Abstract
Toll-like receptor 5 (TLR5) has been widely studied in an inflammatory context, but the effect of TLR5 on the adaptive response to bacterial flagellin has received considerably less attention. Here, we demonstrate that TLR5 expression by DCs allows a 1000-fold enhancement of T cell sensitivity to flagellin, and this enhancement did not require the expression of NLRC4 or Myd88. The effect of TLR5 on CD4 T cell sensitivity was independent of the adjuvant effect of flagellin and TLR5 ligation did not alter the sensitivity of OVA-specific T cells to OVA. In the spleen, the exquisite T cell sensitivity to flagellin was regulated by CD4−CD8α− dendritic cells and was blocked by a monoclonal antibody to TLR5. In the mesenteric lymph nodes, flagellin-specific T cell activation was regulated by a population of CD103−CD11b+ DCs. Thus, TLR5 expression by mucosal and systemic DC subsets controls the sensitivity of the adaptive immune response to flagellated pathogens.
Introduction
Flagellin subunits form the major filament of the flagella and are produced in large quantities by flagellated bacteria1. Different leukocytes populations express surface TLR5, which can recognize extracellular flagellin and initiate an inflammatory response2. As with many TLRs, TLR5 signaling is dependent upon the recruitment of adaptor protein Myd88, but does not utilize TRIF (TIR-domain-containing adaptor-inducing interferon-β)3. The conserved structure of bacterial flagellins ensures that TLR5 can detect a wide array of flagellated bacteria including Listeria, Salmonella, Legionella, and Pseudomonas2, 4. Bacterial flagellins can also be recognized by the inflammasome complex and induce inflammatory responses via NAIP5 and NLRC45–8. Given their proinflammatory properties, flagellins can function as effective adjuvants4, 9 and are currently being tested in clinical trials10, 11.
Unlike most TLR ligands, flagellins are also proteins and can be processed and presented by host MHC molecules and directly recognized by flagellin-specific T cells12. Our laboratory and others have identified natural MHC class-II epitopes of Salmonella flagellin13–15, which has allowed detailed study of flagellin-specific T cell responses in mice12, 16. Flagellin-specific CD4 T cells dominate the early immune response to intestinal Salmonella infection14, and flagellins are also a major target antigen in murine and human inflammatory bowel disease17. Immune reactivity to flagellins correlates with increasingly severe intestinal disease in patients suffering from Crohn’s disease18, 19. Thus, flagellins are unusual bacterial proteins that can be simultaneously recognized by multiple innate and adaptive immune receptors.
Dendritic cells (DCs) are antigen-presenting cells that are uniquely able to integrate signals from TLRs and control the activation of naïve T cells in secondary lymphoid tissues20. It has been thought that murine splenic DCs lack TLR5 expression since they do not produce an inflammatory response to flagellin in vitro21–23. Indeed, a comprehensive analysis of TLR5 expression found that TLR5-expressing DCs were restricted to the intestinal lamina propria (LP)24. However, a more recent study detected a TLR5-dependent adjuvant effect in draining lymph node DCs25, demonstrating that TLR5 can be expressed by DCs outside the intestinal LP.
Recently, we reported a requirement for TLR5 expression for induction of adaptive immune responses to flagellin after immunization or oral infection26–29. Here, we have examined the role of TLR5 in DC antigen presentation of a natural flagellin epitope from Salmonella Typhimurium. We report that TLR5 is essential and NLRC4 dispensable for flagellin-specific CD4 T cell expansion in vivo. Furthermore, expression of TLR5 allowed the host to mount a flagellin-specific immune response to very low amounts of antigen. This exquisite sensitivity to flagellin was also reproduced in vitro where TLR5 expression by DCs conferred a 1000-fold increase in the ability to activate flagellin-specific CD4 T cells, an effect that was distinct from the adjuvant effect of flagellin and did not require NLRC4 or Myd88 expression. In addition, we identified CD4−CD8α− DCs in the spleen as a unique subset that can regulate enhanced activation of flagellin-specific CD4 T cells. Furthermore, a corresponding population of CD103−CD11b+ DCs isolated from the mesenteric lymph nodes was able to regulate T cell sensitivity to bacterial flagellin in intestinal lymphoid tissues.
Results
TLR5 expression is required for the clonal expansion of flagellin-specific T cells in vivo
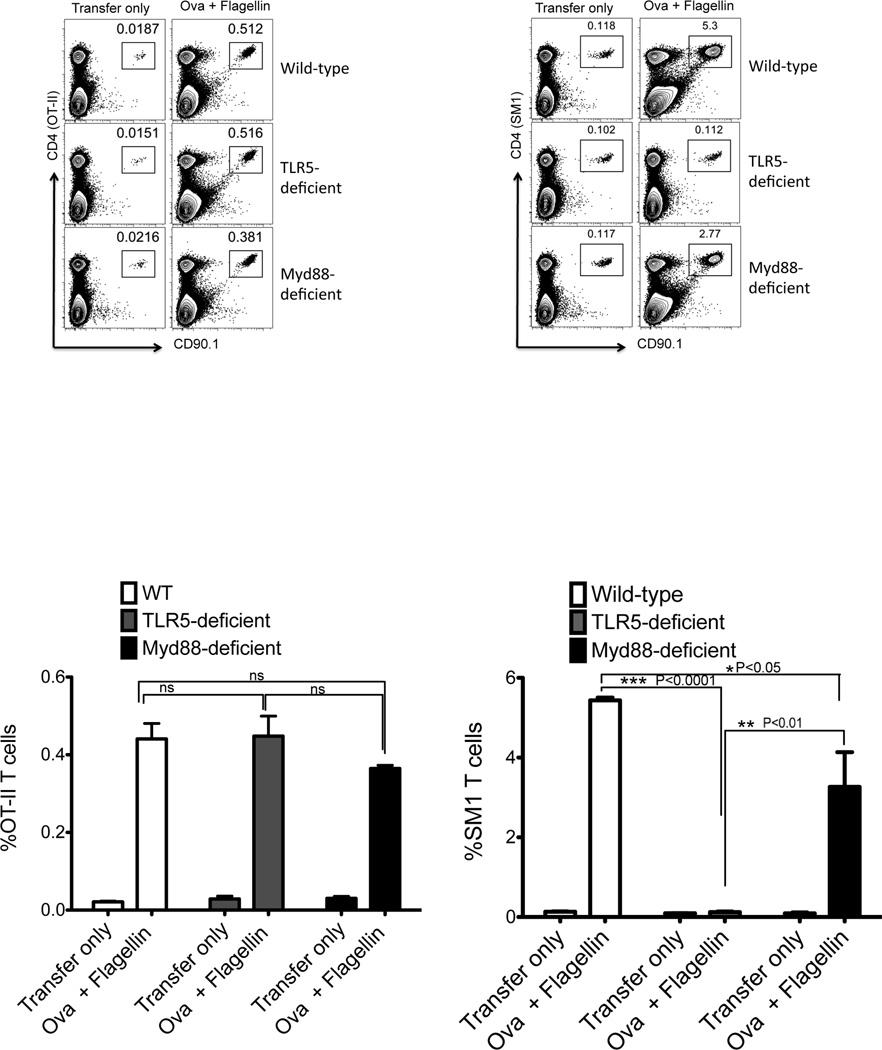
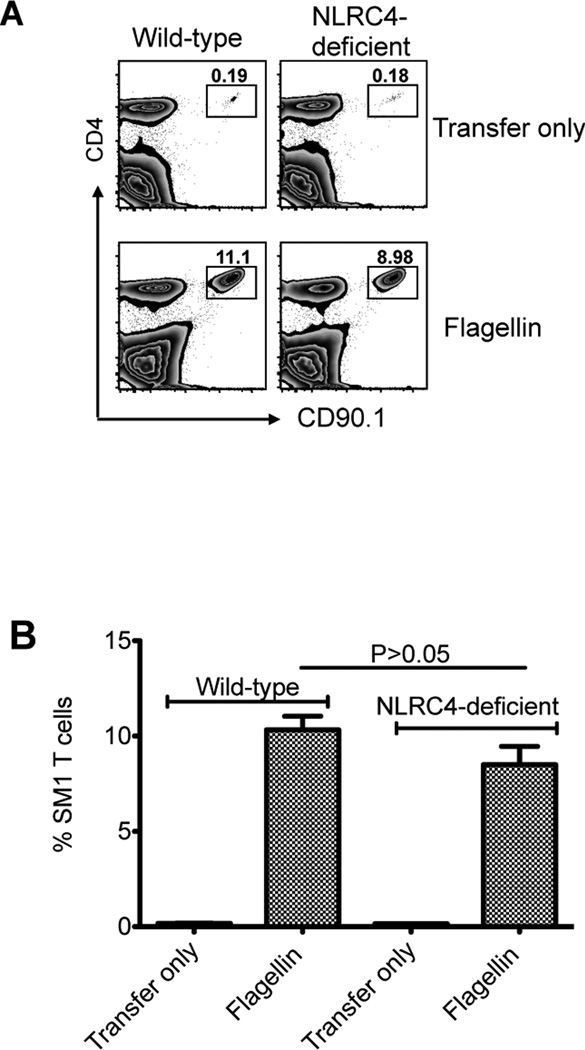
Given the dominance of flagellin-specific T cell responses in infectious and inflammatory disease14, 17, we examined how TLR5 or Myd88 expression affected the initial clonal expansion of OVA-, or flagellin-specific, T cells in vivo. C57BL/6 (wild-type) or TLR5-deficient mice were adoptively transferred with OVA-specific OT-II T cells, or flagellin-specific SM1 T cells, and immunized with a mixture of flagellin and OVA. OT-II T cells expanded similarly in wild-type, TLR5-, and Myd88-deficient mice (Fig. 1), demonstrating that TLR5 or Myd88 are not essential for the initial clonal expansion of OVA-specific T cells. In marked contrast, SM1 T cells expanded in wild-type and Myd88-deficient mice, but failed to expand in TLR5-deficient mice (Fig. 1). Although both SM1 and OT-II T cells expanded in Myd88-deficient mice, the level of expansion was slightly reduced compared to wild-type mice, most likely due to a reduction in the inflammatory response mediated by Myd88 (Fig. 1). NLRC4 is an inflammasome component that allows intracellular recognition of flagellin30, and has previously been implicated in the induction of flagellin-specific antibody responses31. However, wild-type and NLRC4-deficient mice both developed robust SM1 clonal expansion three days after immunization of flagellin, although the level of expansion was slightly lower in NLRC4-deficient recipients (Fig. 2). Together, these experiments demonstrate that TLR5 expression is required for the in vivo expansion of flagellin-specific CD4 T cells to flagellin immunization.
Figure 1. In vivo expansion of flagellin-specific T cells requires TLR5, but not Myd88.
Wild-type, TLR5-deficient, and Myd88-deficient mice were adoptively transferred with 1×106 CD90.1 OT-II or SM1 TCR transgenic T cells and immunized with 100µg OVA plus 1µg flagellin. Three days later clonal expansion of OT-II and SM1 T cells was assessed in the spleen. (A) FACS plots show CD4 and CD90.1 staining of the gating on live cells. (B) Graphs show the mean percentage +/− SEM of OT-II and SM1 T cells, (★★★) denotes a p value <0.0001, (★★) denotes a p value <0.01, and (★) denotes a p value <0.05, as analyzed by two-way ANOVA.
Figure 2. Robust flagellin-specific T cell responses in NLRC4-deficient mice.
Wild-type and NLRC4-deficient mice were adoptively transferred with CFSE labeled SM1 T cells and immunized intravenously the following day with 1µg of flagellin and 10µg of LPS. (A) FACS plot show expansion of SM1 T cells (CD4 and CD90.1 positive) in the spleen of wild-type and NLRC4-deficient mice three days after immunization. (B) Bar plots show the percentage of SM1 T cells in the spleen of wild-type and NLRC4-deficient mice three days after immunization. There is no significant difference between immunized groups, as evaluated by unpaired t-test (p<0.05).
TLR5 regulates adaptive immune sensitivity to bacterial flagellin
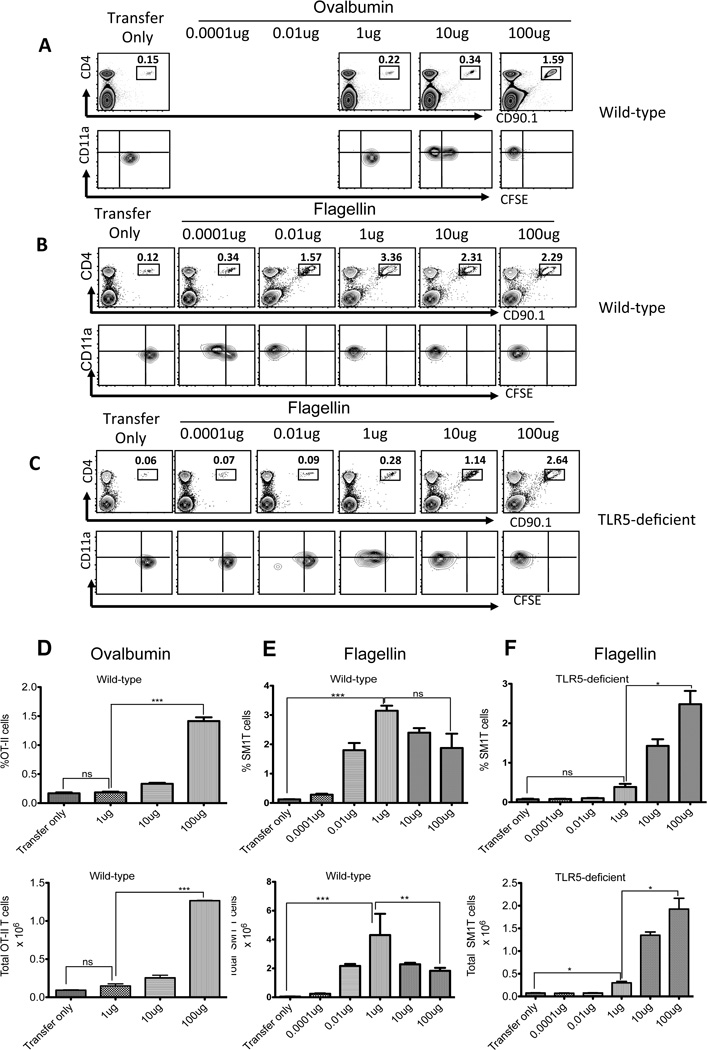
The experiments above suggested that TLR5 controls the host adaptive immune response to bacterial flagellin. In order to determine the in vivo threshold for antigen-specific T cell activation, we immunized mice with varying concentrations of OVA and flagellin and examined the clonal expansion and CFSE-dye dilution of OT-II and SM1 T cells. In wild-type mice, OT-II T cells were activated to undergo CFSE-dye dilution, expansion, and increased surface expression of CD11a, following immunization with 10–100µg OVA (Fig. 3A and D). Importantly, OT-II T cells did not respond to immunization with 1µg OVA, remained CD11alow, and did not dilute CFSE (Fig. 3A and D). In marked contrast, SM1 T cells diluted CFSE, clonally expanded, and increased surface expression of CD11a after immunization with as little as 0.0001µg (100pg) of flagellin (Fig. 3B and E). This exquisite sensitivity to low dose flagellin immunization was absent in mice lacking TLR5 (Fig. 3C and F). Indeed, SM1 T cells in TLR5-deficient mice were only able to respond to flagellin when doses of 1–100µg were used (Fig. 3C and F). This effect was distinct from the intrinsic adjuvant effect of flagellin since the expansion of OT-II T cells was unaffected by TLR5-deficiency, even when flagellin was used as an adjuvant, and T cell expansion was also detected in Myd88-deficient mice (Fig. 1). Thus, host expression of an innate receptor for flagellin (TLR5) allows for a 1000-fold enhancement in the sensitivity of the adaptive immune response to this same antigen.
Figure 3. TLR5 enhances T cell activation to low doses of flagellin.
1×106 CFSE labeled TCR transgenic T cells were adoptively transferred into wild-type and TLR5-deficient mice and the following day were immunized with different doses of ovalbumin (1–100µg) or flagellin (100pg–100µg) plus 10 µg of LPS. Three days later clonal expansion of (A) OT-II or (B–C) SM1 T cells was examined in the spleen. Upper plots show staining of CD4 and CD90.1 to identify transgenic T cells and lower plots show CD11a and CFSE staining after gating on OT-II and SM1 T cells. Each plot is a representative of three mice per group and two independent experiments. (D) Bar plots showing the percentage and total number of OT-II T cells in the spleen of Ova (1–100µg) immunized animals. (E–F) Plots show the percentage and total number (±SEM) of SM1 T cells in the spleen of (E) wild-type and (F) TLR5-deficient mice immunized with flagellin. Graphs combine the results of two independent experiments with three mice per group. Statistical significance was examined using unpaired t-test between the groups immunized with Ova (100µg) or flagellin (1µg) and transfer only. (★★★) denotes a p value <0.0001, (★★) denotes a p value <0.01, and (★) denotes a p value <0.05, as analyzed by unpaired t-test.
DC expression of TLR5 controls T cell sensitivity to flagellin
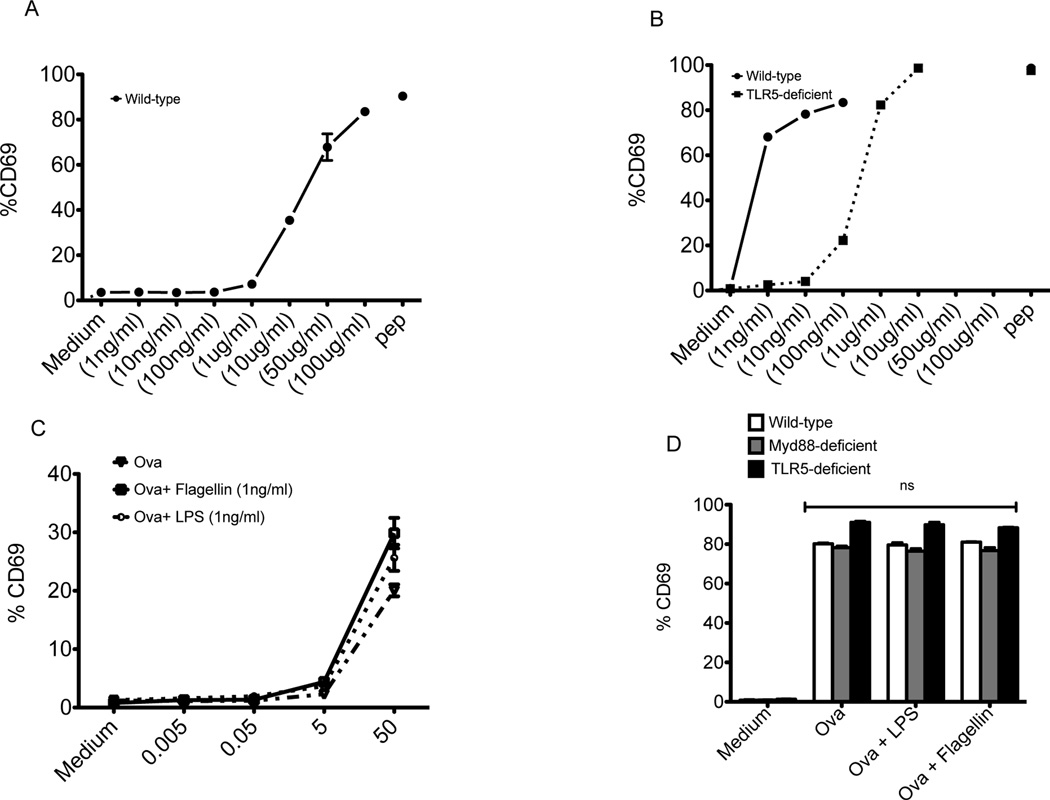
Lymph node and splenic dendritic cells are not thought to express TLR5 since these populations do not generate an inflammatory response to flagellins in vitro21–23. We examined whether TLR5 expression by splenic DCs was responsible for the sensitivity of flagellin specific T cell activation. CD11c+ DCs from the spleens of wild-type and TLR5-deficient mice were enriched by magnetic selection (Fig. S1A). CD11c+ DCs were incubated with OT-II or SM1 T cells in the presence or absence of OVA or flagellin, and T cell activation was subsequently examined. At 16 hours after incubation with 10–100µg/ml of OVA, OT-II T cells displayed increased expression of CD69, while lower doses failed to activate OVA-specific T cells (Fig. 4A-solid line). In a similar manner, SM1 T cells incubated with TLR5-deficient DCs were activated to express CD69 when a range of 1–100µg/ml of flagellin was added to cultures (Fig. 4B-dotted line). In marked contrast, DCs from wild-type mice were capable of activating SM1 T cells to express CD69 even when as little as 1ng/ml of flagellin was used (Fig. 4B-solid line). This difference in antigen presenting capacity at low doses was absent when flagellin peptide was used as a stimulus rather than whole flagellin protein (Fig. S2), indicating that the enhanced sensitivity to flagellin in wild-type DCs is a consequence of TLR5 expression rather than intrinsic differences in the capacity to present antigen. This TLR5-dependent effect was also distinct from the adjuvant effect of flagellin in vitro, since the addition of flagellin in vitro did not improve the sensitivity of the OT-II responses to OVA (Fig. 4C). Furthermore, activation of OT-II cells was largely unaffected by TLR5 deficiency, even when flagellin was added to cultures (Fig. 4D).
Figure 4. TLR5 expression by CD11c+DCs controls the sensitivity of flagellin-specific T cell activation.
DCs isolated from C57BL/6 (wild-type) and TLR5-deficient mice were cultured with TCR transgenic (SM1 or OT-II) T cells and various concentrations of antigen (flagellin or OVA). (A) Graph shows increased expression of CD69 on gated (CD4+CD90.1+) OT-II T cells after 16 hours of incubation with wild-type DCs and OVA (1ng–100µg)/ml. (B) Graph shows increased expression of CD69 on (CD4+CD90.1+) SM1 T cells after 16 hours of incubation in the presence of wild-type (solid line) or TLR5 deficient DCs (dotted line) with flagellin (1ng–10µg)/ml or flagellin peptide (6µM). (C) DCs were cultured with TCR transgenic OT-II T cells and various concentrations of OVA (µg/ml), OVA plus flagellin, or OVA plus LPS. Graph shows increased expression of CD69 on gated (CD4+CD90.1+) OT-II T cells after 16 hours of incubation with wild-type DCs. (D) DCs isolated from wild-type, TLR5-deficient, or Myd88-deficient mice were cultured with OT-II T cells and OVA (100µg/ml) or OVA plus flagellin (1ng/ml), or OVA plus LPS (1ng/ml). Graph shows increased expression of CD69 on (CD4+CD90.1+) OT-II T cells after 16 hours of incubation with wild-type, TLR5-deficient, or Myd88-deficient DCs.
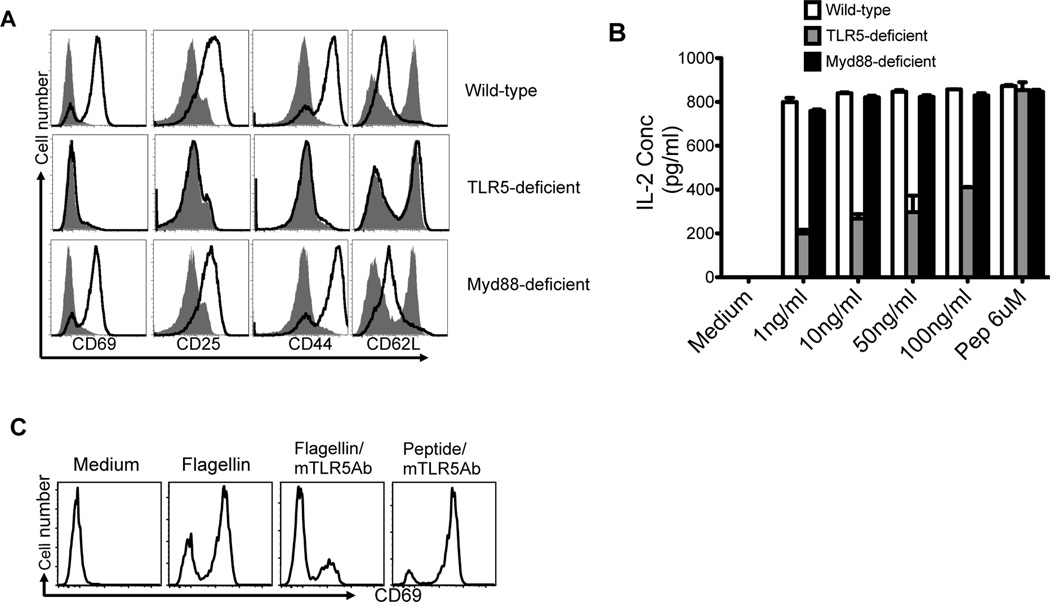
Next we examined the expression of multiple markers of CD4 T cell activation following stimulation of flagellin-specific T cells with low dose (1ng/ml) flagellin. Wild-type DCs induced SM1 T cells to increase the expression of CD25 and CD69, 16 hours after adding flagellin, while TLR5-deficient DCs did not (Fig. 5A). Similarly, at 48 hours, wild-type DCs induced SM1 T cells to increase expression of CD44 and reduce expression of CD62L, while TLR5-deficient DCs did not (Fig. 5A). Wild-type DCs also induced IL-2 production from naïve SM1 T cells at much lower flagellin concentrations than TLR5-deficient DCs (Fig. 5B). In contrast, TLR5-deficient DCs were able to induce an equivalent response if flagellin peptide (427–441) was used to stimulate SM1 cells (Fig. 5B). Since Myd88 expression is required for all previously reported functions of TLR54, we examined the activation of SM1 T cells using Myd88-deficient DCs. Myd88-deficient DCs remained fully capable of activating SM1 T cells to modulate expression of activation markers and secrete IL-2 (Fig. 5A and B). Thus, the sensitivity of flagellin-specific T cells is controlled by DC expression of TLR5, but does not require the expression of Myd88.
Figure 5. DC expression of TLR5, but not Myd88, is required to activate flagellin-specific T cells.
DCs from the spleen of wild-type, TLR5-deficient and Myd88-deficient mice were incubated with flagellin and SM1 T cells to examine T cell activation. (A–B) 1×105 DCs were cultured with 1×105 SM1 T cells for 16 (CD69 and CD25) or 48h (CD44 and CD62L) in the presence of 10 ng/ml of flagellin (solid line) or medium alone (shaded area). (A) Plots show cell surface expression of CD69, CD25, CD44, or CD62L after gating on (CD4+CD90.1+) SM1 T cells. (B) Production of IL-2 in culture supernatants was assessed by ELISA 48 hours after incubation with different concentrations of flagellin or medium alone. Graph shows mean IL-2 +/− SEM in tissue culture replicates after stimulation. (C) Prior treatment of DCs with a monoclonal anti-TLR5 antibody (10µg/ml) for 60 min inhibited flagellin-specific activation of SM1 T cells. Plots show CD69 surface staining after gating on CD4+CD90.1 SM1 T cells. All data are representative of three independent experiments.
Next, we examined whether inhibition of flagellin binding to TLR5 could interfere with the ability of DCs to activate flagellin-specific T cells. Wild-type CD11c+ DCs isolated from the spleen were incubated with SM1 T cells, flagellin, and a blocking TLR5 monoclonal antibody. Inhibition of TLR5 binding eliminated SM1 T cell activation (Fig. 5C), while this same antibody had no effect on the T cell response to processed flagellin peptide 427–441 (Fig. 5C). Thus, physical interaction of flagellin with TLR5 on DCs is a prerequisite for TLR5 to enhance flagellin-specific T cell responses.
Splenic CD4− CD8α− Myeloid DCs control T cell responses to flagellin
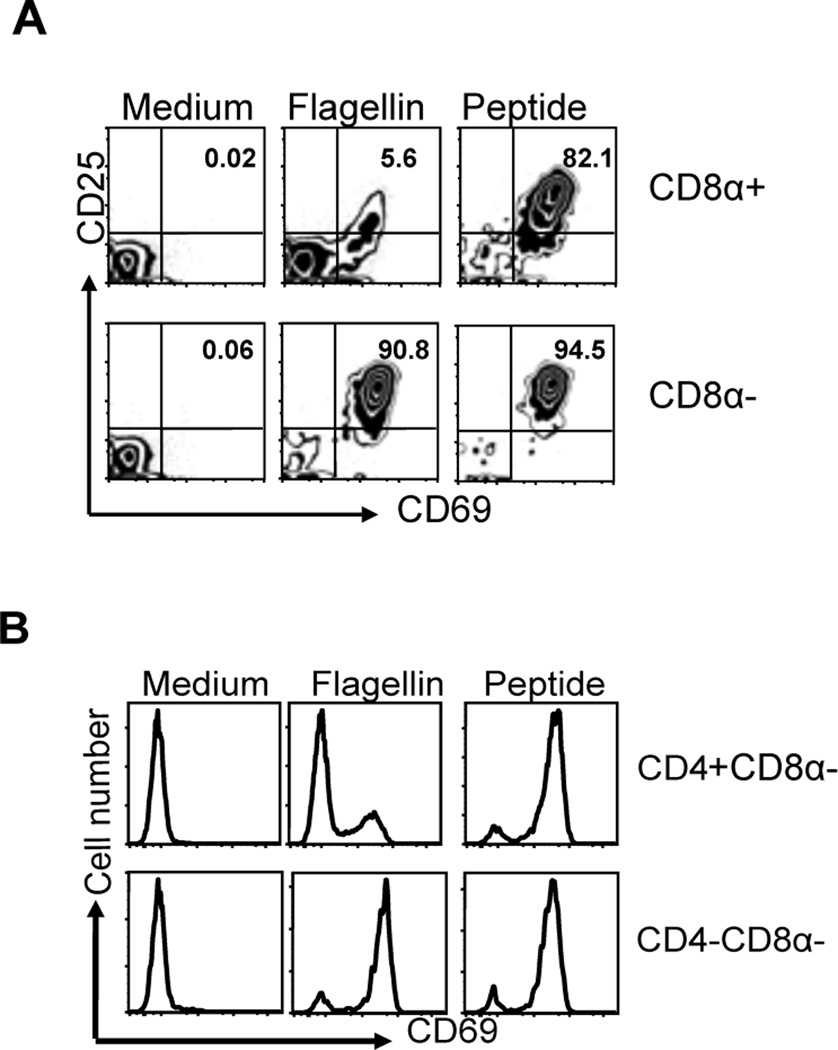
CD11c+ splenic DCs are a heterogeneous population and several different subsets have been defined based on the expression of CD8α, CD11b, and CD432, 33. We examined whether these different splenic DC subsets were differentially able to activate flagellin-specific T cells in vitro. Initially we isolated CD11c+CD8α+ and CD11c+CD8α− DCs and found that only myeloid (CD11c+CD8α−) DCs were capable of activating SM1 T cells to express CD69 and CD25 using flagellin as an antigen (Fig. 6A). Examining this myeloid DC population further, we found that only CD11c+CD8α− DCs that lack CD4 expression were able to efficiently activate SM1 T cells in vitro, while CD4-expressing DCs did not (Fig. 6B). Thus, the ability to sensitively activate flagellin-specific T cells is regulated by a minor population of splenic DCs that lack expression of both CD4 and CD8α.
Figure 6. Splenic DC subsets have differential ability to activate flagellin-specific T cells.
CD8α+, CD8α− CD4+CD8α− and CD4−CD8α− splenic CD11c+ DC subsets were purified using DC enrichment kits and were analyzed for their ability to activate flagellin-specific T cells in vitro. DC subsets were incubated with SM1 T cells at a 1:1 ratio (1×105 cells/well) in the presence of 10ng/ml of flagellin or 6µM of flagellin peptide (427–441). (A) Upper and lower panel showed the activation of SM1 T cells in the presence of CD11c+CD8α+ lymphoid or CD11c+CD8α-myeloid DCs as evaluated by CD69 and CD25 expression on gated SM1 T cells, 16h post incubation. (B) CD4+CD8α− and CD4−CD8α− myeloid DC subsets 1×105 were co-cultured with 1×105 SM1 T cells for 16hours in the presence of flagellin and T cell activation analyzed by examining CD69 expression on gated SM1 T cells. Data are representative of two independent experiments completed in triplicate.
Mesenteric lymph node CD103−CD11b+ DCs regulate T cell responses to flagellin
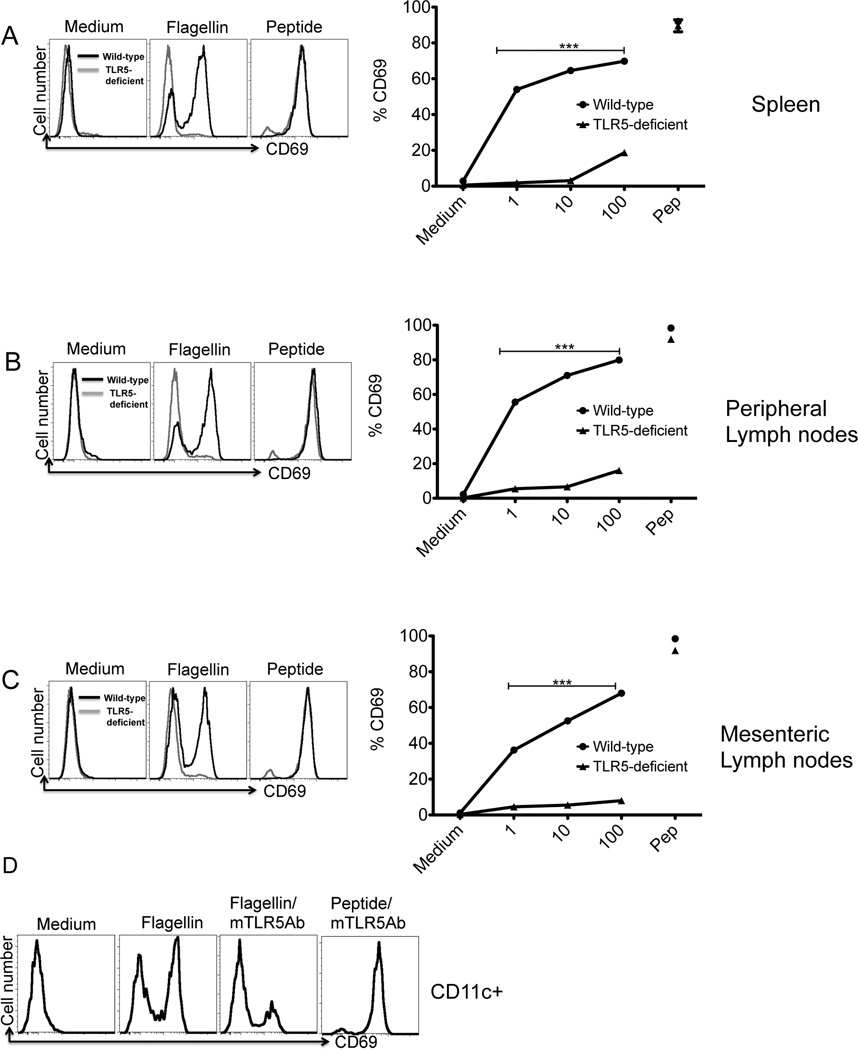
Flagellin-specific T cell responses are often induced in the intestine and thus host immune sensitivity to flagellin is likely to be regulated by intestinal DC subsets26, 34, 35. We therefore compared the ability of DCs from spleen, peripheral lymph nodes, and mesenteric lymph nodes of wild-type and TLR5-deficient mice to activate SM1 T cells in vitro. CD11c+ DCs from each of these locations were able to activate flagellin-specific T cells in vitro to express CD69, and the expression of TLR5 was required in each case (Fig. 7A–C). In contrast, wild-type and TLR5-deficient DCs were equivalently able to activate OT-II cells incubated in the presence of OVA (Fig. S3). In addition, flagellin-specific T cell activation by wild-type DCs was blocked by the addition of an anti-TLR5 monoclonal antibody (Fig. 7D). In contrast, CD11c+ DCs from the Peyer’s Patch or lamina propria were poorly able to activate flagellin-specific T cells (Fig. S4), although the CD11c enrichment efficiency was somewhat lower for both these tissues.
Figure 7. TLR5 is required for splenic and mucosal DCs to activate flagellin specific T cells.
CD11c+ dendritic cells enriched from (A) spleen, (B) peripheral lymph nodes or (C) mesenteric lymph nodes of wild-type and TLR5-deficient mice. FACS plots (left) show activation of SM1 T cells after 16h of culture with wild-type DCs (black) or TLR5-deficient DCs (grey) in the presence or absence of 10ng/ml of flagellin or flagellin peptide (6µM). T cell activation was analyzed by the expression of CD69 on gated SM1 T cells (CD4+CD90.1+). Graphs (right) show increased expression of CD69 on (CD4+CD90.1+) SM1 T cells after 16 hours of incubation in the presence of wild-type (λ) or TLR5-deficient DCs (σ) with flagellin (1ng–100ng)/ml or flagellin peptide (6µM). Each data point is representative of mean of two independent experiments done in triplicate, (★★★) denotes a p value <0.0001, as analyzed by two-way ANOVA. (D) CD11c+ dendritic cells from mesenteric lymph nodes were incubated with monoclonal TLR5 antibody (10µg/ml) for 60 min and then cultured in a 1:1 ratio with SM1 cells in the presence of flagellin (1ng/ml) and flagellin peptide (6µM). 16 hours later, CD69 expression was measured on T cells by flow cytometry. Data are representative of two independent experiments.
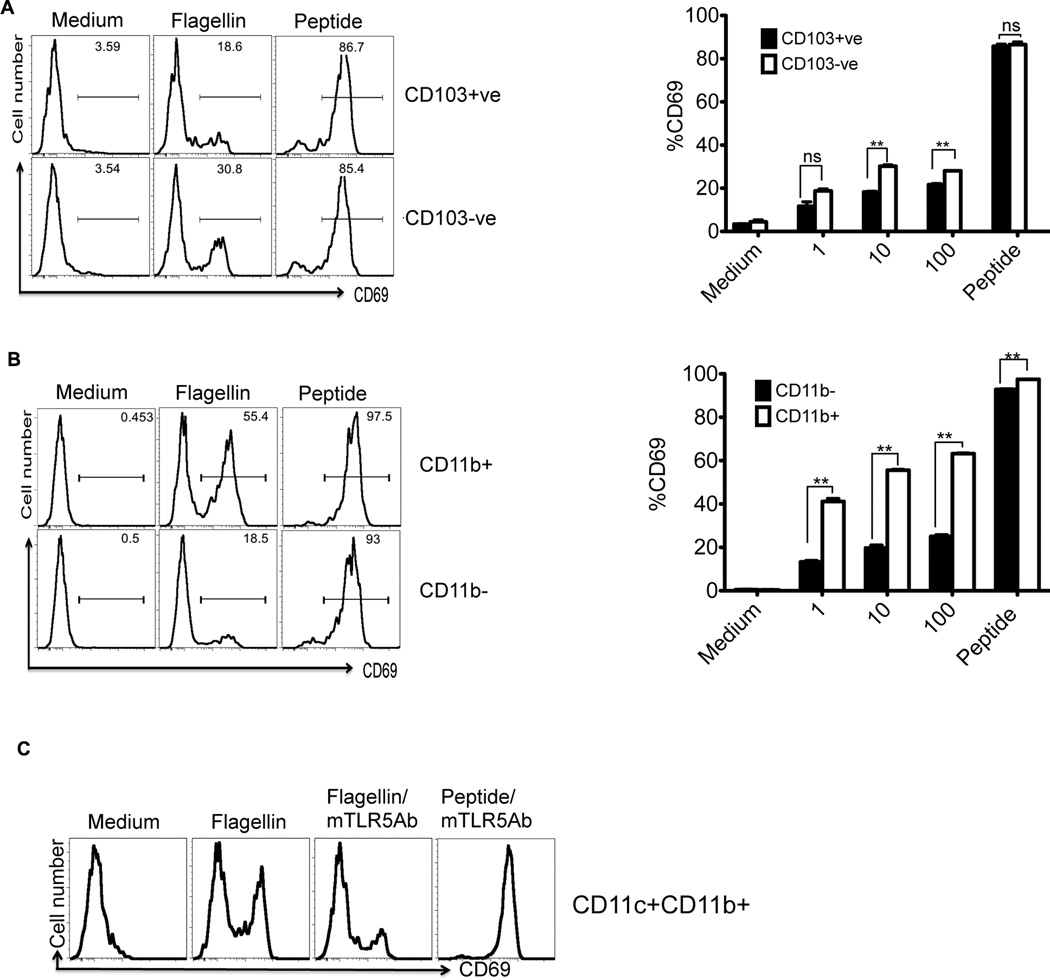
Next we enriched CD103+ and CD103− MLN DCs and examined whether these populations were differentially able to present flagellin (Fig. S1). While CD103+ DCs were relatively poor at activating SM1 T cells, there was a small but significant increase in T cell activation when using CD103− DCs were used (Fig. 8A). Similar to splenic DCs, when MLN DCs were enriched based on expression of CD11b, there was a significant enhancement in the ability to present flagellin to SM1 T cells (Fig. 8B). Furthermore, the ability of MLN CD11b+ DCs to present flagellin was blocked by the addition of a monoclonal antibody against TLR5 (Fig. 8C). The difference in the ability of CD11b+ DCs to activate flagellin-specific T cells also correlated with increased TLR5 expression by this subset, as examined by RT-PCR analysis (Fig. S5). Together, these data suggest that CD103− and CD11b+ expression define a MLN DC subset with enhanced ability to initiate flagellin-specific T cell responses to low doses of flagellin.
Figure 8. Mesenteric lymph node DC subsets have differential ability to activate flagellin specific T cells.
(A) CD103 positive or negative myeloid DCs from mesenteric lymph nodes were enriched from CD11c+ DCs using magnetic beads and cultured with SM1 T cells for 16h in the presence of flagellin (10ng/ml) or flagellin peptide (6µM). FACS plots (left) show CD69 expression on gated SM1 T cells. Bar graphs (right) show dose dependent activation of SM1 T cells cultured with CD103 positive or negative fractions. Each bar shows the mean percentage of CD69+ cells after gating on SM1 T cells. (B) FACS plots show activation of SM1 T cells cultured for 16h with CD11b positive or negative dendritic cells fraction in the presence or absence of flagellin (10ng/ml) or flagellin peptide (6µM). Bar graphs (right) show dose dependent activation of SM1 T cells cultured with CD11b+ or CD11b− MLN DCs. Each bar shows the mean percentage of CD69+ cells after gating on SM1 T cells. (C) CD11b+ dendritic cells pretreated with monoclonal anti-TLR5 antibody were cultured with SM1 T cells and T cell activation was examined 16h later. (★★) denotes a p value <0.01 and ns denotes a p value >0.05 as analyzed by unpaired t-test.
Discussion
Immunologists have historically devoted a significant amount of attention to examining flagellin-specific immune responses due to the immunogenicity of this protein36–39. For example, it was noted more than 40 years ago that immune response in rats can be induced with as little as 10ng of flagellin36, 38. Our data demonstrate that TLR5 expression largely accounts for the exquisite sensitivity of a mammalian host to respond to bacterial flagellin. This finding is important since it also provides a plausible explanation for the underlying dominance of flagellin-specific immune responses in infectious and inflammatory diseases of the intestine, perhaps especially so when overall antigen concentrations are very low, such as during the early stages of bacterial infection. However, it is also important to note that TLR5-deficient mice retain the ability to respond to concentrations of flagellin in the microgram range and this is similar to the threshold for OVA-specific responses. Thus, expression of TLR5 simply confers a unique sensitivity to low doses of flagellin in wild-type mice. Further studies will be required to determine whether the expression of TLR5 is required for directing flagellin-specific adaptive immunity during infection or inflammatory bowel disease.
Bacterial flagellin can also be recognized by NLRC4 (earlier known as Ice like protease activating factor-IPAF) and NAIP5 in the cytosol5–8. Recently NLRC4 was reported to be essential for humoral responses to flagellin31. However, our data demonstrate that NLRC4 plays at most a minor role in directing flagellin-specific T cell activation since the expansion and activation of SM1 T cells was similar in mice expressing or lacking NLRC4. Given the cytosolic location of inflammasome detection, it remains possible that NLRC4 plays a more important role in directing the CD8 T cell responses, however, reagents to study flagellin-specific CD8 T cells are not yet available.
The spleen contains multiple DC subsets including CD4−CD8α+, CD4+CD8α− and CD4−CD8α− populations33. CD8α+ lymphoid DCs are prominent in the white pulp while CD8α− myeloid DCs localize to the marginal zone and bridging channels40. Here, we show that myeloid CD4− DCs are also functionally specialized to activate flagellin-specific T cells at very low antigen concentrations. Similarly, the mesenteric lymph node contains multiple resident and migratory DC subsets and our data pinpoint CD11c+CD103−CD11b+ DCs as a subset that is specifically capable of activating flagellin-specific T cells response at very low antigen concentrations. This subset is likely a blood-derived resident DC population that is found in all secondary lymphoid tissues, although further studies will be required to demonstrate this. The enhanced capacity to activate flagellin-specific T cells required the expression of TLR5 and was blocked by anti-TLR5. It seems likely therefore that TLR5 allows preferential delivery of flagellin to the MHC class-II loading compartment, although this remains to be demonstrated in vitro or in vivo. It is important to note that the ability of these DC subsets to present flagellin to T cells is TLR5-dependent but independent of Myd88 and can be detected in splenic DCs that show low inflammatory responses to flagellin in vitro. Thus, this functionality is distinct from the inflammatory activity of flagellin mediated by TLR5 and could therefore require a co-receptor or unique signaling components. Indeed, a population of CD103+ DCs in the lamina propria have been noted to display inflammatory activity in response to flagellin41, but we detected low responses from lamina propria DCs in T cell stimulation assays. These data suggest that different intestinal DC subsets may use TLR5 differentially to induce inflammatory responses in intestinal tissue or present antigen to CD4 T cells in secondary lymphoid tissues.
It is not yet clear whether the increased sensitivity to antigen conferred by TLR5 expression is typical of other TLRs. TLR11 also recognized a protein ligand, profilin, and indeed, profilin-specific CD4 T cells are induced in a TLR11-dependent manner42. It is possible that any protein ligand that is internalized via surface TLRs benefits from enhanced deliver to the class-II processing pathway and therefore the mechanism of the this enhanced delivery will be of interest to vaccine design. In conclusion our study demonstrates the importance of TLR5 in DC antigen presentation by decreasing the threshold at which flagellin-specific T cells are activated and also suggests that TLR5 expression by distinct populations of splenic and intestinal dendritic cells are responsible for focusing host responses onto this antigen in infectious and inflammatory disease states. Given these findings, it is possible that this novel function of TLR5 can be utilized to improve the immunogenicity of enteric vaccines or prevent flagellin specific responses in inflammatory disease.
Materials and Methods
Mice and reagents
C57BL/6 and B6.SJL-PtprcaPep3b/BoyJ (CD45.1 congenic) mice (6–8 weeks old) were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA) and NCI (Frederick, MD). TLR5-deficient and NLRC4-deficient mice24, 43 were bred from stock originally provided by Dr. A. Gewirtz (Georgia State University, GA). Myd88-deficient mice44, were provided by Dr. S. Way (University of Minnesota Medical School, MN). Rag-deficient, CD90.1 congenic, flagellin-specific SM1 TCR transgenic mice have been described26, 27. Rag-deficient, OT-II TCR transgenic mice45 were backcrossed to a Rag-deficient CD90.1 congenic background in our laboratory. The flagellin epitope recognized by SM1 T cells (flagellin427–441) has been reported14, and this peptide was purchased from Invitrogen Corporation (Carlsbad, CA).
Bacterial strains and flagellin production
LPS-deficient Salmonella serovar Typhimurium X4700, provided by Dr. R Curtiss (Arizona State University, Tempe, AZ), was used to purify flagellin using a modified acid-shock protocol. Overnight LB broth cultures were centrifuged, washed and resuspended in PBS, before acid treatment to liberate flagellin. Monomeric flagellin was prepared by depolymerizing dialyzed samples at 70°C for 1hour and passed through endotoxin removal columns (Pierce Biotechnology, Rockford, IL). Flagellin purity was determined using silver stained SDS gels and endotoxin detection kits (Sigma, St. Louis, MO). Our flagellin preparations have identical biological activity to recombinant flagellin produced by eukaryotic cells22.
Adoptive transfer and immunization
Spleen, inguinal, brachial, cervical, and mesenteric lymph nodes were harvested from SM1 or OT-II mice and red blood cells were lysed using ACK lysis buffer (Lonza, Walkersville, MD) before labeling with CFSE46. The percentage of transgenic T cells was determined and 800,000–1×106 cells transferred intravenously into mice. The following day, mice were injected IV with various doses of flagellin or OVA.
DC enrichment and In vitro stimulation
Spleens, peripheral lymph nodes, and mesenteric lymph nodes were digested using collagenase D (Roche Diagnostics, Indianapolis, IN)22 and DCs enriched to greater than 85–95% purity using CD11c microbeads (Miltenyi Biotech, Inc., Auburn, CA) (Fig. S1). DC subsets were enriched by positive or negative selection using antibodies specific for CD11c, CD11b, CD103 or CD8α− (Miltenyi Biotech, Inc.). Further separation of splenic myeloid CD8α− DCs was accomplished using a CD4 isolation kit (Miltenyi Biotech, Inc.). CD11b+ DCs were enriched using CD11b+ isolation kit (Milteyni Biotech, Inc.) (Fig. S1). CD103+ DCs were isolated from negatively enriched CD11c+ DCs using CD103-specific antibody and streptavidin beads (Milteyni Biotech, Inc.). The unbound CD103− fraction was used to purify CD11b+ DCs. DC subsets (1×105 cell/well) were incubated 1:1 with SM1 or OT-II T cells in the presence or absence of antigen. CD4 T cells were recovered at 16 and 48 hour time points to examine activation by surface staining and cytokine production was detected in the supernatant. In some assays, DCs were also incubated with 10µg/ml neutralizing anti-TLR5 monoclonal antibody (Invivogen) for 60 min before addition of flagellin and SM1 T cells.
Flow cytometric analysis
DC subsets were characterized using antibodies specific for CD11c, CD11b, CD8α, CD103, DEC205 (CD205) and CD4 (eBioscience, Inc., San Diego, CA). T cell activation was examined using antibodies specific for CD4, CD90.1, CD69, CD25, CD44, and CD62L, (eBiosciences). Cells were analyzed using a BD Fortessa and data analyzed using FlowJo software (Treestar, Inc., Ashland, OR).
Measurement of IL-2 cytokine production by ELISA
IL-2 cytokine production in 16 or 48 hour culture supernatants was measured using an mIL-2 BD Opt EIA detection kit (BD Biosciences, San Diego, CA), according to manufacturers protocol. Absorbance was measured at 450 nm using a micro plate reader (Spectra Max M2, Molecular Devices, Inc., Sunnyvale, CA).
RNA isolation, cDNA Synthesis and RT-PCR
Dendritic cells were isolated from spleen and mesenteric lymph nodes as described above. Total RNA was extracted using RNA easy mini Kit (Qiagen) and 100ng RNA was reverse transcribed with taqman (Applied Biosystems) following manufacturer’s protocol. Tlr5 specific primers were purchased from Invivogen and 5ul of cDNA was used for the amplification from each sample. PCR conditions were exactly as suggested by Invivogen and PCR products were separated on a 2% agarose gel. GAPDH amplification was used as an internal control in each case.
Isolation of Dendritic cells from Lamina Propria (LP) and Peyer’s Patch (PP)
The small intestine was removed from at least 5–6 mice and washed twice with PBS to remove fecal contents before PP were excised. DCs were isolated from PPs using CD11c microbeads, as described for other lymphoid tissues above. Mucus was removed from the remaining intestinal tissue and washed with HBSS before incubating in 25 ml HBSS containing 2mM EDTA and 5% FBS for 10 min at 37°C. Supernatant was removed and intestinal tissue cut into small pieces before incubation in a shaking incubator (150 rpm) at 37°C for 1h in 50ml of RPMI-1640 containing 5%FBS, 1mg/ml Collagenase (Life technologies, Invitrogen, Grand Island, NY), 1mg/ml dispase (Life technologies, Invitrogen, Grand Island, NY), 40ug/ml DNaseI (Roche Diagnostics, Indianapolis, IN). Tissue was centrifuged and pellets were resuspended in 10mM EDTA containing EHAA for 5min at 37°C. Cells then passed through a 40µm strainer and washed twice with MACS buffer before CD11c+ DCs enriched using CD11c+ microbeads.
Statistical analysis
Statistical analysis of data was accomplished using InStat (GraphPad Software, La Jolla, CA). Data were compared using unpaired t test between groups and were considered significantly different with a p value of <0.05. Two way ANOVA analysis was also used for statistical analysis where appropriate.
Supplementary Material
(A) Splenic CD11c+ DCs were enriched by positive selection and bound and unbound fractions stained with antibodies specific for CD11c and CD11b. (B) MLN CD11c+ DCs were enriched by negative selection and bound and unbound fractions stained with antibodies specific for CD11c and CD11b. CD11b+ DCs were enriched by positive selection and bound and unbound fractions stained with antibodies specific for CD11c, CD11b, CD103, CD8a and DEC 205 (CD205).
MLN CD11c+ DCs isolated from wild-type (solid line) and TLR5 deficient (dotted line) mice were cultured with flagellin specific SM1 T cells in the presence of varying concentrations of flagellin peptide. SM1 T cell activation was examined by measuring surface CD69 expression 16 hours later. Graph shows mean +/− SEM of triplicate samples at each peptide concentration.
CD11c+ DC (1×105) isolated from MLN of wild-type (upper panel) and TLR5 deficient mice (lower panel) were cultured for 16h with OT-II T cells (1×105) and OVA (10 & 100µg/ml). Facs plots show an increase in CD69 expression after gating on OT-II T cells and are representative of two independent experiments.
CD11c+ DCs enriched from (A) Peyer’s Patch (PP) and (B) lamina propria were cultured with SM1 T cells in the presence of varying concentrations of flagellin or flagellin peptide. FACS plots show SM1 T cell activation 16h after the addition of flagellin or flagellin peptide. Each histogram is representative of two independent experiments.
CD11c+ DCs were isolated from the spleen (positive selection) and MLN by negative selection and CD11b+ DCs enriched by positive selection. Expression of TLR5 mRNA in each DC subset was evaluated by RT-PCR and compared to the expression of GAPDH.
Acknowledgements
The authors would like to acknowledge helpful discussions with current and past members of the McSorley laboratory and the laboratory of Dr. S. Way in completion of these experiments. This work was supported by grants from the National Institutes of Health AI073672 and AI055743.
References
- 1.Macnab RM. Genetics and biogenesis of bacterial flagella. Annu Rev Genet. 1992;26:131–158. doi: 10.1146/annurev.ge.26.120192.001023. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 3.Gohda J, Matsumura T, Inoue J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not toll/IL-1 receptor domain-containing adaptor-inducing IFN-beta (TRIF)-dependent pathway in TLR signaling. J Immunol. 2004;173(5):2913–2917. doi: 10.4049/jimmunol.173.5.2913. [DOI] [PubMed] [Google Scholar]
- 4.Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. J Immunol. 2010;185(10):5677–5682. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7(6):569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 6.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7(6):576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 7.Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA, et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9(10):1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477(7366):596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 9.McSorley SJ, Ehst BD, Yu Y, Gewirtz AT. Bacterial flagellin is an effective adjuvant for CD4 T cells in vivo. J Immunol. 2002;169:3914. doi: 10.4049/jimmunol.169.7.3914. [DOI] [PubMed] [Google Scholar]
- 10.Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine. 2011;29(32):5145–5152. doi: 10.1016/j.vaccine.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 11.Taylor DN, Treanor JJ, Strout C, Johnson C, Fitzgerald T, Kavita U, et al. Induction of a potent immune response in the elderly using the TLR-5 agonist, flagellin, with a recombinant hemagglutinin influenza-flagellin fusion vaccine (VAX125, STF2.HA1 SI) Vaccine. 2011;29(31):4897–4902. doi: 10.1016/j.vaccine.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Salazar-Gonzalez RM, McSorley SJ. Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol Lett. 2005;101(2):117–122. doi: 10.1016/j.imlet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Cookson BT, Bevan MJ. Identification of a natural T cell epitope presented by Salmonella-infected macrophages and recognised by T cells from orally immunised mice. J Immunol. 1997;158:4310–4319. [PubMed] [Google Scholar]
- 14.McSorley SJ, Cookson BT, Jenkins MK. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol. 2000;164(2):986–993. doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- 15.Bergman MA, Cummings LA, Alaniz RC, Mayeda L, Fellnerova I, Cookson BT. CD4+-T-cell responses generated during murine Salmonella enterica serovar Typhimurium infection are directed towards multiple epitopes within the natural antigen FliC. Infect Immun. 2005;73(11):7226–7235. doi: 10.1128/IAI.73.11.7226-7235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cookson BT, Cummings LA, Rassoulian Barrett SL. Bacterial antigens elicit T cell responses via adaptive and transitional immune recognition. Curr Opin Microbiol. 2001;4(3):267–273. doi: 10.1016/s1369-5274(00)00201-0. [DOI] [PubMed] [Google Scholar]
- 17.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113(9):1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn's disease. Gastroenterology. 2005;128(7):2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 19.Dubinsky MC, Lin YC, Dutridge D, Picornell Y, Landers CJ, Farrior S, et al. Serum immune responses predict rapid disease progression among children with Crohn's disease: immune responses predict disease progression. The American journal of gastroenterology. 2006;101(2):360–367. doi: 10.1111/j.1572-0241.2006.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, et al. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 21.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170(10):5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 22.Salazar-Gonzalez RM, Srinivasan A, Griffin A, Muralimohan G, Ertelt JM, Ravindran R, et al. Salmonella flagellin induces bystander activation of splenic dendritic cells and hinders bacterial replication in vivo. J Immunol. 2007;179(9):6169–6175. doi: 10.4049/jimmunol.179.9.6169. [DOI] [PubMed] [Google Scholar]
- 23.Uematsu S, Akira S. Immune responses of TLR5(+) lamina propria dendritic cells in enterobacterial infection. Journal of gastroenterology. 2009;44(8):803–811. doi: 10.1007/s00535-009-0094-y. [DOI] [PubMed] [Google Scholar]
- 24.Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7(8):868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 25.Bates JT, Uematsu S, Akira S, Mizel SB. Direct stimulation of tlr5+/+ CD11c+ cells is necessary for the adjuvant activity of flagellin. J Immunol. 2009;182(12):7539–7547. doi: 10.4049/jimmunol.0804225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McSorley SJ, Asch S, Costalonga M, Rieinhardt RL, Jenkins MK. Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan A, Foley J, Ravindran R, McSorley SJ. Low-dose Salmonella infection evades activation of flagellin-specific CD4 T cells. J Immunol. 2004;173(6):4091–4099. doi: 10.4049/jimmunol.173.6.4091. [DOI] [PubMed] [Google Scholar]
- 28.Letran SE, Lee SJ, Atif SM, Uematsu S, Akira S, McSorley SJ. TLR5 functions as an endocytic receptor to enhance flagellin-specific adaptive immunity. Eur J Immunol. 2011;41(1):29–38. doi: 10.1002/eji.201040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Letran SE, Lee SJ, Atif SM, Flores-Langarica A, Uematsu S, Akira S, et al. TLR5-deficient mice lack basal inflammatory and metabolic defects but exhibit impaired CD4 T cell responses to a flagellated pathogen. J Immunol. 2011;186(9):5406–5412. doi: 10.4049/jimmunol.1003576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Seminars in immunopathology. 2007;29(3):275–288. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 31.Vijay-Kumar M, Carvalho FA, Aitken JD, Fifadara NH, Gewirtz AT. TLR5 or NLRC4 is necessary and sufficient for promotion of humoral immunity by flagellin. Eur J Immunol. 2010;40(12):3528–3534. doi: 10.1002/eji.201040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 33.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2(3):151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 34.Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, Sundberg JP, et al. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187(6):855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SJ, McLachlan JB, Kurtz JR, Fan D, Winter SE, Baumler AJ, et al. Temporal expression of bacterial proteins instructs host CD4 T cell expansion and th17 development. PLoS Path. 2012;8(1):e1002499. doi: 10.1371/journal.ppat.1002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ada GL, Nossal GJ, Pye J, Abbot A. Behaviour of Active Bacterial Antigens during the Induction of the Immune Response. I. Properties of Flagellar Antigens from Salmonella. Nature. 1963;199:1257–1259. doi: 10.1038/1991257a0. [DOI] [PubMed] [Google Scholar]
- 37.Nossal GJ, Ada GL, Austin CM. Behaviour of Active Bacterial Antigens during the Induction of the Immune Response. Ii. Cellular Distribution of Flagellar Antigens Labelled with Iodine-131. Nature. 1963;199:1259–1262. doi: 10.1038/1991259a0. [DOI] [PubMed] [Google Scholar]
- 38.Parish CR. Immune response to chemically modified flagellin. I. Induction of antibody tolerance to flagellin by acetoacetylated derivatives of the protein. The Journal of experimental medicine. 1971;134(1):1–20. doi: 10.1084/jem.134.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parish CR. Immune response to chemically modified flagellin. II. Evidence for a fundamental relationship between humoral and cell-mediated immunity. The Journal of experimental medicine. 1971;134(1):21–47. doi: 10.1084/jem.134.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 41.Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36(2):276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarovinsky F, Kanzler H, Hieny S, Coffman RL, Sher A. Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4+ T cell response. Immunity. 2006;25(4):655–664. doi: 10.1016/j.immuni.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430(6996):213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 44.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 45.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 46.Parish CR. Fluorescent dyes for lymphocyte migration and proliferation studies. Immunol Cell Biol. 1999;77(6):499–508. doi: 10.1046/j.1440-1711.1999.00877.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Splenic CD11c+ DCs were enriched by positive selection and bound and unbound fractions stained with antibodies specific for CD11c and CD11b. (B) MLN CD11c+ DCs were enriched by negative selection and bound and unbound fractions stained with antibodies specific for CD11c and CD11b. CD11b+ DCs were enriched by positive selection and bound and unbound fractions stained with antibodies specific for CD11c, CD11b, CD103, CD8a and DEC 205 (CD205).
MLN CD11c+ DCs isolated from wild-type (solid line) and TLR5 deficient (dotted line) mice were cultured with flagellin specific SM1 T cells in the presence of varying concentrations of flagellin peptide. SM1 T cell activation was examined by measuring surface CD69 expression 16 hours later. Graph shows mean +/− SEM of triplicate samples at each peptide concentration.
CD11c+ DC (1×105) isolated from MLN of wild-type (upper panel) and TLR5 deficient mice (lower panel) were cultured for 16h with OT-II T cells (1×105) and OVA (10 & 100µg/ml). Facs plots show an increase in CD69 expression after gating on OT-II T cells and are representative of two independent experiments.
CD11c+ DCs enriched from (A) Peyer’s Patch (PP) and (B) lamina propria were cultured with SM1 T cells in the presence of varying concentrations of flagellin or flagellin peptide. FACS plots show SM1 T cell activation 16h after the addition of flagellin or flagellin peptide. Each histogram is representative of two independent experiments.
CD11c+ DCs were isolated from the spleen (positive selection) and MLN by negative selection and CD11b+ DCs enriched by positive selection. Expression of TLR5 mRNA in each DC subset was evaluated by RT-PCR and compared to the expression of GAPDH.