memory CD4+ and CD8+ T cells in lymphoid and mucosal tissues obtained from healthy individuals exhibit rapid IL-2 and IFNγ production, respectively, when activated non-specifically with PMA/ionomycin11. It was previously determined that individual circulating memory T cells can produce multiple cytokines, and the presence of these multifunctional or polyfunctional memory T cells correlated with superior recall and protective responses79. There is emerging evidence that TRM can be multifunctional, and also exhibit qualitative functional differences. Human bone marrow TRM cells are polyfunctional for effector cytokines and cytolytic molecules48,80, a substantial fraction of human lung TRM cells produce multiple pro-inflammatory cytokines18, and human intestinal TRM cells are also multifunctional11. Other functions, however, appear to be confined to specific subsets and/or tissue sites. IL-17 is produced by a subset of CD4+ TRM cells in mucosal sites, particularly in intestines in healthy individuals11, by CCR6+ memory T cells in peripheral blood81,82, and also by a subset of CD161+ T cells in inflamed tissue, such as the skin of patients with psoriasis83. A subset of skin memory CD4+ T cells can produce IL-22 (and not IL-17) when cloned and expanded ex vivo84, and IL-22-producing T cells have also been identified in inflamed skin in psoriasis85. Thus, while predominant memory T cell functions, such as IFNγ production, are broadly distributed among multiple memory T cell subsets and tissues, TRM cells in tissue sites can adopt multiple or distinct functional attributes which may also depend on tissue-specific inflammation.
Diverse antigen specificity of human memory T cells
Specificity in the adaptive immune response is intricately linked to the establishment and persistence of memory T cells that record previous antigen experiences via specific T cell receptors (TCRs). In mice, the development of memory T cells in response to viral pathogens is marked by extensive clonal expansion of virus-specific T cells, followed by the contraction and death of >90% of activated or effector T cells, and long-term persistence of virus-specific memory T cells at variable frequencies (<1 to >10% of the total number of T cells) depending on the virus2,86. These mouse models generally examine memory T cell development and responses to one type of virus in otherwise sterile conditions. By contrast, in humans, antigen-specific memory T cells are generated and maintained in the face of thousands of different pathogens introduced at various life stages in the context of a dynamically maintained, heterogeneous T cell population. Assessing the physiological significance of antigen-specific memory T cells in humans has therefore proved challenging.
Pathogen-specific memory generation and maintenance
Our ability to measure the frequency of human antigen-specific memory T cells has increased in accuracy and sensitivity from classical functional recall assays to cytometry-based methods (Box 1). The assessment of human antigen-specific memory T cells has mostly occurred in the context of virus infections that are ubiquitous in healthy humans, including acute infections with influenza virus and chronic infections with viruses such as cytomegalovirus (CMV) and Epstein–Barr virus (EBV). Memory T cells responses to these viruses are generated as a result of a productive immune response that effectively controls the virus. Human T cell responses in chronic HIV infection have been extensively studied; however, mechanisms for their generation and maintenance are more complex, as memory T cells are the targets for chronic infection and virus persistence87–89 and HIV is not cleared by the immune system in most individuals (reviewed elsewhere90–92).
Box 1 | Approaches for analyzing antigen-specific T cell memory.
The techniques for examining antigen-specific T cell responses have increased in sensitivity and specificity in recent years. The classical limiting dilution assay requires ex vivo antigen-driven expansion of human T cell populations, but this approach is limited because non-proliferating T cell clones are under-represented. More recently, development of the ELISPOT assay provided a sensitive, yet robust functional method for detecting antigen-specific memory T cells by their rapid production of effector cytokines, and enabled their precise quantitation without the need for antigen-driven population expansion.
The development of MHC tetramer reagents148 allowed for the visualization and quantitation of epitope-specific T cells based solely on TCR specificity, although this mention lacks a functional readout. TCR staining with tetramers, pentamers or MHC multimers has enabled a more sensitive estimation of memory T cell specificity in humans compared with direct functional recall responses to antigens as measured by ELISPOT or limiting dilution assays149,150. Moreover, new techniques for enrichment of antigen-specific T cells using labelled tetramer reagents and secondary binding reagents coated to magnetic beads115 enables detection of rare antigen-specific T cell populations, even among naïve T cells that have not undergone in vivo expansion.
The most recent technical advance in antigen-specific T cell detection is achieved through the use of cytometry by time-of-flight mass spectrometry (CyTOF) which employs the technique of mass cytometry by using antibodies labelled by different masses, enabling >50 parameters to be assayed on single cells151 without the problems of compensation that occurs in multiparameter flow cytometry. Coupling different mass labels to tetramer reagents allows for the combinatorial assessment of >50 TCR specificities in a single sample152. Use of these new technologies makes the assessment of T cell epitope specificities from small clinical biopsy samples possible, which will be essential for future diagnostic application of antigen-specificities in immune monitoring.
Cross-sectional studies of memory T cell frequency in different age groups demonstrate that most virus-specific memory T cells are generated early in life. Congenital CMV infection was demonstrated to generate virus-specific memory CD8 T cells in utero93. Furthermore, in a large cohort study of children and adults, CMV-specific CD8+ T cells were detected in the blood of infants, and their frequency remained stable throughout early childhood and young adulthood94. Memory T cells specific for adenovirus are detected in early childhood and progressively increase in frequency until age 10, when they remain at a constant level throughout adulthood31. Influenza virus-specific T cells response are also detected in children at levels comparable to those observed in adults95,96. Together, these results suggest that despite the overall increase in circulating memory T cells over a lifetime (Fig. 1), memory T cells generated to ubiquitous pathogens exhibit early increases in frequency and stable maintenance throughout adulthood.
The ability to observe pathogen-specific human memory T cell development through the process of clonal expansion and contraction is not readily accomplished; however, prospective infection and vaccine studies have provided novel glimpses into this process. Circulating T cells specific for EBV or for influenza virus underwent clonal expansion, contraction and persistence as memory T cells following acute infection97–99. Similarly, the administration of yellow fever and smallpox vaccines, which both consist of live viruses, stimulates robust CD8+ T cell clonal expansion, contraction and memory formation that is measurable in peripheral blood100. However, there was no significant increase in the frequency of circulating, virus-specific T cells following acute infection with rotavirus101, which is a gastrointestinal virus, or with the lung pathogen respiratory syncytial virus (RSV)102,103. This variability in observing memory T cell development in human blood may be due to inefficient mobilization of responses during infection104, and/or compartmentalization of pathogen-specific responses at the infected site.
Several studies have demonstrated biased generation and maintenance of virus-specific effector and memory T cells in tissues versus circulation. Intradermal immunization with purified protein derivative (PPD) of M. tuberculosis resulted in the proliferation of antigen-specific T cells in skin but not in the blood105, and similarly, cutaneous challenge with varicella zoster virus results in memory T cell accumulation in skin106, suggesting that the formation of memory may occur at distinct sites. Lung tissue was found to contain an increased frequency of influenza virus-specific memory CD8+ T cells compared to the blood107,108 and spleen47, and influenza virus-specific cells in human lungs exhibit a TRM cell phenotype (that is, CD69+CD103+)47,109. CD8+ T cells specific for HSV2 were found to persist in genital skin, but not in skin from other body regions110, suggesting local maintenance of skin TRM cells. Similarly, memory CD4+ T cells specific for astrovirus, a common enteropathogenic virus, were detected in the small intestines111. Together, these results suggest compartmentalization of pathogen-specific memory T cell responses that are preferentially maintained at the sites of initial effector T cell recruitment. These findings also indicate that accurate assessment of pathogen-specific memory T cell responses requires sampling of the initial infection site.
Memory T cell cross-reactivity
Despite their specificity, human memory T cells exhibit cross-reactivity to antigenic epitopes not previously encountered, which is possibly due to intrinsic properties of TCR recognition112 and to the range and breadth of human antigenic experience. Memory CD4+ and CD8+ T cells specific for unique epitopes of avian influenza strain H5N1 were detected in healthy individuals that were not exposed to H5N1 infection assessed by serology113,114. In addition, HIV-specific memory T cells have been identified in HIV-negative individuals115. Virus-specific memory T cells also show cross-reactivity to alloantigens, autoantigens and unrelated pathogens116,117: EBV-specific human memory T cells generated in HLA-B8 individuals exhibit allogeneic cross-reactivity to HLA-B44118, and influenza virus- and HIV-specific memory CD4+ T cells recognize epitopes from unrelated microbial pathogens115. Furthermore, T cells specific for the autoantigen myelin basic protein (MBP) recognized multiple epitopes from viral and bacterial pathogens117,119. This cross-reactivity may enable memory T cells to mediate protection without initial disease — a phenomenon known as heterologous immunity120. Heterologous immunity has been demonstrated in humans where EBV infection expanded clones of influenza virus-specific T cells121. The role of T cell cross-reactivity in determining how T cell subsets may be compartmentalized in tissue sites is not known, but could be an important mechanism for their homeostatic maintenance (See below).
Role of microbiome in generating memory T cells
Mucosal sites and the skin harbour resident bacteria and viruses, collectively referred to as the microbiome. Humans are exposed to >2,000 microbial species8, of which only a small fraction are pathogenic. It has been suggested that the very purpose of immune memory in vertebrates is to preserve proper immune homeostasis with commensals8. Studies in mouse models show that the presence and the composition of the microbiome are critical in promoting appropriate immune responses to pathogens and maintaining proper immune homeostasis (for a review, see122). Whether the species of commensals present in certain sites influence the type of memory T cells that reside there is not known. In a limited study examining the reactivity to endogenous bacterial flora of T cells expanded from blood and intestinal biopsy samples of two patients, more bacteria-reactive T cells clones were isolated from intestines than from the blood123, suggesting that intestinal T cells may exhibit biased cross-reactivity to intestinal flora. Although memory T cells specific for commensal bacteria have been recently detected in mice124, they are associated with pathogenic infection of the intestines and could be a consequence of dysregulated immunity in inflammatory bowel disease. In humans, memory T cells survive far longer and are exposed to more antigens during their lifetime, and therefore commensal-specific responses may be part of the healthy immune balance between the microflora and the indigenous T cells.
Human memory T cell homeostasis
Specific clones of memory T cells expressing a unique TCR can persist for decades in vivo. Human memory T cell longevity is clearly demonstrated by studies showing that memory T cells specific for vaccinia virus — the etiologic agent of smallpox eradicated four decades ago — persisted in individuals vaccinated 25–70 years previously125. However, mechanisms for memory T cell maintenance in humans remain unclear. In mouse models, the long-term requirements for T cell maintenance and homeostasis have been defined using mice deficient for various cytokines and cytokine receptors, for MHC molecules and/or for components of the TCR signalling cascade (for a review, see126). These studies established that virus-specific memory CD8+ T cells do not require antigen or MHC for their maintenance, but rely on IL-15 for homeostasis and IL-7 for survival, whereas memory CD4+ T cells require TCR signalling and/or MHC class II molecules for their functional maintenance and homeostasis127–129.
An elegant approach to probe human memory T cell turnover or longevity is to administer deuterated water or deuterated glucose to volunteers and subsequently examine the incorporation of deuterium by T cells in vivo130,131. The calculated half-life of human T cells using this approach was found to vary according to the type of labelling, the limited sampling of peripheral blood, the timing of sampling and the mathematical algorithm132. On average, human naive T cells have a longer half-life than memory T cells (1–8 years versus 1–12 months)132; CD4+ T cells have a shorter half-life than CD8+ T cells and TEM cells have a shorter half-life than TCM cells133. Compared with naïve T cells, human memory T cells also have shorter telomeres in individuals of all ages134, which indicates their more extensive replicative history. These studies suggest that memory T cells in the circulation are maintained, in part, by continuous, homeostatic turnover. Turnover and replicative history of human memory T cells in tissue compartments remains completely unexplored and will be important to assess TRM cell stability.
Transcriptome, epigenetic and deep sequencing analysis of human memory T cells provide new evidence into mechanisms for memory T cell longevity135,136, and the potential role of TCR and cytokine signals in this process. Human memory CD4+ and CD8+ T cells exhibit transcriptional upregulation of genes encoding TCR-coupled activation markers compared with naïve T cells, including multiple MHC class II molecules, chemokine receptors, CD95 and effector molecules135. Moreover, activation-induced epigenetic changes in the loci of effector cytokine genes are maintained in circulating human memory CD8+ T cells135,137. Deep sequencing of TCR genes revealed conserved clonotypes and reduced diversity among human memory T cell subsets138,139. Together, these findings implicate tonic TCR signalling in human memory CD4+ and CD8+ T cell maintenance, potentially due to cross-reactivity to self-antigens, environmental antigens and/or resident commensal organisms. Requirements for cytokine signals in this maintenance are not clearly defined, although individuals with mutations in the cytokine-induced transcription factor signal transducer and activation of transcription 3 (STAT3) exhibit reduced memory T cell frequency and responses140. Whether requirements for TRM cell maintenance differ according to the tissue site is not known. For example, memory T cells may be preferentially maintained by cross-reactive TCR-mediated interactions with microbial antigens at mucosal sites due to high antigen density, whereas those in blood, lymphoid tissue, spleen and bone marrow may be maintained preferentially through responses to homeostatic cytokines such as IL-7 or IL-15, which are abundantly expressed in these sites64.
Antigen-specific memory T cells in space and time
A proposed schematic of how T cell subsets and specificities for ubiquitous microbial antigens are distributed in space (from circulation to peripheral tissue sites) and time (from infancy to old age) is presented in Fig. 4. TEM and TRM cell compartmentalization in mucosal and peripheral tissue sites is initiated during infancy and stably maintained throughout life. TRM cells in these sites may be enriched in specificities for pathogenic and non-pathogenic microbial species that populate or infect these tissues. Dynamic changes occur in the circulation and in lymphoid tissue, including the reduction in naïve T cells and increase in TCM and TEM cell subsets with increasing age. Circulating TEM cells may be enriched in specificities for chronic and systemic pathogens, but not for microbiota in tissues. We propose that this sequestration of memory T cell specificities in distinct anatomic compartments could be a mechanism to stabilize and preserve pathogen-specific memory T cells, and maintain immune homeostasis in the body. Through additional tissue sampling and integration of large datasets with computation and statistical approaches, it will be possible in future studies to directly test this model (Fig. 4) and map the organization of T cell subsets and specificities within the human body throughout life.
Figure 4. Model for compartmentalization of antigen-specific memory T cell subsets in space and time.
This schematic shows the relative naïve and memory T cell subset frequencies in the circulation and peripheral sites and at different life stages. The schematic also shows the biased specificity for microbial antigens derived from pathogens that has been observed in specific tissue sites, including cytomegalovirus (CMV) in the blood; Epstein-Barr virus (EBV) in the spleen; vaccinia virus in lymph nodes; influenza virus in the lungs; rotavirus in the intestine and herpes simplex virus (HSV) and varicella zoster virus (VZV) in the skin. In addition, the specificities of some memory T cells at mucosal sites (lungs, intestine and skin) are biased for antigens from the microflora. The relative frequencies of each T cell subset in each tissue site for youths through adults are compiled from Ref. 11, and are extrapolated for infants based on Refs. 21, 22. At birth, there is a preponderance of naïve T cells in the circulation, and an abundance of mucosal microbial antigens are encountered during infancy, resulting in seeding of mucosal sites with TEM cells specific for mucosal pathogens, which could develop into TRM cells in situ. Infant skin has few, if any T cells (R. Clark, personal communication) and therefore estimates for memory T cell content in skin begin during youth. During childhood, exposure to the ubiquitous pathogenic and non-pathogenic microbial species in each site occurs and new memory T cells are formed which are partitioned as TRM cells in skin and mucosal sites, and as TCM cells in lymphoid tissues. This basic partitioning of antigen-specific memory T cell subsets in tissues is maintained during adulthood, with more TEM and TCM cell subsets gradually accumulating in the circulation and lymph nodes that could potentially replenish and/or convert to TRM cells that are lost through attrition.
Implications for Vaccines
The induction of memory T cells through vaccination has great potential to provide efficacious protective immunity to multiple types of pathogens, including viral, intracellular bacterial and parasitic infections, because of their broad specificity for internal and conserved pathogen epitopes, their residence in diverse sites of infection, and their longevity. Immune protection for existing vaccines is largely associated with the generation of neutralizing antibodies, whereas the potential of memory T cells to mediate protection has not been realized141. Several recent human challenge studies have demonstrated a protective correlate for memory T cells. One such study with influenza virus found a direct correlation between the presence of virus-specific memory CD4+ T cells and reduced illness to influenza virus challenge142. In addition, generation of circumsporozoite protein (CSP)-specific memory CD4+ T cells from a malaria vaccine was correlated with protective anti-parasite immunity143. Multifunctional circulating memory CD4+ T cells are similarly associated with HIV-infected non-progressors79. Therefore, while generation of CD8+ memory T cells is often the focus of mouse studies for protection, memory CD4+ T cells may also be a relevant subset to target in humans.
The consideration of anatomic location of vaccine administration appears critical in the design of vaccines to promote generation of pathogen-specific memory T cells at the right place and time. Administering a vaccine or attenuated pathogen at the site of infection in the skin, lung, rectal or genital mucosal surfaces could enhance generation of TRM cells in vivo. Studies in mice have tested a “prime and pull” strategy in which pathogen-specific T cells are primed through systemic vaccines and then recruited specifically to a tissue site through the local administration of chemoattractants53. In humans, the introduction of the smallpox vaccine through skin scarification promotes local T cell responses, and long-term persistence of vaccinia virus-specific memory CD4+ T cells23,125. There are also promising results from HIV vaccine studies in non-human primate models using CMV-mediated vectors to promote polyclonal generation of TEM cells to multiple epitopes144. HIV subunit vaccination using these CMV vectors via multiple routes leads to TEM cell generation and population in mucosal and lymphoid sites, and lasting protective immunity145,146. These studies show that adjusting the route and mode of immunization can enable memory T cell-mediated protective immunity to intractable pathogens.
Timing is another important consideration for memory T cell generation and vaccine design. Memory T cells generated early in life are maintained at a measurable frequency throughout a healthy adulthood, based on studies in peripheral blood31,94, suggesting that priming for memory T cell generation at early life stages, when fewer memory T cells clones are present, enables them to establish a niche for long-term persistence. Peripheral tissues have low numbers of memory T cells at birth, but become populated by memory T cells by early childhood (see above), indicating that site-specific vaccines could promote effective tissue targeting at early life stages. Other considerations, such as targeting of tissue dendritic cells for the development of protective immunity at specific sites71, and the co-administration of immunomodulators to enhance memory T cell generation147, could be integrated into the design of vaccines for generating durable, in situ T cell immunity.
Conclusions and perspective
Improved and more sensitive methods to determine function and antigen specificity of memory T cells, combined with high throughput approaches, are enabling us to define the molecular landscape of human immune cells. When combined with human tissue and blood samples, we have an unprecedented opportunity to obtain a more comprehensive understanding of the immune system in the context of the human lifespan, which is not possible in animal models. Future studies examining site-specific immune responses should focus on understanding how memory T cells are generated in early life and on clarifying the role of the microbiome in these processes to identify novel strategies for promoting effective immunoregulation and pathogen-specific immunity.
Key Points.
Most of our understanding of memory T cell generation, function, and maintenance is based on mouse studies, which cannot recapitulate the decades-long exposure to diverse antigens and microbiota that occurs in humans.
Memory T cell frequency changes dynamically throughout the human lifetime that can be divided into three phases: generation, homeostasis, and immunosenescence.
CD45RO+CD45RA− T cells comprise diverse memory subsets, including central memory (TCM), effector memory (TEM), memory T cells (TSCM) and resident memory (TRM), that are heterogeneous in their generation, distribution, and function.
Antigen-specific memory T cells to ubiquitous pathogens and possibly endogenous flora are generated early in life, and are preferentially compartmentalized at the sites of infection throughout adulthood.
Human memory T cells in diverse tissue sites are homeostatically maintained potentially through tonic TCR signaling, can exhibit extensive cross-reactivity and persist for decades.
Induction of memory CD8+ and CD4+ T cells through vaccination can enhance protection against pathogens, and may be improved by considering anatomic location and timing of administration during early life stages.
Acknowledgements
The authors wish to thank Joseph Thome and Damian Turner for critical review of the manuscript. D.L.F. is supported by NIH grants AI106697, AI100119, and AI083022.
Glossary terms
- Memory homeostasis
The stable maintenance of memory T cell numbers through multiple mechanisms including continuous turnover, responses to homeostatic cytokines and non-cognate T cell receptor interactions.
- immunosenescence
The decreased function of the immune system with age. In particular, the number of naive T cells decreases as thymic function decreases.
- telomeres
Regions of highly repetitive DNA at the end of linear eukaryotic chromosomes. They protect the ends of the chromosome from shortening on replication.
- ELISPOT
An antibody-capture-based method for enumerating specific CD4+ and CD8+ T cells that secrete a particular cytokine (often interferon-γ).
- MHC tetramer
A method of visualizing antigen-specific T cells by flow cytometry. Typically, four MHC molecules with their associated peptides are held together by streptavidin, which has four binding sites for biotin, which is attached to the tail of the MHC molecule. These four-peptide–MHC complexes (tetramers) can bind peptide-specific T cell receptors. The streptavidin molecules are often labelled with a fluorochrome so that binding can be assessed by flow cytometry.
Biographies
Donna L. Farber obtained her Ph.D. in Biochemistry and Molecular Biology and did postdoctoral training in immunology at Yale University. She is currently a Professor of Surgical Sciences and Microbiology and Immunology at Columbia University in the Columbia Center for Translational Immunology. Her research interests are in immunological memory, anti-viral T cell immunity and human immunology. Website: http://www.microbiology.columbia.edu/faculty/farber.html
Naomi Yudanin is a graduate student in the department of Microbiology and Immunology at Columbia University. She received her undergraduate degree in Molecular Biology and Chemistry from Carnegie Mellon University. Since joining Dr. Farber’s laboratory, she has focused on systematically identifying mechanisms underlying memory T cell migration and maintenance.
Nicholas Restifo obtained his M.D. and post-doctoral training in New York before becoming a principal investigator at the NCI/NIH in Bethesda. His primary interests are the differentiation of mouse and human T cells and how this differentiation impacts the therapeutic effectiveness of T cells in patients with infectious diseases and cancer. website: http://ccr.cancer.gov/staff/staff.asp?profileid=5762
References
- 1.Remakus S, Sigal LJ. Memory CD8(+) T cell protection. Adv Exp Med Biol. 2013;785:77–86. doi: 10.1007/978-1-4614-6217-0_9. [DOI] [PubMed] [Google Scholar]
- 2.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teijaro JR, et al. Costimulation modulation uncouples protection from immunopathology in memory T cell responses to influenza virus. J Immunol. 2009;182:6834–6843. doi: 10.4049/jimmunol.0803860. [DOI] [PubMed] [Google Scholar]
- 4. Teijaro JR, et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. This study identified retention of CD4+ TRM cells in mouse lungs and demonstrated superior protective capacity of lung TRM cells compared with circulating spleen memory CD4+ T cells to influenza virus infection.
- 5.Teijaro JR, Verhoeven D, Page CA, Turner D, Farber DL. Memory CD4 T cells direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J Virol. 2010;84:9217–9226. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khader SA, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4(+) T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 7.Anthony RM, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFall-Ngai M. Adaptive immunity: care for the community. Nature. 2007;445:153. doi: 10.1038/445153a. [DOI] [PubMed] [Google Scholar]
- 9. Cossarizza A, et al. CD45 isoforms expression on CD4+ and CD8+ T cells throughout life, from newborns to centenarians: implications for T cell memory. Mech Ageing Dev. 1996;86:173–195. doi: 10.1016/0047-6374(95)01691-0. An early survey of memory T cell frequencies in peripheral blood throughout the human lifespan.
- 10.Christensen KL, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025–1035. doi: 10.1086/605562. [DOI] [PubMed] [Google Scholar]
- 11. Sathaliyawala T, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. This study describes a whole-body analysis of T cell subsets in the blood and in multiple lymphoid and mucosal sites from individual organ donors. It identifies how memory T cell subsets are differentially compartmentalized in tissue sites, which is remarkably conserved between diverse individuals.
- 12. Saule P, et al. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. 2006;127:274–281. doi: 10.1016/j.mad.2005.11.001. An excellent survey of peripheral blood T cell subsets in a large cohort of individuals from birth to old age.
- 13.den Braber I, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolich-Zugich J, Rudd BD. Immune memory and aging: an infinite or finite resource? Curr Opin Immunol. 2010;22:535–540. doi: 10.1016/j.coi.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark RA, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 17.Clark RA, et al. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J Invest Dermatol. 2006;126:1059–1070. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- 18. Purwar R, et al. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. The study describes the phenotype and functional properties of human TRM cells in lung tissue.
- 19. Ganusov VV, De Boer RJ. Do most lymphocytes in humans really reside in the gut? Trends Immunol. 2007;28:514–518. doi: 10.1016/j.it.2007.08.009. This study provides a novel quantitative assessment of human T cell numbers in mucosal and lymphoid tissues.
- 20.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 21.Bunders MJ, et al. Memory CD4(+)CCR5(+) T cells are abundantly present in the gut of newborn infants to facilitate mother-to-child transmission of HIV-1. Blood. 2012;120:4383–4390. doi: 10.1182/blood-2012-06-437566. [DOI] [PubMed] [Google Scholar]
- 22.Dos Santos AB, et al. Immune cell profile in infants' lung tissue. Ann Anat. 2013 doi: 10.1016/j.aanat.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Kupper TS. Old and new: recent innovations in vaccine biology and skin T cells. J Invest Dermatol. 2012;132:829–834. doi: 10.1038/jid.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders ME, et al. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988;140:1401–1407. [PubMed] [Google Scholar]
- 25.Smith SH, Brown MH, Rowe D, Callard RE, Beverley PC. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986;58:63–70. [PMC free article] [PubMed] [Google Scholar]
- 26. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. This publication is a seminal study that described memory T cell heterogeneity in humans and established a new paradigm for memory T cell heterogeneity.
- 27.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 28. Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. This study established the heterogeneous tissue distribution of anti-viral memory CD8+ T cells and the biased distribution of TEM cells in non-lymphoid sites.
- 29.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 30.Wang A, et al. The stoichiometric production of IL-2 and IFN-gamma mRNA defines memory T cells that can self-renew after adoptive transfer in humans. Sci Transl Med. 2012;4:149ra120. doi: 10.1126/scitranslmed.3004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pedron B, et al. Development of cytomegalovirus and adenovirus-specific memory CD4 T-cell functions from birth to adulthood. Pediatr Res. 2011;69:106–111. doi: 10.1203/PDR.0b013e318204e469. The authors provide a comprehensive assessment of CMV- and adenovirus-specific memory T cells in a very large cohort, in cross-sectional studies of individuals from birth throughout adulthood.
- 32.Champagne P, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 33.Ellefsen K, et al. Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections. Eur J Immunol. 2002;32:3756–3764. doi: 10.1002/1521-4141(200212)32:12<3756::AID-IMMU3756>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 34.Fearon DT, Carr JM, Telaranta A, Carrasco MJ, Thaventhiran JE. The rationale for the IL-2-independent generation of the self-renewing central memory CD8+ T cells. Immunol Rev. 2006;211:104–118. doi: 10.1111/j.0105-2896.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 35. Gattinoni L, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. This study identified a new. self-renewing population of memory T cells in human blood, designated TSCM cells, and provides functional and phenotypic characterization and enumerates their potential in immunotherapy.
- 36.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 37.Gattinoni L, Ji Y, Restifo NP. Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res. 2010;16:4695–4701. doi: 10.1158/1078-0432.CCR-10-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12:671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9:662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 42.Buchholz VR, et al. Disparate individual fates compose robust CD8+ T cell immunity. Science. 2013;340:630–635. doi: 10.1126/science.1235454. [DOI] [PubMed] [Google Scholar]
- 43.Gerlach C, et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science. 2013;340:635–639. doi: 10.1126/science.1235487. [DOI] [PubMed] [Google Scholar]
- 44.Stemberger C, et al. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 46.Campbell JJ, et al. CCR7 expression and memory T cell diversity in humans. J Immunol. 2001;166:877–884. doi: 10.4049/jimmunol.166.2.877. [DOI] [PubMed] [Google Scholar]
- 47.Turner DL, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.67. online before print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herndler-Brandstetter D, et al. Human bone marrow hosts polyfunctional memory CD4+ and CD8+ T cells with close contact to IL-15-producing cells. J Immunol. 2011;186:6965–6971. doi: 10.4049/jimmunol.1100243. [DOI] [PubMed] [Google Scholar]
- 49.Klonowski KD, et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 50. Clark RA, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4:117ra117. doi: 10.1126/scitranslmed.3003008. By examining a cohort of patients on T cell-depletion therapy that reduces peripheral T cell numbers, this study provides evidence that TRM cells in human skin can provide protection to virus infection.
- 51.Liu L, et al. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mackay LK, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 56.Anderson KG, et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol. 2012;189:2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casey KA, et al. Antigen-Independent Differentiation and Maintenance of Effector-like Resident Memory T Cells in Tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol. 2012;188:5811–5817. doi: 10.4049/jimmunol.1102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gebhardt T, Mackay LK. Local immunity by tissue-resident CD8(+) memory T cells. Front Immunol. 2012;3:340. doi: 10.3389/fimmu.2012.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jiang X, et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. This study demonstrates in mice that skin TRM do not circulate and also mediate protection to viral infection of the skin.
- 62.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 63.Wakim LM, Gebhardt T, Heath WR, Carbone FR. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J Immunol. 2008;181:5837–5841. doi: 10.4049/jimmunol.181.9.5837. [DOI] [PubMed] [Google Scholar]
- 64.Tokoyoda K, et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 65.Lugli E, et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013;123:594–599. doi: 10.1172/JCI66327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol. 2013;34:27–32. doi: 10.1016/j.it.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130:362–370. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Homey B, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 69.Kunkel EJ, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agace WW. T-cell recruitment to the intestinal mucosa. Trends Immunol. 2008;29:514–522. doi: 10.1016/j.it.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 71.Ruane D, et al. Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. J Exp Med. 2013;210:1871–1888. doi: 10.1084/jem.20122762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edele F, et al. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol. 2008;181:3745–3749. doi: 10.4049/jimmunol.181.6.3745. [DOI] [PubMed] [Google Scholar]
- 73.Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T cell expression of alpha4beta7 integrin. Eur J Immunol. 2002;32:1445–1454. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 74.Cimbro R, et al. IL-7 induces expression and activation of integrin alpha4beta7 promoting naive T-cell homing to the intestinal mucosa. Blood. 2012;120:2610–2619. doi: 10.1182/blood-2012-06-434779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang HH, et al. CCR2 identifies a stable population of human effector memory CD4+ T cells equipped for rapid recall response. J Immunol. 2010;185:6646–6663. doi: 10.4049/jimmunol.0904156. [DOI] [PubMed] [Google Scholar]
- 76.Kim CH, et al. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–1339. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev. 2003;195:58–71. doi: 10.1034/j.1600-065x.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 78.Andrew DP, et al. C-C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J Immunol. 2001;166:103–111. doi: 10.4049/jimmunol.166.1.103. [DOI] [PubMed] [Google Scholar]
- 79.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 80.Zhang X, et al. Human bone marrow: a reservoir for "enhanced effector memory" CD8+ T cells with potent recall function. J Immunol. 2006;177:6730–6737. doi: 10.4049/jimmunol.177.10.6730. [DOI] [PubMed] [Google Scholar]
- 81. Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214–221. doi: 10.4049/jimmunol.180.1.214. This study demonstrates how IL-17-producing human memory T cells are specifically maintained within a CCR6-expressing subset.
- 82.Wan Q, et al. Cytokine signals through PI-3 kinase pathway modulate Th17 cytokine production by CCR6+ human memory T cells. J Exp Med. 2011;208:1875–1887. doi: 10.1084/jem.20102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cosmi L, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. This study identifies a specific subset of IL-17-producing memory T cells in inflamed tissues that expresses CD161.
- 84.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 85.Eyerich S, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murali-Krishna K, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 87.Chomont N, DaFonseca S, Vandergeeten C, Ancuta P, Sekaly RP. Maintenance of CD4+ T-cell memory and HIV persistence: keeping memory, keeping HIV. Curr Opin HIV AIDS. 2011;6:30–36. doi: 10.1097/COH.0b013e3283413775. [DOI] [PubMed] [Google Scholar]
- 88.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eisele E, Siliciano RF. Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity. 2012;37:377–388. doi: 10.1016/j.immuni.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chakrabarti LA, Simon V. Immune mechanisms of HIV control. Curr Opin Immunol. 2010;22:488–496. doi: 10.1016/j.coi.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Youngblood B, Wherry EJ, Ahmed R. Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells. Curr Opin HIV AIDS. 2012;7:50–57. doi: 10.1097/COH.0b013e32834ddcf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walker B, McMichael A. The T-cell response to HIV. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a007054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marchant A, et al. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest. 2003;111:1747–1755. doi: 10.1172/JCI17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Komatsu H, et al. Large scale analysis of pediatric antiviral CD8+ T cell populations reveals sustained, functional and mature responses. Immun Ageing. 2006;3:11. doi: 10.1186/1742-4933-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He XS, et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80:11756–11766. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He XS, et al. Analysis of the frequencies and of the memory T cell phenotypes of human CD8+ T cells specific for influenza A viruses. J Infect Dis. 2003;187:1075–1084. doi: 10.1086/368218. [DOI] [PubMed] [Google Scholar]
- 97.Amyes E, et al. Characterization of the CD4+ T cell response to Epstein-Barr virus during primary and persistent infection. J Exp Med. 2003;198:903–911. doi: 10.1084/jem.20022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Callan MF, et al. CD8(+) T-cell selection, function, and death in the primary immune response in vivo. J Clin Invest. 2000;106:1251–1261. doi: 10.1172/JCI10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hillaire ML, et al. Characterization of the human CD8(+) T cell response following infection with 2009 pandemic influenza H1N1 virus. J Virol. 2011;85:12057–12061. doi: 10.1128/JVI.05204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Miller JD, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. This study demonstrates the generation of virus-specific effector and memory CD8 T cell responses in peripheral blood at sequential timepoints in humans following yellow fever vaccination.
- 101.Jaimes MC, et al. Frequencies of virus-specific CD4(+) and CD8(+) T lymphocytes secreting gamma interferon after acute natural rotavirus infection in children and adults. J Virol. 2002;76:4741–4749. doi: 10.1128/JVI.76.10.4741-4749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bont L, et al. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatr Res. 2002;52:363–367. doi: 10.1203/00006450-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 103.de Waal L, et al. Moderate local and systemic respiratory syncytial virus-specific T-cell responses upon mild or subclinical RSV infection. J Med Virol. 2003;70:309–318. doi: 10.1002/jmv.10396. [DOI] [PubMed] [Google Scholar]
- 104.Gonzalez PA, et al. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc Natl Acad Sci U S A. 2008;105:14999–15004. doi: 10.1073/pnas.0802555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reed JR, et al. Telomere erosion in memory T cells induced by telomerase inhibition at the site of antigenic challenge in vivo. J Exp Med. 2004;199:1433–1443. doi: 10.1084/jem.20040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vukmanovic-Stejic M, et al. Varicella zoster-specific CD4+Foxp3+ T cells accumulate after cutaneous antigen challenge in humans. J Immunol. 2013;190:977–986. doi: 10.4049/jimmunol.1201331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Bree GJ, et al. Characterization of CD4+ memory T cell responses directed against common respiratory pathogens in peripheral blood and lung. J Infect Dis. 2007;195:1718–1725. doi: 10.1086/517612. [DOI] [PubMed] [Google Scholar]
- 108. de Bree GJ, et al. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J Exp Med. 2005;202:1433–1442. doi: 10.1084/jem.20051365. This report describes the initial finding of biased distribution of memory T cells specific for a lung pathogen in the lung compared with peripheral blood.
- 109.Piet B, et al. CD8+ T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J Clin Invest. 2011 doi: 10.1172/JCI44675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhu J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Molberg O, et al. CD4+ T cells with specific reactivity against astrovirus isolated from normal human small intestine. Gastroenterology. 1998;114:115–122. doi: 10.1016/s0016-5085(98)70639-0. [DOI] [PubMed] [Google Scholar]
- 112.Sewell AK. Why must T cells be cross-reactive? Nat Rev Immunol. 2012;12:669–677. doi: 10.1038/nri3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee LY, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008 doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Roti M, et al. Healthy Human Subjects Have CD4+ T Cells Directed against H5N1 Influenza Virus. J Immunol. 2008;180:1758–1768. doi: 10.4049/jimmunol.180.3.1758. References 113 and 114 demonstrate cross-reactivity of memory CD4+ T cells, and identifies pre-existing memory T cells specific for avian influenza in individuals who never were exposed to this virus.
- 115. Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. This article demonstrates cross-reactivity of virus-specific memory T cells using tetramer enrichment and identifies HIV-specific memory T cells in individuals who were never infected.
- 116.D'Orsogna LJ, Roelen DL, Doxiadis II, Claas FH. Alloreactivity from human viral specific memory T-cells. Transpl Immunol. 2010;23:149–155. doi: 10.1016/j.trim.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 117.Wucherpfennig KW. T cell receptor crossreactivity as a general property of T cell recognition. Mol Immunol. 2004;40:1009–1017. doi: 10.1016/j.molimm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 118.Burrows SR, Khanna R, Burrows JM, Moss DJ. An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross-reactive with a single Epstein-Barr virus CTL epitope: implications for graft-versus-host disease. J Exp Med. 1994;179:1155–1161. doi: 10.1084/jem.179.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 121.Clute SC, et al. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J Clin Invest. 2005;115:3602–3612. doi: 10.1172/JCI25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Duchmann R, et al. T cell specificity and cross reactivity towards enterobacteria, bacteroides, bifidobacterium, and antigens from resident intestinal flora in humans. Gut. 1999;44:812–818. doi: 10.1136/gut.44.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hand TW, et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337:1553–1556. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hammarlund E, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. This study provides direct evidence that human memory T cells generated through vaccination are long-lived without repeat exposures.
- 126.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 127.Bushar ND, Corbo E, Schmidt M, Maltzman JS, Farber DL. Ablation of SLP-76 signaling after T cell priming generates memory CD4 T cells impaired in steady-state and cytokine-driven homeostasis. Proc Natl Acad Sci U S A. 2010;107:827–831. doi: 10.1073/pnas.0908126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat Immunol. 2002;3:244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 129.Kassiotis G, Gray D, Kiafard Z, Zwirner J, Stockinger B. Functional specialization of memory Th cells revealed by expression of integrin CD49b. J Immunol. 2006;177:968–975. doi: 10.4049/jimmunol.177.2.968. [DOI] [PubMed] [Google Scholar]
- 130.Macallan DC, et al. Measurement and modeling of human T cell kinetics. Eur J Immunol. 2003;33:2316–2326. doi: 10.1002/eji.200323763. [DOI] [PubMed] [Google Scholar]
- 131.Macallan DC, et al. Rapid turnover of effector-memory CD4(+) T cells in healthy humans. J Exp Med. 2004;200:255–260. doi: 10.1084/jem.20040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. De Boer RJ, Perelson AS. Quantifying T lymphocyte turnover. J Theor Biol. 2013;327:45–87. doi: 10.1016/j.jtbi.2012.12.025. This article contains an interesting mathematical analysis of human T cell proliferation studies.
- 133.Vukmanovic-Stejic M, et al. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Weng NP, Levine BL, June CH, Hodes RJ. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci U S A. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weng NP, Araki Y, Subedi K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat Rev Immunol. 2012;12:306–315. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miconnet I. Probing the T-cell receptor repertoire with deep sequencing. Curr Opin HIV AIDS. 2012;7:64–70. doi: 10.1097/COH.0b013e32834ddcae. [DOI] [PubMed] [Google Scholar]
- 137.Youngblood B, Davis CW, Ahmed R. Making memories that last a lifetime: heritable functions of self-renewing memory CD8 T cells. Int Immunol. 2010;22:797–803. doi: 10.1093/intimm/dxq437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Robins HS, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114:4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Robins HS, et al. Overlap and effective size of the human CD8+ T cell receptor repertoire. Sci Transl Med. 2010;2:47ra64. doi: 10.1126/scitranslmed.3001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Siegel AM, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35:806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zinkernagel RM. Immunological memory not equal protective immunity. Cell Mol Life Sci. 2012;69:1635–1640. doi: 10.1007/s00018-012-0972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wilkinson TM, et al. Preexisting influenza-specific CD4(+) T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–280. doi: 10.1038/nm.2612. The authors conduct a live challenge study with influenza virus in human subjects and establish the protective correlate to reduced illness is the quantity of circulating influenza-specific memory CD4+ T cells.
- 143.Lumsden JM, et al. Protective immunity induced with the RTS,S/AS vaccine is associated with IL-2 and TNF-alpha producing effector and central memory CD4 T cells. PLoS One. 2011;6:e20775. doi: 10.1371/journal.pone.0020775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hansen SG, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013;340:1237874. doi: 10.1126/science.1237874. Intriguing study showing that vaccination with CMV-based vectors expressing HIV gag protein generates robust effector-memory CD8+ T cell responses with remarkably broad specificity to epitopes presented by MHC class I and class II alleles in a non-human primate model.
- 145.Hansen SG, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hansen SG, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013 doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Altman JD, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 149.Bakker AH, Schumacher TN. MHC multimer technology: current status and future prospects. Curr Opin Immunol. 2005;17:428–433. doi: 10.1016/j.coi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 150.Tan LC, et al. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. [PubMed] [Google Scholar]
- 151.Bendall SC, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Newell EW, et al. Combinatorial tetramer staining and mass cytometry analysis facilitate T-cell epitope mapping and characterization. Nat Biotechnol. 2013;31:623–629. doi: 10.1038/nbt.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]