Abstract
Hepatic stellate cells (HSC) are a major source of the immunoregulatory metabolite all-trans retinoic acid (ATRA), which may contribute to the generation of tolerogenic dendritic cells (DCs) in the liver. The present study seeks to clarify the mechanism(s) through which ATRA promotes the development of tolerogenic DCs. Though bone marrow-derived ATRA-treated DCs (RA-DCs) and conventional DCs had comparable surface phenotype, RA-DCs had diminished stimulatory capacity and could directly inhibit the expansion of DC/OVA-stimulated OT-II T cells. Arginase I (Arg-1) was found promote suppression because: a) ATRA was a potent inducer of Arg-1 protein and activity, b) the Arg-1 inhibitor nor-NOHA partially reversed suppression, and c) the suppressive function of RA-DCs was partially compromised using OT-II T cells from GCN2−/− mice, which are insensitive to Arg-1. Inducible nitric oxide synthase (iNOS), however, was found to be a more significant contributor to RA-DC function because: a) ATRA potentiated the expression of IFN-γ induced iNOS, b) suppressive function in RA-DCs was blocked by the iNOS inhibitor L-NMMA, and c) RA-DCs derived from iNOS−/− mice exhibited near complete loss of tolerogenic function, despite sustained Arg-1 activity. The expression of iNOS and the suppressive function of RA-DCs were dependent on both IFN-γ and ATRA. Furthermore, the in vivo behavior of RA-DCs proved to be consistent with their in vitro behavior. Thus, we conclude that ATRA enhances both Arg-1 and iNOS expression in IFN-γ treated DCs, resulting in a tolerogenic phenotype. These findings elucidate mechanisms through which ATRA may contribute to liver immune tolerance.
INTRODUCTION
Hepatic stellate cells (HSCs) have been shown to contribute to the immunoregulatory properties of the liver (1, 2). One of the crucial mechanisms involves the induction of myeloid cells with suppressive functions, generated primarily through the production of soluble factors. The activities of these HSC induced myeloid cells promotes T cell unresponsiveness (3). HSCs serve as the primary storage site for vitamin A (retinol) and can metabolize retinol into all-trans-retinoic acid (ATRA) following activation. ATRA is the predominant isoform found in tissues and has been implicated in various aspects of immune regulation, particularly in the induction of tolerance (4, 5). Therefore, ATRA derived from HSCs may be a critical factor in liver immunity. Recent reports have shown that ATRA produced from HSCs can induce the differentiation of regulatory T cells (Tregs), but only in the presence of TGF-β and dendritic cells (DCs) (6, 7), suggesting that ATRA may be influencing the development of DCs with regulatory function. However, the mechanisms as to how ATRA may be regulating the differentiation and function of DCs with T cell suppressive capacity remain unclear.
Induction of T cell tolerance by suppressive DCs has been reported to involve a number of mechanisms including cell-cell contact, secretion of regulatory cytokines, promotion of Tregs, the catabolism of essential amino acids via Arg-1 or IDO, and the production of NO via iNOS (8, 9). Notably, tolerogenic APCs and myeloid derived suppressor cells (MDSCs) are known to express Arg-1, IDO, and iNOS. We, therefore, reasoned that ATRA may enhance the expression of Arg-1 and iNOS in DCs leading to suppression of T cell proliferation through the depletion of L-arginine and/or generation of NO. Depletion of L-arginine promotes suppression of T cell proliferation through loss of ζ-chain expression in the TCR complex and activation of the general control nondepressible 2 (GCN2) amino acid stress response pathway, which regulates the expression of proteins involved in cell cycle progression (10–12). Alternatively, NO in and of itself can serve as an effective regulatory molecule by restricting T cell proliferation and altering the T cell response (13–15).
Arg-1 and iNOS both have the capacity to utilize L-arginine as a substrate leading to the production of L-ornithine and urea or L-citrulline and NO, respectively. Regulation of the expression of these enzymes is believed to depend upon the cytokine milieu– Arg-1 is induced primarily in response to Th2 cytokines including IL-4, IL-5, IL-10, or IL-13, while iNOS is associated with Th1 cytokines, such as IFN-γ and TNF-α (16, 17). The counter regulation of these enzymes is further mediated by the activities of these enzymes, with increased Arg-1 activity limiting availability of L-arginine as a substrate for iNOS and triggering mechanisms inhibiting translation of iNOS mRNA. Although iNOS and Arg-1 exhibit differences in their affinities for L-arginine and in their catalytic activities, they ultimately metabolize L-arginine at similar same rates (18).
It is noteworthy that RA has been shown to enhance Arg-1 expression in peritoneal macrophage cultures and, in combination with IL-4, can promote Arg-1 expression in DCs (19, 20). Furthermore, several reports suggest that RA can also modulate NO production in peritoneal macrophages during activation by LPS and IFN-γ (21, 22). Here we show that treatment of bone marrow derived DCs with ATRA promotes Arg-1 expression and increases sensitivity to IFN-γ induced iNOS expression in DCs, engaging L-arginine metabolic and NO effector pathways to drive T cell suppression. Collectively, these findings provide a potential mechanism through which HSC-derived ATRA may modulate tolerogenic DC development in the liver and contribute to regulation of the local immune response.
MATERIALS AND METHODS
Animals
Wildtype C57BL/6J (B6), ovalbumin (OVA)-specific T-cell TCR transgenic (OT-II OVA-TCR Tg), iNOS−/−, IFN-γR1−/−, GCN2−/− (B6.129S6) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and used at 10-12 weeks of age. OT-II mice deficient in GCN2 were generated by crossing OT-II mice onto a GCN2 deficient background. All animals were maintained under specific pathogen-free conditions and in accordance with NIH guidelines. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the Lerner Research Institute.
Cell Culture
DCs were generated as previously described (Lu et al., 1995). BM cells were isolated from the femur and tibia of mice under sterile conditions. BM cells were cultured in 24 well plates (2×106 /well) in complete RPMI-1640 medium (Corning, Manassas, VA, USA), which contained 10% FBS (Atlanta Biologicals, Flowery Branch, GA, USA), MEM non-essential amino acids (Gibco, Grand Island, NY, USA), sodium pyruvate (Gibco), L-glutamine (Fisher Scientific, Pittsburgh, PA) penicillin/streptomycin (Fisher Scientific) and 2-mercaptoethanol (Gibco), and in the presence of rmGM-CSF (10 ng/mL; Schering Plough Research Institute, Kenilworth, NJ, USA) and rmIL-4 (1000 U/mL; Schering Plough Research Institute). BM cultures were maintained at 37°C, 5% CO2. Granulocyte contaminants (non-adherent cells) were removed on day 2 by aspiration. Wells were then replenished with half fresh medium containing GM-CSF and IL-4 (10 ng/mL and 1000 U/mL, respectively) and half old medium. Mature DCs were propagated from BM monocytic cells 5 days later. RA-DCs were prepared as above but in the presence of ATRA (100 nM; Sigma Aldrich, St. Louis, MO, USA). Where indicated, LE-135 (1 μM; Tocris Biosciences, Ellisville, MO, USA) was added into culture at day 0. For further stimulation, BM cells were exposed to IFN-γ (100 U/mL; R & D Systems, Minneapolis, MN, USA) for 24 hours.
T-cell proliferation assays
Splenic T cells were isolated from OT-II mice and purified by nylon wool. 0.2×106 CFSE labeled T cells (1.5 μM, Molecular Probes, Eugene, OR, USA) were cultured with WT DCs (1:10, DC/RA-DC:T) and OVA protein (100 μg/mL; Worthington Biochemical Corporation, Lakewood, NJ, USA). For antigen pulsed experiments, DCs and RA-DCs were incubated with OVA protein (400 μg/mL, 18 hrs; Worthington Biochemical Corporation, Lakewood, NJ, USA) or ovalbumin peptide OVA323–339 (0.6 μM, 3hrs). In most conditions, additional DCs or RA-DCs were added into co-cultures (1:10, DC/RA-DC:T). Cultures were maintained in 10% FBS complete RPMI-1640 medium for 3 days at 37°C, 5% CO2. T cell proliferative response was determined by CFSE dilution assay and analyzed by flow cytometry. For anti-CD3/CD28 studies, T cells were added to an anti-CD3 mAb coated plate (1 μg/mL, overnight; eBiosciences, San Diego, CA, USA) followed by addition of anti-CD28 mAb (1μg/mL; eBiosciences). To inhibit Arg-1 or iNOS activity, Nw-hydroxy nor-L-arginine (nor-NOHA) or NG-monomethyl-L-arginine, monoacetate salt (L-NMMA) (Caliobiochem, San Diego, CA, USA) were added at the beginning of co-culture to achieve a final concentration of 300 μM. L-arginine (Sigma Aldrich) was added in excess at the time of co-culture to achieve a final concentration of 3 mM.
Flow cytometric analysis
Cell surface analyses of DCs and RA-DCs were performed by staining with mAbs CD11b, CD11c, I-Ab, and CD86 (BD Pharmingen, San Diego, CA, USA). For proliferation assays, CFSE labeled T cells were stained with CD4 mAb (BD Pharmingen). Intracellular IFN-γ staining was performed by surface staining with CD4 mAb and then permeabilizing the cells in 0.1% saponin, followed by IFN-γ mAb staining (BD Pharmingen).
Western Blotting
Cell lysates (15 μg of protein/well) were loaded onto a SDS-polyacrylamide gel and transferred onto a polyvinylidene difluride membrane. Arg-1 and iNOS were detected by blocking the membrane for 1 h in 5% nonfat dried milk or 5% BSA at room temperature followed by overnight incubation in primary Ab at 4°C and 1 h incubation with HRP anti-mouse IgG (Cell Signaling, Danvers, MA, USA) or HRP-anti rabbit IgG (GE Healthcare, UK) at room temperature. ECL Plus (Perkin Elmer, Waltham, MA, USA) was used for detection. The following primary antibodies were used: Arg-1 (BD Pharmingen), iNOS (Cell Signaling), and GAPDH (Millipore, Billerica, MA, USA).
Amino acid analysis
Amino acids were measured by HPLC (Agilent Technologies, Santa Clara, CA) using pre-column derivatization with O-phthaldialdehyde (OPA). In brief, samples were deproteinized with equal volume of 6% sulfosalicylic acid containing 500 μM ethionine as an internal standard. Chromatographic separations were performed using an OPA-HR column (150 × 4.6mm). The mobile phase consisted of 20mM sodium acetate (pH 5.7) with 4% tetrahydrofuran. Buffer B consisted of methanol with 4% tetrahydrofuran. Samples were measured by FLD using excitation 340 nm and emission 455 nm (24).
Nitrite Assay
Nitrite production was determined using the Griess reaction in which supernatants were mixed with equal volumes of 1% sulfanilamide, 0.1% naphthylethylene diamine dihydrochloride, and 2.5% phosphoric acid and compared to a sodium nitrite standard. The absorbance was measured at 570 nm in an automated plate reader.
In vivo immunization with antigen-pulsed BM-DCs/RA-DCs
WT or GCN2−/− OT-II cells were purified as described above, labeled with CFSE, (3 μM; Molecular Probes) and intravenously (i.v.) injected into B6 mice (5×106/mouse). The following day, OVA protein-pulsed WT or iNOS−/− BM-DCs and BM-RA-DCs (400μg/mL, 18 hrs; Worthington Biochemical Corporation) were injected subcutaneously into the hind footpads of mice (0.4 ×106/mouse) that had been adoptively transferred with OT-II cells. After 3 days, the primary draining popliteal lymph nodes were harvested and single cell suspensions were prepared. Proliferation of OT-II cells was determined by CFSE dilution assay with CD4 mAb staining (BD Pharmingen) and flow cytometric analysis.
Statistical Analysis
Significance between groups was assessed by performing a Mann Whitney test with post hoc Kruskal Wallis testing. p values ≤ 0.05 were considered significant.
RESULTS
RA-DCs have reduced T cell stimulatory capacity
Although RA has been shown to affect the phenotype and function of DCs, there are marked differences in the reported outcomes (25, 26). Therefore, we first sought to investigate differences in the surface characteristics and stimulatory capacity of DCs following treatment with ATRA. Bone marrow cells were cultured with GM-CSF and IL-4 in the presence or absence of ATRA to generate RA-DCs or DCs respectively. RA-DCs and DCs both expressed CD11b and exhibited comparable expression of the key surface molecules CD11c, I-Ab, and CD86 (Fig 1A). The expression of MHC class II and co-stimulatory molecules indicated that RA-DCs possess the potential for antigen presenting function. To evaluate their stimulatory capacity, we cultured DCs or RA-DCs with CFSE-labeled CD4+ T cells isolated from ovalbumin (OVA)-specific, MHC class II-restricted, TCR-transgenic mice (OT-II-mice) and OVA protein. Whereas DCs potently induced the proliferation of OT-II T cells, RA-DCs were poor stimulators (Fig. 1B). Since the reduced stimulatory capacity of RA-DCs could be the result of deficiencies in antigen capture and processing capabilities, we compared the priming abilities of RA-DCs pulsed with OVA peptide, which would not require antigen processing, to RA-DCs pulsed with OVA protein. RA-DCs pulsed with OVA peptide elicited a reduced OT-II T cell proliferative response, comparable to RA-DCs pulsed with OVA protein (Fig. 1C). Thus, the stimulatory function of ATRA treated DCs is compromised by deficiencies beyond MHC class II, co-stimulatory surface phenotype, and antigen processing.
FIGURE 1.
Comparison of surface molecules and stimulatory capacity of RA-DCs and DCs. (A) DCs and RA-DCs were stained for CD11b and the following markers: CD11c, I-Ab, and CD86 and analyzed by FACs. Cells were gated on CD11b+ cells and the respective marker and compared in the form of histograms. The shaded gray lines indicate isotype controls and the black/dotted lines indicate stained cells. Data representative of three experiments. (B) T cells purified from OT-II TCR transgenic mice (0.2×106/well) were labeled with CFSE and cultured with DCs or RA-DCs at a ratio of (1:10, DC/RA-DC: T) in the presence of OVA protein (100 μg/mL) for three days. Proliferating OT-II T cells were measured by FACs, in which cells were gated on live CFSE+ lymphocytes expressing CD4. A representative histogram is provided in addition to a bar graph displaying the mean percentages of dividing CD4+ T lymphocytes + SE from seven independent experiments. (C) OT-II T cells were cultured with DCs or RA-DCs which had been pulsed with either OVA protein or OVA323–339 peptide for three days. Live CFSE+, CD4+ T cells were gated and division was analyzed by FACs. Shown are a representative histogram and the mean percentages of dividing CD4+ T lymphocytes + SE from five independent experiments. *p < 0.05
Suppressive capacity of RA-DCs is related to gain of immunosuppressive function
The diminished capacity of RA-DCs to promote antigen specific T cell responses could reflect either loss of stimulatory function or the acquisition of suppressive function that overrides stimulatory activity. We, therefore, wanted to determine whether RA-DCs could regulate proliferative responses induced by professional antigen presenting cells (APCs), such as DCs. DCs or RA-DCs were added to cultures containing DCs, OVA, and CFSE labeled OT-II T cells. The addition of RA-DCs resulted in marked T cell inhibition (Fig 2A). Since it still remained unclear whether RA-DCs were modulating T cells directly or indirectly by influencing the stimulatory function of DCs, we tested these possibilities by two methods: 1) pulsing the DCs and RA-DCs with OVA protein prior to co-culture, which would exclude potential competition between RA-DCs and DCs for soluble antigen and 2) culturing DCs or RA-DCs with anti-CD3/CD28 stimulated OT-II cells. RA-DCs were able to inhibit proliferation of OT-II T cells stimulated by OVA protein -pulsed DCs (Fig 2B) as well as proliferation of OT-II T cells stimulated by anti-CD3/CD28 mAbs (Fig 2C), demonstrating that RA-DCs are not interfering with the ability of DCs to present antigen and are most likely targeting the T cells. Furthermore, inhibition of T cell activation by RA-DCs was further evidenced by a reduction in the frequency of IFN-γ producing T cells, which declined from 22 to 11% (Fig 2D), reflecting either reduced T cell proliferation or Th1 differentiation.
FIGURE 2.
RA-DCs can inhibit the proliferation of activated OT-II T cells. (A) CFSE labeled OT-II T cells (0.2×106/well) were co-cultured with DCs (1:20, DC:T) and OVA protein. Additional DCs or RA-DCs were added as regulators (1:10, DC/RA-DC:T). Live CFSE+, CD4+ T cells were gated and division was analyzed by FACs. The data show a representative histogram and the mean percentages of dividing CD4+ T cells + SE from ten independent experiments. (B) OT-II T cells were co-cultured with DCs (1:20, DC:T), which had been pulsed with OVA protein (18hrs), and additional OVA-pulsed DCs or RA-DCs added as regulators (1:10, DC/RA-DC:T). Proliferation was analyzed after three days. The data show a representative histogram and the mean percentages of proliferating CD4+ T cells + SE from five independent experiments. (C) OT-II T cells were purified and labeled with CFSE and added to plates coated overnight with anti-CD3 Ab (1μg/mL). DCs or RA-DCs were added (1:10, DC/RA-DC:T) in the presence of anti-CD28 Ab (1ug/mL). Cells were cultured for three days. Proliferation was measured via CFSE dilution and FACs analysis. A representative histogram and the mean percentages of proliferating CD4+ T cells + SE from three experiments are presented. (D) OT-II T cells from DC-OT-II and RA-DC-OT-II co-culture experiments were stained for IFN-γ and analyzed by FACs. Data representative of three experiments. The IFN-γ data shown here corresponds with the same experiment in which T cell proliferation was measured (Fig. 2A). *p < 0.05
ATRA promotes Arg-1 expression in DC precursors
Various myeloid cell populations, including macrophages and MDSCs, regulate T cell function through the actions of the enzymes Arg-1 and iNOS (27, 28). The activities of these enzymes limit L-arginine availability and generate NO. It was recently reported that RA can induce the expression of Arg-1 in DCs and enhance Arg-1 expression promoted by IL-4 (19). Hence, we asked whether the suppressive capacity of RA-DCs is a consequence of the induction of Arg-1 expression by ATRA. Arg-1 expression was weak in BM cultures treated with GM-CSF alone and could be increased modestly when GM-CSF was used in combination with IL-4. ATRA treatment, however, markedly enhanced Arg-1 protein levels independently of IL-4, suggesting that ATRA alone is capable of inducing Arg-1 expression (Fig 3A). Moreover, the induction of Arg-1 expression by ATRA was attenuated in cells co-treated with the retinoic acid receptor antagonist LE-135 (Fig 3B), confirming that Arg-1 expression is dependent on RA signaling.
FIGURE 3.
ATRA is a potent inducer of Arg-1 expression in BM-DCs. (A) BM cells were propagated in GM-CSF (10 ng/mL) with or without IL-4 (1000 U/mL) and/or ATRA (100 nM) and cultured for five days. BM lysates were analyzed for Arg-1 expression by Western blotting. GAPDH was used as a loading control. Data representative of at least seven experiments. (B) BM cells were cultured with GM-CSF (10 ng/mL) and IL-4 (1000 U/mL), with or without ATRA (100 nM) and/or LE-135 (1 μM) for five days. BM lysates were analyzed for Arg-1 expression by Western blotting. GAPDH was used as a loading control. Data representative of three experiments. (C) RA-DC-derived Arg-1 significantly reduces L-arginine levels in supernatants of RA-DC-T-cell co-cultures. The concentration of L-arginine in supernatants of DC/RA-DC-T cell co-cultures was measured at 24, 48 and 72 h by HPLC. Control indicates complete RPMI medium. Data show mean + SE of three independent experiments. *p < 0.05
ATRA induced Arg-1 exhibits significant catabolic activity and contributes to T cell suppression
The functional consequences of elevated Arg-1 protein in ATRA treated DCs was also demonstrated by measuring the kinetics of L-arginine depletion in DC and OVA stimulated OT-II T cell cultures with added DCs or RA-DCs as regulators, hereafter referred to as DC/RA-DC-T cell co-cultures. The supernatants of RA-DC-T cell co-cultures had significantly reduced levels of L-arginine compared to supernatants of DC-T cell co-cultures, with the peak of reduction occurring at 72 hours (Fig 3C). Furthermore, the reduction in L-arginine correlated with an increase in L-ornithine production, a byproduct of Arg-1 mediated L-arginine catabolism (Fig 3D).
To determine the role of Arg-1 in RA-DC-mediated T cell suppression, we added the arginase inhibitor nor-NOHA to RA-DC-T cell co-cultures. Inhibition of Arg-1 activity led to a reduction in the suppressive capacity of RA-DCs (Fig 4A). Furthermore, the addition of excess L-arginine (3 mM) to RA-DC T cell co-cultures (Fig 4B) resulted in a slight increase in proliferation, which showed a trend towards significance (p = 0.08).
FIGURE 4.
Inhibition of Arg-1 activity reduces RA-DC suppressive capacity. (A) Purified, CFSE labeled OT-II T cells were set up as in Figure 2A. The arginase inhibitor nor-NOHA (300 μM) was added to DC/RA-DC-T cell co-cultures. T cell proliferation was assessed thorough FACs. (B) Excess L-arginine (3 mM) was added to DC/RA-DC-T cell co-cultures and proliferation was analyzed by FACs. A representative histogram and the mean percentages of proliferating CD4+ T cells + SE from three independent experiments, with DC controls, are provided. *p < 0.05
T cell suppression by RA-DCs requires GCN2
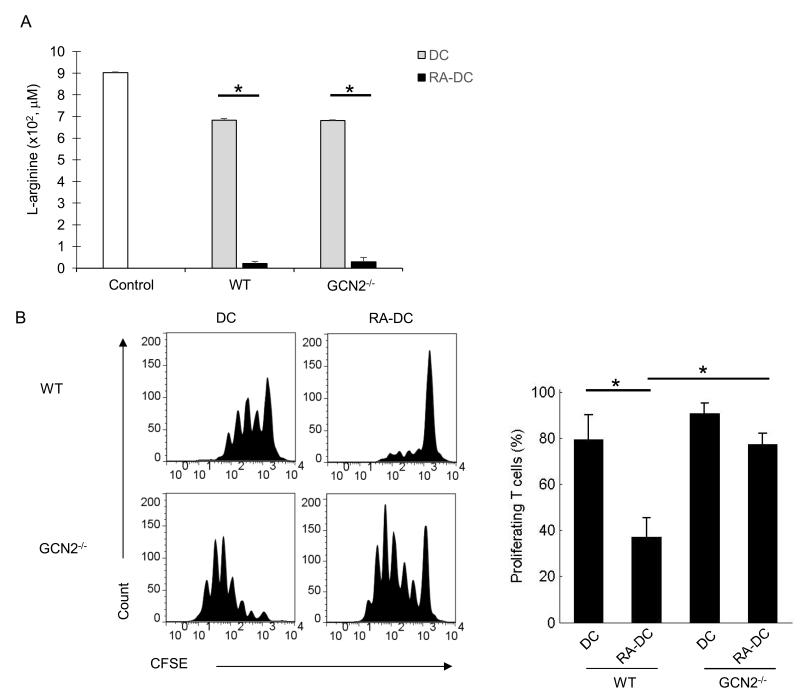
GCN2 is the eIF2α specific protein kinase that senses amino acid deficiency and has been shown to be essential for Arg-1 and IDO-mediated T cell suppression. As an additional measure of the role of Arg-1 and arginine depletion, we tested the relative sensitivity of OT-II T cells from WT or GCN2−/− background to RA-DC mediated suppression. RA-DCs effectively depleted L-arginine in both WT and GCN2−/− T cell co-cultures (Fig. 5A). The suppressive activity of RADCs was, however, significantly reduced in GCN2−/− T cells as compared to WT T cells (Fig 5B). Collectively, these data suggest that RA-DCs can regulate cell cycle progression and T cell suppression through the depletion of L-arginine and utilization of the GCN2 pathway.
FIGURE 5.
Suppression by RA-DCs is GCN2 dependent. (A) T cells were isolated from WT OT-II mice and GCN2−/− OT-II mice, labeled with CFSE, and used as responders in DC/OVA protein (1:20, DC:T) plus DC/RA-DC (1:10, DC/RA-DC:T) co-cultures. Supernatants were harvested at 72 h and L-arginine levels were analyzed by HPLC. Control indicates complete RPMI medium. Data show the mean + SE of three independent experiments. (B) T cell proliferation was assessed by FACs. Shown are a representative histogram and the mean percentages of proliferating CD4+ T cells + SE from three independent experiments. *p < 0.05
iNOS is critical for mediating suppression by RA-DCs
Even though L-arginine can serve as a substrate for iNOS, iNOS has been shown to contribute only modestly to reductions in extracellular L-arginine (10). Rather, iNOS mediates its effects through the generation of NO (29). Hence, we asked if ATRA could also regulate the expression of iNOS in DCs. At baseline, iNOS expression could not be detected in DCs or RA-DCs. However, stimulation with IFN-γ induced greater expression of iNOS in RA-DCs as compared to DCs (Fig. 6A). Inhibition of iNOS activity by L-NMMA reversed RA-DC driven suppression of T cell proliferation by RA-DCs (Fig. 6B). To further evaluate the contribution of iNOS in RADC mediated suppression, we generated RA-DCs from iNOS−/− mice. Consistent with our previous findings, iNOS−/− RA-DCs exhibited reduced suppression of T cell proliferative responses as compared to WT RA-DCs (Fig. 6C). The addition of nor-NOHA to iNOS−/− RA-DC T cell co-cultures did not induce a significantly greater proliferative response compared to iNOS-/- RA-DC co-cultures (Fig 6D), however, this could be explained by the fact that iNOS deficiency alone led to a significant reduction in the suppressive capacity of RA-DCs and resulted in near maximal T cell proliferative response (similar to DC controls). Thus, while Arg-1 does contribute to the suppressive capacity of RA-DCs, the iNOS pathway appears to have a more prominent role.
FIGURE 6.
RA-DC mediated suppression is highly dependent on iNOS. (A) RA-DC and DC cultures were prepared from BM cells cultured in the presence of GM-CSF (10 ng/mL) and IL-4 (1000 U/mL) with or without ATRA (100 nM), respectively. After five days of culture, DCs and RA-DCs were stimulated with IFN-γ for 24 h. Cell lysates were analyzed for iNOS expression by Western blotting. GAPDH was used as a loading control. Data is representative of three experiments. (B) iNOS activity was inhibited by administration of L-NMMA (300 μM) into RA DC-T cell co-cultures (set up as described in Figure 2A). Proliferation was determined by FACs analysis. (C) DCs and RA-DCs were prepared from WT and iNOS−/− mice (as indicated in Figure 6A). CFSE labeled OT-II T cells (0.2×106/well) were cultured with DCs/OVA (1:20, DC:T) and WT or iNOS−/− DCs/RA-DCs (1:10, DC/RA-DC:T) for three days. (D) nor-NOHA (300 μM) was added into WT or iNOS−/− DC/RA-DC-T cell co-cultures (as set up in Figure 6C). Proliferation was measured after three days by CFSE dilution and FACs analysis. All proliferation data show a representative histogram and the mean + SE from three independent experiments. *p < 0.05
IFN-γ signaling is required for iNOS expression and RA-DC mediated suppressive function
Since IFN-γ is a potent inducer of iNOS expression, we next assessed the importance of IFN-γ signaling both in terms of the modulation of iNOS expression by RA-DCs and the ability of RADCs to suppress T cell proliferation. iNOS expression was abrogated in RA-DCs generated from IFN-γR1−/− mice (Fig. 7A) and IFN-γR1−/− RA-DCs were compromised in their ability to inhibit T cell proliferation (Fig. 7B). Analysis of nitrite levels in the supernatants of these co-cultures showed iNOS activity was decreased in IFN-γR1−/− RA-DC-T cell co-cultures compared to WT RA-DC-T cell co-cultures (Fig. 7C). Of interest, even though RA-DC-mediated suppression of T cell proliferation was markedly reduced in IFN-γR1−/− RA-DC-T cell co-cultures as compared to WT RA-DC-T cell co-cultures, IFN-γR1−/− RA-DCs maintained expression of Arg-1 (Fig. 7D) and displayed potent Arg-1 activity (Fig. 7E). It is worth noting that Arg-1 expression was sustained in WT RA-DCs as well as in IFN-γR1−/− RA-DCs (Fig. 7D) in the presence of IFN-γ stimulation. Furthermore, while IFN-γ treatment could suppress the expression of Arg-1 in WT DCs induced by IL-4, it did not compromise expression of Arg-1 driven by ATRA (Fig. 7F), indicating that the pathways connecting IL-4 and ATRA to Arg-1 are mechanistically distinct and, moreover, that RA-DCs can simultaneously express iNOS and Arg-1.
FIGURE 7.
IFN-γ signaling is required for iNOS expression and RA-DC mediated suppressive function. (A) DCs and RA-DCs were prepared from the bone marrow of WT and IFN-γR1−/− mice (as indicated in Figure 6A). WT and IFN-γR1−/− DCs and RA-DCs were stimulated with IFN-γ for 24 h. Cell lysates were analyzed for iNOS expression by Western blotting. GAPDH was used as a loading control. Data representative of three experiments. (B) WT or IFN-γR1−/− DCs/RA-DCs were co-cultured with CFSE labeled OT-II T cells (1:10, DC/RA-DC:T) in the presence of WT DCs (1:20, DC:T) and OVA protein. Cell division was determined by FACs. Data show a representative histogram and the mean percentages of proliferating CD4+ T cells + SE of four experiments. (C) Nitrite levels were measured in the supernatants of these co-cultures at 72 h by Griess assay. Data represented as the mean percentages of dividing CD4+ T cells + SE of three independent experiments. (D) Cell lysates from WT and IFN-γR1−/− DCs/RA-DCs were analyzed for Arg-1 expression by Western blotting. GAPDH was used as a loading control. (E) L-arginine levels in WT or IFN-γR1−/− RA-DC-T cell co-culture (as set up in Figure 7B) supernatants were analyzed by HPLC. Control indicates complete RPMI medium. Data show mean + SE of three experiments. (F) Cell lysates from BM cells cultured in the presence of GM-CSF and/or IL-4 and/or ATRA, with or without IFN-γ stimulation for 24 h. DCs/RA-DCs were analyzed for Arg-1 expression by Western blotting. GAPDH was used as a loading control. Data representative of three experiments. *p < 0.05
RA-DCs exhibit potent suppressive capacity in vivo
To determine whether RA-DCs displayed suppressive capacity and utilized Arg-1 and iNOS mediated mechanisms to regulate T cells in in vivo settings, we adoptively transferred CFSE labeled WT OT-II T cells into B6 recipient mice, which were then immunized in the footpads with OVA protein-pulsed DCs or RA-DCs (unpulsed DCs served as the control). While OT-II T cells recovered from the draining lymph nodes of DC immunized mice exhibited strong proliferative capacity, OT-II T cells from RA-DC immunized mice showed a markedly reduced proliferative response (Fig. 8A). To confirm the role of GCN2 in T cells in RA-DC mediated suppression, B6 mice were adoptively transferred with CFSE labeled WT or GCN2−/− OT-II T cells and immunized with OVA-pulsed DCs or RA-DCs in the footpads. RADCs lost their ability to effectively regulate GCN2−/− OT-II T cells (Fig. 8B). Furthermore, administration of WT OT-II T cells and OVA-pulsed iNOS−/− RA-DCs (Fig. 8C) induced robust proliferation of OT-II T cells, comparable to WT DC controls, suggesting that RA-DCs have intact migratory capacity and maintain their immunosuppressive properties in vivo.
FIGURE 8.
RA-DCs mediate suppression through Arg-1 and iNOS mediated mechanisms in vivo. (A) 5×106 CFSE labeled OT-II T cells were injected i.v. into B6 recipient mice who were then subcutaneously injected in the footpads with DCs or RA-DCs pulsed with OVA protein. Unpulsed DCs were injected in control mice. Transferred OT-II T cells were recovered from the primary draining popliteal lymph nodes 3 days later and proliferation of OT-II T cells was assessed by CD4 staining of CFSE+ T cells. (B) WT or GCN2−/− OT-II T cells were purified and labeled with CFSE (as previously described) and injected i.v. into B6 recipients. WT DCs or RADCs were pulsed with OVA protein and subcutaneously injected into the footpads of recipients. OT-II T cells were recovered after 3 days and proliferation was determined by CFSE dilution assay. (C) B6 recipients were injected i.v. with WT OT-II T cells. Recipients were then immunized with OVA protein pulsed WT or iNOS−/− DCs or RA-DCs at the footpads. After 3 days, OT-II T cells were recovered and assessed for proliferation. In all experiments, cells were gated on live cells expressing CFSE and CD4. Data for 8A-C are presented in the form of a representative histogram and display the mean percentages of dividing CD4+ T cells + SE of four independent experiments. *p < 0.05
DISCUSSION
While it is known that the liver is an immune privileged site, the mechanistic basis is poorly defined and is likely an amalgam of multiple biological processes (30, 31). Emerging evidence suggests that the non-parenchymal cells of the liver, such as the HSCs, are pivotal in conferring immune privilege. One major contributing feature of HSCs is ATRA, which has been shown to modulate the development of DCs with tolerogenic function (7, 32–34). However, the molecular basis for ATRA-mediated immune suppression has not been thoroughly evaluated. Here we report that DCs treated with ATRA exhibited markedly diminished stimulatory capacity as compared to conventional DCs cultured with GM-CSF and IL-4 alone, despite comparable expression of cell surface MHC class II and CD86. Moreover, ATRA-treated DCs could directly suppress T cell proliferation mediated by polyclonal TCR stimulation in the absence of DCs. T cell suppressive function was mediated by the immunoregulatory enzymes Arg-1 and iNOS, whose expression was directly induced or enhanced by RA signaling. Elevated Arg-1 contributed to inhibition of T cell response via depletion of extracellular L-arginine and Arg-1 effects were dependent on GCN2 expression in T cells. Arg-1 activity by itself, however, could not fully account for the suppression observed, and we demonstrate a prominent role for IFN-γ-induced iNOS. Indeed, iNOS appears to be responsible for a major portion of T cell suppressive function following antigen stimulation. Furthermore, adoptive transfer experiments with WT or GCN2−/− OT-II T cells and immunization with OVA-pulsed WT or iNOS−/− DCs or RA-DCs provide direct evidence that our in vitro findings are consistent with our in vivo findings.
The expression of Arg-1 and/or iNOS by macrophages and DCs is well recognized to have immunosuppressive consequences (29, 35–37), however, tolerogenic DCs can also dampen the magnitude of T cell activation through mechanisms beyond Arg-1 and iNOS. We found that expression of IL-12p40, an important contributor to the development of Th1 cells (38, 39), was reduced in RA-DCs (data not shown). Our observations were consistent with the findings of others who also reported diminished production of IL-12 in DCs and macrophages in response to ATRA treatment (25, 40). The reduction in IL-12 production could also contribute to the reduction in IFN-γ producing CD4+ T cells. While this finding might account for loss of stimulatory capacity, it seems unlikely to be the basis for the suppressive functions of RA-DCs since our findings show that RA-DCs have the capacity to directly inhibit T cell expansion driven by anti-CD3/CD28 stimulation. This does not, however, rule out the possibility that RADCs can impact DC APC function and, thereby, T cell proliferation via indirect mechanisms. Additionally, we observed that RA-DCs express high levels of B7 homolog 1 (B7-H1)/ programmed death-ligand 1 (PD-L1) (data not shown), which is a well-established negative regulator of T cells (41, 42). It has been previously reported that DCs cultured in the presence of ATRA can promote the development of Tregs (19) and, furthermore, that ATRA can skew Th1/Th2 development by promoting the expansion of Th2 populations and inhibiting polarization towards a Th1 phenotype (43). Though these mechanisms may be in play, they do not appear to be responsible for the bulk of suppressive function since iNOS appears to account for as much as 90% of RA-DC-mediated suppression, based on results using iNOS deficient bone marrow cells.
Prior studies have shown that the expression of Arg-1 and iNOS are often not coincident but rather subject to competitive counter-regulation by Th1 and Th2 cytokines. Th2 cytokines, including IL-4 and IL-13, are strong inducers of Arg-1 but antagonize the expression of iNOS in response to IFN-γ. Likewise, IFN-γ can interfere with IL-4 and/or IL-13-stimulated Arg-1 expression (44, 45). Moreover, iNOS enzymatic activity may be compromised by co-expression of Arg-1 via restriction of the common substrate L-arginine (46, 47). Other work suggests that the activities of these enzymes are not mutually exclusive since co-expression of Arg-1 and iNOS has been observed in populations of MDSCs (16). Our data demonstrate that ATRA treatment is distinct from cytokine-mediated regulation of these enzymes since expression of both Arg-1 and iNOS was induced and/or sustained in RA-DCs treated with IFN-γ, while Arg-1 expression in DCs, induced via IL-4, was suppressed. Importantly, the findings provide evidence that IFN-γ, presumably derived from DC-stimulated T cells and specifically targeting RA-DCs, is an essential component of the process. The molecular mechanisms through which these often conflicting events are cooperative will be an important issue to clarify in future studies.
We have previously shown that HSCs contribute to local immune regulation through the induction of myeloid cells expressing Arg-1 and iNOS (3). HSCs promoted the development of these suppressive myeloid cells through the production of multiple soluble factors, which could include ATRA. However, since HSCs possess a plethora of suppressive mechanisms it is difficult to specifically examine the contribution of HSC derived ATRA in tolerogenic DC development. Therefore, we added exogenous ATRA to DC cultures and provide evidence that ATRA plays an important role in the regulation of immune responses by promoting the differentiation of DCs with suppressive function. In the resting state, RA signaling in DCs significantly increases production of Arg-1, which contributes to amino acid depletion, activation of the GCN2 pathway, and consequently, T cell inactivation. In inflammatory settings, stimulation by cytokines, such as IFN-γ released by activated T cells, in combination with ATRA results in sustained expression of Arg-1 as well as enhanced expression of iNOS. Thus, although Arg-1 and iNOS contribute to the suppressive capacity of RA-DCs with varying degrees, both play a role in T suppression. These observations provide mechanistic evidence for the potential role of ATRA in liver immunity and strategies for treatment of liver diseases and tolerance induction in transplantation.
ACKNOWLEDGEMENTS
We thank Dr. A. Ghosh for assistance with Griess analysis and Drs. A. Ghodadra and A. Nowacki for statistical guidance.
This work was supported by NIH grants: DK084192 (L.L.), AI090468 (S.Q.), and CA039621 and CA062220 (T.A.H)
Abbreviations
- Arg-1
arginase I
- ATRA
all-trans retinoic acid
- DC
dendritic cell
- GCN2
general control non-depressible 2
- HSC
hepatic stellate cell
- iNOS
inducible nitric oxide synthase
- L-NMMA
NG-monomethyl-L-arginine, monoacetate salt
- MDSC
myeloid derived suppressor cells
- nor-NOHA
Nw-hydroxy nor-L-arginine
- RA-DCs
bone marrow derived DCs cultured with ATRA
- Tregs
regulatory T cells
Footnotes
The authors declare no financial conflicts of interest.
REFERENCES
- 1.Yu M-C, Chen C-H, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 2.Chen C-H, Kuo L-M, Chang Y, Wu W, Goldbach C, Ross MA, Stolz DB, Chen L, Fung JJ, Lu L, Qian S. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44:1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]
- 3.Chou H-S, Hsieh C-C, Yang H-R, Wang L, Arakawa Y, Brown K, Wu Q, Lin F, Peters M, Fung JJ, Lu L, Qian S. Hepatic Stellate Cells Regulate Immune Response via Induction of Myeloid Suppressor Cells. Hepatology. 2011;53:1007–1019. doi: 10.1002/hep.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomhoff R, Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. FASEB J. 1991;5:271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- 5.Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7135–7140. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichikawa S, Mucida D, Tyznik AJ, Kronenberg M, Cheroutre H. Hepatic stellate cells function as regulatory bystanders. J. Immunol. 2011;186:5549–5555. doi: 10.4049/jimmunol.1003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunham RM, Thapa M, Velazquez VM, Elrod EJ, Denning TL, Pulendran B, Grakoui A. Hepatic Stellate Cells Preferentially Induce Foxp3+ Regulatory T Cells by Production of Retinoic Acid. J Immunol. 2013;190:2009–2016. doi: 10.4049/jimmunol.1201937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic Dendritic Cells. Annual Review of Immunology. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 9.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, Ochoa JB, Ochoa AC. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J. Immunol. 2003;171:1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Bingisser RM, Tilbrook PA, Holt PG, Kees UR. Macrophage-Derived Nitric Oxide Regulates T Cell Activation via Reversible Disruption of the Jak3/STAT5 Signaling Pathway. J Immunol. 1998;160:5729–5734. [PubMed] [Google Scholar]
- 14.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid Suppressor Lines Inhibit T Cell Responses by an NO-Dependent Mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 16.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 17.Raber P, Ochoa AC, Rodriguez PC. Metabolism of L-Arginine by Myeloid-Derived Suppressor Cells in Cancer: Mechanisms of T cell suppression and Therapeutic Perspectives. Immunol Invest. 2012;41:614–634. doi: 10.3109/08820139.2012.680634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris SM., Jr. Arginine metabolism: boundaries of our knowledge. J. Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- 19.Chang J, Thangamani S, Kim MH, Ulrich B, Morris SM, Jr, Kim CH. Retinoic acid promotes the development of Arg1-expressing dendritic cells for the regulation of T-cell differentiation. Eur. J. Immunol. 2013;43:967–978. doi: 10.1002/eji.201242772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surace MJ, Li L. Potent suppression of arginase 1 expression in murine macrophages by low dose endotoxin. Am J Clin Exp Immunol. 2013;2:117–123. [PMC free article] [PubMed] [Google Scholar]
- 21.Devaux Y, Grosjean S, Seguin C, David C, Dousset B, Zannad F, Meistelman C, De Talancé N, Mertes P-M, Ungureanu-Longrois D. Retinoic acid and host-pathogen interactions: effects on inducible nitric oxide synthase in vivo. American Journal of Physiology -Endocrinology and Metabolism. 2000;279:E1045–E1053. doi: 10.1152/ajpendo.2000.279.5.E1045. [DOI] [PubMed] [Google Scholar]
- 22.Austenaa LM, Ross AC. Potentiation of interferon-gamma-stimulated nitric oxide production by retinoic acid in RAW 264.7 cells. J. Leukoc. Biol. 2001;70:121–129. [PubMed] [Google Scholar]
- 23.Lu L, McCaslin D, Starzl TE, Thomson AW. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7-1dim, B7-2-) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. 1995;60:1539–1545. doi: 10.1097/00007890-199560120-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turnell DC, Cooper JD. Rapid assay for amino acids in serum or urine by pre-column derivatization and reversed-phase liquid chromatography. Clin. Chem. 1982;28:527–531. [PubMed] [Google Scholar]
- 25.Wada Y, Hisamatsu T, Kamada N, Okamoto S, Hibi T. Retinoic acid contributes to the induction of IL-12-hypoproducing dendritic cells. Inflammatory Bowel Diseases. 2009;15:1548–1556. doi: 10.1002/ibd.20934. [DOI] [PubMed] [Google Scholar]
- 26.Jin C-J, Hong CY, Takei M, Chung S-Y, Park J-S, Pham T-NN, Choi S-J-N, Nam J-H, Chung I-J, Kim H-J, Lee J-J. All-trans retinoic acid inhibits the differentiation, maturation, and function of human monocyte-derived dendritic cells. Leuk. Res. 2010;34:513–520. doi: 10.1016/j.leukres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Gabrilovich DI, Nagaraj S. Myeloid-derived-suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raber P, Ochoa AC, Rodríguez PC. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol. Invest. 2012;41:614–634. doi: 10.3109/08820139.2012.680634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 30.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol. Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 31.Tiegs G, Lohse AW. Immune tolerance: What is unique about the liver. Journal of Autoimmunity. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun C-M, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β– and retinoic acid–dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saurer L, McCullough KC, Summerfield A. In vitro induction of mucosa-type dendritic cells by all-trans retinoic acid. J. Immunol. 2007;179:3504–3514. doi: 10.4049/jimmunol.179.6.3504. [DOI] [PubMed] [Google Scholar]
- 34.Zhu B, Buttrick T, Bassil R, Zhu C, Olah M, Wu C, Xiao S, Orent W, Elyaman W, Khoury SJ. IL-4 and retinoic acid synergistically induce regulatory dendritic cells expressing Aldh1a2. J. Immunol. 2013;191:3139–3151. doi: 10.4049/jimmunol.1300329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu L, Bonham CA, Chambers FG, Watkins SC, Hoffman RA, Simmons RL, Thomson AW. Induction of nitric oxide synthase in mouse dendritic cells by IFN-gamma, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577–3586. [PubMed] [Google Scholar]
- 36.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends in Immunology. 2003;24:301–305. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 37.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 38.Germann T, Gately MK, Schoenhaut DS, Lohoff M, Mattner F, Fischer S, Jin SC, Schmitt E, Rüde E. Interleukin-12/T cell stimulating factor, a cytokine with multiple effects on T helper type 1 (Th1) but not on Th2 cells. Eur. J. Immunol. 1993;23:1762–1770. doi: 10.1002/eji.1830230805. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 40.Kang BY, Chung SW, Kim SH, Kang SN, Choe YK, Kim TS. Retinoid-mediated inhibition of interleukin-12 production in mouse macrophages suppresses Th1 cytokine profile in CD4+ T cells. Br J Pharmacol. 2000;130:581–586. doi: 10.1038/sj.bjp.0703345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu L, Qian S, Hershberger PA, Rudert WA, Lynch DH, Thomson AW. Fas ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. J Immunol. 1997;158:5676–5684. [PubMed] [Google Scholar]
- 42.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 Family Revisited. Annual Review of Immunology. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 43.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The Role of Retinoic Acid in Tolerance and. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J. Immunol. 1998;160:5347–5354. [PubMed] [Google Scholar]
- 45.Munder M, Eichmann K, Morán JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J. Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 46.Chang CI, Liao JC, Kuo L. Arginase modulates nitric oxide production in activated macrophages. Am. J. Physiol. 1998;274:H342–348. doi: 10.1152/ajpheart.1998.274.1.H342. [DOI] [PubMed] [Google Scholar]
- 47.Lee J, Ryu H, Ferrante RJ, Morris SM, Ratan RR. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. PNAS. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]